Abstract
The effects of cold air inhalation and isometric exercise on coronary blood flow are currently unknown, despite the fact that both cold air and acute exertion trigger angina in clinical populations. In this study, we used transthoracic Doppler echocardiography to measure coronary blood flow velocity (CBV; left anterior descending coronary artery) and myocardial function during cold air inhalation and handgrip exercise. Ten young healthy subjects underwent the following protocols: 5 min of inhaling cold air (cold air protocol), 5 min of inhaling thermoneutral air (sham protocol), 2 min of isometric handgrip at 30% of maximal voluntary contraction (grip protocol), and 5 min of isometric handgrip at 30% maximal voluntary contraction while breathing cold air (cold + grip protocol). Heart rate, blood pressure, inspired air temperature, CBV, myocardial function (tissue Doppler imaging), O2 saturation, and pulmonary function were measured. The rate-pressure product (RPP) was used as an index of myocardial O2 demand, whereas CBV was used as an index of myocardial O2 supply. Compared with the sham protocol, the cold air protocol caused a significantly higher RPP, but there was a significant reduction in CBV. The cold + grip protocol caused a significantly greater increase in RPP compared with the grip protocol (P = 0.045), but the increase in CBV was significantly less (P = 0.039). However, myocardial function was not impaired during the cold + grip protocol relative to the grip protocol alone. Collectively, these data indicate that there is a supply-demand mismatch in the coronary vascular bed when cold ambient air is breathed during acute exertion but myocardial function is preserved, suggesting an adequate redistribution of blood flow.
Keywords: blood pressure, sympathetic nervous system, oxygen consumption, handgrip
exposure to cold temperatures causes many physiological adjustments, most of which help prevent a fall in core body temperature. Specifically, peripheral vasoconstriction and shivering both raise blood pressure and effectively increase the work of the heart. These are known triggers of angina in clinical populations (33). Cardiac death is highest in the winter months, even in people who spend little time outdoors (45, 54). While the effects of lowered skin temperature on cardiovascular function have been extensively studied (10, 29, 59, 60), other mechanisms likely play a role in this process.
Physical perturbation of the oropharynx and larynx elevates blood pressure in clinical settings (18, 34), and laryngeal cold receptors have been identified in animals (38, 51). Previous studies (26, 28, 35, 37) have shown that blood pressure, heart rate, and/or muscle sympathetic nerve activity are increased with cold air breathing in healthy humans. Acute exertion is also a known trigger for adverse cardiac events (3), but the effects of cold air breathing during isometric exertion are currently unknown. A better understanding of these phenomena may allow for specific therapies to lessen winter mortality.
Recent advances in Doppler ultrasound have allowed researchers to measure blood flow through the left anterior descending coronary artery (LAD) (21, 30, 43). This is a unique vascular bed because O2 extraction is near maximal at rest, so an increase in O2 demand (e.g., exercise) is primarily met by an increase in flow (56). Moreover, flow predominantly occurs during diastole, which is relatively shorter at higher heart rates. If coronary blood flow were impaired, it is possible that myocardial function would also be compromised. Tissue Doppler imaging (TDI) measures the speed of the myocardium throughout the cardiac cycle, which provides an index of myocardial function (12).
Thus, we conducted three physiological experiments to test three related yet distinct hypotheses: 1) compared with thermoneutral air, cold air inhalation will cause an increase in myocardial O2 demand [as assessed by the rate-pressure product (RPP)] that will not coincide with an increase in myocardial O2 supply [as assessed by coronary blood velocity (CBV)]; 2) cold air inhalation during 2-min isometric handgrip exercise will cause a greater increase in RPP and a smaller increase in CBV compared with 2-min isometric handgrip exercise alone; and 3) myocardial function (as assessed by TDI) will be similar whether handgrip exercise is performed alone or in combination with cold air inhalation. In this report, we demonstrate that there is an adequate redistribution of blood when isometric exercise is performed during cold air breathing. In young healthy humans, it appears that the body can adapt to the combined stimulus of cold air inhalation and exercise.
METHODS
Subjects.
Ten young adults (5 men and 5 women, age: 25 ± 1 yr, height: 175 ± 3 cm, weight 73 ± 4 kg) volunteered to participate in this study. All were normotensive, nonasthmatic, nonsmokers, not taking any medication, and were in good health as determined by history and physical examination. The protocols used in this study were approved by the Institutional Review Board of the Penn State Milton S. Hershey Medical Center and conformed with the Declaration of Helsinki. Each subject had the purposes and risks explained to them before written informed consent was obtained. Subjects refrained from caffeine, alcohol, and exercise for 24 h before the study and arrived to the laboratory in a semifasted state (i.e., 4–6 h after their last meal).
Experimental protocols.
The present investigation used a within-subjects design. Each participant reported to the laboratory for three visits: familiarization (visit 1), coronary blood flow (visit 2), and myocardial function (visit 3). Visit 1 served two purposes: 1) to confirm that cold air breathing did not cause bronchoconstriction and 2) to familiarize the subjects with the procedures. Specifically, participants underwent two 5-min cold air breathing sessions (cold air protocol), one 5-min room temperature air breathing session (sham protocol), a 2-min handgrip bout at 30% of their maximal voluntary contraction (always the right hand), and a 90-s cold pressor test (hand in ice water). The first cold air breathing session was conducted in the seated upright position so that pulmonary function could be measured before and after. All other measurements were obtained in the left lateral position to facilitate the acquisition of echocardiography data.
During visit 2, participants underwent the procedures shown in Fig. 1. The exercise bouts (grip protocol and cold + grip protocol) were performed in a counterbalanced fashion and were separated by ∼1 h to minimize fatigue (49). The 30% grip exercise was always performed for the last 2 min of the 5-min cold + grip protocol. To provide visual feedback to the subject during exercise, a Stoelting dynamometer was interfaced to a custom device using an analog meter display. The cold air and sham protocols were also undertaken in a counterbalanced manner. The cold pressor test (immersion of the hand into ice water, a well-validated cold stimulus that activates the sympathetic nervous system) was always performed last, and at least 15 min separated each protocol.
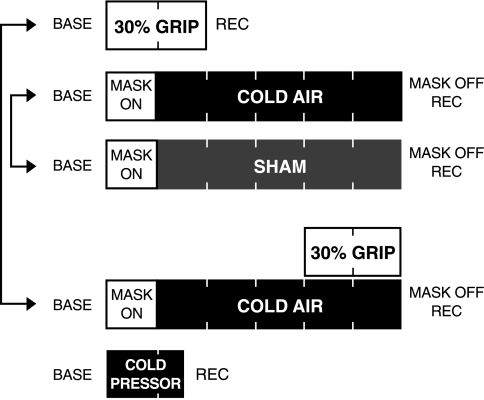
Fig. 1.
Experimental timeline for visit 2. All subjects underwent a 2-min bout of isometric handgrip at 30% maximal voluntary contraction (grip protocol), 5 min of cold air inhalation (cold air protocol), 5 min of thermoneutral air inhalation (sham protocol), and 5 min of cold air inhalation with a 2-min bout of isometric handgrip during minutes 4 and 5 (cold + grip protocol). The arrows signify that connected protocols were counterbalanced. The cold pressor test (hand in 1°C water) was always performed last. Base, baseline.
Based on the RPP and CBV results obtained during visit 2, all 10 subjects returned to undergo visit 3. On this occasion, TDI measurements were obtained during the grip and cold + grip protocols. The protocols were identical to those shown in Fig. 1, and the same mechanical workload was used. Since TDI uses a different transducer (M4S) than CBV (7S) and since the peak exercise responses must be captured within a 10- to 15-s window, we chose to bring subjects back to the laboratory instead of attempting to acquire all data in the same session. Heart rate and blood pressure were acquired continuously during all protocols so that visit-to-visit reliability could be determined (e.g., change in RPP with the grip protocol during visit 2 vs. change in RPP with the grip protocol during visit 3). Within the context of this noninvasive study, we chose RPP as an index of myocardial O2 demand because it can be obtained continuously regardless of the transducer being used.
Cold air breathing apparatus.
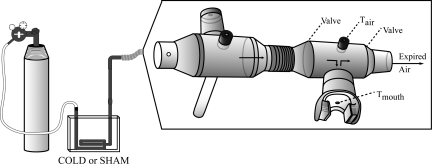
As shown in Fig. 2, a custom system supplied cold air to the mouth of the subject. This system was designed after consulting previous research (11, 22, 26, 36). Specifically, a 150-cm-long closed loop of copper coil (20-mm diameter) was placed into a Styrofoam box (internal dimensions: 15 × 20 × 10 cm) that was either empty (sham protocol) or filled with liquid nitrogen (cold air protocol). The two vertical components of the copper coil exited the lid of the box. Compressed medical air was supplied to the copper coil at a high flow rate (∼25–30 l/min). After exiting the coil, the air passed through a 75-cm vertical copper transport pipe that was connected to a 32-cm piece of flexible plastic tubing, ultimately terminating at a 28-mm Hans Rudolph mouthpiece (model 2700B). Two mouthpieces were connected in series because pilot testing revealed that this extra dead space allowed for a consistent supply of cold air without forcing the first valve open. The mouthpiece more distal to the subject did not contain any valves and had three small holes for sampling. The mouthpiece more proximal to the subject had two one-way valves, a small hole to sample air temperature (Tair) 5 cm in front of the mouth, and a small opening in the rubber mouthpiece itself to sample mouth temperature (Tmouth). Care was taken to match the compressed air flow rate to subject respiration (i.e., air only exited the mouthpiece during physiological expiration and not because the valves were forced open by the compressed air). A noseclip was not worn during the experiment, but the subjects were encouraged to breathe predominantly through the mouth.
Fig. 2.
Cold air breathing apparatus. Compressed medical air was pushed through a copper coil situated in a Styrofoam box containing either liquid nitrogen (cold air protocol) or nothing (sham protocol). Please see the text for specifics.
Measurements.
Blood pressure was recorded on a beat-to-beat basis from a finger via photoplethysmography (Finometer, FMS). These values were corrected to match the blood pressure obtained with an automated sphygmomanometer (Philips SureSigns Vs3). A standard electrocardiogram (Cardiocap/5, General Electric Healthcare) was used to monitor heart rate. Basic pulmonary function (forced expiratory volume in 1 s and forced vital capacity) was determined before and after cold air breathing with a MiniSpirdevice (Medical International Research). Tair was monitored with thermistors (TC-2000, Sable Systems) situated in the breathing apparatus (Fig. 2). O2 saturation was monitored by pulse oximetry on the finger (Philips SureSigns VM4). Thermal sensation of the body (1 = cold to 7 = hot) (20), perception of mouth coldness (0 = neutral to 11 = unbearably cold) (24), perception of mouth pain (0 = no pain to 10 = unbearable pain) (27), and rating of perceived exertion during exercise (6 = very, very light to 20 = maximal exertion) (6) were also quantified.
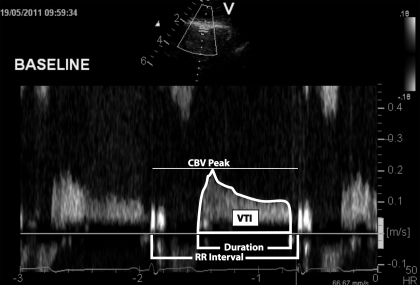
CBV, an index of myocardial O2 supply, was obtained from the apical four-chamber view with a commercially available echocardiography system (Vivid 7, General Electric Healthcare). The specific procedures used in our laboratory have been previously described (21, 44). Briefly, a variable frequency phased-array transducer (7S) was positioned to explore the left ventricular apex. The imaging depth was set at 5 cm, and the focal zones were set at ∼2–3 cm. Color flow mapping was used, and the two-dimensional gain was adjusted to obtain the best blood flow signal of the LAD. Once this was obtained, a 2.0-mm sample volume was placed over the color signal, and CBV was recorded at end expiration. The transducer was held still throughout the protocols, and care was taken to obtain at least one three-beat clip at the end of each minute. The Doppler tracing of the diastolic portion of each cardiac cycle was analyzed using Pro Solv 3.0 to obtain the peak CBV, velocity time integral, flow duration, and relative perfusion time (flow duration/RR interval). Because of the limited spatial resolution and small vessel size, we did not attempt to measure LAD diameter. However, it has been documented that the percent increase in CBV measured via transthoracic Doppler echocardiography is similar to the percent increase in CBV measured by the intracoronary Doppler guidewire (43). Furthermore, intracoronary Doppler guidewire measurements of the percent increase in CBV significantly correlate with the percent increase in coronary blood flow (via angiogram) (50). A representative recording of CBV along with its derived parameters is shown in Fig. 3.
Fig. 3.
Representative coronary blood flow velocity (CBV) recording at baseline. VTI, velocity time integral; HR, heart rate.
TDI was used to measure myocardial tissue velocities during the last 15 s of each minute during the protocols. These velocities were obtained with the transducer in the apical four-chamber view and the sampling volume at the septal mitral annular region. Measurements included the systolic myocardial velocity and early and late diastolic myocardial velocities at the mitral annulus at the end of the respiration. The TDI technique is relatively insensitive to changes in loading condition (1) and is able to detect both systolic and diastolic dysfunction within 5 s after an acute reduction in LAD blood flow (12).
Data collection and statistical analysis.
Blood pressure, heart rate, Tair, and grip force were sampled at 200 Hz by a data-acquisition system (MacLab, AD Instruments). These data were collected on a beat-by-beat basis, and the final 15–20 s of each minute were averaged and reported (i.e., to match when CBV and TDI were obtained). RPP, an index of myocardial O2 demand, was calculated as heart rate × systolic blood pressure.
All statistical analyses were conducted using IBM SPPS 19.0. Normality was confirmed by the Kolmogorov-Smirnov test (i.e., P > 0.05 for all measurements). Data were then screened to ensure that wearing the mouthpiece did not impact any of the measured parameters. To address the first hypothesis, 2 treatment (cold air and sham protocols) × 6 time point (baseline, 1 min, 2 min, 3 min, 4 min, and 5 min) repeated-measures ANOVAs were conducted on the variables of interest followed by paired t-tests when a significant main effect was obtained. To address the second hypothesis, 2 treatment (grip and cold + grip protocols) × 3 time point (baseline, 1 min, and 2 min) repeated-measures ANOVA was conducted for each of the measured parameters. Paired t-tests were also performed between (grip vs. cold + grip protocols) and within (vs. respective baseline) trials. In an effort to remain consistent with previous coronary blood flow studies (43, 50) and compare RPP and CBV in the same units, percent changes (baseline to peak exercise response) were calculated for RPP and CBV. RPP and CBV were also calculated for the cold pressor test. To address the third hypothesis, paired t-tests were performed between (grip vs. cold + grip protocols) and within (vs. respective baseline) trials. Intraclass correlations were conducted to determine test-retest reliability (visit 2 vs. visit 3) of the percent change in RPP during the grip and cold + grip protocols. A consistent change in RPP across visits was essential because all of the data in this report could not be collected at the same time. A Wilcoxon nonparametric test was used to determine differences in the perceptual variables (thermal sensation, pain, and rating of perceived exertion) during the protocols. Data are presented as means ± SE, and P values of <0.05 were considered statistically significant.
RESULTS
All individuals completed the protocols with no reported pain in the mouth. Thermal sensation of the torso ranged from “neutral” to “moderately cool” throughout and was not different between any of the interventions. Tair in the mouth was perceived as being significantly colder during the cold air protocol compared with the sham protocol (z = −2.539, P = 0.011). Perceived exertion was not different between the grip and cold + grip protocols; scores ranged from “somewhat hard” to “hard” at the end of 2 min. Throughout the protocols, O2 saturation was 99 ± 1% and never dropped below 96% in any subject. Pulmonary function was unchanged with the cold air protocol (forced expiratory volume in 1 s from 4.19 ± 0.32 to 4.18 ± 0.30 liters and forced vital capacity from 5.42 ± 0.38 to 5.42 ± 0.38 liters). On average, the 90-s cold pressor test was perceived as “very very cold” and increased RPP by 44 ± 12% and CBV by 57 ± 18%. The mouthpiece also had no effect on hemodynamics or CBV parameters at baseline. Table 1 shows the heart rate and blood pressure responses to the cold air, sham, grip, and cold + grip protocols.
Table 1.
Time course of hemodynamic responses during visit 2
| Baseline | 1 min | 2 min | 3 min | 4 min | 5 min | |
|---|---|---|---|---|---|---|
| Systolic blood pressure, mmHg | ||||||
| Sham protocol | 100 ± 2 | 98 ± 3 | 99 ± 2 | 99 ± 3 | 99 ± 3 | 99 ± 3 |
| Cold air protocol | 100 ± 2 | 99 ± 2 | 99 ± 3 | 99 ± 3 | 100 ± 3 | 102 ± 2 |
| Cold + grip protocol | 99 ± 2 | 98 ± 1 | 100 ± 3 | 102 ± 2 | 111 ± 4* | 128 ± 5* |
| Grip protocol | 101 ± 2 | 112 ± 3* | 128 ± 4* | |||
| Diastolic blood pressure, mmHg | ||||||
| Sham protocol | 59 ± 3 | 58 ± 3 | 60 ± 3 | 60 ± 3 | 59 ± 3 | 60 ± 3 |
| Cold air protocol | 56 ± 2 | 56 ± 2 | 57 ± 2 | 57 ± 2 | 56 ± 2 | 57 ± 2 |
| Cold + grip protocol | 56 ± 2 | 55 ± 2 | 56 ± 2 | 56 ± 2 | 64 ± 2* | 76 ± 3* |
| Grip protocol | 57 ± 3 | 66 ± 3* | 77 ± 4* | |||
| Mean blood pressure, mmHg | ||||||
| Sham protocol | 73 ± 2 | 72 ± 2 | 73 ± 2 | 73 ± 2 | 73 ± 2 | 73 ± 3 |
| Cold air protocol | 72 ± 2 | 73 ± 2 | 72 ± 2 | 71 ± 2 | 73 ± 2 | 72 ± 2 |
| Cold + grip protocol | 72 ± 2 | 71 ± 1 | 73 ± 2 | 72 ± 2 | 82 ± 2* | 97 ± 3* |
| Grip protocol | 72 ± 3 | 82 ± 3* | 97 ± 4* | |||
| Heart rate, beats/min | ||||||
| Sham protocol | 57 ± 2 | 56 ± 2 | 58 ± 3 | 57 ± 3 | 56 ± 3 | 58 ± 2 |
| Cold air protocol | 58 ± 2 | 63 ± 2* | 62 ± 3 | 62 ± 2* | 61 ± 3* | 61 ± 3 |
| Cold + grip protocol | 58 ± 3 | 64 ± 3* | 65 ± 2* | 66 ± 3* | 74 ± 3*† | 77 ± 3* |
| Grip protocol | 60 ± 3 | 68 ± 4* | 73 ± 4* |
Values are means ± SE; n = 10 subjects. Hemodynamic responses to cold air inhalation (cold air protocol), thermoneutral air inhalation (sham protocol), 2-min handgrip exercise at 30% maximal voluntary contraction (grip protocol), and 2-min handgrip exercise at 30% maximal voluntary contraction during cold air inhalation (cold + grip protocol) are shown.
Significantly different from the sham protocol;
significantly different from the grip protocol. Note that the grip protocol was 2 min in duration but is displayed at 4 and 5 min to more easily compare with the cold + grip protocol.
Hypothesis 1: effect of inspired air temperature on RPP and CBV.
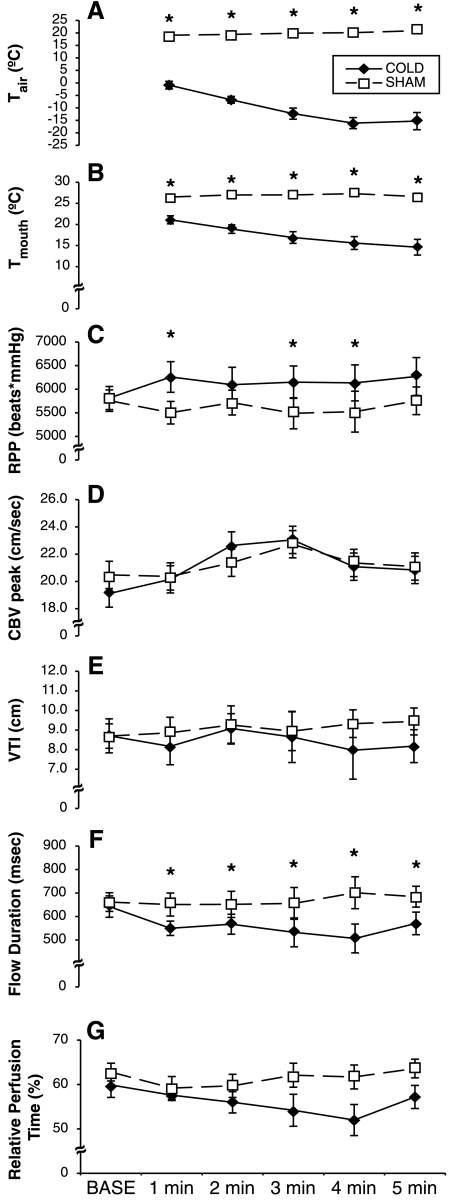
As shown in Fig. 4, both Tair and Tmouth were significantly different between the cold air and sham protocols at all time points (P < 0.001). RPP revealed a main effect for treatment (P = 0.012) and a treatment × time interaction (P = 0.010). Post hoc analyses revealed that the cold air protocol caused a significantly higher RPP at 1 min (P = 0.018), 3 min (P = 0.005), and 4 min (P = 0.013). Peak CBV showed a main effect for time (P = 0.023), but no specific time points were significantly different between the cold air and sham protocols. Flow duration revealed a treatment × time interaction (P = 0.010), and subsequent analyses showed that flow duration was shorter with the cold air protocol at 1 min (P = 0.039), 2 min (P = 0.032), 3 min (P = 0.026), 4 min (P = 0.001), and 5 min (P = 0.034). Relative perfusion time and the velocity time integral did not demonstrate any main or interaction effects. Taken together, the data shown in Fig. 4 demonstrate that myocardial O2 demand was significantly greater with the cold air protocol, but there was a significant reduction in myocardial O2 supply.
Fig. 4.
Effect of the cold air and sham protocols on air temperature (Tair; A), mouth temperature (Tmouth; B), rate-pressure product (RPP; C), peak CBV (D), VTI (E), flow duration (F), and relative perfusion time (G). *Significantly different between conditions (P < 0.05).
Hypothesis 2: effect of inspired air temperature on RPP and CBV during isometric handgrip.
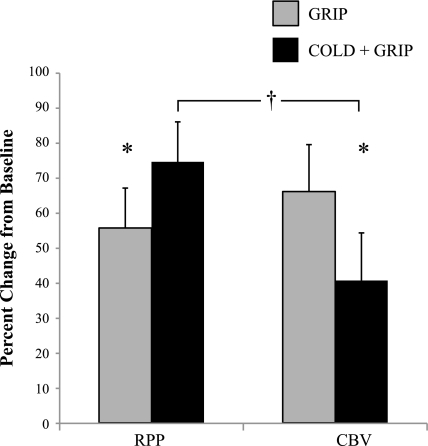
For RPP, 2 treatment (grip and cold + grip protocols) × 3 time point repeated-measures ANOVA (baseline, 1 min, and 2 min) demonstrated a main effect for time (P < 0.001) and a treatment × time interaction (P = 0.031). As shown in Table 2, RPP increased during exercise regardless of treatment. In a similar fashion, flow duration revealed a main effect for time (P = 0.001) such that flow duration decreased during exercise regardless of treatment. However, none of the other CBV parameters showed a main effect for time or treatment or a treatment × time interaction. As shown in Fig. 5, 2-min isometric handgrip exercise during cold air inhalation caused a greater increase in RPP than 2-min isometric handgrip exercise alone (P = 0.045). Based on this finding, a post priori one-tailed t-test revealed that the increase in peak CBV was lower during the cold + grip protocol compared with the grip protocol (P = 0.039). Of note, 8 of the 10 subjects had a greater increase in RPP during the cold + grip protocol, and 8 of the 10 subjects had an attenuated increase in peak CBV during the cold + grip protocol. When comparing the relative changes in RPP and peak CBV during the cold + grip protocol, we observed a much greater rise in RPP than peak CBV (P = 0.024). Force output was not different between the grip (8.53 ± 0.73 kg) and cold + grip (8.52 ± 0.71 kg) protocols. Tair and Tmouth during the cold + grip protocol were qualitatively similar to cold air protocol (data not shown).
Table 2.
Coronary blood flow parameters during isometric handgrip exercise
| Baseline | 1 min | 2 min | |
|---|---|---|---|
| Rate-pressure product, beats · min−1 · mmHg | |||
| Grip protocol | 6,085 ± 363 | 7,672 ± 631* | 9,405 ± 722* |
| Cold + grip protocol | 5,730 ± 286 | 8,237 ± 502* | 9,910 ± 683* |
| Peak coronary blood flow velocity, cm/s | |||
| Grip protocol | 18.3 ± 0.7 | 26.4 ± 1.8* | 30.8 ± 2.8* |
| Cold + grip protocol | 18.7 ± 0.8 | 28.4 ± 3.0* | 26.5 ± 2.6* |
| Velocity time integral, cm | |||
| Grip protocol | 8.1 ± 0.8 | 9.4 ± 1.2 | 9.3 ± 1.0 |
| Cold + grip protocol | 8.7 ± 0.5 | 9.0 ± 0.8 | 8.0 ± 0.6 |
| Flow duration, ms | |||
| Grip protocol | 629 ± 58 | 518 ± 47* | 451 ± 50* |
| Cold + grip protocol | 687 ± 57 | 475 ± 30* | 429 ± 27* |
| Relative perfusion time, % | |||
| Grip protocol | 59 ± 3 | 57 ± 3 | 53 ± 4 |
| Cold + grip protocol | 62 ± 3 | 56 ± 2* | 53 ± 3* |
Values are means ± SE; n = 10 subjects. Coronary blood flow parameters during the grip protocol and cold + grip protocols are shown.
Significantly different from baseline.
Fig. 5.
Effect of cold air inhalation during exercise (cold + grip protocol) on RPP and CBV compared with exercise during thermoneutal air breathing (grip protocol).*Significantly different between conditions (P < 0.05); †significantly different between RPP and CBV during the cold + grip protocol.
Hypothesis 3: effect of inspired Tair on myocardial function during isometric handgrip.
As shown in Table 3, cold air inhalation by itself (i.e., first 3 min of the cold + grip protocol) had no significant effect on TDI indexes of myocardial function. By itself, the grip protocol did not affect systolic myocardial velocity but significantly increased both early (P = 0.008 at 2 min) and late (P = 0.001 at 1 min and P = 0.001 at 2 min) diastolic velocities. Systolic myocardial velocity was significantly greater at the first minute of grip during the cold + grip trial (P = 0.044) compared with the grip trial. Similarly, early diastolic velocity was significantly greater during the second minute of grip during the cold + grip trial (P = 0.042). Taken together, these data indicate that myocardial function was not impaired during the cold + grip trial; in fact, some parameters were even improved relative to the grip trial alone.
Table 3.
Time course of myocardial function during visit 3
| Baseline | 1 min | 2 min | 3 min | 4 min | 5 min | |
|---|---|---|---|---|---|---|
| Systolic myocardial velocity, cm/s | ||||||
| Grip protocol | 8.5 ± 0.4 | 8.3 ± 0.3 | 8.3 ± 0.2 | |||
| Cold + grip protocol | 8.9 ± 0.3 | 8.8 ± 0.3 | 9.1 ± 0.3 | 8.7 ± 0.2 | 8.7 ± 0.2† | 8.5 ± 0.2 |
| Early diastolic myocardial velocity, cm/s | ||||||
| Grip protocol | 13.7 ± 0.4 | 13.3 ± 0.4 | 12.1 ± 0.5* | |||
| Cold + grip protocol | 13.5 ± 0.4 | 14.7 ± 0.5 | 14.3 ± 0.6 | 14.2 ± 0.5 | 14.0 ± 0.5 | 13.5 ± 0.5† |
| Late diastolic myocardial velocity, cm/s | ||||||
| Grip protocol | 7.1 ± 0.4 | 8.4 ± 0.5* | 8.9 ± 0.7* | |||
| Cold + grip protocol | 7.4 ± 0.3 | 7.5 ± 0.4 | 7.8 ± 0.3 | 7.8 ± 0.3 | 8.4 ± 0.5* | 9.2 ± 0.6* |
Values are means ± SE; n = 9 subjects. Tissue Doppler parameters during the grip and cold + grip protocols are shown.
Significantly different from baseline;
significantly different from the grip protocol. Note the grip protocol was 2 min in duration but is displayed at 4 and 5 min to more easily compare with the cold + grip protocol.
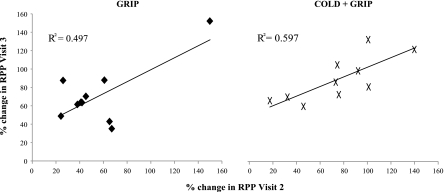
As shown in Fig. 6, the percent change in RPP was highly reproducible from day to day (i.e., high test-retest reliability) for both grip (Cronbach's α = 0.757) and cold + grip (Cronbach's α = 0.813) protocols. An analysis of the raw change in RPP demonstrated a similar effect for both grip (Cronbach's α = 0.757) and cold + grip (Cronbach's α = 0.813) protocols. Thus, individuals who had a large change in RPP on visit 2 had a large change in RPP on visit 3.
Fig. 6.
Test-retest reliability of the change in RPP during the grip (left) and cold + grip (right) protocols on visit 2 and visit 3.
DISCUSSION
These experiments were undertaken to establish the time course and magnitude of physiological responses that occur when healthy young people exercise while breathing cold air. There are three main findings that help inform this clinically relevant scenario. First, despite an increase in myocardial O2 demand (i.e., RPP) with the cold air protocol, myocardial O2 supply (i.e., CBV) was actually impaired. Second, breathing cold air during 2 min of isometric handgrip caused a greater increase in RPP but a smaller increase in peak CBV compared with handgrip alone. Third, despite this apparent supply-demand mismatch in the LAD during the cold + grip protocol, myocardial function was unchanged, suggesting that young healthy humans can adequately redistribute blood to the subendocardium during the combined stimulus of cold air inhalation and handgrip exercise.
Environmental factors such as temperature, smoke, and pollution can trigger acute cardiac events. Epidemiological studies (5, 16a, 40, 55) have shown that cardiovascular mortality peaks in winter. Human cold physiology studies, on the other hand, have not shown consistent results; this is likely attributed to the mode, duration, and intensity of body cooling. Whole body cold exposure evokes peripheral vasoconstriction and increases in central blood volume, presumably to increase the body's insulative capacity and prevent a reduction in core temperature. In the supine posture, skin cooling via a water-perfused suit (i.e., nonshivering, nonnoxious cold stimuli) causes an increase in central venous pressure (10), an increase in pulmonary capillary wedge pressure (58), and a slight but nonsignificant increase in myocardial O2 demand (59) in young healthy adults. Although systolic blood pressure has been shown to increase with skin cooling (15, 29, 58, 60), the concomitant decrease in heart rate minimizes the effects of cooling on RPP. Similarly, whole body exposure to 5°C air has been shown to increase systolic blood pressure, lower heart rate, and increase stroke volume in both supine (48) and seated volunteers (46, 57), but these studies involved mild shivering, which makes a direct comparison with the present study challenging.
While the effects of skin cooling on cardiovascular function have been the focus of much research, the specific effects of cold air inhalation are less clear. This is practically relevant since many outdoor winter activities are relatively short in duration (e.g., walking to the mailbox or carrying groceries into the home) and likely do not reduce skin temperature. During these brief cold exposures, it is possible that breathing cold air alone could provoke symptoms of chest pain. With regard to myocardial O2 demand, the present study suggests that heart rate plays a greater role than systolic blood pressure during the cold air protocol. This is in contrast to a report by Heindl et al. (28), where 5 min of cold air inhalation (−25°C) caused a significant increase in systolic blood pressure but not heart rate in young healthy men. The discrepancy between studies is difficult to explain, but we have confidence in our results because 1) we included a room temperature time control (sham protocol) to account for any intrinsic differences in hemodynamics due to the breathing apparatus itself, 2) our subjects underwent two cold air breathing trials on a prior familiarization day to minimize anxiety, 3) both O2 saturation and pulmonary function were unchanged with the cold air protocol in the present study, suggesting that this phenomenon is localized to the upper airway. Consistent with our data, RPP has been shown to be elevated in patients with cold intolerance during −35°C air breathing (35) as well as in healthy male volunteers during −16°C air breathing (37). Several indexes of coronary blood flow were impaired in the present study during the cold air protocol. In a previous report (26), both RPP and coronary blood flow (catheter in coronary sinus) increased during −20°C air breathing in older patients with exertional angina.
Vigorous exercise is also a risk factor for adverse cardiac events (3). Shoveling snow includes both isometric and isotonic components and causes a rapid increase in heart rate, blood pressure, and O2 consumption (14, 19, 52, 53). Other cold weather activities, such as skiing and deer hunting, similarly increase myocardial O2 demand (4, 8, 25). Within 1–7 days after a heavy snowstorm, the prevalence of myocardial infarction often doubles (9, 23, 32). These acute coronary patients commonly present to the emergency room normothermic but with chest pain and diaphoresis. The increase in winter cardiac events, termed “snow shoveler's ST elevation,” suggests that thermoregulatory reflexes (i.e., core and/or skin temperature reduction) are not the sole factors that trigger adverse events in the winter. We have shown that breathing cold air potentiates the RPP response to a 2-min bout of isometric handgrip without a concomitant increase in peak CBV (Fig. 5). Since O2 extraction in the coronary bed is near maximal at rest, an increase in demand (i.e., RPP) must be met by an increase in supply (CBV). The greater increase in RPP during the cold + grip protocol is not surprising since both the cold air and grip protocols each individually increase this index of myocardial O2 consumption (Table 1). Based on the augmented RPP response to the cold + grip protocol, we would have anticipated a greater increase in peak CBV. Instead, we showed that the percent increase in peak CBV during the cold + grip protocol is significantly less than the peak CBV response to the grip protocol alone. Furthermore, when RPP and peak CBV were compared during the cold + grip protocol, the percent change in peak CBV was significantly impaired. It appears that heart rate, and not systolic blood pressure, is causing the greater rise in RPP. Higher heart rates would shorten the relative perfusion time for CBV. In the present study, relative perfusion time was not significantly different between treatments (cold vs. sham protocols or grip vs. cold + grip protocols), but it is apparent that exercise per se reduces this parameter. Future experiments could investigate the relationship between heart rate and CBV by raising (e.g., atropine) or lowering (e.g., propranolol) heart rates at baseline.
It is interesting to note that animal studies have demonstrated that α-adrenergic coronary vasoconstriction can serve to redistribute blood to the subendocardium during exercise (31). This is particularly important with high systolic blood pressures and/or high heart rates. With high systolic pressures, endocardium compressive faces are greater than those seen in the epicardium and high heart rates lead to a reduction in the percentage of time that the left ventricle spends in diastole (17).
The present TDI data suggest that myocardial function was similar during the grip and cold + grip protocols, even though peak CBV during the cold + grip protocol was not as great as CBV during the grip protocol alone. These findings could be due to a sympathetically mediated redistribution of flow during the cold + grip protocol. However, our data must be viewed cautiously since it is also possible that our findings are due to relative sensitivities/differences in the Doppler flow velocity and tissue Doppler indexes, respectively. To effectively address these issues, new paradigms and approaches aimed at measuring both flow and function during higher levels of exertion will be needed.
Previous studies have shown that patients with stable angina have an earlier onset of chest pain and a reduction in exercise capacity during treadmill (13) and cycling (47) exercise during a cold air protocol. These studies suggest that the cold air protocol might trigger a sympathetic reflex that acts directly on the myocardial resistance vessels, thereby causing a supply-demand mismatch. While there is no direct evidence to confirm this, other studies using exercise in conjunction with whole body cold exposure have reported similar findings in patients with coronary artery disease. Specifically, submaximal exercise performance (7), time to ischemia (33, 41), and O2 demand (7, 16, 33) were higher when exercise was performed in a cold environmental chamber. To our knowledge, the present study is the first to demonstrate that the cold + grip protocol causes an augmented RPP response and an attenuated CBV response in young healthy subjects.
Consistent with a previous study (44) in young healthy humans, the cold pressor test increased both RPP and CBV. An interesting note is that the water temperature for this 90-s protocol was 1 ± 1°C and was perceived as “very, very cold,” whereas the 5-min cold air protocol (temperature from −10 to −20°C) was perceived as “cool” or “moderately cool.” This is not surprising since Tmouth in our study was the product of both inspired (very cold) and expired (warm) air. Moreover, the cold pressor test at this temperature induces a moderate amount of pain, which has been shown to contribute to the increase in RPP (42). As expected, this local cold stimulus to the hand initiated a sympathetic reflex whereby the peak CBV increased to meet the increase in RPP.
Coronary blood flow is controlled by the sympathetic nervous system as well as local vasodilator mechanisms (31). Previous work from our laboratory has shown that 15- to 20-s bouts of isometric handgrip, a preferential activation of central command and the muscle mechanoreflex, cause an increase in coronary vascular resistance (i.e., vasoconstriction) (44). Although the present study does not provide evidence for vasoconstriction during a 2-min bout of the grip protocol (i.e., when the metaboreflex was also activated), a sympathetic reflex originating in the upper airway could explain the impaired increase in peak CBV with the cold + grip protocol. Future studies could block the afferent and efferent arms of this reflex to determine the underlying mechanisms.
Limitations.
Echocardiography-derived measurements of coronary blood flow and myocardial function cannot be measured simultaneously, and they also are unable to quantify mechanical or metabolic adaptations in the myocardium. In this study, we chose to study people on two separate days (visit 2 and visit 3) because the peak exercise responses occur within a 15- to 20-s window and switching transducers would have introduced measurements error. However, the changes in blood pressure and heart rate (i.e., RPP) to the grip and cold + grip protocols were similar across visits (Fig. 6). We chose a 2-min bout of handgrip exercise in the left lateral position because it best allows for the measurement of CBV. Furthermore, isometric gripping and carrying heavy objects are common in everyday life. Future studies could investigate fatiguing grip exercise or dynamic whole body exercise to determine if an expected increase in breathing depth and frequency might cool lower portions of the respiratory tract (39) and further affect CBV. It is well known that Tmouth decreases with inspiration and increases with expiration, so an approximation of respiratory frequency can be made from our data (not shown). However, direct measurements of expired air were not obtained because the turbine and sampling hoses froze during pilot testing.
Summary.
In conclusion, this echocardiographic study in healthy young humans provides three novel findings that lay the foundation for future studies in clinical populations. First, the cold air protocol caused an increase in RPP compared with the sham protocol, but CBV was actually impaired under these conditions. Second, the cold + grip protocol caused a greater increase in RPP and a smaller increase in CBV compared with the grip protocol alone. Third, myocardial function was not impaired during the cold + grip protocol relative to the grip protocol alone, suggesting that a reduction in CBV in the LAD is an appropriate response to redistribute blood to the subendocardium. These data add to current knowledge, although further investigation is required to determine underlying mechanisms.
GRANTS
This work was supported by National Institutes of Health Grants P01-HL-096570 (to L. I. Sinoway), M01-RR-010732, C06-RR-016499, and UL RR033184 as well as a Wilderness Medical Society Research in Training Grant (to M. D. Muller).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.D.M., Z.G., R.C.D., M.D.H., U.A.L., and L.I.S. conception and design of research; M.D.M., Z.G., R.C.D., and M.D.H. performed experiments; M.D.M., Z.G., U.A.L., and L.I.S. analyzed data; M.D.M., Z.G., R.C.D., M.D.H., U.A.L., and L.I.S. interpreted results of experiments; M.D.M. prepared figures; M.D.M., R.C.D., and U.A.L. drafted manuscript; M.D.M., Z.G., R.C.D., M.D.H., U.A.L., and L.I.S. edited and revised manuscript; M.D.M., Z.G., R.C.D., M.D.H., U.A.L., and L.I.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Cheryl Blaha and Jessica Mast for expert study coordination and Anne Muller for preparing the graphics for this study. The authors also appreciate the administrative help of Kris Gray and Jen Stoner.
REFERENCES
- 1. Abali G, Tokgozoglu L, Ozcebe OI, Aytemir K, Nazli N. Which Doppler parameters are load independent? A study in normal volunteers after blood donation. J Am Soc Echocardiogr 18: 1260–1265, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med 343: 1355–1361, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Astrand PO, Saltin B. Maximal oxygen uptake and heart rate in various types of muscular activity. J Appl Physiol 16: 977–981, 1961 [DOI] [PubMed] [Google Scholar]
- 5. Bhaskaran K, Hajat S, Haines A, Herrett E, Wilkinson P, Smeeth L. Short term effects of temperature on risk of myocardial infarction in England and Wales: time series regression analysis of the Myocardial Ischaemia National Audit Project (MINAP) registry. BMJ 341: c3823, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982 [PubMed] [Google Scholar]
- 7. Brown CF, Oldridge NB. Exercise-induced angina in the cold. Med Sci Sports Exerc 17: 607–612, 1985 [PubMed] [Google Scholar]
- 8. Burtscher M, Pachinger O, Mittleman MA, Ulmer H. Prior myocardial infarction is the major risk factor associated with sudden cardiac death during downhill skiing. Int J Sports Med 21: 613–615, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Chowdhury PS, Franklin BA, Boura JA, Dragovic LJ, Kanluen S, Spitz W, Hodak J, O'Neill WW. Sudden cardiac death after manual or automated snow removal. Am J Cardiol 92: 833–835, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Cui J, Durand S, Levine BD, Crandall CG. Effect of skin surface cooling on central venous pressure during orthostatic challenge. Am J Physiol Heart Circ Physiol 289: H2429–H2433, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Deal EC, Jr, McFadden ER, Jr, Ingram RH, Jr, Jaeger JJ. Effects of atropine on potentiation of exercise-induced bronchospasm by cold air. J Appl Physiol 45: 238–243, 1978 [DOI] [PubMed] [Google Scholar]
- 12. Derumeaux G, Ovize M, Loufoua J, Andre-Fouet X, Minaire Y, Cribier A, Letac B. Doppler tissue imaging quantitates regional wall motion during myocardial ischemia and reperfusion. Circulation 97: 1970–1977, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Dodds PA, Bellamy CM, Muirhead RA, Perry RA. Vasoconstrictor peptides and cold intolerance in patients with stable angina pectoris. Br Heart J 73: 25–31, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dougherty SM, Sheldahl LM, Wilke NA, Levandoski SG, Hoffman MD, Tristani FE. Physiologic responses to shoveling and thermal stress in men with cardiac disease. Med Sci Sports Exerc 25: 790–795, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Durand S, Cui J, Williams KD, Crandall CG. Skin surface cooling improves orthostatic tolerance in normothermic individuals. Am J Physiol Regul Integr Comp Physiol 286: R199–R205, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Epstein SE, Stampfer M, Beiser GD, Goldstein RE, Braunwald E. Effects of a reduction in environmental temperature on the circulatory response to exercise in man. Implications concerning angina pectoris. N Engl J Med 280: 7–11, 1969 [DOI] [PubMed] [Google Scholar]
- 16a. The Eurowinter Group Cold exposure and winter mortality from ischaemic heart disease, cerebrovascular disease, respiratory disease, and all causes in warm and cold regions of Europe. The Eurowinter Group. Lancet 349: 1341–1346, 1997 [PubMed] [Google Scholar]
- 17. Feigl EO. The paradox of adrenergic coronary vasoconstriction. Circulation 76: 737–745, 1987 [DOI] [PubMed] [Google Scholar]
- 18. Forbes AM, Dally FG. Acute hypertension during induction of anaesthesia and endotracheal intubation in normotensive man. Br J Anaesth 42: 618–624, 1970 [DOI] [PubMed] [Google Scholar]
- 19. Franklin BA, Hogan P, Bonzheim K, Bakalyar D, Terrien E, Gordon S, Timmis GC. Cardiac demands of heavy snow shoveling. JAMA 273: 880–882, 1995 [PubMed] [Google Scholar]
- 20. Gagge AP, Stolwijk JA, Saltin B. Comfort and thermal sensations and associated physiological responses during exercise at various ambient temperatures. Environ Res 2: 209–229, 1969 [DOI] [PubMed] [Google Scholar]
- 21. Gao Z, Momen A, Novick M, Williams R, Cyran S, Blaha C, Mast J, Spilk S, Leuenberger U, Sinoway L. Weight loss attenuates oxidative stress related-coronary vasoconstriction in obese adolescents (Abstract). FASEB J 23: 1032.3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Geladas N, Banister EW. Effect of cold air inhalation on core temperature in exercising subjects under heat stress. J Appl Physiol 64: 2381–2387, 1988 [DOI] [PubMed] [Google Scholar]
- 23. Glass RI, Zack MM., Jr Increase in deaths from ischaemic heart-disease after blizzards. Lancet 1: 485–487, 1979 [DOI] [PubMed] [Google Scholar]
- 24. Glickman-Weiss EL, Hearon CM, Nelson AG, Robertson RJ. A thermal perception scale for use during resting exposure to cold air. Percept Mot Skills 79: 547–560, 1994 [DOI] [PubMed] [Google Scholar]
- 25. Haapaniemi S, Franklin BA, Wegner JH, Hamar S, Gordon S, Timmis GC, O'Neill WW. Electrocardiographic responses to deer hunting activities in men with and without coronary artery disease. Am J Cardiol 100: 175–179, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Hattenhaur M, Neill WA. The effect of cold air inhalation on again pectoris and myocardial oxygen supply. Circulation 51: 1053–1058, 1975 [DOI] [PubMed] [Google Scholar]
- 27. Havenith G, van de Linde EJ, Heus R. Pain, thermal sensation and cooling rates of hands while touching cold materials. Eur J Appl Physiol Occup Physiol 65: 43–51, 1992 [DOI] [PubMed] [Google Scholar]
- 28. Heindl S, Struck J, Wellhoner P, Sayk F, Dodt C. Effect of facial cooling and cold air inhalation on sympathetic nerve activity in men. Respir Physiol Neurobiol 142: 69–80, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Hess KL, Wilson TE, Sauder CL, Gao Z, Ray CA, Monahan KD. Aging affects the cardiovascular responses to cold stress in humans. J Appl Physiol 107: 1076–1082, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hozumi T, Yoshida K, Ogata Y, Akasaka T, Asami Y, Takagi T, Morioka S. Noninvasive assessment of significant left anterior descending coronary artery stenosis by coronary flow velocity reserve with transthoracic color Doppler echocardiography. Circulation 97: 1557–1562, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Huang AH, Feigl EO. Adrenergic coronary vasoconstriction helps maintain uniform transmural blood flow distribution during exercise. Circ Res 62: 286–298, 1988 [DOI] [PubMed] [Google Scholar]
- 32. Janardhanan R, Henry Z, Hur DJ, Lin CM, Lopez D, Reagan PM, Rudnick SR, Koshko TJ, Keeley EC. The snow-shoveler's ST elevation myocardial infarction. Am J Cardiol 106: 596–600, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Juneau M, Johnstone M, Dempsey E, Waters DD. Exercise-induced myocardial ischemia in a cold environment. Effect of antianginal medications. Circulation 79: 1015–1020, 1989 [DOI] [PubMed] [Google Scholar]
- 34. King BD, Harris LC, Jr, Greifenstein FE, Elder JD, Jr, Dripps RD. Reflex circulatory responses to direct laryngoscopy and tracheal intubation performed during general anesthesia. Anesthesiology 12: 556–566, 1951 [DOI] [PubMed] [Google Scholar]
- 35. Lassvik C, Areskog NH. Angina pectoris during inhalation of cold air. Reactions to exercise. Br Heart J 43: 661–667, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Le Merre C, Kim HH, Chediak AD, Wanner A. Airway blood flow responses to temperature and humidity of inhaled air. Respir Physiol 105: 235–239, 1996 [DOI] [PubMed] [Google Scholar]
- 37. Leon DF, Amidi M, Leonard JJ. Left heart work and temperature responses to cold exposure in man. Am J Cardiol 26: 38–45, 1970 [DOI] [PubMed] [Google Scholar]
- 38. Mathew OP, Sant'Ambrogio FB, Sant'Ambrogio G. Effects of cooling on laryngeal reflexes in the dog. Respir Physiol 66: 61–70, 1986 [DOI] [PubMed] [Google Scholar]
- 39. McFadden ER, Jr, Pichurko BM, Bowman HF, Ingenito E, Burns S, Dowling N, Solway J. Thermal mapping of the airways in humans. J Appl Physiol 58: 564–570, 1985 [DOI] [PubMed] [Google Scholar]
- 40. Mercer JB. Cold–an underrated risk factor for health. Environ Res 92: 8–13, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Meyer P, Guiraud T, Curnier D, Juneau M, Gayda M, Nozza A, Nigam A. Exposure to extreme cold lowers the ischemic threshold in coronary artery disease patients. Can J Cardiol 26: e50–e53, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mitchell LA, MacDonald RA, Brodie EE. Temperature and the cold pressor test. J Pain 5: 233–237, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Momen A, Kozak M, Leuenberger UA, Ettinger S, Blaha C, Mascarenhas V, Lendel V, Herr MD, Sinoway LI. Transthoracic Doppler echocardiography to non-invasively assess coronary vasoconstrictor and dilator responses in humans. Am J Physiol Heart Circ Physiol 298: H524–H529, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Momen A, Mascarenhas V, Gahremanpour A, Gao Z, Moradkhan R, Kunselman A, Boehmer J, Sinoway LI, Leuenberger UA. Coronary blood flow responses to physiological stress in humans. Am J Physiol Heart Circ Physiol 296: H854–H861, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morabito M, Modesti PA, Cecchi L, Crisci A, Orlandini S, Maracchi G, Gensini GF. Relationships between weather and myocardial infarction: a biometeorological approach. Int J Cardiol 105: 288–293, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Muller MD, Ryan EJ, Kim CH, Bellar DM, Blankfield RP, Glickman EL. Reliability of the measurement of stroke volume using impedance cardiography during acute cold exposure. Aviat Space Environ Med 81: 120–124, 2010 [DOI] [PubMed] [Google Scholar]
- 47. Petersen CL, Hansen A, Frandsen E, Strange S, Jonassen O, Nielsen JR, Dige-Petersen H, Hesse B. Endothelin release and enhanced regional myocardial ischemia induced by cold-air inhalation in patients with stable angina. Am Heart J 128: 511–516, 1994 [DOI] [PubMed] [Google Scholar]
- 48. Raven PB, Niki I, Dahms TE, Horvath SM. Compensatory cardiovascular responses during an environmental cold stress, 5°C. J Appl Physiol 29: 417–421, 1970 [DOI] [PubMed] [Google Scholar]
- 49. Ray CA. Interaction between vestibulosympathetic and skeletal muscle reflexes on sympathetic activity in humans. J Appl Physiol 90: 242–247, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Reis SE, Holubkov R, Lee JS, Sharaf B, Reichek N, Rogers WJ, Walsh EG, Fuisz AR, Kerensky R, Detre KM, Sopko G, Pepine CJ. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women's Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol 33: 1469–1475, 1999 [DOI] [PubMed] [Google Scholar]
- 51. Sant'Ambrogio G, Mathew OP, Sant'Ambrogio FB, Fisher JT. Laryngeal cold receptors. Respir Physiol 59: 35–44, 1985 [DOI] [PubMed] [Google Scholar]
- 52. Sheldahl LM, Wilke NA, Dougherty S, Tristani FE. Snow blowing and shoveling in normal and asymptomatic coronary artery diseased men. Int J Cardiol 43: 233–238, 1994 [DOI] [PubMed] [Google Scholar]
- 53. Sheldahl LM, Wilke NA, Dougherty SM, Levandoski SG, Hoffman MD, Tristani FE. Effect of age and coronary artery disease on response to snow shoveling. J Am Coll Cardiol 20: 1111–1117, 1992 [DOI] [PubMed] [Google Scholar]
- 54. Sheth T, Nair C, Muller J, Yusuf S. Increased winter mortality from acute myocardial infarction and stroke: the effect of age. J Am Coll Cardiol 33: 1916–1919, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Spitalnic SJ, Jagminas L, Cox J. An association between snowfall and ED presentation of cardiac arrest. Am J Emerg Med 14: 572–573, 1996 [DOI] [PubMed] [Google Scholar]
- 56. Tune JD, Richmond KN, Gorman MW, Feigl EO. Control of coronary blood flow during exercise. Exp Biol Med (Maywood) 227: 238–250, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Wagner JA, Horvath SM. Cardiovascular reactions to cold exposures differ with age and gender. J Appl Physiol 58: 187–192, 1985 [DOI] [PubMed] [Google Scholar]
- 58. Wilson TE, Brothers RM, Tollund C, Dawson EA, Nissen P, Yoshiga CC, Jons C, Secher NH, Crandall CG. Effect of thermal stress on Frank-Starling relations in humans. J Physiol 587: 3383–3392, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wilson TE, Gao Z, Hess KL, Monahan KD. Effect of aging on cardiac function during cold stress in humans. Am J Physiol Regul Integr Comp Physiol 298: R1627–R1633, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wilson TE, Sauder CL, Kearney ML, Kuipers NT, Leuenberger UA, Monahan KD, Ray CA. Skin-surface cooling elicits peripheral and visceral vasoconstriction in humans. J Appl Physiol 103: 1257–1262, 2007 [DOI] [PubMed] [Google Scholar]