Abstract
The expressions of trypsin and chymotrypsin in the pyloric caeca of Atlantic salmon (Salmo salar L.) were studied in three experiments. Two internal (trypsin phenotypes, life stages) and three common external factors (starvation, feeding, temperatures) influencing growth rates were varied. Growth was stimulated by increased temperature and higher feeding rate, and it was depressed during starvation. The interaction between trypsin phenotype and start-feeding temperature affected specific activity of trypsin, but not of chymotrypsin. Trypsin specific activity and the activity ratio of trypsin to chymotrypsin (T/C ratio) increased when growth was promoted. Chymotrypsin specific activity, on the other hand, increased when there was a reduction in growth rate whereas fish with higher growth had higher chymotrypsin specific activity resulting in lower T/C ratio value. During a rapid growth phase, trypsin specific activity did not correlate with chymotrypsin specific activity. On the other hand, a relationship between specific activities of trypsin and chymotrypsin could be observed when growth declined, such as during food deprivation. Trypsin is the sensitive key protease under conditions favouring growth and genetically and environmentally affected, while chymotrypsin plays a major role when growth is limited or depressed. Trypsin specific activity and the T/C ratio value are shown to be important factors in the digestion process affecting growth rate, and could be applicable as indicators for growth studies of fish in captive cultures and in the wild, especially when food consumption rate cannot be measured.
Keywords: activity ratio of trypsin to chymotrypsin, chymotrypsin, starvation, temperature, trypsin, trypsin phenotype
Introduction
During the past two decades, a series of studies on proteolytic enzymes in fish has improved the knowledge on protein utilisation, and established the importance of trypsin as the key enzyme for feed utilisation and growth through its role in the protein digestion processes (Rungruangsak Torrissen and Male 2000). A major step forward was achieved by a series of studies on trypsin isozymes in the pyloric caeca of salmonids, especially Atlantic salmon (Salmo salar L.). These works are summarised as an illustration in Figure 1, showing a series of growth mechanisms, primarily affected by trypsin expression and thus influencing protein and amino acid utilisation and finally growth. Trypsin is found in different isoforms in the pyloric caeca and intestine (Torrissen 1984; Torrissen and Torrissen 1985), and different distributions of these isoforms could determine genetic variation in protein and feed utilisation and growth performance of individuals in aquaculture (Torrissen 1991; Torrissen et al. 1993; Rungruangsak-Torrissen et al. 1998, 1999a). Variations in trypsin phenotype distributions were also observed in natural marine ecosystem in different isothermal areas where temperature preferences of different phenotypes observed in corresponding to those found in aquaculture (Rungruangsak-Torrissen and Stensholt 2001). Different trypsin phenotypes, induced by variations in temperatures at the very early life, have different temperature preferences for feed conversion efficiency and growth (Rungruangsak-Torrissen et al. 1998). As seen in the Norwegian Sea when food abundance was limited, the pattern 1 salmon possessing trypsin isozyme effectively functioning at high temperature of >8 °C were mainly distributed at an average ambient temperature of 9.3 °C. Pattern 2′ salmon possessing trypsin isozyme effectively functioning at low temperature of ≤ 8 °C were mainly distributed at an average ambient temperature of 7.7 °C, while the heterozygote salmon of pattern 2 were mainly distributed between these two phenotypes at around 8.7 °C (Rungruangsak-Torrissen and Stensholt 2001). The details of different trypsin isozymes were described in Rungruangsak-Torrissen et al. (1998) and Rungruangsak Torrissen and Male (2000). Variations in the expression of trypsin (either by phenotype or enzymatic activity) have been related to feed conversion efficiency and/or growth rate (Torrissen and Shearer 1992; Rungruangsak-Torrissen et al. 1998; Lemieux et al. 1999; Sunde et al. 2001, 2004) by affecting rates of protein digestion, and resulting in differences in amino acid absorption (Torrissen et al. 1994; Rungruangsak-Torrissen and Sundby 2000; Sunde et al. 2001, 2004) and transport (Torrissen et al. 1994; Sunde et al. 2001, 2004). Absorption rate and level of free amino acids in the plasma influence plasma insulin levels (Rungruangsak-Torrissen and Sundby 2000) and consequently the rate of protein synthesis. A decrease in trypsin specific activity in the pyloric caecal tissue, due to an increase in trypsin secretion into the lumen (Rungruangsak Torrissen and Male 2000), accompanied by a general increase in plasma insulin level was observed one month before an increase in growth rate was seen (Rungruangsak-Torrissen et al. 1999a). Similarly, differences in trypsin specific activity were observed 30 days before a detection of differences in feed conversion efficiency (Sunde et al. 2004). Atlantic salmon possessing different trypsin phenotypes also exhibited different responses of some specific and non-specific immune parameters after vaccination (Rungruangsak-Torrissen et al. 1999b). Thus, the differences in trypsin expression may have indirect effects on the immune system as it affects variations in nutrient influx (Rungruangsak-Torrissen et al. 1999b), and dietary nutrients have important roles in relation to the immune function of fish (Waagbø 1994). A question has also been raised on the possible role of trypsinogen isozymes in non-pancreatic tissues, as eight trypsinogen genes were found to constitute 4.6% of the DNA of the human β T cell receptor locus, which has a vital role in immunity (Rowen et al. 1996). Different environmental or external factors, such as temperature (Rungruangsak-Torrissen et al. 1998; Rungruangsak Torrissen and Male 2000), light (Sunde et al. 2001), dietary quality (Torrissen et al. 1995; Rungruangsak-Torrissen et al. 2002; Sunde et al. 2004), growth hormone (Lemieux et al. 1999), and gene manipulation (Sunde et al. 2001; Blier et al. 2002) induced differences in expression of trypsin (as well as chymotrypsin) and thus partly resulted in growth rate variations observed (Figure 1). Possible importance of different trypsin phenotypes on growth of individual Atlantic salmon post-smolts at different temperatures during sea migration in the north-eastern Atlantic Ocean has been shown (Rungruangsak-Torrissen and Stensholt 2001). Off the Hebrides where fish weights were not different among the three phenotypes (due to high food abundance), the pattern 2′ salmon (with trypsin isozyme effectively functioning at low temperature of ≤ 8 °C) were smallest at an average ambient temperature of 10.2 °C, whereas they were biggest in the Norwegian Sea at an average ambient temperature of 7.7 °C, compared to the other trypsin phenotypes (Rungruangsak-Torrissen and Stensholt 2001). Besides our work, little evidence is available on the processes of feed utilisation and growth at different life stages in association with differences in trypsin expression at both molecular and protein levels. Most studies deal with early development and characterisation of trypsin, as reviewed by Rungruangsak Torrissen and Male (2000), and similar studies have been performed more recently. For example, early expression of trypsin has been reported for larvae of different species, such as Walleye pollock Theragra chalcogramma Pallas (Oozeki and Bailey 1995), sea bass Dicentrarchus labrax L. (Peres et al. 1996), Japanese flounder Paralichthys olivaceus Temminck and Schlegel (Kurokawa and Suzuki 1996), red drum Sciaenops ocellatus L. (Lazo et al. 2000b), Atlantic halibut Hippoglossus hippoglossus L. (Gawlicka et al. 2000), as well as in invertebrates, such as pink shrimp Farfantepenaeus paulensis Pérez-Farfante (Lemos et al. 1999). Characterisations of trypsin isozymes have been reported in different species at both protein (Sabapathy and Teo 1995; Outzen et al. 1996; Pavlisko et al. 1997) and molecular (Genicot et al. 1996; Schroder et al. 1998; Spilliaert and Gudmundsdottir 1999; Leiros et al. 2000) levels. The activity of trypsin showed a similar pattern as growth of juvenile Atlantic salmon during seasonal variation, and its secretion rate was related to the amount of feed passing through the gut (Einarsson et al. 1997). A linear relationship between trypsin activity and protein digestibility was observed in rainbow trout Oncorhynchus mykiss Walbaum (Krogdahl et al. 1994). Trypsin activity was reported to relate with growth rate in fish larvae fed high quality diets, such as in goldfish Carassius auratus L. (Abiayad and Kestemont 1994), sea bass (Cahu et al. 1998; Nolting et al. 1999), and red drum (Lazo et al. 2000a). At start feeding, replacements of fish meal by different protein meals affected the physiological status of juvenile channel catfish Ictalurus punctatus Rafinesque, and trypsin was depressed in fish offered isonitrogenous diets with a high replacement of fish meal protein with a low quality protein analogue (El-Saidy et al. 2000). In tilapia Oreochromis niloticus L. kept at different seawater salinities, trypsin activity was highest in fish with highest growth rate and serum thyroxine level (Woo et al. 1997).
Figure 1.
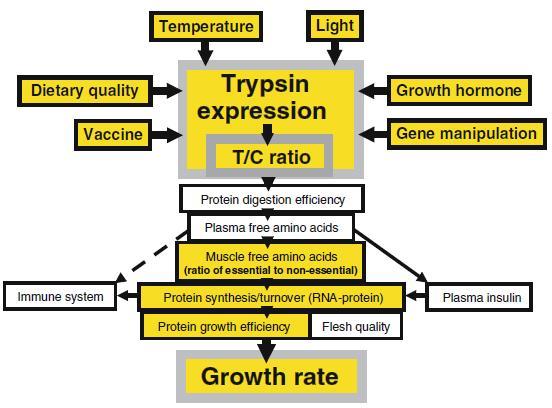
Diagram showing the importance of trypsin for a series of growth mechanisms through its role as the key enzyme in protein digestion process. Trypsin expression and the protease activity ratio of trypsin to chymotrypsin (T/C ratio) in the pyloric caeca, affecting nutrient influx that subsequently influences capacity for protein synthesis and immune system and growth, is influenced by temperature and dietary quality (Rungruangsak Torrissen and Male 2000; Sunde et al. 2004; present work) as well as by light regime (Sunde et al. 2001), growth hormone (Lemieux et al. 1999), gene manipulation (Sunde et al. 2001; Blier et al. 2002), and by vaccine type (unpublished work). Transport rate and level of free amino acids to target tissues (affecting plasma insulin secretion and protein synthesis in the white muscle) indicate protein utilisation efficiency and flesh quality of the fish. Details of the effects of trypsin expression on protein utilisation and muscle capacity for protein synthesis and on growth were described in Rungruangsak Torrissen and Male (2000) and Sunde et al.
Trypsin is the key protease activating other pancreatic proteases including chymotrypsin in mammals (Stryer 1988) and fish (Sunde et al. 2001). We would expect that any factors that affect trypsin activity should also influence chymotrypsin activity in a similar way, since they are the dominating digestive proteases and their activities are related. Interestingly, when trypsin activity correlated to casein levels in the feeds (Le Moullac and Van Wormhoudt 1994; Le Moullac et al. 1996), affected weight gain by different replacements of fish meal protein (El-Saidy et al. 2000), correlated to feed conversion efficiency under growth hormone treatment (Lemieux et al. 1999), and correlated to specific growth rate under light and ploidy treatments (Sunde et al. 2001); chymotrypsin did not show the same relationships as trypsin. This paper presents studies where variations in the expressions of trypsin and chymotrypsin in the pyloric caeca of Atlantic salmon were induced by different internal (trypsin phenotypes, life stages) and external (starvation, feeding, temperatures) factors. The work was performed in order to elucidate how trypsin and chymotrypsin are expressed under different growth conditions, which could be of importance for future growth studies in fish and probably other species in captive cultures and in the wild.
Materials and methods
Starvation effect
The experiment was performed at the Institute of
Marine Research - Matre, for 115 days during winter in three sea cages (5 × 5 × 5 m3) using 2–3 kg Atlantic salmon with 100 fish per sea cage. The sea temperature at 0–5 m depth varied between 3 and 8 °C (February–May 1996). The fish in two cages were starved for 89 days, followed by re-feeding with low (according to the recommendation of National Research Council (NRC), 1993) or high (NRC levels × 10) vitamin-mineral diets for 26 days. The fish in the other cage was used as control and were provided with a commercial diet according to feeding tables (Austreng et al. 1987) during the whole experimental period. Stomach and pyloric caeca were sampled every third week from eight fish per sea cage, altogether seven sampling times during the whole experimental period. The samples were kept frozen at −80 °C for later analyses. The stomach was used for the determination of pepsin specific activity, and the pyloric caeca was used for the analyses of trypsin and chymotrypsin specific activities (see below).
Temperature effect
The experiment was a part of the work described in Rungruangsak-Torrissen et al. (1998) on the effect of temperature on the expression of trypsin isozymes at early life stage, carried out at the Institute of Marine Research - Matre. Mixed fertilised eggs were produced from a total of 17 females and 9 males Atlantic salmon, and randomly divided into two groups for incubation at cold (5.9±1.9 °C) or warm (9.6±1.2 °C) water temperatures. About 350 °C × days after hatching, the alevins from each hatching temperature were further divided into two sub-groups, and transferred to start feeding at cold (5.6±1.3 °C) or warm (12.2±0.5 °C) water temperatures, during February to June 1994. Four groups differing in either hatching and/or start-feeding temperatures were produced (Cold-Cold, Cold-Warm, Warm-Cold and Warm-Warm). Five replicate tanks (1 m3) were used for each temperature group. All fish were cultured at natural water temperatures from July 1994 until sampling in January 1995. The fish were fed a commercial feed by using automatic feeders, 8 h day−1 according to feeding tables (Austreng et al. 1987). Since feed conversion efficiency relates to growth rate when feeding is restricted, the fish were fed at a 50% ration to restrict their growth rates during natural temperature period. In January 1995 (represented the sampling time when the fish had been in cold water temperatures of 6.1±0.5 °C for 3 months), 150 Atlantic salmon parr (30 fish per tank) from each temperature control group were sampled. The weight of individuals was recorded. The pyloric caeca was dissected and kept frozen at −80 °C for later analyses. Trypsin isozyme patterns of the individuals (patterns 1, 2 and 2′) were analysed by isoelectric focusing on agaroseIEF gel (Torrissen 1984). About 20–40% of the samples collected from each trypsin phenotype in each temperature control group were randomly chosen for the determinations of trypsin and chymotrypsin specific activities (see below). The fish of trypsin isozyme pattern 2′ possess a trypsin variant effectively functioning at ≤ 8 °C (especially ≤ 6 °C), whereas the pattern 1 fish do not possess this trypsin variant (Rungruangsak-Torrissen et al. 1998; Rungruangsak Torrissen and Male 2000). The pattern 2 fish are the heterozygotes of pattern 1 and pattern 2′ (Rungruangsak-Torrissen et al. 1998; Rungruangsak Torrissen and Male 2000).
Temperature and feeding effects
The Atlantic salmon parr from the cold temperature start-feeding groups (Cold-Cold and Warm-Cold) from the previous experiment were reared at a density of about 3 kg per m3 in 1 m3 tanks in four replicates from January to August 1995. The fish were fed at a restricted ration of 0.5% body weight day−1. Two sampling times were performed, in January after 3 months rearing in cold temperature (6.1±0.5 °C) and in August after 3 months rearing in warm temperature (9.8±0.3 °C). At each sampling time, the pyloric caeca samples were collected from 30–40 fish per tank, one sample set was taken during routine feeding and the other set during 5–7 h post-feeding (after 7 days starvation following with a continuous re-feeding for 30 min by using automatic feeders). The samples were kept frozen at −80 °C for later analyses. Trypsin isozyme patterns (patterns 1, 2 and 2′) of the individuals (Torrissen 1984; Rungruangsak-Torrissen et al. 1998; Rungruangsak Torrissen and Male 2000), and specific activities of trypsin and chymotrypsin (see below) were determined.
Determination of pepsin in the stomach
Pepsin in the stomach was determined using the method described by Rungruangsak and Utne (1981). Briefly, the stomach was extracted with five times its weight with 10 mM HCl. After homogenising and centrifuging at 15,000 × g for 60 min at 4 °C, the crude enzyme extract in the supernatant was kept at −80 °C. When assaying pepsin activity, 200 µl of the crude enzyme extract (containing 50–200 µg protein ml−1) was added to 200 µl of 1% casein (in 60 mM HCl). The mixture was incubated at 37 °C. The reaction was stopped after exactly 10 min incubation by adding 1 ml of 5% TCA, mixed thoroughly, let stand at room temperature for 30 min, and centrifuged at 5000 × g for 20 min. One ml of 0.5 M NaOH was added to 0.5 ml of the supernatant, following by 0.3 ml of Folin-Ciocalteu reagent (1:3 dilution). After standing for 10 min at room temperature, the absorbancy was measured spectrophotometrically at 720 nm and compared with l-tyrosine standard curve. The protein content of the crude enzyme extract was determined by Lowry method (Lowry et al. 1951). Pepsin specific activity was expressed as µmol tyrosine h−1 mg protein−1.
Determinations of trypsin and chymotrypsin in the pyloric caeca
The whole pyloric caeca sample was extracted with five times its weight with 1 mMHCl (Rungruangsak and Utne 1981). After homogenising and centrifuging at 15,000 × g for 60 min at 4 °C, the crude enzyme extract in the supernatant was kept at −80 °C. Trypsin activity was determined by incubating 0.1 ml of crude enzyme extract with 0.7 ml of trypsin substrate [1.25 mM benzoyl-l-arginine-p-nitroanilide (dissolved with dimethylformamide (5% final concentration) before made up to final volume with 0.2 M Tris-HCl buffer pH 8.4)] at 50 °C for 10 min (Torrissen et al. 1994). The reaction was stopped by the addition of 0.8 ml 30% acetic acid. The production of nitroaniline was measured spectrophotometrically at 410 nm, and compared with a nitroaniline standard curve. Chymotrypsin activity was determined by incubating 0.1 ml of crude enzyme extract with 0.7 ml of chymotrypsin substrate [0.1 mM succinyl-Ala-Ala-Ala-Pro-Phe-p-nitroanilide (dissolved with dimethylformamide (5% final concentration) before made up to final volume with 0.2 M Tris-HCl buffer pH 8.4)] at 40 °C for 10 min (Rungruangsak-Torrissen and Sundby 2000). The reaction was stopped and measured at 410 nm in the same way as trypsin. The protein content of the crude enzyme extract was determined by Lowry method (Lowry et al. 1951). Both trypsin and chymotrypsin specific activities were expressed as lmol p-nitroaniline produced h−1 mg protein−1.
Ethical aspect
The fish were immediately killed by a blow to the head before organ sampling. All experiments and procedures were approved by the local animal experiment authority in accordance with the laws and regulations controlling experiments and procedures on live animals in Norway. The work on starvation effect was under responsibility of the National Institute of Nutrition and Seafood Research, in order to see the effect of starvation during low temperature period. It should not have an ethical effect, as food availability is low in nature during this time of the year.
Statistical analyses
The values are given as mean±standard error of mean throughout. Data were analysed using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) and Statistica 5.1 software for Windows® (StatSoft, Tulsa, OK, USA). Linear regression, analysis of variance, and paired comparisons by Student’s t-test at 95% significance level were performed.
Results
Starvation effect
The starved fish lost their weight by 8.2% after 89 days starvation and had weight gain about 11.4% after 26 days re-feeding, while the control group gained their weight by 51% during the whole experimental period. The effect of starvation on the expression of pepsin in the stomach, and trypsin and chymotrypsin in the pyloric caeca is shown in Figure 2. Pepsin specific activity decreased during starvation period, while it took about 2 weeks before trypsin specific activity decreased and maintained at a lower level than the control-feeding group, in a similar way as pepsin specific activity. Chymotrypsin specific activity, on the other hand, increased and maintained at a higher level during starvation than during feeding period. Differences in the enzyme specific activities were observed after 14, 21 and 42 days starvation for pepsin, chymotrypsin and trypsin, respectively, (p<0.05). This resulted in a significantly lower value of protease activity ratio of trypsin to chymotrypsin (T/C ratio) during 21–89 days starvation (p<0.01). Comparisons between paired data sets indicated differences between starvation and feeding groups in protease specific activities of pepsin (days 0–89: p<0.05), trypsin (days 21–89: p<0.03), chymotrypsin (days 0–89: p<0.02), and of the T/C ratio value (days 0–89: p<0.05).
Figure 2.
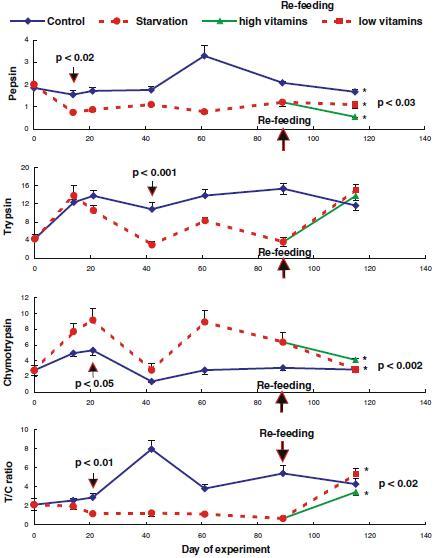
Effect of starvation on expressions of pepsin, trypsin, and chymotrypsin in the digestive tract of Atlantic salmon. Arrows with probability values indicate first observations of significantly changing in the enzyme expressions. The values with asterisk (*) are significantly different (p<0.05). The enzyme specific activities are expressed as µmol tyrosine h−1 mg protein−1 for pepsin in the stomach, and as µmol p-nitroaniline h−1 mg protein−1 for trypsin and chymotrypsin in the pyloric caeca.
After 26 days of re-feeding, the specific activity of chymotrypsin reduced and that of trypsin increased to normal levels as in the control-feeding group without special requirement for high vitamin and mineral concentrations in the diet, while pepsin specific activity was still at a low level. As a consequence, the T/C ratio value that was low during starvation increased to a normal level after re-feeding. Re-feeding with low vitamin-mineral diet resulted in a higher trypsin specific activity with specific activities of pepsin and chymotrypsin closer to the normal feeding than re-feeding with high vitamin-mineral diet.
Temperature effect
About 5% of the sampled fish were of other trypsin phenotypes (see Rungruangsak-Torrissen et al. 1998) and were not included in the analyses. As usual, the fish hatched and start-fed at warmer temperatures grew faster than those hatched and start-fed at colder temperatures (Table 1). Study of protease specific activities of trypsin and chymotrypsin and the T/C ratio value in these fish groups during the first winter indicated that these enzyme values varied according to trypsin phenotypes, past temperature experience during early feeding, and present environmental temperature (Table 1). By performing 3-way ANOVA (Table 2), fish weight was influenced by the temperatures at the early life period (p<0.0001), trypsin phenotypes (p<0.02), and by the interactions between these factors (p<0.03 – 0.0001). Effect on fish weight by the interaction between start-feeding temperature and trypsin phenotype was close to significant (p<0.08). Chymotrypsin specific activity was influenced by both hatching (p<0.02) and start-feeding (p<0.0001) temperatures. Effect on chymotrypsin specific activity by the interaction between hatching temperature and trypsin phenotype was close to significant (p<0.06). Trypsin specific activity, on the other hand, was influenced by the interaction between start-feeding temperature and trypsin phenotype (p<0.03). Thus, the T/C ratio value was affected by start-feeding temperature (p<0.0001). Effect on T/C ratio by hatching temperature (p<0.083) and by the interaction between start-feeding temperature and trypsin phenotype (p<0.07) were close to significant.
Table 1.
Effect of different temperatures during egg incubation and the first-feeding periods on weight and expressions of trypsin and chymotrypsin (expressed as lmol p-nitroaniline h–1 mg protein–1) and on the activity ratio of trypsin to chymotrypsin (T/C ratio) during the first winter in Atlantic salmon parr possessing different trypsin phenotypes
| Temperature (°C) | Parameter (mean±sem) | Trypsin isozyme pattern | ||
|---|---|---|---|---|
| 1 | 2 | 3′ | ||
| Hatching | Number | 24 | 25 | 18 |
| 5.9±1.9 | Weight (g) | 14.6±0.5 | 15.6±0.4 | 15.4±0.5 |
| Start-feeding | Trypsin | 68.5±3.7 | 72.4±4.2 | 80.1±5.7 |
| 5.6±1.3 | Chymotrypsin | 7.9±0.6a | 7.5±0.5a | 9.6±0.7b |
| (Cold-Cold) | T/C ratio | 9.9±1.0 | 10.7±0.9 | 9.3±1.1 |
| Hatching | Number | 7 | 28 | 23 |
| 5.9±1.9 | Weight (g) | 34.5±2.3 | 33.0±1.0 | 34.8±1.4 |
| Start-feeding | Trypsin | 73.5±6.9 | 77.6±3.4 | 72.5±3.7 |
| 12.2±0.5 | Chymotrypsin | 3.5±0.3 | 4.1±0.2 | 4.4±0.2 |
| (Cold-Warm) | T/C ratio | 22.4±3.3 | 20.2±1.4 | 17.4±1.3 |
| Hatching | Number | 32 | 21 | 17 |
| 9.6±1.2 | Weight (g) | 17.5±0.7 | 19.9±1.0 | 16.1±1.0 |
| Start-feeding | Trypsin | 72.3±3.6 | 68.3±3.6 | 73.4±5.3 |
| 5.6±1.3 | Chymotrypsin | 9.1±0.7 | 9.3±0.4 | 8.7±1.0 |
| (Warm-Cold) | T/C ratio | 9.5±0.9 | 7.8±0.6 | 11.7±2.1 |
| Hatching | Number | 11 | 29 | 23 |
| 9.6±1.2 | Weight (g) | 46.3±4.5a | 66.3±3.7b | 61.2±4.8b |
| Start-feeding | Trypsin | 81.9±5.7 | 85.8±4.8 | 70.9±4.5 |
| 12.2±0.5 | Chymotrypsin | 4.9±0.4 | 4.9±0.2 | 4.9±0.3 |
| (Warm-Warm) | T/C ratio | 17.8±1.7 | 18.4±1.4 | 15.8±1.7 |
The values with different superscripts are significantly different (p<0.05). See details of each trypsin pattern in the text and in the legend of Figure 2.
Table 2.
Three-way ANOVA of the effects of hatching temperature (HT), start-feeding temperature (SFT) and trypsin phenotype (TRP) on weight and the expressions of trypsin and chymotrypsin (expressed as lmol p-nitroaniline h–1 mg protein–1) and on the activity ratio of trypsin to chymotrypsin (T/C ratio) during the first winter in Atlantic salmon parr, with significant effects shown by the p values <0.05 (bold values)
| General effect | df | Weight (g) | Trypsin | Chymotrypsin | T/C ratio | ||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | ||
| HT | 1 | 81.97 | 0.0000 | 0.23 | 0.6326 | 6.23 | 0.0132 | 3.04 | 0.0825 |
| SFT | 1 | 407.19 | 0.0000 | 2.64 | 0.1055 | 172.57 | 0.0000 | 106.59 | 0.0000 |
| TRP | 2 | 4.48 | 0.0123 | 0.23 | 0.7936 | 1.12 | 0.3278 | 0.74 | 0.4784 |
| HT×SFT | 1 | 52.39 | 0.0000 | 1.71 | 0.1918 | 0.10 | 0.7508 | 1.85 | 0.1745 |
| HT×TRP | 2 | 4.99 | 0.0075 | 1.06 | 0.3477 | 2.86 | 0.0594 | 1.26 | 0.2843 |
| SFT×TRP | 2 | 2.61 | 0.0755 | 3.72 | 0.0256 | 0.45 | 0.6388 | 2.70 | 0.0690 |
| HT×SFT×TRP | 2 | 3.89 | 0.0216 | 0.24 | 0.7903 | 1.41 | 0.2469 | 1.23 | 0.2941 |
| Error | 246 | ||||||||
The fish possessing trypsin phenotype pattern 2′ with the trypsin variant effectively functioning at temperature <6 °C showed a relatively higher value of either trypsin specific activity or both trypsin specific activity and T/C ratio than the pattern 1 fish without the variant, if both phenotypes had cold start-feeding temperature experience (Table 1). These enzyme values were vice versa (pattern 2′< pattern 1) if the fish had warm start-feeding temperature experience, regardless of hatching temperatures (Table 1). There was no difference in weight during the first winter between salmon parr of different trypsin phenotypes within the same temperature control group, except for the group without cold temperature experience (warm hatching and warm start-feeding temperatures: Warm-Warm) that the fish lacking the cold temperature variant (pattern 1: 46.3±4.5 g) were significantly smaller (p<0.05) than the fish carrying the variant (pattern 2: 66.3±3.7 g, pattern 2′: 61.2± 4.8 g). The heterozygote salmon of pattern 2 showed better performance than the other phenotypes at varying temperature control conditions (Table 1). Interestingly, trypsin specific activity (p<0.05) and T/C ratio (p<0.0001) were significantly higher, while chymotrypsin specific activity (p<0.0001) was significantly lower, in salmon parr with higher growth (48.0±1.9 g) having warm start-feeding temperature experience (Cold-Warm and Warm-Warm) than the fish with slower growth (16.6±0.3 g) having cold start-feeding temperature experience (Cold-Cold and Warm-Cold), regardless of hatching temperatures and trypsin phenotypes (Table 1). The weight of the fish was significantly correlated with the T/C ratio value (R2=0.1739, n=258, p<0.0001) and with trypsin specific activity (R2=0.0352, n=258, p<0.003), regardless of rearing temperatures and trypsin phenotypes (Figure 3). For chymotrypsin, a negative correlation with weight (R2=0.0511, n=137, p<0.01) was observed for the cold temperature start-feeding groups (Cold-Cold and Warm-Cold), while a positive correlation with weight (R2=0.0826, n=121, p<0.002) was observed for the warm temperature start-feeding groups (Cold-Warm and Warm-Warm) (Figure 3).
Figure 3.
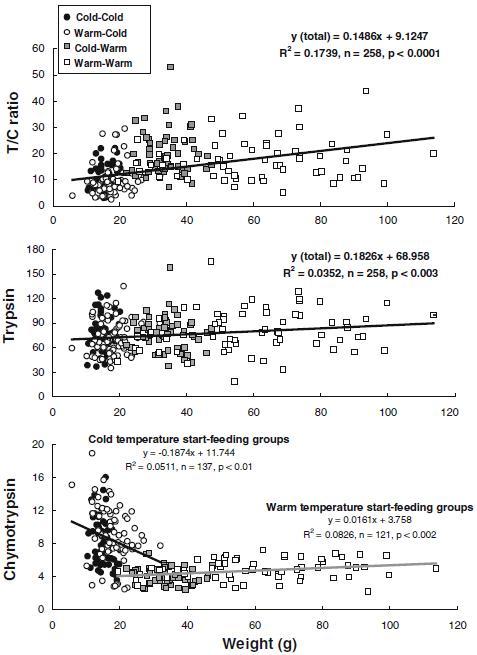
Relationships of weight with trypsin, chymotrypsin, and with the activity ratio of trypsin to chymotrypsin (T/C ratio) during the first winter in Atlantic salmon parr, regardless of rearing temperatures and trypsin phenotypes. The enzyme specific activities of trypsin and chymotrypsin are expressed as µmol p-nitroaniline h−1 mg protein−1.
Temperature and feeding effects
The weights of patterns 1, 2 and 2′ fish were 16.3±0.5 g, 17.7±0.6 g and 15.7±0.6 g in January after 3 months at 6 °C; and 29.1±1.0 g, 27.3±0.9 g and 26.1±0.9 g in August after 3 months at 10 °C, respectively. There was no significant difference in weight among the different trypsin phenotypes in January (6 °C), whereas the fish of pattern 2′ were significantly smaller (p<0.04) than the pattern 1 fish in August (10 °C). During routine feeding, there was no correlation between the enzyme specific activities of trypsin and chymotrypsin at any temperature, and trypsin activated higher chymotrypsin at 6 °C than at 10 °C (p<0.0001, Figure 4a). On the other hand, the relationship between trypsin specific activity and the T/C ratio value was significant at both 6 °C (R2=0.2525, n=138, p<0.0001) and 10 °C (R2=0.0634, n=118, p<0.01) with higher T/C ratio value at 10 °C than at 6 °C (p<0.0001, Figure 4b). The slopes of the relationship between trypsin specific activity and the T/C ratio value were not different among the three trypsin phenotypes at 6 °C (Figure 4c), while the slope was significantly higher (p<0.05) in the pattern 1 fish than in the fish of pattern 2 and pattern 2′ at 10 °C, with a relatively higher T/C ratio value in the fish of pattern 1 (19.8±1.7, p<0.063) and pattern 2 (20.6±2.0, p<0.05) compared to the pattern 2′ fish (15.3±1.5) (Figure 4d).
Figure 4.
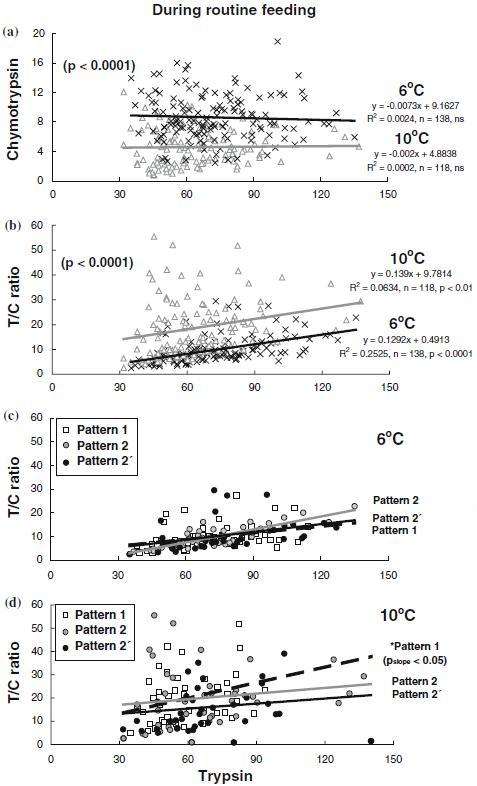
Effects of water temperature and routine feeding on the relationships of trypsin with chymotrypsin and with the activity ratio of trypsin to chymotrypsin (T/C ratio) in Atlantic salmon parr reared at 6 °C (×) and 10 °C (D), regardless of trypsin phenotypes (a) and (b), and according to trypsin phenotypes at 6 °C (c) and 10 °C (d). The enzyme specific activities of trypsin and chymotrypsin are expressed as µmol p-nitroaniline h−1 mg protein−1. ns, not significant.
During 5–7 h post-feeding, there was a significant correlation between trypsin and chymotrypsin specific activities at 10 °C (R2=0.1046, n=130, p<0.0002), but not at 6 °C, with trypsin activated higher chymotrypsin (p<0.0001) at 10 °C than at 6 °C (Figure 5a) in contrast to the values from routine feeding (Figure 4a). Trypsin specific activity correlated with the T/C ratio value at both 6 °C (R2=0.2827, n=68, p<0.0001) and 10 °C (R2=0.762, n=130, p<0.0001), with a higher T/C ratio at 6 °C than at 10 °C (p<0.0001, Figure 5b) in opposite to the values from routine feeding (Figure 4b). At 6 °C, the slope of the postprandial relationship between trypsin specific activity and the T/C ratio values was higher (p<0.05) in the pattern 2′ fish (T/C ratio=22.5±3.5), compared to the fish of pattern 2 (T/C ratio=14.8±1.4) and pattern 1 (T/C ratio=16.0±2.0) (Figure 5c). This post-prandial relationship was similar between the different trypsin phenotypes at 10 °C (Figure 5d).
Figure 5.
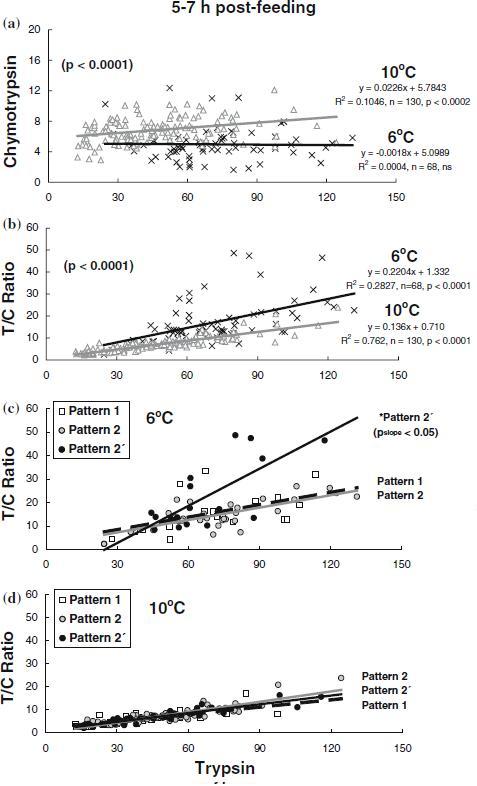
Effects of temperature and post-feeding on the relationships of trypsin with chymotrypsin and with the activity ratio of trypsin to chymotrypsin (T/C ratio) in Atlantic salmon parr reared at 6 °C (×) and 10 °C (D), regardless of trypsin phenotypes (a) and (b), and according to trypsin phenotypes at 6 °C (c) and 10°C (d). The enzyme specific activities of trypsin and chymotrypsin are expressed as µmol p-nitroaniline h−1 mg protein−1.
Discussion
Trypsin activity could be detected during starvation as digestive enzyme gene expression of trypsin is never entirely ‘switched off’ during the feeding cycle, and remains transcriptionally active for digestive enzyme messenger RNAs during periods of starvation (Lehnert and Johnson 2002). After 3 months starvation in tank scale experiment where no natural foods supply through water, trypsin activity, but not chymotrypsin activity, was still detected at a low level (unpublished data). Thus the enzyme activities, especially of chymotrypsin, found in starved fish in the present experiment indicated that the fish had consumption. We do not know why trypsin and chymotrypsin specific activities increased during the early period of starvation (days 0–20 in Figure 2). It might be due to the fact that the fish were reared in sea cages and might feed on either natural food or artificial diet from near-by sea cages. However, increase in protease specific activity during the early period of starvation was not observed for pepsin probably suggesting that pepsin is less sensitive to feeding than trypsin and chymotrypsin (Figure 2). Among the three proteases studied, alkaline proteases (trypsin and chymotrypsin) were affected by different feed qualities while acidic protease (pepsin) was not affected (Rungruangsak and Utne 1981). Regardless of higher protein or lipid deposition, trypsin specific activity increased in potential rapidly growing fish (Sunde et al. 2001, 2004), and this was also seen in the present studies. Trypsin activates chymotrypsin, and their relationship could be observed when growth was limited such as during food deprivation (Figure 5a). Relationship between specific activities of trypsin and chymotrypsin was not found during routine feeding of growing phase (Figure 4a). Significant relationship between trypsin and chymotrypsin in the pyloric caeca during starvation had been previously observed, and the relationship disappeared after feeding (Rungruangsak Torrissen and Male 2000). No relationship between trypsin and chymotrypsin observed during 5–7 h post-feeding at 6 °C (Figure 5a) might be due to the fact that the amount of feed required for growth at 6 °C was less than that at 10 °C, and a 30 min re-feeding might reach this requirement at 6 °C but not at 10 °C. Protease activity ratio of trypsin to chymotrypsin (T/C ratio) was higher during rapid growth and lower during slower growth rate. Relationships between feed conversion efficiency (and/or specific growth rate) with trypsin specific activity (Lemieux et al. 1999; Zabielski et al. 1999; Sunde et al. 2001), and with the T/C ratio value (Sunde et al. 2001) have been previously observed in different fish species. Fish consuming identical quantities of food could grow differently (Carter et al. 1992, 1993) due to variability in food conversion efficiency. It seemed that increased feed conversion efficiency through a similar consumption rate as in diploid fish was due to a decreased chymotrypsin specific activity, while increased feed conversion efficiency through increased consumption rate as in triploid fish was due to a relatively higher increase in trypsin than in chymotrypsin specific activity (Sunde et al. 2001). The T/C ratio value seemed to be more sensitive than trypsin specific activity for comparison between fish with potential different growth rate (Sunde et al. 2001), especially when genetically manipulated fish is concerned, as the enzyme activities may not be directly comparable to diploids (Sunde et al. 2001). Similarly, comparison of fast growing transgenic coho salmon (0+) and control group (1+) of similar body size showed a significantly higher mean T/C ratio value (p<0.05) in the transgenic group (1738±270; mean±SD), compared to the control (1239±154), indicating a potentially higher growth rate of the transgenic fish, whereas the specific activities of trypsin (4.09±0.57 vs. 4.40±1.30 U mg protein−1) and chymotrypsin (0.00245±0.0078 vs. 0.00359±0.00112 U mg protein−1, p<0.05) were not directly comparable (Blier et al. 2002). By calculating the T/C ratio values from the published work of El-Saidy et al. (2000), a high correlation potential (y=0.0167x−4.1468, R2 = 0.5365, n=7, p=0.06) was observed between weight gains (x) and the T/C ratios (y) in channel catfish fed different isonitrogenous starter diets, regardless of fish meal types. Increased fish meal replacements by fish meal analogue reduced weight gain and the calculated T/C ratio value. An increase in the T/C ratio value could be due to a relatively higher increased trypsin than chymotrypsin specific activity or a decreased chymotrypsin specific activity (Rungruangsak-Torrissen and Sundby 2000; calculated from El-Saidy et al. 2000; Sunde et al. 2001; current work).
Unlike trypsin, higher chymotrypsin specific activity was observed when growth was limited or depressed due to starvation or food deprivation. During starvation, specific activity of trypsin decreased while that of chymotrypsin increased resulting in a lower T/C ratio value than during feeding (Figure 2). Correspondingly, when growth was depressed due to food deprivation in the fish that were expected to have a higher growth rate (at 10 °C), trypsin activated a higher chymotrypsin specific activity during 5–7 h post-feeding resulting in a lower T/C ratio value, compared to a slower growth fish (at 6 °C) (Figure 5). During non-growing or steady growth phase due to adaptation to a new feed type, a lower T/C ratio value (resulted from increased chymotrypsin specific activity) was observed in fish with higher feed conversion efficiency (Rungruangsak-Torrissen et al. 2002; Sunde et al. 2004). Similarly, lower T/C ratio value in rapidly growing fish during steady growth was also observed in maturing Atlantic mackerel (Scomber scombrus L.), a fish with high lipid deposition rate (Rungruangsak-Torrissen and Fosseidengen 2002). On the other hand, the expressions of trypsin and chymotrypsin observed in the fish during routine feeding of growing phase showed that the slower growing fish had higher chymotrypsin specific activities (lower T/C ratio) than the faster growing fish (Table 1 and Figure 4).
It is interesting to note that the Atlantic salmon possessing the trypsin variant effectively functioning at low temperature had relatively higher trypsin specific activity and/or the T/C ratio value at low rearing temperatures (6 °C) than the fish lacking the variant (Table 1 and Figure 5), whilst the values were vice versa at high rearing temperatures (≥10 °C) (Table 1 and Figure 4). The explanations about specific trypsin variants (trypsin phenotypes) induced and affecting feed conversion efficiency and growth at different temperatures were described in Rungruangsak-Torrissen et al. (1998), and the effects of them on digestive efficiency and growth at different temperatures are described in the current work. Genetic variation in trypsin phenotype and digestive efficiency is important for optimising food utilisation for optimal growth of individuals in nature, especially when food is not abundance. The fish seem to distribute spatially in the temperature zones suitable to their trypsin phenotypes, as observed in Atlantic salmon post-smolts during summer in the Norwegian Sea (Rungruangsak-Torrissen and Stensholt 2001). Genetic variation in digestive efficiency was dependent on the temperature history of the fish during the very early life stage. The interactions between the temperature experiences during start-feeding and trypsin phenotype were evident, affecting trypsin specific activity (p<0.03), and to some extent the T/C ratio (p<0.07) and fish growth (p<0.08) (Table 2). On the other hand, the interaction between hatching temperature regimes and trypsin phenotype affected fish growth (p<0.01), and chymotrypsin specific activity (p<0.06). When including trypsin phenotype as genetic factor, the temperature regime at hatching seemed to influence chymotrypsin specific activity, whereas the start-feeding temperature regime influenced trypsin specific activity (Tables 1 and 2). The Atlantic salmon alevins should experience both cold (6–8 °C) and warm (10–12 °C) temperatures in order to have a chance to express genetic diversity in digestive ability (pattern 2) at varying living temperatures (Rungruangsak-Torrissen et al. 1998; Table 1). In addition, the levels of vitamins and minerals in the diet could affect the protease specific activities, since excess of micronutrients showed an adverse effect on the digestive efficiency and growth of the fish. Higher chymotrypsin specific activity and lower pepsin and trypsin specific activities and T/C ratio value were observed after re-feeding adult salmon a high vitamin-mineral diet, compared to a low vitamin-mineral diet with micronutrients added according to the requirement (Figure 2). Trypsin specific activity and the T/C ratio value are important digestive efficiency markers in growth studies (Lemieux et al. 1999; calculated from El-Saidy et al. 2000; Rungruangsak Torrissen and Male 2000; Sunde et al. 2001, 2004; Rungruangsak-Torrissen et al. 2002; Rungruangsak-Torrissen and Fosseidengen 2002; present work). By knowing fish weight, we could compare and predict the growth potential of different fish groups by studying these markers and their relationships. The specific activities of trypsin and chymotrypsin are correlated when growth is limited, while the correlation disappears during a rapid growth (i.e. positive correlation between trypsin specific activity and the T/C ratio). In fish with high growth potential, the T/C ratio value would rise during rapid growth (Sunde et al. 2001), while it would decline during no or steady growth (Rungruangsak-Torrissen et al. 2002; Rungruangsak-Torrissen and Fosseidengen 2002; Sunde et al. 2004). Trypsin specific activity would be higher in rapidly growing fish (Abiayad and Kestemont 1994; Le Moullac and Van Wormhoudt 1994; Le Moullac et al. 1996; Woo et al. 1997; Cahu et al. 1998; Lemieux et al. 1999; Nolting et al. 1999; Lazo et al. 2000a/b; El-Saidy et al. 2000; Sunde et al. 2001; present work), and when the fish show similar weight or growth rate, differences in the trypsin specific activity observed seem to predict future differences in growth rate (Rungruangsak-Torrissen et al. 1999a) or feed conversion efficiency (Sunde et al. 2004) one month later. Thus, with experience, both trypsin specific activity and the T/C ratio could be used to compare and predict growth between fish groups within a period of 1–2 months. By knowing more about the chemical quality of the diets fed, these protease markers will increase in their precision in predicting growth and in evaluating the biological quality of the diets (Rungruangsak-Torrissen et al. 2002). Specific activities of trypsin and chymotrypsin were higher in fish fed a higher level of dietary protein, and the T/C ratio values were higher in higher growth fish independent of the specific activity levels of the enzymes (to be published elsewhere).
Trypsin cleaves protein at the carboxyl side of basic amino acids, lysine and arginine (Stryer 1988), which show higher digestibilities than other amino acids (Espe et al. 1993; Skrede et al. 1998). Both lysine and arginine seem to elevate plasma insulin levels in salmonids (Plisetskaya et al. 1991). Arginine is the most potent stimulator of insulin secretion (Plisetskaya et al. 1991), while lysine is more potent than arginine in stimulating the endocrine pancreas in the eel Anguilla anguilla (Ince 1980). Absorption rate and level of free amino acids in the plasma influence plasma insulin secretion (Rungruangsak-Torrissen and Sundby 2000). Insulin stimulates amino acid uptake and protein synthesis especially in the muscle tissues (Murat et al. 1981; Matty 1986), and thereby exerting a growth promoting effect in salmonids (Donaldson et al. 1979; Ablett et al. 1981; Sundby et al. 1991; Rungruangsak-Torrissen et al. 1999a). Increased luminal secretion of trypsin by decreasing the levels of trypsin specific activity in tissues (Rungruangsak Torrissen and Male 2000) accompanied by increased plasma insulin level during a period of similar growth resulted in increased growth in Atlantic salmon one month later (Rungruangsak-Torrissen et al. 1999a). Trypsin itself does not directly induce growth, as illustrated by a low correlation coefficient value between the two parameters (Sunde et al. 2001; Figure 3). A highly significant correlation between trypsin specific activity and growth rate (although at a low correlation coefficient value) indicates that an increase in trypsin expression in the digestion process is a mediator that could stimulate growth mechanisms in animals (Rungruangsak Torrissen and Male 2000; Figure 1). Trypsin activates chymotrypsin in fish (Sunde et al. 2001) as in mammals (Stryer 1988). Chymotrypsin cleaves protein at the carboxyl side of aromatic amino acids phenylalanine, tyrosine, tryptophan, as well as of large hydrophobic residues such as methionine (Stryer 1988). Increased chymotrypsin expression is however associated with a period when there is a reduction in growth rate. It is not known whether the aromatic amino acids phenylalanine and tyrosine in excess might impact biological systems in a similar way as toxic organic pollutants, such as benzene, polychlorinated biphenyls (PCBs) and dioxins, as they contain similar free benzene ring(s). Gene expressions of trypsin and chymotrypsin (as well as of different isozymes) should be studied closely in connection with other important biological factors affecting protein synthesis rate, such as the multitude of endocrine regulators, in order to gain knowledge of how growth is controlled through or parallel to the digestive processes.
Conclusion
Trypsin is the sensitive key protease under condition favouring growth, and genetically and environmentally affected. Chymotrypsin, on the other hand, plays a major role when growth is limited or depressed. Trypsin specific activity and the protease activity ratio of trypsin to chymotrypsin (T/C ratio) have been shown to be important markers in the digestion process, correlating to feed conversion efficiency and growth rate of animals, regardless of higher protein or lipid deposition. They are sensitive and reliable biological indicators for comparison of diet quality, digestive efficiency, and the potential growth rate. Further, analyses of muscle growth and muscle quality (Rungruangsak Torrissen and Male 2000; Sunde et al. 2001, 2004; Rungruangsak-Torrissen and Fosseidengen 2002) would indicate whether any growth differences observed among fish groups are due to higher protein synthesis or retention. Any factors (feed ingredients (Rungruangsak-Torrissen et al. 2002; Rungruangsak-Torrissen and Fosseidengen 2002; Sunde et al. 2004; Figure 2), genetic differences (Sunde et al. 2001; Blier et al. 2002; current work), hormones (Lemieux et al. 1999), light (Sunde et al. 2001), vaccines (unpublished work), drugs, environmental stressors, etc.) that may affect growth rate and welfare of animals through changes in the digestion processes should be carefully evaluated for their effects on both trypsin and chymotrypsin expressions. Without knowing feed consumption, trypsin specific activity and the T/C ratio value could predict the present and/or the future growth, probably over a period of 1–2 months. By studying the interactions between these enzymatic markers and with the weight of the animal, a direction of a future change in growth status could be predicted. Practically, these biological parameters could be applicable for studying food utilisation and growth of individuals with different behaviours and in the wild where individual food consumption rate cannot be measured.
Acknowledgement
The authors would like to thank E. Bakke and M. Berntsen (previously technical assistants) for their contributions to the projects. These works were supported by the Institute of Marine Research in Norway, and by grants from the Ministry of Fisheries and the Research Council of Norway.
References
- Ablett R.F., Sinnhuber R.O., Holmes R.M., Selivon-chick D.P. The effect of prolonged administration of bovine insulin in rainbow trout (Salmo gairdneri R) Gen. Comp. Endocrinol. 1981;43:211–217. doi: 10.1016/0016-6480(81)90314-2. [DOI] [PubMed] [Google Scholar]
- Abiayad A., Kestemont P. Comparison of the nutritional status of goldfish (Carassius auratus) larvae fed with live, mixed or dry diet. Aquaculture. 1994;128:163–176. doi: 10.1016/0044-8486(94)90111-2. [DOI] [Google Scholar]
- Austreng E., Storebakken T., Åsgård T. Growth rate estimates for cultured Atlantic salmon and rainbow trout. Aquaculture. 1987;60:157–160. doi: 10.1016/0044-8486(87)90307-3. [DOI] [Google Scholar]
- Blier P.U., Lemieux H., Devlin R.H. Is the growth rate of fish set by digestive enzymes or metabolic capacity of tissues? Insight from transgenic coho salmon. Aquaculture. 2002;209:379–384. doi: 10.1016/S0044-8486(01)00807-9. [DOI] [Google Scholar]
- Cahu C.L., Infante J.L.Z., Peres A., Quazuguel P., Le Gall M.M. Algal addition in sea bass (Dicentrarchus labrax) larvae rearing: effect on digestive enzymes. Aquaculture. 1998;161:479–489. doi: 10.1016/S0044-8486(97)00295-0. [DOI] [Google Scholar]
- Carter C.G., Houlihan D.F., McCarthy I.D., Brafield A.E. Variation in the food intake of grass carp, Ctenopharyngodon idella (Val.), fed singly or in groups. Aquat. Liv. Res. 1992;5:225–228. doi: 10.1051/alr:1992022. [DOI] [Google Scholar]
- Carter C.G., Houlihan D.F., Brechin J., McCarthy I.D. The relationships between protein intake and protein accretion, synthesis and retention efficiency for individual grass carp, Ctenopharyngodon idella (Val.) Can. J. Zool. 1993;71:392–400. doi: 10.1139/z93-055. [DOI] [Google Scholar]
- Donaldson E.M., Fagerlund U.H.M., Higgs D.A., McBride J.R. Hormonal enhancement of growth. In: Hoar W.S., Randall D.J., Brett R.J., editors. Fish Physiology. New York: Academic Press; 1979. pp. 455–579. [Google Scholar]
- Einarsson S., Jonsson A.C., Davies P.S. Seasonal variation in trypsin activity in juvenile Atlantic salmon upper and lower modal groups. J. Fish Biol. 1997;51:1209–1218. doi: 10.1111/j.1095-8649.1997.tb01137.x. [DOI] [PubMed] [Google Scholar]
- El-Saidy D.M.S.D., Dabrowski K., Bai S.C. Nutritional effects of protein source in starter diets for channel catfish (Ictalurus punctatus Rafinesque) in suboptimal water temperature. Aquacult. Res. 2000;31:885–892. doi: 10.1046/j.1365-2109.2000.00494.x. [DOI] [Google Scholar]
- Espe M., Lied E., Torrissen K.R. In vitro protein synthesis in muscle of Atlantic salmon (Salmo salar) as affected by the degree of proteolysis in feeds. J. Anim. Physiol. Anim. Nutr. 1993;69:260–266. doi: 10.1111/j.1439-0396.1993.tb00813.x. [DOI] [Google Scholar]
- Gawlicka A., Parent B., Horn M.H., Ross N., Opstad I., Torrissen O.J. Activity of digestive enzymes in yolk-sac larvae of Atlantic halibut (Hippoglossus hippoglossus): indication of readiness for first feeding. Aquaculture. 2000;184:303–314. doi: 10.1016/S0044-8486(99)00322-1. [DOI] [Google Scholar]
- Genicot S., Rentier-Delrue F., Edwards D., Van-Beeumen J., Gerday C. Trypsin and trypsinogen from an Antarctic fish: Molecular basis of cold adaptation. Biochim. Biophys. Acta-Prot. Struc. Mol. Enzymol. 1996;1298:45–57. doi: 10.1016/S0167-4838(96)00095-7. [DOI] [PubMed] [Google Scholar]
- Ince B.W. Amino acid stimulation of insulin secretion from the in situ perfused eel pancreas; modifications by somatostatin, adrenaline and theophylline. Gen. Comp. Endocrinol. 1980;40:275–282. doi: 10.1016/0016-6480(80)90276-2. [DOI] [PubMed] [Google Scholar]
- Krogdahl A., Lea T.B., Olli J.L. Soybean proteinase inhibitors affect intestinal trypsin activities and amino acid digestibilities in rainbow trout (Oncorhynchus mykiss) Comp. Biochem. Physiol. 1994;107A:215–219. doi: 10.1016/0300-9629(94)90296-8. [DOI] [Google Scholar]
- Kurokawa T., Suzuki T. Formation of the diffuse pancreas and the development of digestive enzyme synthesis in larvae of the Japanese flounder Paralichthys olivaceus. Aquaculture. 1996;141:267–276. doi: 10.1016/0044-8486(95)01237-0. [DOI] [Google Scholar]
- Lazo J.P., Dinis M.T., Holt G.J., Faulk C., Arnold C.R. Co-feeding microparticulate diets with algae: toward eliminating the need of zooplankton at first feeding in larval red drum (Sciaenops ocellatus) Aquaculture. 2000a;188:339–351. doi: 10.1016/S0044-8486(00)00339-2. [DOI] [Google Scholar]
- Lazo J.P., Holt G.J., Arnold C.R. Ontogeny of pancreatic enzymes in larval red drum Sciaenops ocellatus. Aquacult. Nutr. 2000b;6:183–192. doi: 10.1046/j.1365-2095.2000.00124.x. [DOI] [Google Scholar]
- Le Moullac G., Klein B., Sellos D., Wormhoudt A. Adaptation of trypsin, chymotrypsin and α-amylase to casein level and protein source in Penaeus vannamei (Crustacea, Decapoda) J. Exp. Mar. Biol. Ecol. 1996;208:107–125. doi: 10.1016/S0022-0981(96)02671-8. [DOI] [Google Scholar]
- Le Moullac G., Wormhoudt A. Adaptation of digestive enzymes to dietary protein, carbohydrate and fibre levels and influence of protein and carbohydrate quality in Penaeus vannamei larvae (Crustacea, Decapoda) Aquat. Liv. Res. 1994;7:203–210. doi: 10.1051/alr:1994022. [DOI] [Google Scholar]
- Lehnert S.A., Johnson S.E. Expression of hemocyanin and digestive enzyme messenger RNAs in the hepatopancreas of the black tiger shrimp Penaeus monodon. Comp. Biochem. Physiol. 2002;133B:163–171. doi: 10.1016/s1096-4959(02)00123-9. [DOI] [PubMed] [Google Scholar]
- Leiros H.K.S., Willassen N.P., Smalås A.O. Structural comparison of psychrophilic and mesophilic trypsins – Elucidating the molecular basis of cold-adaptation. Eur. J. Biochem. 2000;267:1039–1049. doi: 10.1046/j.1432-1327.2000.01098.x. [DOI] [PubMed] [Google Scholar]
- Lemieux H., Blier P.U., Dutil J-D. Do digestive enzymes set physiological limit on growth rate and food conversion efficiency in Atlantic cod (Gadus morhua)? Fish Physiol. Biochem. 1999;20:293–303. doi: 10.1023/A:1007791019523. [DOI] [Google Scholar]
- Lemos D., Hernandez-Cortes M.P., Navarrete A., Garcia-Carreno F.L., Phan V.N. Ontogenetic variation in digestive proteinase activity of larvae and postlarvae of the pink shrimp Farfantepenaeus paulensis (Crustacea: Decapoda: Penaeidae) Mar. Biol. 1999;135:653–662. doi: 10.1007/s002270050666. [DOI] [Google Scholar]
- Lowry H.O., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Matty A.J. Nutrition, hormones and growth. Fish Physiol. Biochem. 1986;2:141–150. doi: 10.1007/BF02264082. [DOI] [PubMed] [Google Scholar]
- Murat J.-C., Plisetskaya E.M., Woo N.Y.S. Endocrine control of nutrition in cyclostomes and fish. Comp. Biochem. Physiol. 1981;68A:149–158. doi: 10.1016/0300-9629(81)90335-2. [DOI] [Google Scholar]
- Nutrient Requirements of Fish. Washington DC: National Academy Press; 1993. [Google Scholar]
- Nolting M., Ueberschar B., Rosenthal H. Trypsin activity and physiological aspects in larval rearing of European sea bass (Dicentrarchus labrax) using live prey and compound diets. J. App. Ichthyol. 1999;15:138–142. doi: 10.1046/j.1439-0426.1999.00138.x. [DOI] [Google Scholar]
- Oozeki Y., Bailey K.M. Ontogenic development of digestive enzyme activities in larval Walleye pollock, Theragra chalcogramma. Mar. Biol. 1995;122:177–186. [Google Scholar]
- Outzen H., Berglund G.I., Smalås A.O., Willassen N.P. Temperature and pH sensitivity of trypsins from Atlantic salmon (Salmo salar) in comparison with bovine and porcine trypsin. Comp. Biochem. Physiol. 1996;115:33–45. doi: 10.1016/0305-0491(96)00081-8. [DOI] [PubMed] [Google Scholar]
- Pavlisko A., Rial A., Coppes Z. Characterization of trypsin purified from the pyloric caeca of the southwest Atlantic white croaker Micropogonias furnieri (Sciaenidae) J. Food Biochem. 1997;21:383–400. doi: 10.1111/j.1745-4514.1997.tb00205.x. [DOI] [Google Scholar]
- Peres A., Cahu C.L., Infante J.L.Z., Le Gall M.M., Quazuguel P. Amylase and trypsin responses to intake of dietary carbohydrate and protein depend on the developmental stage in sea bass (Dicentrarchus labrax) larvae. Fish Physiol. Biochem. 1996;15:237–242. doi: 10.1007/BF01875574. [DOI] [PubMed] [Google Scholar]
- Plisetskaya E.M., Buchelli-Narvaez L.I., Hardy R.W., Dickhoff W.W. Effects of injected and dietary arginine on plasma insulin levels and growth of pacific salmon and rainbow trout. Comp. Biochem. Physiol. 1991;98A:165–170. doi: 10.1016/0300-9629(91)90595-4. [DOI] [Google Scholar]
- Rowen L., Koop B.F., Hood L. The complete 685-kilobase DNA sequence of the human β T cell receptor locus. Science. 1996;272:1755–1762. doi: 10.1126/science.272.5269.1755. [DOI] [PubMed] [Google Scholar]
- Rungruangsak K., Utne F. Effect of different acidified wet feeds on protease activities in the digestive tract and on growth rate of rainbow trout (Salmo gairdneri Richardson) Aquaculture. 1981;22:67–79. doi: 10.1016/0044-8486(81)90134-4. [DOI] [Google Scholar]
- Rungruangsak-Torrissen K., Carter C.G., Sundby A., Berg A., Houlihan D.F. Maintenance ration, protein synthesis capacity, plasma insulin and growth of Atlantic salmon (Salmo salar L.) with genetically different trypsin isozymes. Fish Physiol. Biochem. 1999a;21:223–233. doi: 10.1023/A:1007804823932. [DOI] [Google Scholar]
- Rungruangsak-Torrissen, K. and Fosseidengen, J.E. 2002. Domestication effect on digestive ability, muscle growth and oocyte quality of maturing Atlantic mackerel (Scomber scombrus L.). Aquaculture Europe 2002 Conference on seafarming: Today and Tomorrow, 16–9 Oct., Trieste, Italy, pp. 447–49 (Abstract).
- Rungruangsak Torrissen K., Male R. Trypsin isozymes: development, digestion and structure. In: Haard N.F., Simpson B.K., editors. Seafood Enzymes, Utilization and Influence on Postharvest Seafood Quality. New York: Marcel Dekker, Inc.; 2000. pp. 215–269. [Google Scholar]
- Rungruangsak-Torrissen K., Pringle G.M., Moss R., Houlihan D.F. Effects of varying rearing temperatures on expression of different trypsin isozymes, feed conversion efficiency and growth in Atlantic salmon (Salmo salar L.) Fish Physiol. Biochem. 1998;19:247–255. doi: 10.1023/A:1007731717021. [DOI] [Google Scholar]
- Rungruangsak-Torrissen K., Rustad A., Sunde J., Eiane S.A., Jensen H.B., Opstvedt J., Nygård E., Samuelsen T.A., Mundheim H., Luzzana U., Venturini G. In vitro digestibility based on fish crude enzyme extract for prediction of feed quality in growth trials. J. Sci. Food Agric. 2002;82:644–654. doi: 10.1002/jsfa.1089. [DOI] [Google Scholar]
- Rungruangsak-Torrissen K., Stensholt B.K. Spatial distribution of Atlantic salmon post-smolts: association between genetic differences in trypsin isozymes and environmental variables. In: Kruse G.H., Bez N., Booth A., Dorn M.W., Hills S., Lipcius R.N., Pelletier D., Roy C., Smith S.J., Witherell D., editors. Spatial Processes and Management of Marine Populations. Fairbanks: University of Alaska Sea Grant; 2001. pp. 415–429. [Google Scholar]
- Rungruangsak-Torrissen K., Sundby A. Protease activities, plasma free amino acids and insulin at different ages of Atlantic salmon (Salmo salar L.) with genetically different trypsin isozymes. Fish Physiol. Biochem. 2000;22:337–347. doi: 10.1023/A:1007864413112. [DOI] [Google Scholar]
- Rungruangsak-Torrissen K., Wergeland H.I., Glette J., Waagbø R. Disease resistance and immune parameters in Atlantic salmon (Salmo salar L.) with genetically different trypsin isozymes. Fish Shellfish Immunol. 1999b;9:557–568. doi: 10.1006/fsim.1999.0214. [DOI] [Google Scholar]
- Sabapathy U., Teo L.H. Some properties of the intestinal proteases of the rabbitfish, Siganus canaliculatus (Park) Fish Physiol. Biochem. 1995;14:215–221. doi: 10.1007/BF00004312. [DOI] [PubMed] [Google Scholar]
- Schroder H.K., Willassen N.P., Smalås A.O. Structure of a non-psychrophilic trypsin from a cold-adapted fish species. Acta Crystallogr. D- Biol. crystallogr. 1998;54:780–798. doi: 10.1107/S0907444997018611. [DOI] [PubMed] [Google Scholar]
- Skrede A., Berge G.M., Storebakken T., Herstad O., Aarstad K.G., Sundstol F. Digestibility of bacterial protein grown on natural gas in mink, pigs, chicken and Atlantic salmon. Anim. Feed Sci. Technol. 1998;76:103–116. doi: 10.1016/S0377-8401(98)00208-9. [DOI] [Google Scholar]
- Spilliaert R., Gudmundsdottir A. Atlantic cod trypsin Y -- Member of a novel trypsin group. Mar. Biotechnol. 1999;1:598–607. doi: 10.1007/PL00011815. [DOI] [PubMed] [Google Scholar]
- Stryer L. Biochemistry. 3. New York: WH Freeman; 1988. [Google Scholar]
- Sundby A., Eliassen K., Refstie T., Plisetskaya E.M. Plasma levels of insulin, glucagon and glucagon-like peptide in salmonids of different weights. Fish Physiol. Biochem. 1991;9:223–230. doi: 10.1007/BF02265143. [DOI] [PubMed] [Google Scholar]
- Sunde J., Eiane S.A., Rustad A., Jensen H.B., Opstvedt J., Nygård E., Venturini G., Rungruangsak-Torrissen K. Effect of fish feed processing conditions on digestive protease activities, free amino acid pools, feed conversion efficiency and growth in Atlantic salmon (Salmo salar L.) Aquacult. Nutr. 2004;10:261–277. doi: 10.1111/j.1365-2095.2004.00300.x. [DOI] [Google Scholar]
- Sunde J., Taranger G.L., Rungruangsak-Torrissen K. Digestive protease activities and free amino acids in white muscle as indicators for feed conversion efficiency and growth rate in Atlantic salmon (Salmo salar L.) Fish Physiol. Biochem. 2001;25:335–345. doi: 10.1023/A:1023233024001. [DOI] [Google Scholar]
- Torrissen K.R. Characterization of proteases in the digestive tract of Atlantic salmon (Salmo salar) in comparison with rainbow trout (Salmo gairdneri) Comp. Biochem. Physiol. 1984;77B:669–674. [Google Scholar]
- Torrissen K.R. Genetic variation in growth rate of Atlantic salmon with different trypsin-like isozyme patterns. Aquaculture. 1991;93:299–312. doi: 10.1016/0044-8486(91)90222-S. [DOI] [Google Scholar]
- Torrissen K.R., Lied E., Espe M. Differences in digestion and absorption of dietary protein in Atlantic salmon (Salmo salar) with genetically different trypsin isozymes. J. Fish Biol. 1994;45:1087–1104. doi: 10.1111/j.1095-8649.1994.tb01075.x. [DOI] [Google Scholar]
- Torrissen, K.R., Lied, E. and Espe, M. 1995. Differences in utilization of dietary proteins with varying degrees of partial pre-hydrolysis in Atlantic salmon (Salmo salar L.) with genetically different trypsin isozymes. In: Proceedings of the 11th FAOBMB Symposium (IUBMB Symposium No. 239) on Biopolymers and Bioproducts: Structure, Function and Applications. pp. 432–42. Edited by J. Svasti, V. Rimphanitchayakit, A. Tassanakajorn, P. Pongsawasdi, B. Sonthayanon, K. Packdibamrung, S. Soontaros, T. Limpaseni, P. Wilairat, J. Boonjawat and S. Kamolsiripichaiporn. Samakkhisan (dokya) Public Company Limited, Bangkok.
- Torrissen K.R., Male R., Nævdal G. Trypsin isozymes in Atlantic salmon, Salmo salar L.: studies of heredity, egg quality and effect on growth of three different populations. Aquacult. Fish. Managem. 1993;24:407–415. [Google Scholar]
- Torrissen K.R., Shearer K.D. Protein digestion, growth and food conversion in Atlantic salmon and Arctic charr with different trypsin-like isozyme patterns. J. Fish Biol. 1992;41:409–415. doi: 10.1111/j.1095-8649.1992.tb02669.x. [DOI] [Google Scholar]
- Torrissen K.R., Torrissen O.J. Protease activity and carotenoid levels during the sexual maturation of Atlantic salmon (Salmo salar) Aquaculture. 1985;50:113–122. doi: 10.1016/0044-8486(85)90157-7. [DOI] [Google Scholar]
- Waagbø R. The impact of nutritional factors on the immune system in Atlantic salmon, Salmo salar L.: a review. Aquacult. Fish. Managem. 1994;25:175–197. [Google Scholar]
- Woo N.Y.S., Ng T.B., Leung T.C., Chow C.Y. Enhancement of growth of tilapia Oreochromis niloticus in iso-osmotic medium. J. Appl. Ichthyol. 1997;13:67–71. doi: 10.1111/j.1439-0426.1997.tb00103.x. [DOI] [Google Scholar]
- Zabielski R., Huerou-Luron I.L., Guilloteau P. Development of gastrointestinal and pancreatic functions in mammalians (mainly bovine and porcine species): influence of age and ingested food. Rep. Nutr. Dev. 1999;39:5–26. doi: 10.1051/rnd:19990101. [DOI] [PubMed] [Google Scholar]


