Abstract
Reductions in oxygen availability (O2) by either reduced arterial O2 content or reduced perfusion pressure can have profound influences on the circulation, including vasodilation in skeletal muscle vascular beds. The purpose of this review is to put into context the present evidence regarding mechanisms responsible for the local control of blood flow during acute systemic hypoxia and/or local hypoperfusion in contracting muscle. The combination of submaximal exercise and hypoxia produces a “compensatory” vasodilation and augmented blood flow in contracting muscles relative to the same level of exercise under normoxic conditions. A similar compensatory vasodilation is observed in response to local reductions in oxygen availability (i.e., hypoperfusion) during normoxic exercise. Available evidence suggests that nitric oxide (NO) contributes to the compensatory dilator response under each of these conditions, whereas adenosine appears to only play a role during hypoperfusion. During systemic hypoxia the NO-mediated component of the compensatory vasodilation is regulated through a β-adrenergic receptor mechanism at low-intensity exercise, while an additional (not yet identified) source of NO is likely to be engaged as exercise intensity increases during hypoxia. Potential candidates for stimulating and/or interacting with NO at higher exercise intensities include prostaglandins and/or ATP. Conversely, prostaglandins do not appear to play a role in the compensatory vasodilation during exercise with hypoperfusion. Taken together, the data for both hypoxia and hypoperfusion suggest NO is important in the compensatory vasodilation seen when oxygen availability is limited. This is important from a basic biological perspective and also has pathophysiological implications for diseases associated with either hypoxia or hypoperfusion.
Keywords: hypoxia, hypoperfusion, vasodilation
during dynamic muscle contractions there is an increased metabolic demand that is matched closely by increases in skeletal muscle blood flow (exercise hyperemia) and oxygen delivery. Blood flow increases rapidly at the onset of contractions and can increase up to 100-fold over resting values during intense exercise (2). The mechanisms responsible for increasing blood flow at the onset of exercise as well as maintaining it over time involve a complex interaction between mechanical factors and various local metabolic and endothelial derived substances that influence vascular tone (132). Over the last several decades exercise hyperemia in general and the potential mechanisms responsible for the regulation of flow to contracting skeletal muscles have been reviewed extensively (13, 33, 35, 68, 75, 80, 81, 131, 152). These reviews have focused primarily on blood flow responses during exercise under normoxic conditions. However, reductions in oxygen (O2) availability (i.e., hypoxia) bring about several cardiovascular adjustments and can have profound influences on the circulation. Along these lines, the mechanisms responsible for the local regulation of skeletal muscle blood flow during exercise are likely enhanced and possibly different than those during normoxic exercise.
Therefore, the purpose of this review is to discuss how O2 availability can impact blood flow and vasodilation in contracting skeletal muscles. Although cardiac pumping capacity (especially during large muscle mass and/or high intensity exercise) can be important in the hyperemic response to exercise, the emphasis in this review is on the local mechanisms regulating active muscle blood flow under conditions of reduced O2 availability during submaximal exercise. Specifically, this review will focus on 1) how acute hypoxia and hypoperfusion interact with exercise to govern the hyperemic responses in contracting muscles of humans and 2) the local vasodilator signals involved in the regulation of muscle blood flow during acute systemic (hypoxia) and local (hypoperfusion) reductions in O2 availability. We will also discuss the potential role of any systemic pressor response under these conditions.
SYSTEMIC HYPOXIA
Effect on Blood Flow to Resting Muscle
At rest acute exposure to moderate hypoxia elicits vasodilation and an augmented blood flow in skeletal muscle vascular beds of young apparently healthy humans (9–11, 25, 26, 37, 84, 124, 158, 160, 161). This vasodilation occurs despite the resting muscle being relatively overperfused, as evidenced by limited O2 extraction (25, 26, 59). The augmented skeletal muscle vasodilation in response to hypoxia also occurs despite an enhanced sympathetic vasoconstrictor activity (52, 83, 130, 143). Evidence suggests that a reduced α-adrenergic responsiveness associated with hypoxia is not responsible for this dilation (37, 161). Local blockade of α-adrenergic receptors in the forearm reveals a substantially greater vasodilator response during systemic hypoxia (158, 160). Thus activation of sympathetic vasoconstrictor nerves during hypoxia likely masks the degree of vasodilation. It should be noted that other studies have reported that hypoxia-mediated forearm vascular conductance was unaffected by local blockade of sympathetic nerves (9) and α-adrenergic blockade (118). Taken together, vasodilation prevails over the vasoconstrictor response in determining vasomotor tone during acute hypoxia in resting skeletal muscle.
A number of vasodilator substances or systems, including but not limited to β-adrenergic receptor activation, nitric oxide (NO), prostaglandins, and adenosine, have been implicated to be involved in hypoxic vasodilation at rest and have been previously reviewed in detail (51). Briefly, we and others (10, 158, 160) have demonstrated that β-adrenergic receptor activation is responsible for a substantial portion of the hypoxic vasodilation at rest. Moreover, a large portion of the β-adrenergic receptor-mediated hypoxic vasodilation at rest occurs through a NO pathway (158). This is consistent with the NO-dependent nature of at least some of the vasodilator responses to β2-mediated vasodilation (31, 45). Along these lines, NO synthase (NOS) inhibition attenuates the hypoxic vasodilator response in the human forearm in some studies (11, 25) but not others (90). In the latter study, the investigators locally blocked β-adrenergic receptors via intra-arterial infusions of propranolol prior to the experimental trials with NOS inhibition and therefore potentially masked the NO-mediated vasodilator response that is observed in other studies without concurrent β-adrenergic blockade. Under similar conditions, single inhibition of prostaglandin synthesis also did not attenuate the vasodilator response to systemic hypoxia (90). However, the combined inhibition of NO and prostaglandins abolished the vasodilator response to hypoxia at rest, thus indicating a synergistic role between these two pathways in regulating peripheral vascular tone and ultimately blood flow (90). Recent evidence suggests both interstitial and plasma adenosine stimulates NO and prostacyclin formation in the human leg under normoxic conditions (103). Moreover, there is strong evidence in animals that adenosine plays a major role in hypoxia-induced skeletal muscle vasodilation (15, 100, 114, 115, 141). However, available data related to the contribution of adenosine in the hypoxic vasodilator response in human studies are conflicting (25, 26, 84).
Hypoxic Exercise
The combination of submaximal dynamic exercise and hypoxia produces a “compensatory” vasodilation and augmented blood flow in contracting muscles (both quadriceps and forearm) relative to the same level of exercise under normoxic conditions (16, 26, 32, 122, 126, 160, 161). This augmented vasodilation exceeds that predicted by a simple sum of the individual dilator responses to hypoxia alone and normoxic exercise. Additionally, this enhanced hypoxic exercise hyperemia is proportional to the hypoxia-induced fall in arterial O2 content, thus preserving muscle O2 delivery and ensuring it is matched to demand (32, 49, 122, 126). Compensatory vasodilation is also observed when O2 availability is reduced by carbon monoxide administration and/or experimentally induced anemia (48, 49, 121, 122). In this context, O2 delivery, rather than blood flow, appears to be the regulated variable producing the augmented hyperemia during hypoxic exercise (47).
Potential Causes of Compensatory Vasodilation
Acute exposure to systemic hypoxia results in substantial reductions in arterial O2 content (CaO2) as well as arterial O2 tension (PaO2). Theoretically, reductions in each of these variables could serve as a signal for the compensatory vasodilation observed during hypoxic exercise (77, 78, 122). Roach and colleagues (122) reported that blood flow and O2 delivery to the lower limbs during rhythmic two-legged knee extension exercise with normoxia, anemia, hypoxia, and anemia + hypoxia were dependent on CaO2 not PaO2. Evidence that a low PaO2 alone does not cause vasodilation was based on the observation of similar limb blood flows in the two conditions (hypoxia and anemia) with nearly identical CaO2 but widely different PaO2 (∼40 vs. ∼105 mmHg). In a subsequent study, leg blood flow and vascular conductance were shown to be tightly coupled to the level of arterial oxyhemoglobin, regardless of a normal or an 11-fold difference in PaO2 (47–538 mmHg) (49). Taken together, these results strongly suggest that factors sensitive to CaO2 serve as the signal for the compensatory increases in skeletal muscle blood flow and vasodilation during hypoxic exercise.
Regulation of the Vascular Tone During Hypoxic Exercise
Vasodilator line-up: usual suspects?
Several vasodilator pathways have been proposed and examined as likely regulators of skeletal muscle blood flow in response to changes in CaO2. Increases in arterial epinephrine are evident with acute exposure to systemic hypoxia and are increased further during submaximal cycling exercise (92). Additionally, an intensity-dependent rise in arterial epinephrine occurs during incremental forearm exercise, suggesting that β-adrenergic receptor-mediated vasodilation might contribute to the augmented hypoxic exercise hyperemia (161). Wolfel and colleagues (163) originally demonstrated that β-adrenergic blockade reduced O2 delivery to the contracting muscles during submaximal exercise at altitude, but was compensated for by an enhanced tissue O2 extraction. However, the use of systemic β-blockade reduced cardiac output and likely engaged vasoconstricting cardiovascular reflexes, therefore making it difficult to determine whether alterations in β-adrenergic receptor-mediated vasodilation within skeletal muscle vasculature contributed to the reduced O2 delivery. Along these lines, local inhibition of β-adrenergic receptors (via intra-arterial infusion of propranolol) has demonstrated that in the absence of overlying sympathetic vasoconstriction, β-adrenergic receptor activation contributes to the hypoxic compensatory vasodilation during mild (10% maximum voluntary contraction; MVC) forearm exercise (160). However, the β-adrenergic component decreases with increased exercise intensity (20% MVC). Taken together, this suggests that other local vasodilating factors are likely responsible for the compensatory vasodilation during hypoxic exercise, especially at higher exercise intensities.
NO release from an endothelial source and/or desaturation of hemoglobin in erythrocytes (85, 109, 140, 147) has been proposed during hypoxia. Along these lines, there is a significant blunting of the compensatory vasodilation in the forearm during mild to moderate (10–20% MVC) hypoxic exercise after NOS inhibition (25). Although NO-mediated mechanisms contribute to the compensatory vasodilation during hypoxic exercise, it appears that it is regulated through different pathways with increasing exercise intensity. At lower-intensity forearm exercise (10% MVC) NO contributes to the compensatory vasodilation via β-adrenergic receptor activation. At higher intensity exercise the contribution of NO becomes independent of the β-adrenergic receptors (19). Therefore, an alternative and currently unknown stimulus for NO-mediated vasodilation occurs during higher intensity hypoxic exercise.
Increases in plasma but not skeletal muscle interstitial NO during hypoxia in humans suggest an endovascular or endothelial NO source (85). Therefore, it is possible that the augmented vasodilation during hypoxic exercise is a result of the direct release of NO from endothelial cells and/or erythrocytes. In this context, luminal hypoxia can elicit the direct release of NO from the endothelium (109) and from erythrocytes in the form S-nitrosohemoglobin (147). However, based on the location for intraluminal NG-monomethyl-l-arginine (NOS inhibitor) administration and the fact that NOS inhibition is effective in reducing forearm blood flow during hypoxic exercise (25), it is reasonable to suspect that the NO responsible for the compensatory vasodilatation is from endothelial sources vs. an erythrocyte source. Furthermore, the effectiveness of NOS inhibition in reducing the compensatory vasodilatation during hypoxic exercise also argues against the idea that nitrite and/or formation of erythrocyte nitroso species is a key vasodilator in this response (39, 46). Along these lines, if nitrite were responsible, either directly or indirectly (via reduction to NO), the compensatory vasodilation would have been maintained despite NOS inhibition.
In animals adenosine is thought to be a key mediator of skeletal muscle blood flow during normoxic exercise (95, 107, 110, 111). However, adenosine receptor blockade appears to only modestly attenuate the exercise-induced skeletal muscle vasodilation in human limbs, but these findings are not consistent (26, 91, 98, 112). The classic adenosine hypothesis proposes that adenosine mediates vasodilation and helps to ensure adequate blood flow to metabolically active tissue during periods of reduced O2 availability (i.e., hypoxia and/or ischemia) (7). This concept would therefore suggest that adenosine may contribute to the compensatory vasodilation during hypoxic exercise. However, our group demonstrated that adenosine is not obligatory for the compensatory vasodilation during hypoxic exercise in humans (26). This was observed with and without overlying sympathetic α-adrenergic vasoconstriction, suggesting that the enhanced sympathetic outflow associated with systemic hypoxia did not mask any underlying adenosine-mediated vasodilation. Moreover, adenosine does not contribute to the compensatory vasodilation after NOS inhibition, thus indicating that adenosine does not act through an NO-independent pathway in this response (25). Using positron emission tomography (PET) imaging, Heinonen and colleagues (59) recently demonstrated that adenosine receptor antagonism (via aminophylline) did not affect blood flow in exercising human quadriceps femoris muscle, suggesting no contribution of adenosine to capillary blood flow during hypoxic exercise. Additionally, adenosine receptor blockade did not change blood flow heterogeneity within the active and inactive muscles during hypoxic exercise.
Deoxygenation (i.e., hypoxia) and mechanical deformation (i.e., exercise) of red blood cells can lead to ATP release from erythrocytes (6, 64, 144, 145). Moreover, venous plasma levels of ATP are elevated during hypoxia at rest and during exercise compared with normoxic conditions (48, 99). Both in vitro and in vivo studies suggest that ATP-induced vasodilation is mediated through a NO pathway (28, 94, 96), thus an ATP/NO-mediated component to the compensatory vasodilation during hypoxic exercise is an attractive hypothesis. It is believed that the vasodilator actions of ATP are mediated through the purinergic P2y receptors located on vascular endothelial cells (113). Unfortunately, specific pharmacological antagonists for P2 receptors to address the role of ATP in the hypoxia-induced compensatory vasodilation are currently unavailable for human use. However, it is important to note that experimentally increasing limb vasodilation during hypoxic exercise (via intra-arterial ATP infusion) does not improve leg and systemic O2 consumption due to a reduction in O2 extraction across the exercising leg (17). Another potential candidate for stimulating and/or interacting with NO in the compensatory vasodilator response might be prostaglandins. There is strong evidence that suggests an interaction between prostaglandins and NO in the regulation of skeletal muscle blood flow at rest and during normoxic exercise (97, 101, 133, 134). As indicated earlier, there is also evidence to suggest a synergistic role between these two pathways in the compensatory vasodilation during hypoxia at rest (90). Recent evidence suggests that combined NO/PG inhibition substantially reduces forearm blood flow and vasodilation during hypoxic exercise in young healthy adults (29). It is important to note that the aforementioned study did not include single inhibition trials of each vasodilator pathway of interest (NO and prostaglandins). Therefore, it is difficult to discern the relative contribution of PGs and its interaction with NO in the compensatory vasodilation during hypoxic exercise.
Sympathetic Restraint of Hypoxia-Induced Compensatory Vasodilation
In young healthy humans the sympathetic response to exercise is greater in hypoxia than it is in normoxia (54, 69, 136). The increased sympathetic vasoconstrictor activity has been postulated to be needed to match O2 delivery with O2 demand (88) and maintain arterial blood pressure during the hypoxic stress and in turn limit the degree of compensatory vasodilation (148). Along these lines, α-adrenergic receptor blockade in the human forearm (160) and in the hindlimbs of dogs (148) reveals a greater vasodilation during hypoxic exercise compared with control hypoxic exercise conditions (i.e., during saline infusion). Despite the augmented sympathetic vasoconstrictor activity a substantial compensatory vasodilation persists during hypoxic exercise. It is well documented that sympathetic vasoconstrictor responses are blunted in the vascular beds of contracting skeletal muscle of animals (150, 151) and humans (36, 56, 57, 117, 153), a phenomenon referred to as “functional sympatholysis.” Moreover, there is evidence to suggest that hypoxia can attenuate vasoconstrictor responses to sympathetic nerve activation and exogenous norepinephrine in resting skeletal muscle of animals and humans (12, 60, 61). Therefore, it is possible that an augmented functional sympatholysis might partially explain the compensatory vasodilation during hypoxic exercise. In this context, Hansen et al. (55) demonstrated a reduced forearm vasoconstrictor responsiveness to lower body negative pressure during moderate hypoxic exercise compared with normoxic exercise. However, our laboratory (161) has demonstrated that the greater vasodilation observed during hypoxic forearm exercise was not due to a reduced postjunctional vasoconstrictor responsiveness (augmented functional sympatholysis) to endogenous norepinephrine (via intra-arterial infusion of tyramine).
Compensatory Vasodilation: Is it Always Present?
It is clear that compensatory vasodilation occurs in response to acute hypoxia during submaximal forearm (19, 25, 26, 29, 160, 161) and single and/or two-legged knee extension (16, 32, 77, 122, 126) exercise. That is, during submaximal exercise, alterations in O2 availability are compensated for by an augmented muscle blood flow to help maintain O2 delivery to the active muscles. However, this compensatory vasodilation appears to be absent during maximal exercise, especially when performed with a large muscle mass (74, 77, 78, 120). The muscle blood flow and vasodilator response to maximal exercise during moderate and severe hypoxia are likely limited to a certain degree by a reduction in maximal cardiac output (16) and not fully capable of compensating when the maximal pumping capacity of the heart is taxed. Under these circumstances it seems likely baroreflex-mediated vasoconstrictor responses are engaged. However, it is important to note that even in well trained cyclists who have a high cardiovascular reserve, leg blood flow and leg O2 consumption is reduced at peak exercise using the one-legged knee extension model (119). The lack of a compensatory vasodilation is also observed during submaximal exercise after acclimatization (long-term exposure to hypoxia), which may be due to an increase in CaO2.
In summary, the studies discussed here clearly demonstrate that reductions in available O2 via systemic hypoxia promote compensatory vasodilation and an augmented blood flow in skeletal muscle vascular beds at rest and during submaximal exercise. The compensatory vasodilation during hypoxic exercise is essential to ensure the maintenance of oxygen delivery to the active muscles. It appears that factors sensitive to CaO2 serve as the signal for the compensatory increases in skeletal muscle blood flow and vasodilation during hypoxic exercise. In humans, NO-mediated vasodilation plays an obligatory role in the augmented blood flow during incremental hypoxic exercise. However, the NO-mediated component of the compensatory vasodilation during hypoxic exercise is regulated through different pathways with increasing exercise intensity. That is, a β-adrenergic receptor-stimulated NO component exists during low-intensity hypoxic exercise, whereas the source of NO contributing to compensatory dilation is less dependent on β-adrenergic mechanisms as exercise intensity increases. It is currently unclear what the stimulus of NO release is with increasing intensity of muscle contraction but does not appear to be adenosine. ATP released from erythrocytes and/or endothelial-derived prostaglandins remain attractive candidates for stimulating NO during higher intensity hypoxic exercise.
The vasodilator response to acute hypoxia detailed above in young healthy humans has been demonstrated to be attenuated in older adults at rest and during exercise (27, 79). Additionally, limited reports suggest that compensatory vasodilation is impaired in some populations with cardiovascular risk factors (5), whereas it is preserved in others (86, 157). From a clinical perspective, if an attenuated vasodilator response occurs in other vascular beds (i.e., coronary circulation) with aging and cardiovascular risk factors, certain individuals may be at risk of reduced perfusion of the myocardium and consequently myocardial injury during exercise with hypoxia.
HYPEROXIA: THE OPPOSITE SIDE OF THE COIN
It is apparent that compromises in O2 availability (i.e., hypoxia) elicit a compensatory vasodilator response in skeletal muscle vascular beds to ensure adequate O2 delivery. Conversely, increased O2 availability (i.e., hyperoxia) reduces resting muscle blood flow in humans (8, 58, 116, 165). Additionally, muscle blood flow is reduced during and after hyperoxic exercise compared with normoxic conditions (24, 48, 116, 159). The challenge of increasing O2 availability by increasing the fraction of inspired O2 (i.e., FiO2 = 100% O2) under normobaric environments is that CaO2 can only be elevated 5–10%. Additional approaches using hyperoxia (FiO2 = 100% O2) superimposed onto experimentally induced polycythemia have also been employed to study the effects of increased O2 availability on muscle blood flow during knee-extensor exercise (47). However, this approach only resulted in an ∼11% increase in CaO2. These relatively small increases in CaO2 are near the signal-to-noise ratio for muscle blood flow measurements. To address this potential limitation, studies on the blood flow response in the exercising forearm to larger increases in estimated CaO2 via hyperbaric hyperoxia have recently been performed (24). Large increases in estimated O2 content (∼25–30%) resulted in a substantial reduction in exercising forearm blood flow despite significantly higher perfusion pressures, thus indicating a greater increase in vascular resistance compared with normoxic conditions (24). The blunted forearm vascular conductance during hyperoxic exercise (∼25% lower) mirrored the estimated increase in arterial O2 content (∼25–30%). These findings during hyperbaric hyperoxic exercise parallel previous findings in which hypoxic-mediated increases in forearm vascular conductance during exercise mirrors the fall in O2 content (25, 26).
The mechanisms responsible for the increased vascular resistance and reduced blood flow during hyperoxic exercise are currently unclear. In general, the potential mechanisms include but are not limited to 1) greater sympathetic restraint and/or 2) decreased release of or responsiveness to local vasodilators within the contracting muscle. Although hyperoxia reduces muscle sympathetic nerve activity at rest (62, 135, 165), it is unclear whether this holds true during exercise and contributes to the reduced muscle blood flow and vasodilation. Limited evidence suggests that hyperoxia does not appear to enhance the magnitude of change in sympathetic nerve activity to non-active skeletal muscle during dynamic exercise (135). Additionally, it is possible that increased oxygen levels may attenuate functional sympatholysis and therefore enhance vasoconstriction in the contracting muscles (55). Finally, some evidence suggests that the vascular response (i.e., constriction) to hyperoxia in the hindlimbs of dogs is not mediated by the autonomic nervous system but is rather attributed to a direct action of high arterial O2 tension (4). Therefore, it is possible that the increase in vascular resistance under hyperoxic exercise conditions may be a direct vasoconstrictor action of O2 on the arterial and arteriolar wall. As discussed earlier, erythrocytes have the ability to sense changes in O2 during hypoxic conditions and modulate vascular tone via release of ATP, thus leading to appropriate changes in blood flow and matching O2 delivery with metabolic need (42). Therefore, large increases in O2 availability during hyperbaric hyperoxic exercise could theoretically attenuate the release of ATP from erythrocytes (Fig. 1) (48).
Fig. 1.
Thigh plasma venous ATP responses at rest and during incremental knee-extensor exercise with exposure to normoxia, hypoxia, hyperoxia, and carbon monoxide (CO) + normoxia. *Significantly different from resting values (P < 0.05). [Adapted with permission from Ref. 48].
A shift in the vasodilator/vasoconstrictor balance due to an attenuated release of or responsiveness to local vasodilators within the contracting muscle could also promote the reduced blood flow during hyperoxic exercise. In this context, prostaglandin-mediated vasodilation following isometric exercise is reduced during hyperoxia (162). Additionally, vasoconstriction via a reduction in basal NO production has been observed in porcine coronary arteries exposed to elevated levels of O2 (106). Along the same lines, an increase in free radical production (i.e., superoxide anions) during hyperbaric oxygenation causes vasoconstriction in the cerebral circulation of rats via inactivation of NO (167). However, recent evidence suggests that NO metabolites are unaffected in humans during normobaric hyperoxic exercise (39). Therefore it is unclear whether alterations in the local vasodilator milieu are responsible for the blunted blood flow response to hyperoxic exercise.
LOCAL REDUCTIONS IN OXYGEN (VIA HYPOPERFUSION)
Over the past several years our laboratory has been interested in addressing whether the compensatory vasodilator signals that maintain oxygen delivery to active muscles during exercise with systemic hypoxia are the same or different than those that cause compensatory vasodilation during normoxic exercise with hypoperfusion. During conditions of insufficient oxygen delivery to the active muscle (e.g., hypoperfusion or ischemia), metabolites can accumulate that either stimulate blood pressure, raising sensory nerves within the muscle, or evoke vasodilation (1, 30, 67, 70, 137, 155, 156). Either of these mechanisms might act to restore blood flow to the contracting muscle and relieve the flow/metabolism mismatch.
Reflex Pressor Response
Activation of chemosensitive afferent nerves in the active muscle evokes reflex increases in sympathetic outflow and arterial pressure, termed the muscle chemoreflex or metaboreflex (1, 30, 67, 70, 155, 156). The increased pressure is thought to augment blood flow to the contracting muscle and partially restore the flow/metabolism matching. Evidence for restoration of blood flow to active muscle via a reflex pressor response comes primarily from studies in conscious animals. In dogs, the hemodynamic consequences of muscle chemoreflex activation, via graded reductions in flow and perfusion pressure, have been studied extensively using the “Seattle model” (53, 104, 105, 138, 139, 164). In this model, a terminal aortic occluder in combination with a flow probe in close proximity is used to suddenly reduce blood flow to the hindlimb muscles of dogs running on a treadmill. Using this model, imposed reductions in blood flow and oxygen delivery to active hindlimb muscles during dynamic exercise can evoke a powerful pressor response that restores ∼50% of the flow deficit (105). The reflex pressor response to acute hypoperfusion during exercise in dogs is dependent on the intensity of the exercise (123) and apparently generated when O2 delivery falls below some critical level causing accumulation of a pressor substance (Fig. 2) (139). The reflex pressor response in normal dogs is generated primarily by a rise in cardiac output (3, 53, 72, 104, 164), whereas in dogs with congestive heart failure the systemic pressor response is caused primarily by peripheral vasoconstriction (53). Taken together, the Seattle model clearly demonstrates that the reflex pressor response contributes to the restoration of blood flow in the hindlimbs of dogs during exercise. However, it should be noted that Britton et al. (14) demonstrated that autoregulation in the hindlimbs of dogs also contributes significantly to the regulation of blood flow in response to acute hypoperfusion during exercise.
Fig. 2.
Effects of CO on the rise of systemic arterial pressure to graded reductions in hindlimb perfusion during exercise (2 mph, 0% grade). Squares and solid lines are control (no CO) exercise data, and circles and dashed lines are exercise with CO data. Filled symbols represent free-flow data; open symbols represent data collected during reduced hindlimb perfusion. These data demonstrate that when terminal aortic flow is decreased during mild exercise the reflex rise in systemic arterial pressure occurs at higher flows after arterial oxygen content is reduced with CO. [Adapted with permission from Ref. 139].
Is the Pressor Response Apparent in Humans?
In humans, large increases in blood pressure during ischemic exercise were first described by Alam and Smirk (1). These investigators observed that the activity of even a small group of muscles may give rise to a substantial increase in systolic blood pressure (up to 70 mmHg) provided that the circulation of the blood through the exercising muscles was arrested completely. Lorentsen (87) later demonstrated that the blood pressure response to plantar flexion in patients with unilateral intermittent claudication due to atherosclerosis obliterans was significantly greater during exercise with the diseased limbs compared with exercise with nondiseased limbs. Taken together, these studies suggest that the reflex pressor response in humans is engaged during exercise with ischemia and/or hypoperfusion. However, stimulation of the muscle chemoreflex evokes a large increase in vasoconstricting sympathetic nerve traffic directed toward the skeletal muscle (30, 89, 154). While the reflex increase in arterial pressure has the potential to restore blood flow and O2 delivery to the active muscles, the reflex increase in vasoconstricting sympathetic outflow might also restrain blood flow to some degree. Whether the pressor response improves blood flow to the hypoperfused active human skeletal muscle has not been extensively studied (relative to that in animals) and is not well understood.
To evaluate the blood flow response to hypoperfusion in contracting muscles the majority of studies in humans have relied on the use of external pressure to reduce limb blood flow during exercise, which have produced conflicting results (30, 66, 127, 149). Using O2 saturation of venous blood (%SvO2) as an indirect method of measuring blood flow, studies in the forearm reported that the reflex pressor response did not appear to restore blood flow to rhythmically contracting muscles (66). In contrast, Rowell et al. (127) concluded that the reflex pressor response partially restored the decrease in leg blood flow (%SvO2) induced by external positive pressure. Using a more direct measurement of flow (brachial artery mean blood velocity), Daley et al. (30) demonstrated that flow is not restored by a reflex pressor response at higher levels of external pressure (+50 mmHg) due to sympathetic vasoconstriction limiting the ability of the pressor response to restore the flow. Potential problems of these earlier studies mainly revolve around the indirect measures of blood flow (66, 127) and the method used to reduce limb blood flow (positive pressure) during exercise (30, 66, 127, 149). While external positive pressure clearly reduces perfusion pressure to the contracting muscles, it differs fundamentally with the Seattle model because it affects all elements of the vascular tree, including the microcirculation and veins, and might either limit expression of local vasodilatation or limit the ability of changes in arterial pressure to improve blood flow.
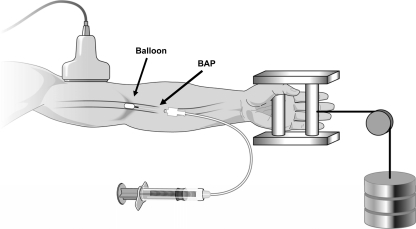
To address the limitations of external pressure, our laboratory recently developed an experimental model in humans to directly measure the blood flow response in hypoperfused skeletal muscle produced by forearm exercise in the presence of a brachial artery occlusion. For this purpose a small balloon catheter is inserted into the brachial artery and inflated to reduce forearm blood flow and vascular conductance during handgrip exercise (Fig. 3) (23). With this experimental model there is a substantial restoration of flow to hypoperfused exercising human muscle. Interestingly, the restoration of flow occurs in the absence of a significant pressor response, thus suggesting local vasodilator and/or myogenic mechanisms are likely responsible. The absence of a significant pressor response (above exercise alone) during hypoperfusion suggests that a reflex increase in pressure is not necessary to restore blood flow to hypoperfused contracting human muscle. The lack of a pressor response in our model may be explained by the small muscle mass (forearm), moderate exercise intensity (20% MVC), magnitude of the reduction in forearm blood flow (∼50%) induced by balloon inflation, or a combination of these being not sufficient enough to augment the pressor response beyond that of exercise alone. However, recent evidence suggests that using an upper arm cuff to reduce flow by ∼40% during forearm exercise at 15% MVC was sufficient to evoke a reflex pressor response (63). It should be noted that the use of an upper arm cuff likely blocked venous outflow, leading to increased intramuscular pressure, which could activate sympathoexcitatory mechanosensitive afferents (93, 125). Therefore, the reflex pressor response observed might have been due to an enhanced mechanoreflex, which is not likely engaged using an intra-arterial balloon catheter to induce hypoperfusion.
Fig. 3.
Schematic of intra-arterial balloon catheter system. Forearm blood flow is reduced by partially occluding the brachial artery via inflation of a Fogarty balloon catheter with saline using a calibrated microsyringe for tight control of balloon volume. Brachial artery blood velocity is measured (via Doppler ultrasound) proximal to the balloon. The configuration of the balloon upstream from the lumen of the introducer allows measurement of the brachial arterial pressure (BAP) distal to the balloon that is perfusing the contracting forearm muscles. Rhythmic forearm exercise is performed with a hand grip device by lifting a weight 4–5 cm over a pulley system at a duty cycle of 1 s contraction/ and 2-s relaxation (20 contractions/min).
Local Vasodilation
During systemic hypoxia there is a compensatory vasodilation that mirrors the decrease in O2 content (25, 26, 47, 160, 161). However, during local reductions in O2 availability (via hypoperfusion) the compensatory response appears to be less than perfect in humans (20–23). That is, flow does not return completely (100% recovery) to preinflation levels. One potential explanation for the incomplete compensatory vasodilation during acute hypoperfusion may be related to an initial rise in vascular resistance at the onset of balloon inflation. The initial rise in vascular resistance in these studies is in contrast to the classic view of autoregulation (a decrease in perfusion pressure is followed by a reduction in resistance to blood flow through the muscle) (14, 50, 108, 146). However, the initial increase in vascular resistance is not unprecedented in that an immediate increase in vascular resistance has also been reported to occur in isolated perfused skeletal muscle of dogs (65). The reason for the acute increase in vascular resistance at the onset of balloon inflation in our model of hypoperfusion is not completely clear but may be related to dampening of pulsatile flow by balloon inflation. One possibility is that pulsatile flow is a critical component for the release of endothelium-derived vasodilators (128). Another possibility is that a sudden drop in perfusion pressure causes the resistance vessels to recoil before autoregulatory vasodilation, a response that was also noted by Koch et al. (76) during mild exercise in dogs. Alterations in vascular bed compliance at the onset of balloon inflation may also contribute to the acute increase in vascular resistance observed in our model of hypoperfusion (166). Since no major pressor response is observed at the onset of balloon inflation (20–23) it is unlikely that a marked rise in sympathetic outflow explains the acute increase in vascular resistance.
The increase in vascular resistance at the onset of balloon inflation is followed by gradual autoregulatory compensation such that the vascular resistance decreases over time toward and, in some cases, below preinflation values. In this context the magnitude of blood flow restoration following acute hypoperfusion in contracting human skeletal muscle is predicted by the reduction in downstream forearm vascular resistance (Fig. 4). Additionally, the recovery of flow is not associated with changes in balloon resistance and systemic arterial pressure (i.e., pressor response). Therefore, local vasodilator mechanisms must be responsible for the reduction in vascular resistance and restoration of flow to hypoperfused contracting forearm muscles.
Fig. 4.
Linear regression analysis of the relationship between % recovery of forearm blood flow (FBF) and reduction in vascular resistance (%) during balloon inflation (n = 151) from all trials performed in references (20–23). The correlation demonstrates that the magnitude of flow restoration is predicted by the reduction in vascular resistance during forearm exercise with hypoperfusion.
VASODILATOR MECHANISMS: USUAL SUSPECTS REVISITED?
The vasodilator mechanisms in the microcirculation that contribute to the regulation of flow distal to the occlusion likely include a myogenic response of the microcirculation and all the mechanisms that are involved in vasodilation in response to increased metabolic needs, including endothelial factors and metabolic messengers released from the muscle. As previously mentioned, NO appears to contribute to the regulation of skeletal muscle blood flow during systemic hypoxia (11, 19, 25, 29, 158). Similarly NOS inhibition during moderate intensity handgrip exercise blunts the magnitude of restoration of forearm blood flow and vascular conductance in hypoperfused contracting muscles (21). Moreover, the compensatory vasodilation to hypoperfusion in the contracting human forearm was slowed with NOS inhibition. To our knowledge this is the only study to examine the contribution of NO to the restoration of blood flow in hypoperfused contracting skeletal muscle. However, parallel findings have been reported in the coronary circulation (40, 142). Duncker and Bache (40) assessed the contribution of NO to the vasodilation of coronary resistance arteries during exercise in the presence of a flow-limiting coronary stenosis. Inhibition of NO production during partial inflation of a hydraulic coronary occluder in dogs performing moderate treadmill exercise resulted in a decreased mean myocardial blood flow in the region perfused by the stenotic artery, but not in the normally perfused control region.
A substantial recovery of forearm blood flow still remains after NOS inhibition, thus suggesting that additional vasodilator mechanisms are involved in the compensatory response. Previous evidence in the Seattle model suggests that adenosine receptor antagonism blunts the recovery of hindlimb blood flow in dogs during high-intensity exercise in response to partial vascular occlusion (76). Additionally, active hyperemia in dog skeletal muscle is potentiated during experimentally restricted flow in the presence of dipyridamole, thus suggesting a role of adenosine in the regulation of blood flow under conditions of arterial occlusion (73). Furthermore, adenosine production is positively correlated to the degree of hypoperfusion in the coronary circulation of dogs (34) and appears to contribute to vasodilation of the coronary resistance vessels during exercise in the presence of a flow-limiting stenosis (82). In agreement with the aforementioned studies in dogs, data in the human forearm suggest 1) adenosine contributes to the overall magnitude of compensatory vasodilation during exercise with acute hypoperfusion in a manner that can be independent of NO-mediated mechanisms and 2) combined inhibition of adenosine receptors and NO formation results in additive reduction in the compensatory vasodilation (20).
Lastly, prostaglandins have been reported to be involved in the regulation of skeletal muscle blood flow following periods of ischemia (18, 43, 71, 102) and during systemic hypoxia (29, 90, 114). However, non-selective cyclooxygenase (COX) inhibition with Ketorolac to block the production of prostaglandins failed to reduce the recovery of forearm blood flow and vascular conductance during exercise with acute hypoperfusion (22). These findings are in agreement with available data in the coronary circulation of pigs where COX inhibition with indomethacin did not alter coronary blood flow in response to an experimentally induced flow-limiting stenosis (129). However, COX inhibition in patients with coronary artery disease causes coronary vasoconstriction (38, 44), thus suggesting that vasodilator prostaglandins may play greater role in the regulation of coronary vasomotor control in chronic vs. acute ischemia and in blood vessels subjected to atherosclerotic disease processes. Taken together, the mechanisms involved in the compensatory vasodilator response to acute hypoperfusion in contracting human muscle suggests that adenosine and NO contribute, whereas prostaglandins are not obligatory (Fig. 5).
Fig. 5.
Reduction in compensatory vasodilation [i.e., % recovery of forearm vascular conductance (FVC) compared with respective control (no drug) trial] during various pharmacological trials. Single inhibition of NOS with NG-monomethyl-l-arginine (white bar) and adenosine receptor blockade with aminophylline (black bar) substantially attenuates the compensatory vasodilation during exercise with acute hypoperfusion. Combined NOS/ADO inhibition (dark gray bar) reveals an even greater reduction in %FVC compared with blockade of each factor alone. Single inhibition of COX with Ketorolac has minimal effect on the compensatory vasodilation (light gray bar). Combined NOS/COX inhibition (hatched bar) has minimal effect on the compensatory vasodilation compared with NOS inhibition alone (white bar). Data were derived from Refs. 20–22. ADO, adenosine; COX, cyclooxygenase; NOS, nitric oxide synthase; PG, prostaglandin.
SUMMARY AND PERSPECTIVES
This review highlights the key vasodilator signals and/or pathways that contribute to the local control of skeletal muscle blood flow during exercise under conditions with reduced oxygen availability (Table 1). Both acute systemic (hypoxia) and local (hypoperfusion) mediated reductions in O2 availability elicit a compensatory vasodilator response. Although NO appears to be a common link between the hypoxia and hypoperfusion-induced vasodilation (Table 1), its stimulus and/or interactions with other vasodilator systems varies between the two conditions. Both NO and adenosine contribute to the compensatory vasodilator responses to hypoperfusion (20, 21). By contrast, adenosine does not seem to play a major role in the compensatory vasodilator responses to hypoxia (25, 26, 59). Thus the story with adenosine is mixed; during hypoperfusion it appears to contribute in a synergistic manner with nitric oxide to compensatory vasodilation. By contrast, during hypoxia it does not contribute. Although combined NOS/COX inhibition has been demonstrated to reduce the compensatory vasodilator response during hypoxic forearm exercise (29), it is unclear what the independent role of prostaglandins are in this response. Available evidence suggests prostaglandins are not obligatory to the compensatory dilation observed during forearm exercise with hypoperfusion (22).
Table 1.
Systemic vs. local reductions in oxygen availability: contribution of local vasodilators in the control of human skeletal muscle blood flow during exercise
| Systemic Hypoxia | Local Hypoperfusion | |
|---|---|---|
| (Low O2) | (Low O2 + ↓ perfusion) | |
| Perfect (proportional to the fall in arterial O2 content) | Compensation | Imperfect (<100% recovery) |
| ++ | β-adrenergic receptor activation* | ? |
| ++ | Nitric oxide | ++ |
| − | Adenosine | ++ |
| ? | Prostaglandins | − |
Is exercise intensity dependent; contribution decreases with increased exercise intensity. ++Contributes to the compensatory vasodilation; − is not obligatory in the compensatory; vasodilation; ?lack of evidence for or against a role in the compensatory vasodilation.
Despite the majority of the data presented in this review being from studies in young apparently healthy adults, it has important implications for understanding conditions associated with impaired endothelial function or reduced NO bioavailability. In this context, data from aging humans clearly demonstrate an attenuated compensatory vasodilation during hypoxic exercise, which appears to be from reduced NO signaling (27). Additionally, the observation that NO contributes to the compensatory flow response in the microcirculation during an acute proximal occlusion (21) raises concerns that the ability to restore flow might be impaired in conditions with endothelial dysfunction and/or reduced NO bioavailability (i.e., vascular disease). Therefore, patients with impaired endothelium-dependent dilation are likely to be more susceptible to hypoperfusion and/or ischemia during exercise, especially in vascular regions distal to a stenosis. Finally, there appears to be a great deal of similarity between the regulation of resistance vessel tone in human skeletal muscle (20–22) with those observed in the coronary circulation of dogs and pigs during exercise with acute hypoperfusion (34, 40, 41, 82, 129). Thus an impaired compensatory vasodilator response in the forearm might reflect impairments in other vascular beds.
In conclusion, the available literature reviewed here shows that under most circumstances skeletal muscle vasodilation is tightly coupled to changes in available O2. Indeed, the compensatory vasodilation observed during hypoxic exercise generally mirrors the fall in CaO2. Conversely, under conditions of increased O2 availability (i.e., hyperoxia), vascular conductance is reduced and mirrors the increase in CaO2. Taken together, these findings suggest that skeletal muscle blood flow is perfectly matched to changes in systemic O2 availability. In contrast, the compensatory vasodilation in response to local reductions in O2 availability via hypoperfusion appears to be less than perfect in human skeletal muscle.
GRANTS
This work was supported by the National Institutes of Health research Grants HL-46493 (to M. J. Joyner) and AR-55819 (to D.P. Casey) and by CTSA RR-024150. The Caywood Professorship via the Mayo Foundation also supported this research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.P.C. prepared figures; D.P.C. and M.J.J. drafted manuscript; D.P.C. and M.J.J. edited and revised manuscript; D.P.C. and M.J.J. approved final version of manuscript.
REFERENCES
- 1. Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O'Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 280: H1645–H1652, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Bachofen M, Gage A, Bachofen H. Vascular response to changes in blood oxygen tension under various blood flow rates. Am J Physiol 220: 1786–1792, 1971 [DOI] [PubMed] [Google Scholar]
- 5. Barreto-Filho JA, Consolim-Colombo FM, Guerra-Riccio GM, Santos RD, Chacra AP, Lopes HF, Teixeira SH, Martinez T, Krieger JE, Krieger EM. Hypercholesterolemia blunts forearm vasorelaxation and enhances the pressor response during acute systemic hypoxia. Arterioscler Thromb Vasc Biol 23: 1660–1666, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 26: 40–47, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Berne RM. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol 204: 317–322, 1963 [DOI] [PubMed] [Google Scholar]
- 8. Bird AD, Telfer AB. The effect of oxygen at 1 and 2 atmospheres on resting forearm blood flow. Surg Gynecol Obstet 123: 260–268, 1966 [PubMed] [Google Scholar]
- 9. Black JE, Roddie IC. The mechanism of the changes in forearm vascular resistance during hypoxia. J Physiol 143: 226–235, 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blauw GJ, Westendorp RG, Simons M, Chang PC, Frolich M, Meinders AE. beta-Adrenergic receptors contribute to hypoxaemia induced vasodilation in man. Br J Clin Pharmacol 40: 453–458, 1995 [PMC free article] [PubMed] [Google Scholar]
- 11. Blitzer ML, Lee SD, Creager MA. Endothelium-derived nitric oxide mediates hypoxic vasodilation of resistance vessels in humans. Am J Physiol Heart Circ Physiol 271: H1182–H1185, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Boegehold MA, Johnson PC. Periarteriolar and tissue PO2 during sympathetic escape in skeletal muscle. Am J Physiol Heart Circ Physiol 254: H929–H936, 1988 [DOI] [PubMed] [Google Scholar]
- 13. Boushel R, Langberg H, Risum N, Kjaer M. Regulation of blood flow by prostaglandins. Current Vascular Pharmacol 2: 191–197, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Britton SL, Metting PJ, Ronau TF, Strader JR, Weldy DL. Autoregulation of hind-limb blood flow in conscious dogs. J Physiol 368: 409–422, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bryan PT, Marshall JM. Adenosine receptor subtypes and vasodilatation in rat skeletal muscle during systemic hypoxia: a role for A1 receptors. J Physiol 514: 151–162, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol Regul Integr Comp Physiol 284: R291–R303, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Calbet JA, Lundby C, Sander M, Robach P, Saltin B, Boushel R. Effects of ATP-induced leg vasodilation on VO2 peak and leg O2 extraction during maximal exercise in humans. Am J Physiol Regul Integr Comp Physiol 291: R447–R453, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Carlsson I, Wennmalm A. Effect of different prostaglandin synthesis inhibitors on post-occlusive blood flow in human forearm. Prostaglandins 26: 241–252, 1983 [DOI] [PubMed] [Google Scholar]
- 19. Casey DP, Curry TB, Wilkins BW, Joyner MJ. Nitric oxide mediated vasodilation becomes independent of β-adrenergic receptor activation with increased intensity of hypoxic exercise. J Appl Physiol 110: 687–694, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Casey DP, Joyner MJ. Contribution of adenosine to the compensatory dilation in hypoperfused contracting human muscles is independent of nitric oxide. J Appl Physiol 110: 1181–1189, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Casey DP, Joyner MJ. NOS inhibition blunts and delays the compensatory dilation in hypoperfused contracting human muscles. J Appl Physiol 107: 1685–1692, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Casey DP, Joyner MJ. Prostaglandins do not contribute to the nitric oxide-mediated compensatory vasodilation in hypoperfused exercising muscle. Am J Physiol Heart Circ Physiol 301: H261–H268, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Casey DP, Joyner MJ. Skeletal muscle blood flow responses to hypoperfusion at rest and during rhythmic exercise in humans. J Appl Physiol 107: 429–437, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Casey DP, Joyner MJ, Claus PL, Curry TB. Hyperbaric hyperoxia reduces exercising forearm blood flow in humans. Am J Physiol Heart Circ Physiol 300: H1892–H1897, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Casey DP, Madery BD, Curry TB, Eisenach JH, Wilkins BW, Joyner MJ. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J Physiol 588: 373–385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Casey DP, Madery BD, Pike TL, Eisenach JH, Dietz NM, Joyner MJ, Wilkins BW. Adenosine receptor antagonist and augmented vasodilation during hypoxic exercise. J Appl Physiol 107: 1128–1137, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Casey DP, Walker BG, Curry TB, Joyner MJ. Ageing reduces the compensatory vasodilatation during hypoxic exercise: the role of nitric oxide. J Physiol 589: 1477–1488, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collins DM, McCullough WT, Ellsworth ML. Conducted vascular responses: communication across the capillary bed. Microvasc Res 56: 43–53, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Augmented skeletal muscle hyperaemia during hypoxic exercise in humans is blunted by combined inhibition of nitric oxide and vasodilating prostaglandins. J Physiol 589: 3671–3683, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Daley JC, 3rd, Khan MH, Hogeman CS, Sinoway LI. Autonomic and vascular responses to reduced limb perfusion. J Appl Physiol 95: 1493–1498, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Dawes M, Chowienczyk PJ, Ritter JM. Effects of inhibition of the L-arginine/nitric oxide pathway on vasodilation caused by beta-adrenergic agonists in human forearm. Circulation 95: 2293–2297, 1997 [DOI] [PubMed] [Google Scholar]
- 32. DeLorey DS, Shaw CN, Shoemaker JK, Kowalchuk JM, Paterson DH. The effect of hypoxia on pulmonary O2 uptake, leg blood flow and muscle deoxygenation during single-leg knee-extension exercise. Exp Physiol 89: 293–302, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Delp MD, Laughlin MH. Regulation of skeletal muscle perfusion during exercise. Acta Physiol Scand 162: 411–419, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Deussen A, Moser G, Schrader J. Contribution of coronary endothelial cells to cardiac adenosine production. Pflügers Arch 406: 608–614, 1986 [DOI] [PubMed] [Google Scholar]
- 35. Dinenno FA, Joyner MJ. Alpha-adrenergic control of skeletal muscle circulation at rest and during exercise in aging humans. Microcirculation 13: 329–341, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol 553: 281–292, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dinenno FA, Joyner MJ, Halliwill JR. Failure of systemic hypoxia to blunt alpha-adrenergic vasoconstriction in the human forearm. J Physiol 549: 985–994, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duffy SJ, Castle SF, Harper RW, Meredith IT. Contribution of vasodilator prostanoids and nitric oxide to resting flow, metabolic vasodilation, and flow-mediated dilation in human coronary circulation. Circulation 100: 1951–1957, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Dufour SP, Patel RP, Brandon A, Teng X, Pearson J, Barker H, Ali L, Yuen AH, Smolenski RT, Gonzalez-Alonso J. Erythrocyte-dependent regulation of human skeletal muscle blood flow: role of varied oxyhemoglobin and exercise on nitrite, S-nitrosohemoglobin, and ATP. Am J Physiol Heart Circ Physiol 299: H1936–H1946, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Duncker DJ, Bache RJ. Inhibition of nitric oxide production aggravates myocardial hypoperfusion during exercise in the presence of a coronary artery stenosis. Circ Res 74: 629–640, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 88: 1009–1086, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology 24: 107–116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol 81: 1807–1814, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Friedman PL, Brown EJ, Jr, Gunther S, Alexander RW, Barry WH, Mudge GH, Jr, Grossman W. Coronary vasoconstrictor effect of indomethacin in patients with coronary-artery disease. N Engl J Med 305: 1171–1175, 1981 [DOI] [PubMed] [Google Scholar]
- 45. Garovic VD, Joyner MJ, Dietz NM, Boerwinkle E, Turner ST. Beta(2)-adrenergic receptor polymorphism and nitric oxide-dependent forearm blood flow responses to isoproterenol in humans. J Physiol 546: 583–589, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gladwin MT. Evidence mounts that nitrite contributes to hypoxic vasodilation in the human circulation. Circulation 117: 594–597, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Gonzalez-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: role of erythrocyte count and oxygenation state of haemoglobin. J Physiol 572: 295–305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91: 1046–1055, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Gonzalez-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol 530: 331–341, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Granger HJ, Goodman AH, Granger DN. Role of resistance and exchange vessels in local microvascular control of skeletal muscle oxygenation in the dog. Circ Res 38: 379–385, 1976 [DOI] [PubMed] [Google Scholar]
- 51. Halliwill JR. Hypoxic regulation of blood flow in humans. Skeletal muscle circulation and the role of epinephrine. Adv Exp Med Biol 543: 223–236, 2003 [PubMed] [Google Scholar]
- 52. Halliwill JR, Minson CT. Effect of hypoxia on arterial baroreflex control of heart rate and muscle sympathetic nerve activity in humans. J Appl Physiol 93: 857–864, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O'Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000 [DOI] [PubMed] [Google Scholar]
- 54. Hanada A, Sander M, Gonzalez-Alonso J. Human skeletal muscle sympathetic nerve activity, heart rate and limb haemodynamics with reduced blood oxygenation and exercise. J Physiol 551: 635–647, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hansen J, Sander M, Hald CF, Victor RG, Thomas GD. Metabolic modulation of sympathetic vasoconstriction in human skeletal muscle: role of tissue hypoxia. J Physiol 527: 387–396, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hansen J, Sayad D, Thomas GD, Clarke GD, Peshock RM, Victor RG. Exercise-induced attenuation of alpha-adrenoceptor mediated vasoconstriction in humans: evidence from phase-contrast MRI. Cardiovasc Res 41: 220–228, 1999 [DOI] [PubMed] [Google Scholar]
- 57. Hansen J, Thomas GD, Harris SA, Parsons WJ, Victor RG. Differential sympathetic neural control of oxygenation in resting and exercising human skeletal muscle. J Clin Invest 98: 584–596, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hansen M, Madsen J. Estimation of relative changes in resting muscle blood flow by 133Xe washout: the effect of oxygen. Scand J Clin Lab Invest 31: 133–139, 1973 [DOI] [PubMed] [Google Scholar]
- 59. Heinonen IH, Kemppainen J, Kaskinoro K, Peltonen JE, Borra R, Lindroos M, Oikonen V, Nuutila P, Knuuti J, Boushel R, Kalliokoski KK. Regulation of human skeletal muscle perfusion and its heterogeneity during exercise in moderate hypoxia. Am J Physiol Regul Integr Comp Physiol 299: R72–R79, 2010 [DOI] [PubMed] [Google Scholar]
- 60. Heistad DD, Abboud FM, Mark AL, Schmid PG. Effect of hypoxemia on responses to norepinephrine and angiotensin in coronary and muscular vessels. J Pharmacol Exp Ther 193: 941–950, 1975 [PubMed] [Google Scholar]
- 61. Heistad DD, Wheeler RC. Effect of acute hypoxia on vascular responsiveness in man. I. Responsiveness to lower body negative pressure and ice on the forehead. II. Responses to norepinephrine and angiotensin 3. Effect of hypoxia and hypocapnia. J Clin Invest 49: 1252–1265, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Houssiere A, Najem B, Cuylits N, Cuypers S, Naeije R, van de Borne P. Hyperoxia enhances metaboreflex sensitivity during static exercise in humans. Am J Physiol Heart Circ Physiol 291: H210–H215, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Ichinose M, Delliaux S, Watanabe K, Fujii N, Nishiyasu T. Evaluation of muscle metaboreflex function through graded reduction in forearm blood flow during rhythmic handgrip exercise in humans. Am J Physiol Heart Circ Physiol 301: H609–H616, 2011 [DOI] [PubMed] [Google Scholar]
- 64. Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol 280: H2833–H2839, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Jones RD, Berne RM. Intrinsic regulation of skeletal muscle blood flow. Circ Res 14: 126–138, 1964 [DOI] [PubMed] [Google Scholar]
- 66. Joyner MJ. Does the pressor response to ischemic exercise improve blood flow to contracting muscles in humans? J Appl Physiol 71: 1496–1501, 1991 [DOI] [PubMed] [Google Scholar]
- 67. Joyner MJ. Muscle chemoreflexes and exercise in humans. Clin Auton Res 2: 201–208, 1992 [DOI] [PubMed] [Google Scholar]
- 68. Joyner MJ, Wilkins BW. Exercise hyperaemia: is anything obligatory but the hyperaemia? J Physiol 583: 855–860, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Katayama K, Ishida K, Iwamoto E, Iemitsu M, Koike T, Saito M. Hypoxia augments muscle sympathetic neural response to leg cycling. Am J Physiol Regul Integr Comp Physiol 301: R456–R464, 2011 [DOI] [PubMed] [Google Scholar]
- 70. Kaufman MP, Hayes SG. The exercise pressor reflex. Clin Auton Res 12: 429–439, 2002 [DOI] [PubMed] [Google Scholar]
- 71. Kilbom A, Wennmalm A. Endogenous prostaglandins as local regulators of blood flow in man: effect of indomethacin on reactive and functional hyperaemia. J Physiol 257: 109–121, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim JK, Sala-Mercado JA, Rodriguez J, Scislo TJ, O'Leary DS. Arterial baroreflex alters strength and mechanisms of muscle metaboreflex during dynamic exercise. Am J Physiol Heart Circ Physiol 288: H1374–H1380, 2005 [DOI] [PubMed] [Google Scholar]
- 73. Klabunde RE. Conditions for dipyridamole potentiation of skeletal muscle active hyperemia. Am J Physiol Heart Circ Physiol 250: H62–H67, 1986 [DOI] [PubMed] [Google Scholar]
- 74. Knight DR, Schaffartzik W, Poole DC, Hogan MC, Bebout DE, Wagner PD. Effects of hyperoxia on maximal leg O2 supply and utilization in men. J Appl Physiol 75: 2586–2594, 1993 [DOI] [PubMed] [Google Scholar]
- 75. Koch DW, Newcomer SC, Proctor DN. Blood flow to exercising limbs varies with age, gender, and training status. Can J Appl Physiol 30: 554–575, 2005 [DOI] [PubMed] [Google Scholar]
- 76. Koch LG, Strick DM, Britton SL, Metting PJ. Reflex versus autoregulatory control of hindlimb blood flow during treadmill exercise in dogs. Am J Physiol Heart Circ Physiol 260: H436–H444, 1991 [DOI] [PubMed] [Google Scholar]
- 77. Koskolou MD, Calbet JA, Radegran G, Roach RC. Hypoxia and the cardiovascular response to dynamic knee-extensor exercise. Am J Physiol Heart Circ Physiol 272: H2655–H2663, 1997 [DOI] [PubMed] [Google Scholar]
- 78. Koskolou MD, Roach RC, Calbet JA, Radegran G, Saltin B. Cardiovascular responses to dynamic exercise with acute anemia in humans. Am J Physiol Heart Circ Physiol 273: H1787–H1793, 1997 [DOI] [PubMed] [Google Scholar]
- 79. Kravec TF, Eggers GW, Jr, Kettel LJ. Influence of patient age on forearm and systemic vascular response to hypoxaemia. Clin Sci 42: 555–565, 1972 [DOI] [PubMed] [Google Scholar]
- 80. Laughlin MH. Skeletal muscle blood flow capacity: role of muscle pump in exercise hyperemia. Am J Physiol Heart Circ Physiol 253: H993–H1004, 1987 [DOI] [PubMed] [Google Scholar]
- 81. Laughlin MH, Armstrong RB. Muscle blood flow during locomotory exercise. Exerc Sport Sci Rev 13: 95–136, 1985 [PubMed] [Google Scholar]
- 82. Laxson DD, Homans DC, Bache RJ. Inhibition of adenosine-mediated coronary vasodilation exacerbates myocardial ischemia during exercise. Am J Physiol Heart Circ Physiol 265: H1471–H1477, 1993 [DOI] [PubMed] [Google Scholar]
- 83. Leuenberger U, Gleeson K, Wroblewski K, Prophet S, Zelis R, Zwillich C, Sinoway L. Norepinephrine clearance is increased during acute hypoxemia in humans. Am J Physiol Heart Circ Physiol 261: H1659–H1664, 1991 [DOI] [PubMed] [Google Scholar]
- 84. Leuenberger UA, Gray K, Herr MD. Adenosine contributes to hypoxia-induced forearm vasodilation in humans. J Appl Physiol 87: 2218–2224, 1999 [DOI] [PubMed] [Google Scholar]
- 85. Leuenberger UA, Johnson D, Loomis J, Gray KS, MacLean DA. Venous but not skeletal muscle interstitial nitric oxide is increased during hypobaric hypoxia. Eur J Appl Physiol 102: 457–461, 2008 [DOI] [PubMed] [Google Scholar]
- 86. Limberg JK, Evans TD, Blain GM, Pegelow DF, Danielson JR, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG. Effect of obesity and metabolic syndrome on hypoxic vasodilation. Eur J Appl Physiol (June 9, 2011) Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lorentsen E. Systemic arterial blood pressure during exercise in patients with atherosclerosis obliterans of the lower limbs. Circulation 46: 257–263, 1972 [DOI] [PubMed] [Google Scholar]
- 88. Lundby C, Boushel R, Robach P, Moller K, Saltin B, Calbet JA. During hypoxic exercise some vasoconstriction is needed to match O2 delivery with O2 demand at the microcirculatory level. J Physiol 586: 123–130, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 57: 461–469, 1985 [DOI] [PubMed] [Google Scholar]
- 90. Markwald RR, Kirby BS, Crecelius AR, Carlson RE, Voyles WF, Dinenno FA. Combined inhibition of nitric oxide and vasodilating prostaglandins abolishes forearm vasodilatation to systemic hypoxia in healthy humans. J Physiol 589: 1979–1990, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Martin EA, Nicholson WT, Eisenach JH, Charkoudian N, Joyner MJ. Influences of adenosine receptor antagonism on vasodilator responses to adenosine and exercise in adenosine responders and nonresponders. J Appl Physiol 101: 1678–1684, 2006 [DOI] [PubMed] [Google Scholar]
- 92. Mazzeo RS, Bender PR, Brooks GA, Butterfield GE, Groves BM, Sutton JR, Wolfel EE, Reeves JT. Arterial catecholamine responses during exercise with acute and chronic high-altitude exposure. Am J Physiol Endocrinol Metab 261: E419–E424, 1991 [DOI] [PubMed] [Google Scholar]
- 93. McClain J, Hardy C, Enders B, Smith M, Sinoway L. Limb congestion and sympathoexcitation during exercise. Implications for congestive heart failure. J Clin Invest 92: 2353–2359, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. McCullough WT, Collins DM, Ellsworth ML. Arteriolar responses to extracellular ATP in striated muscle. Am J Physiol Heart Circ Physiol 272: H1886–H1891, 1997 [DOI] [PubMed] [Google Scholar]
- 95. Metting PJ, Weldy DL, Ronau TF, Britton SL. Effect of aminophylline on hindlimb blood flow autoregulation during increased metabolism in dogs. J Appl Physiol 60: 1857–1864, 1986 [DOI] [PubMed] [Google Scholar]
- 96. Mortensen SP, Gonzalez-Alonso J, Bune LT, Saltin B, Pilegaard H, Hellsten Y. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol 296: R1140–R1148, 2009 [DOI] [PubMed] [Google Scholar]
- 97. Mortensen SP, Gonzalez-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol 581: 853–861, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mortensen SP, Nyberg M, Thaning P, Saltin B, Hellsten Y. Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension 53: 993–999, 2009 [DOI] [PubMed] [Google Scholar]
- 99. Mortensen SP, Thaning P, Nyberg M, Saltin B, Hellsten Y. Local release of ATP into the arterial inflow and venous drainage of human skeletal muscle: insight from ATP determination with the intravascular microdialysis technique. J Physiol 589: 1847–1857, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Neylon M, Marshall JM. The role of adenosine in the respiratory and cardiovascular response to systemic hypoxia in the rat. J Physiol 440: 529–545, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nicholson WT, Vaa B, Hesse C, Eisenach JH, Joyner MJ. Aging is associated with reduced prostacyclin-mediated dilation in the human forearm. Hypertension 53: 973–978, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Nowak J, Wennmalm A. A study on the role of endogenous prostaglandins in the development of exercise-induced and post-occlusive hyperemia in human limbs. Acta Physiol Scand 106: 365–369, 1979 [DOI] [PubMed] [Google Scholar]
- 103. Nyberg M, Mortensen SP, Thaning P, Saltin B, Hellsten Y. Interstitial and plasma adenosine stimulate nitric oxide and prostacyclin formation in human skeletal muscle. Hypertension 56: 1102–1108, 2010 [DOI] [PubMed] [Google Scholar]
- 104. O'Leary DS, Augustyniak RA. Muscle metaboreflex increases ventricular performance in conscious dogs. Am J Physiol Heart Circ Physiol 275: H220–H224, 1998 [DOI] [PubMed] [Google Scholar]
- 105. O'Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle in dogs? Am J Physiol Heart Circ Physiol 268: H980–H986, 1995 [DOI] [PubMed] [Google Scholar]
- 106. Pasgaard T, Stankevicius E, Jorgensen MM, Ostergaard L, Simonsen U, Frobert O. Hyperoxia reduces basal release of nitric oxide and contracts porcine coronary arteries. Acta Physiol Scand 191: 285–296, 2007 [DOI] [PubMed] [Google Scholar]
- 107. Persson MG, Ohlen A, Lindbom L, Hedqvist P, Gustafsson LE. Role of adenosine in functional hyperemia in skeletal muscle as indicated by pharmacological tools. Naunyn Schmiedebergs Arch Pharmacol 343: 52–57, 1991 [DOI] [PubMed] [Google Scholar]
- 108. Ping P, Johnson PC. Role of myogenic response in enhancing autoregulation of flow during sympathetic nerve stimulation. Am J Physiol Heart Circ Physiol 263: H1177–H1184, 1992 [DOI] [PubMed] [Google Scholar]
- 109. Pohl U, Busse R. Hypoxia stimulates release of endothelium-derived relaxant factor. Am J Physiol Heart Circ Physiol 256: H1595–H1600, 1989 [DOI] [PubMed] [Google Scholar]
- 110. Poucher SM. The role of the A(2A) adenosine receptor subtype in functional hyperaemia in the hindlimb of anaesthetized cats. J Physiol 492: 495–503, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Poucher SM, Nowell CG, Collis MG. The role of adenosine in exercise hyperaemia of the gracilis muscle in anaesthetized cats. J Physiol 427: 19–29, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Radegran G, Calbet JA. Role of adenosine in exercise-induced human skeletal muscle vasodilatation. Acta Physiol Scand 171: 177–185, 2001 [DOI] [PubMed] [Google Scholar]
- 113. Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998 [PubMed] [Google Scholar]
- 114. Ray CJ, Abbas MR, Coney AM, Marshall JM. Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies. J Physiol 544: 195–209, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ray CJ, Marshall JM. Measurement of nitric oxide release evoked by systemic hypoxia and adenosine from rat skeletal muscle in vivo. J Physiol 568: 967–978, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Reich T, Tuckman J, Naftchi NE, Jacobson JH. Effect of normo- and hyperbaric oxygenation on resting and postexercise calf blood flow. J Appl Physiol 28: 275–278, 1970 [DOI] [PubMed] [Google Scholar]
- 117. Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res 11: 370–380, 1962 [DOI] [PubMed] [Google Scholar]
- 118. Richardson DW, Kontos HA, Raper AJ, Patterson JL., Jr Modification by beta-adrenergic blockade of the circulatory response to acute hypoxia in man. J Clin Invest 46: 77–85, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J, Wagner PD. Evidence of O2 supply-dependent VO2 max in the exercise-trained human quadriceps. J Appl Physiol 86: 1048–1053, 1999 [DOI] [PubMed] [Google Scholar]
- 120. Richardson RS, Knight DR, Poole DC, Kurdak SS, Hogan MC, Grassi B, Wagner PD. Determinants of maximal exercise VO2 during single leg knee-extensor exercise in humans. Am J Physiol Heart Circ Physiol 268: H1453–H1461, 1995 [DOI] [PubMed] [Google Scholar]
- 121. Richardson RS, Noyszewski EA, Saltin B, Gonzalez-Alonso J. Effect of mild carboxy-hemoglobin on exercising skeletal muscle: intravascular and intracellular evidence. Am J Physiol Regul Integr Comp Physiol 283: R1131–R1139, 2002 [DOI] [PubMed] [Google Scholar]
- 122. Roach RC, Koskolou MD, Calbet JA, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol Heart Circ Physiol 276: H438–H445, 1999 [DOI] [PubMed] [Google Scholar]
- 123. Rowell LB. How are cardiovascular and metabolic functions matched during exercise: what is the exercise stimulus? In: Human Circulation: Regulation During Physical Stress. New York: Oxford Univ. Press, 1986, p. 287–327 [Google Scholar]
- 124. Rowell LB, Blackmon JR. Human cardiovascular adjustments to acute hypoxaemia. Clin Physiol 7: 349–376, 1987 [DOI] [PubMed] [Google Scholar]
- 125. Rowell LB, O'Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol 69: 407–418, 1990 [DOI] [PubMed] [Google Scholar]
- 126. Rowell LB, Saltin B, Kiens B, Christensen NJ. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? Am J Physiol Heart Circ Physiol 251: H1038–H1044, 1986 [DOI] [PubMed] [Google Scholar]
- 127. Rowell LB, Savage MV, Chambers J, Blackmon JR. Cardiovascular responses to graded reductions in leg perfusion in exercising humans. Am J Physiol Heart Circ Physiol 261: H1545–H1553, 1991 [DOI] [PubMed] [Google Scholar]
- 128. Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol 250: H1145–H1149, 1986 [DOI] [PubMed] [Google Scholar]
- 129. Ruocco NA, Most AS, Sasken H, Steiner M, Gewirtz H. Role of endogenous prostacyclin in myocardial blood flow regulation distal to a severe coronary stenosis. Cardiovasc Res 22: 511–519, 1988 [DOI] [PubMed] [Google Scholar]
- 130. Saito M, Mano T, Iwase S, Koga K, Abe H, Yamazaki Y. Responses in muscle sympathetic activity to acute hypoxia in humans. J Appl Physiol 65: 1548–1552, 1988 [DOI] [PubMed] [Google Scholar]
- 131. Saltin B. Exercise hyperaemia: magnitude and aspects on regulation in humans. J Physiol 583: 819–823, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand 162: 421–436, 1998 [DOI] [PubMed] [Google Scholar]
- 133. Saunders NR, Dinenno FA, Pyke KE, Rogers AM, Tschakovsky ME. Impact of combined NO and PG blockade on rapid vasodilation in a forearm mild-to-moderate exercise transition in humans. Am J Physiol Heart Circ Physiol 288: H214–H220, 2005 [DOI] [PubMed] [Google Scholar]
- 134. Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol 557: 599–611, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Seals DR, Johnson DG, Fregosi RF. Hyperoxia lowers sympathetic activity at rest but not during exercise in humans. Am J Physiol Regul Integr Comp Physiol 260: R873–R878, 1991 [DOI] [PubMed] [Google Scholar]
- 136. Seals DR, Johnson DG, Fregosi RF. Hypoxia potentiates exercise-induced sympathetic neural activation in humans. J Appl Physiol 71: 1032–1040, 1991 [DOI] [PubMed] [Google Scholar]
- 137. Shepherd JT. Circulation to skeletal muscle. In: Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Blood Flow. Bethesda, MD: Am Physiol Soc, 1983, sect 2, vol. 3, p. 319–370 [Google Scholar]
- 138. Sheriff DD. Latency of muscle chemoreflex to vascular occlusion of active muscle during dynamic exercise. Am J Physiol Heart Circ Physiol 272: H1981–H1985, 1997 [DOI] [PubMed] [Google Scholar]
- 139. Sheriff DD, Wyss CR, Rowell LB, Scher AM. Does inadequate oxygen delivery trigger pressor response to muscle hypoperfusion during exercise? Am J Physiol Heart Circ Physiol 253: H1199–H1207, 1987 [DOI] [PubMed] [Google Scholar]
- 140. Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol 67: 99–145, 2005 [DOI] [PubMed] [Google Scholar]
- 141. Skinner MR, Marshall JM. Studies on the roles of ATP, adenosine and nitric oxide in mediating muscle vasodilatation induced in the rat by acute systemic hypoxia. J Physiol 495: 553–560, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Smith TP, Jr, Canty JM., Jr Modulation of coronary autoregulatory responses by nitric oxide Evidence for flow-dependent resistance adjustments in conscious dogs. Circ Res 73: 232–240, 1993 [DOI] [PubMed] [Google Scholar]
- 143. Somers VK, Mark AL, Zavala DC, Abboud FM. Influence of ventilation and hypocapnia on sympathetic nerve responses to hypoxia in normal humans. J Appl Physiol 67: 2095–2100, 1989 [DOI] [PubMed] [Google Scholar]
- 144. Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart Circ Physiol 275: H1726–H1732, 1998 [DOI] [PubMed] [Google Scholar]
- 145. Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol 281: C1158–C1164, 2001 [DOI] [PubMed] [Google Scholar]
- 146. Stainsby WN. Autoregulation of blood flow in skeletal muscle during increased metabolic activity. Am J Physiol 202: 273–276, 1962 [DOI] [PubMed] [Google Scholar]
- 147. Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 276: 2034–2037, 1997 [DOI] [PubMed] [Google Scholar]
- 148. Stickland MK, Smith CA, Soriano BJ, Dempsey JA. Sympathetic restraint of muscle blood flow during hypoxic exercise. Am J Physiol Regul Integr Comp Physiol 296: R1538–R1546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Sundberg CJ, Kaijser L. Effects of graded restriction of perfusion on circulation and metabolism in the working leg; quantification of a human ischaemia-model. Acta Physiol Scand 146: 1–9, 1992 [DOI] [PubMed] [Google Scholar]
- 150. Thomas GD, Hansen J, Victor RG. Inhibition of alpha 2-adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hindlimb muscle. Am J Physiol Heart Circ Physiol 266: H920–H929, 1994 [DOI] [PubMed] [Google Scholar]
- 151. Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol 506: 817–826, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Tschakovsky ME, Joyner MJ. Nitric oxide and muscle blood flow in exercise. Appl Physiol Nutr Metab 33: 151–161, 2008 [DOI] [PubMed] [Google Scholar]
- 153. Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol 541: 623–635, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Victor RG, Bertocci LA, Pryor SL, Nunnally RL. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest 82: 1301–1305, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Victor RG, Seals DR. Reflex stimulation of sympathetic outflow during rhythmic exercise in humans. Am J Physiol Heart Circ Physiol 257: H2017–H2024, 1989 [DOI] [PubMed] [Google Scholar]
- 156. Wallin BG, Victor RG, Mark AL. Sympathetic outflow to resting muscles during static handgrip and postcontraction muscle ischemia. Am J Physiol Heart Circ Physiol 256: H105–H110, 1989 [DOI] [PubMed] [Google Scholar]
- 157. Weisbrod CJ, Eastwood PR, O'Driscoll G, Walsh JH, Best M, Halliwill JR, Green DJ. Vasomotor responses to hypoxia in type 2 diabetes. Diabetes 53: 2073–2078, 2004 [DOI] [PubMed] [Google Scholar]
- 158. Weisbrod CJ, Minson CT, Joyner MJ, Halliwill JR. Effects of regional phentolamine on hypoxic vasodilatation in healthy humans. J Physiol 537: 613–621, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Welch HG, Bonde-Petersen F, Graham T, Klausen K, Secher N. Effects of hyperoxia on leg blood flow and metabolism during exercise. J Appl Physiol 42: 385–390, 1977 [DOI] [PubMed] [Google Scholar]
- 160. Wilkins BW, Pike TL, Martin EA, Curry TB, Ceridon ML, Joyner MJ. Exercise intensity-dependent contribution of beta-adrenergic receptor-mediated vasodilatation in hypoxic humans. J Physiol 586: 1195–1205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Wilkins BW, Schrage WG, Liu Z, Hancock KC, Joyner MJ. Systemic hypoxia and vasoconstrictor responsiveness in exercising human muscle. J Appl Physiol 101: 1343–1350, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Win TS, Marshall JM. Contribution of prostaglandins to the dilation that follows isometric forearm contraction in human subjects: effects of aspirin and hyperoxia. J Appl Physiol 99: 45–52, 2005 [DOI] [PubMed] [Google Scholar]
- 163. Wolfel EE, Selland MA, Cymerman A, Brooks GA, Butterfield GE, Mazzeo RS, Grover RF, Reeves JT. O2 extraction maintains O2 uptake during submaximal exercise with beta-adrenergic blockade at 4,300 m. J Appl Physiol 85: 1092–1102, 1998 [DOI] [PubMed] [Google Scholar]
- 164. Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol 245: H481–H486, 1983 [DOI] [PubMed] [Google Scholar]
- 165. Yamauchi K, Tsutsui Y, Endo Y, Sagawa S, Yamazaki F, Shiraki K. Sympathetic nervous and hemodynamic responses to lower body negative pressure in hyperbaria in men. Am J Physiol Regul Integr Comp Physiol 282: R38–R45, 2002 [DOI] [PubMed] [Google Scholar]
- 166. Zamir M, Goswami R, Salzer D, Shoemaker JK. Role of vascular bed compliance in vasomotor control in human skeletal muscle. Exp Physiol 92: 841–848, 2007 [DOI] [PubMed] [Google Scholar]
- 167. Zhilyaev SY, Moskvin AN, Platonova TF, Gutsaeva DR, Churilina IV, Demchenko IT. Hyperoxic vasoconstriction in the brain is mediated by inactivation of nitric oxide by superoxide anions. Neurosci Behav Physiol 33: 783–787, 2003 [DOI] [PubMed] [Google Scholar]