Abstract
Activation of ADP-sensitive P2Y1 receptors has been proposed as an integral step in the putative “nucleotide axis” regulating coronary blood flow. However, the specific mechanism(s) and overall contribution of P2Y1 receptors to the control of coronary blood flow have not been clearly defined. Using vertically integrative studies in isolated coronary arterioles and open-chest anesthetized dogs, we examined the hypothesis that P2Y1 receptors induce coronary vasodilation via an endothelium-dependent mechanism and contribute to coronary pressure-flow autoregulation and/or ischemic coronary vasodilation. Immunohistochemistry revealed P2Y1 receptor expression in coronary arteriolar endothelial and vascular smooth muscle cells. The ADP analog 2-methylthio-ADP induced arteriolar dilation in vitro and in vivo that was abolished by the selective P2Y1 antagonist MRS-2179 and the nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester. MRS-2179 did not alter baseline coronary flow in vivo but significantly attenuated coronary vasodilation to ATP in vitro and in vivo and the nonhydrolyzable ATP analog ATPγS in vitro. Coronary blood flow responses to alterations in coronary perfusion pressure (40–100 mmHg) or to a brief 15-s coronary artery occlusion were unaffected by MRS-2179. Our data reveal that P2Y1 receptors are functionally expressed in the coronary circulation and that activation produces coronary vasodilation via an endothelium/nitric oxide-dependent mechanism. Although these receptors represent a critical component of purinergic coronary vasodilation, our findings indicate that P2Y1 receptor activation is not required for coronary pressure-flow autoregulation or reactive hyperemia.
Keywords: pressure-flow autoregulation, reactive hyperemia, ischemia, isolated arteriole
the release of purinergic compounds from cells within and around the coronary vasculature has been proposed to be a mechanism responsible for the coupling of coronary blood flow with myocardial metabolism (8, 19, 23). Indeed, the release of ATP from red blood cells and cardiac myocytes is sensitive to cardiac metabolism, as ATP release across the coronary circulation increases in proportion to cardiac work (19, 47). ATP released in this fashion is an initial step in a putative “nucleotide axis,” whereby extracellular ATP and its metabolites modulate coronary vascular resistance via activation of P2 purinoceptors (8). While the components of this axis have been demonstrated to induce coronary vasodilation in vitro and in vivo (18, 22, 41), the functional relevance of coronary P2 receptor subtypes in mediating physiological changes in coronary flow remains unclear.
The P2Y1 receptor is a member of the G protein-coupled family of P2Y receptors, for which ATP and ADP are endogenous ligands (41, 48). In general, the P2Y1 receptor demonstrates greater sensitivity to ADP and differs from the P2Y2 receptor, which is readily activated by triphosphates such as ATP and UTP (41, 48). In the vasculature, both receptors are expressed in endothelial and vascular smooth muscle cells, and their activation induces vasodilation in normal whole vessel preparations (18, 41). However, the relative contribution of endothelium-dependent vs. -independent pathways to ADP-mediated vasodilation varies considerably across circulations (5, 7, 24, 26). In particular, Brayden (5) reported that removal of the endothelium eliminates ADP-mediated vasodilation in middle cerebral arteries but only attenuates this response ∼40% in skeletal muscle arterioles and ∼10% in mesenteric resistance arteries. Previous work in the coronary circulation yielded conflicting results regarding the mechanism(s) of ADP-induced dilation, with some studies supporting primary endothelial dependence (31, 36) and others suggesting little to no involvement of the endothelium (17, 21, 22, 30, 42). These equivocal findings could be related to the use of nonselective and low-potency agonists such as the ATP analog 2-methylthio-ATP, which also activates P2X receptors (17, 33, 35, 37, 46) and subsequent confounding endothelium-dependent responses (25, 26, 51, 52). Thus the specific mechanisms and relative contribution of P2Y1 receptors to the control of coronary blood flow have not been clearly defined.
The purpose of this investigation was to test the hypothesis that P2Y1 receptors induce coronary vasodilation via an endothelium-dependent mechanism and contribute to physiological coronary responses to changes in perfusion pressure (autoregulation) and/or cardiac ischemia (reactive hyperemia). This hypothesis was examined by vertically integrated experiments in isolated, pressurized coronary arterioles in vitro and open-chest anesthetized dogs in vivo. Expression and localization of P2Y1 receptors were also assessed by Western blotting and immunohistochemistry.
METHODS
All protocols were approved by an Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publ. No. 85-23, Revised 1996). Male mongrel dogs (20–30 kg) were sedated with morphine (3 mg/kg sc) and anesthetized with α-chloralose (100 mg/kg iv). After completion of experimental protocols, hearts were fibrillated and excised as recommended by the American Veterinary Medical Association guidelines on euthanasia (June 2007).
Surgical preparation.
The methods for these procedures are described in detail elsewhere (4, 13). Briefly, after induction of anesthesia, intubation, and ventilation, the left femoral artery was catheterized to supply blood to an extracorporeal perfusion system used to perfuse the left anterior descending (LAD) coronary artery. The right femoral vein was also catheterized for injection of supplemental anesthetic, heparin, and sodium bicarbonate as necessary based on periodic arterial blood gas determination. After a left lateral thoracotomy and heparin administration (500 U/kg), a proximal portion of the LAD was isolated and cannulated with a stainless steel cannula attached to the extracorporeal perfusion system. Coronary perfusion pressure was maintained at 100 mmHg throughout experimental protocols, unless otherwise noted, by a servo-controlled roller pump. Blood flow in the coronary perfusion line was measured by an in-line flow transducer (Transonic, Ithaca, NY). Data were collected using Iox acquisition software (Emka Technologies, Falls Church, VA). Hemodynamic parameters were allowed to stabilize for ∼30 min before initiation of experimental protocols.
In vivo experimental protocols.
ATP (1–10 μg·kg−1·min−1) and the selective P2Y1 receptor agonist 2-methyl-ADP trisodium salt (2-MeS-ADP) were infused at a constant rate into the LAD perfusion circuit before and during administration of the selective P2Y1 receptor antagonist MRS-2179 (0.5 mg/min ic). Infusion of MRS-2179 was initiated 5 min prior to agonist infusions. The infusion rate of 2-MeS-ADP was adjusted for each animal based on basal coronary flow and hematocrit to achieve a calculated plasma concentration of 10 μM. In separate animals, coronary flow responses to 2-MeS-ADP were examined after administration of the nitric oxide (NO) synthase (NOS) inhibitor NG-nitro-l-arginine-methyl ester (l-NAME, 150 μg/min ic). Coronary reactive hyperemia was assessed by a 15-s occlusion of the LAD coronary artery before and after administration of MRS-2179 (0.5 mg/min ic). Coronary pressure-flow autoregulation was examined via stepwise reductions in coronary perfusion pressure between 100 and 40 mmHg before and during administration of MRS-2179 (0.5 mg/min ic). All drugs were prepared in 0.9% saline. LAD coronary artery perfusion territory was estimated as previously described by Feigl et al. (20).
Isolated arterioles.
For in vitro studies, separate dogs were euthanized with pentobarbital sodium, and the heart was excised. An apical portion of the left ventricle was immediately removed and placed in ice-cold physiological salt solution (PSS) containing 145 mM NaCl, 4.7 mM KCl, 1.2 mM NaH2PO4, 1.17 mM MgSO4, 2 mM CaCl2, 5 mM glucose, 2 mM pyruvate, 0.02 mM EDTA, 3 mM MOPS, and 1% bovine serum albumin, pH 7.4. The sample was shipped overnight to the University of Missouri for in vitro experiments, similar to previous studies (2, 38). Tissue was used within 24 h from removal of the heart. Subepicardial coronary arterioles (<200 μm) were isolated, cleaned, and secured to glass micropipettes with 11-0 suture, as previously described (2). The preparation was transferred to the stage of an inverted microscope, where arterioles were pressurized to 60 cmH2O and displayed on a video monitor equipped with a calibrated video micrometer for the measurement of intraluminal diameter. Arterioles that were free from leaks were equilibrated for ∼1 h, during which bath temperature was raised to 37°C and the bathing PSS solution was changed every 15 min. Constriction to 80 mM KCl and dilation to 10 mM ACh were determined to verify functional vessels. Arterioles were preconstricted to 30–60% tone with endothelin-1 (ET-1) for examination of dilator responses.
In vitro experimental protocols.
Coronary arteriolar dilation was assessed in response to the selective P2Y1 receptor agonist 2-MeS-ADP (1 nM–100 μM), the nonselective purinergic agonist ATP (1 nM–100 μM), and ATPγS, a nonhydrolyzable ATP analog (1 nM–100 μM). Dilator responses were examined before and after treatment of arterioles with the selective P2Y1 receptor antagonist MRS-2179 (10 μM) for 30 min. The role of NOS-generated NO in 2-MeS-ADP-induced dilation was examined in a separate set of arterioles following 20 min of pretreatment with l-NAME (300 μM). Maximum passive diameter was determined for each vessel at the end of the experiment by replacement of the bath PSS with calcium-free PSS. Time control experiments demonstrated similar dose-dependent dilator responses following repeated administration of each agonist (i.e., no tachyphylaxis; data not shown).
Immunoblotting.
Receptor protein expression and antibody selectivity were also determined by immunoblot analysis, similar to a previous study (2). A section of left circumflex (LCX) coronary artery was prepared by digestion in Laemmli buffer and intermittent heating and agitation. Total sample protein content (in μg/μl) was determined using a NanoOrange protein quantitation kit (Invitrogen). Protein (30 and 50 μg/lane) was separated by 12% SDS-PAGE and transferred to polyvinylidene difluoride membranes. Membranes were blocked in a 5% nonfat milk-Tris-buffered saline-Tween 20 solution and incubated overnight at 4°C with an anti-P2Y1 receptor antibody (catalog no. APR-009, Alomone Labs) alone or after prior incubation of the antibody with the synthetic control peptide, according to the manufacturer's instructions. The primary antibody was prepared in PBS containing 1% bovine serum albumin. Membranes were then incubated for 1 h with secondary antibody conjugated to horseradish peroxidase, and protein expression was detected by enhanced chemiluminescence on a Kodak Image Station.
Immunohistochemistry.
Cellular localization of P2Y1 receptors was determined via immunohistochemical staining of coronary arterioles in dog myocardial sections, similar to a previous study (27). An apical section of myocardium was immersion-fixed in 10% buffered formalin for >24 h and processed through standard paraffin embedding. Myocardial sections (5 μm thick) were cut with an automated microtome, floated onto positively charged slides, and deparaffinized. Slides were then steamed in citrate buffer at pH 6.0 (target retrieval solution S1699, Dako) for 30 min for antigen retrieval and then cooled for 30 min. The slides were washed sequentially with Tris buffer and water after each step in the staining protocol. Sections were incubated with avidin-biotin two-step blocking solution (Vector SP-2001) to inhibit background staining and in 3% hydrogen peroxide to inhibit endogenous peroxidase. Nonserum protein block (catalog no. X909, Dako) was applied to inhibit nonspecific protein binding, and slides were incubated in primary rabbit polyclonal anti-P2Y1 receptor antibody (catalog no. APR-009, Alomone Labs) at 1:800 dilution overnight at 4°C. After appropriate wash steps were completed, the sections were incubated with biotinylated secondary antibody in PBS containing 15 mM sodium azide and peroxidase-labeled streptavidin (LSAB+ kit, peroxidase; catalog no. K0690, Dako). Diaminobenzidine (Dako) was applied for 5 min to allow visualization of primary antibody staining. Sections were counterstained with Mayer's hematoxylin stain for 1 min and dehydrated, and coverslips were applied. Omission of the primary antibody or prior incubation of the primary antibody with the synthetic control peptide according to the manufacturer's instructions resulted in a lack of staining (not shown). Sections were examined and photographed using an Olympus BX61 photomicroscope.
Drugs and solutions.
ATP, ATPγS, l-NAME, and PSS components were purchased from Sigma (St. Louis, MO). 2-MeS-ADP and MRS-2179 were purchased from Tocris (Ellisville, MO). Stock solutions of all agonists and antagonists for in vitro studies were prepared in distilled water, frozen, thawed on experiment days, and diluted to appropriate working concentrations in PSS.
Data analysis.
In vitro arteriolar diameter results were normalized to maximum passive diameter and presented as percent maximal dilation, calculated as follows: [(Dd − Db)/(Dmax − Db) × 100], where Dd is diameter after a drug intervention, Db is baseline preconstricted diameter, and Dmax is maximum passive diameter. EC50 values (pD2 = −log EC50) were determined for individual concentration-response curves of each agonist, and reactive hyperemic volumes were calculated as the area under the curve using Prism software (GraphPad, San Diego, CA). Hyperemic volumes were normalized to the amount of coronary flow debt incurred during coronary occlusion (i.e., repayment-to-debt ratio). The duration of hyperemia was evaluated at the point when coronary flow returned to within 5% of baseline. Statistical analysis was performed using a t-test or one- or two-way ANOVA for repeated measures (when appropriate) with Tukey's post hoc analysis in SigmaStat. P < 0.05 was considered significant. Values are means ± SE.
RESULTS
Coronary P2Y1 protein expression.
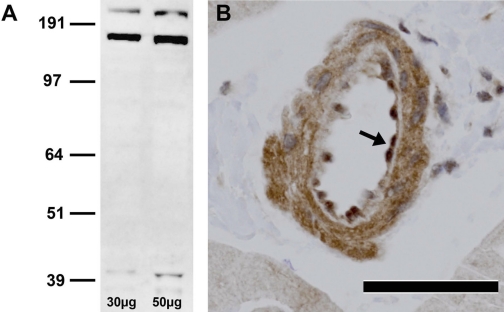
Western blots of canine LCX coronary artery revealed three bands for the P2Y1 receptor (∼42, 168, and 210 kDa; Fig. 1A), similar to findings with the same primary antibody in human and porcine vascular tissue (42, 49). P2Y1 receptor oligomerization has been suggested to account for these multiple bands, such that the band at 168 kDa represents a P2Y1 tetramer (49, 54). Immunohistochemistry revealed abundant localization of P2Y1 receptors to endothelial and vascular smooth muscle cells in canine coronary arterioles (Fig. 1B). Prior incubation of the primary antibody with the synthetic control peptide eliminated all three immunoblot bands and positive staining of arterioles, confirming P2Y1 specificity (not shown). Omission of the primary antibody also eliminated positive staining of arterioles (not shown).
Fig. 1.
Expression of P2Y1 receptors in canine coronary circulation. A: immunoblot demonstrating P2Y1 receptor immunoreactivity in canine left circumflex coronary artery extract; 30 and 50 μg of total protein were added to consecutive lanes. Molecular weight markers are shown at left. B: representative photomicrograph of canine left ventricular tissue immunostained for the P2Y1 receptor. Note strong positive staining (brown) in arteriolar endothelial (arrow) and vascular smooth muscle cells. Magnification ×40. Scale bar, 50 μm.
Mechanism of P2Y1-mediated coronary vasodilation.
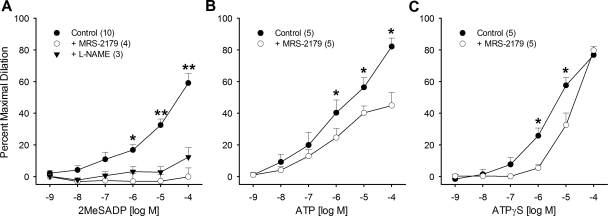
Coronary arterioles with a passive internal diameter of 129 ± 7 μm were used in this study. Twenty-six arterioles from 17 dogs were used (i.e., multiple arterioles were isolated from several dogs, each for a different agonist). ET-1-induced tone averaged 45 ± 3%. All purinergic agonists induced dose-dependent coronary arteriolar dilation with order of potency as follows: ATP ∼ ATPγS > 2-MeS-ADP; pD2 values were 5.2 ± 0.3, 5.1 ± 0.2, and 4.2 ± 0.1 for ATP, ATPγS, and 2-MeS-ADP, respectively (P < 0.05 for 2-MeS-ADP vs. other agonists). Activation of P2Y1 receptors with 2-MeS-ADP induced maximal dilation of 59 ± 6% at 100 μM, and this dilation was abolished by the selective P2Y1 antagonist MRS-2179 (10 μM; Fig. 2A). Treatment of arterioles with MRS-2179 tended to reduce basal ET-1-induced tone (−18 ± 9%, P = 0.055 vs. baseline). Administration of the NOS inhibitor l-NAME (300 μM) abrogated coronary dilation to 2-MeS-ADP to an extent similar to MRS-2179 (Fig. 2A). Inhibition of P2Y1 receptors with MRS-2179 also significantly attenuated dilation to ATP (1 μM–100 μM), reducing maximal dilation from 82 ± 5% to 45 ± 8% (Fig. 2B). To address whether activation of P2Y1 receptors occurs directly by ATP or via its breakdown products, additional vasoreactivity studies were conducted with the nonhydrolyzable ATP analog ATPγS. MRS-2179 shifted the ATPγS concentration-response curve to the right but did not affect the maximal dilatory response (74 ± 6% vs. 80 ± 3%; Fig. 2C), indicating modest direct P2Y1 activation by ATP.
Fig. 2.
Mechanism of P2Y1-mediated coronary arteriolar dilation. A: ADP analog 2-methylthio-ADP (2-MeS-ADP) produced dose-dependent vasodilation under control conditions. This vasodilation was abolished by the P2Y1 antagonist MRS-2179 (10 μM) or the nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME, 300 μM). B: ATP produced dose-dependent vasodilation under control conditions that was attenuated by MRS-2179. C: nonhydrolyzable ATP analog ATPγS produced dose-dependent vasodilation under control conditions that was rightward shifted by MRS-2179. Values are means ± SE of number of samples in parentheses. Where error bars are not shown, they are within the symbol. *P < 0.05 vs. MRS-2179. **P < 0.05 vs. all other groups.
In vivo P2Y1-mediated coronary vasodilation.
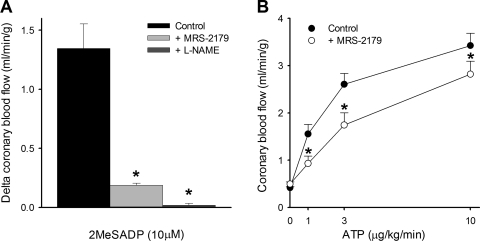
Twelve dogs were dedicated for in vivo blood flow studies (i.e., no arterioles isolated for in vitro work). Infusion of the selective P2Y1 agonist 2-MeS-ADP (plasma concentration = 10 μM) into the LAD coronary artery increased coronary flow by 1.3 ± 0.2 ml·min−1·g−1 (Fig. 3A). In agreement with our findings in isolated arterioles, this increase in coronary flow was markedly attenuated by inhibition of P2Y1 receptors with MRS-2179 (0.5 mg/min ic) or NO production with l-NAME (150 μg/min ic; Fig. 3A). Intracoronary infusion of MRS-2179 or l-NAME did not alter baseline coronary flow (transiently or persistently), mean arterial pressure, or heart rate (Table 1). Infusion of MRS-2179 also had no effect on cardiac work, indicated by no change in rate-pressure product (systolic blood pressure × heart rate), before and after infusion (9,219 ± 974 and 10,328 ± 1,139, respectively, P = 0.11). Inhibition of P2Y1 receptors with MRS-2179 also reduced coronary vasodilation to ATP (1–10 μg·kg−1·min−1 ic) in vivo by ∼20% (Fig. 3B).
Fig. 3.
Coronary blood flow responses to 2-MeS-ADP (A, n = 3) and ATP (B, n = 7). Coronary vasodilation induced by 2-MeS-ADP (plasma concentration ∼10 μM) was markedly attenuated by MRS-2179 (0.5 mg/min ic) or l-NAME (150 μg/min ic). ATP-induced coronary vasodilation was attenuated by MRS-2179. Values are means ± SE. *P < 0.05 vs. control.
Table 1.
Effect of antagonists on baseline hemodynamic variables in anesthetized open-chest dogs
| MAP, mmHg | HR, beats/min | CBF, ml/min | |
|---|---|---|---|
| Baseline | 102 ± 5 | 70 ± 5 | 26 ± 2 |
| +MRS-2179 | 104 ± 4 | 89 ± 11 | 25 ± 2 |
| +l-NAME | 101 ± 9 | 82 ± 7 | 26 ± 5 |
Values are means ± SE. MAP, mean arterial blood pressure; HR, heart rate; CBF, coronary blood flow; l-NAME, NG-nitro-l-arginine methyl ester.
Contribution of P2Y1 receptors to pressure-flow autoregulation and coronary reactive hyperemia.
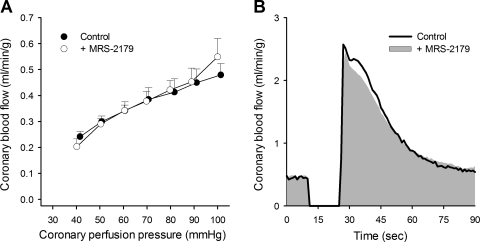
Pressure-flow autoregulation was assessed via servo-control of coronary perfusion pressure over a range of 100 to 40 mmHg. Under untreated-control conditions, coronary blood flow averaged 0.48 ± 0.04 ml·min−1·g−1 at a perfusion pressure of 100 mmHg and 0.34 ± 0.03 ml·min−1·g−1 at a perfusion pressure of 60 mmHg. Inhibition of P2Y1 receptors with MRS-2179 did not significantly affect flow transiently at each pressure step or the relationship between coronary blood flow and coronary perfusion pressure, i.e., autoregulatory capability (Fig. 4A). Coronary zero flow pressure was also unchanged by MRS-2179 (26 ± 1 vs. 28 ± 3 mmHg).
Fig. 4.
Blockade of P2Y1 receptors with MRS-2179 (0.5 mg/min ic) had no significant effect on coronary pressure-flow autoregulation (A, n = 4) or ischemia-induced coronary vasodilation (B, n = 7). Values are means ± SE.
The role of P2Y1 receptors in coronary reactive hyperemia was assessed by the flow response following a 15-s coronary artery occlusion before and during infusion of MRS-2179 (Fig. 4B). Inhibition of P2Y1 receptors did not significantly alter coronary vasodilation in response to this brief episode of cardiac ischemia, as MRS-2179 did not affect the peak hyperemic response (2.6 ± 0.2 vs. 2.5 ± 0.2 ml·min−1·g−1), the duration of hyperemia (52 ± 4 vs. 52 ± 3 s), the volume of repayment (0.7 ± 0.1 vs. 0.8 ± 0.1 ml/g), or the repayment of coronary flow debt (636 ± 33% vs. 737 ± 74%).
DISCUSSION
A nucleotide axis involving the activation of P2 purinergic receptors by ATP and its degradation product ADP has been proposed as a mechanism for the moment-to-moment regulation of coronary blood flow (8, 23). However, a role for endogenous purinergic compounds, notably ATP and ADP, in the control of coronary blood flow has remained an area of substantive debate. Activation of endothelial P2Y1 receptors by ADP may therefore represent an integral step in this cascade. Vasodilation to ADP has been linked to the release of NO, prostacyclin, and endothelium-derived hyperpolarizing factor (5, 18, 41). In this context, the present study was designed to examine the mechanisms underlying P2Y1 receptor-mediated dilation in the coronary circulation, as well as the functional contribution of these receptors to coronary pressure-flow autoregulation and reactive hyperemia. The major findings of this study are as follows: 1) P2Y1 receptors are expressed in coronary microvascular endothelial and vascular smooth muscle cells; 2) ADP-induced coronary arteriolar dilation is predominantly mediated via a P2Y1- and NO-dependent mechanism; and 3) P2Y1 receptors contribute only partially (∼20%) to ATP-induced coronary dilation. Despite these effects, inhibition of P2Y1 receptors did not affect coronary blood flow under baseline-resting conditions, in response to changes in coronary perfusion pressure (autoregulation), or in response to a brief coronary artery occlusion (reactive hyperemia). Taken together, these data indicate that although P2Y1 receptors represent a critical component of purinergic coronary vasodilation, they do not play an essential role in physiological coronary responses to alterations in perfusion pressure or cardiac ischemia.
Mechanisms of P2Y1-mediated coronary vasodilation.
Vascular P2Y1 receptors demonstrate high affinity for ADP relative to P2Y2 receptors, which more readily bind triphosphates such as ATP and UTP (41). Previous studies demonstrated that the mechanism(s) of ADP-induced dilation varies substantially across vascular beds (5, 7, 24, 34). Some, but not all, previous work in the coronary circulation demonstrated that ADP dilation is largely endothelium-dependent, as it is markedly attenuated by endothelial denudation (17, 21, 22, 30, 31, 36, 40, 42). However, the specific endothelial pathways involved remain unclear. Our results extend these findings by demonstrating that coronary arteriolar dilation to the ADP analog 2-MeS-ADP is P2Y1 receptor- and NO-dependent, as it is similarly blocked by MRS-2179 and l-NAME in vivo and in vitro. These findings are consistent with the coronary vasodilator effect of dinucleotide polyphosphates such as Ap5A and Ap6A (46) and indicate that coronary endothelial P2Y1 receptors signal primarily through NO, with potentially minor roles for prostacyclin or endothelium-derived hyperpolarizing factor. ADP and P2Y1 signaling to NOS has been linked to rapid (<1-min) endothelial NOS activation in cultured endothelial cells through phosphorylation of stimulatory serine residues (Ser1179 and Ser635) and concomitant dephosphorylation of the inhibitory Ser116 site (10, 28). This effect is consistent with the time course of ADP-induced vasodilation in the present study and that reported for NO release after P2Y1 stimulation in the mesenteric and placental endothelium (6, 7). The endothelial dependence of ADP-mediated coronary vasodilation is also consistent with other vascular beds such as the cerebral circulation (5, 55) but differs from others, i.e., mesenteric and skeletal muscle (5). Our data are intriguing in this regard, as we found strong P2Y1 immunoreactivity in coronary microvascular smooth muscle, despite little to no vasomotor effect of 2-MeS-ADP in the presence of the NOS inhibitor l-NAME. To our knowledge, this is the first study to demonstrate the specific endothelial pathway involved in ADP-mediated coronary vasodilation in vivo.
The putative role of P2Y1 receptors in the regulation of coronary blood flow in vivo would likely involve the breakdown of endogenous ATP to ADP (19, 23). Therefore, we conducted additional experiments to examine the contribution of P2Y1 receptors to ATP-mediated coronary vasodilation. ATP has long been recognized as a potent coronary vasodilator through direct effects on vascular cells and indirect effects via breakdown products such as ADP (18, 30, 41). Regarding the P2Y1 receptor, our data support both of these mechanisms, as P2Y1 inhibition abolished ADP-mediated coronary vasodilation while modestly attenuating ATP-mediated vasodilation in vitro and in vivo. This finding is consistent with other reports (22, 23) and indicates robust P2Y1 activation by ADP, with a more modest role for P2Y1-mediated NO production in ATP-induced coronary vasodilation (29). Our in vitro data indicate that coronary P2Y1 receptors are also directly activated by ATP, as dilation to the nonhydrolyzable ATP analog ATPγS was attenuated by inhibition of P2Y1 receptors with MRS-2179. Maximal dilation to ATPγS was not altered by P2Y1 blockade, however, indicating that the direct effects of ATP involve other dilator receptors/systems. We propose that this direct effect is related to the activation of P2Y2 receptors, as Gorman et al. (23) recently reported that the highly selective P2Y2 agonist MRS-2768 induces coronary vasodilation in the in situ dog heart.
P2Y1 receptor involvement in coronary blood flow control.
It has been proposed that the local liberation of ATP from various sources (i.e., red blood cells, endothelial cells, and myocytes) is sensitive to myocardial metabolism and, thus, may contribute to the matching of coronary flow to myocardial metabolism (12, 19, 23). This hypothesis is supported by previous reports that coronary venous plasma ATP levels increase in proportion to cardiac work during exercise (19) and that inhibition of purinergic vasodilation (combined blockade with adenosine and P2Y1 receptor antagonists and l-NAME) decreases the balance between coronary blood flow and myocardial metabolism at rest and during exercise in dogs (23). These findings implicate ATP and its breakdown products in the negative-feedback control of the coronary circulation (8, 23, 53). However, the present data importantly indicate that P2Y1 receptors do not significantly contribute to the regulation of basal coronary tone, as MRS-2179 did not significantly reduce resting coronary flow. These data are consistent with previous in vivo studies in dogs and swine (23, 39). Since P2Y1 receptor activation stimulates NO production and numerous earlier studies demonstrated little to no effect of NOS inhibition on baseline coronary flow, the lack of effect of P2Y1 inhibition on baseline coronary flow is not surprising (1, 3, 14, 43, 44).
Coronary blood flow responses to changes in perfusion pressure and following brief coronary artery occlusion have previously been reported to be modulated, at least in part, by NO (9, 11, 13, 43, 50). The production of NO upon endogenous stimulation of coronary P2Y1 receptors may therefore contribute to coronary pressure-flow autoregulation and/or reactive hyperemia. Our data are the first to examine the contribution of P2Y1 receptors to coronary pressure-flow autoregulation and demonstrate that blockade of these receptors does not modulate the autoregulation of coronary flow in response to changes in perfusion pressure. Additionally, inhibition of P2Y1 receptors with MRS-2179 had little to no effect on the coronary reactive hyperemic response. This finding differs from a previous study by Olivecrona et al. (39), who reported a ∼50% decrease in the coronary hyperemic response to 10 min of ischemia following an intracoronary bolus of MRS-2179 in swine. Besides potential species differences, these discrepant findings may be the result of the significant difference in the duration of ischemia: 15 s in the present study and 10 min in the study of Olivecrona et al. Longer durations (5–20 min) of ischemia have been associated with ultrastructural changes in the myocardium, which are absent following acute (<5 min) ischemia (32). Taken together, these data suggest that P2Y1 involvement in coronary ischemic dilation may be dependent on the accumulation of endogenous P2Y1 agonists over time, i.e., modulation of vasodilation in response to prolonged (rather than acute) bouts of myocardial ischemia. This contention is supported by studies that reported increased release of ATP from red blood cells and cardiac myocytes in response to reductions in blood Po2 and tissue Po2 after minutes of ischemia, as opposed to seconds of ischemia (15, 16, 47). The potential time course of P2Y1 activation following cardiac ischemia warrants further examination. In summary, the present data demonstrate that endogenous activation of P2Y1 receptors is not required for the acute regulation of coronary flow. Thus the contribution of NO to coronary autoregulation and reactive hyperemia does not require activation of these receptors (9, 13, 50). Additional studies are needed, however, to address whether the negligible effect of P2Y1 receptor inhibition could be related to the activation of compensatory pathways or other purinergic receptor subtypes such as endothelial P2Y2 or P2Y6, which could mask the effect of P2Y1 receptor inhibition on coronary blood flow regulation. The fact that P2Y1 blockade inhibits ∼20% of in vivo coronary dilation to ATP suggests significant redundancy in the nucleotide axis. Alternatively, recent evidence also suggests a potential role for endothelial P2X receptors in the regulation of vascular tone; however, expression of these receptors in the coronary vasculature has not been determined (25, 26, 51, 52). It is also possible that the production of ADP and ATP increased to overcome the competitive receptor blockade with MRS-2179, as would be predicted if these metabolites are part of a high-gain negative-feedback system. We propose that this hypothesis is unlikely, as Tune et al. (45) demonstrated little change in coronary venous levels of adenosine following inhibition of adenosine receptors.
In conclusion, data from the present study demonstrate that P2Y1 receptors are functionally expressed in the canine coronary microcirculation and mediate vasodilation via a NO-dependent mechanism upon activation by ADP and, to a lesser extent, ATP. Thus, P2Y1 receptors represent a critical component of purinergic coronary vasodilation. However, our findings indicate that endogenous activation of these receptors does not significantly contribute to the autoregulation of coronary blood flow or the vasodilation following a brief coronary artery occlusion. Further work is necessary to delineate the potential involvement of other purinergic receptors. However, such studies are hampered by a lack of potent and selective receptor agonists and antagonists.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-092245 (to J. D. Tune) and HL-52490 (to M. H. Laughlin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.B.B., M.H.L., and J.D.T. are responsible for conception and design of the research; S.B.B., Z.C.B., and J.D.T. performed the experiments; S.B.B., Z.C.B., and J.D.T. analyzed the data; S.B.B., M.H.L., and J.D.T. interpreted the results of the experiments; S.B.B. prepared the figures; S.B.B. drafted the manuscript; S.B.B., Z.C.B., M.H.L., and J.D.T. edited and revised the manuscript; S.B.B., Z.C.B., M.H.L., and J.D.T. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the expert assistance of Pam Thorne, Ann Melloh, Jennifer Casati, Don Connor, Michelle Kurian, and Meredith Kohr.
REFERENCES
- 1. Altman JD, Kinn J, Duncker DJ, Bache RJ. Effect of inhibition of nitric oxide formation on coronary blood flow during exercise in the dog. Cardiovasc Res 28: 119–124, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Bender SB, Tune JD, Borbouse L, Long X, Sturek M, Laughlin MH. Altered mechanism of adenosine-induced coronary arteriolar dilation in early-stage metabolic syndrome. Exp Biol Med (Maywood) 234: 683–692, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bernstein RD, Ochoa FY, Xu X, Forfia P, Shen W, Thompson CI, Hintze TH. Function and production of nitric oxide in the coronary circulation of the conscious dog during exercise. Circ Res 79: 840–848, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Berwick ZC, Payne GA, Lynch B, Dick GM, Sturek M, Tune JD. Contribution of adenosine A2A and A2B receptors to ischemic coronary dilation: role of KV and KATP channels. Microcirculation 17: 600–607, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brayden JE. Hyperpolarization and relaxation of resistance arteries in response to adenosine diphosphate. Distribution and mechanism of action. Circ Res 69: 1415–1420, 1991 [DOI] [PubMed] [Google Scholar]
- 6. Buvinic S, Briones R, Huidobro-Toro JP. P2Y1 and P2Y2 receptors are coupled to the NO/cGMP pathway to vasodilate the rat arterial mesenteric bed. Br J Pharmacol 136: 847–856, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buvinic S, Poblete MI, Donoso MV, Delpiano AM, Briones R, Miranda R, Huidobro-Toro JP. P2Y1 and P2Y2 receptor distribution varies along the human placental vascular tree: role of nucleotides in vascular tone regulation. J Physiol 573: 427–443, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buxton IL, Kaiser RA, Oxhorn BC, Cheek DJ. Evidence supporting the nucleotide axis hypothesis: ATP release and metabolism by coronary endothelium. Am J Physiol Heart Circ Physiol 281: H1657–H1666, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Canty JM, Jr, Smith TP., Jr Modulation of coronary autoregulatory responses by endothelium-derived nitric oxide. Int J Cardiol 50: 207–215, 1995 [DOI] [PubMed] [Google Scholar]
- 10. da Silva CG, Specht A, Wegiel B, Ferran C, Kaczmarek E. Mechanism of purinergic activation of endothelial nitric oxide synthase in endothelial cells. Circulation 119: 871–879, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeFily DV, Chilian WM. Coronary microcirculation: autoregulation and metabolic control. Basic Res Cardiol 90: 112–118, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Deussen A, Brand M, Pexa A, Weichsel J. Metabolic coronary flow regulation—current concepts. Basic Res Cardiol 101: 453–464, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Dick GM, Bratz IN, Borbouse L, Payne GA, Dincer UD, Knudson JD, Rogers PA, Tune JD. Voltage-dependent K+ channels regulate the duration of reactive hyperemia in the canine coronary circulation. Am J Physiol Heart Circ Physiol 294: H2371–H2381, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Duncker DJ, Bache RJ. Inhibition of nitric oxide production aggravates myocardial hypoperfusion during exercise in the presence of a coronary artery stenosis. Circ Res 74: 629–640, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology (Bethesda) 24: 107–116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol 269: H2155–H2161, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Erga KS, Seubert CN, Liang HX, Wu L, Shryock JC, Belardinelli L. Role of A2A-adenosine receptor activation for ATP-mediated coronary vasodilation in guinea-pig isolated heart. Br J Pharmacol 130: 1065–1075, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal 4: 1–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Farias M, 3rd, Gorman MW, Savage MV, Feigl EO. Plasma ATP during exercise: possible role in regulation of coronary blood flow. Am J Physiol Heart Circ Physiol 288: H1586–H1590, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Feigl EO, Neat GW, Huang AH. Interrelations between coronary artery pressure, myocardial metabolism and coronary blood flow. J Mol Cell Cardiol 22: 375–390, 1990 [DOI] [PubMed] [Google Scholar]
- 21. Fleetwood G, Gordon JL. Purinoceptors in the rat heart. Br J Pharmacol 90: 219–227, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gorman MW, Ogimoto K, Savage MV, Jacobson KA, Feigl EO. Nucleotide coronary vasodilation in guinea pig hearts. Am J Physiol Heart Circ Physiol 285: H1040–H1047, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gorman MW, Rooke GA, Savage MV, Jayasekara MP, Jacobson KA, Feigl EO. Adenine nucleotide control of coronary blood flow during exercise. Am J Physiol Heart Circ Physiol 299: H1981–H1989, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guns PJ, Korda A, Crauwels HM, Van Assche T, Robaye B, Boeynaems JM, Bult H. Pharmacological characterization of nucleotide P2Y receptors on endothelial cells of the mouse aorta. Br J Pharmacol 146: 288–295, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harrington LS, Evans RJ, Wray J, Norling L, Swales KE, Vial C, Ali F, Carrier MJ, Mitchell JA. Purinergic 2X1 receptors mediate endothelial dependent vasodilation to ATP. Mol Pharmacol 72: 1132–1136, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Harrington LS, Mitchell JA. Novel role for P2X receptor activation in endothelium-dependent vasodilation. Br J Pharmacol 143: 611–617, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Henderson KK, Turk JR, Rush JW, Laughlin MH. Endothelial function in coronary arterioles from pigs with early-stage coronary disease induced by high-fat, high-cholesterol diet: effect of exercise. J Appl Physiol 97: 1159–1168, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Hess CN, Kou R, Johnson RP, Li GK, Michel T. ADP signaling in vascular endothelial cells: ADP-dependent activation of the endothelial isoform of nitric-oxide synthase requires the expression but not the kinase activity of AMP-activated protein kinase. J Biol Chem 284: 32209–32224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kitakaze M, Node K, Komamura K, Minamino T, Kosaka H, Kuzuya T, Hori M. Intracoronary administration of adenosine triphosphate increases coronary blood flow and attenuates the severity of myocardial ischemic injury in dogs. Cardiovasc Drugs Ther 13: 407–414, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Korchazhkina O, Wright G, Exley C. Intravascular ATP and coronary vasodilation in the isolated working rat heart. Br J Pharmacol 127: 701–708, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuo L, Davis MJ, Cannon MS, Chilian WM. Pathophysiological consequences of atherosclerosis extend into the coronary microcirculation. Restoration of endothelium-dependent responses by l-arginine. Circ Res 70: 465–476, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Lichtig C, Brooks H. Myocardial ultrastructure and function during progressive early ischemia in the intact heart. J Thorac Cardiovasc Surg 70: 309–315, 1975 [PubMed] [Google Scholar]
- 33. Liu SF, McCormack DG, Evans TW, Barnes PJ. Characterization and distribution of P2-purinoceptor subtypes in rat pulmonary vessels. J Pharmacol Exp Ther 251: 1204–1210, 1989 [PubMed] [Google Scholar]
- 34. Malmsjo M, Erlinge D, Hogestatt ED, Zygmunt PM. Endothelial P2Y receptors induce hyperpolarisation of vascular smooth muscle by release of endothelium-derived hyperpolarising factor. Eur J Pharmacol 364: 169–173, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Marrelli SP. Mechanisms of endothelial P2Y1- and P2Y2-mediated vasodilatation involve differential [Ca2+]i responses. Am J Physiol Heart Circ Physiol 281: H1759–H1766, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Miller FJ, Jr, Dellsperger KC, Gutterman DD. Pharmacologic activation of the human coronary microcirculation in vitro: endothelium-dependent dilation and differential responses to acetylcholine. Cardiovasc Res 38: 744–750, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Moccia F, Baruffi S, Spaggiari S, Coltrini D, Berra-Romani R, Signorelli S, Castelli L, Taglietti V, Tanzi F. P2Y1 and P2Y2 receptor-operated Ca2+ signals in primary cultures of cardiac microvascular endothelial cells. Microvasc Res 61: 240–252, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Mokelke EA, Dietz NJ, Eckman DM, Nelson MT, Sturek M. Diabetic dyslipidemia and exercise affect coronary tone and differential regulation of conduit and microvessel K+ current. Am J Physiol Heart Circ Physiol 288: H1233–H1241, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Olivecrona GK, Gotberg M, Harnek J, Wang L, Jacobson KA, Erlinge D. Coronary artery reperfusion: the ADP receptor P2Y1 mediates early reactive hyperemia in vivo in pigs. Purinergic Signal 1: 59–65, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pohl U, Ogilvie A, Lamontagne D, Busse R. Potent effects of Ap3A and Ap4A on coronary resistance and autacoid release of intact rabbit hearts. Am J Physiol Heart Circ Physiol 260: H1692–H1697, 1991 [DOI] [PubMed] [Google Scholar]
- 41. Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998 [PubMed] [Google Scholar]
- 42. Rayment SJ, Ralevic V, Barrett DA, Cordell R, Alexander SP. A novel mechanism of vasoregulation: ADP-induced relaxation of the porcine isolated coronary artery is mediated via adenosine release. FASEB J 21: 577–585, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Solzbach U, Liao J, Eigler NL, Zeiher AM. Effects of inhibition of nitric oxide formation on the regulation of coronary blood flow in anesthetized dogs. Basic Res Cardiol 90: 489–497, 1995 [DOI] [PubMed] [Google Scholar]
- 44. Tune JD, Richmond KN, Gorman MW, Feigl EO. Role of nitric oxide and adenosine in control of coronary blood flow in exercising dogs. Circulation 101: 2942–2948, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Tune JD, Richmond KN, Gorman MW, Olsson RA, Feigl EO. Adenosine is not responsible for local metabolic control of coronary blood flow in dogs during exercise. Am J Physiol Heart Circ Physiol 278: H74–H84, 2000 [DOI] [PubMed] [Google Scholar]
- 46. van der Giet M, Schmidt S, Tolle M, Jankowski J, Schluter H, Zidek W, Tepel M. Effects of dinucleoside polyphosphates on regulation of coronary vascular tone. Eur J Pharmacol 448: 207–213, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Vassort G. Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev 81: 767–806, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Waldo GL, Harden TK. Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol Pharmacol 65: 426–436, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Wang L, Karlsson L, Moses S, Hultgardh-Nilsson A, Andersson M, Borna C, Gudbjartsson T, Jern S, Erlinge D. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol 40: 841–853, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Yamabe H, Okumura K, Ishizaka H, Tsuchiya T, Yasue H. Role of endothelium-derived nitric oxide in myocardial reactive hyperemia. Am J Physiol Heart Circ Physiol 263: H8–H14, 1992 [DOI] [PubMed] [Google Scholar]
- 51. Yamamoto K, Korenaga R, Kamiya A, Qi Z, Sokabe M, Ando J. P2X4 receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am J Physiol Heart Circ Physiol 279: H285–H292, 2000 [DOI] [PubMed] [Google Scholar]
- 52. Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, Fukuda T, Sato T, Sekine K, Kato S, Isshiki M, Fujita T, Kobayashi M, Kawamura K, Masuda H, Kamiya A, Ando J. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med 12: 133–137, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Yang S, Cheek DJ, Westfall DP, Buxton IL. Purinergic axis in cardiac blood vessels. Agonist-mediated release of ATP from cardiac endothelial cells. Circ Res 74: 401–407, 1994 [DOI] [PubMed] [Google Scholar]
- 54. Yoshioka K, Saitoh O, Nakata H. Heteromeric association creates a P2Y-like adenosine receptor. Proc Natl Acad Sci USA 98: 7617–7622, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. You J, Johnson TD, Childres WF, Bryan RM., Jr Endothelial-mediated dilations of rat middle cerebral arteries by ATP and ADP. Am J Physiol Heart Circ Physiol 273: H1472–H1477, 1997 [DOI] [PubMed] [Google Scholar]