Abstract
The eel has long been esteemed as an important food fish in the world, especially in Japan, and has been used as an experimental fish for many fields of fish physiology. However, the decreases in eel resources have been a serious concern in recent years. The catches of glass eels as seedlings for aquaculture have shown a long-term decrease in both Europe and East Asia. To increase eel resources, the development of techniques for artificial induction of maturation and spawning and rearing their larvae have been eagerly desired. Recent progress of reproductive physiology of fish, especially mechanisms of oocyte maturation and ovulation in female and of spermatozoa maturation in male, facilitate to establish techniques for hormonal induction of maturation and spawning in sexually immature eels. With persistent effort to development of rearing techniques of larvae, we have first succeeded to produce glass eel. These applied techniques are may contribute to understand the basic reproductive physiology of the eel.
Keywords: Japanese eel, Artificial maturation, Oogenesis, Spermatogenesis, Artificial insemination, Larval rearing, Leptocephalus, Glass eel
Introduction
The eel has long been esteemed not only in Japan but also in European countries as an important food fish. The aquacultural production of Japanese eel, Anguilla japonica, in Japan is about 30,000 tons a year in recent years. Japan also imports a total of 80,000 tons of eel from mainly China and Taiwan, and other countries. Seedlings for eel aquaculture are totally dependent on glass eels, natural juveniles of eel which captured in estuaries. However, in both East Asia and Europe the catches of glass eels differ greatly from year to year and especially in the past 25 years, have been decreasing, resulting in a sharp rise in its price. Therefore, to maintain the natural glass eel resources and to obtain reliable supplies of glass eels for aquaculture, development of an artificially induced breeding procedure for eels has been eagerly desired.
Techniques for artificial breeding of the Japanese eel have been studied intensively since the 1960s. Yamamoto and Yamauchi (1974) first succeeded in obtaining fertilized eggs and larvae of the Japanese eel by hormone treatment, and preleptocephalus larvae were reared for 2 weeks (Yamauchi et al. 1976). Thereafter, many researchers have succeeded in obtaining eel larvae, but preleptocephalus larvae could not survive beyond the depletion of their yolk and oil droplet stores. Failures of production of the glass eel may be caused by incomplete techniques for inducing sexual maturation of both male and female Japanese eels and incomplete rearing techniques of larvae. Basic science on reproductive biology of fish has remarkably progressed, especially in the fields of endocrine mechanisms of oogenesis and spermatogenesis. Therefore, it is expected that information from the basic science facilitate to develop techniques for induction of sexual maturation and rearing techniques of the Japanese eel larvae. Therefore, in this paper, our recent researches on glass eel production, mainly on induction of sexual maturation, are reviewed. Using newly developed techniques, our group has succeeded for the first time to obtain glass eels.
Maturation and ovulation
Cultivated female eels or silver eels that migrate down river are sexually immature and their gonadosomatic index slightly increase to around 1–2% in fall and their ovaries contain oocytes at the oil stage or oocytes at the primary yolk globule stage (Kagawa et al. 1998). However, cultivated and silver eels do not mature and ovulate under normal culture conditions. Yamamoto and Yamauchi (1974) succeeded in inducing oocyte growth and obtaining full-grown oocytes in almost of all fish by repeated injections of salmon pituitary extracts. Since then, repeated injections of piscine pituitary (mainly carp or salmon pituitary) extracts are now routinely used for induction of vitellogenesis in female eels. However, the percentage of ovulated females is very low, and even if ovulated eggs are obtained, these eggs show low fertility and hatchability.
After 8–13 injections of salmon pituitary extracts (20 mg/fish/week), females have ovaries containing the full-grown oocytes which complete vitellogenesis and attain the migratory nucleus stage (about 750–800 µm in diameter in Japanese eel, Ohta et al. 1997; Kagawa et al. 1998). However, their oocytes do not undergo maturation and become over-ripe with oocyte cytoplasmic degeneration in response to further injections of salmon pituitary extracts.
After the oocytes complete vitellogenesis, oocyte maturation occurs prior to ovulation and is a prerequisite for successful fertilization; it consists of breakdown of germinal vesicle (GVBD), the resumption of meiosis, and oocyte cytoplamic maturation (see Nagahama et al. 1995). These processes of oocyte maturation are regulated by the pituitary gonadotropin (primary endocrine factor responsible for triggering meiotic maturation) and maturation-inducing steroid which is synthesized in the ovarian follicle under the influence of gonadotropin. The maturation-inducing steroid directly acts on the oocyte to initiate the process of oocyte maturation (Nagahama et al. 1995). The maturation-inducing steroid ( 17; 20β-dihydroxy-4-pregnen-3-one, DHP) of a salmonid fish, amago salmon (Oncorhynchus rhodurus), was isolated for the first time in vertebrate species (Nagahama and Adachi 1985) and thereafter DHP has been found to be the most effective steroid in inducing oocyte maturation of many fish species. Yamauchi and Yamamoto (1982) reported that in vitro administration of DHP into the incubation medium induced oocyte maturation in the Japanese eel. From these fundamental researches, it is expected that in vivo administration of DHP could induce oocyte maturation and ovulation.
For application of DHP on artificial induction of maturation and ovulation in the Japanese eel, other valuable information on oocyte maturation and ovulation have been obtained. From basic researches of red seabream (Kagawa et al. 1994a, b; Patino et al. 2001), we have found that process of oocyte maturation consists of two critical periods. During the first stage of maturation, oocytes acquire ability to respond to maturation inducing steroid, whereas in the second stage the follicles produce maturation-inducing steroid and, consequently, the oocyte undergo germinal vesicle breakdown. In vitro incubation of oocyte of the Japanese eel at various developmental stages revealed (Kagawa et al. 1995) that oocytes undergo GVBD in response to both 17α-hydroxyprogesterone and DHP when oocytes diameter attain the migratory nucleus stage (approximately 700–800 µm in diameter). These results suggest that oocytes acquire the ability to respond to maturation-inducing steroid (maturational competence) at the migratory nucleus stage. However, the production of maturation-inducing steroid is not induced by further injections of salmon pituitary extracts, possibly due to a lack of precursor synthesis (Ijiri et al. 1995), resulting in failure of induction of maturation and ovulation by injection of salmon pituitary extracts. Our data also indicate that DHP can induce not only in vitro maturation but also in vitro ovulation (Kagawa et al. 2003). Moreover, DHP-induced in vitro ovulation occurred earlier in larger oocytes more than 860 µm in diameter compared to smaller oocytes less than 860 µm in diameter (Fig. 1). Data obtained from one female eel show that ovulation rates rapidly decreased after oocytes attain more than 860 µm in diameter (Fig. 2). These data suggest that not only oocyte maturation but also ovulation can be induced by in vivo administration of DHP, when female eels have ovaries containing maturational competent oocytes at the migratory nucleus stage.
Fig. 1.
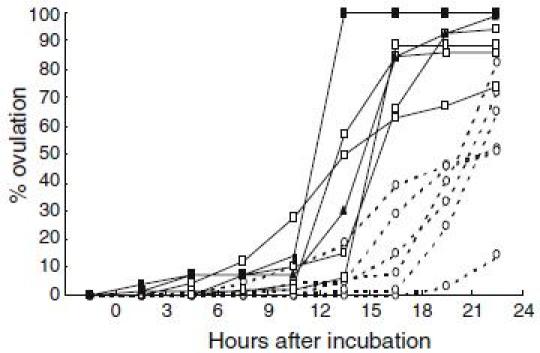
Effects of 17; 20β-dihydroxy-4-pregnen-3-one (DHP) on in vitro ovulation in the Japanese eel. Oocytes with follicular layers obtained from female eels were incubated in 1 ml L-15 incubation media with DHP (100 ng/ml). Dotted line and solid line indicate changes in % ovulation of oocytes less than 860 µm in diameter and more than 860 µm in diameter respectively
Fig. 2.
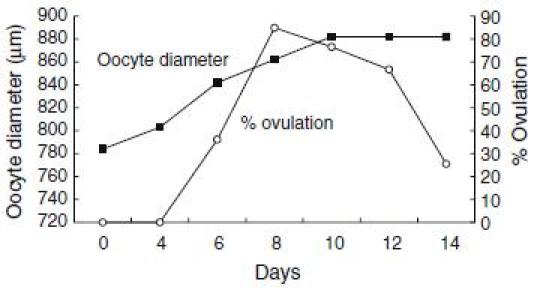
Changes in oocyte diameter and in vitro ovulation rates of oocytes with follicle layers obtained from one female Japanese eel. Oocytes with follicle layers sequentially obtained from one female which was induce by repeated injection of salmon pituitary extracts were incubated in L-15 incubation media with DHP (100 ng/ml). Ovulation rates were observed 24 h after incubation
From these in vitro experiments, the following techniques of induction of maturation and ovulation were developed (Fig. 3). Female eels that possess oocytes at the migratory nucleus stage (approximately 800–850 µm in diameter) were injected with salmon pituitary extracts (20 mg/fish) as a priming dose followed 24 h later by an injection of DHP (2 µg/g body weight) (Ohta et al. 1996a; Kagawa 2003). Injection of DHP succeeded in inducing oocyte maturation and ovulation in almost all females used in the experiments. Fertilization and hatching rates are approximately 60 and 40% respectively (Kagawa et al. 1997). Both fertilization and hatching rates of ovulated females decreased rapidly to almost 0% by 6 h after ovulation, suggesting that artificial fertilization must be carried out immediately after ovulation in order to obtain good quality eggs (Ohta et al. 1996a).
Fig. 3.
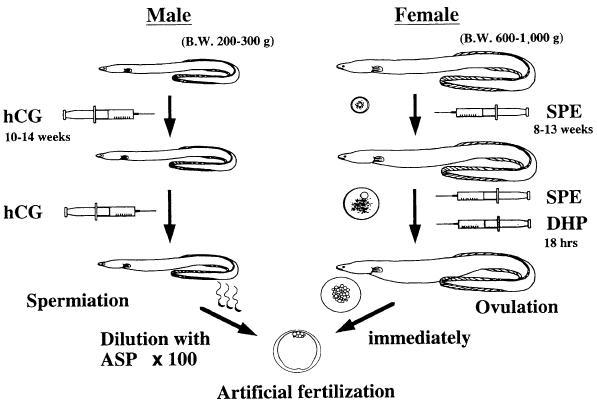
Summary of the artificial induction method of maturation and fertilization in the Japanese eel. SPE, salmon pituitary extract; DHP, 17; 20β-dihydroxy-4-pregnen-3-one; ASP, artificial seminal plasma; hCG, human chorionic gonadotropin. Slightly modified Ohta et al. (1997) with permission
Induction of spermatogenesis and spermiation
Cultivated male Japanese eels are also sexually immature and do not mature under normal culture conditions. Injecting them with human chorionic gonadotropin (HCG) can easily induce maturation (Miura et al. 1991) and spermiation (Ohta et al. 1996b). After 6 weekly injections of HCG at a dose of 1 IU/g BW, most fish spermiate small volumes of milt, and milt volume increase and become stable after 11th injection. However, there are major differences in the extent of maturity induced in individual fish after the same treatment with HCG. The effectiveness of HCG injection in inducing spermatogenesis is to be a considerable extent influenced by individual difference in the rates of HCG clearance among fish (Ohta and Tanaka 1997). Moreover, individual differences in the rate of sperm motility are significant problems among hormonally matured males, ranging between less than 10% and more than 90% (Ohta et al. 2001), leading to an obvious varying degree of successful fertilization. Therefore, techniques for successful fertilization with a small quantity of inadequate quality of sperm need to be developed.
Recent progress of basic science on mechanism of spermatogenesis in the Japanese eel revealed that DHP is related to the regulation of sperm maturation (Miura et al. 2003). DHP increases pH in the seminal plasma, which in turn increases the sperm content of cAMP, thereby allowing the acquisition of sperm motility. Our data also indicate that injection of hCG affects the aqueous environment around the spermatozoa in the sperm duct and the potential for motility in the spermatozoa fluctuates with environmental factor(s) (Ohta and Unuma 2003). Milt with higher pH and potassium concentration of seminal plasma shows higher rate of motility in the spermiated male eel injected with hCG. From these fundamental researches, the effects of an artificial seminal plasma (ASP), which was produced based on the measurements of seminal plasma, on acquisition of the potential for motility of spermatozoa were examined. After immotile spermatozoa from testis were incubated with ASP in vitro, significant increase of the motility rate is observed (Ohta and Unuma 2003). Moreover, the rate of motility of the spermatozoa was controlled by adjusting the concentration of potassium ions and hydrogen bicarbonate ions in ASP. Therefore, from these studies, diluted spermatozoa with the ASP were stocked in refrigerator one day before fertilization and artificial fertilization was performed using the diluted spermatozoa and ovulated eggs (Fig. 3).
Techniques for larval rearing
Fertilized eggs begin to hatch about 40 h after fertilization at a water temperature of 22–23°C. By 7 days after hatching, several marked changes are observed, such as pigmentation of eye and synthesis of digestive enzymes in the pancreas, suggesting that larvae acquire the ability to take feeds and digest them (Kurokawa et al. 1995). We first reported that preleptocephalus larvae at 13 day after hatching ingested rotifers, the most common initial feed used in the production of marine fish fry (Tanaka et al. 1995). However, even the larvae that ingest rotifers do not grow much larger and their survival period does not extend. Among feed items examined, finally, we found that eel larvae actively ingest a slurry-type diet made from shark egg powder. Eel larvae grow up from 3.7 mm long at just after hatch to about 8.1 mm in total length and the survival rate was 56% on 18th day. Therefore, we newly mixed soybean peptide, extract of krill, vitamin and mineral mixture to shark egg powder and made slurry diet. The eel larvae continued to survive and grow up linearly up to 50 days after hatching, their total length attained about 16 mm on the day and body depth gradually increased to yield the willow leaf-like form typical of wild leptocephali (Tanaka et al. 2001). Thereafter, larvae fed on further modified diet become fully grown leptocephali 50–60 mm in TL and have begun to metamorphose into glass eels approximately 250 after hatching (Tanaka 2003) (Fig. 4).
Fig. 4.
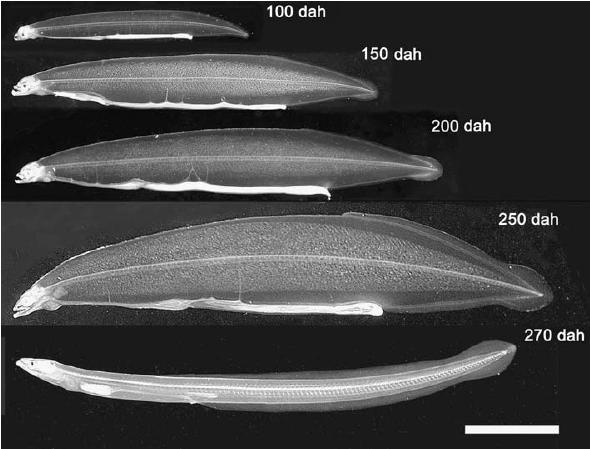
Growth of captive-bred Anguilla japonica larvae. Age in days after hatching (dah), total length: 100 dah, 24.8 mm; 150 dah, 39.0 mm; 200 dah, 45.1 mm; 250 dah, 57.1 mm; 270 dah (glass eel), 52.6 mm. Scale bar=10 mm. Reproduced from Tanaka (2003) with permission
Conclusion
We developed the method for induction of maturation and ovulation in the Japanese eel by using the maturation-inducing steroid (DHP). In the male eels, in vitro incubation of the immotile spermatozoa with ASP shows significant increase of the motility rate. However, there are major differences in the extent of egg quality of the female eels induced in individual fish after the same hormonal treatments. The reason of inadequate quality of egg have not been clarified. Recent progress of researches on hypothalamus (gonadotropin-releasing hormone, GnRH)-pituitary (gonadotropin) axis (Suetake et al. 2003) and mechanisms of steroid production in eel ovary (Adachi et al. 2003) would facilitate to develop new techniques for inducing maturation of the Japanese eel. For example, synthetic GnRH will be able to use effectively for inducing sexual maturation of the Japanese eel, since they are effective in inducing sexual maturation in several commercially important fish (Matsuyama et al. 1995; Kumakura et al. 2003), including eels (Hirose 1992). Moreover, biotechnology for production of recombinant eel gonadotropins (Kamei et al. 2003) would be also useful tools for controlling all processes of spermatogenesis and oogenesis normally.
References
- Adachi S, Ijiri S, Kazeto Y, Yamauchi K. Oogenesis in the Japanese eel, Anguilla japonica. In: Aida K, Tsukamoto K, Yamauchi K, editors. Eel biology. Melbourne: Blackwell Publishing Asia; 2003. pp. 301–317. [Google Scholar]
- Hirose K. Induced spawning of Japanese eel with LHRH-A copolymer pellet. NOAA Tech Rep NMFS. 1992;106:43–48. [Google Scholar]
- Ijiri S, Kazeto Y, Takeda N, Chiba H, Adachi S, Yamauchi K. Changes in serum steroid hormones and steroidogenic ovarian follicles during artificial maturation of cultivated Japanese eel, Anguilla japonica. Aquaculture. 1995;135:3–16. doi: 10.1016/0044-8486(96)81292-0. [DOI] [Google Scholar]
- Kagawa H. Artificial induction of oocyte maturation and ovulation. In: Aida K, Tsukamoto K, Yamauchi K, editors. Eel biology. Melbourne: Blackwell Publishing Asia; 2003. pp. 401–414. [Google Scholar]
- Kagawa H, Iinuma N, Tanaka H, Ohta H, Okuzawa K. Effects of rearing period in seawater on induced maturation in female Japanese eel Anguilla japonica. Fish Sci. 1998;64:77–82. [Google Scholar]
- Kagawa H, Kobayashi M, Hasegawa Y, Aida K. Insulin and insulin-like growth factors I and II induce final maturation of oocytes of red seabream, Pagrus major, in vitro. Gen Comp Endocrinol. 1994a;95:293–300. doi: 10.1006/gcen.1994.1126. [DOI] [PubMed] [Google Scholar]
- Kagawa H, Tanaka H, Okuzawa K, Hirose K. Development of maturational competence of oocytes of red seabream, Pagrus major, after human chorionic gonadotropin treatment in vitro requires RNA and protein synthesis. Gen Comp Endocrinol. 1994b;94:199–206. doi: 10.1006/gcen.1994.1076. [DOI] [PubMed] [Google Scholar]
- Kagawa H, Tanaka H, Ohta H, Okuzawa K, Hirose K. In vitro effects of 17α-hydroxyprogesterone and 17α, 20β-dihydroxy-4-pregnen-3-one on final maturation of oocytes at various developmental stages in artificially matured Japanese eel Anguilla japonica. Fish Sci. 1995;61:1012–1015. [Google Scholar]
- Kagawa H, Tanaka H, Ohta H, Okuzawa K, Iinuma N. Induced ovulation of 17, 20β-dihydroxy-4-pregnen-3-one in the artificially matured Japanese eel, with special reference to ovulation time. Fish Sci. 1997;63:356–367. [Google Scholar]
- Kagawa H, Tanaka H, Unuma T, Ohta H, Gen K, Okuzawa K. Role of prostaglandin in the control of ovulation in the Japanese eel Anguilla japonica. Fish Sci. 2003;69:234–241. doi: 10.1046/j.1444-2906.2003.00613.x. [DOI] [Google Scholar]
- Kamei H, Ohira T, Yoshiura Y, Uchida N, Nagasawa H, Aida K. Expression of a biologically active recombinant follicle stimulating hormone of Japanese eel Anguilla japonica using methylotropic yeast, Pichia pastoris. Gen Comp Endocrinol. 2003;134:2444–2254. doi: 10.1016/S0016-6480(03)00259-4. [DOI] [PubMed] [Google Scholar]
- Kumakura N, Okuzawa K, Gen K, Kagawa H. Effects of gonadotropin-releasing hormone agonist and dopamine antagonist on hypothalamus-pituitary-gonadal axis of pre-pubertal female red seabream (Pagurs major) Gen Comp Endocrinol. 2003;131:264–273. doi: 10.1016/S0016-6480(03)00012-1. [DOI] [PubMed] [Google Scholar]
- Kurokawa H, Kagawa H, Ohta H, Tanaka H, Okuzawa K, Hirose K. Development of digestive organs and feeding ability in larvae of Japanese eel (Anguilla japonica) Can J Fish Aquat Sci. 1995;52:1030–1036. doi: 10.1139/f95-101. [DOI] [Google Scholar]
- Matsuyama M, Takeuchi H, Kashiwagi M, Hirose K, Kagawa H. Induced gonadal development and spawning of immature red sea bream Pagurus major with LHRH-a administration in different ways during winter season. Fish Sci. 1995;61:472–477. [Google Scholar]
- Miura T, Miura C, Yamauchi K. Spermatogenesis in the Japanese eel. In: Aida K, Tsukamoto K, Yamauchi K, editors. Eel biology. Melbourne: Blackwell Publishing Asia; 2003. pp. 319–329. [Google Scholar]
- Miura T, Yamauchi K, Nagahama Y, Takahashi H. Induction of spermatogenesis in male Japanese eel, Anguilla japonica, by a single injection of human chorionic gonadotropin. Zool Sci. 1991;8:63–73. [Google Scholar]
- Nagahama Y, Adachi S. Identification of a maturation-inducing steroid in a teleost, the amago salmon (Oncorhynchus rhodurus) Dev Biol. 1985;109:428–435. doi: 10.1016/0012-1606(85)90469-5. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Yamashita M, Tokumoto T, Katsu Y. Regulation of oocyte maturation in fish. Curr Top Dev Biol. 1995;30:103–145. doi: 10.1016/S0070-2153(08)60565-7. [DOI] [PubMed] [Google Scholar]
- Ohta H, Kagawa H, Tanaka H, Okuzawa K, Hirose K. Changes in fertilization and hatching rates with time after ovulation induced by 17, 20β-dihydroxy-4-pregnen-3-one in the Japanese eel, Anguilla japonica. Aquaculture. 1996a;139:291–301. doi: 10.1016/0044-8486(95)01167-6. [DOI] [Google Scholar]
- Ohta H, Kagawa H, Tanaka H, Okuzawa K, Hirose K. Milt production in the Japanese eel Anguilla japonica induced by repeated injections of human chorionic gonadotropin. Fish Sci. 1996b;62:44–49. [Google Scholar]
- Ohta H, Kagawa H, Tanaka H, Okuzawa K, Iinuma N, Hirose K. Artificial induction of maturation and fertilization in the Japanese eel, Anguilla japonica. Fish Physiol Biochem. 1997;17:163–169. doi: 10.1023/A:1007720600588. [DOI] [Google Scholar]
- Ohta H, Kagawa H, Tanaka H, Unuma T. Control by the environmental concentration of ions of the potential for motility in Japanese eel spermatozoa. Aquaculture. 2001;198:339–351. doi: 10.1016/S0044-8486(00)00597-4. [DOI] [Google Scholar]
- Ohta H, Tanaka H. Relationship between serum levels of human chorionic gonadotropin (hCG) and 11-ketotestosterone after a single injection of hCG and induced maturity in the male Japanese eel, Anguilla japonica. Aquaculture. 1997;153:123–134. doi: 10.1016/S0044-8486(97)00020-3. [DOI] [Google Scholar]
- Ohta H, Unuma T. Induction of sperm maturation. In: Aida K, Tsukamoto K, Yamauchi K, editors. Eel biology. Melbourne: Blackwell Publishing Asia; 2003. pp. 415–423. [Google Scholar]
- Patino R, Yoshizaki G, Thomas P, Kagawa H. Gonadotropic control of ovarian follicle maturation: the two-stage concept and its mechanisms. Com Biochem Physiol. 2001;129B:427–439. doi: 10.1016/S1096-4959(01)00344-X. [DOI] [PubMed] [Google Scholar]
- Suetake H, Okubo K, Yoshiura Y, Aida K. GTH and GnRH molecules and their expression in the Japanese eel. In: Aida K, Tsukamoto K, Yamauchi K, editors. Eel biology. Melbourne: Blackwell Publishing Asia; 2003. pp. 351–372. [Google Scholar]
- Tanaka H. Techniques for larval rearing. In: Aida K, Tsukamoto K, Yamauchi K, editors. Eel biology. Melbourne: Blackwell Publishing Asia; 2003. pp. 427–434. [Google Scholar]
- Tanaka H, Kagawa H, Ohta H. Production of leptocephali of Japanese eel (Anguilla japonica) in captivity. Aquaculture. 2001;201:55–60. doi: 10.1016/S0044-8486(01)00553-1. [DOI] [Google Scholar]
- Tanaka H, Kagawa H, Ohta H, Okuzawa K, Hirose K. The first report of eel larvae ingesting rotifers. Fish Sci. 1995;61:171–172. [Google Scholar]
- Yamauchi K, Nakamura M, Takahashi H, Takano K. Cultivation of larvae of Japanese eel. Nature. 1976;263:412. doi: 10.1038/263412a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Yamauchi K. Sexual maturation of Japanese eel and production of eel larvae in the aquarium. Nature. 1974;251:220–222. doi: 10.1038/251220a0. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Yamamoto K. Experiments on artificial maturation and fertilization of the Japanese eel (Anguilla japonica) In: Richter CJ, Goos GJTh, editors. Reproductive physiology of fish. Wargeningen: Pudoc Press; 1982. pp. 185–189. [Google Scholar]


