Abstract
Species of Penicillium section Citrina have a worldwide distribution and occur commonly in soils. The section is here delimited using a combination of phenotypic characters and sequences of the nuclear ribosomal RNA gene operon, including the internal transcribed spacer regions ITS1 and ITS2, the 5.8S nrDNA (ITS) and partial RPB2 sequences. Species assigned to section Citrina share the production of symmetrically biverticillate conidiophores, flask shaped phialides (7.0–9.0 μm long) and relatively small conidia (2.0–3.0 μm diam). Some species can produce greyish-brown coloured cleistothecia containing flanged ascospores. In the present study, more than 250 isolates presumably belonging to section Citrina were examined using a combined analysis of phenotypic and physiological characters, extrolite profiles and ITS, β-tubulin and/or calmodulin sequences. Section Citrina includes 39 species, and 17 of those are described here as new. The most important phenotypic characters for distinguishing species are growth rates and colony reverse colours on the agar media CYA, MEA and YES; shape, size and ornamentation of conidia and the production of sclerotia or cleistothecia. Temperature-growth profiles were made for all examined species and are a valuable character characters for species identification. Species centered around P. citrinum generally have a higher maximum growth temperature (33–36 °C) than species related to P. westlingii (27–33 °C). Extrolite patterns and partial calmodulin and β-tubulin sequences can be used for sequence based identification and resolved all species. In contrast, ITS sequences were less variable and only 55 % of the species could be unambiguously identified with this locus.
Taxonomic novelties:
Penicillium argentinense Houbraken, Frisvad & Samson, P. atrofulvum Houbraken, Frisvad & Samson, P. aurantiacobrunneum Houbraken, Frisvad & Samson, P. cairnsense Houbraken, Frisvad & Samson, P. christenseniae Houbraken, Frisvad & Samson, P. copticola Houbraken, Frisvad & Samson, P. cosmopolitanum Houbraken, Frisvad & Samson, P. neomiczynskii Cole, Houbraken, Frisvad & Samson, P. nothofagi Houbraken, Frisvad & Samson, P. pancosmium Houbraken, Frisvad & Samson, P. pasqualense Houbraken, Frisvad & Samson, P. quebecense Seifert, Houbraken, Frisvad & Samson, P. raphiae Houbraken, Frisvad & Samson, P. terrigenum Seifert, Houbraken, Frisvad & Samson, P. ubiquetum Houbraken, Frisvad & Samson, P. vancouverense Houbraken, Frisvad & Samson, P. wellingtonense Cole, Houbraken, Frisvad & Samson.
Keywords: citreoviridin, citrinin, soil fungi, taxonomy, phylogeny
INTRODUCTION
Raper & Thom (1949) introduced the “Penicillium citrinum series” for Penicillium species with restricted growth on Czapek's agar and producing terminal verticils of metulae in combination with relatively small conidia (2.5–3.2 μm). Penicillium citrinum, P. corylophilum and P. steckii were classified in this series. Ramírez (1982) followed Raper & Thom's concept, and added P. matritii. Pitt (1980) formalised series Citrina, and using similar criteria as Raper & Thom, he accepted seven species: P. citrinum, P. corylophilum, P. miczynskii, P. humuli, P. herquei, P. paxilli and P. inflatum. In his description of series Citrina, Pitt (1980) noted that it encompasses a rather diverse collection of species, which in some cases show relatively little affinity with each other. This observation was supported by the taxonomic and phylogenetic study of Houbraken et al. (2010). Seven species were recognised in series Citrina, and of all the species mentioned above, only P. citrinum and P. steckii were maintained. Peterson (2000) was among the first to study the phylogeny of Penicillium with sequence data. Using ITS sequences, he constructed a phylogeny of Penicillium and showed that P. citrinum is related to P. westlingii, P. sumatrense, P. paxilli, P. waksmanii, P. miczynskii, Eupenicillium anatolicum and E. shearii. Recently, a new sectional classification for Penicillium was proposed and section Citrina was introduced (Houbraken & Samson 2011). This classification was based on a combined analysis of sequence data of four loci and the species belonging to section Citrina are the same as those belonging to Peterson's group 1. Peterson et al. (2004) and Houbraken et al. (2010) studied certain species of this section in more detail, however, a modern overview of species and their synonyms is lacking.
Members of section Citrina are very abundant and have a worldwide distribution. It is even claimed that P. citrinum may well be one of the most commonly occurring eukaryotic life forms on earth (Pitt 1980). Species of this section are very common in soil, but are also isolated from indoor environments and foodstuffs (Pitt & Hocking 2009, Samson et al. 2010). The distribution of species appears to be climate-related. Penicillium citrinum is more common in (sub)tropical soils, and present only in low numbers in soils from temperate regions (the Netherlands, Poland, Canada), where P. westlingii and related species predominate.
Members of section Citrina are also known for their ability to produce the mycotoxins citrinin and citreoviridin. The nephrotoxic compound citrinin is consistently produced by P. citrinum, but also by other related species including P. gorlenkoanum, P. hetheringtonii, P. miczynskii, P. chrzaszczii, P. manginii and P. westlingii, and citreoviridin is produced by P. miczynskii and P. manginii (Pollock 1947, Frisvad 1989, Frisvad & Filtenborg 1990, Frisvad et al. 2006, Houbraken et al. 2010). Many other extrolites are reported to be produced by members of section Citrina; however, some of these extrolites are erroneously linked to certain species (Frisvad 1989, Frisvad & Filtenborg 1990, Houbraken et al. 2010).
In this study, we delimited Penicillium section Citrina using a combination of ITS (internal spacer region and 5.8S rDNA gene) and partial RPB2 gene sequences. After delimitation, the taxonomy of this section was studied in-depth using a polyphasic approach. Over 250 strains belonging to section Citrina, including type and freshly isolated strains, were included. Sequences of a part of the β-tubulin and calmodulin gene in combination with extrolite profiles, physiological and macro- and micromorphological characters were used for species delimitation.
MATERIAL AND METHODS
Strains
Data on the strains used in this study are listed in Table 1. More detailed information can be found in the on-line database of the CBS. These fungi are permanently preserved in the culture collection of the CBS-KNAW Fungal Biodiversity Centre, Utrecht, the Netherlands and placed in the working collection of the department of Applied and Industrial Mycology (DTO), housed at CBS.
Table 1.
Isolates in Penicillium section Citrina examined in this study.
| Species | CBS no. | Other numbers | Substrate and locality |
|---|---|---|---|
| P. anatolicum | DTO 23A1 = IBT 30775 | Contaminant of CBS 316.67 | |
| CBS 308.89 | CBS H-20648 = DTO 23E6 = IBT 30768 | Soil, Keewadin Island, Florida, USA | |
| CBS 467.67 | CBS H-20647 = DTO 23A2 = CSIR 1095 = IBT 30763 | Sandy soil, Kosi Bay, Natal, South Africa | |
| CBS 478.66T | DTO 22I5 = DTO 22I6 = ATCC 18621 = CSIR 940 = IFO 31729 = IMI 136242 = IBT 30765 | Soil, Turkey | |
| CBS 479.66 | DTO 22I6 = IBT 16177 = IBT 30764 | Soil, Turkey | |
| P. argentinense | CBS 130371T | CBS H-20641 = DTO 16B7 = IBT 30761 | Soil, Valdes Peninsula peninsula, prov. Chubet, Argentinia |
| CBS 130373 | DTO 18B1 = IBT 30760 | Soil, Spaanderswoud, Bussum, the Netherlands | |
| CBS 130374 | DTO 18B6 = IBT 30761 | Soil, Spaanderswoud, Bussum, the Netherlands | |
| CBS 130381 | DTO 132D5 | Phaenocoma leaf bracts, South Africa | |
| P. atrofulvum | CBS 109.66T | CBS H-20650 = DTO 31B2 = FRR 799 = 30032 = IBT 29667 IBT | Soil, Katanga, Zaire |
| CBS 126331 | DTO 120G7 | Soil of oak forest; Ras Rajel, Tunesia | |
| CBS 126332 | DTO 118D4 | Soil of oak forest; Fey el Rih, Tunesia | |
| CBS 261.64 | DTO 22H4 = IBT 16171 | Unrecorded source, the Netherlands | |
| P. aurantiacobrunneum | CBS 126228T | CBS H-20662 = DTO 78G2 = IBT 18753 | Air sample, Cake factory, Give, Denmark |
| CBS 126229 | DTO 82C3 = IBT 23001 | Soil, Nothofagus sp., Chile | |
| CBS 126230 | DTO 82C9 = IBT 29145 | Wood litter, Eves Bush, Marlborough, New Zealand | |
| CBS 126277 | DTO 76D1 = IBT 29115 | Soil, New Zealand | |
| P. cairnsense | CBS 117962 | DTO 55A5 = KAS 2100 = IBT 29675 | Decaying basidioma of Lactarius sp.; Algonquin Park, Ontario, Canada, 45.593086° -78.519914° |
| CBS 117982 | DTO 5A7 = KAS 2122 = IBT 29857 | Nut of Carya cordiformis (bitternut); Fireman's Park, Niagara Falls, Ontario, Canada, 43.142051° -79.115903° | |
| CBS 118028 | CBS H-20653 = DTO 55B2 = KAS 2178 | Ants (Camponotus spp.), New Brunswick, Canada | |
| CBS 124324 | DTO 30B9 = IBT 29068 | Soil, near lake Barrine, Australia | |
| CBS 124325T | DTO 30E6 = IBT 29042 | Soil, Atherton Tableland, Australia | |
| CBS 124326 | DTO 30E8 = IBT 29069 | Soil, Atherton Tableland, Australia | |
| CBS 126225 | DTO 82B6 = IBT 18352 = CCRC 33163 | Soil, Sun-Moon Lake, Nantou County, Taiwan | |
| CBS 126226 | DTO 85A4 = IBT 30006 | Soil, 2 mtr. from road, Ranomafana, Madagascar | |
| P. christenseniae | CBS 126236T | CBS H-20656 = DTO 76C3 = IBT 23355 | Soil in native forest near base of aerial tram. “Lowland forest” east / north east side of Costa Rica about 30 km inland from Limon and the Caribbean |
| CBS 126237 | DTO 78A5 = RMF 9554 = IBT 18183 | Litter of Manilkara bidenta or Guarea guidonia, rainforest, El verde in the Luquillo Experimental Forest, Caribbean National Forest, Puerto Rico | |
| P. chrzaszczii | CBS 124320 | DTO 42A8 = IBT 30635 | Soil, Poland |
| CBS 126430 | DTO 42G9 = IBT 30634 | Soil, Poland | |
| CBS 176.81 | DTO 23D7 = ATCC 42242 = IJFM 7097 = F-2198 = IBT 16265 VKM | Type of P. turolense; leaves litter of Fagus silvatica, near Nancy, France | |
| CBS 217.28T | 22E4 = FRR 903 = MUCL 29167 = NRRL 903 = NRRL 1741 = IBT 18226 = IBT 11222 = IBT 16409 | Woodland soil, Puszcza Bialowieska Forest, Poland | |
| P. citrinum | CBS 101275 | DTO 23G2 = IBT 29060 | Leaf, Panama |
| CBS 115992 | DTO 23G6 | Compost, the Netherlands | |
| CBS 117.64 | DTO 22H3 = IBT 30003 | Epoxy softener, the Netherlands | |
| CBS 122394 | DTO 7B8 | Soil, Malaysia | |
| CBS 122395 | DTO 20A3 | Coconut milk; produced in Indonesia, imported into the Netherlands | |
| CBS 122397 | DTO 6D6 | Soil, Treasure Island, Florida, USA | |
| CBS 122398 | DTO 31F9 | Peanut, Indonesia | |
| CBS 122451 | DTO 48C2 = NRRL 2145 = IBT 16140 | Color mutant; unrecorded source | |
| CBS 122452 | DTO 32B6 = IBT 30061 | Color mutant, coffee beans, Thailand | |
| CBS 122726 | DTO 58A4 = NRRL 783 = IBT 16149 | Representative of P. sartoryi, unrecorded source | |
| P. citrinum | CBS 139.45T | DTO 22F3 = ATCC 1109 = ATCC 36382 = CECT 2269 = FRR 1841 = IMI 091961 = IMI 092196 = MUCL 29781 = NRRL 1841 = IBT 16200 = NRRL 1842 = IBT 16207 | Type of P. citrinum and P. aurifluum, unrecorded source |
| CBS 232.38 | DTO 37B7 = Thom 4733.73 = IBT 21675 | Type of P. implicatum; unrecorded source | |
| CBS 241.85 | IMI 092267 = MUCL 29788 = IBT 21934 | Type of P. phaeojanthinellum; unrecorded source | |
| CBS 252.55 | DTO 22G4 = ATCC 12068 = FRR 3463 = NRRL 3463 = QM 6946 = IBT 19474 | Isotype of P. botryosum; herbarium specimen, Recife, Brazil | |
| CBS 865.97 | DTO 23F8 | Patient with acute myeloid leukemia, autopsy of lung and pericardium | |
| P. copticola | CBS 127355T | CBS H-20643 = DTO 19H7 = IBT 30771 | Tortilla, USA |
| CBS 127356 | DTO 104E8 = IBT 30772 | Dried flower of Cannabis, the Netherlands | |
| CBS 130382 | DTO 162G5 | Air of a toilet in a kindergarten, Trier, Germany | |
| P. cosmopolitanum | DTO 82C8 = IBT 29104 | Forest soil, Hokitika, New Zealand | |
| DTO 42G4 = IBT 29692 | Soil, Poland | ||
| CBS 122406 | DTO 17E3 | Soil under oak, Spaanderswoud, Bussum, the Netherlands | |
| CBS 122435 | DTO 38D6 = IBT 29040 | Organic soil of mixed forest, Rijnsweerd, Utrecht | |
| CBS 124315 | DTO 42F6 = IBT 30684 | Soil, Poland | |
| CBS 124316 | DTO 42D3 = IBT 29677 | Soil, Poland | |
| CBS 126990 | DTO 42F4 = IBT 30691 | Soil, Poland | |
| CBS 126991 | DTO 42G6 = IBT 30693 | Soil, Poland | |
| CBS 126992 | DTO 41B1 = IBT 30719 | Soil, Poland | |
| CBS 126993 | DTO 40E9 = IBT 30690 | Soil, Poland | |
| CBS 126994 | DTO 40I4 = IBT 30697 | Soil, Poland | |
| CBS 126995T | CBS H-20665 = DTO 92E8 = IBT 30681 | Soil heathland, Cartier heide, Eersel, the Netherlands | |
| CBS 126996 | DTO 42G1 = IBT 30683 | Soil, Poland | |
| CBS 126997 | DTO 42A1 = IBT 29690 | Soil, Poland | |
| CBS 126998 | DTO 41A4 = IBT 30757 | Soil, Poland | |
| CBS 126999 | DTO 39D5 = IBT 30687 | Soil, Poland | |
| CBS 127000 | DTO 92G6 = IBT 30678 | Soil heathland, Cartier heide, Eersel, the Netherlands | |
| CBS 127001 | DTO 92E9 = IBT 30682 | Soil heathland, Cartier heide, Eersel, the Netherlands | |
| CBS 127002 | DTO 42E1 = IBT 30680 | Soil, Poland | |
| CBS 127038 | DTO 76B6 = IBT 21692 | Soil, near Lyngby Lake, Denmark | |
| CBS 200.86 | DTO 23E4 = IBT 16144 = IBT 29697 | Root of Pseudotsuga menziesii, the Netherlands | |
| CBS 251.70 | DTO 23B1 = IBT 29071 | Root of gymnosperm, Denmark | |
| CBS 552.86 | DTO 23E5 = IBT 29681 = IBT 30689 | Root of Pseudotsuga menziesii, the Netherlands | |
| CBS 586.70 | DTO 23B5 = IBT 30686 | Root of gymnosperm, Denmark | |
| CBS 637.70 | DTO 23B6 | Root of gymnosperm, Denmark | |
| P. decaturense | CBS 117504 | DTO 3A9 = IBT 27057 = NRRL 29675 | Trichaptum biformis, on dead hardwood branch, Chehaw Park, Albany, Georgia, USA |
| CBS 117505 | DTO 3B1 = IBT 27058 = NRRL 29708 | Basidiomycete on dead hardwood, Reed Bingham State park (hardwood swamp area), Adel, Georgia, USA | |
| CBS 117506 | DTO 3B2 = IBT 27059 = NRRL 29828 | Trichaptum biformis, on dead hardwood branch, Wakulla Springs State Park, Crawfordsville, Florida, USA | |
| CBS 117507 | DTO 3F5 = IBT 27111 = NRRL 28160 | Ischnoderma, old basidiomata, found on dead hardwood log, North Picture Ridge Road, Peoria, Illinois, USA | |
| CBS 117508 | DTO 3F6 = IBT 27114 = NRRL 29840 | Polypore found on a dead pine branch, Blountstown, Torreya State Park, Illinois, USA | |
| CBS 117509T | DTO 3F7 = IBT 27117 = NRRL 28152 | Old resupinate fungus, Ramsey Lake State Park, Decatur, Illinois, USA | |
| CBS 117510 | DTO 3F8 = IBT 27120 = NRRL 28119 | Wood decaying fungus | |
| CBS 119390 | DTO 9F2 = IBT 27868 = NRRL 29807 | Pyrenomycete stroma on dead hardwood; sabal palm swamp, Hickory Mounds, Florida, USA | |
| P. euglaucum | CBS 130372 | DTO 16G1 = IBT 30776 | Soil, Azul, prov. Buenos Aires, Argentina |
| CBS 323.71NT | DTO 23B9 = IBT 30767 | Soil, Argentina | |
| P. gallaicum | CBS 164.81 | DTO 34G2 = IJFM 7026 = IMI 253797 = VKM F-2193 = IBT 22014 | Type of P. alicantinum; air, Madrid, Spain |
| CBS 167.81T | DT 34G3 = IJFM 5597 = DTO 34G3 = ATCC 42232 = IMI 253794 = VKM F-2190 = IBT 22016 | Air, Madrid, Spain | |
| CBS 418.69 | DTO 23A9 = NRRL 3759 = IBT 30046 = IMI 140303 = FRR 519 | Type of P. syriacum nomen dubium; soil, Berza, Damascus, Syria | |
| P. godlewskii | CBS 117273 | DTO 2H8 = IBT 29661 | Butter, the Netherlands |
| CBS 124319 | DTO 39C7 = IBT 29678 | Soil, Bialowieska, Poland | |
| CBS 126419 | DTO 40E3 = IBT 30692 | Soil, Bialowieska, Poland | |
| CBS 126420 | DTO 39C4 = IBT 30637 | Soil, Bialowieska, Poland | |
| CBS 126421 | DTO 42G2 = IBT 30636 | Soil, Bialowieska, Poland | |
| CBS 126422 | DTO 76B5 = IBT 21219 | Sand under pine, summit of Eagle Rock, Medicine Bow National Forest near Laramie, Wyoming, USA | |
| CBS 126423 | DTO 42E7 = IBT 30638 | Soil, Bialowieska, Poland | |
| CBS 126424 | DTO 58C6 = IBT 30640 | Unknown substrate, Germany | |
| CBS 215.28T | DTO 22E2 = ATCC 10449 = ATCC 48714 = FRR 2111 = I FO 7724 = IMI 040591 = MUCL 29243 = NRRL 2111 = QM 7566 = VKM F-1826 | Soil under pine, Bialowieska, Poland | |
| CBS 218.28 | ATCC 10457 = FRR 2147 = IFO 30869 = IFO 7674 = IMI 040567 = MUCL 29245 = NRRL 2147 = QM 7588 = IBT 4998 = IBT 5045 | Type strain of P. kapuscinskii, ex sandy soil, Baltic, Poland | |
| P. gorlenkoanum | CBS 408.69IsoT | DTO 34E3 = FRR 511 = IMI 140339 = VKM F-1079 = IBT 19235 | Soil, Syria |
| CBS 411.69 | DTO 23A6 = IMI 140337 = VKM F-1070 = IBT 16117 | Type strain of P. damascenum; soil, Ima, Damascus region, Syria | |
| P. hetheringtonii | DTO 30H7 | Soil, Lookout Kuranda, Australia | |
| CBS 122392T | DTO 5H9 = IBT 29057 | Soil, Treasure Island, Florida, USA | |
| CBS 124286 | DTO 30H5 = IBT 29061 | Soil, Lookout Kuranda, Australia | |
| CBS 124287 | DTO 32E3 | Soil, Lake Easchem, Australia | |
| P. manginii | CBS 108.66 | DTO 22I3 = IBT 16132 = IBT 30406 | Soil, Latosol, near Kipushi, Katanga, Congo |
| CBS 122403 | DTO 21B2 | Indoor air of house, Eindhoven | |
| CBS 126232 | DTO 87E5 | Soil of rainforest, Ranoma fana, Madagascar | |
| CBS 126233 | CBS H-20654 = DTO 76B7 = IBT 22405 | Soil under Cyathea tree ferns, on Rio Jaba Trail near Quebrada Culebra, Wilson Botanical Garden/ La Cruces Biological Station, Costa Rica | |
| CBS 253.31NT | DTO 22E9 = NRRL 2134 = IMI 191732 = FRR 2134 = IBT 18224 | Soil, unknown locality | |
| CBS 265.65 | DTO 22H6 = ATCC 18334 = IMI 143926 = NRRL 3379 = IBT 18186 | Type of P. pedemontanum, mycorrhizae of Fagus silvatica, Italy | |
| CBS 327.79 | DTO 23D5 = IJFM 3782 = IBT 29651 | Air, Madrid, Spain | |
| CBS 343.52 | DTO 22G2 = BRL 111A = IBT 16157 | Soil, Norway | |
| CBS 378.65 | DTO 22H8 = NRRL 3555 = IBT 18223 = IBT 30412 = IBT 29064 | Soil, near Baya, Katanga, Congo | |
| CBS 407.65 | DTO 22H9 = IMI 096225 | Hay, Haslemere, Surrey, UK | |
| CBS 408.65 | DTO 22I1 = FRR 1836 = IMI 099085 = IBT 3998 | Soil, Cambridge, England, UK | |
| CBS 409.65 | DTO 22I2 = IMI 096290 | Rhizosphere of Triticum aestivum, Rothamsted, UK | |
| P. miczynskii | CBS 124323 | DTO 42F2 = IBT 30584 | Soil, Bialowieza National Park, Poland |
| CBS 126222 | DTO 16A2 = IBT 29054 | Soil, Los Alerces National Park, Chubut, Argentina | |
| CBS 126223 | DTO 76B2 = IBT 18227 = RMF 7771 | A1 horizon soil in conifer forest (lodgepole pine), Cinnabar Park, Wyoming, USA | |
| CBS 126224 | DTO 82C7 = IBT 26903 | Soil, Spread Creek, Wyoming, USA | |
| CBS 220.28T | DTO 22E5 = ATCC 10470 = DSM 2437 = FRR 1077 = IFO 7730 = IMI 040030 = MUCL 29228 = NRRL 1077 = IBT 5491 | Soil under conifer, Tatry mountains, Poland | |
| P. neomiczynskii | CBS 126231T | CBS H-20661 = DTO 78C2 = IBT 23560 | Soil, New Zealand |
| P. nothofagi | CBS 127004 | DTO 80D2 = IBT 17235 | Soil, Brazil |
| CBS 130383 | CBS H-20655 = DTO 76C2 = IBT 23018 | Soil under Nothofagus, Chile | |
| P. pancosmium | DTO 82D1 = IBT 29160 | Unknown source, New Zealand | |
| CBS 118007 | DTO 55A9 = KAS 2150 = IBT 29670 | Porcupine dung, Dufferin, Dufferin County Forest, 1 km N. of Mansfield, Ontario, Canada | |
| CBS 118018 | DTO 55B1 = KAS 2163 = IBT 29871 | Nut of Juglans cinerea (butternut); Fireman's Park, Niagara Falls, Ontario, Canada, 43.142051° -79.115903° | |
| CBS 124293 | DTO 84H4 = IBT 22166 | Growth on Piptosphaeria (on Betula sp), Lambs Lane, New Jersey, USA | |
| CBS 126431 | DTO 118I8 = IBT 30707 | Soil of oak forest; Fey el Rih, Tunesia | |
| CBS 126432 | DTO 100A1 | Soil, Portugal | |
| CBS 126433 | DTO 82C2 = IBT 22969 | Soil under Nothofagus, Chile | |
| CBS 126434 | DTO 120A1 = IBT 30648 | Soil; Ras Rajel, Tunesia | |
| CBS 126435 | DTO 119A4 = IBT 30643 | Soil of oak forest, Fey el Rih, Tunesia | |
| CBS 276.75T | CBS H-20651 = DTO 31B4 = DAOM 147467 = IBT 29991 | Old Armillaria mellea, on hardwood log; Meach Lake, Gatineau Park, Gatineau County, Quebec, Canada | |
| P. pasqualense | CBS 122402 | DTO 28C2 = IBT 29047 | Air in bakery, Averhorn, the Netherlands |
| CBS 124327 | DTO 57D3 | Soil, Katandra Nature Reserve, NSW, Australia | |
| CBS 126329 | DTO 78B3 = IBT 17865 | Soil and debris under Juniperus sp., Wind River canyon, 10 km south of Thermopolis, Wyoming, USA | |
| CBS 126330T | CBS H-20663 = DTO 80D5 = IBT 14235 | Soil, Easter Island, Chile | |
| P. paxilli | CBS 101273 | DTO 23F9 = IBT 30832 | Leaf, Panama |
| CBS 117190 | DTO 31A8 = IBT 16459 | Soil, Galapagos Islands, Ecuador | |
| CBS 117191 | DTO 31A9 = IBT 20977 = IBT 21034 = IBT 21005 | Mangrove, Venezuela | |
| CBS 118002 | KAS 2144 | Coustania superba, Panama | |
| CBS 118052 | KAS 2206 = IBT 29839 | Nut of Carya cordiformis (bitternut); Fireman's Park, Niagara Falls, Ontario, Canada, 43.142051° -79.115903° | |
| CBS 127360 | DTO 52F9 = IBT 30839 | Melon imported in the Netherlands, Brazil | |
| CBS 127361 | DTO 30A6 = IBT 29070 | Soil, near lake Cratez, Barrine, Queensland, Australia | |
| CBS 162.96 | DTO 23F3 = IBT 30847 | Wood in tropical rainforest, Madang Province, Finisterre Range, Papua-New Guinea | |
| CBS 360.48T | DTO 31A6 = ATCC 10480 = FRR 2008 = IMI 040226 = NRRL 2008 = QM 725 = IBT 16202 | Optical instrument, Barro Colorado Island, Panama | |
| CBS 547.77 | DTO 31A7 = ATCC 26601 = FRR 1900 = IBT 3128 = IBT 3329 = IBT 5531 | Carya illinoensis, Juglandaceae, Georgia, USA | |
| P. quebecense | CBS 101623T | CBS H-20666 = DTO 9B8 = IBT 29050 | Air in sawmill, Quebec, Canada |
| P. raphiae | CBS 126234T | CBS H-20660 = DTO 78B8 = IBT 22407 | Soil under Raphia (?) palm in primary forest, Las Alturas, elev. 1530 m, Costa Rica |
| CBS 126235 | CBS H-20664 = DTO 84I9 = IBT 30001 | Soil under baobab tree; Montagne d'Ambre National Park, Madagascar | |
| P. roseopurpureum | CBS 127025 | DTO 28F5 = IBT 30782 | Indoor air of house, Eindhoven, the Netherlands |
| CBS 127026 | DTO 28F6 = IBT 30781 | Indoor air of house, Eindhoven, the Netherlands | |
| CBS 127027 | DTO 76C9 = IBT 27944 | Soil under Pinus flexilis, Bear Mountain, Wyoming, USA | |
| CBS 127028 | DTO 76D3 = IBT 27930 | Soil under Artemisia cana, Bear Mountain, Wyoming, USA | |
| CBS 266.29NT | DTO 9E3 = ATCC 10492 = ATHUM 2895 = FRR 2064 = IMI 040573 = MUCL 28654 = MUCL 29237 = NRRL 2064 = NRRL 2064A | Unrecorded source | |
| CBS 281.39 | DTO 9E7 = FRR 2066 = MUCL 28670 = MUCL 29240 = NRRL 2066 = IBT 30783 | Type of P. carminoviolaceum; plant material in ethanol, unknown location | |
| P. sanguifluum | CBS 110.64 | DTO 9E6 = IBT 29045 | Soil, Erzurum, Turkey |
| CBS 118020 | DTO 128C8 = KAS 2165 | Ants (Camponotus spp.), New Brunswick, Canada | |
| CBS 118024 | DTO 128C9 = KAS 2171 | Ants (Camponotus spp.), New Brunswick, Canada | |
| CBS 127029 | DTO 15H6 = IBT 30793 | Soil, Parque Nacional Los Alerces, Argentina | |
| CBS 127030 | DTO 6D7 = IBT 30759 | Chestnut, Corsica, France | |
| CBS 127031 | CBS H-20642 = DTO 17G5 = IBT 29051 | Soil, Calahonda, Costa del Sol, Spain | |
| CBS 127032NT | CBS H-20645 = DTO 20B7 = IBT 29041 | Soil, Calahonda, Costa del Sol, Spain | |
| CBS 127033 | DTO 99I9 = IBT 30786 | Unknown, Catia Rodriguez | |
| CBS 127034 | DTO 119I1 = IBT 30785 | Soil, Ras Rajel, Tunesia | |
| P. sanguifluum | CBS 127035 | DTO 120G9 = IBT 30784 | Soil, Ras Rajel, Tunesia |
| CBS 127036 | DTO 121D8 | Soil, Ras Rajel, Tunesia | |
| CBS 148.83 | DTO 9E2 = CECT 2753 | Type of P. vaccaeorum; sandy soil under pine tree, Valladolid, Spain | |
| CBS 300.67 | DTO 9E5 = IBT 30787 | Sandy greenhouse soil, the Netherlands | |
| CBS 643.73 | DTO 9E4 = IBT 30789 | Soil, sandy beach ridge, Manitoba, Canada | |
| CBS 685.85 | DTO 36B9 = IJFM 19078 = IBT 4904 = IBT 10578 = IBT 10579 | Type of P. lacussarmientei, sandy soil, National Park of Torres del Paine, near Lake Sarmiento, Tierra del Fuego, Chile | |
| P. shearii | DTO 78C5 = IBT 28734 | Unknown source, Brazil | |
| CBS 118059 | DTO 23H7 = KAS 2214 = IBT 30164 | Soil eaten by chimpanzees, Mahale Mountains National Park, Tanzania | |
| CBS 127358 | DTO 54B8 = IBT 30837 | Soil, Langkawi, Malaysia | |
| CBS 127359 | DTO 99H1 = IBT 30821 | Soil, Portugal | |
| CBS 290.48T | DTO 22F6 = IMI 39739 = ATCC 10410 = NRRL 715 = IFO 6088 = IBT 24588 | Soil, Tela, Honduras | |
| CBS 342.68 | DTO 23A3 = IBT 14785 = IBT 14786 | Soil, Congo | |
| CBS 343.54 | DTO 22G3 = NRRL 3325 = IBT 14695 | Soil, Congo | |
| CBS 502.78 | DTO 23D4 = IBT 24589 | Cassava field soil, Colombia | |
| CBS 513.73 | NHL 6444 = IBT 14698 | Soil, Cape Hoskins, Waississi, New Britain Island, Papua-New Guinea | |
| CBS 578.70 | DTO 23B4 = IBT 30815 | Soil, San Blas, Nayarit State, Mexico | |
| P. sizovae | CBS 115968 | DTO 23G5 | Cropped soil, Italy |
| CBS 117183 | DTO 23H2 | Papaver somniferum, the Netherlands | |
| CBS 117184 | DTO 23H3 = IBT 22812 | Salty water in saltern, Slovenia | |
| CBS 122386 | DTO 5C5 | Glue, the Netherlands | |
| CBS 122387 | DTO 19H1 | Margarine, the Netherlands | |
| CBS 139.65 | DTO 22H5 | Sea salt, Portugal | |
| CBS 413.69NT | DTO 23A7 = FRR 518 = IMI 140344 = VKM F-1073 | Soil, Syria | |
| P. steckii | DTO 49G1 = IBT 14692 = NRRL 2142 | Exposed fabric, Panama | |
| CBS 122388 | DTO 49F9 = IBT 14691 = NRRL 6336 | Baled coastal grass hay, Bermuda | |
| CBS 122389 | DTO 49F8 = IBT 19353 = IFO 6024 | Unrecorded source | |
| CBS 122390 | DTO 48D3 = IBT 21096 | Caranx crysos (blue runner, fish), sand bottoms with corals, surface water 23°C, dept 2–3 m at Cabruta, Mochima Bay, Venezuela | |
| CBS 122391 | DTO 7D2 | Potting soil, the Netherlands | |
| CBS 122417 | DTO 48D2 = IBT 20952 | Ascidie (tunicate, urochordata), sand bottoms with corals, surface water 23 °C, dept 2–3 m at Cabruta, Mochima Bay, Venezuela | |
| CBS 122418 | DTO 48D1 = IBT 6452 | Cynara scolymus (Artichoke), Egypt | |
| CBS 260.55NT | DTO 22G5 = ATCC 10499 = CECT 2268 = DSM 1252 = IMI 040583 = NRRL 2140 = QM 6413 | Cotton fabric treated with copper naphthenate; Panama | |
| CBS 325.59 | DTO 22G7 = ATCC 20203 = ATCC 18307 = CECT 2273 = FRR 636 = IFO 6227 = IMI 068229 = QM 7291 | Type of P. corylophiloides; soil, Japan | |
| CBS 789.70 | DTO 23B7 = IBT 3145 | Unrecorded source | |
| P. sumatrense | CBS 115708 | DTO 23G4 = IBT 29691 | Soil, Presicce, Apulia, Italy |
| CBS 117185 | DTO 23H4 = IBT 24845 = IBT 29668 | Bromeliad leaf tissue, Orthophyton burle-marxii, Selby Botanical Garden, Sarasota, Florida, USA | |
| CBS 127362 | DTO 5I2 = IBT 29048 | Soil, Land's end Garden, Treasure Island, Florida, USA | |
| CBS 127363 | DTO 15E6 = IBT 30841 | Packaging material, imported into the Netherlands | |
| CBS 127364 | DTO 30H8 = IBT 29059 | Soil, Lookout Kuranda, Queensland, Australia | |
| CBS 127365 | DTO 99B6 = IBT 30840 | Soil, Portugal | |
| CBS 127366 | DTO 120H3 = IBT 30831 | Soil, Ras Rajel, Tunisia | |
| CBS 130377 | DTO 78A8 = IBT 27264 | Bromeliad leaf, Aechmia magdalenae, Panama | |
| CBS 130378 | DTO 78B2 = IBT 28809 | Forest fruit, Uganda | |
| CBS 130380 | DTO 80D6 = IBT 13201 | Utility Pole, USA (no. JP 923, as P. steckii) | |
| P. sumatrense | CBS 281.36T | DTO 22F1 = NRRL 779 = FRR 779 = ATCC 48669 = IBT 29658 = IBT 4978 | Soil, Toba Heath, Sumatra, Indonesia |
| CBS 335.59 | DTO 31B8 = ATCC 18378 = FAT 803 = FRR 639 = IFO 6232 = IMI 068232 = QM 7313 = IBT 14696 | Type of P. meleagrinum var. viridiflavum; soil, Japan | |
| CBS 416.69 | DTO 23A8 = FRR 508 = IMI 140336 = VKM F-1069 = IBT 29648 | Isotype of P. baradicum; soil under cornel, Damascus, Syria | |
| P. terrigenum | CBS 117967 | KAS 2104 = IBT 29807 | Mushroom fairy ring, Oshawa, Ontario, Canada |
| CBS 117993 | KAS 2133 = IBT 29908 | Leaf surface, Puerto Rico | |
| CBS 127354T | CBS H-20667 = DTO 9D4 = IBT 30769 | Soil, Hawaii, USA | |
| P. cf. terrigenum | CBS 127357 | CBS H-20644 = DTO 19H8 = IBT 30770 | Tortilla, USA |
| P. tropicoides | CBS 122410T | DTO 10C4 = IBT 29043 | Type; soil rainforest, near Hua-Hin, Thailand |
| CBS 122436 | DTO 10C8 | Soil rainforest, near Hua-Hin, Thailand | |
| P. tropicum | DTO 78C4 = IBT 27056 | Leaf, Florida, USA | |
| CBS 112584T | DTO 31B1 = IBT 24580 | Soil under Coffea arabica, Mertha Subbagudigy, Karnataka, India | |
| CBS 130379 | DTO 80D3 = IBT 16462 = DMG 1004 | Soil, Galapagos Islands, Ecuador | |
| P. ubiquetum | CBS 124317 | DTO 30A8 = IBT 30705 | Soil near lake Cratez, Barrine, Queensland, Australia |
| CBS 124318 | DTO 32D7 = IBT 30704 | Soil, Lake Easchem, Queensland, Australia | |
| CBS 124450 | DTO 84G8 = IBT 13179 = WSF 2210 | A1 horizon soil, maple-elm-ash forest, Wisconsin, USA | |
| CBS 126436 | DTO 30E2 = IBT 30397 | Soil, wet forest, Atherton Tableland, Queensland, Australia | |
| CBS 126437T | CBS H-20659 = DTO 78B5 = IBT 22226 | Soil, Wilson Botanical Garden, Costa Rica | |
| CBS 126438 | DTO 87B4 = IBT 30011 | Soil under tree; Montagne d'Ambre, Madagascar | |
| CBS 126439 | DTO 85B6 = IBT 30644 | Soil, Ranoma fana, Madagascar | |
| P. vancouverense | CBS 117962 | DTO 55A4 = KAS 2098 = IBT 29801 | Nut of Juglans cinerea (butternut); Fireman's Park, Niagara Falls, Ontario, Canada, 43.142051° -79.115903° |
| CBS 122400 | DTO 38F5 | Organic soil, mixed forest Rijnsweerd, Utrecht, the Netherlands | |
| CBS 122401 | DTO 21B1 = IBT 29063 | Indoor air of house, Eindhoven | |
| CBS 124328 | DTO 30D3 = IBT 29736 | Soil, wet forest, Atherton Tableland, QLD, Australia | |
| CBS 124329 | DTO 38D2 = IBT 30044 | Organic soil, mixed forest Rijnsweerd, Utrecht, the Netherlands; dilution plate | |
| CBS 126321 | DTO 78B6 = IBT 22265 | Soil, Pacific slope of Volcan Barva at ca. 2000 m, just above Porrosati, in Heredia Province, under Ticodendron in wet montane forest, Costa Rica, November 2000 | |
| CBS 126322 | DTO 76B4 = IBT 20820 | Soil under Maple tree, Vancouver, BC, Canada | |
| CBS 126323T | CBS H-20646 = DTO 82B8 = IBT 20700 | Soil under Maple tree, Vancouver, BC, Canada | |
| CBS 126324 | DTO 76B9 = IBT 22472 | Type; soil under Nothofagus glauca, Costa Azul School Forest of Universidad Catolica del Maule (35 37c / 72 c45w), Chile | |
| CBS 126325 | DTO 30D1 = IBT 29058 | Soil, wet forest, Atherton Tableland, QLD, Australia | |
| CBS 126326 | DTO 76D2 = IBT 29309 | Soil under Cypress, Pebble beach, Asilomar, California, USA | |
| CBS 126327 | DTO 82C4 = IBT 20692 | Soil under Maple tree, Vancouver, BC, Canada | |
| CBS 126328 | DTO 85B2 = IBT 30004 | Soil rainforest, Ranoma Fana, Madagascar | |
| CBS 130376 | DTO 78A4 = IBT 16486 | Soil under fern on slope on the way to the beach, “path 3”, University of Vancouver, Vancouver, BC, Canada | |
| P. waksmanii | DTO 78C1 = IBT 23508 | Soil, New Zealand | |
| CBS 117502 | DTO 3A8 = IBT 27053 = ATCC 48699 = FRR 906 = NRRL 906 | Type of P. rivolii; forest soil, Poland | |
| CBS 117525 | DTO 3A7 = IBT 27052 = NRRL 28095 | Dead polypore, New Mexico, USA | |
| CBS 124295 | DTO 84H6 = IBT 24654 | Soil under conifer, Selatræd, Osterøy, Faroe Islands | |
| CBS 124321 | DTO 42F8 = IBT 29680 | Soil, Poland | |
| CBS 124322 | CBS H-20652 = DTO 42G7 = IBT 29993 | Soil, Poland | |
| CBS 126425 | DTO 76A7 = IBT 13531 | Tilia swamp, Denmark | |
| CBS 126426 | CBS H-20658 = DTO 78A3 = IBT 15841 = DAOM 174586 | Washed organic soil particle, Alberta, Canada | |
| CBS 126427 | DTO 42A6 = IBT 29674 | Soil, Poland | |
| P. waksmanii | CBS 126428 | DTO 82C6 = IBT 24649 | Soil under tax tree, Selatræd, Osterøy, Faroe Islands |
| CBS 126429 | DTO 76C7 = IBT 23558x | Culture contaminant of IBT 23558 | |
| CBS 230.28T | DTO 22E6 = ATCC 10516 = FRR 777 = IFO 7737 = IMI 039746 = IMI 039746i = MUCL 29120 = NRRL 777 = QM 7681 = IBT 5003 = IBT 6994 | Woodland soil, Purczcza Bialowieska Forest, Poland | |
| P. wellingtonense | CBS 130375 | CBS H-20657 = DTO 76C6 = IBT 23557 | Soil, New Zealand |
| P. westlingii | CBS 118037 | KAS 2189 = IBT 29822 | Moose dung, Haliburton, Algonquin Park, Wildlife Research Station, Ontario, Canada |
| CBS 118051 | KAS 2205 = IBT 29838 | Nut of Juglans nigra (black walnut); Fireman's Park, Niagara Falls, Ontario, Canada, 43.142051° -79.115903° | |
| CBS 118166 | KAS 2117 = IBT 29853 | Acorns of Quercus, Simcoe, Cawaja Beach, Ontario, Canada | |
| CBS 122407 | DTO 28F9 = IBT 30688 | Indoor air of house, Eindhoven, the Netherlands | |
| CBS 122408 | DTO 18D7 = IBT 30677 | Soil under oak, Spaanderswoud, Bussum, the Netherlands | |
| CBS 122409 | DTO 17H7 = IBT 29062 | Soil under oak, Spaanderswoud, Bussum, the Netherlands | |
| CBS 124311 | DTO 39D4 = IBT 30774 | Soil, Poland | |
| CBS 124312 | DTO 30D6 = IBT 29067 | Soil of rainforest, Atherton Tableland, Queensland, Australia | |
| CBS 124313 | CBS H-20649 = DTO 30E3 = IBT 29992 | Soil, Atherton Tableland, Queensland, Australia | |
| CBS 127003 | DTO 32E1 = IBT 29659 | Soil, Lake Easchem, Queensland, Australia | |
| CBS 127005 | DTO 39D8 = IBT 30758 | Soil, Poland | |
| CBS 127006 | DTO 92G3 | Soil heathland, Cartier heide, Eersel, the Netherlands | |
| CBS 127007 | DTO 42H1 = IBT 30756 | Soil, Poland | |
| CBS 127008 | DTO 80I4 = IBT 30685 | Indoor environment, Germany | |
| CBS 127037 | DTO 78B7 = IBT 22399 | Soil under Cyathea fern tree, on Rio Jaba trail, near Quebrada, Culebra, Wilson Botanical garden, Las Cruces Biological state park, Costa Rica | |
| CBS 127039 | DTO 78B4 = IBT 22164 | On Ganoderma lucidum, Turkey Swamp, New Jersey, USA | |
| CBS 127040 | DTO 78G4 = DTO 78G3 = IBT 22985 | Soil, St. Teresa Forest reserve, Brazil | |
| CBS 231.28T | DTO 22E7 = IMI 092272 = IBT 15088 | Soil under conifer, Denga Goolina, Poznan, Poland | |
| CBS 688.77 | DTO 23D2 = IJFM 3046 = IBT 19471 | Type of P. citrinum var. pseudopaxilli; andosol soil, Navarra, Spain |
DNA extraction, PCR amplification and sequencing
Strains were grown for 7 to 14 d on MEA prior to DNA extraction. DNA extraction was performed using the UltracleanTM Microbial DNA isolation Kit (MoBio, Solana Beach, U.S.A.) according to the manufacturer's instructions. The extracted DNA was stored at -20 °C until used. The ITS regions and parts of the β-tubulin, calmodulin and RPB2 genes were amplified and sequenced according the method described previously (Houbraken et al. 2007, 2011a, 2011b, Houbraken & Samson 2011).
Data analysis
The sequence data was optimised using the software package Seqman from DNAStar Inc. Sequences were aligned using the software Muscle in the MEGA5 programme (Tamura et al. 2011). The RAxML (randomised axelerated maximum likelihood) software (Stamatakis et al. 2008) was used in order to perform the Maximum Likelihood (ML) analysis on the combined data sets. Combined data sets were analysed as two distinct data partitions and individual branch length optimisation was applied per partition. Maximum Likelihood analysis on the individual data sets was performed with the MEGA5 software. Trees were redrawn from tree files using TREEVIEW (Page 1996). Section Citrina was delimitated using a combination of ITS and RPB2 sequences. Coccidioides immitis (strain RS) was used as an outgroup for this analysis. The phylogeny of different lineages within section Citrina was studied using a combination of partial β-tubulin and calmodulin sequences. These phylograms were rooted with P. corylophilum CBS 330.79, a member of section Exilicaulis (Houbraken & Samson 2011). Also the ITS region was sequenced for the majority of strains, and this locus was used to determine the effectiveness for species recognition. Unique, newly generated sequences were deposited in GenBank with accession numbers JN606358–JN606858.
Morphological analysis
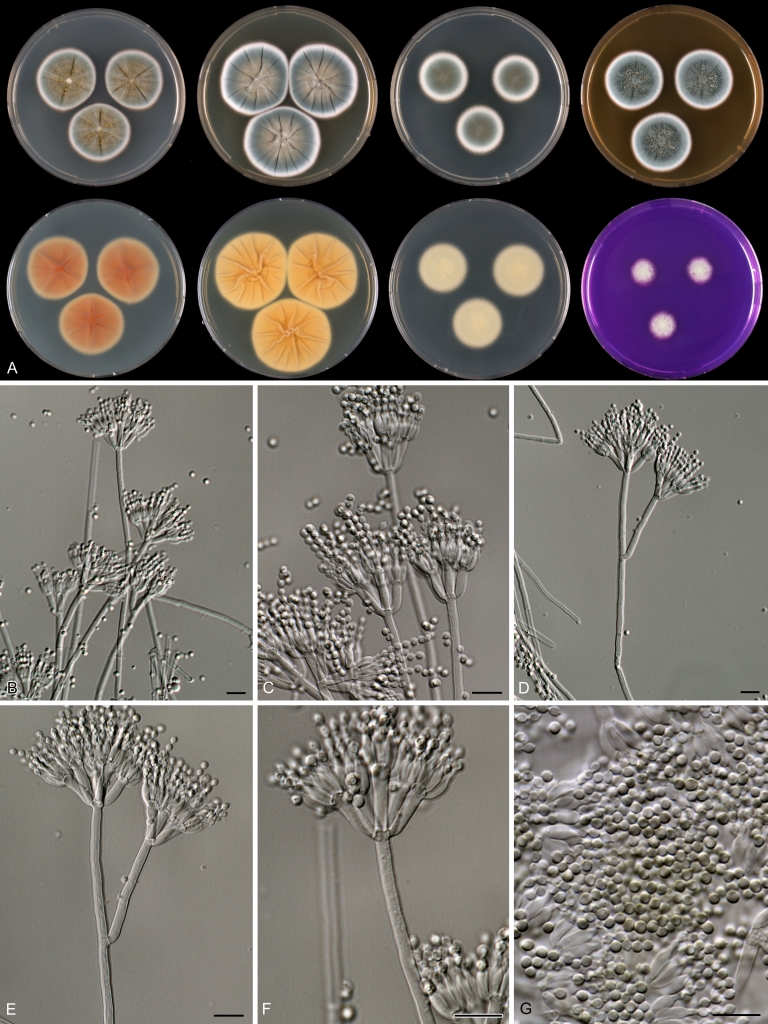
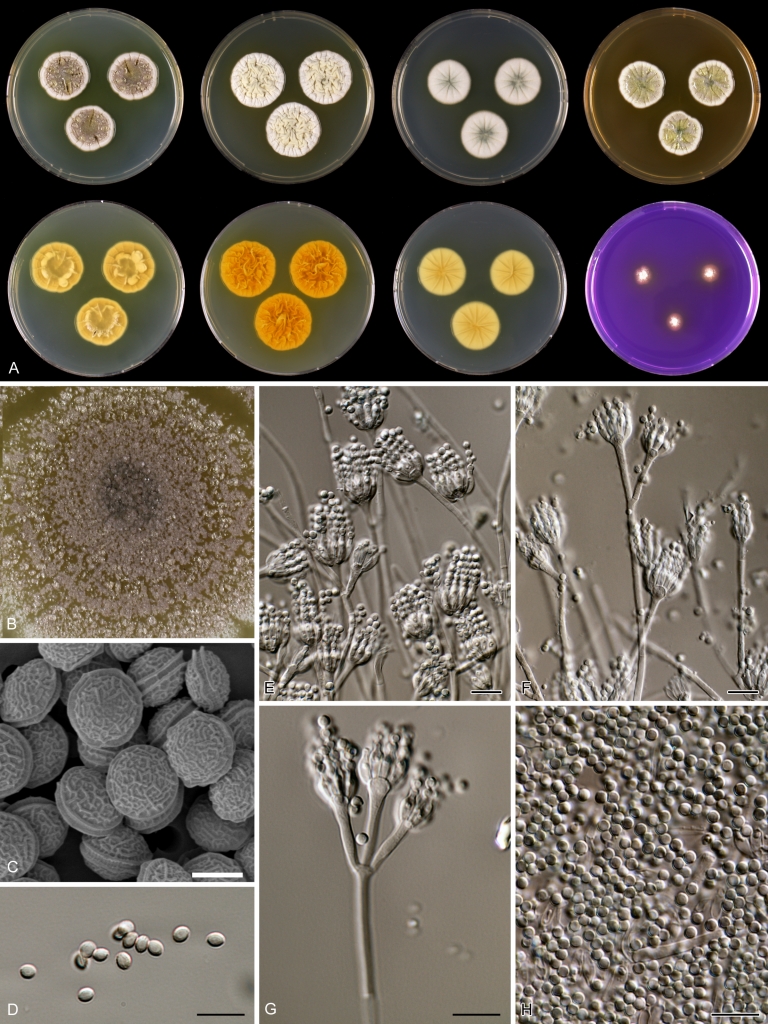
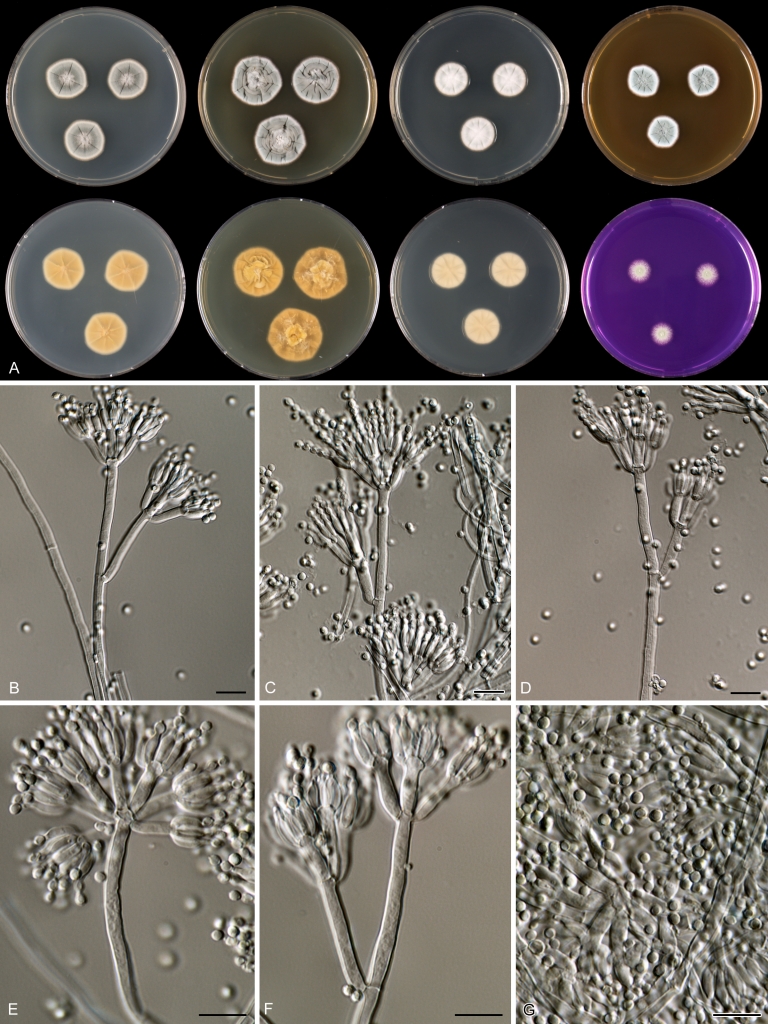
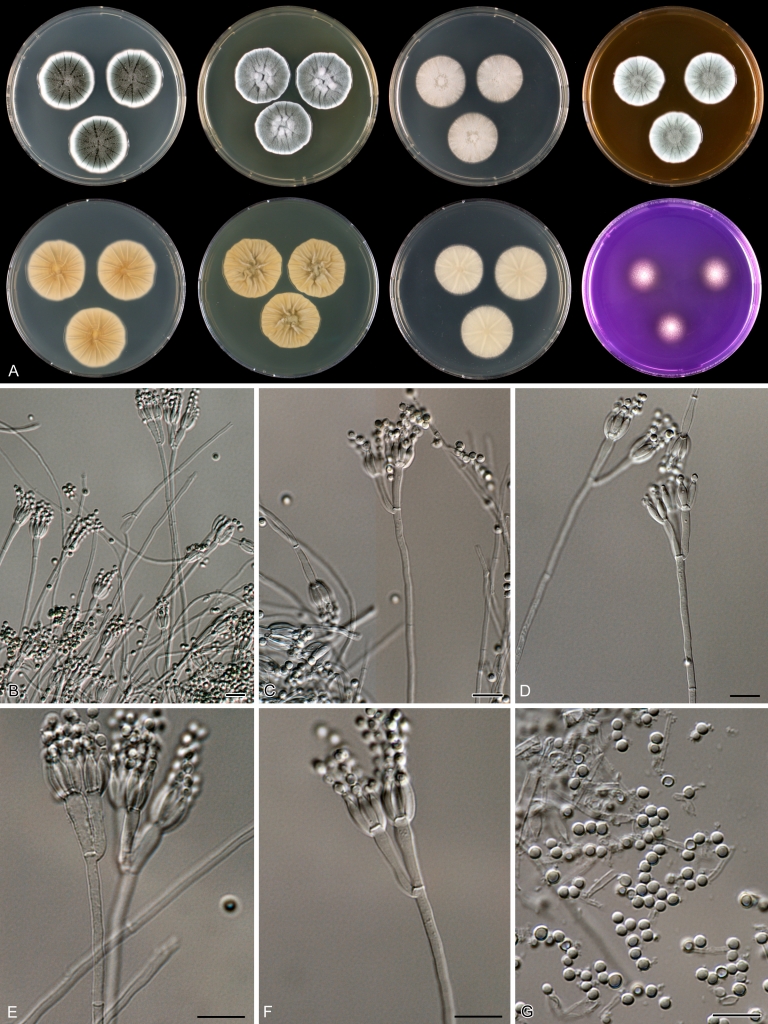
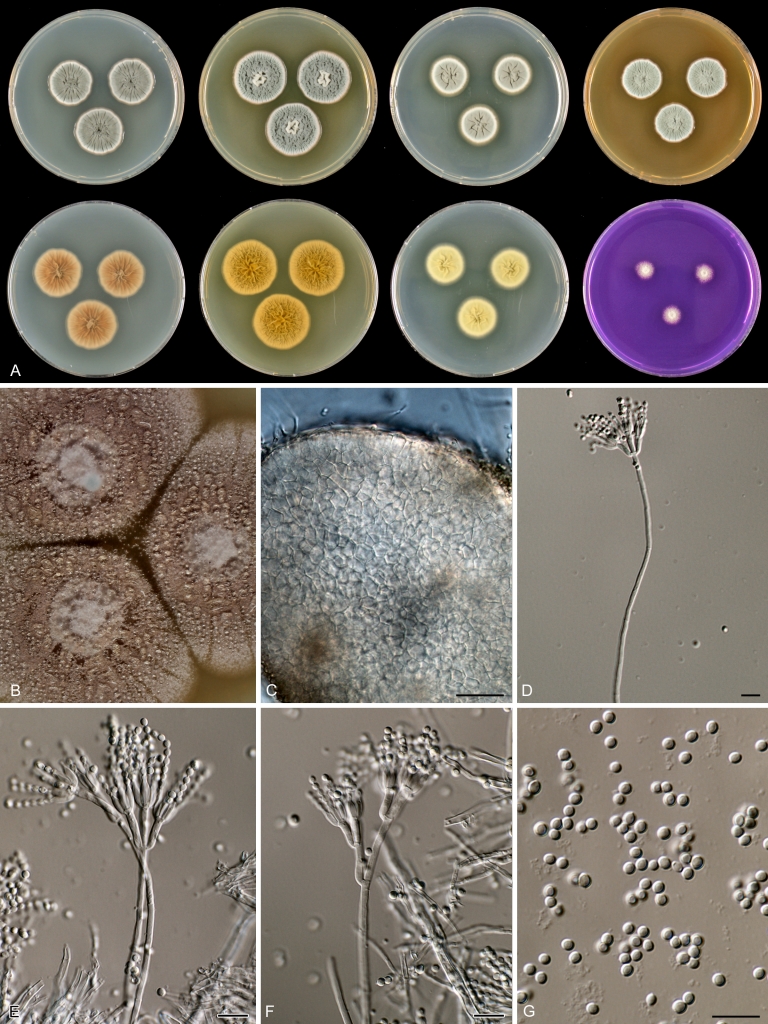
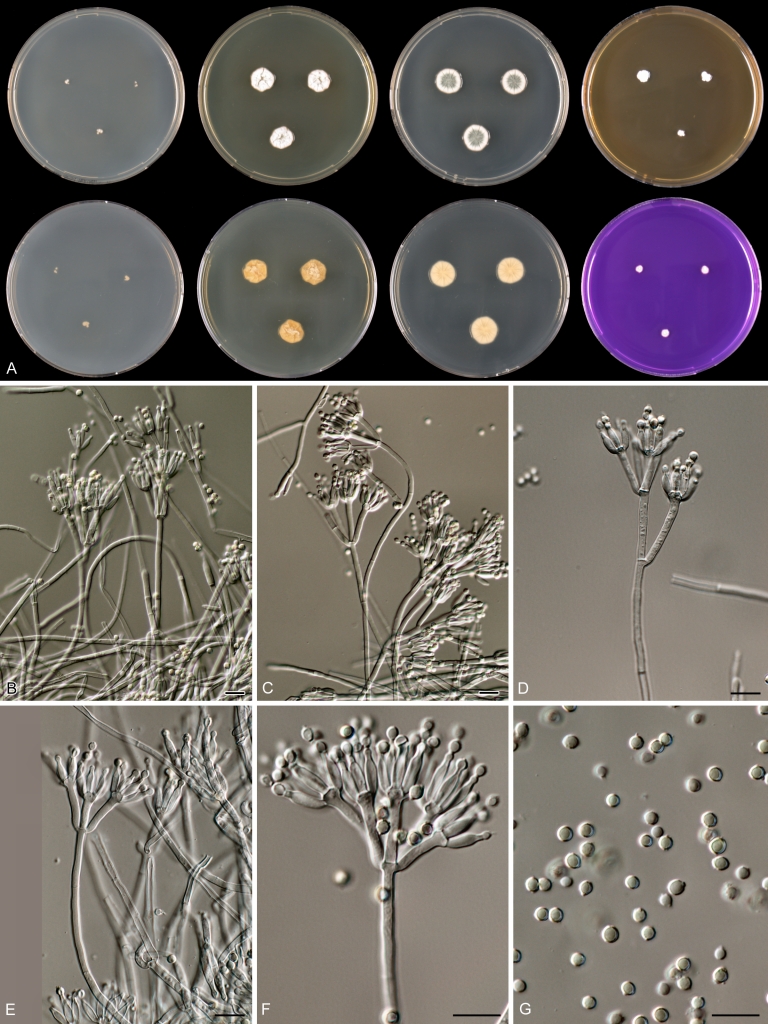
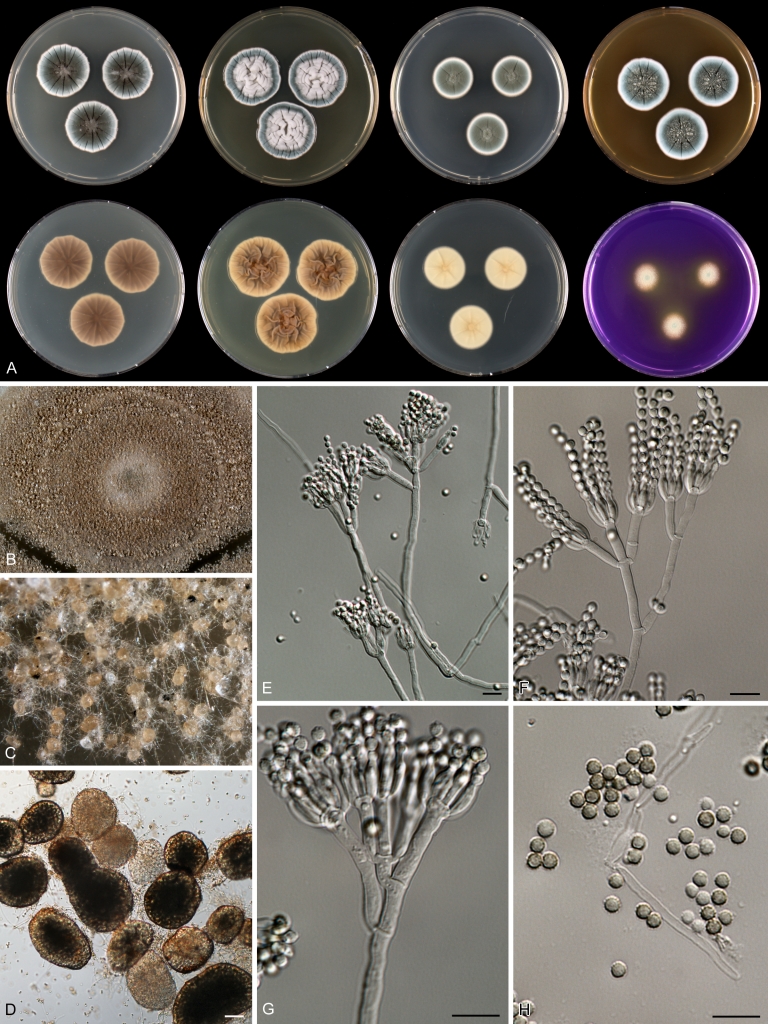
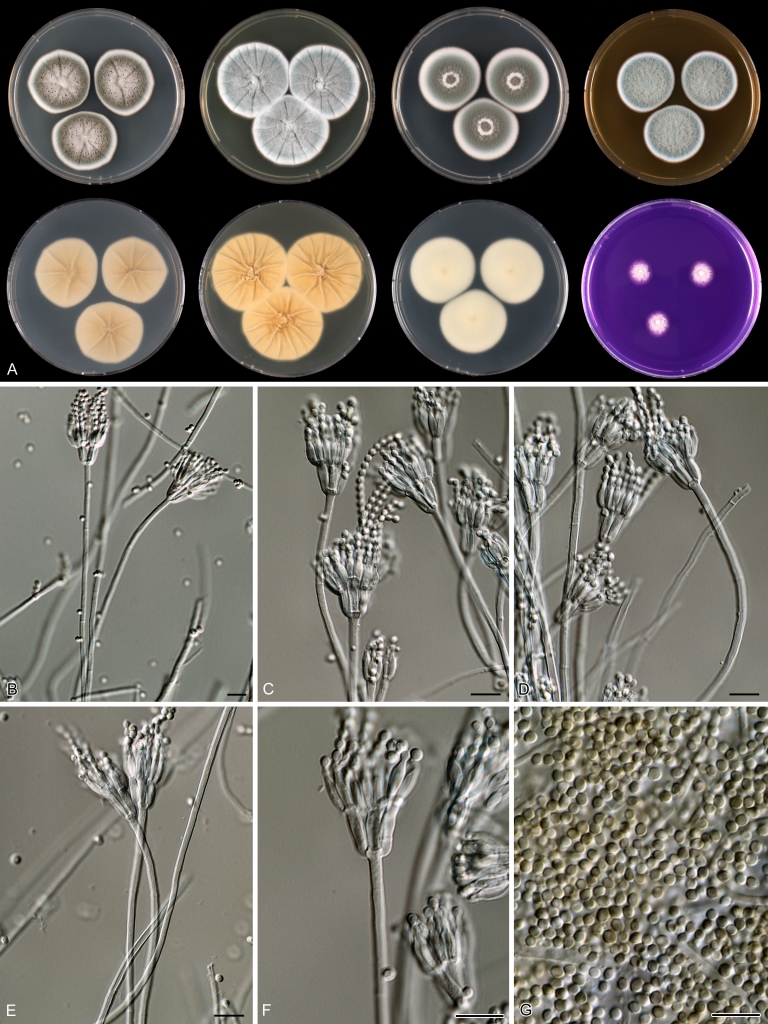
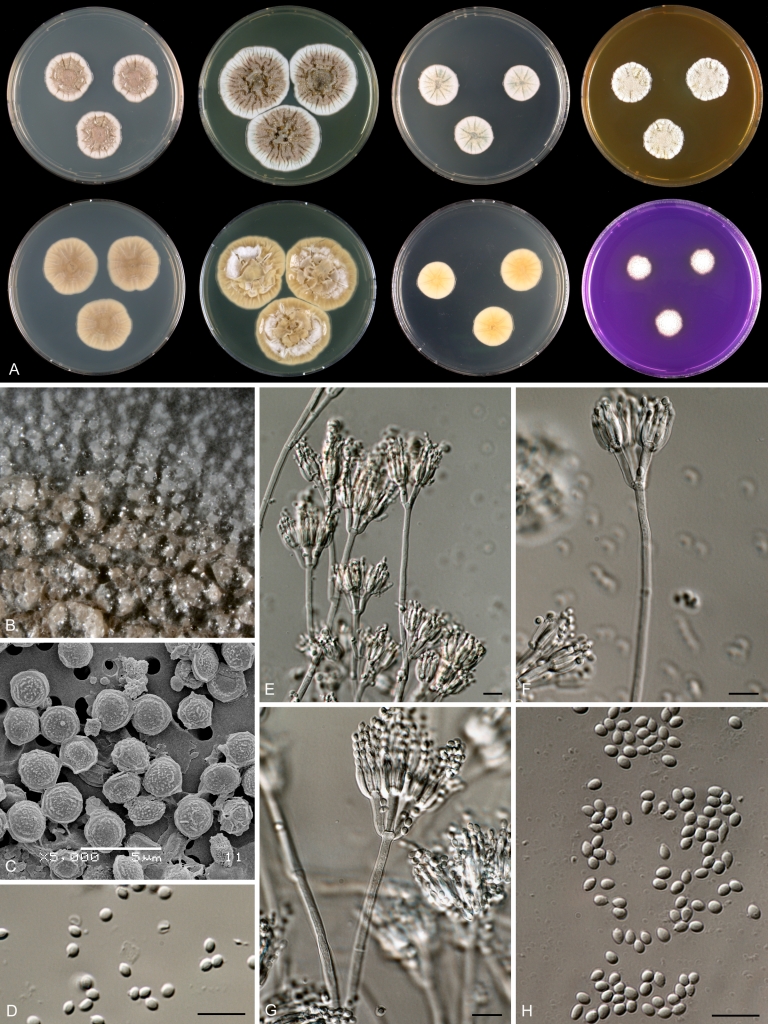
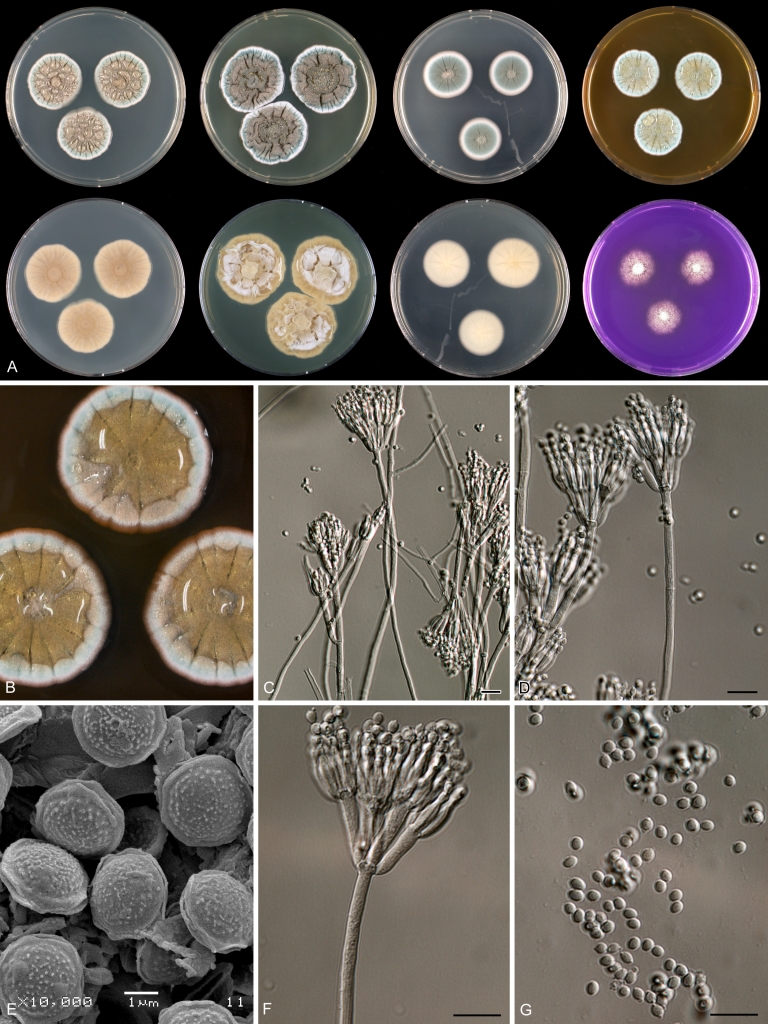
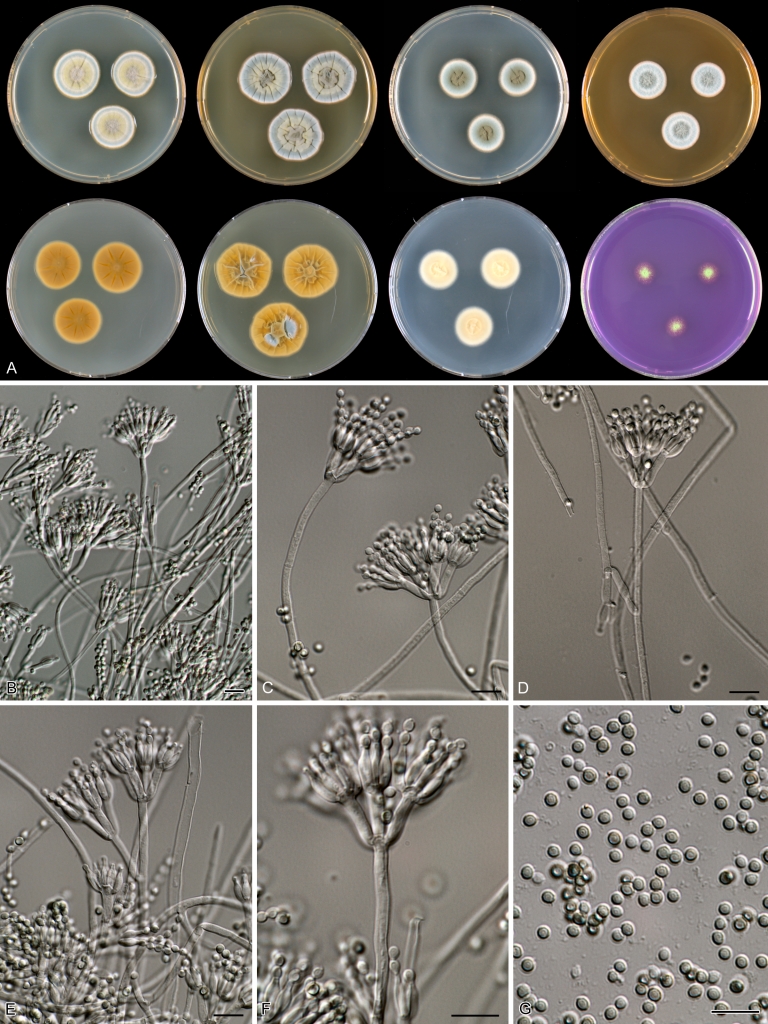
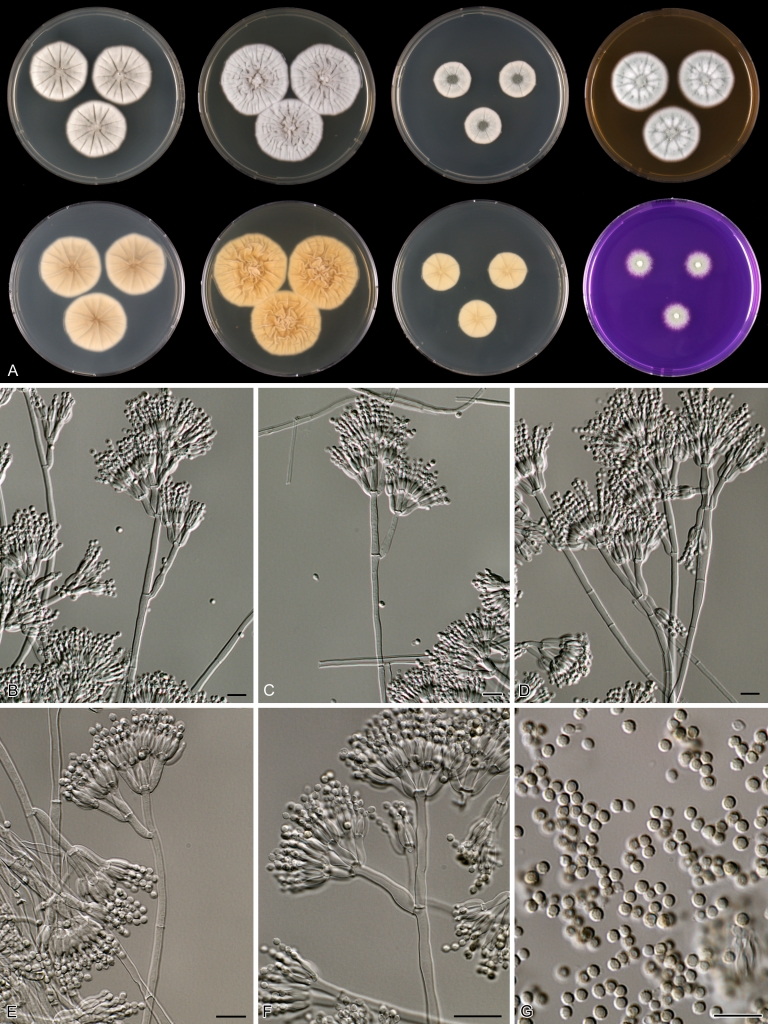
Macroscopical characters were studied on the agar media Czapek yeast extract agar (CYA), CYA supplemented with 5 % NaCl (CYAS), yeast extract sucrose agar (YES), creatine sucrose agar (CREA), dichloran 18 % glycerol agar (DG18), oatmeal agar (OA) and malt extract agar (Oxoid) (MEA). The strains were inoculated at three points on 90-mm Petri dishes and incubated for 7 d at 25 °C in darkness. In addition, CYA plates were inoculated and incubated for 7 d at 15, 30 and 37 °C (CYA15°C, CYA30°C and CYA37°C, respectively). All media were prepared as described by Samson et al. (2010). The temperature-growth response of the strains was studied on CYA. Strains were inoculated at 3 points and incubated at 18, 21, 24, 27, 30, 33, 36 and 40 °C for 7 d in darkness. After incubation, the colony diameter on the various agar media was measured. Also the degree of sporulation, obverse and reverse colony colours and the production of soluble pigments was determined. Colony colours were not described using colour standards as good colour charts are rarely available and frequently used colour plates differ between the various copies of the same book. Instead, we choose to take pictures of the colonies with a Nikon Coolpix 990. The isolates were also examined for production of alkaloids reacting with Ehrlich reagent using a filter paper method (Lund 1995). The appearance of a violet ring within 10 min was regarded as a positive reaction, all other colours were considered negative.
Fungal material was examined using light microscopy (Olympus BH2 or Zeiss Axioskop 2 Plus). Microscopic mounts were prepared in 85 % lactic acid from MEA or OA and a drop of alcohol was added to remove air bubbles and excess conidia. Detailed examination of the ornamentation of the ascospores was performed by scanning electron microscopy (SEM). A quick sample preparation method was developed (J. Dijksterhuis unpubl. data), and this method is explained here in brief. Fungal cultures with ripe ascomata were flooded with 10 mM ACES buffer (pH 6.8, N-[2-acetamido]-2-aminoethane-sulfonic acid) supplemented with 0.05 % Tween 80. The ascomata were disconnected by vortexing with glass beads (1 mm) and filtered through sterile glass wool. Ascospores were spun down at 1,100×g (10 min) and washed twice in ACES buffer. In the last washing step, sterile demineralised water was used and the suspension was sonicated for 30 s prior to centrifugation. Filter disks with 1 μm pore size were placed on a Whatman filter paper (grade no. 1). Small aliquots of the ascospore-suspension were transferred on the filter disk, resulting in a quick removal of the water. The filter disks with the ascospores were fixed on aluminium stubs with carbon conductive double-sided tape and air-dried. Samples were examined in a JEOL 5600LV scanning electron microscope (JEOL, Tokyo, Japan).
Extrolite analysis
Strains listed in Table 1 were grown for 7 d at 25 °C on YES and CYA prior to extrolite extraction. Five agar plugs were taken along a diameter of the fungal colony and pooled together into the same vial. The extraction solvent ethyl acetate / dichloromethane / methanol (3:2:1, v/v/v) with 1 % (v/v) formic acid was added to the vial and subsequently ultrasonicated for 50 min. The extracts were transferred to 1.5 ml autosampler screw-cap vials, evaporated to dryness and re-dissolved in 400 μl methanol by ultrasonication for 10 min. Subsequently, the extracts were filtered through 0.45 μm filter (Minisart RC4, Sartorius, Germany) and kept at -18° C prior to analysis. The extracts were analysed by ultra high performance liquid chromatography (U-HPLC) using alkylphenone retention indices and diode array UV-VIS detection as described by Frisvad & Thrane (1987) and Nielsen et al. (2011). Identification of extrolites was performed by comparison of the UV-Visible spectra and retention times of the extrolites with those present in the collection at Department of Systems Biology, Kgs. Lyngby, Denmark. During our investigations many compounds were found, which could not be chemically identified. However, these extrolites proved to be important components for the species extrolite profile and they are listed between quotation marks.
RESULTS
Delimitation of section Citrina
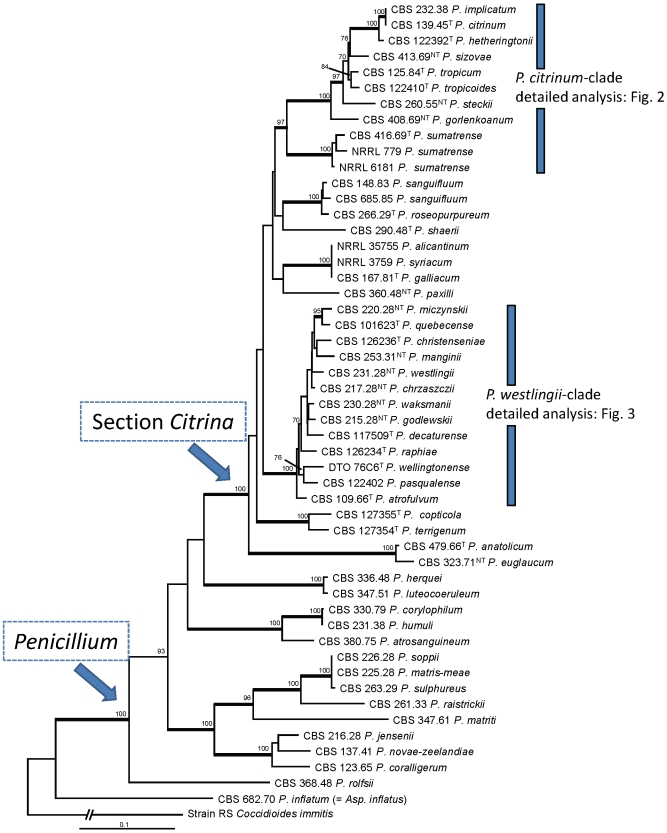
In order to determine the species belonging to section Citrina, a phylogenetic study using combined sequence data of two loci (ITS and RPB2) was performed. 52 taxa were included in the analysis and the total length of the alignment was 1491 characters. The ITS partition was 575 characters long and had 174 variable sites, while the RPB2 partition included 915 base pairs and 424 of them were variable. Figure 1 shows the results of this analysis. Members of section Citrina form a well-supported lineage on the phylogram (100 %). The majority of the branches in the backbone of this section are poorly supported. Two species-rich lineages are present in this section: one lineage is centered on P. citrinum and the other on P. westlingii. Three other well-supported lineages are present and these are centered on P. sanguifluum/P. roseopurpureum, P. copticola/P. terrigenum and P. anatolicum/P. euglaucum. These lineages appear to be less species-rich than those centered on P. citrinum and P. westlingii. Penicillium shearii and P. paxilli occurred on single branches and the relationship with other members of section Citrina remains unsolved. An overview of species classified by other authors in the P. citrinum series (Raper & Thom 1949, Ramírez 1982) or series Citrina (Pitt 1980) is presented in Table 2. Several of these species do not phylogenetically belong to section Citrina (Fig. 1), including P. corylophilum (synonyms: P. obscurum, P. chloroleucon, P. citreovirens, P. humuli), P. soppii (synonym: P. matris-meae), P. herquei (synonym: P. luteocoeruleum nom. inval.), P. coralligerum, P. atrosanguineum, P. matriti and Aspergillus inflatus (basionym: P. inflatum, R.A. Samson, unpublished data).
Fig. 1.
Best-scoring Maximum Likelihood tree using RAxML based on a combination of partial RPB2 and ITS sequences. Members of section Citrina are in a well-supported lineage (100 % bs) and some species previously belonging to series Citrina are placed in other lineages. Bootstrap percentages of the Maximum Likelihood (ML) analysis are presented at the nodes. Values less than 70 % supported in the ML are not shown and branches with more than 95 % bootstrap support are thickened. The bar indicates the number of substitutions per site. The phylogram is rooted with Coccidioides immitis (Strain RS).
Table 2.
Overview of species classified by Raper & Thom (1949), Pitt (1980) and Ramírez (1982) in the series P. citrinum or related P. miczynskii (Christensen et al. 1999). The names in bold are excluded from section Citrina in the current study.
| Raper & Thom (1949>) | Pitt (1980) | Ramírez (1982) | Christensen et al. (1999) |
|---|---|---|---|
| P. citrinum | P. citrinum | P. citrinum | P. miczynskii |
| P. corylophilum | P. corylophilum | P. corylophilum | P. manginii |
| P. steckii | P. miczynskii | P. steckii | P. atrosanguineum |
| P. inflatum | P. matriti | P. soppii | |
| P. paxilli | P. syriacum nomen ambiguum | ||
| P. herquei | P. chrzaszcii nomen ambiguum | ||
| P. humuli | P. sulphureum nomen dubium | ||
| (P. rolfsii)* | |||
| (P. raistrickii)* |
P. raistrickii and P. rolfsii were included in this study for comparison purposes and were not claimed to be related to P. miczynskii.
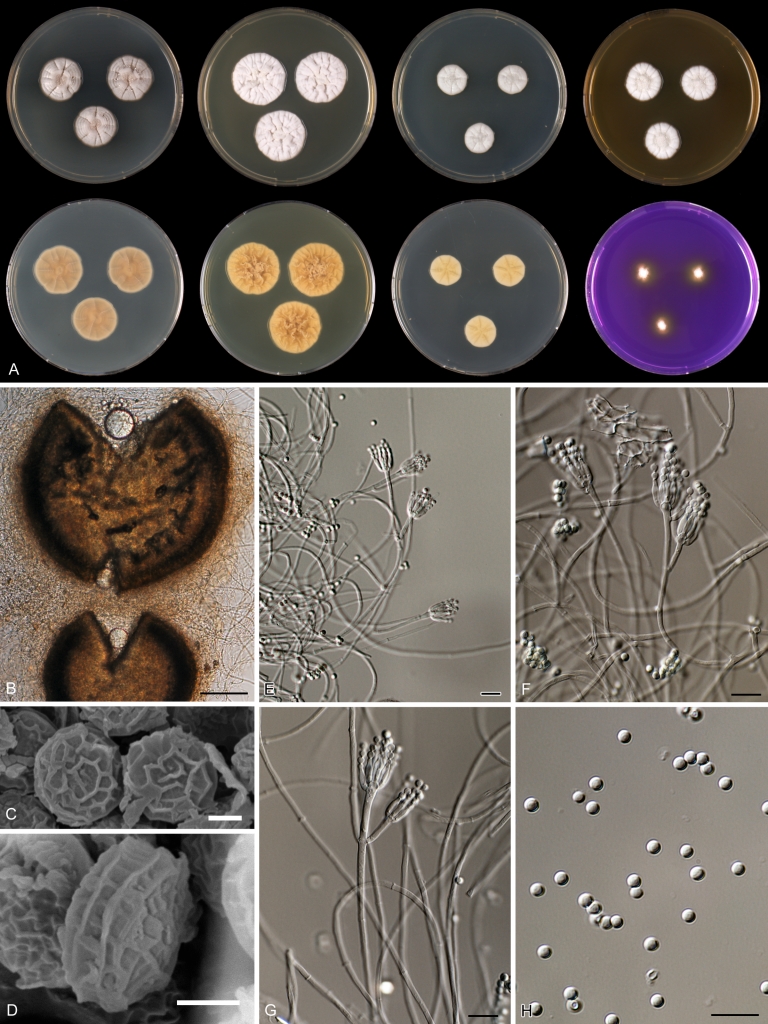
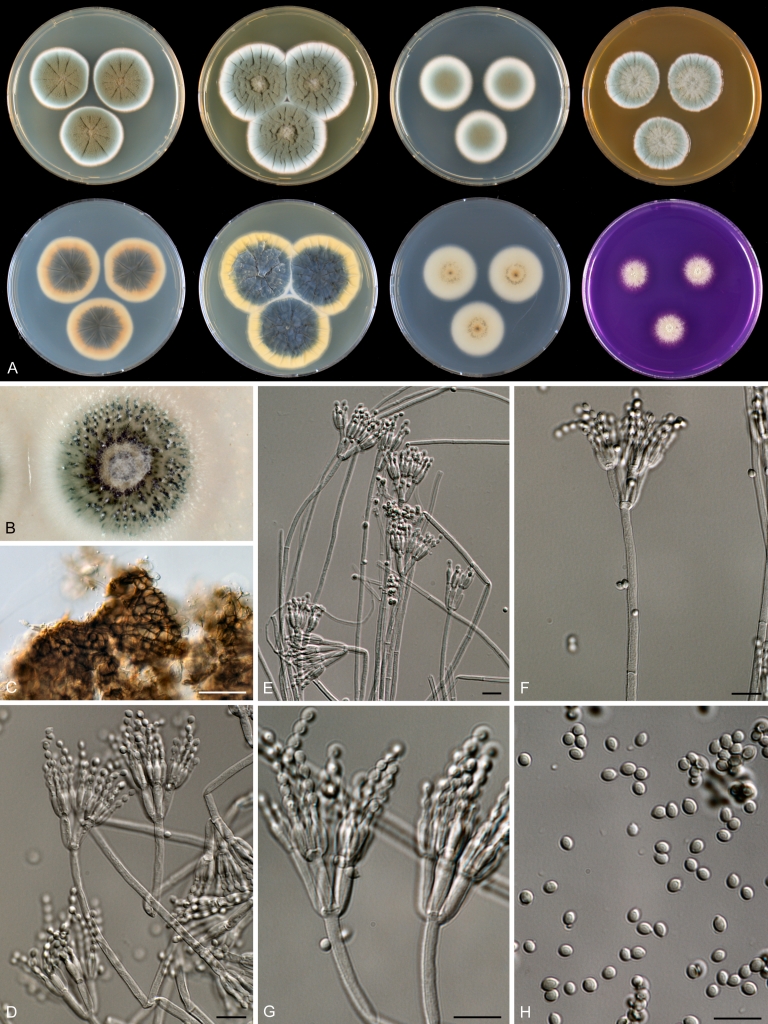
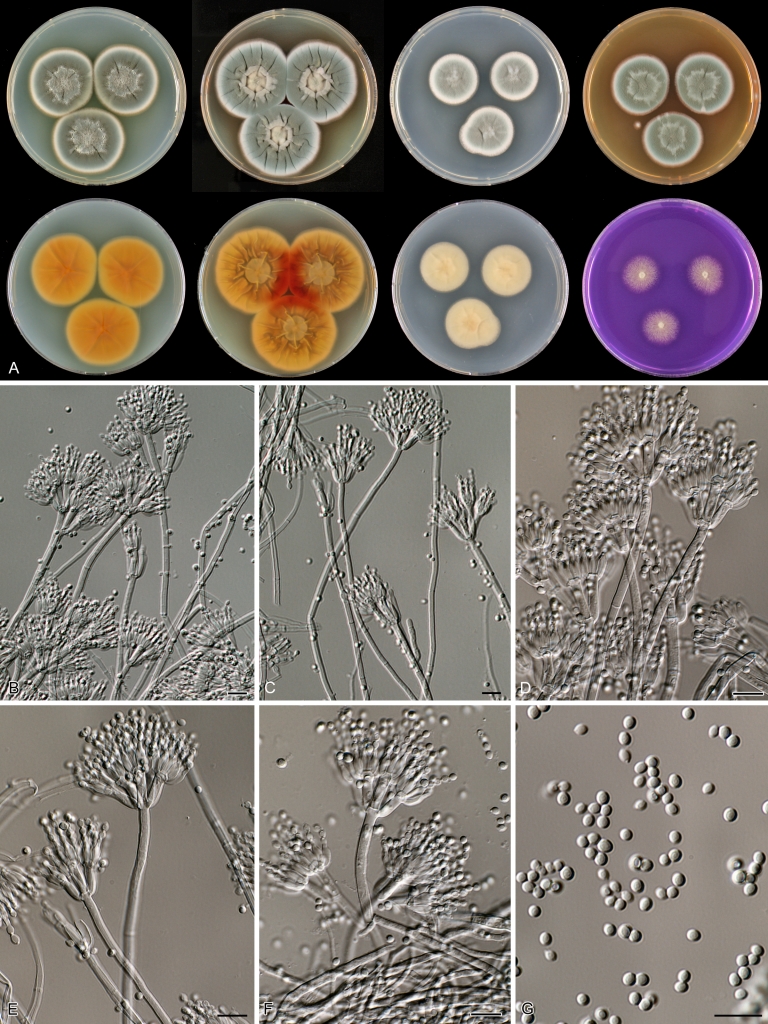
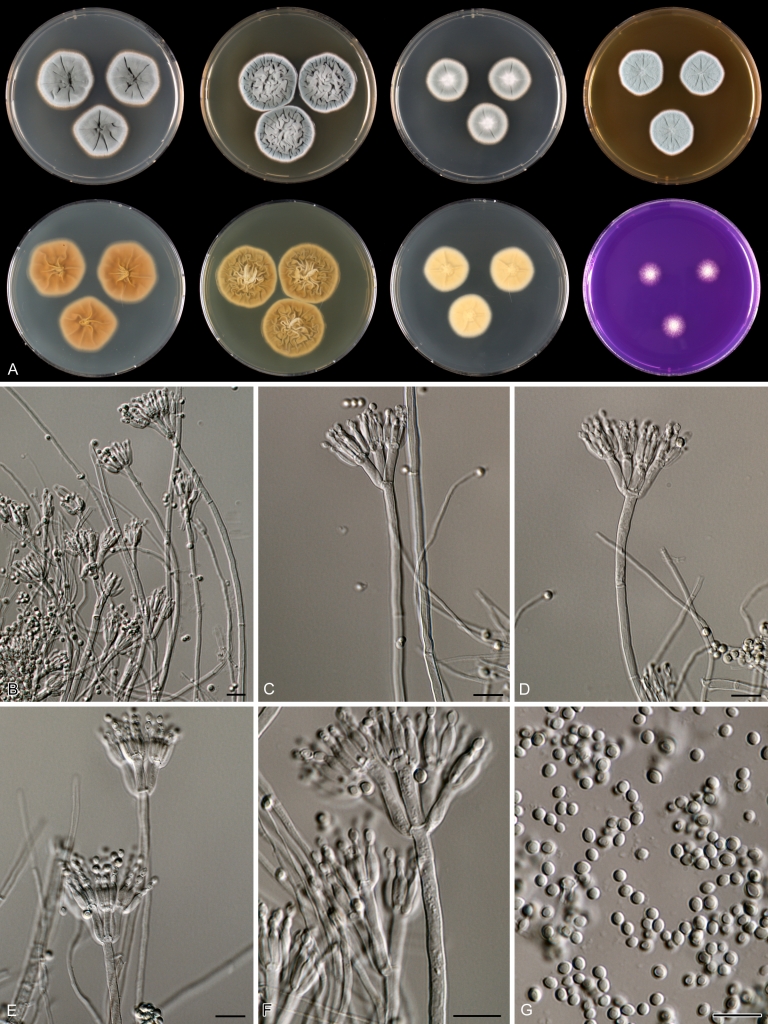
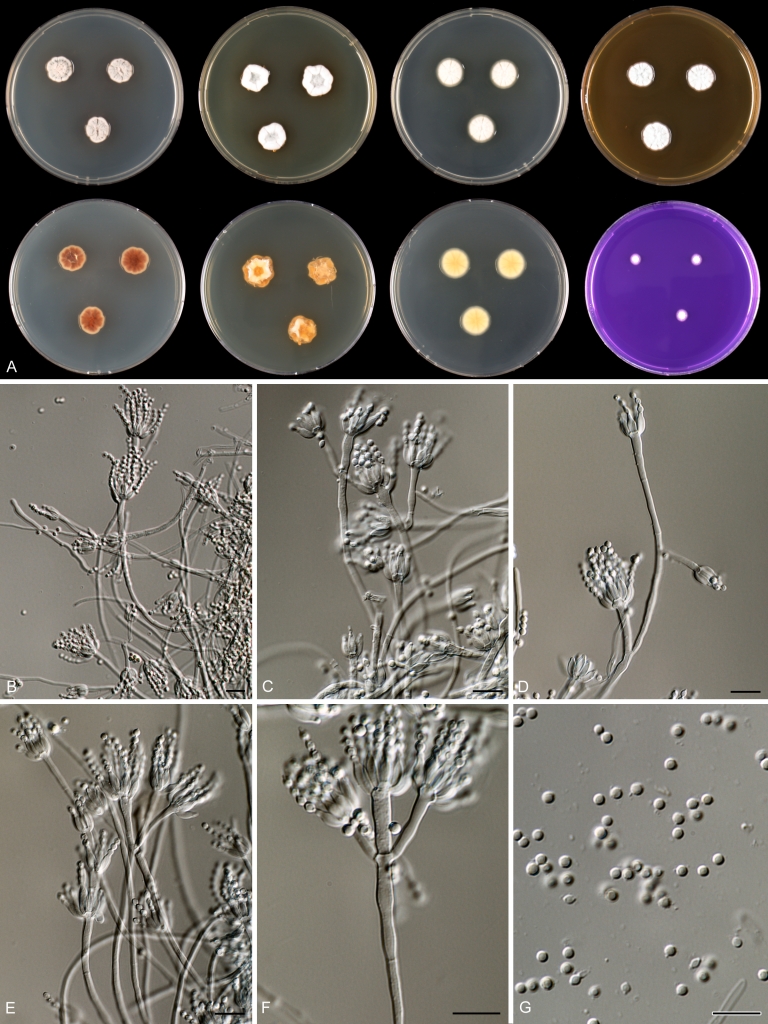
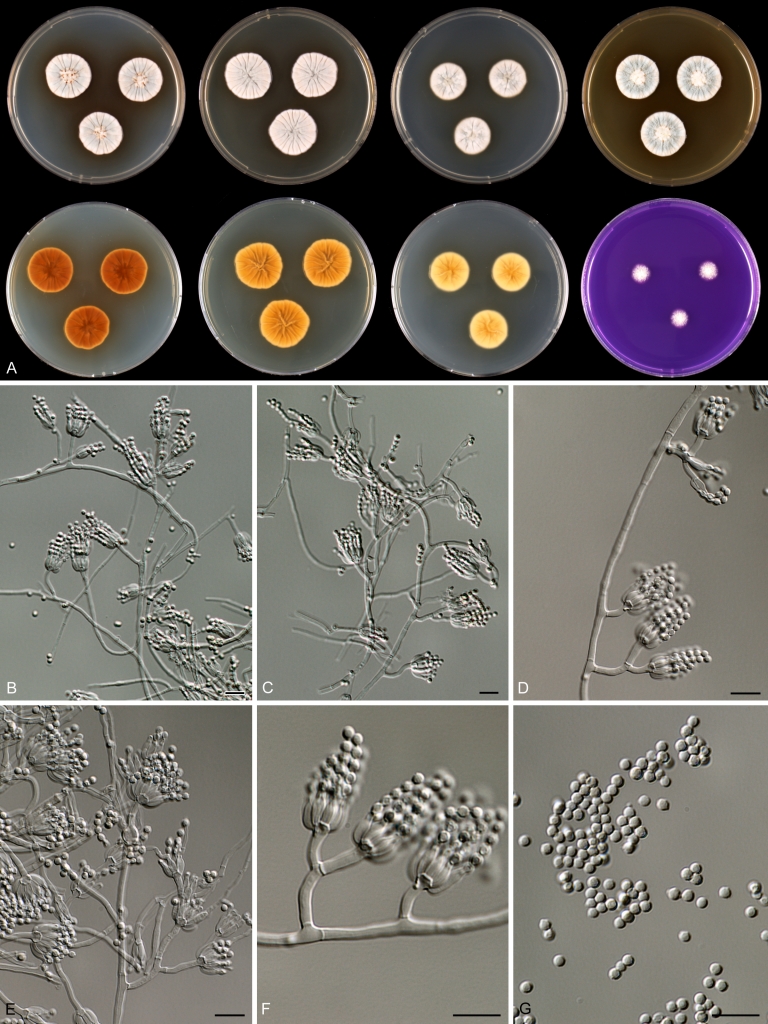
Species belonging to section Citrina share several characters. The majority of species produce symmetrically biverticillate conidiophores, flask shaped phialides (7.0–9.0 μm long) and relatively small-sized conidia (2.0–3.0 μm diam). The conidiophores of some species have an additional branch, which itself can also be biverticillate branched. Six of the 39 species produced greyish brown cleistothecia and these cleistothecia contain flanged ascospores. The extrolite citrinin was produced by 16 of the 39 species and was most commonly produced by species belonging to section Citrina. The majority of the species grows poorly on CREA and do not have a violet reaction with Ehrlich reagent.
Phylogeny of section Citrina
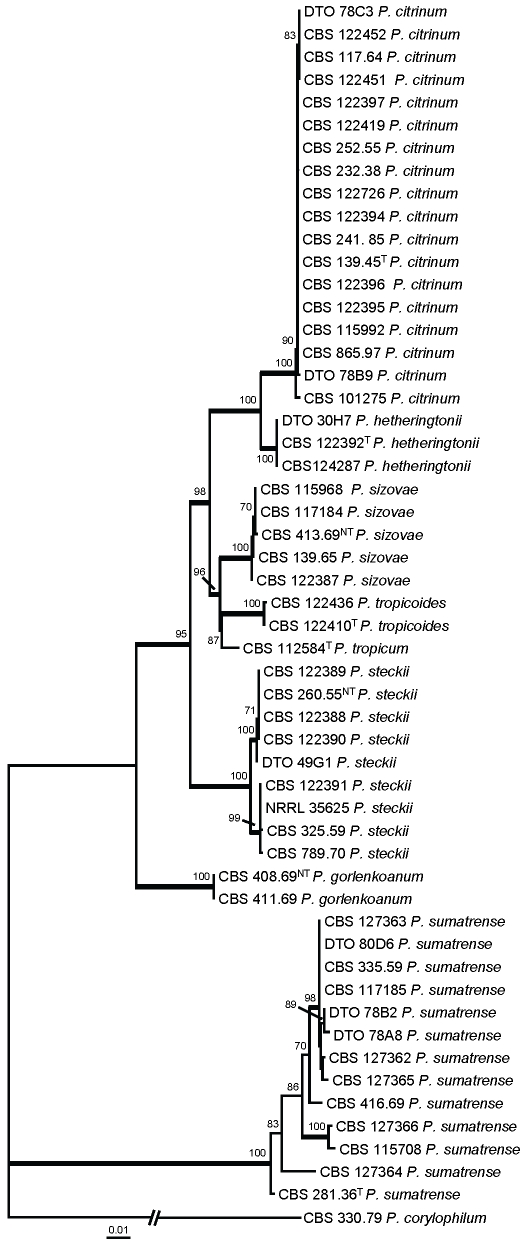
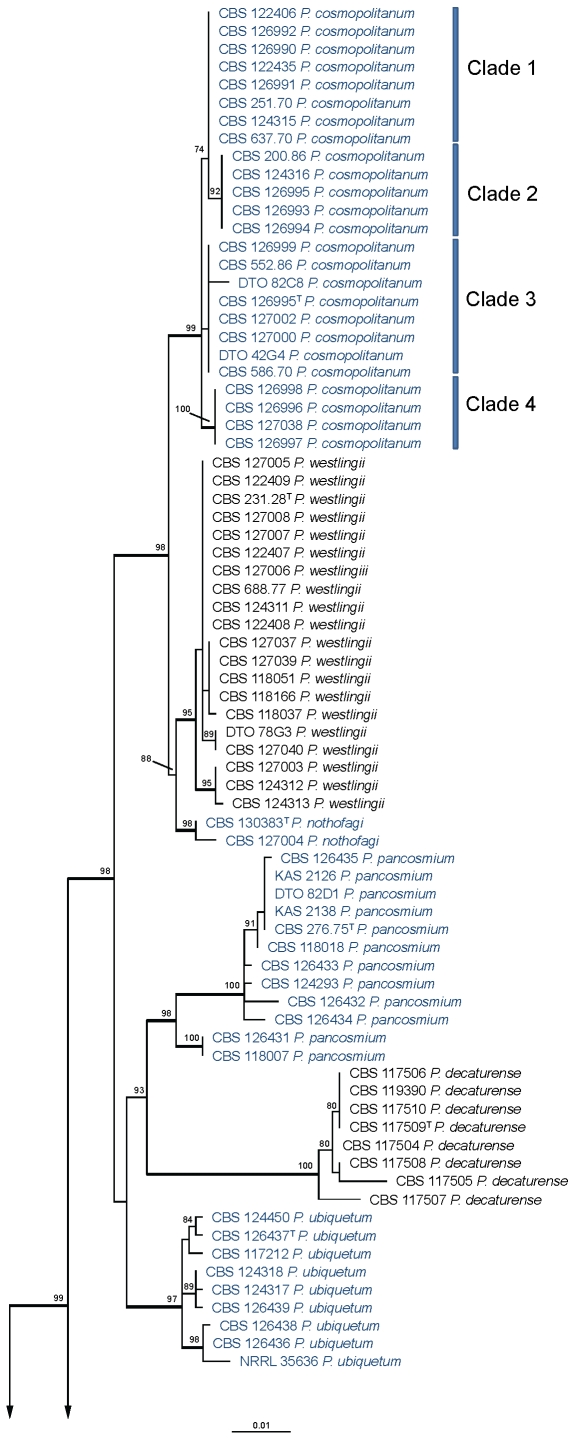
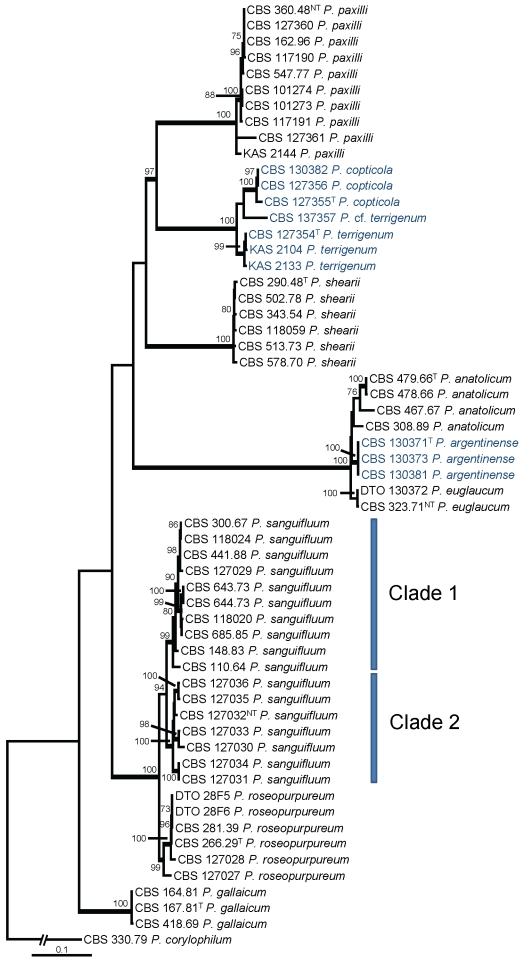
Section Citrina was studied in detail with partial β-tubulin and calmodulin sequences. Three separated analyses were performed: one with species related to P. citrinum (= P. citrinum-clade) (Fig. 2), one with species related to P. westlingii (P. westlingii-clade) (Fig. 3) and one with all the other members of section Citrina (Fig. 4). Details on the partitions and variable sites are given in Table 3. Individual gene trees can be found in supplementary Figs 1, 2, 3, 4, 5, 6.
Fig. 2.
Best-scoring Maximum Likelihood tree using RAxML based on a combination of partial β-tubulin and calmodulin sequences, showing the relationship among members of the P. citrinum-clade. Bootstrap percentages of the maximum likelihood (ML) analysis are presented at the nodes. Values less than 70 % supported in the ML are not shown and branches with more than 95 % bootstrap support are thickened. The bar indicates the number of substitutions per site. The phylogram is rooted with P. corylophilum (CBS 330.79).
Fig. 3.
Best-scoring Maximum Likelihood tree using RAxML based on a combination of partial β-tubulin and calmodulin sequences, showing the phylogenetic relationship among members of the P. westlingii-clade. Newly described species belonging to this section are presented in dark blue. Bootstrap percentages of the maximum likelihood (ML) analysis are presented at the nodes. Values less than 70 % supported in the ML are not shown and branches with more than 95 % bootstrap support are thickened. The bar indicates the number of substitutions per site. The phylogram is rooted with P. corylophilum (CBS 330.79).
Fig. 4.
Best-scoring Maximum Likelihood tree using RAxML based on a combination of partial β-tubulin and calmodulin sequences, showing the phylogenetic relationship among selected members of section Citrina. Newly described species belonging to this section are presented in dark blue. Bootstrap percentages of the maximum likelihood (ML) analysis are presented at the nodes. Values less than 70 % supported in the ML are not shown and branches with more than 95 % bootstrap support are thickened. The bar indicates the number of substitutions per site. The phylogram is rooted with P. corylophilum (CBS 330.79).
Table 3.
Parameters of matrices used to generate phylogenies.
| Figure | No. species | β-tubulin |
Calmodulin |
||
|---|---|---|---|---|---|
| Length | Variable sites | Length | Variable sites | ||
| Fig. 2, P. citrinum-clade | 8 | 474 | 149 | 464 | 178 |
| Fig. 3, P. westlingii-clade | 21 | 452 | 148 | 469 | 225 |
| Fig. 4, other sect. Citrina species | 10 | 475 | 212 | 733 | 349 |
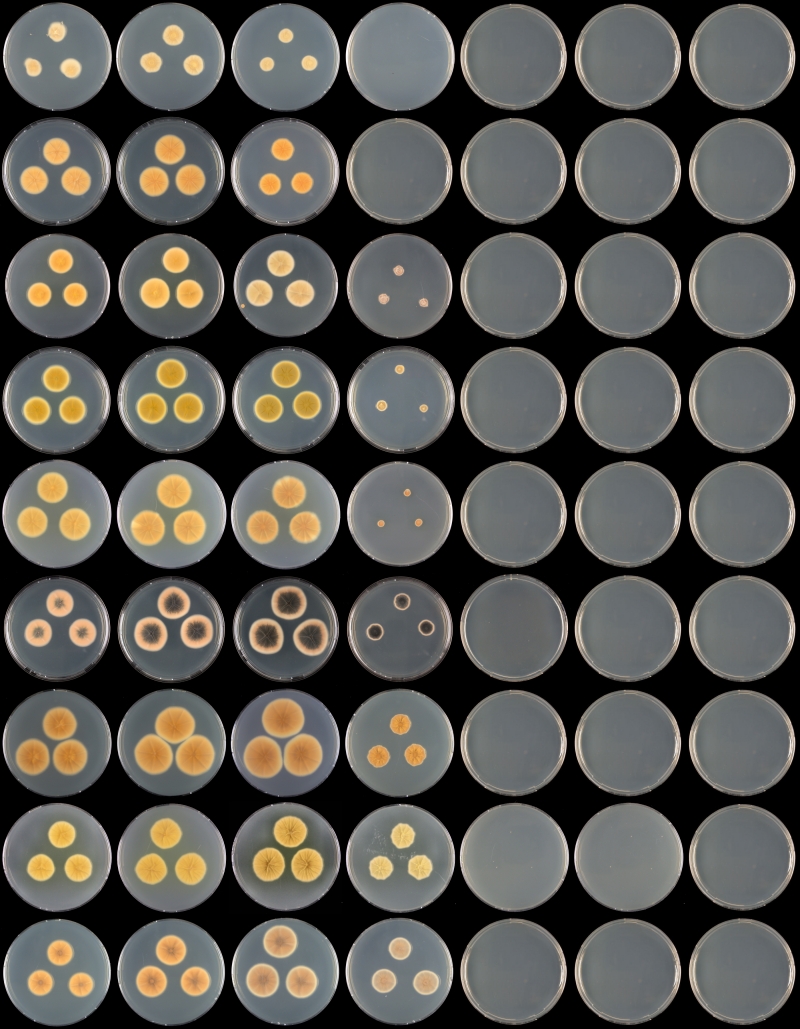
Fig. 5.
Overview of growth rates on CYA (reverse) after 7 d at various temperatures. Row, left to right: 21, 24, 27, 30, 33, 36 °C; columns, top to bottom: P. nothofagi, P. wellingtonense, P. godlewskii, P. vancouverense, P. neomiczynskii, P. atrofulvum, P. christenseniae, P. miczynskii, P. waksmanii.
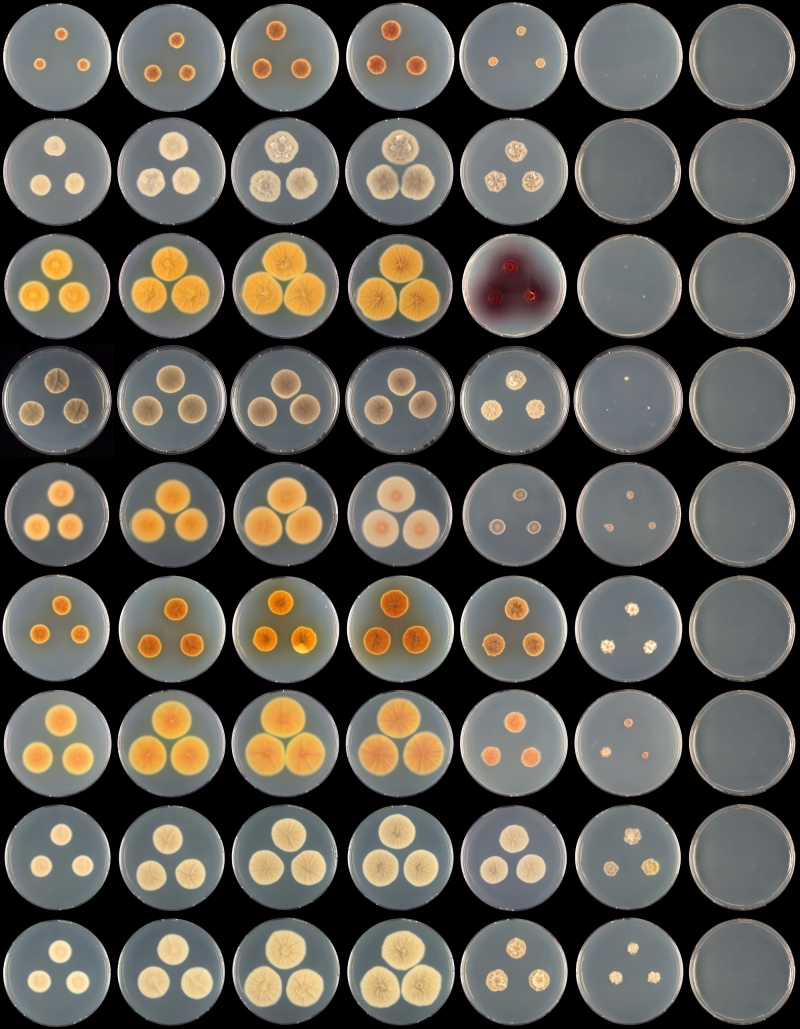
Fig. 6.
Overview of growth rates on CYA (reverse) after 7 d at various temperatures. Row, left to right: 21, 24, 27, 30, 33, 36 °C; columns, top to bottom: P. raphiae, P. chrzaszczii, P. ubiquetum, P. aurantiacobrunneum, P. pancosmium, P. cosmopolitanum, P. westlingii, P. manginii, P. manginii.
Fifty-three strains were included in the analysis of the members belonging to the P. citrinum-clade and the total length of the alignment was 938 characters. This clade includes eight accepted species: P. citrinum, P. hetheringtonii, P. sizovae, P. tropicoides, P. tropicum, P. steckii, P. gorlenkoanum and P. sumatrense. The former seven species are accommodated in a well-supported lineage (100 %), and statistical support for the relationship of the latter species is lacking. However, this species was included in this analysis based on the results presented in Fig. 1, which confidently included this species in this clade (97 %).
One hundred and sixty-six isolates were included in the analysis of the P. westlingii-clade, and the total length of the alignment was 921 characters. Twenty-one species are present in this clade, and 14 of those are newly described here. The P. westlingii-clade can be subdivided into different subclades. Penicillium cosmopolitanum, P. westlingii, P. nothofagi, P. pancosmium, P. decaturense, P. ubiquetum, P. waksmanii, P. godlewskii and P. chrzaszczii are on a well-supported lineage (99 %). Another subclade only includes the newly described species P. vancouverense, P. wellingtonense, P. pasqualense, P. atrofulvum (96 %); P. raphiae and P. christenseniae are basal to this clade (82 %). Penicillium cairnsense, P. quebecense, P. miczynskii, P. aurantiacobrunneum and P. neomiczynskii are on another well-supported branch (98 %) and P. manginii is on a separate well-supported branch (100 %).
The phylogenetic relationships of the species not belonging to the P. citrinum or P. westlingii-clades are shown in Fig. 4. Sixty strains were included and the total length of the alignment was 1208 characters long. Six different lineages are present and comprise 10 species. Penicillium paxilli formed one clade, and this clade is related to a lineage containing the new species P. copticola and P. terrigenum (97 %). Penicillium shearii and P. gallaicum formed single lineages, while P. sanguifluum and P. roseopurpureum were together on a well-supported branch (100 %). Penicillium euglaucum, P. anatolicum and P. argentinense were also together on a well-supported branch (100 %).
Morphology and physiology
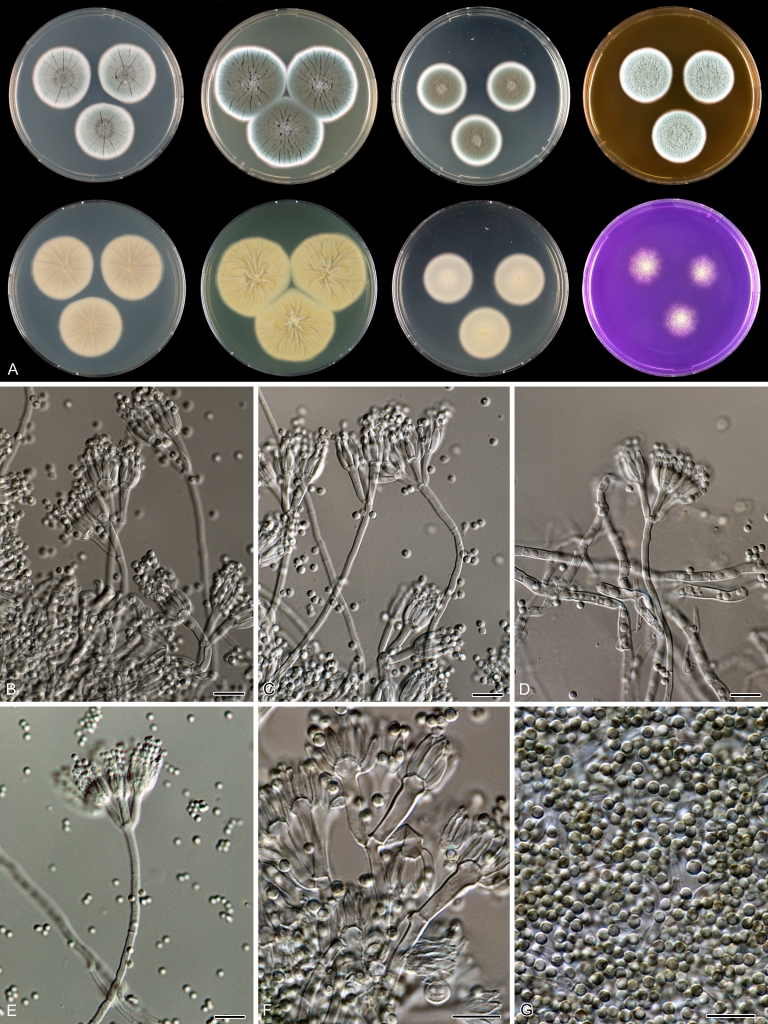
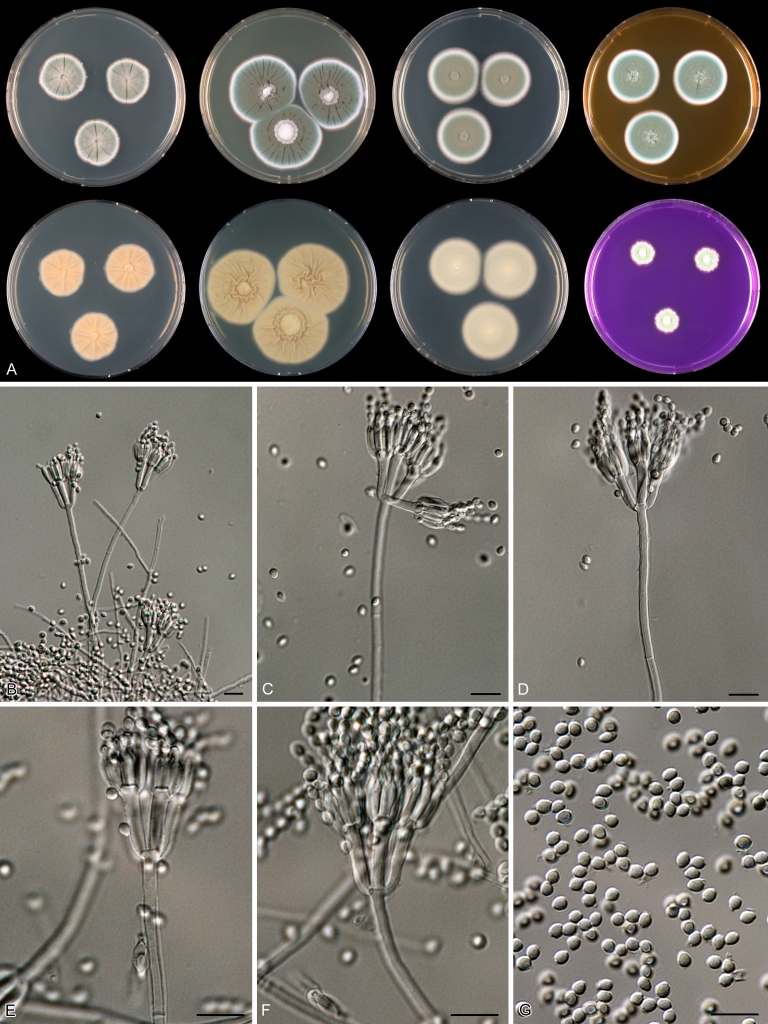
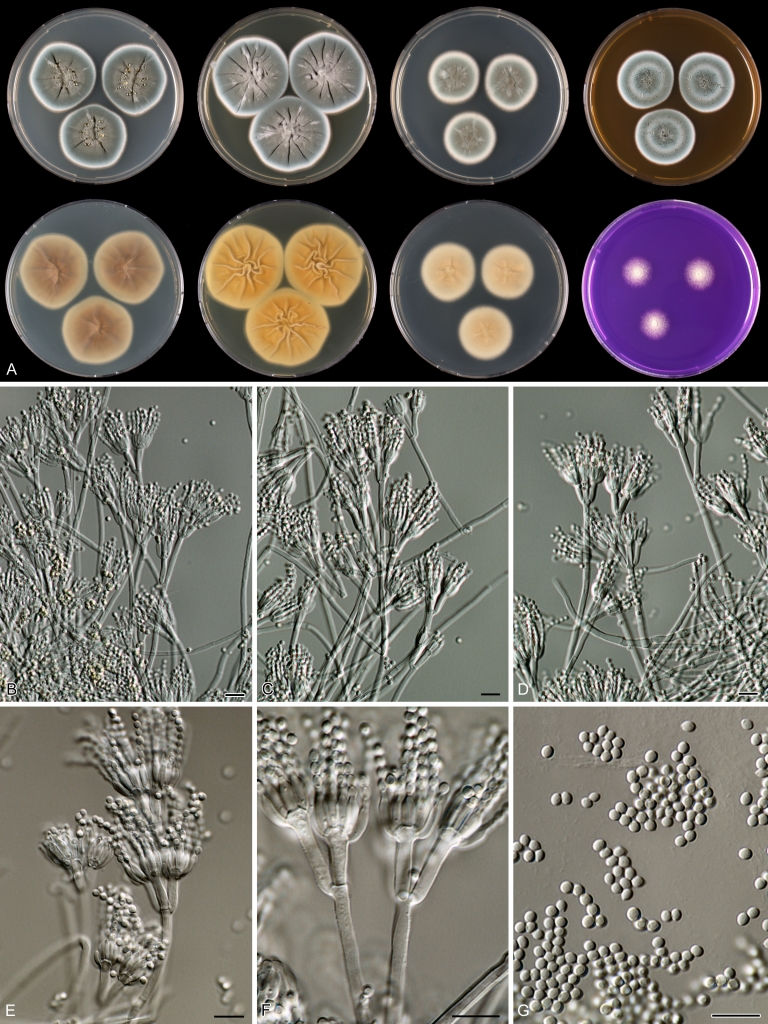
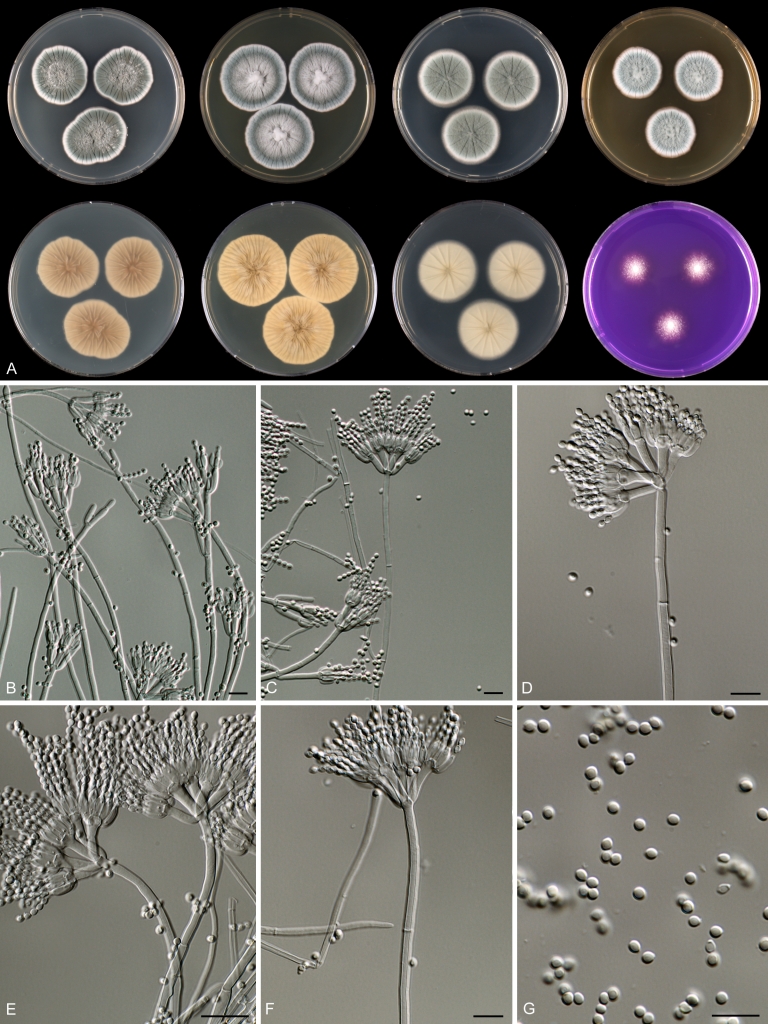
Macro-morphology
Various phenotypic differences were observed among the investigated species. Growth rates on CYA, MEA, YES and DG18 are useful diagnostic features for species recognition. Some species, e.g. P. wellingtonense, P. nothofagi grow very restricted on CYA (5–15 mm), while others grow rapidly (P. sumatrense, P. decaturense, P. quebecense, 30–45 mm). Reverse colours on CYA and YES and the production of soluble pigments were also useful characters for differentiating species belonging to section Citrina. The colour of the mycelium was white and inconspicuous in most species, but certain species had (light) yellow coloured mycelium (e.g. P. vancouverense, P. miczynskii, P. cairnsense). Creatine agar, which is used for identification of species belonging to subgenus Penicillium (Frisvad 1985, Frisvad & Samson 2004) was also tested, but had little discriminatory power. Most species showed weak growth with no or weak acid production. Exceptions are P. christenseniae, P. steckii and P. copticola and certain strains of P. pasqualense, P. tropicoides, P. tropicum and P. atrofulvum. Another important feature was the production of sclerotia or cleistothecia. Six species formed cleistothecia on OA: P. shearii, P. euglaucum, P. anatolicum, P. argentinense, P. tropicum and P. tropicoides. These cleistothecia were coloured in greyish-brown shades and often took more than 6 wk to ripen. The ascospores of these species were ellipsoidal, with two narrow, closely appressed equatorial ridges. The ornamentation of the valves varied among the species, from finely roughened (P. anatolicum, P. tropicum) to warted (P. tropicoides) or reticulate (P. argentinense, P. euglaucum). Eight species produced sclerotia and these structures remained sterile after prolonged incubation up to 6 mo on OA, MEA and CYA. The production of sclerotia was species specific and most prominently present in freshly isolated strains. With exception of P. gallaicum, all sclerotium producing species belong to the P. westlingii-clade (P. atrofulvum, P. aurantiacobrunneum, P. cairnsense, P. manginii, P. miczynskii, P. pasqualense, P. quebecense). Some of the sclerotia of the latter six species were flecked, caused by short segments of pigmented external hyphae (Christensen et al. 1999). Penicillium atrofulvum produces black sclerotia, and all others were in shades of orange-brown. The Ehrlich reaction was of poor added value for differentiating among species of section Citrina. With exception of P. aurantiacobrunneum, all strains were negative in their Ehrlich reaction.
Micro-morphology
The micro-morphology was similar for most species and the majority has symmetrically branched biverticillate condiophores. Some species have additional branches and in some species these branches have the same branching pattern as the main axis (“double symmetrically biverticillate”, e.g. in P. pasqualense). Penicillium roseopurpureum, P. sanguifluum and P. galliacum are exceptions in section Citrina and these species do not produce symmetrically branched conidiophores. They are predominantly monoverticillate, however, examination of older parts of the culture showed presence of divergent lower branch-like metulae or symmetrically biverticillate structures. The majority of the members of section Citrina have smooth walled stipes; however, there are exceptions, e.g. P. paxilli and certain isolates of P. manginii and P. atrofulvum. Conidia generally measure 2.0–3.0 μm and vary from smooth to rough-walled and from globose to ellipsoidal.
Temperature-growth curves
One of the main characters for identification of species in section Citrina is the optimum and maximum growth temperature on CYA. Temperature-growth curves were made, if possible, for at least four strains of each species. An overview of typical growth profiles is shown in Figs 5, 6, 7, 8, 9 and Table 4. The result of this analysis shows that optimum and maximum growth temperature is a species-specific character and an important feature for identification of members of section Citrina. Often phylogenetically related species also have similar optimum and maximum growth temperatures. Members of the P. westlingii-clade generally have maximum growth temperatures at or below 30 °C and an optimum between 21 and 24 °C. The exceptions in this clade are P. pasqualense, P. quebecense and P. decaturense. These species grow well at 30 °C (5–15 mm), and some strains can even grow at 33 °C. Members of the P. citrinum-clade, in contrast, have higher optimum and maximum growth temperatures. With exception of P. tropicoides, all species were able to grow at 33 °C. Furthermore, all examined P. citrinum strains consistently grew at 37 °C. Some strains of P. sizovae (five of seven) and P. hetheringtonii (one of four) were able to grow at this temperature, though more restrictedly than P. citrinum. Not only members of the P. citrinum-clade were able to grow at 37 °C. This feature is shared by P. shearii, P. gallaicum and P. euglaucum and related species.
Fig. 7.
Overview of growth rates on CYA (reverse) after 7 d at various temperatures. Row, left to right: 21, 24, 27, 30, 33, 36 °C; columns, top to bottom: P. roseopurpureum, P. tropicoides, P. cairnsense, P. pasqualense, P. decaturense, P. sanguifluum, P. quebecense, P. terrigenum, P. copticola.
Fig. 8.
Overview of growth rates on CYA (reverse) after 7 d at various temperatures. Row, left to right: 21, 24, 27, 30, 33, 36 °C; columns, top to bottom: P. paxilli, P. tropicum, P. sumatrense, P. gorlenkoanum, P. steckii, P. sizovae, P. argentinense, P. euglaucum, P. anatolicum.
Fig. 9.
Overview of growth rates on CYA (reverse) after 7 d at various temperatures. Row, left to right: 21, 24, 27, 30, 33, 36 °C; columns, top to bottom: P. gallaicum, P. hetheringtonii, P. citrinum, P. shearii.
Table 4.
Overview of main characters for identification of species belonging to section Citrina.
| Penicillium sp. | Colony diameter (mm) |
Cleistothecia / sclerotia | Maximum growth temperature (colony diameter, mm)* | Shape, ornamentation and size conidia | Typical feature(s) | Similar species | |
|---|---|---|---|---|---|---|---|
| CYA | MEA | ||||||
| P. anatolicum | 21–30 | 15–21 | Cleistothecia | 33 °C (15–25; 1/4) | Globose to subglobose, finely roughened, 2.0–2.5 μm | Yellow soluble pigments | P. argentinense, P. euglaucum, P. gallaicum |
| 36 °C (0–15; 3/4) | |||||||
| P. argentinense | 21–27 | 20–25 | Cleistothecia | 36 °C (mc–10) | Globose, smooth, 2.0–2.5 μm | Soluble pigment absent | P. anatolicum, P. euglaucum, P. gallaicum |
| P. atrofulvum | 30–40 | 28–38 | Sclerotia | 27 °C (13–21) | Ellipsoidal, smooth, 2.0–3.0 × 2.0–2.5 μm | Dark sclerotia | None |
| P. aurantiacobrunneum | 24–30 | 22–28 | Sclerotia | 27 °C (15–20; 2/4) | (Sub)globose, smooth, 2.0–3.0 μm | Ehrlich reaction positive | P. miczynskii, P. neomiczynskii |
| 30 °C (0–mc; 2/4) | |||||||
| P. cairnsense | 29–39 | 28–38 | Sclerotia | 30 °C (5–10; 1/4) | (Sub)globose to broadly ellipsoidal, smooth, 2.0–3.0 × 1.8–2.5 μm | Red or blackish reverse on YES and/or DG18 | P. quebecense |
| 33 °C (0–5; 3/4) | |||||||
| P. christenseniae | 31–37 | 21–28 | Absent | 27 °C (15–22) | Globose to subglobose, finely roughened, 2.0–3.0 μm | Short stipes, moderate growth on CREA | P. cosmopolitanum, P. pancosmium, P. ubiquetum, P. westlingii |
| P. chrzaszczii | 25–33 | 21–28 | Absent | 27 °C (15–25) | (Sub)globose, finely roughened, 2.0–3.0 μm | No sporulation of CYA, yellow soluble pigments on CYA, reverse on DG18 in shades of yellow | P. cosmopolitanum, P. waksmanii, P. westlingii |
| P. citrinum | 27–33 | 18–25 | Absent | 36 °C (8–17) | (Sub)globose, smooth, 1.8–2.5 μm | Growth at 37 °C, yellow reverse on CYA, soluble pigment on CYA and YES | P. gorlenkoanum, P. hetheringtonii |
| P. copticola | 31–37 | 25–34 | Absent | 33 °C (5–10) | Broadly ellipsoidal, smooth, 2.5–3.0 × 2.0–2.5 μm | Good growth on CREA | P. christenseniae, P. steckii, P. terrigenum, |
| P. cosmopolitanum | 25–32 | 20–29 | Absent | 27 °C ((8–) 18–28) | Globose, roughened, 2.5–3.0 μm | No or weak sporulation on CYA and YES; reverse CYA beige-brown with orange coloured sulcations | P. chrzaszczii, P. pancosmium, P. ubiquetum, P. westlingii |
| P. decaturense | 32–40 | 27–34 | Absent | 30 °C (5–15; 3/5) | (Sub)globose, finely roughened, 2.0–2.5 μm | Colony diameters on CYA30°C 5–15 mm | P. cosmopolitanum, P. pancosmium, P. ubiquetum, P. westlingii |
| 33 °C (0–10; 2/5) | |||||||
| P. euglaucum | 23–29 | 22–26 | Cleistothecia | 36 °C (5–15) | Globose, finely roughened, 2.0–2.5 μm | Ascospores 3.0–4.0 × 2.5–3.0 μm | P. anatolicum, P. argentinense, P. gallaicum |
| P. gallaicum | 19–25 | 24–30 | Sclerotia | 36 °C (3–10) | (Sub)globose, smooth, 2.0–2.5 μm | Monoverticillate conidiophores | P. anatolicum, P. argentinense, P. euglaucum |
| P. godlewskii | 15–25 | 12–20 | Absent | 27 °C (mc–10) | (Sub)globose, finely roughened, 2.0–2.5 μm | No growth at 30 °C and small colonies at 27 °C | None |
| P. gorlenkoanum | 26–31 | 20–27 | Absent | 33 °C (6–12; 1/3) | (Sub)globose, finely roughened, 2.0–2.5 (–3.0) μm | Crème-brown reverse on CYA | P. citrinum, P. hetheringtonii |
| 36 °C (0–mc; 2/3) | |||||||
| P. hetheringtonii | 26–32 | 17–23 | Absent | 36 °C (7–14) | (Sub)globose, smooth to finely roughened, 2.0–2.5 μm | Growth at 36 °C | P. citrinum, P. gorlenkoanum |
| P. manginii | 28–40 | 25–37 | Sclerotia | 27 °C (20–35; 5/8) | (Broadly) ellipsoidal, smooth, 2.5–3.0 × 2.0–2.5 μm | Yellow mycelium on CYA15°C, fast growth rate on YES with red soluble pigments | P. miczynskii |
| 30 °C (0–10; 3/8) | |||||||
| P. miczynskii | 21–27 | 17–25 | Sclerotia | 27 °C (12–25) | Subglobose to broadly ellipsoidal, smooth, 2.0–3.0 × 2.0–2.5 μm | Soluble pigments, if produced, yellow | P. aurantiacobrunneum, P. manginii, P. neomiczynskii |
| P. neomiczynskii | 21–27 | 12–18 | Absent | 27 °C (8–15) | Subglobose-broadly ellipsoidal, smooth, 2.0–3.0 × 2.0–2.5 μm | Reverse on CYA yellowish brown, soluble pigments yellow-brown | P. aurantiacobrunneum, P. miczynskii |
| P. nothofagi | 5–10 | 4–8 | Absent | 24 °C (10–15) | Globose to subglobose, finely roughened, 2.5–3.5 μm | Restricted growth on CYA, MEA and YES | P. wellingtonense |
| P. pancosmium | (23–)28–35 | (20–)25–31 | Absent | 27 °C (15–25; 3/5) | Globose to subglobose, finely roughened, 2.0–3.0 μm | Reverse on YES yellow-orange or orange, dull-green or grey-green conidia on CYA | P. ubiquetum |
| 30 °C (0–mc; 2/5) | |||||||
| P. pasqualense | 25–35 | (15–)25–30 | Sclerotia | 30 °C (6–15; 2/4) | (Sub)globose, spinose, 2.5–3.5 μm | Dark brown reverse on CYA, conidia (dark) blue green, spinose | None |
| 33 °C (0–mc, 2/4) | |||||||
| P. paxilli | 30–37 | 28–35 | Absent | 33 °C (mc–15) | Subglobose-broadly ellipsoidal, smooth or nearly so, 2.0–3.0 μm | Rough walled stipes, predominantly biverticillate with appressed terminal whorl of 4-8 metulae | P. raphiae |
| P. quebecense | 38–42 | 30–35 | Sclerotia | 33 °C (3–10) | Subglobose, smooth, 2.0–3.0 μm | Dark red reverse on YES | P. cairnsense |
| P. raphiae | 32–36 | 21–25 | Absent | 27 °C (15–22) | Broadly ellipsoidal, smooth or finely roughened, 2.0–2.5 × 1.8–2.5 μm | Symmetrically biverticillate conidiophores, broadly ellipsoidal conidia | P. paxilli |
| P. roseopurpureum | 7–16 | 9–19 | Absent | 30 °C (mc–15) | (Sub)globose, smooth to finely roughened, 1.8–2.5 μm | Monoverticillate conidiophores, reverse on CYA in shades of red with red-brown diffusible pigments | P. sanguifluum |
| P. sanguifluum | (15–)18–26 | 17–26 | Absent | 33 °C (mc–10) | Globose to subglobose, smooth to finely roughened, 2.0–2.5 μm | Monoverticillate conidiophores, reverse on CYA in shades of red with red-brown diffusible pigments | P. roseopurpureum |
| P. shearii | 28–40 | 26–37 | Cleistothecia | 36 °C (8–20) | Subglobose-broadly ellipsoidal, smooth, 2.5–3.0 × 1.8–2.5 μm | Abundant production of dark grey coloured cleistothecia, growth at 37 °C | P. tropicum, P. tropicoides |
| P. sizovae | 28–39 | 27–35 | Absent | 36 °C (4–8) | (Sub)globose, finely roughened, 2.0–2.5 μm | Fast growth rate on MEA and YES, pale reverse on CYA, growth at 36 °C | P. steckii |
| P. steckii | 24–32 | 21–30 | Absent | 33 °C (mc–12 (–24); 5/6) | Broadly ellipsoidal towards fusiform, smooth, 2.3–3.0 × 2.0–2.5 μm. | Weak to moderate growth on CREA | P. christenseniae, P. copticola, P. sizovae, P. sumatrense, P. terrigenum |
| 36°C (0–12; 1/6) | |||||||
| P. sumatrense | 33–42 | 27–36 | Absent | 30 °C (10–30; 2/9) | Subglobose-broadly ellipsoidal, finely roughened, 2.0–2.5 μm | Good growth on YES with yellow reverse | P. steckii |
| 33°C ((0–) 5–20; 7/9) | |||||||
| P. terrigenum | 28–36 | 25–32 | Absent | 33 °C (10–15) | Broadly ellipsoidal, smooth, 2.0–3.0 × 2.0–2.5 μm | Poor growth on CREA | P. copticola, P. steckii |
| P. tropicoides | 24–30 | 18–23 | Cleistothecia | 30 °C (10–16) | Broadly ellipsoidal, smooth, 2.0–3.0 × 1.8–2.5 μm | Abundant production of drab-grey cleitothecia, no growth at 33 °C | P. shearii, P. tropicum |
| P. tropicum | 24–31 | 23–27 | Cleistothecia | 33 °C (8–18) | Broadly ellipsoidal, smooth, 2.0–3.0 × 2.0–2.5 μm | Abundant production of brownish-grey cleitothecia, growth at 33 °C | P. shearii, P. tropicoides |
| P. ubiquetum | 24–34 | 18–26 | Absent | 27 °C (15–25) | (Sub)globose, finely roughened, 1.8–2.5 μm | Reverse on YES orange to pinkish-red, conidia dull green or dark green on CYA | P. pancosmium |
| P. vancouverense | 20–30 | 16–23 | Absent | 27 °C (mc–15) | Subglobose, finely roughened, 2.0–3.0 μm | Light yellow mycelium (most pronouncedly on YES), colonies restricted | P. manginii P. miczynskii |
| P. waksmanii | (20–)25–32 | 18–24(–30) | Absent | 27 °C (10–25) | (Sub)globose, finely roughened, 2.0–3.0 μm | Beige-brown reverse on CYA | P. chrzaszczii, P. godlewskii |
| P. wellingtonense | 10–15 | 8–13 | Absent | 24 °C (15–20) | Subglobose to broadly ellipsoidal, smooth to finely roughened 2.5–3.5 μm | Slow growth on MEA and CYA, reverse on CYA in shade of orange | P. nothofagi |
| P. westlingii | (25–)30–36 | 25–34 | Absent | 27 °C ((8–) 15–27; 11/14) 30 °C (0–mc; 3/14) |
Globose, roughened, 1.8–2.5 μm | No or weak sporulation on CYA and YES; reverse CYA pale, pale-beige or pinkish-beige | P. chrzaszczii, P. cosmopolitanum, P. pancosmium, P. ubiquetum |
mc = micro colonies, 1–2 mm in diam.
The maximum growth temperature is determined at intervals of 3 °C (see material & methods). The highest temperature with visible growth is listed and the colony diameter is mentioned between brackets. If the maximum growth varied within a species, then both temperatures are listed together with the number of isolates showing growth at that temperature.
Extrolites
Extrolite analysis showed that all species have a unique profile of metabolites. An overview of extrolites produced by all section Citrina species is given in Table 5. The extrolite profiles of each species are included in the species descriptions (see Taxonomy). Citrinin was most frequently detected and 41 % of the Citrina species were able to produce this extrolite. These citrinin producing strains were not present in a certain clade within section Citrina. In contrast, the tentatively named extrolite “MIF” (26 %) was only produced by species belonging to the P. westlingii-clade, and citreoviridin (23 %) and terrein (26 %) were almost exclusively produced by this clade. These extrolites could have been present in a common ancestor for all the species in the P. westlingii-clade. In general, the extrolite profiles were congruent with phenotype and phylogeny. Exceptions are in e.g. P. manginii, P. vancouverense, P. waksmanii, where strains could be divided in different subgroups based on extrolite profiles. More detailed chemical investigations are needed and these species might actually represent species complexes.
Table 5.
Extrolites produced by species assigned to Penicillium section Citrina.
| Species | Extrolites produced |
|---|---|
| P. anatolicum | Anthraquinones, bisanthrons, curvularin, dehydrocurvularin, sorbicillins, “POTO”, “3-T” |
| P. argentinense | Curvularin, dehydrocurvularin, “AURANMUF”, “OXIM” |
| P. atrofulvum | “ALK”, “GULLA”, “SOLIS”, “3T” |
| P. aurantiacobrunneum | Benzomalvins, citreoviridin, terrein, “OTOT” |
| P. cairnsense | CBS 126226, CBS 117982, CBS 118028 and CBS 117962: benzomalvins, citreoviridin, phoenicin, decaturin; CBS 124325, CBS 126225 and DTO 87B9: citreoviridin, terrein and/or quinolactacin; other extrolites: “KUM”, “MIF”, “MIM”, “RAI”, “SENGA” |
| P. christenseniae | Citrinin, quinolactacin, “FON”, “KUM”, “MIF”, “RYLA” |
| P. chrzaszczii | Citrinin, terrein, “MIF”, “MIM”, “RAI”, “3T”, “VERN” |
| P. citrinum | Citrinin, quinolactacins, citrinadins, perinadine, several anthraquinones, “CITY”, “met k”, “shamix” |
| P. copticola | “GULLA”, “HAEN”, “PRS”, “VERSI” |
| P. cosmopolitanum | Citrinin, okaramin, perinadine, territrems, “CURVO”, “HAEN”, “PHOE”, “ROTO”, “SENGA”, “TRIP”, “VERSI”, “XANTHOC” |
| P. decaturense | Daldinin D, decaturin A, deoxyoxalicine B, terrein, “SENGA”, “SNIL”, “SVOL”, “VERSI”, “XANTHOC” |
| P. euglaucum | Terrein, “ALK”, “FRIL”, “GLAD”, “RAI”, “SPOKO”, “3-T” |
| P. gallaicum | Citreoviridin, “KOKSO”, “3-S”, “TIDL”, “VYL” |
| P. godlewskii | Citrinin, citreoviridin, decaturin, okaramin, perinadine, “TRIP” |
| P. gorlenkoanum | Citrinin, costaclavin, chanoclavine-I, “KUSK”, “PHOE”, “WK”, “WS”, “WT” and “WØ” |
| P. hetheringtonii | Citrinadine, citrinin, quinolactacin, anthraquinones, “SHAMIX”, “FON”, “CITY”, “PR1-x” |
| P. manginii | Citrinin, citreomontanin, citreoviridin A, citreoviridinol A1 and A2, epicitreoviridinol, phoenicin, “MIF”, “MIM” |
| P. miczynskii | Citreoviridin, cyclopiazonic acid, quinolactacin, terrein, “met OE”, “MIF”, “TERRIT”, “XANTHOC” |
| P. neomiczynskii | Citreoviridin, terrein, “MIF”, “OFSO” |
| P. nothofagi | Citrinin, “CURVU”, “SENTRIP”, “SKAEM” |
| P. pancosmium | Citrinin, daldinin D, decaturin, terrein, “MELI”, “ORAN”, “SENGA”, “XANTHOC” |
| P. pasqualense | Pyrenocines, indol alkaloids, “PAS” |
| P. paxilli | Paxillin, dehydroxypaxillin, 1’-O-acetylpaxillin, meleagrin, “PU”, “PUX”, “TOTO” |
| P. quebecense | Citreoviridin, phoenicin, terrein, “SENOE” (verrucofortine-type molecule), “MIF”, “MIM”, “SENGA”, “alk-770” |
| P. raphiae | CBS 126234T: citrinin, “FON”, “MIF”,“KUM”, “LOST”, “PHOE”, and “TRIP”; CBS 126235: citrinin, quinolactacin, “FON”, “MIF”, “KUM”, “MIM”, “REJS”, “SENGA”, and “XANTHOC” |
| P. roseopurpureum | Bisanthrons, roseopurpurin, sorbicillins, “AQ”, “SEL” |
| P. sanguifluum | Bisanthrons, roseopurpurin, β-hydroxycurvularin, dehydrocurvularin, curvularin, “FOSI”, “FYKS”, “SNIT”, “TIDL”, “VERN” |
| P. shearii | Paxillin, paspalinine, shearinin A & B, “XX” and several indole alkaloids |
| P. sizovae | Quinolactacin, tanzawaic acid E, verrucolone, “AFSI”, “CHAE and “PNUF” |
| P. steckii | Isochromantoxins, quinolactacin, tanzawaic acid E, “ALTI”, “EXPO”, “FON”, “FOS”, “GLOO”, “GYF”, “PHOE”, “RAI”, “STOK”, “SVUL”, “VERN” |
| P. sumatrense | Curvularin, dehydrocurvularin, “POTO”, “SAAT”, “TERRIT”, “TIDL”, “VOX” |
| P. terrigenum | “HAEN”, “ISOC”, “PRS”, “VERSI” |
| P. tropicoides | Isochromantoxins, several apolar indol-alkaloids, “CITY”, “HOLOX”, “PR1-x”, “RAIMO” |
| P. tropicum | Several apolar indol-alkaloids, “CITY”,“EMON”, “HOLOX” and “RAIMO |
| P. ubiquetum | Citrinin, terrein, “ALK”, “GLYF”, “RAI”, “TRIP”, “XANTHOC”; CBS 126438, CBS 126436: anthraquinone bisanthorns, citrinin, okaramins, and “SENGA” |
| P. vancouverense | Citrinin, citreoviridin, “MIF”, “PAS”, “met OE” |
| P. waksmanii | Citrinin, cyclopiamin, meleagrin (only produced by one isolate), “GLYF”, “PAS”, “SENGA”. |
| P. wellingtonense | Citrinin, decaturin, “MIF”, “met Q”, “POF”, “RAI”, “TRIP”, “XANTHOC” |
| P. westlingii | Citrinin, curvularin, dehydrocurvularin,“PHOE”, “TRIP”, “XANTHOC” |
DISCUSSION
The species in section Citrina are very common in soil, but are also found in foods, indoor air and many other substrates. The description of 17 new species may help determining more accurately the mycobiota of soils, which may be important for biodiversity, ecological and climate change studies. Even though the species treated here are both phylogenetically and ecologically related, section Citrina was treated very differently in previous taxonomic studies (Raper & Thom 1949, Pitt 1980, Ramírez 1982). The inclusion of physiological, chemical and nucleotide sequence based data has changed the perception of series and sections in filamentous fungi and these taxonomic groupings are now both phylogenetically and ecologically consistent. Disregarding the many different species concepts proposed, a polyphasic approach to taxonomy has proven to give clear results that are predictive (e.g. Frisvad & Samson 2004). The species in section Citrina grow optimally at 23–26 °C, can grow at low water activities, in substrates containing NaCl, and often produce citrinin, citreoviridin, anthraquinones, indol-alkaloids, paxillin, and/or isochromantoxins. On the other hand, no species in section Citrina produce asperentins, atpenins, austins, brevianamides, chaetoglobosins, chrysogines, communesins, compactins, curvulic acids, cycloaspeptides, expansolides, fumitremorgins, fumagillins, gliotoxins, griseofulvins, kojic acids, mycophenolic acids, ochratoxins, paraherquamides, patulins, penicillic acids, penicillins, penigequinolones, penitrems, psychrophilins, pyripyropens, terrestric acids, tryptoquialanins, tryptoquivalins, viridicatumtoxins, verruculones, viridicatins, xanthocillins, xanthoepocins, xanthomegnins, and several other extrolites, often found in Penicillium subgenus Penicillium or section Lanata-divaricata (as series Simplicissima) (Frisvad & Filtenborg 1990, Frisvad & Samson 2004). Despite this, a large number of extrolites could not be identified (Table 5) and may prove to be new interesting drug leads.
TAXONOMY
Species delimitation
In this study, we applied a polyphasic approach for species recognition. Phenotypic and physiological characters combined with extrolite profiles and DNA sequences were used for species delimitation. New species were introduced when the results of these approaches were congruent. In some cases, these approaches were incongruent. In general, the phylogenetic analysis based on partial β-tubulin and calmodulin sequences generated more taxonomic units (clades) than the analyses based on phenotypic and physiological characters. If no distinct differences in phenotype and/or extrolite patterns were detected between those closely related clades, then we decided to keep them as one species, until more evidence becomes available to warrant describing them as species. More details on these decisions are given in the “taxonomy and phylogeny” part in the species descriptions.
Identification
As mentioned above, current species delimitation is based on a combination of characters. An overview of useful phenotypic and physiological characters for identification is given in Table 4. Although there are differences in phenotype and physiology among these species, identification based on these features remains difficult for non-specialists. Molecular based identification (sequencing) is nowadays common practice. Currently, ITS is the accepted barcode (C. Schoch et al., unpubl. data); however, this locus is inadequate for species recognition in section Citrina. 55 % of the species could be unambiguously identified using ITS sequences. Especially in the P. westlingii-clade, many species share the same ITS sequence. Partial calmodulin and β-tubulin sequences had sufficient discriminatory power to differentiate all species of section Citrina. It is therefore recommended to sequence either gene for correct species identification.
List of accepted species and their synonyms
Our polyphasic taxonomic approach revealed that Penicillium section Citrina includes 39 species including 17 new species. An overview of species belonging to section Citrina is presented in Table 4. Species belonging section Citrina and their synonyms are listed in Table 6 and the current classification is compared with those of Pitt (1980), Ramírez (1982) and Pitt et al. (2000).
Table 6.
Taxonomic disposition of members of section Citrina in different studies of Penicillium.
| Original species name | Pitt (1980) | Ramirez (1982) | Pitt et al. (2000) | Current study |
|---|---|---|---|---|
| Citromyces cesiae | P. roseopurpureum | P. cyaneum, p.655 | P. roseopurpureum | P. roseopurpureum |
| Citromyces sanguifluus | P. roseopurpureum | P. roseopurpureum | P. roseopurpureum | P. sanguifluum |
| Citromyces subtilis | P. citrinum | P. sartoryi | P. citrinum | P. citrinum |
| E. anatolicum | E. anatolicum | Not treated | E. anatolicum | P. anatolicum |
| E. euglaucum | Not treated | Not treated | Not treated | P. euglaucum |
| E. shearii | E. shearii | Not treated | E. shearii | P. shearii |
| E. tropicum | Not treated | Not treated | Not treated | P. tropicum |
| P. alicantinum | Not treated | P. alicantinum | P. citreonigrum | P. gallaicum |
| P. aurifluum | P. citrinum | P. citrinum | P. citrinum | P. citrinum |
| P. baradicum | P. citrinum | P. baradicum | P. citrinum | P. sumatrense |
| P. botryosum | P. citrinum | P. botryosum | P. citrinum | P. citrinum |
| P. carminoviolaceum | P. roseopurpureum | P. roseopurpureum | P. roseopurpureum | P. roseopurpureum |
| P. chrzaszczii | P. miczynskii | P. jensenii | P. miczynskii | P. chrzaszczii |
| P. citrinum | P. citrinum | P. citrinum | P. citrinum | P. citrinum |
| P. corylophiloides nom. inval. | P. jensenii | P. corylophilum | P. jensenii | P. steckii |
| P. damascenum | P. melinii | P. damascenum | P. melinii | P. gorlenkoanum |
| P. decaturense | Not treated | Not treated | Not treated | P. decaturense |
| P. gallaicum | Not treated | P. gallaicum | P. citreonigrum | P. gallaicum |
| P. godlewskii | P. jensenii | P. godlewskii | P. jensenii | P. godlewskii |
| P. gorlenkoanum | P. citrinum | P. gorlenkoanum | P. citrinum | P. gorlenkoanum |
| P. hetheringtonii | Not treated | Not treated | Not treated | P. hetheringtonii |
| P. implicatum | P. implicatum | P. implicatum | P. implicatum | P. citrinum |
| P. kapuscinskii | P. canescens | P. kapuscinskii | P. canescens | P. godlewskii |
| P. lacussarmientei | Not treated | Not treated | P. roseopurpureum | P. sanguifluum |
| P. manginii | P. miczynskii | P. miczynskii | P. manginii | P. manginii |
| P. meleagrinum var. viridiflavum | P. janthinellum | P. janthinellum | P. janthinellum | P. sumatrense |
| P. miczynskii | P. miczynskii | P. miczynskii | P. miczynskii | P. miczynskii |
| P. paxilli | P. paxilli | P. paxilli | P. paxilli | P. paxilli |
| P. pedemontanum | P. miczynskii | P. pedemontanum | P. pedemontanum | P. manginii |
| P. phaeojanthinellum | P. fellutanum | P. fellutanum | P. fellutanum | P. citrinum |
| P. rivolii | P. jensenii | P. janthinellum | P. jensenii | P. waksmanii |
| P. roseopurpureum | P. roseopurpureum | P. roseopurpureum | P. roseopurpureum | P. roseopurpureum |
| P. sartoryi | P. citrinum | P. sartoryi | P. citrinum | P. citrinum |
| P. sizovae | P. fellutanum | P. sizovae | P. sizovae | P. sizovae |
| P. steckii | P. citrinum | P. steckii | P. steckii | P. steckii |
| P. sumatrense | P. corylophilum | P. corylophilum | P. corylophilum | P. sumatrense |
| P. tropicoides | Not treated | Not treated | Not treated | P. tropicoides |
| P. turolense | Not treated | P. turolense | P. westlingii | P. chrzaszczii |
| P. vaccaeorum | Not treated | Not treated | P. roseopurpureum | P. sanguifluum |
| P. waksmanii | P. waksmanii | P. waksmanii | P. waksmanii | P. waksmanii |
| P. westlingii | P. waksmanii | P. waksmanii | P. westlingii | P. westlingii |
Species descriptions
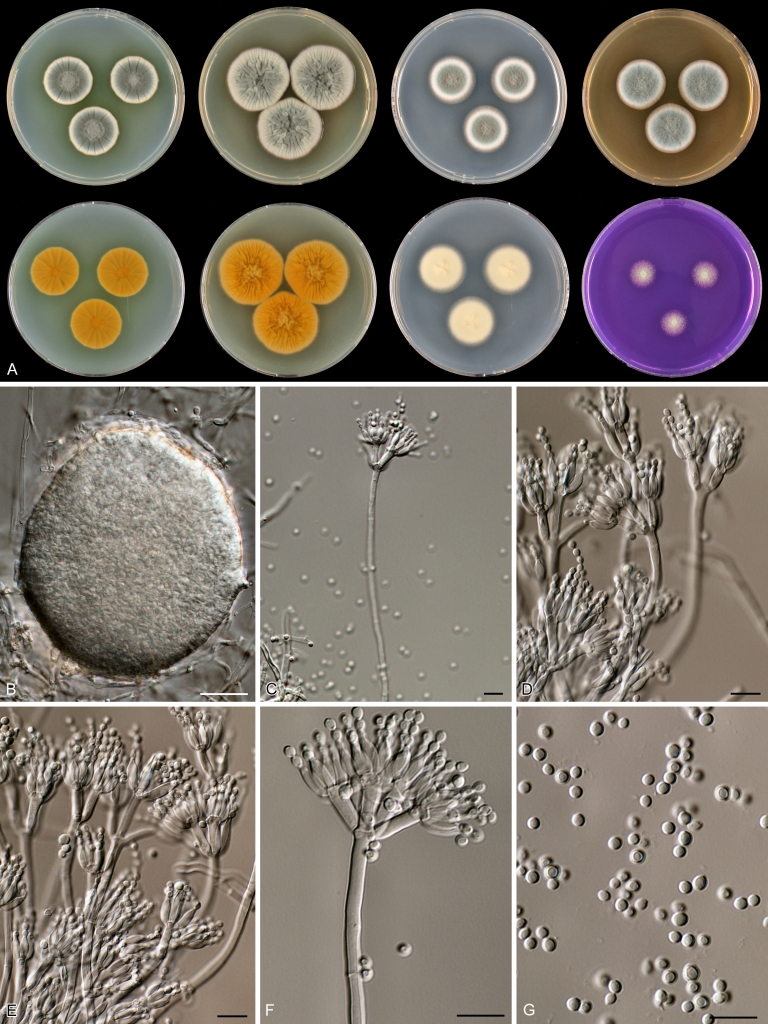
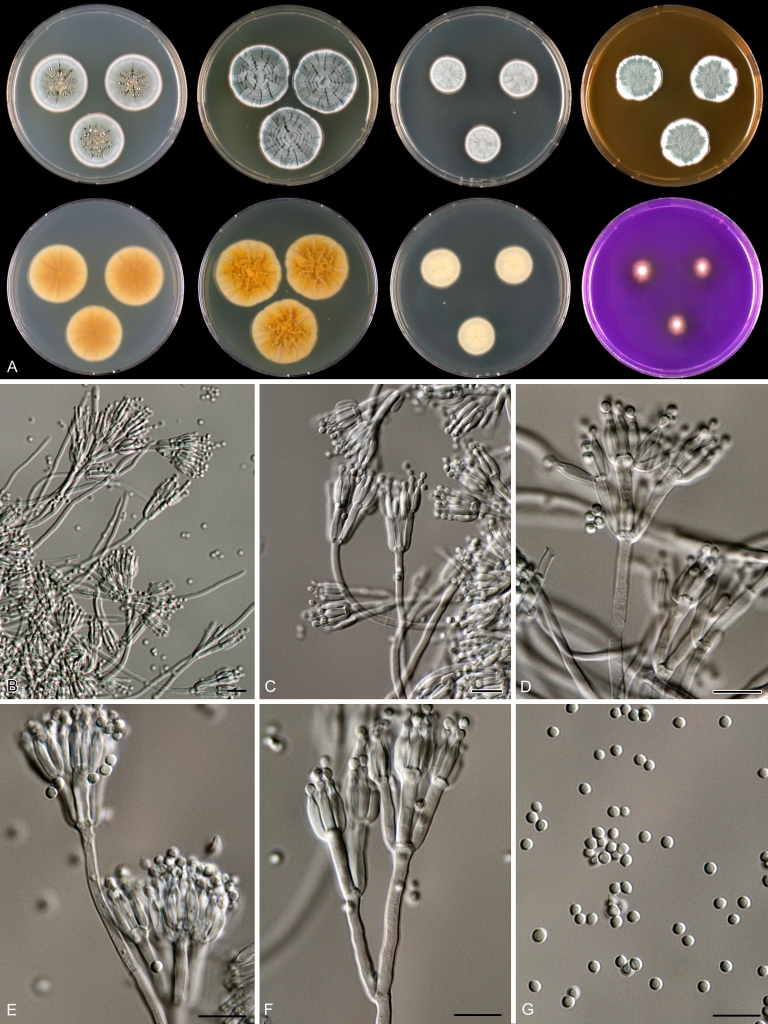
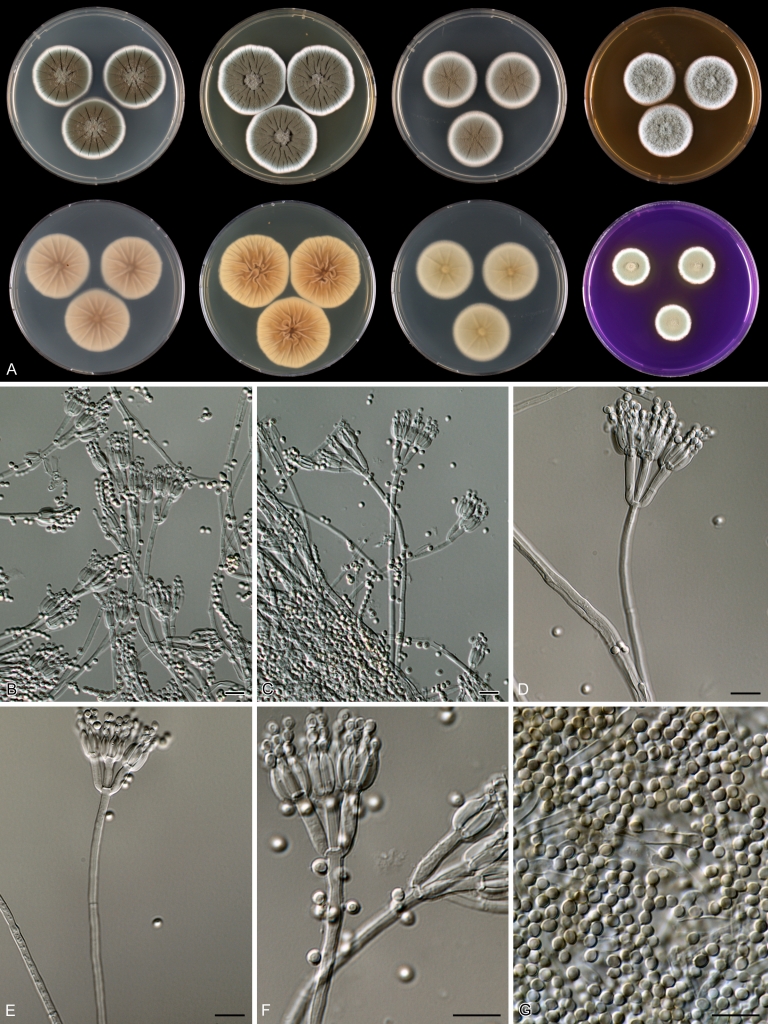
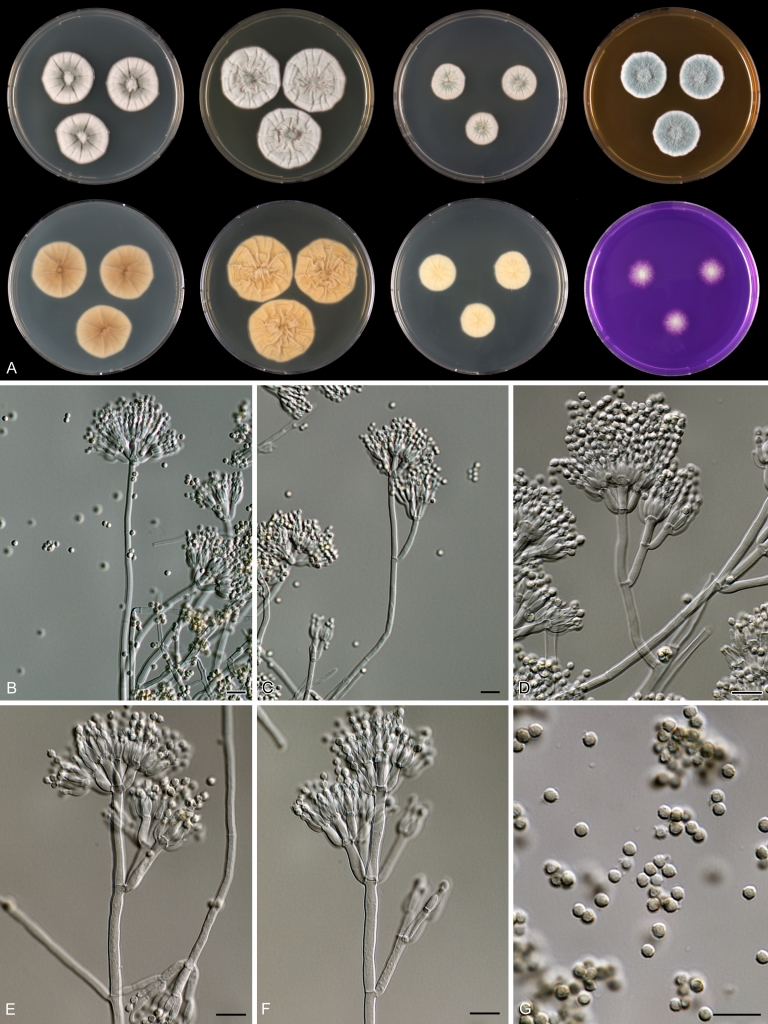
Penicillium anatolicum Stolk, Ant. van Leeuwenhoek 34: 46. 1968. Fig. 10.
Fig. 10.
Penicillium anatolicum. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–C. Ascomata. D–E. Ascospores. F–H. Conidiophores. I. Conidia. Scale bars = 10 μm.
= Eupenicillium anatolicum Stolk, Ant. van Leeuwenhoek 34: 46. 1968.
Typus: ex soil, Turkey (CBS 479.66, holotype; cultures ex-type CBS H-20647 = DTO 16B7 = IBT 16177 = IBT 30764).
Description: Colony diam, 7 d, in mm: CYA 18–30; CYA15°C 4–8; CYA30°C 23–32; CYA37°C 0–5; MEA 15–21; YES 23–30; DG18 20–28; ratio CYAS:CYA 0.85–1.0; creatine agar 11–18, weak growth, weak or no acid, and no base production.
Sporulation on CYA moderate, conidia grey green, cleistothecia abundantly produced in freshly isolated strains and covered under a felt of conidiophores, mycelium inconspicuous, clear exudate produced in small droplets, soluble pigment strong yellow, margin entire, reverse yellow-brown. Sporulation on YES moderate, conidia blue-green, mycelium pale-yellow, soluble pigments yellow, reverse (vivid) yellow. Sporulation on DG18 weak to moderate, conidia blue-green, mycelium white, reverse vivid yellow. Moderate sporulation on MEA, conidia dull-green with a blue element, colony texture slightly floccose, mycelium white. Ehrlich reaction negative.
Cleistothecia produced on most agar media, yellow-brown when young, becoming brown at age; globose or subglobose, up to 200 μm diam, occasionally larger, consisting of sclerotioid masses of polygonal cells, ripening after 4–5 wk or more. Ascospores ellipsoidal, with 2 distinct, appressed equatorial ridges, smooth to slightly roughened valves under light microscope, but showing warts and small ridges when viewed with SEM, 2.5–3.5 × 2.0–3.0 μm. Conidiophores predominantly biverticillate, stipes variable in length 20–200 μm, smooth walled, 2.0–3.0 μm wide. Metulae in verticils of 2–3 (–4), unequal in length, divaricate, slightly inflated at the apex, 10–20 × 2.0–4.0 μm. Phialides ampulliform, 6.0–8.0 × 2–3 μm. Conidia globose, finely roughened, 2.3–2.8 μm diam.
Extrolites: Anthraquinones, bisanthrons, curvularin, dehydro-curvularin, sorbicillins, “POTO”, “3-T”.
Diagnostic characters: Yellow soluble pigments (sorbicillins), metulae of unequal length, with inflated apex.
Similar species: Penicillium anatolicum is phylogenetically related to P. euglaucum and P. argentinense. The ascospores of P. euglaucum are larger than those of P. anatolicum and P. argentinense. In addition, P. argentinense does not produce yellow soluble pigments. Penicillium gallaicum also yellow soluble pigments (citreoviridins), but forms predominantly monoverticillate conidiophores and produces sclerotia instead of ascomata.
Distribution and ecology: Soil seems to be the primary habitat; isolated in Turkey, Florida, USA and South-Africa.
Barcode & molecular based ID: GenBank no. GU944598. This species can be identified with ITS, β-tubulin and calmodulin sequences.
Taxonomy and phylogeny: Stolk & Samson (1983) reduced E. anatolicum to synonymy with E. euglaucum, and P. citreonigrum was considered to be the anamorph. Peterson (2000) found that E. anatolicum is phylogenetically distinct from E. euglaucum and not closely related to P. citreonigrum. In contrast, our data show that P. anatolicum and P. euglaucum are closely related and both species are phylogenetically distinct from P. citreonigrum (Houbraken & Samson 2011). CBS 308.89 warrants further attention. This strain is phylogenetically related to P. anatolicum (Fig. 4), though without statistical support. This strain resembles P. anatolicum in many aspects, but differs in having a more restricted growth rate on DG18.
Penicillium argentinense Houbraken, Frisvad & Samson, sp. nov. MycoBank MB563185. Fig. 11.
Fig. 11.
Penicillium argentinense. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B. Ascomata. C–D. Ascospores. E–G. Conidiophores. H. Conidia. Scale bars = 10 μm.
Etymology: Named after Argentina, the location of the type culture.
Differt ab omnibus speciebus affinibus coloniis ad 37 °C haud crescentibus, reverso pallido vel psammocolorato coloniae in agaro CYA et YES, sine pigmentis solubilibus.
Typus: ex soil, Valdes Peninsula, Chubet, Argentinia, M.B. Pildain. (CBS H-20641 – holotypus, cultures ex-type CBS 130371 = DTO 16B7 = IBT 30761).
Description: Colony diam, 7 d, in mm: CYA 21–27; CYA15°C 3–7; CYA30°C 22–30; no growth on CYA37°C; MEA 20–25; YES 22–29; DG18 14–20; ratio CYAS:CYA 1.0–1.1; creatine agar 8–14, weak growth, weak acid and no base production.
Sporulation on CYA absent after 7 d and sporulation sparsely after prolonged incubation, cleistothecia sparsely produced and inconspicuous when young and becoming brownish grey in age, mycelium white, exudate clear, produced in small droplets, soluble pigments absent, margin entire, reverse pale or beige. Sporulation on YES absent, mycelium white, soluble pigments absent, reverse in shades of pale-beige, becoming brown in the centre after prolonged incubation. Sporulation on DG18 absent, mycelium white, reverse pale to pale-cream. Sporulation on MEA absent, remaining sterile after prolong incubation, mycelium white. Ehrlich reaction negative.
Cleistothecia only produced on CYA and oatmeal agar, globose or subglobose, up to 100–200 μm diam, consisting of sclerotioid masses of polygonal cells, slowly ripening in more than 6 wk. Ascospores ellipsoidal, with 2 inconspicuous equatorial ridges, roughened valves under light microscope, reticulate when viewed with SEM, 2.5–3.0 × 2.0–2.5 μm. Conidiophores monoverticillate or biverticillate, stipes variable in length 30–200 μm, smooth walled, thin, measuring 1.5–2.5 μm, ending with a slightly inflated apex, 2.0–4.0 μm. Metulae, when present, as additional branch, 10–20 × 1.5–3.0 μm wide. Phialides ampulliform, occasionally positioned subapically, 7.0–9.0 × 2–3 μm. Conidia globose, smooth, 2.0–2.5 μm diam.
Extrolites: Curvularin, dehydrocurvularin, “AURANMUF”, “OXIM”.
Diagnostic characters: No growth at 37 °C, pale or beige reverse on CYA and YES, soluble pigments absent.
Similar species: See P. anatolicum.
Distribution and ecology: This species has a worldwide distribution. It has been isolated from soil in Argentina and the Netherlands and Phaenocoma leaf bracts from South Africa.
Barcode & molecular based ID: JN831359. This species can be identified with ITS, β-tubulin and calmodulin sequences.
Taxonomy and phylogeny: None.
Penicillium atrofulvum Houbraken, Frisvad & Samson, sp. nov. MycoBank MB563183. Fig. 12.
Fig. 12.
Penicillium atrofulvum. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–C. Sclerotia. D–G. Conidiophores. H. Conidia. Scale bars = 10 μm.
Etymology: Named after the black coloured sclerotia produced by this species.
Differt ab omnibus speciebus affinibus formatione sclerotiorum atratorum, reverso atrocolorato coloniae in agaro diverso et conidiophoris symmetricis biverticillatis.
Typus: ex soil, Katanga near Kipushi, Zaire; No. 153, C. Lanneau (CBS H-20650 – holotypus, cultures ex-type CBS 109.66 = DTO 31B2 = FRR 799 = IBT 30032).
Description: Colony diam, 7 d, in mm: CYA 30–40; CYA15°C 15–25; CYA30°C and CYA37°C: no growth; MEA 28–38; YES 40–47; DG18 28–35; ratio CYAS:CYA 1.0–1.2; creatine agar 13–22, weak to moderate growth and no acid production.
Moderate to good sporulation on CYA, velvety, conidia dark-green or dull green, mycelium inconspicuous, exudate absent or sparsely produced as small clear droplets, soluble pigment absent, margin entire, reverse dark brown to dark green and almost black underneath the sclerotia. Good sporulation on YES, conidia dull green, mycelium inconspicuous, soluble pigments absent, reverse black with beige margins. Good sporulation on on DG18, conidia grey green, reverse pale with a black centre. Colonies on MEA grey or dull-grey green, colony texture floccose, mycelium white. Ehrlich reaction negative.
Sclerotia black, partly embedded in the agar, irregular in shape, up to 50–800 μm diam, often produced under a thick felt of conidiophores, rather soft, confluent and forming coriaceous masses, sometimes concentrated along radial lines, consisting of dark pigmented, polygonal, thick walled cells. Asci and ascospores are not observed. Conidiophores predominantly symmetrically biverticillate, stipes 300–500 μm long, smooth or finely rough walled, 2.5–3.5 μm wide; metulae in a compact terminal whorls of 3–5, equal in length, 10–14 × 2.5–3.5 μm; phialides ampulliform, 7–9 × 2.0–3.0 μm. Conidia ellipsoidal, smooth walled, variable in size, but not in shape, 2.0–3.0 × 2.0–2.5 μm.
Extrolites: “ALK”, “GULLA”, “SOLIS”, “3T”.
Diagnostic characters: The formation of dark sclerotia, black coloured reverse on various agar media, symmetrical biverticillate conidiophores.
Similar species: None; this species is unique because of the formation of dark coloured sclerotia.
Distribution and ecology: This species has a worldwide occurrence and is isolated from soil in the Netherlands, Zaire and Tunisia.
Barcode & molecular based ID: GenBank no. JN617663. This species has unique ITS, β-tubulin and calmodulin sequences.
Taxonomy and phylogeny: Penicillium atrofulvum phenotypically resembles P. novae-zeelandiae by the production of black coloured sclerotia and symmetrical biverticillate conidiophores. The lectotype of P. novae-zeelandiae (CBS 137.41T = NRRL 2128T) is related to P. canescens, P. jensenii and P. coralligerum in section Canescentia (Peterson & Horn 2009, Houbraken & Samson 2011). According to the original description of van Beyma (1940), this ex-type strain of P. novae-zeelandiae (CBS 137.41) produces black coloured sclerotia. However, this strain no longer shows this diagnostic feature. Phenotypically, P. novae-zeelandiae can be differentiated from P. atrofulvum by the formation of warted stipes and globose conidia. Furthermore, P. novae-zeelandiae produces patulin, an extrolite not formed by P. atrofulvum (Frisvad & Filtenborg 1990).
Penicillium aurantiacobrunneum Houbraken, Frisvad & Samson, sp. nov. MycoBank MB563206. Fig. 13.
Fig. 13.
Penicillium aurantiacobrunneum. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B. Sclerotia. C–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Etymology: Named after the orange-brown coloured sclerotia, produced by this species.
Differt ab omnibus speciebus affinibus divisione elementorum “Ehrlich” roseoviolacea, ratione CYAS:CYA 1.0–1.2, sclerotiis pallide aurantiacis.
Typus: ex air sample of cake factory, Give, Denmark, A. Svendsen (CBS H-20662 – holotype, cultures ex-type CBS 126228 = DTO 78G2 = IBT 18753).
Description: Colony diam, 7 d, in mm: CYA 24–30; CYA30°C germination–3; CYA37°C: no growth; MEA 22–28 mm; YES 31–35 mm; DG18 21–29; ratio CYAS:CYA 1.0–1.2; creatine agar 12–18 mm, weak growth and no acid production.
Good sporulation on CYA with velvety to floccose surface, conidia dull blue green, mycelium inconspicuous or pale-yellow, exudate absent or sparsely produced as small clear droplets, soluble pigments yellow, margin entire or slightly polygonal, reverse yellow-orange. Moderate to good sporulation on YES, conidia light green, soluble pigments yellow, reverse yellow-orange or yellow brown. Good sporulation on DG18, conidia dull-grey green, reverse pale or pale yellow. Good sporulation on MEA, conidia grey green or bluish grey green, colony texture velvety to floccose. Ehrlich reaction positive (pinkish-violet).
Sclerotia white when young, becoming pale orange to orange-brown, 150–250 μm, sparsely produced on oatmeal agar under a layer of conidiophores and large exudates droplets; hard, consisting of polygonal cells; no asci or ascospores observed. Conidiophores 200–400 μm long, predominant biverticillate, occasionally terverticillate, stipes smooth, 2.5–3.5 μm wide. Metulae in terminal whorls of 3–6 and mostly equal in length, 10–14 × 2.5–3.5 μm. Phialides ampulliform with short neck, 7–9 × 2.5–3.5 μm. Conidia subglobose, smooth, rather large variation in size within an isolate, 2.0–3.0 μm diam.
Extrolites: Benzomalvins, citreoviridin, terrein, “OTOT”.
Diagnostic characters: Ehrlich reaction pinkish-violet, ratio CYAS:CYA 1.0–1.2, pale orange sclerotia.
Similar species: See P. miczynskii. Penicillium aurantiacobrunneum morphologically resembles P. miczynskii and P. neomiczynskii, but can be differentiated by the pinkish-violet Ehrlich reaction of the former species.
Distribution and ecology: Worldwide distribution; from soil (New Zealand, Chile) and air (Denmark).
Barcode & molecular based ID: GenBank no. JN617670. Penicillium aurantiacobrunneum and P. miczynskii share the same ITS sequence. Partial β-tubulin and/or calmodulin sequences can be used to identify these species.
Taxonomy and phylogeny: None.
Penicillium cairnsense Houbraken, Frisvad & Samson, sp. nov. MycoBank MB563184. Fig. 14.
Fig. 14.
Penicillium cairnsense. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–C. Sclerotia. D–G. Conidiophores. H. Conidia. Scale bars = 10 μm.
Etymology: Named after Cairns (Australia), the city near the location where the type culture was collected.
Differt ab omnibus speciebus affinibus reverso rubro vel subnigro coloniae in agaro YES et/vel DG18, coloniis in agaro CYA 29–39 mm, constrictis, sed in agaro CYA30 continenter crescentibus, ratione CYAS:CYA 1.0–1.2, sclerotiis pallide aurantiacis vel aurantiaco-brunneis.
Typus: ex soil, Atherton Tableland, Australia, J. Houbraken (CBS H-20686 – holotype, cultures ex-type CBS 124325 = DTO 30E6 = IBT 29042).
Description: Colony diam, 7 d, in mm: CYA 29–39; CYA30°C 5–12; CYA37°C: no growth; MEA 28–38 mm; YES 40–50 mm; DG18 25–34; ratio CYAS:CYA 1.0–1.2; creatine agar 17–26 mm, weak growth and no acid production.
Good sporulation on CYA, velvety to slightly floccose, conidia dull green, mycelium light yellow, exudate produced in many minute droplets and clear to light yellow coloured, soluble pigments yellow, margin polygonal, reverse yellow-orange or orange, but also in shades of yellow brown, light brown or brown. Good sporulation on YES, conidia dull green, soluble pigments produced in most isolates and red, reverse brownish red or blackish red. Good sporulation on DG18, conidia dull-grey green, mycelium white, reverse (dark) red with red soluble pigments diffusing into the agar or pale yellow. Good sporulation on MEA, conidia dull-grey green, colony texture velvety. Ehrlich reaction negative.
Sclerotia white when young, becoming pale orange to orange-brown in age, 125–250 (–300) μm, produced on oatmeal agar in a velvety layer with small exudate droplets; consisting of polygonal cells and red-brown pigmented spots are present on the surface of the sclerotia; asci and ascospores not observed. Conidiophores predominantly biverticillate, but also a large portion terverticillate, additional branch both direct under terminal whorl and further down the stipe, 200–400 μm long, stipes smooth or occasionally finely roughened, 2.0–3.5 μm wide. Metulae in terminal whorls of 3–6 (–8) and often unequal in length, 9–13 (–15) × 2.5–3.5 μm. Phialides ampulliform, with short neck, 7–9 × 2–3 μm. Conidia smooth walled, subglobose to broadly ellipsoidal, 2.0–3.0 × 2.0–2.5 μm; a small portion of the conidia larger, globose, 3.0–3.5 μm diam.
Extrolites: The extrolite pattern of P. cairnsense is rather diverse. CBS 126226, CBS 117982, CBS 118028 and CBS 117962 produce the extrolites benzomalvins, citreoviridin, phoenicin and decaturin; CBS 124325, CBS 126225 and DTO 87B9 produce citreoviridin, terrein and/or quinolactacin. Other extrolites: “KUM”, “MIF”, “MIM”, “RAI”, “SENGA”.
Diagnostic characters: Red or blackish reverse on YES and/or DG18, colonies on CYA 29–39 mm, restricted, but consistent growth on CYA30, ratio CYAS:CYA 1.0–1.2, pale orange to orange-brown sclerotia.
Similar species: See P. miczynskii. Penicillium quebecense is morphologically similar, but has a CYAS:CYA ratio lower than 1.
Barcode & molecular based ID: GenBank no. JN617669 (CBS 124325T), JN617664 (CBS 117982). The strains CBS 124324, CBS 124326, CBS 124325T, CBS 126225 have identical and unique ITS sequences. The P. cairnsense strains isolated nuts of Carya cordiformis (bitternut), Niagara Falls, Ontario, Canada (CBS 117982 and CBS 117962) differ one base pair from CBS 124325T and share ITS sequences with the type cultures of P. quebecense and P. neomiczynskii.
Distribution and ecology: Worldwide; isolated from soil, ants (Camponotus sp.), decaying basidioma of Lactarius sp. and nut of Carya cordiformis (bitternut).
Taxonomy and phylogeny: Pitt (1980) mentioned in the description of P. miczynskii that some isolates of this species can produce red soluble pigment on MEA. These isolates were probably P. cairnsense, P. quebecense or P. manginii.
Penicillium christenseniae Houbraken, Frisvad & Samson, sp. nov. MycoBank MB563187. Fig. 15.
Fig. 15.
Penicillium christenseniae. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Etymology: Named after Martha Christensen who collected and isolated the type culture of this species.
Differt ab omnibus speciebus affinibus stipitibus brevibus et conidiophoris compactis, coloniis in agaro MEA velutinis, in agaro CREA modice crescentibus et haud crescentibus in agaro CYA ad 30 °C.
Typus: ex soil in native forest, east/north east side of Costa Rica, about 30 km inland from Limon and the Caribbean, M. Christensen (CBS H-20656 – holotypus, cultures ex-type CBS 126236 = DTO 76C3 = IBT 23355).
Description: Colony diam, 7 d, in mm: CYA 31–37; CYA15°C 20–26; CYA30°C and CYA37°C no growth; MEA 21–28; YES 33–38; DG18 21–26; ratio CYAS:CYA 1.0–1.2 creatine agar 16–22, moderate growth and no acid production.
Good sporulation on CYA, velvety, conidia dull green, mycelium white, exudate produced in clear droplets, soluble pigments absent, margin entire, reverse light brown with orange sulcations in centre. Good sporulation on YES, conidia dull green, mycelium inconspicuous, soluble pigments absent. Good sporulation on DG18, conidia dull or dull-grey green, reverse bright yellow or yellow-orange. Good sporulation on MEA, conidia dull-grey green, colony texture velvety, mycelium inconspicious. Ehrlich reaction negative.
Sclerotia absent. Conidiophores predominantly symmetrically biverticillate and occasionally with an additional branch; stipes relatively short, up to 250 μm, smooth walled, width, 2.0–3.0 μm; metulae in a compact terminal whorls of 4–8 (–10), rather equal in length, vesiculate, 10–15 × 2.0–3.0 μm; phialides ampulliform, 7–9 × 2.0–3.0 μm. Conidia globose to subglobose, finely roughened, 2.0–3.0 μm diam.
Extrolites: Citrinin, quinolactacin, “FON”, “KUM”, “MIF”, “RYLA”.
Diagnostic characters: This species is characterised by its short stipes (compared with other related species) and compact conidiophores, velvety colonies on MEA, moderate growth on CREA and no growth on CYA incubated at 30 °C.
Similar species: This species produces finely rough walled globose conidia and does not grow at 30 °C, which is also observed in species such as P. westlingii, P. waksmanii and P. godlewskii. However, the moderate growth on CREA, the velvety colonies on MEA and short stipes are characteristic for this species.
Distribution and ecology: Soil of a native forest and litter of Manilkara bidenta or Guarea guidonia; Costa Rica and Puerto Rico, USA.
Barcode & molecular based ID: GenBank no. JN617674. Penicillium christenseniae has unique β-tubulin, calmodulin and ITS sequences.
Taxonomy and phylogeny: This species is phylogenetically unique and belongs to the P. westlingii-clade. However, it does shares the production of quinolactacin and “FON” with P. steckii.
Penicillium chrzaszczii Zaleski, Bull. Int. Acad. pol. Sci. Lett., Sér. B.: 464. 1927. Fig. 16.
Fig. 16.
Penicillium chrzaszczii. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
= P. turolense Ramírez & Martínez, Mycopathol. 74: 36. 1981.
Typus: ex woodland soil, Puszcza Bialowieska Forest, Poland (CBS 217.28 – lectotype, designated here; cultures ex-type IBT 18226 = IBT 11222 = IBT 16409 = DTO 22E4 = FRR 903 = MUCL 29167 = NRRL 903 = NRRL 1741).
Description: Colony diam, 7 d, in mm: CYA 25–33; CYA15°C 16–22; CYA30°C and CYA37°C no growth; MEA 21–28; YES 28–36; DG18 20–27; ratio CYAS:CYA 0.95–1.1; creatine agar 15–20, weak growth and no acid production.
No or weak sporulation on CYA, velvety, conidia grey-green, mycelium inconspicuous, exudate absent or sparsely present as minute clear droplets, soluble pigments present in fresh isolates and weak yellow coloured, margin slightly polygonal, reverse (pale) yellow-orange. Sporulation on YES absent, mycelium white, soluble pigments absent, reverse vivid yellow or yellow-orange. No or poor sporulation on DG18, white mycelium, yellow soluble pigments produced in time, reverse pale or vivid yellow. Weak to moderate sporulation on MEA, conidia grey green when young and becoming dull green in age, colony texture floccose. Ehrlich reaction negative.
Sclerotia absent. Conidiophores symmetrically biverticillate and often with an divergent branch, often starting 40–60 μm below the terminal verticil; stipes rather long, up to 500 μm, smooth, 2.5–3.5 μm wide; metulae in a compact terminal verticil, 4–7 (–9), unequal in length, vesiculate, 10–14 × 2.5–3.5 μm; phialides ampulliform, 7–9 × 2–3 μm. Conidia globose to subglobose, finely roughened, 2.0–3.0 μm diam.
Extrolites: Citrinin, terrein, “MIF”, “MIM”, “RAI”, “3T”, “VERN” (also see Christensen et al. 1999).
Diagnostic characters: No or poor sporulation on CYA, finely roughened conidia, no growth at 30 °C, often with terverticillate structures, yellow soluble pigment production on CYA, reverse on DG18 in shades of yellow (pale or vivid).
Similar species: Phylogenetically, P. chrzaszczii is related to P. godlewskii and P. waksmanii. The reverse on CYA of P. waksmanii is in shades of beige-brown, while P. godlewskii and P. chrzaszczii have reverses in shades of yellow and/or orange. Penicillium godlewskii is more restricted in its growth on CYA (15–25 mm) than P. chrzaszczii (25–33 mm). Penicillium chrzaszczii can be distinguished from P. miczynskii and related species by the formation of globose, roughened conidia.
Distribution and ecology: This species is not commonly occurring and was previously isolated from soil in Poland and France.
Barcode & molecular based ID: GenBank no. GU944603. This species shares identical ITS sequences with P. decaturense, but can be identified based on partial β-tubulin and calmodulin sequences.
Taxonomy and phylogeny: Penicillium chrzaszczii was described by Zaleski (1927) in the subsection “concentrice-undulata”, which is characterised by concentric sulcated colonies. This feature was not observed on the agar media used in this study. Raper & Thom (1949) placed P. chrzaszczii in synonymy with P. jensenii and Pitt (1980) synonymised this species with P. miczynskii. Molecular data indicate that P. turolense (CBS 176.81) is a synonym of P. chrzaszczii. Our strain of P. turolense is degenerated and sporulates weakly on most agar media. The original description shows typical ornamented conidia and biverticillate conidiophores (Ramírez & Martinez 1981) and therefore this species can be confidentially placed in synonymy.
Penicillium citrinum Thom, Bull. U.S. Dep. Agric., Bur. Animal Indus. 118: 61. 1910. Fig. 17.
Fig. 17.
Penicillium citrinum. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
= Citromyces subtilis Bainier & Sartory, Saccardo's Syll. fung. XXV: 684. 1912.
= Penicillium subtile (Bainier & Sartory) Biourge, Cellule 33: 106. 1923. (nom. illegit.,Art. 64; non Berk. 1841.
= Penicillium aurifluum Biourge, Cellule 33: 250. 1923.
= Penicillium phaeojanthinellum Biourge, Cellule 33: 289. 1923.
= Penicillium implicatum Biourge, Cellule 33: 278. 1923.
= Penicillium sartoryi Thom [as ‘sartorii’], The Penicillia: 233. 1930.
= Penicillium botryosum Bat. & H. Maia, Anais Soc. Biol. Pernambuco 15: 157. 1957.
Typus: unrecorded source (IMI 92196ii, type of both P. citrinum and P. aurifluum; cultures ex-type DTO 22F3 = CBS 139.45 = Biourge 53 = Thom 4733.14 = ATCC 1109 = ATCC 36382 = CECT 2269 = FRR 1841 = IMI 091961 = IMI 092196 = LSHB P25 = LSHB P6 = LSHB Ad95 = MUCL 29781 = NRRL 1841 = NRRL 1842).
Description: Colony diam, 7 d, in mm: CYA 27–33; CYA15°C 5–10; CYA30°C 27–40; CYA37°C 2–12; MEA 18–25; YES 29–37; DG18 15–23; ratio CYAS:CYA 0.9–1.2; creatine agar 10–19, poor growth, no or weak acid.
Moderate sporulation on CYA, conidia grey green or blueish grey green, mycelium inconspicuous, small exudate droplets produced by some strains and clear or pale yellow coloured, soluble pigments yellow, margin entire, reverse brownish-yellow. Moderate to good sporulation on YES, conidial colour variable: grey green to dark green, soluble pigment present in majority of strains and strong yellow or yellow-orange coloured, reverse yellow to yellow-orange. Moderate to good sporulation on DG18, conidia grey green, reverse pale and occasionally pale with yellow centre. Moderate to good sporulation on MEA, conidia grey green with a strong blue element, colony texture velvety. Ehrlich reaction negative.
Sclerotia absent. Conidiophores arising from mycelial mat, predominant symmetrically biverticillate, terverticillate structures abundantly produced in fresh isolates; stipes smooth, 100–300 × 2.0–3.0 μm. Metulae in whorls of 3–4 (–6), 12–16 × 2.0–3.0 μm. Phialides ampulliform, 7.5–10 × 2.0–2.5 μm. Conidia globose to subglobose, smooth, 2.0–2.5 × 2.0–2.5 μm.
Extrolites: Citrinin, quinolactacins, citrinadins, perinadine, several anthraquinones, “CITY”, “met k” and “shamix” (Houbraken et al. 2010).
Diagnostic characters: Growth on CYA when incubated at 37 °C, 2–12 mm in diam, reverse on CYA in shades of yellow, soluble pigment production on CYA and YES, globose, smooth walled conidia.
Similar species: Penicillium citrinum belongs to the P. citrinum-clade and can be differentiated by other members of this series by its ability to grow at 37 °C and the formation of yellow or yellow-orange soluble pigments on YES.
Distribution and ecology: This species has a worldwide distribution and occurs more frequently in the (sub)tropics than in temperate regions. Penicillium citrinum is isolated from soils, but also from indoor air, food and as an endophyte of root, stem, leaves of coffee plants (Posada et al. 2007) and roots of Ixeris repens (Khan et al. 2008; identity based on ITS sequences deposited in GenBank).
Barcode & molecular based ID: GenBank no. GU944562. A gap of 36–38 bp was observed in the alignment of the ITS1 region of all P. citrinum isolates, when compared to most other species of this series. This species has unique ITS, partial β-tubulin and calmodulin sequences.
Taxonomy and phylogeny: Penicillium implicatum is synonymised with P. citrinum (Houbraken et al. 2010). Pitt (1980) considered the type strain of P. implicatum lost and designated IMI 190235 (= CBS 184.81) as the neotype. However, the type culture of P. implicatum, deposited by Thom, is maintained at the CBS under CBS 232.38 and resembles P. citrinum in many aspects (Frisvad et al. 1990a, Houbraken et al. 2010). Houbraken et al. (2010) placed P. phaeojanthinellum and P. botryosum in synonymy with P. citrinum and more details about the taxonomy of P. citrinum can be found there.
Penicillium copticola Houbraken, Frisvad & Samson, sp. nov. MycoBank MB563205. Fig. 18.
Fig. 18.
Penicillium copticola. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Etymology: Referring to pastery, the substrate where the type strain was growing on.
Differt ab omnibus speciebus affinibus coloniis in agaro CREA bene crescentibus, in agaro CYA ad 33 °C quoque crescentibus, coloniis in agaro MEA floccosis, conidiophoris biverticillatis.
Typus: ex tortilla, USA, J. Murray (CBS H-20643 – holotypus, cultures ex-type CBS 127355 = DTO 19H7 = IBT 30771).
Description: Colony diam, 7 d, in mm: CYA 31–37; CYA15°C 7–11; CYA30°C 13–17; CYA37°C no growth; MEA 25–34; YES 35–41; DG18 27–35; ratio CYAS:CYA 1.0–1.2; creatine agar 18–25, good growth, weak acid production followed by (delayed) base reaction.
Moderate or good sporulation on CYA, velvety, conidia dull green or dull-pure green, mycelium inconspicuous, exudate produced as minute clear droplets, soluble pigments absent, reverse pale beige or crème. Good sporulation on YES, conidia dull green, soluble pigment absent, reverse yellow to dark beige, with a darker greenish centre. Dull green conidia on DG18, reverse transparent or pale with a pale-cream centre. Colonies on MEA pure green or dull-pure green, colony texture floccose. Ehrlich reaction negative.
Sclerotia absent. Conidiophores predominantly symmetrically biverticillate, young conidophores monoverticillate, stipes up to 500 μm long, smooth, 2.0–3.0 μm wide; metulae in a compact terminal whorls of 2–4, equal or unequal in length, 12–16 × 2.0–3.5 μm, occasionally vesiculate. Phialides ampulliform to cylindrical, 7.5–9 × 2.0–3.0 μm. Conidia broadly ellipsoidal, smooth, 2.5–3.0 × 2.0–2.5 μm.
Extrolites: “GULLA”, “HAEN”, “PRS”, “VERSI”.
Diagnostic characters: Good growth on CREA with base production, growth on CYA incubated at 33 °C, floccose colonies on MEA, biverticillate conidiophores.
Similar species: See P. terrigenum.
Distribution and ecology: This species has a worldwide distribution. It is isolated from tortillas (USA), seed from ripe coffee berry (Hawaii, USA; NRRL 32575, GenBank DQ123664ITS), dried flowers of Cannabis sativa, the Netherlands and air of a toilet in Germany.
Barcode & molecular based ID: GenBank JN617685. This species can be identified with ITS, partial β-tubulin and/or calmodulin sequences.
Taxonomy and phylogeny: Isolate NRRL 32575 was listed as Penicillium sp. by Vega et al. (2006) and comparison of the ITS sequence of this strain deposited in GenBank (DQ123664) shows that it is P. copticola.
Penicillium cosmopolitanum Houbraken, Frisvad & Samson, sp. nov. MycoBank MB563188. Fig. 19.
Fig. 19.
Penicillium cosmopolitanum. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Etymology: Named after the worldwide distribution of this species.
Differt ab omnibus speciebus affinibus conidiis exasperatis, coloniis haud crescentibus ad 30 °C, reverso psammocolorato-brunneo in agaro CYA, reverso pallide flavido vel eburneo in agaro YES, conidiis in agaro CYA et YES haud vel vix formantibus.
Typus: ex heathland soil, Cartier heide, Eersel, the Netherlands, J. Houbraken (CBS H-20665 – holotypus, cultures ex-type CBS 126995 = DTO 92E8 = IBT 30681).
Description: Colony diam, 7 d, in mm: CYA 25–32; CYA15°C 15–20; CYA30°C and CYA37°C no growth; MEA 20–29; YES 27–36; DG18 16–25; ratio CYAS:CYA 0.8–1.0 (–1.1); creatine agar 10–18, weak growth and no acid production.
Sporulation on CYA in most isolates absent or weak, occasionally moderate to good, velvety, conidia dull green, mycelium white, exudate occasionally present as small clear droplets, soluble pigments absent, margin polygonal or entire, reverse beige-brown with orange coloured sulcations giving the colony a pinkish tinge, some isolates pale beige or beige (CBS 200.86 and CBS 124316). Sporulation on YES mostly absent, mycelium white, soluble pigments absent, reverse cream, cream-buff or light beige. Sporulation on DG18 moderate to good, conidia dull or grey green, reverse transparent, pale beige or cream. Colonies on MEA poorly sporulating, conidia blueish green or dull green, colony texture floccose. Ehrlich reaction negative.
Sclerotia absent. Conidiophores symmetrically biverticillate, often with an divergent branch that is shorter than the main axis, occasionally quaterverticillate; stipes long, up to 500 μm, smooth, 2.5–4.0 μm wide; metulae in a compact terminal verticil, 3–6 (–8), more or less even in length, vesiculate and non-vesiculate, 9–13 (–15) × 2.0–3.5 μm; phialides ampulliform, 6.5–8.5 × 2–3 μm. Conidia globose, rough, 2.5–3.0 μm diam.
Extrolites: Citrinin, okaramin, perinadine, territrems, “CURVO”, “HAEN”, “PHOE”, “ROTO”, “SENGA”, “TRIP”, “VERSI”, “XANTHOC”.
Diagnostic characters: Rough walled conidia, no growth at 30 °C, reverse on CYA beige-brown, reverse on YES pale yellow to cream, no or weak sporulation on CYA and YES.
Similar species: See P. westlingii.
Distribution and ecology: This species is frequently isolated from soils in the Netherlands, Poland, Denmark and New Zealand.
Barcode & molecular based ID: GenBank no. JN617691 (Fig. 3, clade 1, 2 and 3), JN617682 (Fig. 3; clade 4). Clades 1, 2 and 3 in P. cosmopolitanum have identical ITS sequences, and these sequences are also shared by certain isolates of P. westlingii (CBS 124312, CBS 124313, CBS 127003, CBS 127040, see also description of P. westlingii). Members of clade 4 share ITS sequences with certain strains of P. godlewskii and P. nothofagi.
Taxonomy and phylogeny: Molecular analysis of partial β-tubulin and calmodulin data shows that this species can be subdivided into four subclades (Fig. 3). No clear morphological differences were observed among these clades, although strains of clade 2 have slightly paler reverse colours on CYA (e.g. CBS 126995T, CBS 200.86).
Penicillium decaturense S.W Peterson, Bayer & Wicklow, Mycologia 96: 1290. 2004. Fig. 20.
Fig. 20.
Penicillium decaturense. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Typus: ex old resupinate fungus, Ramsey Lake State Park, Decatur, Illinois, USA (BPI 842267 – holotypus, cultures ex-type CBS 117509 = IBT 27117 = DTO 3F7 = NRRL 28152).
Description: Colony diam, 7 d, in mm: CYA 32–40; CYA15°C 12–18; CYA30°C 5–15; CYA37°C no growth; MEA 27–34; YES 39–47; DG18 23–30; ratio CYAS:CYA 0.9–1.1; creatine agar 11–18, weak growth and no acid production.
Good sporulation on CYA, velvety, condia dark-green or blue-grey-green, mycelium inconspicuous, exudate production variable, absent, sparsely or predominant, clear or light yellow, soluble pigments absent, margin entire, reverse orange-beige to skin coloured, occasionally with beige-brown centre. Good sporulation on YES, conidia dark green, mycelium white, soluble pigments absent, reverse yellow-orange, in some isolates yellow. Good sporulation on DG18, conidia dull-grey green, reverse pale, cream or pale yellow. Good sporulation on MEA, conidia blue-green or blueish-dark green, colony texture floccose to velvely. Ehrlich reaction negative.
Sclerotia absent. Conidiophores symmetrically biverticillate, occasionally with an divergent branch that is shorter than the main axis; stipes up to 300 μm, smooth to very finely rough, 2.0–3.5 μm; metulae in a compact terminal verticil, 3–5 (–7), unequal in length, vesiculate, 10–16 x 2.0–3.5 μm; phialides ampulliform, broad, 7.0–9.0 × 2.0–3.5 μm. Conidia globose to subglobose, finely roughened, 2.0–2.5 μm diam.
Extrolites: Daldinin D, decaturin A and deoxyoxalicine B (Zhang et al. 2003), terrein, “SENGA”, “SNIL”, “SVOL”, “VERSI”, “XANTHOC”.
Diagnostic characters: Finely roughened conidia, all examined isolates grow at 30 °C and some up to 33 °C, fast growing: 32–40 mm on CYA in 7 d at 25 °C.
Similar species: Penicillium decaturense forms finely roughened (sub)globose conidia. This feature is shared by several other species, such as P. godlewskii, P. pancosmium, P. ubiquetum, P. cosmopolitanum, P. westlingii and P. chrzaszczii. This species can be differentiated from the above mentioned species by its ability to grow consistently at 30 °C (5–15 mm).
Distribution and ecology: This species has been isolated as a colonist of fungal sporocarps (Trichaptum biformis and Ischnoderma sp.), collected in Illinois, Georgia and Florida, USA (Peterson et al. 2004).
Barcode & molecular based ID: GenBank no. GU944604. This species shares ITS sequences with P. chrzaszczii. Partial β-tubulin and calmodulin sequences can be used for identification.
Taxonomy and phylogeny: This species is a unique member of the P. westlingii-clade, because it is able to grow up to 33 °C. Penicillium decaturense is phylogenetically related to P. pancosmium.
Penicillium euglaucum van Beyma, Ant. van Leeuwenhoek 6: 269. 1940. Fig. 21.
Fig. 21.
Penicillium euglaucum. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B. Ascomata. C–D. Ascospores. E–G. Conidiophores. H. Conidia. Scale bars = 10 μm.
= Eupenicillium euglaucum (van Beyma) Stolk & Samson, Stud. Mycol. 23: 90. 1983.
Typus: ex soil, Argentina (CBS 323.71 – neotype, Stolk & Samson 1983; cultures ex-type DTO 23B9 = IBT 30767).
Description: Colony diam, 7 d, in mm: CYA 23–29; CYA15°C 3–8; CYA30°C 21–30; CYA37°C (0–)5–15; MEA 22–26; YES 23–30; DG18 23–29; ratio CYAS:CYA 0.9–1.1; creatine agar 8–16, weak growth, weak acid and no base production.
Sporulation on CYA absent or inconspicuous in fresh isolates, moderate to good sporulation in cultures maintained for longer periods in culture, conidia blue-grey green, cleistothecia abundantly produced, pale yellow when young, becoming warm grey in age, mycelium inconspicuous or light yellow, exudate produced in large clear or light yellow coloured droplets, soluble pigment production strong, yellow coloured, margin entire, reverse yellow or yellow-brown and becoming dark brown in age. Sporulation on YES inconspicuous in fresh cultures, cleistothecia abundantly produced in age, warm-grey coloured, mycelium light yellow, strong yellow soluble pigments production, reverse in shades of yellow-brown. Sporulation on DG18 weak in fresh cultures and strong in degenerated cultures, conidia grey-green, mycelium white, reverse yellow. Sporulation on MEA inconspicuous and not influencing the colony colour, cleistothecia abundantly produced light yellow to grey coloured when young and becoming warm grey in age. Ehrlich reaction negative.
Cleistothecia abundantly produced on CYA, MEA and YES, globose or subglobose, up to 400 μm diam, consisting of sclerotioid masses of polygonal cells, ripening after 4–5 wk or more; warm-grey on MEA and CYA, grayish-brown on oatmeal agar. Ascospores ellipsoidal, with 2 appressed equatorial ridges, finely roughened valves in light microscope, reticulate with SEM, 3.0–4.0 x 2.5–3.0 μm. Conidiophores simple when young becoming biverticillate in age, stipes 5–60 (–100) μm long, occasionally longer, smooth walled or nearly so, 1.5–3.0 μm wide. Metulae, when present, in verticils of 2–3 (–4), unequal in length, 10–20 × 1.5–3.0 μm, often inflated at the apex, 2.5–5.0 μm wide. Phialides ampulliform, 7.0–9.0 × 2–3 μm. Conidia globose, finely roughened, 2.0–2.5 μm diam.
Extrolites: Terrein, “ALK”, “FRIL”, “GLAD”, “RAI”, “SPOKO”, “3-T”.
Diagnostic characters: Penicillium euglaucum is characterised by the production of warm-grey coloured cleistothecia, strong yellow soluble pigment production, good growth at 30 °C and ascospores 3.0–4.0 x 2.5–3.0 μm.
Similar species: See P. anatolicum.
Distribution and ecology: Penicillium euglaucum is isolated from Argentinean soil.
Barcode & molecular based ID: GenBank no. JN617699. This species has unique ITS, β-tubulin and calmodulin sequences.
Taxonomy and phylogeny: Penicillium euglaucum was neotypified by Stolk & Samson (1983) with CBS 323.71, which resembles van Beyma's original notes of P. euglaucum. They noted that Penicillium citreonigrum is the anamorph of E. euglaucum and thirty-seven species were placed in synonymy with these two species (Stolk & Samson 1983). Houbraken & Samson (2011) show that the type culture of P. citreonigrum, CBS 258.29, is phylogenetically unrelated to P. euglaucum. Analysis of the other synonyms mentioned shows that P. euglaucum is unrelated to any of those species (J. Houbraken, unpublished results) and therefore P. euglaucum is not as commonly occurring as suggested by Stolk & Samson (1983).
Penicillium gallaicum Ramírez, Martínez & Berenguer, Mycopathol. 72: 29. 1980. Fig. 22.
Fig. 22.
Penicillium gallaicum. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B. Sclerotia. C–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
= Penicillium alicantinum Ramírez & Martínez, Mycopathol. 72: 185. 1980.
= Penicillium syriacum Baghdadi, Novosti Sist. Nizs. Rast. 1968: 111. 1968 (pro parte).
Typus: ex air, Madrid, Spain (IJFM 5597 – holotype, cultures ex type DTO 34G3 = CBS 167.81 = ATCC 42232 = IMI 253794 = VKM F-2190 = IBT 22016).
Description: Colony diam, 7 d, in mm: CYA 19–25; CYA15°C 3–6; CYA30°C 18–25; CYA37°C 0–5; MEA 24–30; YES 26–32; DG18 24–30; ratio CYAS:CYA 0.9–1.1; creatine agar 7–17, weak growth and acid production absent or only beneath the colony.
Sporulation on CYA absent, weak or moderate, conidia dull or pale grey green, mycelium pale yellow or pale crème, exudates present as droplets pale yellow, strong yellow-orange soluble pigments production, margin entire, reverse yellow-orange and becoming yellow brown in age or yellow-brown. Sporulation on YES absent or weak, conidia grey-green, mycelium white or pale beige, soluble pigments yellow-orange, reverse orange or orange-brown. Sporulation on DG18 absent or weak, obverse dull-grey green because of conidia or as white mycelium, reverse vivid yellow or yellow with conidial colour visible through the colony. Colonies on MEA weakly sporulating, mycelium white, crème or light grey coloured. Ehrlich reaction negative.
Sclerotia inconspicuously formed under a layer of mycelium or conidiophores; white and soft when young, becoming hard and orange-brown in age, 60–100 (–150) μm; asci and ascospores not observed after prolonged incubation. Conidiophores monoverticillate occasionally with additional branch, stipes up to 50 μm, smooth walled, 2.0–3.0 μm. Phialides ampulliform, 8.0–10 × 2–3.5 μm. Conidia globose or subglobose, smooth, 2.0–2.5 μm diam.
Extrolites: Citreoviridin (Frisvad et al. 1990b), “KOKSO”, “3-S”, “TIDL”, “VYL”.
Diagnostic characters: Short monoverticillate conidiophores, yellow-orange soluble pigments on CYA and YES, and yellow-orange reverse becoming brown yellow brown on CYA, sclerotia production.
Similar species: Penicillium gallaicum is unique in section Citrina and shares monoverticillate conidiophores with P. roseopurpureum and P. sanguifluum. However, these species produce reddish soluble pigments. Macromorphologically, P. gallaicum resembles P. citreonigrum and both species produce citreoviridin (Frisvad et al. 1990b).
Distribution and ecology: Three strains were studied, two from air in Madrid, Spain and one from soil in Syria.
Barcode & molecular based ID: GenBank no. JN617690. This species has unique ITS, β-tubulin and calmodulin sequences.
Taxonomy and phylogeny: Christensen et al. (1999) examined ex-type material of P. syriacum and indicated that this strain is a mixed culture. One of the isolates originating from the type of P. syriacum (CBS 418.69) is a P. galliacum. This strain has monoverticillate conidiophores and does not resemble Baghdadi's original description (Baghdadi 1968).
Penicillium godlewskii Zaleski, Bull. Int. Acad. pol. Sci. Lett., Sér. B.: 466. 1927. Fig. 23.
Fig. 23.
Penicillium godlewskii. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
= Penicillium kapuscinskii Zaleski, Bull. Int. Acad. pol. Sci. Lett., Sér. B: 484. 1927.
Typus: ex soil under pine, Bialowieska, Poland (CBS 215.28 – lectotype, designated here; cultures ex type DTO 22E2 = ATCC 10449 = ATCC 48714 = FRR 2111 = IFO 7724 = IMI 040591 = MUCL 29243 = NRRL 2111 = QM 7566 = VKM F-1826).
Description: Colony diam, 7 d, in mm: CYA 15–25; CYA15°C 13–20; CYA30°C and CYA37°C no growth; MEA 12–20; YES 20–30; DG18 15–23; ratio CYAS:CYA 1.0–1.4; creatine agar 10–17, weak growth and no acid production.
Moderate to good sporulation on CYA, velvety, conidia grey-green, mycelium inconspicuous, exudate absent, soluble pigment absent, margin entire to slightly polygonal, reverse in shades of orange, often beige-orange. Sporulation on YES variable, absent to good, mycelium white, soluble pigments absent, reverse beige, beige-orange or yellow-orange. Moderate to good sporulation on DG18, conidia dull-green or grey-green, reverse pale. Moderate to good sporulation on MEA, conidia grey green, becoming blue-grey green in age, colony texture velvety with floccose centre. No reaction with Ehrlich test.
Sclerotia absent. Conidiophores symmetrically biverticillate and often with an divergent branch, starting often 30–50 μm under terminal verticil; stipes long, up to 700 μm, smooth and rather broad, 2.5–4.0 μm; metulae in a compact terminal verticil, 5–8 (–10), unequal in length, vesiculate, 9–13 (–15) × 2.5–3.5 μm; phialides ampulliform, 6.5–8.5 × 2–3 μm. Conidia globose to subglobose, finely roughened, 2.0–2.5 μm diam.
Extrolites: Citrinin, citreoviridin, decaturin, an okaramin, perinadine, “TRIP”.
Diagnostic characters: Finely roughened conidia, weak growth on CYA incubated at 27 °C (0–5 mm), reverse on CYA in shades of yellow-orange.
Similar species: See P. chrzaszczii.
Distribution and ecology: Soil appears to be the primary habitat, but also isolated from butter; known from Poland, Germany and the Netherlands.
Barcode & molecular based ID: GenBank no. JN617692. Penicillium godlewskii shares ITS sequences with P. nothofagi and certain strains of P. cosmopolitanum (Fig. 3, clade 4) (CBS 126997, CBS 127038). Penicillium godlewskii can be identified using partial β-tubulin and/or calmodulin sequences.
Taxonomy and phylogeny: Penicillium godlewskii was described by Zaleski (1927). Raper & Thom (1949) gave it as a separate species status in their monograph, while Pitt (1980) placed this species in synonymy with P. jensenii. Type material of this species (CBS 215.28T) is degenerated. Sequences generated from this strain indicate that P. godlewskii is distinct and belongs to the P. westlingii-clade. Raper & Thom (1949) placed P. kapuscinskii in the Penicillium nigricans series and Pitt (1980) accommodated this species in the Canescentia series. The main reason for this was the formation of ornamented conidia. However, molecular data indicate that this species is a synonym of P. godlewskii, a species that also forms (finely) roughened conidia. Furthermore, the original drawing of Zaleski (1927:55) shows that P. kapuscinskii produces symmetrically biverticillate structures, indicating a relation with section Citrina. The isolate maintained in the CBS collection is degenerated and produces conidiophores sparsely.
Penicillium gorlenkoanum Baghdadi, Nov. sist. Niz. Rast., 1968: 97. 1968. Fig. 24.
Fig. 24.
Penicillium gorlenkoanum. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
= Penicillium damascenum Baghdadi, Nov. sist. Niz. Rast., 1968: 101. 1968.
Typus: ex soil, Syria (CBS 408.69 – type; cultures ex-type DTO 34E3 = FRR 511 = IMI 140339 = VKM F-1079).
Description: Colony diam, 7 d, in mm: CYA 26–31; CYA15°C 8–12; CYA30°C 20–30; CYA37°C no growth; MEA 20–27; YES 26–30; DG18 18–26; ratio CYAS:CYA 1.0–1.1; creatine agar 13–19, weak growth and no or weak acid production.
Moderate or good sporulation on CYA, velvety with floccose centre, conidia grey, dull green or dark green, mycelium inconspicuous, exudate droplets minute and clear or weak yellow coloured, soluble pigments absent, margin entire, reverse pale yellow or crème-brown. Degree of sporulation on YES variable: weak (CBS 409.69) to strong (CBS 408.69), conidia grey green, soluble pigment absent, reverse pale yellow. Sporulation on DG18 variable, absent to strong, condia grey green or dark dull green, reverse pale or pale-light yellow. Variable sporulation on MEA, conidia grey green, colony texture velvety to floccose. Ehrlich reaction negative.
Sclerotia absent. Conidiophores from aerial hyphae, predominantly irregularly biverticillate, stipes smooth, width 2.0–2.7. Metulae terminal in whorls of 2–3, 12–17 × 2.2–3.0 μm. Phialides ampulliform, 7.5–9.0 × 2.0–3.0 μm. Conidia globose to subglobose, smooth to finely roughened, variable in size, predominantly 2.0–2.5 μm, smaller portion of conidia larger, 2.5–3.0 μm diam.
Extrolites: Citrinin, costaclavin, chanoclavine-I (Kozlovskiĭ et al. 1981a, 1981b), “KUSK”, “PHOE”, “WK”, “WS”, “WT” and “WØ” (Houbraken et al. 2010).
Diagnostic characters: No growth at 37 °C, production of chanoclavine-I.
Similar species: Penicillium gorlenkoanum is related to P. citrinum and related species. It can be distinguished from these species by the production of chanoclavine-I, a crème-brown reverse on CYA, absence of cleistothecia, and no growth at 37 °C.
Distribution and ecology: This species is only known from Syrian soil.
Barcode & molecular based ID: GenBank no. GU944581. This species has unique ITS, β-tubulin and calmodulin sequences.
Taxonomy and phylogeny: Only two strains of this species were available for examination (CBS 408.69 and CBS 409.69) and both lacked typical terminal metulae in whorls of 5–8, as reported and shown in the original descriptions (Baghdadi 1969). This might be a result of degeneration of these cultures during preservation. The conidial size and the original drawings of the conidiophores indicate that this species belongs to section Citrina. Combined morphological, molecular and extrolite data show that Penicillium gorlenkoanum is conspecific with and P. damascenum.
Penicillium hetheringtonii Houbraken, Frisvad & Samson, Fung. Divers. 44: 125. 2010. Fig. 25.
Fig. 25.
Penicillium hetheringtonii. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Typus: ex soil, Treasure Island, Florida, USA, R.A. Samson (CBS 122392 – holotype, cultures ex-type DTO 5H9 = IBT 29057).
Description: Colony diam, 7 d, in mm: CYA 26–32; CYA15°C 7–11; CYA30°C 26–34; CYA37°C 0–2; MEA 17–23; YES 27–35; DG18 16–25; ratio CYAS:CYA 0.8–1.0; creatine agar 13–17, poor growth on creatine agar, no acid production.
Moderate to good sporulation on CYA, velvety, conidia dull green or dark green, mycelium inconspicuous, small hyaline exudate droplets, diffusible pigments absent, margin entire, reverse colour crème-brown. Moderate to good sporulation on YES, conidia dark green, mycelium inconspicuous, soluble pigments absent, reverse orange. Good sporulation on DG18, conidia grey green, reverse in shades of yellow (varying from pale to bright). Good sporulation on MEA, conidia dark grey green, colony texture velvety and floccose in centre. Ehrlich reaction negative.
Sclerotia absent. Conidiophores borne from surface hyphae, predominant symmetrically biverticillate, terverticillate conidiophores occasionally present; stipes smooth, 2.5–3.5 μm wide. Metulae in compact whorls of 4–8 (–12), 11–15 × 2.5–3.5 μm, vesticulated, even in length. Phialides ampulliform, 7.0–9.2 × 2.0–3.0 μm. Conidia globose to subglobose, smooth to finely roughened, 2.0–2.5 μm diam.
Extrolites: Citrinadine, citrinin, quinolactacin, two anthraquinones,“SHAMIX”, “FON”, “CITY”, “PR1-x” (Houbraken et al. 2010).
Diagnostic characters: Metulae in verticils of 4–8 (–12), crème-brown reverse on YES, lacking diffusible soluble pigments on YES and CYA, CYAS:CYA 0.8–1.0, production of uncharacterised metabolite PR1-x.
Similar species: Penicillium hetheringtonii resembles P. citrinum in having similar growth rates on agar media and an orange reverse on YES, but differs from P. citrinum in having broader stipes, 4–8 closely appressed metulae and lacking the production of soluble pigments on YES and CYA.
Distribution and ecology: This species probably has a worldwide distribution and has a preference for warmer climates. It has been isolated from soil in Florida, USA and Queensland, Australia.
Barcode & molecular based ID: GenBank no. GU944558. This species can be identified with ITS sequences. It has a 36–38 bp deletion in the ITS1 when compared with other members of section Citrina. This deletion was also observed in all isolates of P. citrinum and CBS 327.79 (P. manginii).
Taxonomy and phylogeny: None.
Penicillium manginii Duché & Heim, Recl. Trav. Cryptog. Louis Mangin: 20. 1931. Fig. 26.
Fig. 26.
Penicillium manginii. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–C. Sclerotia. D–G. Conidiophores. H. Conidia. Scale bars = 10 μm.
= Penicillium pedemontanum Mosca & Fontana, Allionia 9: 40. 1963.
Typus: unrecorded source (CBS 253.31 – neotype, designated by Pitt et al. 2000; cultures ex-type DTO 22E9 = NRRL 2134 = IMI 191732 = FRR 2134 = IBT 18224).
Description: Colony diam, 7 d, in mm: CYA 28–40; CYA15°C 19–27; CYA30°C 0–8; CYA37°C no growth; MEA 25–37; YES 35–47; DG18 18–27; ratio CYAS:CYA (0.85–) 1.0–1.3; creatine agar 16–22, weak growth and no acid production.
Moderate to good sporulation on CYA, velvety, conidia grey-green, mycelium light-yellow, exudate in some strains produced as minute clear or yellow droplets, soluble pigment yellow, margin in most isolates entire, in some strains polygonal, reverse orange or orange with red centre. Moderate to good sporulation on YES, conidia grey green, mycelium light-yellow, strong red soluble pigment production, reverse blackish-red or dark red-brown. Moderate to good sporulation on DG18, conidia grey green, reverse in most isolates deep-red with red soluble pigments, occasionally yellow or pale, conidia. Sporulation variable on MEA, varying from absent to good, conidia grey green, colony texture velvety, becoming floccose in age. Ehrlich reaction negative.
Sclerotia light yellow-brown and soft when young, becoming orange brown and hard in age, consisting of large polygonal cells, with red brown pigmented hyphe present on the sclerotial body, 100–250 μm, irregular in shape. No ascospores observed after incubation on OA for 3 mo. Conidiophores predominantly symmetrically biverticillate and, depending on the isolate, additional branches can occur; stipes 200–500 μm long, finely rough walled occasionally smooth walled, width variable, 2.0–4.0 μm; metulae in a compact terminal whorls of 2–4 (–6), even in length, non-vesiculate, 10–14 × 2.0–3.5 μm; phialides ampulliform, 7–9 × 2.0–3.0 μm. Conidia (broadly) ellipsoidal, smooth, 2.5–3.0 × 2.0–2.5 μm.
Extrolites: Citrinin, citreomontanin (Rebuffat et al. 1980), citreoviridin A (Nagel et al. 1972, Rebuffat et al. 1984, Frisvad & Filtenborg 1990), citreoviridinol A1 and A2 (Rebuffat et al. 1984), epicitreoviridinol (Lai et al. 1990), phoenicin, “MIF”,”MIM”. Phoenicin was not detected in CBS 235.31, CBS 263.29, CBS 378.65 and CBS 126233, but all other 20 strains of P. manginii examined produced this compound. This compound contributes to the red colour of the diffusible pigment of P. manginii, but the species also produces some red anthraquinone secondary metabolites. Citrinin was produced by CBS 235.31, CBS 265.65, CBS 263.29, CBS 408.65, CBS 409.65, CBS 122403, CBS 126232 and seven additional strains. Citreoviridin was produced by all strains examined. CBS 126233 shares extrolites with other strains of P. manginii, but is unique in producing decaturins and aflavinin-type apolar sclerotial indolterpenes.
Diagnostic characters: Yellow mycelium (citreoviridins) (CYA15°C), fast growth rate on YES with red soluble pigments, light brown or orange brown sclerotia.
Similar species: The production of yellow mycelium is shared with P. vancouverense, but P. manginii grows faster, produces red soluble pigment and can have finely rough walled stipes.
Distribution and ecology: Worldwide. Isolated from soil in Norway, Congo, Madagascar and UK; air in the Netherlands and Spain, mycorrhizae of Fagus sylvatica, Italy and rhizosphere of Triticum aestivum, UK.
Barcode & molecular based ID: GenBank no. GU944599. The ITS region of the majority of the analysed P. manginii isolates were invariable. Isolate CBS 327.79 was an exception and had 37 bp deletion in the ITS1 region. This deletion in also observed in P. citrinum and P. hetheringtonii, but not in other strains of the P. westlingii-clade. Phylogenetic analysis of partial β-tubulin and calmodulin data shows that isolates CBS 378.65, CBS 108.66 and CBS 126233 are deviating from the majority of the analysed P. manginii isolates and each strain has a unique sequence. However, these three strains have similar ITS sequences (0, 1 and 4 bp difference, respectively) as the type of P. manginii, CBS 253.31T.
Taxonomy and phylogeny: Penicillium manginii was placed in synonymy with P. miczynskii by Raper & Thom (1949), Pitt (1980) and Ramírez (1982), but this was not followed by Stolk & Samson (1983), who maintained it as a separate species on the basis of conidiophore ornamentation and conidial shape. Molecular data supports the conclusions of Stolk & Samson (1983). Penicillium pedemontanum is synonymised with P. manginii. The type of P. pedemontanum (CBS 265.65T) once produced large light brown sclerotia, but the culture maintained at CBS has lost this ability.
Molecular data shows variation among the analysed P. manginii isolates and this species is probably a complex. Of all P. manginii strains analysed, CBS 378.65 was the only strain with a CYAS:CYA ratio lower than 1 (0.85). CBS 126233 produced decaturins and aflavinin-type apolar sclerotial indolterpenes and did not produce red soluble pigments. However, this latter feature was also observed in some other P. manginii strains. These strains might represent new species, but we wait with the description until more strains are collected and investigated. Several strains originally identified as P. pulvillorum (Nagel et al. 1972) proved to be P. manginii (CSIR 1405, CSIR 1406, IMI 059911, IMI 089983, IMI 096225, IMI 096290 and IMI 099085). Comparison of deposited calmodulin sequences of NRRL 29865 (AY443481) and NRRL 29736 (AY443483) suggests that these strains are closely related to P. manginii and might represent a new species.
Penicillium miczynskii Zaleski, Bull. Int. Acad. pol. Sci. Lett., Sér. B.: 482. 1927. Fig. 27.
Fig. 27.
Penicillium miczynskii. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–C. Sclerotia. D–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Typus: ex soil under conifer, Tatry mountains, Poland (IMI 40030 – lectotype, Pitt 1980; cultures ex-type CBS 220.28 = ATCC 10470 = DSM 2437 = FRR 1077 = IFO 7730 = IMI 040030 = MUCL 29228 = NRRL 1077 = QM 1957 = IBT 5491)
Description: Colony diam, 7 d, in mm: CYA 21–27; CYA15°C 15–23; CYA30°C and CYA37°C: no growth; MEA 17–25 mm; YES 26–33 mm; DG18 18–25; ratio CYAS:CYA 0.85–1.0; creatine agar 9–13 mm, weak growth and no acid production.
Degree of sporulation on CYA generally poor, occasionally good sporulation (CBS 126223), velvety, conidia grey green, mycelium white or light yellow, exudate absent or sparsely produced as small clear droplets, soluble pigments absent and in some strains yellow, margin of most isolates polygonal, occasionally entire, reverse beige to beige brown in the majority of strains, occasionally yellow-orange (CBS 126222). No or weak sporulation on YES, soluble pigments absent (except CBS 126223, which has strong sporulation and yellow soluble pigments), reverse yellow-orange or yellow-brown. Moderate to good sporulation on DG18, conidia grey green, reverse (bright) yellow. Sporulation on MEA variable, conidia grey green, colony texture velvety to slightly floccose. No reaction with Ehrlich test; with exception of CBS 126222.
Sclerotia produced on oatmeal agar under large, clear exudate droplets and a thin layer of conidiophores. Sclerotia pale orange becoming orange-brown in age, 125–250 (–300 μm), soft when young becoming hard with age, consisting of polygonal cells, red-brown pigmented spots often present on the surface. Asci and ascospores not observed. Conidiophores predominantly symmetrical biverticillate with occasionally an additional branch, stipes 200–400 μm long, smooth walled, 2.5–4.0 μm wide; metulae in terminal whorls of 3–6 (–8) and often uneven in length, 10–12 × 2.5–4.0 μm; phialides ampulliform, 7–9 × 2.0–3.0 μm. Conidia subglobose to broadly ellipsoidal, smooth, 2.0–3.0 × 2.0–2.5 μm.
Extrolites: Citreoviridin, cyclopiazonic acid, quinolactacin, terrein, “met OE”, “MIF”, “TERRIT”, “XANTHOC”.
Diagnostic characters: Colonies on CYA 21–27 mm; no growth on CYA30, ratio CYAS:CYA 0.85–1.0, orange-brown sclerotia.
Similar species: This species is phylogenetically related to P. cairnsense, P. aurantiacobrunneum, P. neomiczynskii and P. quebecense. Penicillium miczynskii deviates from P. cairnsense and P. quebecense in having smaller colony diameters on YES, MEA and CYA and does not grow at 30 °C. In addition, P. cairnsense and P. quebecense often produce red soluble pigments and have many exudate droplets on CYA. The ratio CYAS:CYA of P. miczynskii is lower than 1 and this character can be used to distinguish P. miczynskii from the morphologically similar species P. aurantiacobrunneum and P. neomiczynskii.
Distribution and ecology: Worldwide, commonly occurring in soil.
Barcode & molecular based ID: GenBank no. GU944600. Penicillium miczynksii and P. aurantiacobrunneum share the same ITS sequence. These species can be distinguished by partial β-tubulin and/or calmodulin sequences.
Taxonomy and phylogeny: Penicillium miczynskii was described by Zaleski (1927) and the taxonomy of this species was considered in various taxonomic studies (Raper & Thom 1949, Pitt 1980, Ramírez 1982, Christensen et al. 1999). Thom (1930: 488) placed this species in a miscellaneous group after his section Biverticillata-Symmetica, while Raper & Thom (1949) included it in the P. janthinellum series. Subsequently, Pitt (1980) placed this species in the series Citrina and broadened the species concept to include sclerotigenic strains. The ex-type culture CBS 220.28 does not produce sclerotia, but most recently isolated strains do. This feature appears to be quite common for P. miczynskii isolates and other phylogenetically related species. Although Pitt (1980) synonymised P. chrzaszczii, P. soppii, P. matris-meae, P. manginii, P. pedemontanum, P. atrosanguineum and P. syriacum with P. miczynskii, our study shows that none of these species are conspecific with P. miczynskii.
Penicillium neomiczynskii AJL Cole, Houbraken, Frisvad & Samson, sp. nov. MycoBank MB563192. Fig. 28.
Fig. 28.
Penicillium neomiczynskii. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Etymology: This species is closely related to P. miczynskii.
Differt ab omnibus speciebus affinibus ratione CYAS:CYA 1.1–1.2, coloniis in agaro CYA ad 30 °C haud crescentibus, coloniis in agaro MEA 12–18 mm.
Typus: ex soil, New Zealand, T. Cole (CBS H-20661 – holotypus, cultures ex-type CBS 126231 = DTO 78C2 = IBT 23560).
Description: Colony diam, 7 d, in mm: CYA 21–27; CYA30°C no growth; CYA37°C: no growth; MEA 12–18 mm; YES 25–31 mm; DG18 16–22; ratio CYAS:CYA 1.1–1.2; creatine agar 9–13 mm, weak growth and no acid production.
Good sporulation on CYA, velvety to floccose, conidia grey-blue green, mycelium inconspicuous, exudate in minute clear droplets, soluble pigments yellow-brown, margin irregular, reverse yellowish brown. Good sporulation on YES, conidia grey green, soluble pigments absent, reverse yellow-beige. Good sporulation on DG18, conidia dull green, mycelium inconspicuous, reverse pale. Good sporulation on MEA, conidia dull green, colony texture velvety. Ehrlich reaction negative.
Sclerotia absent. Conidiophores 200–400 μm long, both symmetrically biverticillate and terverticillate, stipes smooth, 2.5–3.5 μm wide. Metulae in a terminal whorl of 3–6 metulae, often unequal in length, 10–13 × 2.5–3.5 μm. Phialides ampulliform, 7–9 × 2.5–3.0 μm. Conidia subglobose to broadly ellipsoidal, smooth, 2.0–3.0 × 2.0–2.5 μm, larger conidia also present, globose, 3.0–3.5 μm diam.
Extrolites: Citreoviridin, terrein, “MIF”, “OFSO”.
Diagnostic characters: CYAS:CYA ratio 1.1–1.2, no growth on CYA30°C, colonies on MEA 12–18 mm.
Similar species: Penicillium neomiczynskii resembles P. miczynskii and P. aurantiacobrunneum. It differs from P. aurantiacobrunneum in its negative Ehrlich reaction and can be differentiated from P. miczynskii by its CYAS:CYA ratio of 1.1–1.2.
Distribution and ecology: Penicillium neomiczynskii is only known from its type culture, which was isolated from soil from New Zealand.
Barcode & molecular based ID: GenBank no. JN617671. The sequences of the ITS regions of P. neomiczynskii are identical to those of the type of P. cairnsense (CBS 124325T) and P. quebecense (CBS 101623T). Partial β-tubulin and calmodulin sequences can be used for identification of this species.
Taxonomy and phylogeny: This species is phylogenetically and morphologically related to P. miczynskii and P. aurantiacobrunneum.
Penicillium nothofagi Houbraken, Frisvad & Samson, sp. nov. MycoBank MB563189. Fig. 29.
Fig. 29.
Penicillium nothofagi. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Etymology: Isolated from soil under Nothofagus sp.
Differt ab omnibus speciebus affinibus coloniis in agaro CYA, MEA et YES restricte crescentibus, conidiis leniter vel distincte exasperates.
Typus: ex soil under Nothofagus sp., Chile (CBS H-20655 – holotypus, cultures ex-type CBS 130383 = DTO 76C2 = IBT 23018).
Description: Colony diam, 7 d, in mm: CYA 5–10; CYA15°C 8–14; CYA30°C and CYA37°C 0; MEA 4–8; YES 10–15; DG18 10–15; ratio CYAS:CYA 2.0–3.0; creatine agar 3–6, weak growth and no acid production.
Moderate sporulation on CYA, velvety, conidia dark green conidia, mycelium inconspicuous, exudates absent, soluble pigment absent, margin entire, reverse pale beige. Sporulation on YES absent, mycelium white, soluble pigments absent, reverse beige. Moderate sporulation on DG18, conidia dull green to grey green, reverse pale to pale-cream. No sporulation on MEA after 7 d of incubation, after 14 d moderate sporulation, conidia blue green, colony texture velvety to granulose. Ehrlich reaction negative.
Sclerotia absent. Conidiophores mostly symmetrically biverticillate and occasionally with an additional divergent branch; stipes variable in length, 50–400 μm long, smooth, 2.0–3.0 μm wide; metulae in a divergent terminal verticil, 2–4 (–7), unequal in length, with a distinct vesicle, long compared to related species, 11–17 × 2.5–3.5 μm, additional branches up to 25 μm; phialides ampulliform, 7.5–10 × 2.5–3.5 μm. Conidia globose to subglobose, finely to distinct roughened, 2.5–3.5 μm diam.
Extrolites: Citrinin, “CURVU”, “SENTRIP”, “SKAEM”.
Diagnostic characters: Restricted growth on CYA, MEA and YES, finely to distinct rough walled conidia.
Similar species: This species is phylogenetically related to P. westlingii and P. cosmopolitanum. It differs from those species by a slower growth rate on the CYA, YES and MEA. Penicillium wellingtonense is phenotypically similar, but produces has an orange coloured reverse on CYA and subglobose to broadly ellipsoidal conidia.
Distribution and ecology: Soil under Nothofagus sp. in Chile and soil, Brazil.
Barcode & molecular based ID: GenBank no. JN617712. CBS 130383T shares ITS sequences with P. godlewskii and with P. cosmopolitanum strains belonging to subclade 4 (Fig. 3).
Taxonomy and phylogeny: Phylogenetically related to P. westlingii and P. cosmopolitanum.
Penicillium pancosmium Houbraken, Frisvad & Samson, sp. nov. MycoBank MB563191. Fig. 30.
Fig. 30.
Penicillium pancosmium. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Etymology: Referring to the worldwide distribution of this species.
Differt ab omnibus speciebus affinibus conidiis subtiliter exasperatis, coloniis ad 30 °C haud crescentibus, ad 28–35 mm diam post hebdomatem, reverso flavo-aurantiaco vel aurantiaco in agaro YES.
Typus: ex old Armillaria mellea, on hardwood log; Meach Lake, Gatineau Park, Gatineau County, Quebec, Canada (CBS H-20651 – holotypus, cultures ex-type CBS 276.75 = DTO 31B4 = DAOM 147467 = IBT 29991).
Description: Colony diam, 7 d, in mm: CYA (23–) 28–35; CYA15°C 15–21; CYA30°C 0 or germination; CYA37°C no growth; MEA (20–)25–31; YES (26–) 30–40; DG18 (16–) 22–30; ratio CYAS:CYA 0.9–1.1; creatine agar 15–20, weak growth and no acid production.
Good sporulation on CYA, velvety or floccose, conidia dull-green or grey-green, mycelium inconspicuous, exudate absent or sparsely present as minute clear droplets, soluble pigments absent in most isolates, except CBS 118007, which produces light red pigments, margin entire or polygonal, reverse pale, light beige or pinkish beige (towards skin colour), often with orange pigments in sulcations. Sporulation on YES variable, absent to strong, mycelium white, soluble pigments absent or yellow, reverse yellow-orange or orange. Variable sporulation on DG18, condia dull green, reverse pale or cream. Good sporulation on MEA, conidia blue-green or blueish-grey green, colony texture floccose. Ehrlich reaction negative.
Sclerotia absent. Conidiophores symmetrically biverticillate, often with an divergent branch that is shorter than the main axis; stipes long, up to 500 μm, smooth, 2.5–4.0 μm; metulae in a compact terminal verticil, 4–6 (–8), unequal in length, vesiculate, 9–13 (–15) × 2.0–3.5 μm; phialides ampulliform, broad, 6.5–9 × 2–3 μm. Conidia globose to subglobose, finely roughened, 2.0–3.0 μm diam, except CBS 126432, which has finely roughened ellipsoidal conidia, 2.5–3.0 × 1.8–2.5 μm.
Extrolites: Citrinin, daldinin D, decaturin, terrein, “MELI”, “ORAN”, “SENGA”, “XANTHOC”.
Diagnostic characters: Finely roughened conidia, no growth at 30 °C, colonies attaining a diameter of 28–35 mm in 7 d at 25 °C, reverse on YES yellow-orange or orange.
Similar species: Penicillium pancosmium is phylogenetically related to P. ubiquetum. The species are phenotypically similar, but the latter has an orange-red reverse on YES and dark-dull green conidia on CYA, while P. pancosmium forms a yellow-orange or orange reverse on YES and has dull green or grey green conidia on CYA. Futhermore, P. pancosmium tends to grow faster on MEA than P. ubiquetum. Penicillium chrzaszczii produces yellow reverse on DG18 and sporulation on CYA is absent or poor, while P. pancosmium and P. ubiquetum isolates sporulate well on CYA and have a pale (or very pale yellow) reverse on DG18.
Distribution and ecology: Isolated from soil, old Armillaria mellea on a hardwood log, Piptoporus (on Betula sp), nut of Juglans cinerea (butternut) and porcupine dung. This species has a worldwide distribution and was isolated in Tunisia, Canada (Ontario and Quebec) and USA (New Jersey).
Barcode & molecular based ID: GenBank no. JN617660. This species has unique ITS, β-tubulin and calmodulin sequences.
Taxonomy and phylogeny: Based on partial β-tubulin and calmodulin data, CBS 118007 and CBS 126431 are phylogenetically closely related, but distinct from CBS 276.75T. These isolates differ from the other P. pancosmium strains in having smaller colonies and a pinkish-brown reverse on CYA. CBS 126432 differs in having ellipsoidal conidia and a different β-tubulin, calmodulin and ITS sequence than CBS 276.75T. It needs to be noted that the variation within P. pancosmium is large, and it could be that this species represents a complex.
Penicillium pasqualense Houbraken, Frisvad & Samson, sp. nov. MycoBank MB563190. Fig. 31.
Fig. 31.
Penicillium pasqualense. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–D. Sclerotia. E–G. Conidiophores. H. Conidia. Scale bars = 10 μm.
Etymology: Referring to Easter Island, the locality of the type strain.
Differt ab omnibus speciebus affinibus (sect. Citrina) coloniis in agaro CYA30 crescentibus, reverse atro-brunneo in agaro CYA, conidiis leviter majoribus.
Typus: ex soil, Easter Island, Chile (CBS H-20663 – holotypus, cultures ex-type CBS 126330 = DTO 80D5 = IBT 14235).
Description: Colony diam, 7 d, in mm: CYA 25–35; CYA15°C 15–20; CYA30°C 5–15; CYA37°C 0; MEA (15–) 25–30; YES 25–35; DG18 17–25; ratio CYAS:CYA 0.75–0.95; creatine agar 13–18, varying from weak (CBS 122402 & CBS 126330) to moderate (CBS 124327) growth, no or weak acid production.
Good sporulation on CYA (except CBS 126329), velvety, conidia dull dark green, mycelium inconspicuous, exudate produced in both small and large droplets, which are clear or pale yellow coloured, soluble pigment absent, margin entire, reverse dark brown or blackish brown. Weak to moderate sporulation on YES, conidia dull green, soluble pigments absent, reverse beige-brown or brown. Good sporulation on DG18, conidia dull-green, reverse pale with a cream centre. Good sporulation on MEA, conidia dark green or dark-blue green, colony texture velvety to floccose, reverse medium-brown. Ehrlich reaction negative.
Sclerotia orange brown or brown, hard, consisting of hyaline polygonal cells with very thick walls, red-brown mycelium strands present present on the sclerotium. Asci and ascospores not observed. Conidiophores predominantly symmetrically biverticillate and often additional branches occur which are equal in length as the main axis and also consist of symmetrically biverticillate structures (“double symmetrically biverticillate”); stipes rather long, 200–400 μm, smooth, 2.5–3.0 μm wide; metulae in a divergent terminal vertical, 2–4, unequal in length, longer than in related species, 11–17 × 2.5–3.5 μm, branches longer up to 25 μm; phialides ampulliform, 7.5–10 × 2.5–3.5 μm. Conidia globose to subglobose, spinose, 2.5–3.5 μm diam.
Extrolites: Pyrenocines, indol alkaloids, “PAS”.
Diagnostic characters: Growth on CYA30, dark brown reverse on CYA, orange brown or brown sclerotia, dark blue green spinose conidia on MEA, slightly larger conidia than most other members of section Citrina.
Similar species: The formation of orange-brown sclerotia indicates a relationship with P. miczynskii and related species, but P. pasqualense is less colourful, has a dark brown reverse on CYA and forms typical dark blue green, spinose conidia on MEA.
Distribution and ecology: This species was isolated from various soils and indoor air of a bakery. World-wide distribution: Easter Island, Chile, NSW, Australia, the Netherlands and Wyoming, USA.
Barcode & molecular based ID: GenBank no. JN617676. Penicillium pasqualense can be identified using ITS, partial β-tubulin and calmodulin sequences.
Taxonomy and phylogeny: Phylogenetic analysis of partial β-tubulin and calmodulin data shows that this species is related to P. vancouverense and P. wellingtonense (99 % bs), but can differentiated by various phenotypic characters, such as growth at 30 °C, spinose conidia and sclerotium formation. The divergent long metulae and branching pattern of this species superficially resemble some species related to P. simplicissimum and P. janthinellum. It shares the production of of pyrenocines with P. paxilli.
Penicillium paxilli Banier, Bull. trimest. Soc. mycol. Fr. 23: 95. 1907. Fig. 32.
Fig. 32.
Penicillium paxilli. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Typus: ex optical instrument, Barro Colorado Island, Panama (IMI 40226 – neotype, Pitt 1980; cultures ex-type CBS 360.48 = DTO 31A6 = ATCC 10480 = FRR 2008 = NRRL 2008 = QM 725 = IBT 16202).
Description: Colony diam, 7 d, in mm: CYA 30–37; CYA15°C 10–20; CYA30°C (9–) 18–25; CYA37°C no growth; MEA 28–35; YES 38–46; DG18 (25–) 30–37; ratio CYAS:CYA 1.0–1.3; creatine agar 11–20, weak growth and no acid production.
Good sporulation on CYA, velvety, conidia dull green or dull-blue green, mycelium white, exudate droplets clear, occasionally absent, soluble pigments absent, margin slightly polygonal, reverse pale or pale with pale beige centre. Strong sporulation on YES, conidia dull green or dull-blue green, mycelium white, soluble pigments absent, reverse (pale) crème or pale yellow. Strong sporulation on DG18, conidia dull green, reverse commonly pale, occasionally pale with pale yellow centre. Strong sporulation on MEA, conidia dull green, also dull green and blue green, colony texture floccose. Ehrlich reaction negative.
Extrolites: Paxillin, dehydroxypaxillin, 1'-O-acetylpaxillin (Frisvad & Filtenborg 1990), meleagrin, pyrenocines, “PU”, “PUX”, “TOTO”. The paxillin biosynthetic pathway of P. paxilli (ATCC 26601 = CBS 547.77) was intensively studied (e.g. Young et al. 2001, McMillan et al. 2003).
Diagnostic characters: Rough walled stipes, predominantly biverticillate with appressed terminal whorl of 4–8 metulae, good growth on CYA incubated at 30 °C and good growth on DG18.
Similar species: Penicillium paxilli can be distinguished from P. citrinum by its inability to grow at 37 °C; from P. sumatrense and P. hetheringtonii by its pale reverse on CYA, and from P. steckii by its rough walled stipes.
Distribution and ecology: This species has a worldwide distribution and has a preference for (sub)tropic regions. Penicillium paxilli was isolated from various substrates, such as soil, wood in a tropical rainforest, the surface of a melon, mangrove, leaves, nut of Carya cordiformis (bitternut), termite mounds, Garcinia sp. (Rungjindamai et al. 2006) and as an endophyte of wild rubber trees (Hevea brasiliensis) (Gazis & Chaverri 2010).
Barcode & molecular based ID: GenBank no. GU944577. This species can be identified with ITS and partial β-tubulin and calmodulin sequences.
Taxonomy and phylogeny: Analysis of partial β-tubulin and calmodulin sequences shows variation among various isolates of P. paxilli and this species might be a complex. A thorough population study is needed to clarify the taxonomy of this species.
Penicillium quebecense Seifert, Houbraken, Frisvad & Samson, sp. nov. MycoBank MB563202. Fig. 33.
Fig. 33.
Penicillium quebecense. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Etymology: Named after the location where the type strain was isolated, Quebec (Canada).
Differt ab omnibus speciebus affinibus reverso atro-rubro coloniae in agaro YES, coloniis in agaro CYA usque ad 38–42 mm et in agaro CYA30 16–20 mm, ratione CYAS:CYA 0.85–1.0, sclerotiis pallide aurantiacis efferentibus.
Typus: ex air in sawmill, Quebec, Canada (CBS H-20666 – holotypus, cultures ex-type CBS 101623 = DTO 9B8 = IBT 29050).
Description: Colony diam, 7 d, in mm: CYA 38–42; CYA30°C 16–20; CYA37°C: no growth; MEA 30–35 mm; YES 42–48 mm; DG18 24–29; ratio CYAS:CYA 0.85–1.0; creatine agar 17–24 mm, weak growth and no acid production.
Good sporulation on CYA, velvety, conidia dull grey green, many minute clear exudate droplets, soluble pigments yellow, margin entire, reverse yellow, yellow-orange in the centre. Good sporulation on YES, conidia dull green, soluble pigments red, reverse deep dark red in center with brown edge. Good sporulation on DG18, conidia grey green, reverse pale. Moderate to good sporulation on MEA, conidia grey green, colony texture velvety. Ehrlich reaction negative.
Sclerotia white when young, becoming pale orange in age, 150–250 μm, inconspicuously, produced on oatmeal agar under a dense layer of conidiophores, hard, consisting of polygonal cells; no asci or ascospores observed. Conidiophores 200–400 μm long, predominantly biverticillate, rarely terverticillate, stipes smooth, 2.5–3.5 μm wide. Metulae, in terminal whorl of 3–7, mostly equal in length, 10–14 x 2.5–3.5 μm. Phialides ampulliform, 7–9 × 2–3 μm. Conidia subglobose, smooth, 2.0–2.5 × 2.0–3.0 μm diam.
Extrolites: Citreoviridin, phoenicin, terrein, “SENOE” (verrucofortine-type molecule), “MIF”, “MIM”, “SENGA”, “alk-770”.
Diagnostic characters: Dark red reverse on YES, colonies on CYA 38–42 mm, colonies on CYA30 16–20 mm, ratio CYAS:CYA 0.85–1.0, pale orange sclerotia.
Similar species: Penicillium quebecense morphologically resembles P. cairnsense, but differs in a higher growth rate at CYA30°C. Additional strains should be compared to determine if this character is consistent among multiple strains.
Distribution and ecology: This species is only known from its type culture, isolated from the air in a sawmill in Quebec, Canada.
Barcode & molecular based ID: GenBank no. JN617661. The ITS regions of P. quebecense are identical to those of the type of P. cairnsense (CBS 124325T) and P. neomiczynskii (CBS 126231T). Partial β-tubulin and calmodulin sequences can be used for the identification of this species.
Taxonomy and phylogeny: None.
Penicillium raphiae Houbraken, Frisvad & Samson, sp. nov. MycoBank MB563203. Fig. 34.
Fig. 34.
Penicillium raphiae. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Etymology: This species was isolated from soil under Raphia palm.
Differt ab omnibus speciebus affinibus coloniis in agaro CYA30 haud crescentibus, conidiis late ellipsoideis, conidiophoris saepe symmetrice biverticillatis.
Typus: ex soil under Raphia palm in primary forest, Las Alturas, Costa Rica (CBS H-20660 – holotypus, cultures ex-type CBS 126234 = DTO 78B8 = IBT 22407).
Description: Colony diam, 7 d, in mm: CYA 32–36; CYA15°C 18–22; CYA30°C and CYA37°C no growth; MEA 21–25; YES 31–35; DG18 23–27; ratio CYAS:CYA 1.0–1.2; creatine agar 10–15, weak growth and no acid production.
Good sporulation on CYA, velvety, conidia blue-green, mycelium inconspicuous, exudate absent, soluble pigments absent, margin slightly irregular or polygonal, reverse creme to light-brown. Good sporulation on YES, conidia grey green, soluble pigments absent, reverse (light-) brown. Good sporulation on DG18, conidia light blue green or dull green, reverse cream or light yellow. Moderate to good sporulation on MEA, conidia light-blue green, colony texture velvety. Ehrlich test negative.
Sclerotia absent. Conidiophores predominantly symmetrically biverticillate and occasionally with additional branch; stipes up to 300–500 μm long, smooth or finely rough walled, 2.0–3.0 μm wide; metulae in compact terminal whorls of 4–8 (–10), equal in length, non-vesiculate, 10–14 × 2.0–3.5 μm. Phialides ampulliform, 7–9 × 2.0–3.0 μm. Conidia smooth or finely rough walled, broadly ellipsoidal, 1.8–2.5 × 2.0–2.5 μm.
Extrolites: CBS 126234T produces citrinin, “FON”, “MIF”,”KUM”, “LOST”,“PHOE”, and “TRIP”; CBS 126235, possibly a P. raphiae, produces citrinin, quinolactacin,”FON”, “MIF”, “KUM”, “MIM”, “REJS”, “SENGA”, and “XANTHOC”.
Diagnostic characters: No growth on CYA30, broadly ellipsoidal conidia, predominantly symmetrically biverticillate conidiophores.
Similar species: The species is phenotypically related to P. steckii, P. copticola and P. terrigenum. Penicillium raphiae does not grow at 30 °C, while the other related species do grow at this temperature.
Distribution and ecology: This species is only known from its type strain, which was isolated from soil in a primary forest under Raphia palm in Costa Rica.
Barcode & molecular based ID: GenBank no. JN617673. This species has unique ITS, partial β-tubulin and calmodulin sequences.
Taxonomy and phylogeny: This species is phylogenetically unique in the P. westlingii-clade. Sequence and extrolite data indicate that CBS 126235 is a new species. However, CBS 126234T and CBS 126235 are phenotypically similar and we wait with the description of this species until more strains are collected and studied.
Penicillium roseopurpureum Dierckx, Annls Soc. Scient. Brux. 25: 86. 1901. Fig. 35.
Fig. 35.
Penicillium roseopurpureum. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
= Penicillium carminoviolaceum Dierckx Annls Soc. Scient. Brux. 25: 86. 1901.
= Citromyces cesiae Bainier & Sartory, Bull. Trimest. Soc. Mycol. Fr. 29:148. 1913.
= Penicillium cesiae (Bainier & Sartory) Biourge, La Cellule 33: 101. 1923.
Typus: unrecorded source (IMI 40573 – neotype, cultures ex-type CBS 266.29 = DTO 9E3 = ATCC 10492 = ATHUM 2895 = FRR 2064 = IMI 040573 = MUCL 28654 = MUCL 29237 = NRRL 2064 = NRRL 2064A).
Description: Colony diam, 7 d, in mm: CYA 7–16; CYA15°C 7–13; CYA30°C and CYA37°C no growth; MEA 9–19; YES 12–18; DG18 14–22; ratio CYAS:CYA 1.2–1.9; creatine agar 3–6, weak growth and no acid production.
Sporulation absent or sparse on CYA and becoming velvety in time, with pale grey green conidia, mycelium white or pale yellow, exudate absent or sparsely present as dark red brown droplets, soluble pigments orange, reverse red brown or orange brown, margin varying form entire to irregular, reverse in shades of brown (red-brown, caramel or yellow-brown). Sporulation on YES variable or poor, mycelium white or pale yellow, soluble pigments absent or yellow-brown, reverse yellow with red-brown centre, orange-red or yellow. No sporulation on DG18, white mycelium, reverse yellow-orange, vivid yellow or pale yellow. Sporulation on MEA sparsely, becoming grey-green in time, colony texture velvety or floccose. Ehrlich reaction negative.
Sclerotia absent. Conidiophores monoverticillate when young, becoming irregularly branched with divergent lower branch-like metulae or symmetrically biverticillate in age; length of stipe of main conidiophore 50–150 μm, lower branch-like metulae shorter, smooth, 2.0–3.0 μm wide; metulae branch-like, irregularly formed and in some cases difficult to distinguish from stipes, when produces terminally, then unequal in length, often gradually enlarging at the apex, giving a clavate appearance or distinct vesiculate, 15–25 × 2.0–3.5 μm at base, terminal up to 5.0 μm diam; phialides ampulliform, formed terminally and subterminally, 6.0–8.0 × 2–3 μm. Conidia globose to subglobose, smooth or very finely roughened, 1.8–2.5 μm diam.
Extrolites: Bisanthrons, roseopurpurin, sorbicillins (produced by some isolates), “AQ” (other anthraquinones apart from roseopurpurin), “SEL”.
Diagnostic characters: Monoverticillate or furcate conidiophores with lower branch-like metulae, reverse on CYA in shades of red, often with red-brown diffusible pigments, restricted growth on agar media and no growth on CYA30°C.
Similar species: Penicillium sanguifluum is related to P. roseopurpureum, but the latter grows slower on CYA and does not grow on CYA incubated at 30 °C. Furthermore, P. roseopurpureum has a higher CYAS:CYA ratio than P. sanguifluum.
Distribution and ecology: This species was isolated from soil (Wyoming, USA) and indoor air (the Netherlands).
Barcode & molecular based ID: GenBank no. GU944605. This species shares ITS sequences with most members of clade 1 of P. sanguifluum (Fig. 4).
Taxonomy and phylogeny: Phylogenetic analysis shows that P. roseopurpureum belongs to section Citrina. This species is characterised by the formation of monoverticillate conidiophores, becoming irregularly branched with divergent lower branch-like metulae or symmetrically biverticillate condiophores in older parts of the colony. This branching pattern is unusual for members of section Citrina, which are in general symmetrically biverticillate. Figure 4 shows that the type strain of P. carminoviolaceum (CBS 281.39) belongs to P. roseopurpureum. No cultures of P. cesiae were available for analysis. Raper & Thom (1949) and Pitt (1980) are followed here and this species is considered as a synonym of P. roseopurpureum.
Penicillium sanguifluum (Sopp) Biourge, La Cellule 33: 105. 1923. Fig. 36.
Fig. 36.
Penicillium sanguifluum. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Basionym: Citromyces sanguifluus Sopp, Skr. udgivne Videnskabs-Selsk. Christiania 11: 115. 1912.
= Penicillium lacussarmientei Ramírez, Mycopathol. 96: 29. 1986.
= Penicillium vaccaeorumQuintanilla, Mycopathol. 80: 77. 1982.
Typus: ex soil, Calahonda, Costa del Sol, Spain, L. Janson (CBS H-20645 – neotype, designated here; cultures ex-type CBS 127032 = DTO 20B7 = IBT 29041).
Description: Colony diam, 7 d, in mm: CYA (15–) 18–26; CYA15°C 7–14; CYA30°C microcolony–13; CYA37°C no growth; MEA 17–26; YES 18–28; DG18 16–22; ratio CYAS:CYA 0.9–1.2; creatine agar 5–14, weak growth and no or poor acid production.
Sporulation on CYA variable, absent to moderate, velvety, conidia grey-green, mycelium white or pale beige, exudate absent or dark red brown, strong red soluble pigment production, margin entire or irregular, reverse dark red brown or red. Sporulation on YES absent or sparse, mycelium white, pale beige or pale yellow, soluble pigments absent or orange, reverse orange, orange-brown with or without orange-red centre. Sporulation absent or sparse on DG18, conidia dull-green, reverse in shades or yellow (yellow-orange, yellow or pale yellow). Colonies on MEA sporulating sparsely, colony texture floccose. Ehrlich reaction negative.
Sclerotia not produced. Conidiophores produced on trailing hyphe, monoverticillate, short, 15–50 μm, smooth, or with branch-like metulae scarcely formed, 1–3, unequal in length, strongly vesiculate, (10–) 13–18 × 2.0–3.0 μm at base, vesicle up to 5 μm; phialides ampulliform, terminally and subterminally formed, 6.5–8 × 2–3 μm. Conidia globose to subglobose, smooth or finely roughened, 2.0–2.5 μm diam.
Extrolites: Bisanthrons, roseopurpurin, β-hydroxycurvularin, dehydrocurvularin, curvularin, “FOSI”, “FYKS”, “SNIT”, “TIDL”, “VERN”.
Diagnostic characters: Monoverticillate conidiophores, dark red brown reverse on CYA with red brown soluble pigment production, growth on CYA30°C.
Similar species: See P. roseopurpureum.
Distribution and ecology: This species appears to have preference for sandy soils and has a worldwide distribution. Penicillium sanguifluum is isolated from soils in Spain, Manitoba, Canada, the Netherlands, Turkey, Chile and Argentina.
Barcode & molecular based ID: GenBank no. JN617711 (clade 1) and JN617681 (clade 2). Two subclades are present in P. sanguifluum (Fig. 4). CBS 127032T is positioned in clade 2 and shares identical ITS sequences with other members of this clade. Members of clade 1 share ITS sequences with P. roseopurpureum. This clade also includes the type cultures of P. lacussarmientei and P. vaccaeorum.
Taxonomy and phylogeny: Penicillium sanguifluum was considered a synonym of P. roseopurpureum (Raper & Thom 1949, Pitt 1980). Examination of the protologue of Citreomyces sanguifluus showed that this species is not P. roseopurpureum (Sopp 1912: 115). It has an optimal growth between 25 and 30 °C and the published Figure (Sopp 1912: XXII, Fig. 3) shows rather well developed colonies. These characters fit better with P. sanguifluum than with P. roseopurpureum. CBS 127032T approximates the orginal description of P. sanguifluum and it is designated here as the neotype of this species. This study shows that the faster growth rate is a good feature for distinguishing P. roseopurpureum and P. sanguifluum. Penicillium vaccaeorum and P. lacussarmientei were considered synonyms of P. roseopurpureum by Frisvad et al. (1990b). They noted that both species are fast growing variants of P. roseopurpureum, and these species are treated here as synonyms of P. sanguifluum. Penicillium sanguifluum and P. roseopurpureum deviate from other members of section Citrina by its monoverticillate or furcate conidiophores. Partial β-tubulin and calmodulin sequences show that two subclades are present in P. sanguifluum (Fig. 4). No phenotypic differences were observed between these two clades, and therefore we did not describe them as two distinct species.
Penicillium shearii Stolk & Scott, Persoonia 4: 396. 1967. Fig. 37.
Fig. 37.
Penicillium shearii. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B. Sclerotia. C–D. Ascospores. E–G. Conidiophores. H. Conidia. Scale bars = 10 μm.
= Carpenteles asperum Shear, Mycologia 26: 107. 1934 (misapplied).
= Penicillium asperum (Shear) Raper & Thom Man. Penicillia: 263. 1949 (misapplied).
= Eupenicillium shearii Stolk & Scott, Persoonia 4: 396. 1967.
Typus: ex soil, Tela, Honduras (CBS 290.48 – holotypus, cultures ex-type DTO 22F6 = IMI 39739 = ATCC 10410 = NRRL 715 = IFO 6088 = IBT 24588).
Description: Colony diam, 7 d, in mm: CYA 28–40; CYA15°C 6–10; CYA30°C 22–36; CYA37°C (0–) 5–19; MEA 26–37; YES 25–37; DG18 28–37; ratio CYAS:CYA (0.7–) 0.9–1.1; creatine agar 10–20, weak growth, acid and base production absent.
Sporulation on CYA absent or sparse; cleistothecia abundantly produced, dark grey coloured, mycelium inconspicuous, large clear exudate droplets, soluble pigments absent, margin entire, reverse (light) brown. Sporulation on YES absent or weak, cleistothecia abundantly produced and dark-grey, mycelium white, soluble pigments absent, reverse (pale) yellow-brown. Sporulation on DG18 absent in fresh isolates or weakly produced in older cultures, conidia grey green, mycelium white, reverse pale or pale yellow. Sporulation on MEA absent or weak, not influencing the colony colour, cleistothecia abundantly produced and grey. Ehrlich reaction negative.
Cleistothecia abundantly produced on CYA, MEA and YES, globose or subglobose, up to 500 μm diam, consisting of sclerotioid masses of polygonal cells, ripening after 4–5 wk or more. Ascospores ellipsoidal, 2.5–3.5 × 2.0–2.5 μm, with 2 appressed equatorial ridges up to 0.5 μm wide, valves roughened (towards warted). Conidiophores biverticillate, occasionally with additional branch, stipes 100–500 μm long, smooth walled or nearly so, 2.0–3.0 μm wide. Metulae in verticils of 2–5 (–8), unequal in length, 10–14 × 2.0–3.0 μm. Phialides ampulliform, 7.0–9.0 × 2–3 μm. Conidia subglobose or broadly ellipsoidal, smooth or nearly so, 2.5–3.0 × 1.8–2.5 μm.
Extrolites: Paxillin, paspalinine, shearinin A & B, “XX” and several indole alkaloids (Belofsky et al. 1995, Tuthill & Frisvad 2004).
Diagnostic characters: Abundant production of dark grey coloured cleistothecia, growth at 37 °C, ascospores produced after prolonged incubation.
Similar species: Penicillium tropicum and P. tropicoides are phenotypically similar species; however, these two species do not grow on CYA at 37 °C. Penicillium shearii can be differentiated from P. euglaucum and P. anatolicum by the absence of yellow soluble pigments and from P. argentinense by its ability to grow at 37 °C.
Distribution and ecology: Penicillium shearii has a worldwide distribution and has a preference for tropical and subtropical soils (Honduras, Colombia, Mexico, Congo, Papua-New Guinea, Tanzania, Malaysia; Tuthill & Frisvad (2004) also isolated this species in Venezuela, Ivory Coast, Australia, Costa Rica and India).
Barcode & molecular based ID: GenBank no. GU944606. This species can be identified with ITS, partial β-tubulin and/or calmodulin sequences.
Taxonomy and phylogeny: According to Shear (1934), the type strain of P. shearii (CBS 290.48) represented Brefeld's ascosporic species “Penicillium glaucum Link”. He proposed Carpenteles asperum as a new name for Brefeld's fungus. However, CBS 290.48 does not produce asci in chains, as described and figured by Brefeld (1874), and consequently C. asperum Shear as well as the combination P. asperum (Shear) Raper & Thom are interpreted as misapplied names. That Shear's fungus differs from Brefeld's is nomenclaturally irrelevant because Shear clearly regarded Brefeld's organism to be the type for his new name. Stolk & Scott (1967) proposed the name Eupenicillium shearii for Shear's fungus and named the anamorph P. shearii.
Stolk & Samson (1983) described P. soppii as the anamorph of E. shearii (= P. shearii) and as a consequence P. shearii was synonymised with P. soppii. However, molecular data shows that P. soppii is distinct (Fig. 1) and phylogenetically unrelated to P. shearii. Furthermore, P. soppii does not grow at 37 °C and no ascospores or asci are produced in the sclerotia of this species.
Penicillium sizovae Baghdadi, Nov. sist. Niz. Rast., 1968: 103. 1968. Fig. 38.
Fig. 38.
Penicillium sizovae. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Typus: ex soil, Syria (CBS 413.69 – neotype, designated by Pitt et al. 2000; cultures ex-type DTO 23A7 = FRR 518 = IMI 140344 = VKM F-1073).
Description: Colony diam, 7 d, in mm: CYA 28–39; CYA15°C 8–15; CYA30°C 28–34; CYA37°C 0–4; MEA 27–35; YES 40–50; DG18 23–32; ratio CYAS:CYA 0.95–1.2; creatine agar 15–23, poor growth, weak acid production.
Good sporulation on CYA, velvety, conidia grey green, mycelium inconspicuous, small clear exudate droplets, soluble pigments absent, margin entire, reverse pale and occasionally pale crème-brown. Moderate to good sporulation on YES, conidia dark green, soluble pigments absent, reverse pale or pale yellow-crème. Most isolates moderate to good sporulation on DG18, occasionally absent or poor, conidia dull green or grey green, reverse pale and conidial colour shining through the agar. Good sporulation on MEA, conidia grey green, colony texture floccose. No reaction with Ehrlich reagent.
Sclerotia absent. Conidiophores from aerial hyphae and the mycelial mat, predominantly symmetrically biverticillate, occasionally with an additional branch; stipes smooth, 100–300 × 2.5–3.2 μm. Metulae in whorls of 2–5, 11–16 × 2.5–3.2 μm, uniform in length. Phialides ampulliform, 7.0–9.5 × 2.0–3.0 μm. Conidia globose to subglobose, finely roughened, 2.0–2.5 μm diam.
Extrolites: Quinolactacin, tanzawaic acid E, verrucolone, “AFSI”, “CHAE and “PNUF” (Houbraken et al. 2010).
Diagnostic characters: Fast growing on MEA and YES, pale reverse on CYA, finely roughened conidia.
Similar species: Penicillium sizovae is phylogenetically related to P. citrinum, P. hetheringtonii, P. steckii and P. gorlenkoanum. It can be differentiated from these species by the formation of finely roughened conidia and its high growth rate on MEA and YES.
Distribution and ecology: This species has been isolated from soil, margarine, sea salt, salty water in saltern, glue and Papaver somniferum in the Netherlands, Portugal, Syria, Italy, Slovenia.
Barcode & molecular based ID: GenBank no. GU944588. This species has unique ITS, β-tubulin and calmodulin sequences.
Taxonomy and phylogeny: Pitt (1980) placed P. sizovae in synonymy with P. fellutanum, but this species was later accepted and reinstated by Pitt & Samson (1993). CBS 413.69NT is degenerated and shows both conidiophores with terminal metulae, as well as subterminal and intercalary metulae. These features could explain the earlier proposed synonymy in P. fellutanum (Houbraken et al. 2010).
Penicillium steckii Zaleski, Bull. Int. Acad. pol. Sci. Lett., Sér. B.: 469. 1927. Fig. 39.
Fig. 39.
Penicillium steckii. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
= Penicillium corylophiloides S. Abe, J. gen. appl. Microbiol, Tokyo 2: 89. 1956 (nom. inval., Art. 36).
Typus: ex cotton fabric treated with copper naphthenate, Panama (IMI 40583 – neotype, designated by Pitt et al. 2000; cultures ex-type CBS 260.55 = DTO 22G5 = ATCC 10499 = CECT 2268 = DSM 1252 = NRRL 2140 = QM 6413 = NDRC 52B4C).
Description: Colony diam, 7 d, in mm: CYA 24–32; CYA15°C 7–13; CYA30°C 15–23; CYA37°C no growth; MEA 21–30; YES 29–40; DG18 24–36; ratio CYAS:CYA 1.0–1.2; creatine agar 11–19, weak to moderate growth, no or weak acid production.
Moderate or good sporulation on CYA, velvety, conidia grey green, mycelium inconspicuous, small clear or weak yellow exudate droplets, soluble pigments absent, reverse in shades of crème (crème, pale crème, yellow-crème or brown crème). Moderate to good sporulation on YES, conidia grey green, occasionally dull green, soluble pigments absent, reverse in most isolates (pale) yellow, sometimes orange. Good sporulation on DG18, conidia grey green conidia, reverse variable, pale, cream, (bright) yellow or yellow-orange. Good sporulation on MEA, conidia grey green or dull green, colony texture velvety. Ehrlich reaction negative, with the exception of CBS 122391.
Sclerotia absent. Conidiophores borne from surface hyphae, predominantly symmetrically biverticillate, occasionally with an additional branch; stipes smooth, 100–300 × 2.2–3.0 μm. Metulae in whorls of 3–6, 13–18 × 2.5–3.3 μm, equal in length. Phialides ampulliform, 7.0–10 × 2.2–3.0 μm. Conidia broadly ellipsoidal, in some strains slightly fusiform, smooth, 2.3–3.0× 2.0–2.5 μm.
Extrolites: Isochromantoxins (Cox et al. 1979, Malmstrøm et al. 2000), quinolactacin, tanzawaic acid E, “ALTI”, “EXPO”, “FON”, “FOS”, “GLOO”, “GYF”, “PHOE”, “RAI”, “STOK”, “SVUL”, and “VERN” (Houbraken et al. 2010).
Diagnostic characters: No growth at 37 °C, moderate growth at 33 °C; reverse colours on CYA in shades of crème, broadly ellipsoidal conidia.
Similar species: Penicillium steckii is phylogenetically related to P. sizovae, P. citrinum, P. hetheringtonii and P. gorlenkoanum. This species is characterised by the formation of broadly ellipsoidal conidia, which are not formed by any of the other species mentioned. Penicillium tropicoides and P. tropicum also form broadly ellipsoidal conidia, but also produce cleistothecia and ascospores.
Distribution and ecology: This species has a worldwide distribution and has been isolated in Japan, the Netherlands, Panama, Venezuela, Bermuda, Egypt, Venezuela, Indonesia and Slovenia. Penicillium steckii is isolated from cotton fabric treated with copper naphthenate, (potting) soil, hypersaline water, blue runner fish, baled coastal grass hay, artichokes, Ascidie (tunicate, urochordata), and as an endophyte of root of coffee plant (Posada et al. 2007).
Barcode & molecular based ID: GenBank no. GU944597. This species has a unique ITS sequence. A subgroup in the P. steckii clade was observed. This subgroup, characterised by a single basepair difference on position 164 of the ITS2 region, included the type strain of P. corylophiloides nom. inval. (CBS 325.59).
Taxonomy and phylogeny: Abe (1956) described P. corylophiloides without a Latin diagnosis and designation of a holotype. According to Abe (1956), P. corylophiloides could be differentiated from P. citrinum and P. steckii by the formation of ellipsoidal conidia. Houbraken et al. (2010) showed that P. steckii also formed broadly ellipsoidal conidia and both species were placed in synonymy. Following the phylogenetic species concept, P. steckii and P. corylophiloides are separate species; however, no differences in morphology, physiology or extrolites patterns could be observed and are therefore they are placed in synonymy (Houbraken et al. 2010).
Penicillium sumatrense von Szilvinyi, Archiv. Hydrobiol. 14, Suppl 6: 533. 1936. Fig. 40.
Fig. 40.
Penicillium sumatrense. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
= Penicillium baradicum Baghdadi, Novosti Sistematiki Nizshikh Rastenii 5: 107. 1968.
= Penicillium meleagrinum var. viridiflavum Abe, J. Gen. Appl. Microbiol., Tokyo 2: 92. 1956 (nom. inval.).
Typus: ex soil, Toba Heath, Sumatra, Indonesia (CBS 281.36 – lectotype, designated here; cultures ex-type DTO 22F1 = NRRL 779 = FRR 779 = ATCC 48669 = IBT 29658 = IBT 4978).
Description: Colony diam, 7 d, in mm: CYA 33–42; CYA15°C 10–16; CYA30°C (10–) 15–25; CYA37°C no growth; MEA 27–36; YES (26–) 32–42 (–47); DG18 (20–) 25–34; ratio CYAS:CYA 0.9–1.1; creatine agar 15–23, weak growth and acid production absent.
Moderate or good sporulation on CYA, occasionally absent, velvety, conidia dull-green or dark-green, mycelium inconspicuous, exudate absent or present as small or large (pale)-yellow droplets, occasionally clear or light brown, soluble pigments in most strains absent, in some isolates weakly produced and light brown coloured, margin entire, reverse in shades of beige, beige-brown or brown. Good sporulation on YES, conidia dull-green, mycelium inconspicuous, soluble pigments absent, reverse yellow. Good sporulation on DG18; conidia grey-green or dull-green, reverse pale or pale yellow. Sporulation on MEA variable, conidia blue-green, light green or grayish-green, mycelium inconspicuous, floccose colony texture in fresh isolates, velvety in strains maintained for longer periods in the collection. Ehrlich reaction negative.
Sclerotia absent. Conidiophores predominantly biverticillate, occasionally with an additional branch, stipes up to 200 μm long with smooth or finely rough walls, 2.0–3.0 μm wide. vMetulae in terminal verticils and fairly compact, 3–6, uneven in length, vesiculate and rather long (10–) 12–16 × 2.0–3.0 μm. Phialides ampulliform, 8.0–10 × 2–3.5 μm. Conidia subglobose or broadly ellipsoidal, finely roughened, occasionally smooth, 2.0–2.5 μm diam.
Extrolites: Curvularin (Vesonder et al. 1976, Malmstrøm et al. 2000), dehydrocurvularin, “POTO”, “SAAT”, “TERRIT”, “TIDL”, “VOX”.
Diagnostic characters: Growth on CYA incubated at 33 °C (cultures, which are maintained for long periods in culture collections, have a lower maximum growth temperature), beige-brown reverse on CYA, high growth rate on YES with yellow reverse.
Similar species: Penicillium sumatrense is phylogenetically distinct and differs from P. citrinum and P. hetheringtonii by its inability to grow at 37 °C. Penicillium paxilli has a pale reverse on CYA, appressed whorls of metulae and roughened stipes; P. steckii and P. sizovae lack a distinct yellow reverse on YES.
Distribution and ecology: This species has a worldwide distribution, but has a preference for (sub)tropical regions. Its main habitat is soil, but it has also been isolated from marine environments (Malmstrøm et al. 2000), as an endophyte of Vitis vinifera (Z. Wang & X. Qian, unpublished, GenBank no. EU030367), cork (Serra et al. 2008), packaging material imported into the Netherlands, pomegranates and bromeliad leaf tissue.
Barcode & molecular based ID: GenBank no. GU944578. This species has unique ITS, tubulin and calmodulin sequences.
Taxonomy and phylogeny: Penicillium sumatrense was formally considered a synonym of P. corylophilum (Pitt 1980), but Peterson (2000) and Houbraken & Samson (2011) showed that these two species are phylogenetically unrelated. The former species belongs to section Citrina (Peterson's group 1), and the latter to section Exilicaulis (Peterson's group 4). Penicillium meleagrinum var. viridiflavum was described without a Latin diagnosis, making the description invalid (Art. 36). Pitt et al. (2000) synonymised this species with P. janthinellum; however, Serra et al. (2008) showed that P. meleagrinum var. viridiflavum is genetically close to the type strain of P. sumatrense. The congruence of the phylograms from four different loci indicated that this could be a separate species (Serra et al. 2008). Our data (Fig. 4) also shows sequence variation among the analysed P. sumatrense strains. However, no differences in phenotype and extrolite patterns were detected among these strains and therefore they are maintained as one species. More research is needed to clarify the population structure of this species.
Penicillium terrigenum Seifert, Houbraken, Frisvad & Samson, sp. nov. MycoBank MB563204. Fig. 41.
Fig. 41.
Penicillium terrigenum. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Etymology: Referring to soil, the substrate from where the type strain was isolated.
Differt ab omnibus speciebus affinibus coloniis in agaro CYA bene crescentibus ad 33 °C, conidiis ellipsoideis, laevibus, conidiophoris biverticillatis.
Typus: ex soil, Hawaii, USA, R. A. Samson (CBS H-20667 – holotypus, cultures ex-type CBS 127354 = DTO 9D4 = IBT 30769).
Description: Colony diam, 7 d, in mm: CYA 28–36; CYA15°C 7–15; CYA30°C 18–23; CYA37°C no growth; MEA 25–32; YES 34–41; DG18 28–34; ratio CYAS:CYA 1.0–1.3; creatine agar 15–22, weak growth and no acid production.
Sporulation on CYA variable, velvety and floccose at the centre, conidia dull-grey green, mycelium white, exudate produced as minute clear droplets, soluble pigments absent, margin entire to slightly irregular, reverse pale or crème. Weak to moderate sporulation on YES, mycelium white, soluble pigment absent, reverse creme-yellow, occasionally with a green shade. Good sporulation on DG18, conidia dull green, reverse pale. Moderate to good sporulation on MEA, conidia dull or dull-grey green, colony texture floccose. Ehrlich reaction negative.
Sclerotia absent. Conidiophores predominantly symmetrically biverticillate, occasionally with an additional branch, stipes long, up to 500 μm, smooth to finely rough walled or distinctly rough walled (CBS 117967), 2.5–3.5 μm wide; metulae in a compact terminal whorls of 3–7, slightly vesiculate, equal in length, (10–) 12–16 × 2.0–3.5 μm; phialides ampulliform to cylindrical, 7.5–9 × 2.5–3.5 μm. Conidia broadly ellipsoidal, smooth, 2.0–3.0 × 2.0–2.5 μm.
Extrolites: “HAEN”, “ISOC”, “PRS”, “VERSI”.
Diagnostic characters: Good on CYA incubated at 33 °C, broadly ellipsoidal smooth walled conidia, biverticillate conidiophores.
Similar species: This species is phylogenetically related to P. copticola, but can be distinguished by its poor growth on CREA. Morphologically, this species is similar to P. steckii, which also forms broadly ellipsoidal conidia and is also able to grow at 33 °C. Penicillium steckii sporulates better on CYA and YES and has velvety colonies.
Distribution and ecology: This species is isolated from Hawaiian soil, a leaf surface, USA, a mushroom fairy ring in Oshawa, Ontario, Canada and soil in Portugal. A BLAST analysis showed that this species was also isolated from a French pastry product (brioche) (GenBank FJ471589, ITS).
Barcode & molecular based ID: GenBank no. JN617684. This species can be identified with ITS, β-tubulin and calmodulin sequences.
Taxonomy and phylogeny: CBS 127357 has an intermediate position between P. copticola and P. terrigenum (Fig. 4) and represents a new species. This strain resembles P. terrigenum in many aspects such as poor grow on CREA and the formation of broadly ellipsoidal conidia. However, it differs from P. copticola and P. terrigenum in its inability to grow at 33 °C. A more in-depth study with more P. copticola and P. terrigenum strains is needed to elucidate the taxonomy of this clade.
Penicillium tropicoides Houbraken, Frisvad & Samson, Fung. Divers. 44: 127. 2010. Fig. 42.
Fig. 42.
Penicillium tropicoides. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B. Ascomata. C–D. Ascospores. E–G. Conidiophores. H. Conidia. Scale bars = 10 μm.
Typus: ex rainforest soil, near Hua-Hin, Thailand (CBS 122410 – holotypus, cultures ex-type DTO 10C4 = IBT 29043).
Description: Colony diam, 7 d, in mm: CYA 24–30; CYA15°C 5–11; CYA30°C 15–25; CYA37°C no growth; MEA 18–23; YES 36–43; DG18 16–23; ratio CYAS:CYA 1.1–1.4; creatine agar 13–16, poor to moderate growth and weak acid production (under colony).
Sporulation on CYA inconspicuous or sparsely produced in fresh isolated strains, becoming more conidial after several transfers, conidia blue grey green, cleistothecia abundantly produced and determining colony colour, drab grey, mycelium inconspicuously, colonies typical with large hyaline exudate droplets, soluble pigments absent, margin slightly irregular, reverse crème-brown. Weak sporulation on YES, cleistothecia abundant present, drab-grey, soluble pigment absent, mycelium inconspicuously, reverse yellow. Weak to good sporulation on DG18, reverse yellow. Colonies on MEA ascomatal and sporulation absent or sparse, cleistothecia darb grey. Ehrlich reaction negative.
Cleistothecia sclerotioid, 200–300 μm diam, ripening slowly and mature after 3 mo on MEA and OA. Ascospores ellipsoidal, 2.5–3.5 × 1.5–2.5 μm, with two narrow, closely appressed equatorial ridges, valves smooth by light microscopy, warted with anastomosing ribs by SEM. Conidiophores arising from the mycelial mat, symmetrically biverticillate, stipes smooth, 100–250 μm long, 2.5–3.5 μm wide. Metulae in whorls of 2–5, 13–17 × 3.0–4.0 μm, uniform in length. Phialides ampulliform, 8.5–10.5 x 2.0–3.0 μm. Conidia broadly ellipsoidal, smooth, 2.0–3.0 × 2.0–2.5 μm.
Extrolites: Isochromantoxins, several apolar indol-alkaloids, “CITY”, “HOLOX”, “PR1-x”, “RAIMO” (Houbraken et al. 2010).
Diagnostic characters: Slow growth at 30 °C, no growth at 37 °C, abundant production of drab-grey cleistothecia, maturing after prolonged incubation, over 3 months.
Similar species: Penicillium tropicoides morphologically resembles P. tropicum. The difference between P. tropicoides and P. tropicum is the slower maturation of the cleistothecia, slower growth rate at 30 °C and the production of isochromantoxins by P. tropicoides. Penicillium shearii is related, but can be differentiated by a higher maximum growth temperature than P. tropicoides and P. tropicum.
Distribution and ecology: Penicillium tropicoides is isolated form rainforest soil in Thailand.
Barcode & molecular based ID: GenBank no. GU944584. Penicillium tropicoides and P. tropicum have no differences in their ITS regions, but these species can be differentiated with β-tubulin and calmodulin sequences.
Taxonomy and phylogeny: None.
Penicillium tropicum Houbraken, Frisvad & Samson, Fung. Divers. 44: 129. 2010. Fig. 43.
Fig. 43.
Penicillium tropicum. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B. Sclerotia. C. Ascospores. D–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
= Eupenicillium tropicum Tuthill & Frisvad Mycological Progress 3: 14. 2004.
Typus: ex soil beneath Coffea arabica, Karnataka, India (SC42-1 – holotype, cultures ex-type DTO 31B1 = CBS 112584 = IBT 24580).
Description: Colony diam, 7 d, in mm: CYA 24–31; CYA15°C 9–13; CYA30°C 25–30; CYA37°C no growth; MEA 23–27; YES 33–37; DG18 20–25; ratio CYAS:CYA 1.0–1.1; creatine agar 16–20, poor growth and weak acid production.
Sporulation on CYA sparse, conidia blue grey green, cleistothecia abundantly produced, orange-tan, becoming warm shades of grey (brownish-grey) in age, mycelium inconspicuous; exudate copious produced in large, hyaline droplets, soluble pigments absent, reverse crème coloured. Weak sporulation on YES, cleistothecia abundantly produced, deep dull grey, mycelium inconspicuous, soluble pigment absent, reverse crème-yellow. Good sporulation on DG18, conidia blue-grey green, reverse pale or very pale yellow. Colonies on MEA ascomatal, in shades of grey, sporulation absent or inconspicuous. Ehrlich reaction negative.
Cleistothecia sclerotioid, 200–300 μm diam, ripening within 3–6 wk on MEA and OA. Ascospores ellipsoidal, 2.5–3 × 2–2.5 μm, with two narrow, closely appressed equatorial flanges and finely roughened valves. Conidiophores arising from the mycelial mat, symmetrically biverticillate, stipes smooth, 2.5–3.5 μm wide; metulae in whorls of 2–5 (–8), 12–16 × 2.5–3.5 μm. Phialides ampulliform, 8.0–10.5 × 2.0–3.0 μm. Conidia broadly ellipsoidal, smooth, 2.3–3.0 × 2.0–2.5 μm.
Extrolites: Several apolar indol-alkaloids, “CITY”,”EMON”,”HOLOX” and “RAIMO” (Tuthill & Frisvad 2004, Houbraken et al. 2010).
Diagnostic characters: No growth at 37 °C, abundant production of cleistothecia in warm shades of grey (brownish grey), maturing within 2–5 wk, colonies on CYA incubated at 30 °C reaching a diameter of 25–30 mm.
Similar species: See P. tropicoides.
Distribution and ecology: Penicillium tropicum has been isolated from (sub)tropical soils (e.g. India, Costa Rica, Ecuador and Galapagos Islands).
Barcode & molecular based ID: GenBank no. GU944582. Penicillium tropicum and P. tropicoides have no differences in their ITS regions, but these species can be differentiated with β-tubulin and calmodulin sequences.
Taxonomy and phylogeny: None.
Penicillium ubiquetum Houbraken, Frisvad & Samson, sp. nov. MycoBank MB563201. Fig. 44.
Fig. 44.
Penicillium ubiquetum. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Etymology: Named after the worldwide distribution of this species.
Differt ab omnibus speciebus affinibus conidiis subtiliter exasperatis, coloniis ad 30 °C haud crescentibus, coloniis in agaro MEA ad 25 °C post hebdomatem usque ad 18–26 mm, reverso plus minusve aurantiaco vel roseo-rubro in agaro YES.
Typus: ex soil, Wilson Botanical Garden, Costa Rica, M. Christensen (CBS H-20659 – holotypus, cultures ex-type CBS 126437 = DTO 78B5 = IBT 22226).
Description: Colony diam, 7 d, in mm: CYA 24–34; CYA15°C 14–18; CYA30°C and CYA37°C no growth; MEA 18–26; YES 30–36; DG18 18–27; ratio CYAS:CYA 1–1.3; creatine agar 13–18, weak to moderate growth and no acid production.
Good sporulation on CYA, velvety, conidia dull-green to dark green, mycelium inconspicuous, exudates clear, soluble pigments absent, margin entire, reverse flesh coloured, pinkish-brown with orange centre, often with orange pigmented sulcations. Good sporulation on YES, mycelium white, soluble pigments absent, reverse in shades of orange to pinkish-red. Good sporulation on DG18, conidia in shades of dull green, reverse pale, pale with yellow centre or bright yellow. Moderate to good sporulation MEA, conidial colour variable: blue-green or bluish-grey green or dark-blue green, colony texture floccose. Ehrlich reaction negative.
Sclerotia absent. Conidiophores symmetrically biverticillate, occasionally with an divergent branch that is shorter than the main axis; stipes shorter than most related species, up to 300 μm, smooth, 2.5–4.0 μm wide; metulae in a compact terminal verticil, 3–7 (–9), unequal in length, vesiculate, (8–) 10–15 × 2.0–3.5 μm. Phialides ampulliform, stout, 6.0–8.0 × 1.5–3.0 μm. Conidia globose to subglobose, finely roughened, strongly pigmented cell wall, 1.8–2.5 μm diam.
Extrolites: Citrinin, terrein, “ALK”, “GLYF”, “RAI”, “TRIP”, “XANTHOC”, isolates in one subclade, CBS 126438 & CBS 126436 produce anthraquinone bisanthrons, citrinin, okaramins, and “SENGA”.
Diagnostic characters: Finely roughened conidia, no growth at 30 °C, colonies on MEA attaining a diameter of 18–26 mm in 7 d at 25 °C, reverse on YES in shades of orange to pinkish-red.
Similar species: See P. pancosmium.
Distribution and ecology: Soil appears to be the primary habitat, but this species is also isolated from cork bark (GenBank no.v EF198586, as P. decaturense). Worldwide distribution: Queensland, Australia, Wisconsin, USA, Madagascar, Costa Rica, Italy, Portugal.
Barcode & molecular based ID: GenBank no. JN617680 (1) and JN617677 (2). Penicillium ubiquetum can be divided in two groups based on ITS sequences. ITS sequences of group 1 are unique (incl. CBS 126437T); group 2 ITS sequences of P. ubiquetum are identical with P. waksmanii (CBS 126438, CBS 126436 and NRRL 35636). Figure 3 shows that the latter isolates also form a subgroup based on partial β-tubulin and calmodulin sequences.
Taxonomy and phylogeny: CBS 126438 and CBS 126436 have a distinct extrolite pattern and differ in their ITS sequence with other P. ubiquetum strains. However, no phenotypic or physiological differences were observed among these and other P. ubiquetum strains, and therefore these strains are all regarded as one species.
Penicillium vancouverense Houbraken, Frisvad & Samson, sp. nov. MycoBank MB563207. Fig. 45.
Fig. 45.
Penicillium vancouverense. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Etymology: Named after the location of the type strain, Vancouver (Canada).
Differt ab omnibus speciebus affinibus mycelio flavido (vulgo in CYA15°C et/vel YES), conidiis glaucoviridibus in agaro MEA et conidiis subtiliter exasperatis, crassitunicatis.
Typus: ex soil under Maple tree, Vancouver, BC, Canada, J.C. Frisvad (CBS H-20646 – holotypus, cultures ex-type. CBS 126323 = DTO 82B8 = IBT 20700).
Description: Colony diam, 7 d, in mm: CYA 20–30; CYA15°C 17–25; CYA30°C and CYA37°C: no growth; MEA 16–23; YES 23–33; DG18 17–25; ratio CYAS:CYA 1.0–1.3; creatine agar 8–17, weak growth and no acid production.
Weak to moderate sporulation on CYA, velvety to floccose, conidia grey-green, mycelium light-yellow, often with minute clear or yellow exudates droplets, soluble pigment production variable, if produced yellow coloured, margin entire, reverse in shades of orange-brown or brown. Moderate to good sporulation on YES, conidia dull green, occasionally dull-blue green, mycelium in shades of yellow, soluble pigments absent, reverse beige or beige-brown. Good sporulation on DG18, conidia dull-green, reverse pale or yellow. Moderate to good sporulation on MEA, conidia blue green, colony texture velvety to floccose. Ehrlich reaction negative, with exception of CBS 126324.
Sclerotia absent. Conidiophores predominantly symmetrically biverticillate and, depending on the isolate, additional branches occur; stipes 200–400 μm long, smooth or finely rough walled, width variable, 2.0–4.0 μm; metulae in a compact terminal whorls of 3–6 (–7), unequal in length, often vesiculate, 10–14 × 2.5–3.5 μm. Phialides ampulliform, 7–9 × 2.0–3.5 μm. Conidia subglobose, finely roughened and with a distinct thick and pigmented cell wall, 2.0–3.0 μm diam.
Extrolites: The extrolite patterns of P. vancouverense isolates are somewhat diverse. All strains produce citrinin, citreoviridin, “MIF”, “PAS” and “met OE”. Some strains also produce “CANOT”, “MIM”, “PHOE” and “XANTHOC”.
Diagnostic characters: Light yellow mycelium (especially on CYA15°C and/or YES), blue green conidia on MEA and finely roughened, thick walled conidia.
Similar species: Penicillium vancouverense is phylogentically related to P. pasqualense, but the latter species does not have yellow mycelium and has a dark-brown reverse on CYA. Penicillium manginii and some strains of P. miczynskii and related species also form yellow mycelium; P. manginii can be differentiated by the faster growth rate on CYA and the red soluble on YES; P. miczynskii and related species have smooth walled, subglobose to broadly ellipsoidal conidia.
Distribution and ecology: Penicillium vancouverense has a worldwide distribution (the Netherlands, Costa Rica, Chile, California, USA, Queensland, Australia, Madagascar, BC and Ontario, Canada). Soil appears to be the primary habitat, but this species is also isolated from indoor air of a house and a nut of Juglans cinerea (butternut).
Barcode & molecular based ID: GenBank no. JN617675. With the exception of isolate CBS 126321, all investigated strains have the same unique ITS sequence. Isolate CBS 126321 has one base pair difference in the ITS region compared with the type isolate CBS 126324T.
Taxonomy and phylogeny: Penicillium miczynskii is characterised by the production of yellow pigmented mycelium, exudates and reverses (Pitt 1980, Christensen et al. 1999). These features are also characteristic for P. vancouverense and it is therefore likely that P. vancouversense isolates were previously identified as P. miczynskii. This study shows that P. miczynskii forms smooth walled conidia and stipes. There is some variation in extrolite profiles and sequences detected among the P. vancouverense isolates. The different extrolite profiles do not correlate with the clustering observed in the phylogenetic study.
Penicillium waksmanii Zaleski, Bull. Int. Acad. pol. Sci. Lett., Sér. B.: 468. 1927. Fig. 46.
Fig. 46.
Penicillium waksmanii. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
= Penicillium rivolii Zaleski, Bull. Int. Acad. pol. Sci. Lett., Sér. B: 471. 1927.
Typus: ex woodland soil, Purczcza Bialowieska Forest, Poland (Herb. IMI 39746i – lectotype, Pitt 1980; cultures ex-type CBS 230.28 = DTO 22E6 = ATCC 10516 = FRR 777 = IFO 7737 = IMI 039746 = MUCL 29120 = NRRL 777 = QM 7681 = IBT 5003 = IBT 6994).
Description: Colony diam, 7 d, in mm: CYA (20–) 25–32; CYA15°C 10–19; CYA30°C and CYA37°C no growth; MEA 18–24(–30); YES 25–33; DG18 16–27; ratio CYAS:CYA 1.0–1.2; creatine agar 10–18, weak growth and no acid production.
Moderate sporulation on CYA, velvety, conidia dull green, mycelium inconspicuous, exudate absent or as very small clear droplets, soluble pigment absent, entire margins, reverse beige or beige-brown. Sporulation on YES moderate, mycelium white, conidia dull green, soluble pigments absent, reverse beige or beige-brown. Grey green conidia on DG18, reverse pale. Colonies on MEA dull-grey green, colony texture velvety with floccose centre. Ehrlich reaction negative.
Sclerotia absent. Conidiophores symmetrically biverticillate and often with a divergent branch, stipes up to 200–500 μm long, smooth, 2.5–3.5 μm wide; metulae in a compact terminal verticil, 5–6 (–8), unequal in length, vesiculate, 10–14 × 2.5–3.5 μm; phialides ampulliform, 7.0–9.0 × 2–3 μm. Conidia globose to subglobose, finely roughened, 2.0–2.5 μm diam.
Extrolites: Citrinin, cyclopiamin, meleagrin (only produced by one isolate), “GLYF”, “PAS”, “SENGA”.
Diagnostic characters: Finely roughened conidia, no growth at 30 °C, reverse on CYA in shades of brown and a pale reverse on DG18.
Similar species: See P. chrzaszczii.
Distribution and ecology: Soil appears to be primary habitat, but this species was also isolated from a dead polypore; strains have been isolated from Poland, New Mexico, USA and New Zeeland.
Barcode & molecular based ID: GenBank no. GU944602. Some strains of P. ubiquetum (NRRL 35636 and CBS 126436) share ITS sequences with P. waksmanii. Partial β-tubulin and calmodulin sequences can be used for identification.
Taxonomy and phylogeny: Pitt (1980) accommodated P. waksmanii in the series Fellutana of the subgenus Furcatum based on the production of irregular conidiophores, while members of the series Citrina produce regular, terminal penicilli. Microscopical analysis of freshly isolated P. waksmanii strains from Polish soil show that this species also forms regularly biverticillate structures, often with an additional branch. Furthermore, phylogenetical analysis clearly indicates a close relationship with P. godlewskii. Peterson (2000) suggested that P. rivolii was a distinct species, because 2 nucleotide differences were observed between the ITS2 region of P. waksmanii and P. rivolii. However, this observation could not be confirmed and our data suggests that the names are conspecific. Zaleski (1927) described the production of orange pigment in this species. This is not observed in our ex-type strain and recent isolated strains of P. waksmanii. CBS 126426 produces, as the only isolate in this species, an anthraquinone, which may be the orange pigment.
Penicillium wellingtonense AJL Cole, Houbraken, Frisvad & Samson, sp. nov. MycoBank MB563208. Fig. 47.
Fig. 47.
Penicillium wellingtonense. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Etymology: Named after location of the type strain, Wellington (New Zealand).
Differt ab omnibus speciebus affinibus, coloniis in agaro CYA, MEA et YES constricte crescentibus, ratione incrementi meliore ad 15 °C quam 25 °C.
Typus: ex soil, Wellington, New Zealand, A.J.L. Cole (CBS H-20657 – holotypus, cultures ex-type CBS 130375 = DTO 76C6 = IBT 23557).
Description: Colony diam, 7 d, in mm: CYA 10–15; CYA15°C 18–23; CYA30°C and CYA37°C no growth; MEA 8–13; YES 20–25; DG18 13–17; ratio CYAS:CYA 1.2–1.4; creatine agar 8–12, weak growth and no acid production.
Moderate sporulation on CYA, velvety, conidia grey-green, mycelium inconspicuous, exudate absent, soluble pigment absent, reverse orange. Good sporulation on YES, conidia grey-green, soluble pigments absent, reverse beige-brown. Dull-green colonies on DG18, soluble pigments yellow, reverse reverse bright yellow. Colonies on MEA blue-green, colony texture velvety and wrinkled surface. Ehrlich reaction negative.
Sclerotia absent. Conidiophores predominantly symmetrically biverticillate and occasionally with a branch; stipes rather long, 200–400 μm, smooth, 2.5–3.5 μm wide; metulae in a compact terminal vertical, 3–7, unequal in length, short and stout, 9–12 × 3.0–4.0 μm; phialides ampulliform, 7.5–9.5 × 2.5–3.5 μm. Conidia subglobose to broadly ellipsoidal, smooth to finely roughened, variable in size, 2.5–3.0 × 2.5–3.0 μm.
Extrolites: Citrinin, decaturin, “MIF”, “met Q”, “POF”, “RAI”, “TRIP”, “XANTHOC”.
Diagnostic characters: Restricted growth on CYA, MEA and YES and a higher growth rate at 15 °C than at 25 °C.
Similar species: This species is unique in its slow growth rate. Penicillium nothofagi is phenotypically similar. This species has a pale-beige reverse on CYA and P. wellingtonense has an orange reverse on CYA.
Distribution and ecology: This species is only known from its type culture, isolated from soil, New Zealand.
Barcode & molecular based ID: GenBank no. JN617713. This species has a unique β-tubulin, calmodulin and ITS sequence.
Taxonomy and phylogeny: Penicillium wellingtonense is phylogenetically basal to P. vancouverense.
Penicillium westlingii Zaleski, Bull. Int. Acad. pol. Sci. Lett., Sér. B.: 473. 1927. Fig. 48.
Fig. 48.
Penicillium westlingii. A. 7 d old cultures, 25 °C, left to right; first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, DG18 reverse, CREA obverse. B–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
= P. citrinum var. pseudopaxilli Martínez & Ramírez, nomen nudum.
Typus: ex soil under conifer, Denga Goolina, Poznan, Poland (IMI 92272 – neotype, designated by Pitt et al. 2000; cultures ex-type CBS 231.28 = DTO 22E7 = IBT 15088).
Description: Colony diam, 7 d, in mm: CYA (25–) 30–36; CYA15°C 15–22; CYA30°C no growth or germination (0–3); CYA37°C no growth; MEA 25–34; YES 33–40; DG18 16–28; ratio CYAS:CYA 0.9–1.1; creatine agar 10–17, weak or moderate growth and no acid production.
No or sparse sporulation on CYA, white mycelium, exudate absent, soluble pigments absent, margin polygonal, reverse pale, pale-beige or pinkish beige. Sporulation on YES absent, mycelium white, soluble pigments absent, reverse pale yellow (cream) to cream-buff. Variable sporulation on DG18, conidia dull green or grey green, reverse pale or pale-cream. Colonies on MEA poorly sporulating, conidia blueish-dark green, colony texture floccose. Ehrlich reaction negative.
Sclerotia absent. Conidiophores symmetrically biverticillate, often with a divergent branch that is shorter than the main axis, occasionally quaterverticillate, stipes up to 500 μm long, smooth, 2.5–4.0 μm wide; metulae in a compact terminal verticil, 3–6 (–8), mostly uniform in length, both vesiculate and non-vesiculate, 8–14 (–16) × 2.0–3.5 μm. Phialides ampulliform, 6.5–8.5 × 2–3 μm. Conidia globose, finely or distinct roughened, 1.8–2.5 μm diam.
Extrolites: Citrinin, curvularin, dehydrocurvularin,”PHOE”, “TRIP”, “XANTHOC”.
Diagnostic characters: Finely roughened conidia, no (or at most very restricted) growth at 30 °C, reverse on CYA pale or pale-beige or pinkish beige, YES pale yellow to cream, no sporulation on CYA and YES.
Similar species: Penicillium westlingii is phylogenetically related to P. nothofagi and P. cosmopolitanum. It differs from P. nothofagi by its faster growth rate on CYA, YES and MEA. Penicillium cosmopolitanum generally has warmer reverse colours on CYA (with orange coloured sulcations) and larger conidia (2.5–3.0 μm diam). Penicillium westlingii is morphologically similar to P. pancosmium and P. ubiquetum, but the latter two species sporulate well on CYA. Penicillium waksmanii is also similar, but P. westlingii has a lighter reverse on CYA and a faster growth rate on CYA and YES.
Distribution and ecology: This species commonly occurs in soils in temperature regions, but is also isolated from a nut of Juglans nigra (black walnut), acorns of Quercus, moose dung and indoor environments.
Barcode & molecular based ID: GenBank no. GU944601. The majority of the investigated P. westlingii isolates have the same and unique ITS sequence, though several P. westlingii isolates (CBS 124312, CBS 124313, CBS 127003, CBS 127040) share sequences with certain isolates of P. cosmopolitanum. These strains also appear in separate subclades in Fig. 4.
Taxonomy and phylogeny: Raper & Thom (1949) and Pitt (1980) placed P. westlingii in synonymy of P. waksmanii. Pitt (1980) noted that P. westlingii grows faster than P. waksmanii, but decided that this was insufficient to describe P. westlingii as a separate species. Peterson (2000) showed that P. westlingii and P. waksmanii are genetically distinct and numerous (99 total) nucleotide differences were detected. Re-examination of these sequences shows that the deposited sequence of P. westlingii NRRL 800T (GenBank no. AF033423) is not the same as CBS 231.28T. Comparison of the sequence of NRRL 800 shows that P. westlingii is the same or very closely related to P. citrinum, while the sequence obtained in this study indicates a relation with P. waksmanii.
Supplemental Information
Acknowledgments
Barbara Byskal is thanked for collecting soil samples of Polish soil and Martin Meijer and Edith de Rijk are thanked for the isolation and sequencing of Penicillium species from those samples. Jelle Bos is acknowledged for performing the temperature growth experiments and Ellen Kirstine Lyhne for her technical support. We are also grateful to Gerry Louis-Seize for generating sequences, Keith Seifert, Dorothy Tuthill and Martha Christensen for sharing strains and sequences, and Marjan Vermaas for preparing the photographic plates. Uwe Braun is acknowledged for providing Latin diagnoses. Research grants from Natural Sciences and Engineering Research Council of Canada (NSERC) supported David Malloch, who shared Penicillium strains used in this study.
REFERENCES
- Abe S. (1956). Studies on the classification of the Penicillia. Journal of General and Applied Microbiology 2: 1–193 [Google Scholar]
- Baghdadi VC. (1968). De speciebus novis Penicilli Fr. et Aspergilli Fr. E terries Syriae isolatis notula. Novitates Systematicae Plantarum non Vascularium 7: 96–114 [Google Scholar]
- Belofsky GN, Gloer JB, Wicklow DT, Dowd PF. (1995). Anti-insectan alkaloids. Shearinines A-C and a new paxilline derivative from the ascostromata of Eupenicillium shearii. Tetrahedron 51: 3959–3968 [Google Scholar]
- Beyma Thoe Kingma FH van. (1940). Beschreibung einiger neuer Pilzarten aus dem Centraalbureau voor Schimmelcultures, Baarn (Nederland) VI. Antonie van Leeuwenhoek 6: 263–290 [PubMed] [Google Scholar]
- Brefeld O. (1874). Botanische Untersuchungen über Schimmelpilze. Heft 2. “Die Entwicklungsgeschichte von Penicillium”. Leipzig: A. Felix; [Google Scholar]
- Christensen M, Frisvad JC, Tuthill D. (1999). Penicillium miczynskii and related species. Mycological Research 103: 527–541 [Google Scholar]
- Cox RH, Hernandez O, Dorner JW, Cole RJ, Fennell DI. (1979) A new isochroman mycotoxin isolated from Penicillium steckii. Journal of Agricultural and Food Chemistry 5: 999–1001 [DOI] [PubMed] [Google Scholar]
- Frisvad JC. (1985). Creatine-sucrose agar, a differential medium for mycotoxin producing terverticillate Penicillium species. Letters in Applied Microbiology 1: 109–113 [Google Scholar]
- Frisvad JC. (1989). The connection between the penicillia and aspergilla and mycotoxins with special emphasis on misidentified isolates. Archives of Environmental Contamination and Toxicology 18: 452–467 [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Filtenborg O. (1990). Revision of Penicillium subgenus Furcatum based on secondary metabolites and conventional characters. In: Modern Concepts in Penicillium and Aspergillus Classification (Samson RA, Pitt JI, eds) Plenum Press, New York: 159–172 [Google Scholar]
- Frisvad JC, Samson RA. (2004). Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of the food and air-borne terverticillate Penicillia and their mycotoxins. Studies in Mycology 49: 1–173 [Google Scholar]
- Frisvad JC, Samson RA, Stolk AC. (1990). Notes on the typification of some species of Penicillium. Persoonia 14: 193–202 [Google Scholar]
- Frisvad JC, Samson RA, Stolk AC. (1990). Disposition of recently described species of Penicillium. Persoonia 14: 209–232 [Google Scholar]
- Frisvad JC, Thrane U. (1987). Standardized high-performance liquid chromatography of 182 mycotoxins and other fungal metabolites based on alkylphenone retention indices and UV-VIS spectra (diode array detection). Journal of Chromatography 404: 195–214 [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Thrane U, Samson RA, Pitt JI. (2006). Important mycotoxins and the fungi which produce them. Advances in Experimental Medicine and Biology 571: 3–31 [DOI] [PubMed] [Google Scholar]
- Gazis R, Chaverria P. (2010). Diversity of fungal endophytes in leaves and stems of wild rubber trees (Hevea brasiliensis) in Peru. Fungal Ecology 3: 240–254 [Google Scholar]
- Houbraken J, Due M, Varga J, Meijer M, Frisvad JC, Samson RA. (2007). Polyphasic taxonomy of Aspergillus section Usti. Studies in Mycology 59: 107–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbraken J, Frisvad JC, Samson RA. (2010). Taxonomy of Penicillium citrinum and related species. Fungal Diversity 44: 117–133 [Google Scholar]
- Houbraken J, López Quintero CA, Frisvad JC, Boekhout T, Theelen B, et al. (2011a). Five new Penicillium species, P. araracuarense, P. elleniae, P. penarojense, P. vanderhammenii and P. wotroi, from Colombian leaf litter. International Journal of Systematic and Evolutionary Microbiology 61: 1462–1475 [DOI] [PubMed] [Google Scholar]
- Houbraken J, Samson RA. (2011). Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Studies in Mycology 70: 1–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbraken J, Spierenburg H, Frisvad JC. (2011b). Rasamsonia, a new genus comprising thermotolerant and thermophilic Talaromyces and Geosmithia species. Antonie van Leeuwenhoek. DOI: 10.1007/s10482-011-9647-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Hamayun M, Yoon H, Kim H-Y, Suh S-J, et al. (2008). Plant growth promotion and Penicillium citrinum. BMC Microbiology 8: 231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlovskiĭ AG, Stefanmova-Avramova LR, Reshitilova TA. (1981a). The effect of culture age and medium composition on the biosynthesis of alkaloids in Penicillium gorlenkoanum. Microbiologiya 50: 1046–1052 [PubMed] [Google Scholar]
- Kozlovskiĭ AG, Stefanmova-Avramova LR, Reshitilova TA, Sakharovskiĭ VG, Adanin VM. (1981b). Clavine ergot alkaloids, metabolites of Penicillium gorlenkoanum. Prikladnaia Biokhimiia i Mikrobiologiia 17: 806–812 [PubMed] [Google Scholar]
- Lai S, Matsunaga K, Shizuri Y, Yamamura S. (1990). Biomimetic synthesis of citreoviridin-type compounds and isolation of epicitreoviridinol, a new metabolite of Penicillium pedemontanum IFO 9583. Tetrahedron Letters 31: 5503–5506 [Google Scholar]
- Lund F. (1995). Differentiating Penicillium species by detection of indole metabolites using a filter paper method. Letters in Applied Microbiology 20: 228–231 [Google Scholar]
- Malmstrøm J, Christophersen C, Frisvad JC. (2000). Secondary metabolites characteristic of Penicillium citrinum, Penicillium steckii and related species. Phytochemistry 54: 301–309 [DOI] [PubMed] [Google Scholar]
- McMillan LK, Carr RL, Young CA, Astin JW, Lowe RG, et al. (2003). Molecular analysis of two cytochrome P450 monooxygenase genes required for paxilline biosynthesis in Penicillium paxilli, and effects of paxilline intermediates on mammalian maxi-K ion channels. Molecular Genetics and Genomics 270: 9–23 [DOI] [PubMed] [Google Scholar]
- Nagel DW, Steyn PS, Scott DB. (1972). Production of citreoviridin by Penicillium pulvillorum. Phytochemistry 11: 627–630 [Google Scholar]
- Nielsen KF, Månsson M, Rank C, Frisvad JC, Larsen TO. (2011). Dereplication of microbial natural products by LC-DAD-TOFMS. Journal of Natural Products. Doi: 10.1021/np200254t. In press. [DOI] [PubMed] [Google Scholar]
- Page RDM. (1996). TREEVIEW: an application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences 12: 357–358 [DOI] [PubMed] [Google Scholar]
- Peterson SW. (2000a). Phylogenetic analysis of Penicillium species based on ITS and LSU-rDNA nucleotide sequences. In: Integration of modern taxonomic methods for Penicillium and Aspergillus classification (Samson RA, Pitt JI, eds) Plenum Press, New York: 163–178 [Google Scholar]
- Peterson SW, Horn BW. (2009). Penicillium parvulum and Penicillium georgiense, sp. nov., isolated from the conidial heads of Aspergillus species. Mycologia 101: 71–83 [DOI] [PubMed] [Google Scholar]
- Peterson SW, Bayer EM, Wicklow DT. (2004). Penicillium thiersii, Penicillium angulare and Penicillium decaturense, new species isolated from wood-decay fungi in North America and their phylogenetic placement from multilocus DNA sequence analysis. Mycologia 96: 1280–1293 [PubMed] [Google Scholar]
- Pitt JI. (1980). The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press, London: [Google Scholar]
- Pitt JI, Hocking AD. (2009). Fungi and food spoilage. New York: Springer; [Google Scholar]
- Pitt JI, Samson RA. (1993). Species names in current use in the Trichocomaceae (Fungi, Eurotiales). Koeltz Scientific Books, Königstein: [Google Scholar]
- Pitt JI, Samson RA, Frisvad JC. (2000). List of accepted species and their synonyms in the family Trichocomaceae. In: Integration of modern methods for Penicillium and Aspergillus classification (Samson RA, Pitt JI, eds). Harwood Academic Publishers: Amsterdam: 9–49 [Google Scholar]
- Pollock AV. (1947). Production of citrinin by five species of Penicillium. Nature 160: 331–332 [DOI] [PubMed] [Google Scholar]
- Posada F, Aime M, Peterson SW, Rehner SA, Vega F. (2007). Inoculation of coffee plants with the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales). Mycological Research 111: 748–757 [DOI] [PubMed] [Google Scholar]
- Raper KB, Thom C. (1949). Manual of the Penicillia, Williams & Wilkins; [Google Scholar]
- Ramírez C. (1982). Manual and atlas of the Penicillia. Amsterdam: Elsevier Biomedical Press; [Google Scholar]
- Ramírez C, Martínez AT. (1981). Seven new species of Penicillium and a new variety of Penicillium novae-caledoniae Smith. Mycopathologia 74: 35–49 [Google Scholar]
- Rebuffat S, Davoust D, Molho L, Molho D. (1980). La citréomontanine, nouvelle α-pyrone polyéthylénique isolée de Penicillium pedemontanum. Phytochemistry 19: 427–431 [Google Scholar]
- Rebuffat S, Molho D, Dizabo P. (1984). Mass spectra of citreoviridin A and related mycotoxins. Organic Mass Spectrometry 19: 349–351 [Google Scholar]
- Samson RA, Houbraken J, Thrane U, Frisvad JC, Andersen B. (2010). Food and Indoor Fungi, CBS laboratory manual series 2, CBS-Fungal Biodiversity Centre, Utrecht: [Google Scholar]
- Shear CL. (1934). Penicillium glaucum of Brefeld (Carpenteles of Langeron) refound. Mycologia 26: 104–107 [Google Scholar]
- Serra R, Peterson SWCTCOR Venâncio A. (2008). Multilocus sequence identification of Penicillium species in cork bark during plank preparation for the manufacture of stoppers. Research in Microbiology 159: 178–186 [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. (2008). A rapid bootstrap algorithm for the RAxML Web-Servers. Systematic Biology 75: 758–771 [DOI] [PubMed] [Google Scholar]
- Stolk AC, Samson RA. (1983). The ascomycete genus Eupenicillium and related Penicillium anamorphs. Studies in Mycology 23: 1–149 [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. (2011). MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom C. (1930). The Penicillia. Williams & Wilkins, Baltimore: 1–644 [Google Scholar]
- Tuthill DE, Frisvad JC. (2004). A new species from tropical soils, Eupenicillium tropicum. Mycological Progress 3: 13–18 [Google Scholar]
- Vega FE, Posada F, Peterson SW, Gianfagna TJ, Chaves F. (2006). Penicillium species endophytic in coffee plants and ochratoxin A production. Mycologia 98: 31–42 [DOI] [PubMed] [Google Scholar]
- Young C, McMillan L, Telfer E, Scott B. (2001). Molecular cloning and genetic analysis of an indole-diterpene gene cluster from Penicillium paxilli. Molecular Microbiology 39: 754–764 [DOI] [PubMed] [Google Scholar]
- Zaleski KM. (1927). Über die in Polen gefundenen Arten der Gruppe Penicillium Link. I, II and III Teil. Bulletin de l’Académie Polonaise des Sciences et des Lettres, Classe des Sciences Mathématiques et Naturelles – Série B: Sciences Naturelles: 417–563, pls 36–44 (printed in 1928) [Google Scholar]
- Zhang Y, Li C, Swenson DC, Gloer JB, Wicklow DT, Dowd PF. (2003). Novel antiinsectan oxalicine alkaloids from two undescribed fungicolous Penicillium spp. Organic Letters 5: 773–776 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.