Abstract
The negative effects of sympathetic overactivity on long-term cardiovascular health are becoming increasingly clear. Moreover, recent work done in animal models of cardiovascular disease suggests that sympathetic tone to the splanchnic vasculature may play an important role in the development and maintenance of these disease states. Work from our laboratory and others led us to hypothesize that a lack of chronic physical activity increases resting and reflex-mediated splanchnic sympathetic nerve activity, possibly through changes occurring in a key brain stem center involved in sympathetic regulation, the rostral ventrolateral medulla (RVLM). To address this hypothesis, we recorded mean arterial pressure (MAP) and splanchnic sympathetic nerve activity (SSNA) in a group of active and sedentary animals that had been housed for 10–13 wk with or without running wheels, respectively. In experiments performed under Inactin anesthesia, we tested responses to RVLM microinjections of glutamate, responses to baroreceptor unloading, and vascular reactivity, the latter of which was performed under conditions of autonomic blockade. Sedentary animals exhibited enhanced resting SSNA and MAP, augmented increases in SSNA to RVLM activation and baroreceptor unloading, and enhanced vascular reactivity to α1-receptor mediated vasoconstriction. Our results suggest that a sedentary lifestyle increases the risk of cardiovascular disease by augmenting resting and reflex-mediated sympathetic output to the splanchnic circulation and also by increasing vascular sensitivity to adrenergic stimulation. We speculate that regular physical exercise offsets or reverses the progression of these disease processes via similar or disparate mechanisms and warrant further examination into physical (in)activity-induced sympathetic nervous system plasticity.
Keywords: sympathetic nervous system, sedentary lifestyle, blood pressure
cardiovascular disease is currently the leading cause of death in the United States and has an economic impact in the hundreds of billions of dollars (35, 58). One of the primary risk factors for cardiovascular disease is a lack of regular exercise (35). The detrimental effects of a sedentary lifestyle on cardiovascular health have been studied and documented for over 50 yr (2, 11, 48). However, only recently have many conditions linked to physical inactivity, such as the metabolic syndrome, heart failure, and hypertension, been associated with overactivity of the sympathetic nervous system (7, 13, 14, 22, 29, 63, 68). Since sympathetic overactivity can have deleterious effects on the cardiovascular system both directly and indirectly (16, 17), it is important to study the mechanisms by which physical inactivity can raise sympathetic output and negatively impact the cardiovascular system.
Sympathetic activity is generated by sympathetic preganglionic neurons in the spinal cord (10, 22). Sympathetic preganglionic neurons receive direct excitatory input from tonically active neurons in the rostral ventrolateral medulla (RVLM) (23, 34). These spinally projecting RVLM neurons are regulated by the major inhibitory and excitatory neurotransmitters GABA and glutamate, respectively (19, 43). Microinjection of glutamate and glutamate receptor agonists into the RVLM results in large increases in sympathetic nerve activity and blood pressure (1, 18, 39, 52, 60, 62). Our laboratory and others have shown that sedentary vs. active rats exhibit greater indexes of sympathoexcitation when RVLM neurons are activated with glutamate (41, 50). It is possible that chronic physical inactivity affects the excitability of RVLM neurons directly and contributes to sympathetic overactivity and increased risk of cardiovascular disease in sedentary individuals.
RVLM neurons regulate sympathetic nerve activity to various target organs and vascular beds (22). Moreover, sympathetic outflow to separate targets may be differentially altered in disease states (31, 56, 71). Sympathetic outflow to the gut through the splanchnic sympathetic nerve is increased in animal models of hypertension (25, 26, 38, 66). Furthermore, enhanced resting SNA is believed to have direct effects on the vasculature (16, 17). Indeed, in vitro studies have shown that blood vessels from sedentary vs. active subjects have increased vascular reactivity (33). Thus, in addition to enhancement in resting and reflex-mediated sympathetic nerve activity, physical inactivity may also enhance vasoconstrictor reactivity in the vasculature.
The purpose of the present study was to test the hypothesis that chronic physical inactivity leads to augmented resting and reflex-mediated splanchnic sympathetic nerve activity (SSNA), resting blood pressure, and vascular reactivity. We tested these hypotheses by measuring the following parameters and responses in a group of sedentary and physically active rats: 1) resting arterial pressure and SSNA, 2) responses to direct activation of RVLM neurons with glutamate and to baroreceptor unloading, and 3) blood pressure responses to an intravenously injected vasoconstrictor agent in the absence of baroreflex-mediated compensation. Our data indicate for the first time that chronic physical inactivity produces enhanced sympathetic nervous system activity to the splanchnic circulation. We suggest that this effect is mediated at least in part by neuroplastic changes occurring in RVLM neurons.
METHODS
All surgical and experimental procedures were approved by the Institutional Animal Care and Use Committee of Wayne State University and conducted in accordance with the American Physiological Society (APS) publication Guiding Principles in the Care and Use of Animals. All animals received food (Purina LabDiet 5001, Purina Mills, Richmond, IN) and tap water ad libitum.
Daily Spontaneous Running
Male Sprague-Dawley rats (Harlan, Indianapolis, IN, n = 33) weighing 75–100 g at the time of arrival were used for these studies. Animals were housed individually in standard cages outfitted with running wheels (physically active group, n = 16) or without running wheels (sedentary group, n = 17) for 10–13 wk. Running wheels were obtained commercially (Tecniplast, Eaton, PA; wheel diameter 47 cm). Daily running distances and cumulative running distances were recorded via bicycle computers (Sigma Sport, Olney, IL) calibrated to the diameter of the running wheel.
Surgical Procedures
Arterial pressure and sympathetic nerve recordings were performed similar to our previous work (50, 51) and others (25, 26, 65). Briefly, animals were anesthetized with isoflurane (2–3% in 100% O2) and catheters were implanted in the femoral artery and vein. Femoral arterial and venous catheters were used to determine arterial pressure and for the administration of drugs, respectively. The trachea was cannulated and the animals were ventilated artificially with a mixture of isoflurane and oxygen (2–3% in 100% O2). To expose splanchnic sympathetic nerves, a flank incision was made ∼1.5–2.0 cm lateral to the midline. A second incision was made through the underlying muscle to expose the retroperitoneal organs and fat. The incision was retracted on both sides and the retroperitoneal organs were dissected gently from the posterior abdominal muscle wall. Retractors were used to carefully displace the kidney and the adrenal gland, and the adrenal vascular bundle was traced proximally to identify the celiac ganglia. The postganglionic portion of the splanchnic sympathetic nerve originates from the celiac ganglia and courses caudal and medial. A dissected portion of the splanchnic sympathetic nerve was placed on electrodes and covered with Kwik-Sil gel (World Precision Instruments, Sarasota, FL). Once hardened, the gel complex was affixed to retroperitoneal muscle with a small amount of super glue gel (Loctite, Westlake, OH) to minimize motion effects on the electrode. The flank incision was closed with wound clips. Rats were then placed in a stereotaxic apparatus (Kopf, Tujunga, CA). The bite bar was placed on top of the animal's nose and adjusted to +17 mm anteroposterior and −10 mm dorsoventral. A midline incision was performed at the back of the head, and muscle overlying the base of the skull was dissected. Following a partial occipital craniotomy, an incision was made through the atlanto-occipital membrane to expose the brain stem.
Following all surgical procedures, Inactin (0.025 ml/min, 100 mg/kg iv) was given intravenously over 30–60 min and isoflurane was slowly reduced to zero by the end of the infusion. After the infusion was complete, supplemental doses (5 mg iv) were given as needed to prevent withdrawal to toe pinch and maintain steady-state anesthesia. Animals were ventilated (60–80 breaths/min) with 100% O2 for the remainder of the experiment. Arterial blood gases were measured periodically and maintained within normal limits (Po2 > 100 mmHg, Pco2 between 35 and 40 mmHg) by adjusting the rate or volume of the ventilator. Body temperature was monitored with a rectal probe and maintained near 37°C with a heating pad. All experiments were performed within a Faraday cage to reduce electrical noise.
Splanchnic Sympathetic Nerve Recordings
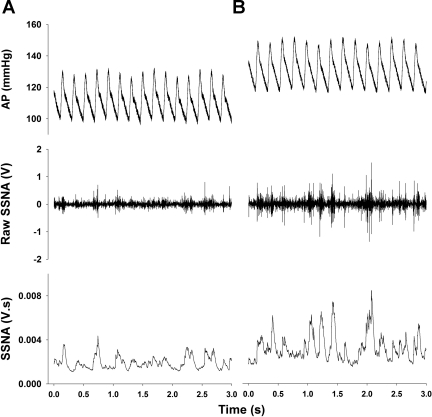
The quality of sympathetic nerve recordings was evaluated in each animal prior to the initiation of any subsequent protocol. As seen in Fig. 1, recordings of amplified (20,000×) nerve activity showed bursting activity that was associated with the diastolic phase of the cardiac cycle. Generally, bursts appeared to be of greater amplitude and frequency in sedentary animals. We made successful recordings in 15 sedentary and 14 active rats. Electrical noise determined post mortem was similar between sedentary and active animals (1.06 ± 0.03 vs. 1.05 ± 0.04 mV/s, respectively, P > 0.05).
Fig. 1.
Mean arterial pressure (MAP) and splanchnic sympathetic nerve recordings. A and B show representative examples of a 3-s window of splanchnic sympathetic nerve activity (SSNA) recorded in 1 active (A) and 1 sedentary rat (B). A raw arterial pressure (AP) tracing is shown at top with raw SSNA recording in the middle. Integrated SSNA calculated from raw activity is shown at the bottom. Post mortem noise has not been subtracted from resting SSNA in this figure. V, volts; V·s, units of rectified, integrated, and amplified SSNA.
Microinjections.
Microinjections were performed similar to previous studies (50, 51). Once steady-state anesthesia was achieved, the animal's head was adjusted to position the caudal tip of area postrema 2.4 mm posterior to the interaural line (30, 47). Triple-barrel glass micropipettes (outside tip diameter 30–60 μm) were inserted into the brain stem using a Kopf stereotactic micromanipulator and surgical microscope. Initial stereotactic coordinates used to locate the RVLM were 0.9–1.1 mm rostral and 1.7–2.2 mm lateral to the caudal tip of area postrema, and 3.5 to 3.7 mm ventral to the dorsal surface of the medulla. Microinjections were achieved with the aid of a commercially available pressure microinjection system with a 3 nl resolution (Parker, Cleveland, OH). Microinjection volumes (30 nl) were measured directly by monitoring the fluid meniscus in each micropipette barrel using a ×150 compound microscope fitted with a calibrated reticule. In all studies, the RVLM was identified both functionally with glutamate and histologically with dye injections. Glutamate (10 mM, 30 nl, 300 pmol) was microinjected to confirm the location of sympathoexcitatory neurons in the RVLM. If the initial microinjection did not produce sympathoexcitation and a pressor response of ≥10 mmHg, additional microinjections were made in surrounding locations until RVLM sympathoexcitatory neurons were localized. We performed RVLM microinjections in 14 sedentary and 13 active rats. At the end of every experiment, microinjection sites in the RVLM were marked with 2% Chicago Sky blue dye. Animals were then overdosed with euthanasia solution (Fatal Plus, Vortech, Dearborn, MI, 0.2 ml) and brains were removed and placed in 4% phosphate buffered formalin solution. Following fixation, brains were transferred to 30% sucrose for cryoprotection. After a minimum of 48 h, the hindbrain was frozen and cut into 50 μm sections on a cryostat. Every other coronal section was mounted on a gel-coated slide and stained with neutral red or mounted on a separate gel-coated slide and left unstained. A compound microscope was used to determine the center of the dye spot, and its location was marked on modified diagrams from a rat atlas (57).
Microinjection protocol—concentration response curves to glutamate-mediated excitation of the RVLM.
Glutamate was microinjected unilaterally into the RVLM at different concentrations (1, 10, and 100 mM in 30 nl or 30, 300, 3,000 pmol). Each concentration of glutamate was microinjected in a random order from separate barrels of the same pipette, and a minimum of 5 min of recovery was allowed between responses. To reduce the overall number of rats needed for this study, we performed more than one experimental protocol in most animals.
Intravenous injections.
Femoral venous catheters were used to inject vasoactive drugs. Sodium nitroprusside (SNP; 0.5–13 μg/kg in 10–100 μl) was used to produce decreases in blood pressure and test baroreflex-mediated excitation of splanchnic sympathetic nerve activity (SSNA). We carried out this protocol in nine sedentary and nine active animals following glutamate microinjections and an additional one sedentary and one active animal independent of the microinjections.
Phenylephrine (PE; 0.25–25 μg/kg in 10–100 μl) was injected after ganglionic blockade with hexamethonium (30 mg/kg) and methyl-atropine (1 mg/kg) to test α1-adrenergic vascular reactivity in the absence of autonomic reflexes. Drugs were injected as a bolus in ascending order of dose. Values of MAP and SSNA were allowed to return to baseline level between injections, typically within 5–10 min. We carried out this protocol in six sedentary and nine active animals following glutamate microinjections and an additional two sedentary and two active animals independent of the microinjections.
Data collection and analysis.
A computer data acquisition system (Power Lab, ADInstruments, Colorado Springs, CO) was used to collect all experimental data. Sympathetic bursting patterns and cardiac synchrony of raw SSNA were verified on an oscilloscope and with an audio monitor. The analog and digital signals were also monitored continuously for irregular or unusual background electrical noise. Only recordings that satisfied these criteria were used in this study. A Grass preamplifier (P511) was used to filter (3 kHz high pass, 30 Hz low pass) and amplify (20,000 times) SSNA. SSNA was electronically rectified and integrated using a time constant of 28 ms. Background noise determined post mortem was subtracted from neural activity in each animal. In all instances, SSNA is reported in millivolts·seconds and represents the rectified, integrated, and amplified signal.
Decreases in blood pressure produced by bolus doses of SNP were averaged within each group as were the respective SSNA responses. Blood pressure decreases were then plotted against corresponding averaged SSNA responses.
Statistical analysis.
Baseline hemodynamic variables, body weights, and organ weights were analyzed by unpaired two-tailed Student's t-tests. The following were analyzed by two-way ANOVA with repeated measures: MAP and SSNA changes at various doses of glutamate, MAP changes at various doses of SNP, and SSNA responses to different levels of MAP. When the two-way ANOVA indicated a significant interaction, differences between individual doses were assessed by post hoc Holm-Sidak test according to a commercially available software package (SigmaStat 3.5, SPSS, Chicago, IL). A probability of P < 0.05 was considered statistically significant and P values above 0.05 but below 0.1 were reported as trends according to guidelines written for APS journals (9). Data are expressed as means ± SE.
Drugs.
Inactin, l-glutamate, PE, and sodium nitroprusside were obtained from Sigma Chemical (St. Louis, MO). Glutamate was dissolved directly in artificial cerebrospinal fluid. Sodium nitroprusside and PE were made fresh each day from concentrated stock solutions by diluting with sterile 0.9% saline. All solutions were pH adjusted to 7.3–7.5 using sodium hydroxide or hydrochloric acid. Glutamate microinjections were relatively short acting with recovery typically within 5 min or less. Recovery from intravenous injections occurred within 5–10 min.
RESULTS
Effects of Chronic Physical (In)Activity on Body Composition and Cardiovascular Variables
Table 1 shows baseline mean arterial pressure (MAP), heart rate (HR), and SSNA and MAP and HR after ganglionic blockade. Also included are body weights recorded just prior to anesthetizing the animal. Inactive rats had slightly but significantly greater body mass. Compared with their physically active counterparts, sedentary rats had significantly elevated resting MAP, and SSNA was elevated more than twofold. Resting HR was not significantly different between groups. Following ganglionic blockade (PostHex) with hexamethonium and methyl-atropine, differences in resting MAP were eliminated. The lack of differences in HR persisted in the presence of ganglionic blockade.
Table 1.
Effects of chronic physical activity on body weight and resting cardiovascular variables
| Group | Sedentary | Active |
|---|---|---|
| n | 17 | 16 |
| Total running distance, km | 203 ± 40 | |
| Body weight, g | 416 ± 6 | 395 ± 6* |
| Resting MAP, mmHg | 121 ± 3 | 112 ± 3* |
| Resting HR, beats/min | 316 ± 7 | 304 ± 12 |
| Resting SSNA, mV · s | 2.10 ± 0.41 (n = 15) | 0.94 ± 0.16* (n = 14) |
| PostHex MAP, mmHg | 74 ± 4 | 69 ± 3 |
| PostHex HR, beats/min | 295 ± 7 | 285 ± 8 |
Values are expressed as means ± SE; n, number of animals. MAP, mean arterial pressure; HR, heart rate; SSNA, splanchnic sympathetic nerve activity; mV · s, units of rectified, integrated, and amplified SSNA; PostHex, values following ganglionic blockade with hexamethonium (30 mg/kg) and methyl atropine (1 mg/kg). Post mortem electrical noise has been subtracted out of resting SSNA values.
P < 0.05.
Effect of Chronic Physical (In)Activity on Responses to Direct Activation of the RVLM
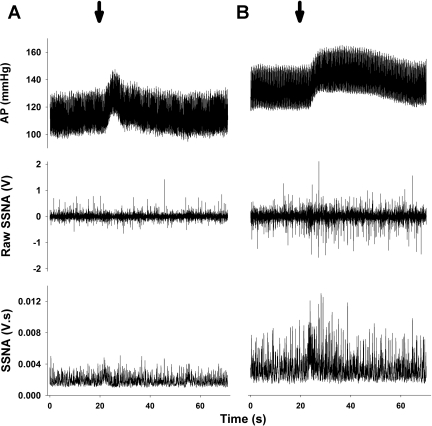
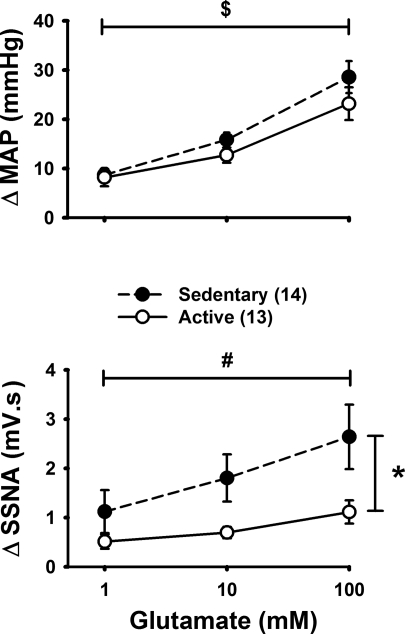
To test the responsiveness of the splanchnic sympathetic nerves to generalized excitation of the RVLM, glutamate was microinjected unilaterally into the RVLM at different concentrations (1, 10, and 100 mM in 30 nl or 30, 300, 3,000 pmol). Example microinjections are shown in Fig. 2 and average data from sedentary and active groups are shown in Fig. 3. Changes in HR were generally small, variable, and not significant between sedentary and active animals (i.e., 8 ± 5 vs. 3 ± 4 beats/min, respectively, P > 0.05 at the highest dose of glutamate). Glutamate produced increases in MAP within both groups that were dose dependent and not statistically different between groups. Similar to MAP responses, glutamate produced dose-dependent increases in SSNA in sedentary animals. However, dose-dependent increases in SSNA were not observed in the active group (Fig. 3) and post hoc analysis revealed a significant enhancement in the response to 100 mM glutamate, and a trend for an enhanced response to 10 mM glutamate (P = 0.064). Nerve activity responses as a percent change from baseline were similar between sedentary and active animals all doses (P > 0.05). For example, at the 100 mM dose, glutamate increased SSNA 156 ± 23% and 172 ± 32%, in sedentary and active animals, respectively. As in our previous studies (50, 51), vehicle control microinjections of aCSF were performed (n = 5) and produced only variable changes in MAP and SSNA that were similar to the natural fluctuations of these parameters.
Fig. 2.
Representative examples of glutamate microinjections (arrows, 10 mM, 30 nl) into the rostral ventrolateral medulla (RVLM) of the active (A) and sedentary (B) rat from Fig. 1. Microinjections of glutamate produced pressor and sympathoexcitatory responses in both animals. Post mortem noise has not been subtracted from resting SSNA in this figure. Abbreviations as defined in Fig. 1 legend.
Fig. 3.
Average data for pressor and sympathoexcitatory response to glutamate (1–100 mM, 30 nl, or 3–3,000 pmol) microinjected into the RVLM of sedentary (n = 14, ●, dashed line) and active (n = 13, ○, solid line) animals. There was a significant difference at 100 mM glutamate (*P < 0.05), and a trend for an enhanced response was seen at 10 mM (P = 0.064). Two-way repeated-measures ANOVA showed a significant interaction between and physical activity level and different doses of glutamate. Post hoc tests showed a significant effect of concentration for sedentary group only (#P < 0.05). There was a significant enhancement in the blood pressure response for active and sedentary groups ($P < 0.05).
Effect of Chronic Physical (In)Activity on Baroreflex-Mediated Activation of SSNA
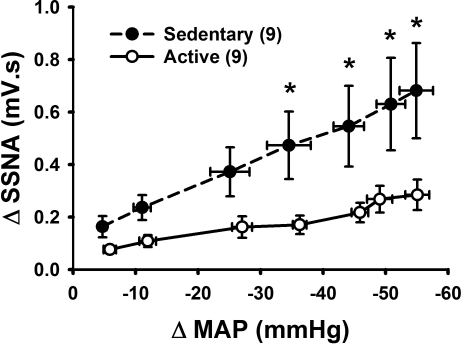
To test the hypothesis that sedentary animals have greater baroreflex-mediated excitation of SSNA, we injected sodium nitroprusside to produce decreases in MAP in sedentary and active animals while recording SSNA. Graded decreases in MAP were statistically similar between groups (P > 0.05) but produced significantly greater increases in SSNA in the sedentary but not active group (Fig. 4). Similarly, there was an overall significant effect of physical activity. Post hoc analysis revealed a significant enhancement in the response to decreases in blood pressure above 30 mmHg. Additionally, SSNA responses expressed as percent change from baseline were significantly enhanced in the sedentary vs. active group at various levels (i.e., 46 ± 8 vs. 28 ± 5%, P < 0.05 for BP decreases of 55 ± 3 and 55 ± 2 mmHg, respectively). These differences occurred although baseline SSNA in sedentary rats was elevated more than twofold compared with their active counterparts. Baroreflex activation also produced increases in HR that were significantly enhanced in sedentary relative to active animals at various levels (i.e., 20 ± 2 vs. 9 ± 1 beats/min, P < 0.05 for BP decrease of 55 ± 3 and 55 ± 2 mmHg, respectively).
Fig. 4.
Average data for SSNA responses to activation of the baroreflex produced by decreases in arterial pressure. Seven doses of sodium nitroprusside were injected intravenously in order of increasing dose and the blood pressure decrease was averaged within groups at each individual dose (SNP, 0.5–13 μg/kg iv). SSNA activation was greater in sedentary (n = 9, ●, dashed line) compared with active (n = 9, ○, solid line) animals. Average reductions in blood pressure at each dose were similar in magnitude between groups by 2-way repeated-measures ANOVA. There was an overall effect of physical activity level on SSNA responses to various levels of MAP, and there was an interaction between physical activity level and decreases in MAP. *P < 0.05 for individual points.
Effect of Chronic Physical (In)Activity on Vascular Reactivity to α1-Adrenergic Receptor-Mediated Vasoconstriction
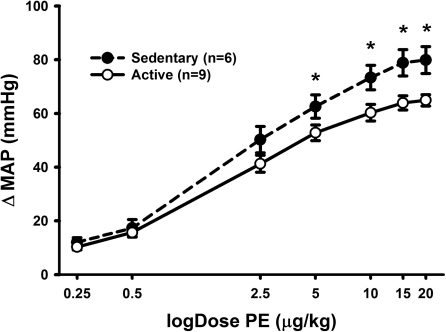
To test the hypothesis that sedentary animals possess greater vascular reactivity, we injected increasing doses of the α1-adrenergic receptor agonist PE intravenously in sedentary and active animals while recording MAP. This was done in the presence of ganglionic blockade to minimize baroreflex compensation. PE produced dose-dependent increases in MAP within both groups (Fig. 5). Sedentary vs. active animals showed significantly enhanced MAP responses. Post hoc testing revealed a significant enhancement in the response to intermediate and higher doses of PE (Fig. 5).
Fig. 5.
Average data for MAP responses to phenylephrine (PE; 0.5–13 μg/kg iv) following ganglionic blockade with hexamethonium (30 mg/kg iv) and methyl-atropine (1 mg/kg). Activation of α1-adrenergic receptors with PE produced dose-dependent increases in MAP in both groups but the response was significantly enhanced in sedentary (n = 6, ●, dashed line) vs. active (n = 9, ○, solid line) animals. Two-way repeated-measures ANOVA showed an overall effect of physical activity level and an interaction between physical activity level and doses of PE (P < 0.05 both); *P < 0.05 specific difference between MAP responses at individual doses.
Histological Identification of Injection Sites
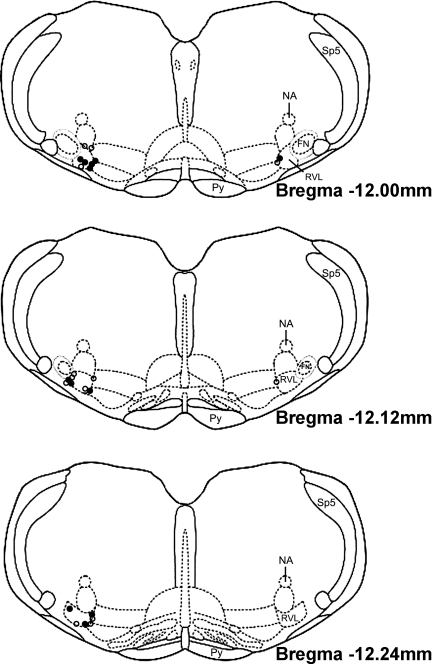
We confirmed our microinjection sites histologically by analyzing injections of 2% Chicago Sky blue dye made at the end of every experiment. We recovered and identified injection sites in 12 sedentary and 13 active animals. A composite representation of the injection sites is shown in Fig. 6. All microinjection sites were located within the 240 μm caudal to the caudal pole of the facial nucleus, lateral to the pyramidal tract, medial to the spinal trigeminal tract, and ventral to the nucleus ambiguus. This area has been described previously to contain barosensitive, spinally projecting cardiovascular neurons (22, 64).
Fig. 6.
Representation of microinjection sites modified from a rat brain atlas (57). Injection sites were marked at the end of every experiment with a 30 nl of 2% Chicago Sky blue dye. ●, Injection sites from sedentary animals (n = 12); ○, injection sites from active animals (n = 13). All injection sites fell within 240 μm of the caudal pole of the facial nucleus. (SP5, spinal trigeminal tract; NA, nucleus ambiguus; FN, facial nucleus; Py, pyramidal tract; RVL, rostral ventrolateral medulla).
DISCUSSION
Using otherwise “normal” rats, we set out to identify the effects of sedentary conditions on sympathetic nervous system regulation of the splanchnic vascular bed. Since regulation of arterial pressure is complex, a secondary purpose of this study was to test whether sedentary animals also exhibit enhanced vascular reactivity to vasoconstriction. When sedentary rats were compared with their physically active counterparts, we discovered an enhancement in 1) resting blood pressure and SSNA, 2) responses to direct and baroreflex-mediated activation of RVLM neurons that regulate SSNA, and 3) reactivity of the peripheral vasculature. Our results suggest that resting and reflex-mediated sympathetic activity and its effect on the vasculature are enhanced by physical inactivity. These data agree with recent findings from other labs that suggest that there is enhanced SNS output to the splanchnic circulation in models of hypertension (25, 26, 31, 71). Taken together, we speculate that increased activation of RVLM neurons controlling the splanchnic vascular bed contributes to the development of sympathetic overactivity in chronically sedentary individuals.
To our knowledge this study is the first to show that directly recorded sympathetic nerve activity to the splanchnic circulation is higher at rest in sedentary compared with physically active animals. The splanchnic vascular bed holds between one-quarter and one-third of the total blood volume in a resting individual (15), so the level of resting sympathetic outflow to this bed likely plays a key role in blood pressure and blood volume regulation. In addition to the current study, previous work has shown that elevations in resting SSNA are associated with elevated blood pressure in animal models of cardiovascular disease (25, 38). Furthermore, surgical removal of sympathetic ganglia controlling the splanchnic bed ameliorates hypertension in an angiotensin II-based rat model (31). Therefore, we hypothesize that sympathetic overactivity in the splanchnic bed may contribute to the development (and perhaps maintenance) of cardiovascular disease in sedentary individuals via one or more mechanisms. Increased tone to splanchnic arteries and veins may increase disease risk in sedentary subjects through an increase in resting blood pressure and “effective” blood volume, respectively (15). In addition, sympathetic overactivity to any vascular bed may lead to detrimental alterations in blood vessel structure and function (16, 17).
It is possible that resting SNA is nonspecifically elevated in sedentary individuals. Meredith and colleagues (46) and Negrao et al. (54) reported an increase in resting renal sympathetic activity in sedentary humans and rats, respectively. Burgi and colleagues (4) more recently reported decreased lumbar sympathetic nerve activity in treadmill-trained Wistar-Kyoto rats However, Meredith and colleagues also observed that cardiac sympathetic activity was unchanged in human subjects from their study, indicating a more specific effect of physical (in)activity. In addition, studies utilizing direct recordings of muscle SNA in sedentary vs. physically active humans have reported equivocal results (49, 59). Despite these controversies, the current study provides direct evidence that resting sympathetic activity to the splanchnic circulation is elevated in sedentary vs. physically active rats and may contribute to the increased incidence of cardiovascular disease in individuals leading a sedentary lifestyle.
The RVLM is an important brain region involved in control of sympathetic outflow (22). In our study we found that generalized activation of the RVLM with glutamate results in enhanced sympathetic activation directed to the splanchnic circulation in sedentary compared with physically active animals. Similar to other laboratories, we suggested that changes occurring in RVLM neurons contribute to pathophysiological states (1, 22, 25, 49, 69). Specifically, the present study and others have provided evidence consistent with changes at the level of the RVLM producing enhanced sympathoexcitatory responses in sedentary animals (41, 50). Similarly, other laboratories have shown that, after a single bout of exercise or physical stress, a greater number of neurons in the RVLM are activated in sedentary vs. active subjects (20, 27). In contrast, studies by Iwamoto and colleagues (55) reported no effect of sedentary conditions on the structure of neurons in the anatomically defined region of the RVLM. It is important to note that RVLM neurons are heterogeneous with only specific subpopulations involved in blood pressure regulation (22). In the absence of evidence for overt structural changes, alterations in glutamate receptors or intracellular signaling pathways within RVLM neurons may be involved in the enhanced functional responses we observe (49). Finally, it is also possible that differences at the level of the spinal cord or sympathetic ganglia may contribute to the responses we observed. The available evidence thus far supports our hypothesis that neuroplasticity in the RVLM itself contributes to enhanced sympathetic responses in sedentary individuals (49).
Reflex-mediated sympathoexcitation is often enhanced in animal models of obesity and hypertension (26, 32, 37, 61). In addition to showing increased resting SSNA, our study is the first to show that reflex SSNA responses are enhanced in sedentary subjects. Previous studies have demonstrated that baroreflex-mediated sympathoexcitation in the renal nerve is enhanced in sedentary animals (6, 12). Studies performed in humans have lead to equivocal results regarding the effect of physical (in)activity on sympathetic reflexes (59, 70). Unfortunately, the lack of available technology to directly measure sympathetic nerve activity to nonskeletal muscle targets may limit conclusions regarding the brain's ability to regulate sympathetic outflow to other vascular beds in humans. As mentioned above, it is also possible that sedentary vs. physically active conditions affect sympathetic outflow to different target organs. In any case, the new evidence in the present study reinforces and extends the previous findings in animals to suggest that enhanced reflex-mediated activation of SSNA contributes to the detrimental effects of being sedentary on the cardiovascular system.
Intrinsic vascular reactivity to vasoconstriction is increased in sedentary animals when assessed in vitro (33). Data from the present study suggest that this manifests in vivo as an enhanced blood pressure response when stimulating the vasculature directly with an α1-adrenergic agonist. Thus alterations at the level of the vasculature likely contribute to increased resting MAP in sedentary animals. In fact, in the present study, sedentary animals did exhibit higher blood pressure at rest, which was normalized following ganglionic blockade with hexamethonium. This suggests that the sympathetic nervous system supports enhanced blood pressure in our model at least in part via two mechanisms: enhanced SSNA and enhanced vascular reactivity to the elevated SNA. Although other studies have shown differential effects of physical activity on resting blood pressure in normotensive animals (12, 42, 50, 51, 54) and humans (8, 28), obvious differences in the design of our study and others (i.e., mode and duration of exercise, anesthetized vs. conscious, humans vs. animals) may account for these disparities. Finally, since blood pressure is a complex variable with many contributing factors, this study highlights the importance of examining the effect of (in)activity on regional sympathetic nerve activity and vascular sensitivity as important components of resting arterial pressure.
Sedentary and physically active animals in our study showed similar blood pressure responses to activation of RVLM neurons despite greater activation of SNA to the splanchnic circulation. Since generalized activation of the RVLM affects multiple sympathetic outputs (45, 52), it is likely that similar blood pressure responses are due to additional differences between sedentary and active animals not directly addressed in this study. For example, enhanced vasoconstriction in sedentary animals may be offset by relatively reduced myocardial contractility responses produced during sympathetic stimulation (3, 67). Alternatively, activation of the RVLM has been shown to increase sympathetic outflow to the adrenal gland, which has been associated with epinephrine release (44, 53) and hindlimb vasodilation (36). This complexity in arterial pressure regulation again reinforces our contention above that regional sympathetic nerve responses need to be examined in determining the overall arterial pressure response to a given stimulus.
Because ionotropic glutamate receptors in the RVLM mediate the majority of sympathoexcitatory reflexes (22), we used glutamate microinjections to produce a direct but generalized activation of RVLM neurons involved in the sympathetic regulation of the splanchnic circulation. Similarly, we utilized baroreceptor unloading as a means of activating SSNA via a physiological reflex. Since baroreflex-mediated sympathoexcitation is typically associated with withdrawal of GABAergic inhibition of RVLM neurons (i.e., disinhibition) (22), it is possible that alterations in both glutamate and GABAergic neurotransmission in the RVLM (and the interaction between the two) are responsible for difference between physically active and sedentary animals.
Technical Considerations
To perform precise RVLM microinjections and record SSNA in the same animals we employed the use of anesthesia. Although technically challenging, one study has performed RVLM microinjections in conscious animals while recording sympathetic nerve activity to the kidney (22, 62). However, we are unaware of any studies that have performed RVLM microinjections in the rat after it has fully recovered from anesthesia and at the same time has maintained high quality sympathetic nerve recordings. Our opinion is that the advantage of having such a technically demanding combination of procedures would seem to be offset by less than optimal success rates, experimental design issues during surgical recovery, and the challenge of performing these in a chronic activity/inactivity animal model.
Quantification of sympathetic activity from direct nerve recordings has been a matter of debate for quite some time (5, 21, 24). In fact, we argued recently that comparing voltages across functionally different nerves may not be physiologically relevant (52). However, in the present study we chose to report our SSNA data in volts, as has been done by previous investigators (25, 26, 38, 40). This not only allowed us to highlight differences in resting sympathetic activity to the splanchnic circulation in sedentary vs. physically active animals but also allowed us to calculate absolute changes and changes as a percent of control when performing RVLM microinjections and baroreceptor unloading. Since the specific recording conditions can affect the recorded voltage (5), we were careful to ensure that these variables were tightly controlled from experiment to experiment by using the same recording equipment, amplification and filter settings, and only one person to implant electrodes for every experiment. Despite the inherent variability in SNA signals in general, we and others have been able to demonstrate statistically significant differences in resting SNA, an important step in identifying disease states of sympathetic overactivity (25, 38).
Conclusions
The results of the present study suggest that chronic physical inactivity elevates resting blood pressure and sympathetic nerve activity to the splanchnic circulation. Additionally, increases in sympathetic nerve activity to splanchnic circulation produced by either activation of RVLM neurons or decreases in arterial pressure are enhanced in inactive rats. Consistent with in vitro studies in models of physical inactivity, overall vascular reactivity is increased in sedentary animals. In summary, we conclude that chronic inactivity enhances resting and reflex-mediated sympathetic outflow to the splanchnic vascular bed and resting blood pressure, possibly through changes occurring in RVLM neurons themselves.
GRANTS
This work was supported by grants from the National Institutes of Health (R01-HL-096787-PJM; F30-HL-105003-NAM).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: N.A.M. and P.J.M. conception and design of research; N.A.M. and P.J.M. performed experiments; N.A.M. and P.J.M. analyzed data; N.A.M. and P.J.M. interpreted results of experiments; N.A.M. and P.J.M. prepared figures; N.A.M. and P.J.M. drafted manuscript; N.A.M. and P.J.M. edited and revised manuscript; N.A.M. and P.J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the following individuals for their help and input on this project: Toni Azar, Rita Kashat, and Jessica Speirs from the Mueller laboratory. We also thank the Neural Control of Cardiorespiratory Function Group at Wayne State University School of Medicine, especially Dr. Tadeusz Scislo for his helpful comments on this manuscript. Finally, we thank Zeljka Minic from Dr. Scislo's laboratory.
REFERENCES
- 1. Adams JM, Madden CJ, Sved AF, Stocker SD. Increased dietary salt enhances sympathoexcitatory and sympathoinhibitory responses from the rostral ventrolateral medulla. Hypertension 50: 354–359, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Blair SN. Physical inactivity: the biggest public health problem of the 21st century. Br J Sports Med 43: 1–2, 2009 [PubMed] [Google Scholar]
- 3. Blomqvist CG, Saltin B. Cardiovascular adaptations to physical training. Ann Rev Physiol 45: 169–189, 1983 [DOI] [PubMed] [Google Scholar]
- 4. Burgi K, Cavalleri MT, Alves AS, Britto LR, Antunes VR, Michelini LC. Tyrosine hydroxylase immunoreactivity as indicator of sympathetic activity: simultaneous evaluation in different tissues of hypertensive rats. Am J Physiol Regul Integr Comp Physiol 300: R264–R271, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Burke SL, Lambert E, Head GA. New approaches to quantifying sympathetic nerve activity. Curr Hypertens Rep 13: 249–257, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Chen CY, DiCarlo SE. Daily exercise and gender influence arterial baroreflex regulation of heart rate and nerve activity. Am J Physiol Heart Circ Physiol 271: H1840–H1848, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension 45: 667–675, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Cornelissen VA, Verheyden B, Aubert AE, Fagard RH. Effects of aerobic training intensity on resting, exercise and post-exercise blood pressure, heart rate and heart-rate variability. J Hum Hypertens 24: 175–182, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Curran-Everett D, Benos D. Guidelines for reporting statistics in journals published by the American Physiological Society. J Appl Physiol 97: 457–459, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med 6: e1000058, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DiCarlo SE, Bishop VS. Exercise training attenuates baroreflex regulation of nerve activity in rabbits. Am J Physiol Heart Circ Physiol 255: H974–H979, 1988 [DOI] [PubMed] [Google Scholar]
- 13. Esler M, Rumantir M, Kaye D. Sympathetic nerve biology in essential hypertension. Clin Exp Pharmacol Physiol 28: 986–989, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Fagard RH, Cornelissen VA. Effect of exercise on blood pressure control in hypertensive patients. Eur J Cardiovasc Prev Rehabil 14: 12–17, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Fink GD, Arthur C. Corcoran Memorial Lecture. Sympathetic activity, vascular capacitance, and long-term regulation of arterial pressure. Hypertension 53: 307–312, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fisher JP, Paton JF. The sympathetic nervous system and blood pressure in humans: implications for hypertension. J Hum Hypertens 2011. July 7 doi: 10.1038/jhh.2011.66. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17. Fisher JP, Young CN, Fadel PJ. Central sympathetic overactivity: maladies and mechanisms. Auton Neurosci 148: 5–15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goodchild AK, Dampney RAL, Bandler R. A method for evoking physiological responses by stimulation of cell bodies, but not axons of passage, within localized regions of the central nervous system. J Neurosci Methods 6: 351–363, 1982 [DOI] [PubMed] [Google Scholar]
- 19. Granata AR. Effects of γ-aminobutyric acid on putative sympatho-excitatory neurons in the rat rostral ventrolateral medulla in vitro: intracellular study. Neurosci Lett 300: 49–53, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Greenwood BN, Kennedy S, Smith TP, Campeau S, Day HEW, Fleshner M. Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience 120: 269–281, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Guild SJ, Barrett CJ, McBryde FD, Van Vliet BN, Head GA, Burke SL, Malpas SC. Quantifying sympathetic nerve activity: problems, pitfalls and the need for standardization. Exp Physiol 95: 41–50, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Guyenet PG, Koshiya N, Huangfu D, Baraban SC, Stornetta RL, Li YW. Role of medulla oblongata in generation of sympathetic and vagal outflows. Prog Brain Res 107: 127–144, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Hopp FA, Seagard JL, Kampine JP. Comparison of four methods of averaging nerve activity. Am J Physiol Regul Integr Comp Physiol 251: R700–R711, 1986 [DOI] [PubMed] [Google Scholar]
- 25. Huber DA, Schreihofer AM. Altered regulation of the rostral ventrolateral medulla in hypertensive obese Zucker rats. Am J Physiol Heart Circ Physiol 301: H230–H240, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huber DA, Schreihofer AM. Attenuated baroreflex control of sympathetic nerve activity in obese Zucker rats by central mechanisms. J Physiol 588: 1515–1525, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ichiyama RM, Gilbert AB, Waldrop TG, Iwamoto GA. Changes in the exercise activation of diencephalic and brainstem cardiorespiratory areas after training. Brain Res 947: 225–233, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Iwasaki KI, Zhang R, Zuckerman JH, Levine BD. Dose-response relationship of the cardiovascular adaptation to endurance training in healthy adults: how much training for what benefit? J Appl Physiol 95: 1575–1583, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Julius S, Valentini M. Consequences of the increased autonomic nervous drive in hypertension, heart failure, and diabetes. Blood Press 7: 5–13, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Kiely JM, Gordon FJ. Role of rostral ventrolateral medulla centrally mediated pressor responses. Am J Physiol Heart Circ Physiol 267: H1549–H1556, 1994 [DOI] [PubMed] [Google Scholar]
- 31. King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension 50: 547–556, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Kumagai H, Suzuki H, Ryuzaki M, Matsukawa S, Saruta T. Baroreflex control of renal sympathetic nerve activity is potentiated at early phase of two-kidney, one-clip Goldblatt hypertension in conscious rabbits. Circ Res 67: 1309–1322, 1990 [DOI] [PubMed] [Google Scholar]
- 33. Laughlin MH, Roseguini B. Mechanisms for exercise training-induced increases in skeletal muscle blood flow capacity: differences with interval sprint training versus aerobic endurance training. J Physiol Pharmacol 59, Suppl 7: 71–88, 2008 [PMC free article] [PubMed] [Google Scholar]
- 34. Llewellyn-Smith IJ. Anatomy of synaptic circuits controlling the activity of sympathetic preganglionic neurons. J Chem Neuroanat 38: 231–239, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De SG, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics—2010 update. A report from the American Heart Association. Circulation 121: 948–954, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Lovick TA. Differential control of cardiac and vasomotor activity by neurones in nucleus paragigantocellularis lateralis in the cat. J Physiol 389: 23–35, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luft FC, Demmert G, Rohmeiss P, Unger T. Baroreceptor reflex effect on sympathetic nerve activity in stroke-prone spontaneously hypertensive rats. J Auton Nerv Syst 17: 199–209, 1986 [DOI] [PubMed] [Google Scholar]
- 38. Luft FC, Wilcox CS, Unger T, Kuhn R, Demmert G, Rohmeiss P, Ganten D, Sterzel RB. Angiotensin-induced hypertension in the rat. Sympathetic nerve activity and prostaglandins. Hypertension 14: 396–403, 1989 [DOI] [PubMed] [Google Scholar]
- 39. Machado BH, Bonagamba LG, Dun SL, Kwok EH, Dun NJ. Pressor response to microinjection of orexin/hypocretin into rostral ventrolateral medulla of awake rats. Regul Pept 104: 75–81, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Maliszewska-Scislo M, Scislo TJ, Rossi NF. Effect of blockade of endogenous angiotensin II on baroreflex function in conscious diabetic rats. Am J Physiol Heart Circ Physiol 284: H1601–H1611, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Martins-Pinge MC, Becker LK, Garcia MR, Zoccal DB, Neto RV, Basso LS, de Souza HC, Lopes OU. Attenuated pressor responses to amino acids in the rostral ventrolateral medulla after swimming training in conscious rats. Auton Neurosci 122: 21–28, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Mastelari RB, de Souza HC, Lenhard A, de Aguiar Correa FM, Martins-Pinge MC. Nitric oxide inhibition in paraventricular nucleus on cardiovascular and autonomic modulation after exercise training in unanesthetized rats. Brain Res 1375: 68–76, 2011 [DOI] [PubMed] [Google Scholar]
- 43. Mayorov DN, Head GA. Ionotropic glutamate receptors in the rostral ventrolateral medulla mediate sympathetic responses to acute stress in conscious rabbits. Auton Neurosci 98: 20–23, 2002 [DOI] [PubMed] [Google Scholar]
- 44. McAllen RM. Action and specificity of ventral medullary vasopressor neurones in the cat. Neuroscience 18: 51–59, 1986 [DOI] [PubMed] [Google Scholar]
- 45. McAllen RM, May CN. Differential drives from rostral ventrolateral medullary neurons to three identified sympathetic outflows. Am J Physiol Regul Integr Comp Physiol 267: R935–R944, 1994 [DOI] [PubMed] [Google Scholar]
- 46. Meredith IT, Friberg P, Jennings GL, Dewar EM, Fazio VA, Lambert GW, Esler MD. Exercise training lowers resting renal but not cardiac sympathetic activity in humans. Hypertension 18: 575–582, 1991 [DOI] [PubMed] [Google Scholar]
- 47. Moffitt JA, Heesch CM, Hasser EM. Increased GABAA inhibition of the RVLM following hindlimb unloading in rats. Am J Physiol Regul Integr Comp Physiol 283: R604–R614, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Morris JN, Heady JA, Raffle PA, Roberts CG, Parks JW. Coronary heart-disease and physical activity of work. Lancet 265: 1111–1120, 1953 [DOI] [PubMed] [Google Scholar]
- 49. Mueller PJ. Physical (in)activity-dependent alterations at the rostral ventrolateral medulla: influence on sympathetic nervous system regulation. Am J Physiol Regul Integr Comp Physiol 298: R1468–R1474, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mueller PJ. Exercise training attenuates increases in lumbar sympathetic nerve activity produced by stimulation of the rostral ventrolateral medulla. J Appl Physiol 102: 803–813, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Mueller PJ, Hasser EM. Putative role of the NTS in alterations in neural control of the circulation following exercise training in rats. Am J Physiol Regul Integr Comp Physiol 290: R383–R392, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Mueller PJ, Mischel NA, Scislo TJ. Differential activation of adrenal, renal, and lumbar sympathetic nerves following stimulation of the rostral ventrolateral medulla of the rat. Am J Physiol Regul Integr Comp Physiol 300: R1230–R1240, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Natarajan M, Morrison SF. Adrenal epinephrine secretion is not regulated by sympathoinhibitory neurons in the caudal ventrolateral medulla. Brain Res 827: 169–175, 1999 [DOI] [PubMed] [Google Scholar]
- 54. Negrao CE, Irigoyen MC, Moreira ED, Brum PC, Freire PM, Krieger EM. Effect of exercise training on RSNA, baroreflex control, and blood pressure responsiveness. Am J Physiol Regul Integr Comp Physiol 265: R365–R370, 1993 [DOI] [PubMed] [Google Scholar]
- 55. Nelson AJ, Juraska JM, Ragan BG, Iwamoto GA. Effects of exercise training on dendritic morphology in the cardiorespiratory and locomotor centers of the mature rat brain. J Appl Physiol 108: 1582–1590, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Osborn JW, Fink GD, Kuroki MT. Neural mechanisms of angiotensin II-salt hypertension: implications for therapies targeting neural control of the splanchnic circulation. Curr Hypertens Rep 13: 221–228, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Burlington, MA: Elsevier, 2007 [Google Scholar]
- 58. Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA, and American College of Sports Medicine American College of Sports Medicine position stand, Exercise and hypertension. Med Sci Sports Exerc 36: 533–553, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Ray CA, Hume KM. Sympathetic neural adaptation to exercise training in humans: insights from microneurography. Med Sci Sports Exerc 30: 387–391, 1998 [DOI] [PubMed] [Google Scholar]
- 60. Ross CA, Ruggiero DA, Park DH, Joh TH, Sved AF, Fernandex-Pardal J, Saavedra JM, Reis DJ. Tonic vasomotor control by the rostal ventrolateral medulla: effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressin. J Neurosci 4: 474–494, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ruggeri P, Cogo GE, Picchio V, Ermirio R, Mary DASG. Reflex cardiovascular responses to somatic stimulation in spontaneously hypertensive rats. J Hypertens 18: 595–600, 2000 [DOI] [PubMed] [Google Scholar]
- 62. Sakima A, Yamazato M, Sesoko S, Muratani H, Fukiyama K. Cardiovascular and sympathetic effects of l-glutamate and glycine injected into the rostral ventrolateral medulla of conscious rats. Hypertens Res 23: 633–641, 2000 [DOI] [PubMed] [Google Scholar]
- 63. Schlaich MP, Lambert E, Kaye D, Krozowski Z, Campbell DJ, Lambert G, Hastings J, Aggarwal A, Esler M. Sympathetic augmentation in hypertension. Role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension 43: 169–175, 2004 [DOI] [PubMed] [Google Scholar]
- 64. Schreihofer AM, Guyenet PG. The baroreflex and beyond: control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol 29: 514–521, 2002 [DOI] [PubMed] [Google Scholar]
- 65. Schreihofer AM, Mandel DA, Mobley SC, Stepp DW. Impairment of sympathetic baroreceptor reflexes in obese Zucker rats. Am J Physiol Heart Circ Physiol 293: H2543–H2549, 2007 [DOI] [PubMed] [Google Scholar]
- 66. Silva AQ, Schreihofer AM. Altered sympathetic reflexes and vascular reactivity in rats after exposure to chronic intermittent hypoxia. J Physiol 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Spina RJ, Ogawa T, Coggan AR, Holloszy JO, Ehsani AA. Exercise training improves left ventricular contractile response to beta-adrenergic agonist. J Appl Physiol 72: 307–311, 1992 [DOI] [PubMed] [Google Scholar]
- 68. Straznicky N, Lambert E, Lambert G, Masuo K, Esler M, Nestel P. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab 90: 5998–6005, 2005 [DOI] [PubMed] [Google Scholar]
- 69. Sved AF, Ito S, Sved JC. Brainstem mechanisms of hypertension: role of the rostral ventrolateral medulla. Curr Hypertens Rep 5: 262–268, 2003 [DOI] [PubMed] [Google Scholar]
- 70. Wallin BG. Interindividual differences in muscle sympathetic nerve activity: a key to new insight into cardiovascular regulation? Acta Physiol (Oxf) 190: 265–275, 2007 [DOI] [PubMed] [Google Scholar]
- 71. Yoshimoto M, Miki K, Fink GD, King A, Osborn JW. Chronic angiotensin II infusion causes differential responses in regional sympathetic nerve activity in rats. Hypertension 55: 644–651, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]