Abstract
The taxonomic history of anamorphic species attributed to Penicillium subgenus Biverticillium is reviewed, along with evidence supporting their relationship with teleomorphic species classified in Talaromyces. To supplement previous conclusions based on ITS, SSU and/or LSU sequencing that Talaromyces and subgenus Biverticillium comprise a monophyletic group that is distinct from Penicillium at the generic level, the phylogenetic relationships of these two groups with other genera of Trichocomaceae was further studied by sequencing a part of the RPB1 (RNA polymerase II largest subunit) gene. Talaromyces species and most species of Penicillium subgenus Biverticillium sensu Pitt reside in a monophyletic clade distant from species of other subgenera of Penicillium. For detailed phylogenetic analysis of species relationships, the ITS region (incl. 5.8S nrDNA) was sequenced for the available type strains and/or representative isolates of Talaromyces and related biverticillate anamorphic species. Extrolite profiles were compiled for all type strains and many supplementary cultures. All evidence supports our conclusions that Penicillium subgenus Biverticillium is distinct from other subgenera in Penicillium and should be taxonomically unified with the Talaromyces species that reside in the same clade. Following the concepts of nomenclatural priority and single name nomenclature, we transfer all accepted species of Penicillium subgenus Biverticillium to Talaromyces. A holomorphic generic diagnosis for the expanded concept of Talaromyces, including teleomorph and anamorph characters, is provided. A list of accepted Talaromyces names and newly combined Penicillium names is given. Species of biotechnological and medical importance, such as P. funiculosum and P. marneffei, are now combined in Talaromyces. Excluded species and taxa that need further taxonomic study are discussed. An appendix lists other generic names, usually considered synonyms of Penicillium sensu lato that were considered prior to our adoption of the name Talaromyces.
Taxonomic novelties:
Taxonomic novelties: New species – Talaromyces apiculatus Samson, Yilmaz & Frisvad, sp. nov. New combinations and names – Talaromyces aculeatus (Raper & Fennell) Samson, Yilmaz, Frisvad & Seifert, T. albobiverticillius (H.-M. Hsieh, Y.-M. Ju & S.-Y. Hsieh) Samson, Yilmaz, Frisvad & Seifert, T. allahabadensis (B.S. Mehrotra & D. Kumar) Samson, Yilmaz & Frisvad, T. aurantiacus (J.H. Mill., Giddens & A.A. Foster) Samson, Yilmaz, & Frisvad, T. boninensis (Yaguchi & Udagawa) Samson, Yilmaz, & Frisvad, T. brunneus (Udagawa) Samson, Yilmaz & Frisvad, T. calidicanius (J.L. Chen) Samson, Yilmaz & Frisvad, T. cecidicola (Seifert, Hoekstra & Frisvad) Samson, Yilmaz, Frisvad & Seifert, T. coalescens (Quintan.) Samson, Yilmaz & Frisvad, T. dendriticus (Pitt) Samson, Yilmaz, Frisvad & Seifert, T. diversus (Raper & Fennell) Samson, Yilmaz & Frisvad, T. duclauxii (Delacr.) Samson, Yilmaz, Frisvad & Seifert, T. echinosporus (Nehira) Samson, Yilmaz & Frisvad, comb. nov. T. erythromellis (A.D. Hocking) Samson, Yilmaz, Frisvad & Seifert, T. funiculosus (Thom) Samson, Yilmaz, Frisvad & Seifert, T. islandicus (Sopp) Samson, Yilmaz, Frisvad & Seifert, T. loliensis (Pitt) Samson, Yilmaz & Frisvad, T. marneffei (Segretain, Capponi & Sureau) Samson, Yilmaz, Frisvad & Seifert, T. minioluteus (Dierckx) Samson, Yilmaz, Frisvad & Seifert, T. palmae (Samson, Stolk & Frisvad) Samson, Yilmaz, Frisvad & Seifert, T. panamensis (Samson, Stolk & Frisvad) Samson, Yilmaz, Frisvad & Seifert, T. paucisporus (Yaguchi, Someya & Udagawa) Samson & Houbraken T. phialosporus (Udagawa) Samson, Yilmaz & Frisvad, T. piceus (Raper & Fennell) Samson, Yilmaz, Frisvad & Seifert, T. pinophilus (Hedgcock) Samson, Yilmaz, Frisvad & Seifert, T. pittii (Quintan.) Samson, Yilmaz, Frisvad & Seifert, T. primulinus (Pitt) Samson, Yilmaz & Frisvad, T. proteolyticus (Kamyschko) Samson, Yilmaz & Frisvad, T. pseudostromaticus (Hodges, G.M. Warner, Rogerson) Samson, Yilmaz, Frisvad & Seifert, T. purpurogenus (Stoll) Samson, Yilmaz, Frisvad & Seifert, T. rademirici (Quintan.) Samson, Yilmaz & Frisvad, T. radicus (A.D. Hocking & Whitelaw) Samson, Yilmaz, Frisvad & Seifert, T. ramulosus (Visagie & K. Jacobs) Samson, Yilmaz, Frisvad & Seifert, T. rubicundus (J.H. Mill., Giddens & A.A. Foster) Samson, Yilmaz, Frisvad & Seifert, T. rugulosus (Thom) Samson, Yilmaz, Frisvad & Seifert, T. sabulosus (Pitt & A.D. Hocking) Samson, Yilmaz & Frisvad, T. siamensis (Manoch & C. Ramírez) Samson, Yilmaz & Frisvad, T. sublevisporus (Yaguchi & Udagawa) Samson, Yilmaz & Frisvad, T. variabilis (Sopp) Samson, Yilmaz, Frisvad & Seifert, T. varians (G. Sm.) Samson, Yilmaz & Frisvad, T. verruculosus (Peyronel) Samson, Yilmaz, Frisvad & Seifert, T. viridulus Samson, Yilmaz & Frisvad.
Keywords: anamorph, DNA phylogeny, single name nomenclature, teleomorph, Trichocomaceae
INTRODUCTION
The modern concept of Penicillium (referred to in this paper as Penicillium sensu lato), was derived from the pioneering monographic revisions of Thom (1930), Raper & Thom (1949), and formalised by the recognition of four subgenera, Aspergilloides, Furcatum, Penicillium and Biverticillium by Pitt (1980). Over the past decade, the realisation has grown that Penicillium subgenus Biverticillium is phylogenetically distinct from other subgenera of Penicillium and that this distinctiveness should be reflected in its formal taxonomy. Because of their usually symmetrical, biverticillate conidiophores, the group has been recognised since Wehmer (1914) segregated them in an informal subdivision of Penicillium that he called “Verticillatae”. The delineation, species composition and taxonomic rank of this group were modified in subsequent monographs by Thom (1930), Raper & Thom (1949), Pitt (1980), and Ramírez (1982), culminating in the widespread recognition of subgenus Biverticillium and the use of this name in many taxonomic and phylogenetic studies. Malloch (1985), based on a consideration of morphological and ecological factors, and anamorph-teleomorph connections, may have been the first to speculate that subgenus Biverticillium should be removed from Penicillium as a separate genus.
The teleomorph genera historically associated with Penicillium sensu lato are Talaromyces and Eupenicillium (in single name nomenclature, the latter is now considered a synonym of Penicillium sensu stricto, see Houbraken & Samson 2011). The teleomorphs of these two groups produce distinctive ascomata. In Talaromyces, the soft ascomatal walls are comprised of multiple layers of interwoven hyphae and the ascomata mature quickly, usually within a few weeks in agar culture. In Penicillium sensu stricto, the sclerotium-like ascomata have rigid walls of thick-walled, isodiametric cells and the ascomatal maturity can take months and often ascospores do not form at all. Furthermore, in Talaromyces the ascus initials sometimes have morphologically distinguishable gametangia and the mature asci are produced in chains (Stolk & Samson 1972), while the ascomatal initials in Penicillium sensu stricto are irregularly interwoven, loosely branched hyphae masses (Emmons 1935), and the mature asci are single. Raper & Thom (1949) already recognised that there was considerable evidence that Penicillium subgenus Biverticillium constituted a natural and homogenous group. A comparison of the anamorphs of these two teleomorph types reveals a correlation with phialide shape, with anamorphs of Talaromyces (until now classified in Penicillium subgenus Biverticillium) having narrower phialides that are aculeate or lanceolate, and anamorphs in Penicillium sensu stricto having broader, ampulliform or flask-shaped phialides. One consequence of the differences in phialide shape is that the symmetrical nature of the conidiophores of species allied with Talaromyces tends to be emphasised, because in general the phialides are more densely packed. The colonies of subgenus Biverticillium can often be distinguished from those of Penicillium sensu stricto by the naked eye. They often have darker green conidia, more or less yellow pigmented and encrusted aerial hyphae, and colony reverses in yellow, orange or red to purplish red shades.
Once DNA-based studies of fungal phylogeny began, it quickly became apparent that the differences between Penicillium sensu stricto and Talaromyces were more than a matter of degree, and that there might be a significant problem with the generic concept of Penicillium sensu lato. Penicillium sensu stricto and Talaromyces occur as distinct clades within Trichocomaceae, which could be considered subfamilies (LoBuglio et al., 1993, LoBuglio & Taylor 1993). Using small subunit nuclear ribosomal DNA sequences (18S), Berbee et al. (1995) showed that Penicillium is polyphyletic if subgenus Biverticillium is included, a conclusion reconfirmed in one of the first reviews of the impact of molecular phylogenetics on Ascomycete taxonomy (Sugiyama 1998) using an analysis of 18S rDNA sequences. Removal of subgenus Biverticillium transforms Penicillium sensu stricto into a monophyletic group. This dichotomy between Penicillium sensu stricto and Talaromyces was shown repeatedly in studies employing nuclear ribosomal RNA genes, for example by Peterson (2000), who analysed a combination of the nuclear ribosomal internal transcribed spacer regions (ITS) and large subunit ribosomal DNA (28S) sequences (Ogawa et al. 1997, Ogawa & Sugiyama 2000), and by Wang & Zhuang (2007) in a phylogeny based on calmodulin sequences. The results of these analyses are all confirmed in the multigene phylogenetic analyses presented elsewhere in this volume by Houbraken & Samson (2011), using genes selected for their ability to accurately reflect molecular phylogeny. As indicated by Houbraken & Samson (2011), when other genera assigned to Trichocomaceae are included in phylogenetic analyses, the division between subgenus Biverticillium and Penicillium sensu stricto becomes even clearer. In that study, intervening genera include Aspergillus, Paecilomyces sensu stricto (with Byssochlamys as a synonym), and several small and less well-known genera such as Thermoascus, Penicilliopsis, Thermomyces and the recently described Rasamsonia (Houbraken et al. 2011).
In a molecularly defined, phylogenetically accurate taxonomic system, maintaining subgenus Biverticillium in Penicillium sensu stricto is untenable. However, almost every aspect of the biology, biochemistry, and physiology of these two groups emphasises their fundamental distinctiveness, although sometimes with limited taxon sampling. For example, Pitt (1980) emphasised the distinctiveness of subgenus Biverticillium by using a low water-activity medium, G25N (which includes 25 % glycerol) in his standard plating regime. Strains assigned to this subgenus grow slowly on this medium, less than 10 mm diam at 25 °C in 7 d, whereas species of the other subgenera are more xerophilic and grow faster. Cell-wall components seem to differ significantly. Leal & Bernabé (1998) reported on the complex glucomannogalactan components of the water soluble polysaccharide fraction of several species of Trichocomaceae, suggesting that a characteristic heteropolysaccharide composed of 4 galactose: 1 mannose: 1 glucose was unique to species of subgenus Biverticillium. Species of Penicillium sensu stricto species were characterised by the presence of a β-(1-5)(1-6)-galactofuran polysaccharide in the same fraction. Cell wall components as reflected by their exoantigens were screened in about 50 species of Penicillium sensu lato using an ELISA reaction to antibodies raised to P. digitatum (subgenus Penicillium). These antibodies reacted well with all the species of subgenera Furcatum, Penicillium and Aspergilloides, but did not react with the four species of subgenus Biverticillium tested (P. funiculosum, P. islandicum, P. rubrum, and P. tardum) (Notermans et al. 1998). Kuraishi et al. (1991) first noted that the pattern of ubiquinones in Penicillium sensu lato and showed a distinct pattern in subgenus Biverticillium. Paterson (1998) examined 335 strains and 118 species of Penicillium sensu lato and determined that the Q9 ubiqinone type was predominant in the species of Penicillium sensu stricto. In contrast, species of Talaromyces, Trichocoma and subgenus Biverticillium had different versions of the Q10 ubiquinone type. Exceptions to these patterns can be explained by the small number of species whose classification in, or elimination from, subgenus Biverticillium has been uncertain or controversial. Frisvad et al. (1990a) provided an overview of the extrolites of Talaromyces species, and demonstrated the occurrence of characteristic extrolites such as mitorubins, bisanthaquinones such as rugulosin and skyrin, vermicellin, vermistatin, vermiculine, duclauxin and glauconic acid. None of these compounds were found in cultures of Penicillium sensu stricto (Frisvad et al. 1990b).
The soon to be published International Code of Nomenclature for Algae, Fungi and Plants removes the primacy of teleomorph-over anamorph-typified names, leaving both kinds of names competing equally for priority (Norvell 2011). Because of these changes, we apply the principle of ‘one fungus - one name’ and in the nomenclatural revision, priority is given to the oldest genus and species name irrespective of whether they were originally described for teleomorphs or anamorphs (Hawksworth et al. 2011). In this respect, Penicillium returns to the single named, but pleomorphic, nomenclatural and taxonomic system used by many of the founders of its taxonomy, and actively promoted by the Peoria school (Thom 1930, Raper & Thom 1949). Talaromyces, now also defined as a pleomorphic genus, is adopted for the anamorphic species formerly included in Penicillium subgenus Biverticillium. In this study, the phylogenetic relationships of species of subgenus Biverticillium and other members of the Trichocomaceae were studied by sequencing a part of the RPB1 (RNA polymerase II largest subunit) gene. Furthermore, we discuss the taxonomy and nomenclature of species of this expanded concept of Talaromyces, based on phylogenetic, phenotypic and extrolite data. For detailed phylogenetic analysis below genus level, the ITS regions (including the 5.8S nrDNA) of ex-type strains and/or representatives were sequenced. As discussed below, this paper is not meant as a monographic treatment, because many complexes have not yet been studied comprehensively.
MATERIALS AND METHODS
Sources of cultures
The fungi examined include type strains or representatives of all available species of Talaromyces and Biverticillium. The strains are maintained in the CBS-KNAW Fungal Biodiversity Centre (CBS) culture collection and an overview of strains used for phylogenetic analysis is shown in Table 1. In a few cases, the ex-type strain was unavailable and sequence data present in GenBank were used.
Table 1.
Strains used in phylogenetic analysis of Talaromyces.
| Name | Collection no. | Origin | GenBank Accession number |
|
|---|---|---|---|---|
| RPB1 | ITS | |||
| “Aphanoascus cinnabarinus” | CBS 267.72 = ATCC 26215 | Soil, Japan | JN121625 | JN899376 |
| Aspergillus aculeatus | CBS 172.66T = ATCC 16872 = IMI 211388 | Tropical soil | JN121590 | |
| Aspergillus clavatoflavus | CBS 473.65NT = ATCC 16866 = IMI 124937 | Rain forest soil, Tulley, Queensland, Australia | JN121686 | |
| Aspergillus flavus | NRRL 3357 = CBS 128202 = ATCC 200026 | Peanut cotyledons, USA | Unpublished | |
| Aspergillus fumigatus | Af293 | Patient with invasive aspergillosis | Nierman et al. (2005) | |
| Aspergillus niger | CBS 513.88 | Derived from NRRL 3122 and currently used as enzyme production strain | Pel et al. (2007) | |
| Aspergillus ochraceoroseus | CBS 101887 = ATCC 42001 = IBT 14580 | Soil, Tai National Forest, Ivory Coast | JN121557 | |
| Aspergillus ochraceus | CBS 108.08NT = ATCC 1008 = CBS 547.65 = IMI 016247 = IMI 016247iii = IMI 016247iv = NRRL 1642 = NRRL 398 | Unknown source | JN121562 | |
| Aspergillus penicillioides | CBS 130294 | Indoor environment, Germany | JN121578 | |
| Aspergillus robustus | CBS 649.93T = CBS 428.77 = IBT 14305 | Surface soil from thorn-forest, near Mombasa, Kenya | JN121711 | |
| Aspergillus sparsus | CBS 139.61NT = ATCC 16851 = IMI 019394 = IMI 019394ii = MUCL 31314 = NRRL 1933 | Soil, Costa Rica | JN121586 | |
| Aspergillus steynii | CBS 112812T = IBT 23096 | Dried arabica green coffee bean, on parchment, internal infection, Chamumdeshuran Estata, Karnataka, district Giris, India | JN121569 | |
| Aspergillus sydowii | CBS 264.81 | Grains and milling fractions, Triticum aestivum, India | JN121624 | |
| Aspergillus versicolor | CBS 245.65 = ATCC 11730 = ATCC 16020 = IMI 045554 = IMI 045554ii = IMI 045554iii = IMI 045554iv = MUCL 19008 | Cellophane, Indiana, USA | JN121614 | |
| Aspergillus zonatus | CBS 506.65NT = ATCC 16867 = IMI 124936 | Forest soil, Province of Linon, Fortuna, Costa Rica | JN121691 | |
| Byssochlamys nivea | CBS 100.11T = ATCC 22260 | Unknown source | JN121511 | |
| Byssochlamys spectabilis | CBS 101075T = ATCC 90900 = FRR 5219 | Heat processed fruit beverage, Tokyo, Japan | JN121554 | |
| Byssochlamys verrucosa | CBS 605.74T = ATCC 34163 | Nesting material of Leipoa ocellata (Malleefowl), Pulletop Nature Reserve, New South Wales, Australia | JN680311 | |
| Chrysosporium inops | CBS 132.31T = IMI 096729 = UAMH 802 | Skin of man, Italy | JN121584 | |
| Coccidioides immitis | Strain “RS” | Vaccine strain - origin unknown | Sharpton et al. (2009) | |
| Emericella nidulans | FGSC A4 (= ATCC 38163 = CBS 112.46) | Unknown source | Galagan et al. (2005) | |
| Eurotium herbariorum | CBS 516.65NT = ATCC 16469 = IMI 211383 = NRRL 116 | Unpainted board, Washington, USA | JN121693 | |
| Geosmithia viridis | CBS 252.87T = FRR 1863 = IMI 288716 | Soil, bank of creek flowing into Little River, New South Wales | JN680284 | JN899314 |
| Hamigera avellanea | CBS 295.48T = ATCC 10414 = IMI 040230 = NRRL 1938 | Soil, San Antonio, Texas, USA | JN121632 | |
| Hamigera striata | CBS 377.48NT = ATCC 10501 = IMI 039741 = NRRL 717 | Canned blueberries, USA | JN121665 | |
| Monascus purpureus | CBS 109.07T = ATCC 16365 = ATCC 16426 = IMI 210765 = NRRL 1596 | Fermented rice grain, ‘ang-quac’ (purple coloured rice), Kagok-Tegal, imported from China, Prov. Quouan-toung, Java, Indonesia | JN121563 | |
| Paecilomyces aerugineus | CBS 350.66T = IMI 105412 | Debris of Glyceria maxima, Attenborough, Notts., UK | JN121657 | JN899388 |
| Paecilomyces pascuus | CBS 253.87T = FRR 1925 | Pasture grass, Otara, New Zealand | JN899292 | JN899321 |
| Penicilliopsis clavariiformis | CBS 761.68 = CSIR 1135 | Unknown source, Pretoria, South Africa | JN121716 | |
| Penicillium aculeatum | CBS 100105 = CBS 289.48 = ATCC 10409 = IMI 040588 = NRRL 2129 = NRRL A-1474 | Textile, USA | JN899389 | |
| CBS 289.48NT = ATCC 10409 = IMI 040588 = NRRL 2129 = NRRL A-1474 | Textile, USA | JN899378 | ||
| Penicillium aculeatum var. apiculatum | CBS 312.59T = ATCC 18315 = FRR 635 = IMI 068239 | Soil, Japan | JN680293 | JN899375 |
| Penicillium allahabadense | CBS 453.93T = ATCC 15067 = CBS 304.63 | Soil of cultivated field, pH 6.9, Allahabad, India | JN680309 | JN899345 |
| Penicillium arenicola | CBS 220.66T = ATCC 18321 = ATCC 18330 = IMI 117658 = NRRL 3392 | Soil from pine forest, Kiev, Ukraine | JN121601 | |
| Penicillium aurantiacum | CBS 314.59T = ATCC 13216 = IMI 099722 = NRRL 3398 | Soil, Georgia | JN899380 | |
| Penicillium aureocephalum | CBS 102801T | Quercus ruber, Gerona, Selva de Mar, Catalania, Spain | JN899392 | |
| Penicillium brunneum | CBS 227.60T = ATCC 18229 = FRR 646 = IFO 6438 = IHEM 3907 = IMI 078259 = MUCL 31318 | Milled rice imported into Japan, Thailand | JN680281 | JN899365 |
| Penicillium calidicanium | CBS 112002T | Soil, Nantou County, Taiwan | JN899305 | JN899319 |
| Penicillium canescens | CBS 300.48NT = ATCC 10419 = IMI 028260 = MUCL 29169 = NRRL 910 | Soil, England | JN121636 | |
| Penicillium catenatum | CBS 352.67T = ATCC 18543 = IMI 136241 | Desert soil, Upington, Cape Province, South Africa | JN121659 | |
| Penicillium cinnamopurpureum | CBS 490.66 = ATCC 18337 = IMI 114483 | Cultivated soil, South Africa | JN121690 | |
| Penicillium citrinum | CBS 139.45T = ATCC 1109 = IMI 091961 = MUCL 29781 = NRRL 1841 | Unknown source | JN121585 | |
| Penicillium coalescens | CBS 103.83T | Soil under Pinus sp., near Vulladolid, Spain | JN899366 | |
| Penicillium concavorugulosum | CBS 898.73T = ATCC 20202 | Unknown substrate, Japan | JN899304 | JN899390 |
| Penicillium crateriforme | CBS 184.27T = FRR 1057 = IMI 094165 = LSHB P164 = MUCL 29224 = NRRL 1057 | Soil, Luisiana | JN680270 | JN899373 |
| Penicillium dendriticum | CBS 660.80T = IMI 216897 | Leaf litter of Eucalyptus pauciflora, Kosciusko National Park, New South Wales, Australia | JN121714 | JN899339 |
| Penicillium diversum | CBS 320.48T = ATCC 10437 = DSM 2212 = IMI 040579 = IMI 040579ii = NRRL 2121 | Leather, USA | JN680297 | JN899341 |
| Penicillium duclauxii | CBS 322.48T = ATCC 10439 = IMI 040044 = MUCL 28672 = MUCL 29094 = MUCL 29212 = NRRL 1030 | Canvas, France | JN121643 | JN899342 |
| Penicillium echinosporum | CBS 293.62T = ATCC 18319 = DSM 2230 = FRR 3411 = IMI 080450 = IMI 101214 | Wood pulp, Surrey, Kenley, UK | JN899363 | |
| Penicillium erythromellis | CBS 644.80T = FRR 1868 = IMI 216899 | Soil from creek bank, Little River, New South Wales, Australia | JN680315 | JN899383 |
| Penicillium euglaucum | CBS 323.71NT | Soil, Argentina | JN121644 | |
| Penicillium expansum | CBS 325.48 = ATCC 7861 = IBT 5101 = IMI 039761= MUCL 29192 = NRRL 976 | Fruit of Malus sylvestris, USA | JN121645 | |
| Penicillium fellutanum | CBS 229.81NT = ATCC 10443 = CBS 326.48 = FRR 746 = IFO 5761 = IMI 039734 = IMI 039734iii = NRRL 746 | Unknown source, USA | JN121605 | |
| Penicillium funiculosum | CBS 272.86NT = IMI 193019 | Lagenaria vulgaris, India | JN680288 | JN899377 |
| Penicillium glabrum | CBS 125543NT = IBT 22658 = IMI 91944 | Unknown source | JN121717 | |
| Penicillium herquei | CBS 336.48T = ATCC 10118 = FRR 1040 = IMI 028809 = MUCL 29213 = NRRL 1040 | Leaf, France | JN121647 | |
| Penicillium ilerdanum | CBS 168.81T = IJFM 5596 = IMI 253793 | Air, Madrid, Spain | JN899311 | |
| Penicillium isariiforme | CBS 247.56T = ATCC 18425 = IMI 060371 = MUCL 31191 = MUCL 31323 = NRRL 2638 | Woodland soil, Zaire | JN121616 | |
| Penicillium islandicum | CBS 338.48NT = ATCC 10127 = IMI 040042 = MUCL 31324 = NRRL 1036 | Unknown source, Cape Town, South Africa | JN121648 | JN899318 |
| Penicillium janthinellum | CBS 340.48NT = ATCC 10455 = IMI 040238 = NRRL 2016 | Soil, Nicaragua | JN131650 | |
| Penicillium javanicum | CBS 341.48T = ATCC 9099 = IMI 039733 = MUCL 29099 = NRRL 707 | Root of Camellia sinensis, Indonesia, Java | JN121651 | |
| Penicillium kewense | CBS 344.61T = ATCC 18240 = IMI 086561 = MUCL 2685 = NRRL 3332 | Culture contaminant of mineral oil CMI 1959; Kew, Surrey, UK | JN121654 | |
| Penicillium korosum | CBS 762.68T | Rhizosphere, India | JN899347 | |
| Penicillium lapidosum | CBS 343.48T = ATCC 10462 = IMI 039743 = NRRL 718 | Canned blueberry, Washington, USA | JN121653 | |
| Penicillium liani | CBS 225.66T = ATCC 18325 = ATCC 18331 = IMI 098480 = NRRL 3380 = VKM F-301 | Soil, China | JN680280 | JN899395 |
| Penicillium loliense | CBS 643.80T = ATCC 52252 = FRR 1798 = IMI 216901 = MUCL 31325 | Lolium, Palmerston North, New Zealand | JN680314 | JN899379 |
| Penicillium marneffei | CBS 388.87T = ATCC 18224= CBS 334.59 = IMI 068794ii = IMI 068794iii | Rhizomys sinensis (bamboo rat), Vietnam | JN899298 | JN899344 |
| Penicillium minioluteum | CBS 642.68T = IMI 089377 = MUCL 28666 | Unknown source | JN121709 | JN899346 |
| Penicillium mirabile | CBS 624.72T = CCRC 31665 = FRR 1959 = IMI 167383 = MUCL 31206 | Forest soil, Crimea, Ukraine | JN680312 | JN899322 |
| Penicillium namylowskii | CBS 353.48T = ATCC 11127 = IMI 040033 = MUCL 29226 = NRRL 1070 | Soil under Pinus sp., Puszceza Bialowieska, square “652”, Poland | JN121660 | |
| Penicillium oblatum | CBS 258.87T = FRR 2234 | Spoiled baby food, Sydney, New South Wales, Australia | JN680285 | JN899364 |
| Penicillium ochrosalmoneum | CBS 489.66 = ATCC 18338 = IMI 116248ii | Cornmeal, South Africa | JN121689 | |
| Penicillium osmophilum | CBS 462.72T = IBT 14679 | Agricultural soil, Wageningen, Netherlands | JN121683 | |
| Penicillium palmae | CBS 442.88T = IMI 343640 | Seed, Wageningen, Netherlands | JN680308 | JN899396 |
| Penicillium panamense | CBS 128.89T = IMI 297546 | Soil, Barro Colorado Island, Panama | JN899291 | JN899362 |
| Penicillium phialosporum | CBS 233.60T = ATCC 18481 = FRR 203 = IMI 078256 | Milled Californian rice, California, USA | JN680282 | JN899340 |
| Penicillium piceum | CBS 361.48T = ATCC 10519 = IMI 040038 = NRRL 1051 | Unknown source | JN899370 | |
| Penicillium pinophilum | CBS 631.66NT = ATCC 36839 = CECT 2809 = DSM 1944 = IAM 7013 =IMI 114933 | PVC, Centre d'Études du Bouchet, M. Magnoux, France | JN680313 | JN899382 |
| Penicillium pittii | CBS 139.84T = IMI 327871 | Clay soil, under poplar trees, bank of Duero River, Valladolid, Spain | JN680274 | JN899325 |
| Penicillium primulinum | CBS 321.48T = ATCC 10438 = CBS 439.88 = FRR 1074 = IMI 040031 = MUCL 31321 = MUCL 31330 = NRRL 1074 | USA | JN680298 | JN899317 |
| Penicillium proteolyticum | CBS 303.67T = ATCC 18326 = NRRL 3378 | Granite soil, Ukraine | JN680292 | JN899387 |
| Penicillium pseudostromaticum | CBS 470.70T = ATCC 18919 = FRR 2039 | Feather, near Itasca State Park, Hubbard Co., Minnesota, USA | JN899300 | JN899371 |
| Penicillium purpurogenum | CBS 286.36T = IMI 091926 | Unknown source | JN680271 | JN899372 |
| Penicillium purpurogenum var. rubisclerotium | CBS 274.95 | Sculpture, castle Troja, Prague, Czech Republic | JN899295 | JN899316 |
| CBS 270.35T = ATCC 4713 = ATCC 52244 = FRR 1064 = IBT 4302 = MUCL 29225 = NRRL 1064 = NRRL 1142 | Zea mays, Castle Rock, Virginia, USA | JN680287 | JN899381 | |
| Penicillium rademirici | CBS 140.84T = CECT 2771 = IMI 282406 = IMI 327870 | Air under willow tree, bank of river Duero, Herrera, Valladolid, Spain | JN899386 | |
| Penicillium radicum | CBS 100489T = FRR 4718 | Root of seedling of Triticum aestivum, Wagga Wagga, New South Wales, Australia | JN899324 | |
| Penicillium rotundum | CBS 369.48T = ATCC 10493 = IMI 040589 = NRRL 2107 | Wood, Chiriqui Prov., Panama | JN899353 | |
| Penicillium rubicundum | CBS 342.59T = ATCC 13217 = IMI 099723 = NRRL 3400 | Soil, Georgia, USA | JN680301 | JN899384 |
| “Penicillium rubrum” | CBS 196.88 = FRR1714 | Unknown source | JN680278 | JN899312 |
| CBS 206.89 = IFO 6580 | Japan | JN680279 | JN899313 | |
| CBS 263.93 | Bronchoalveolair lavage of immunecompetent female patient with pneumonia by Nocardia | JN680286 | JN899315 | |
| Penicillium rugulosum | CBS 371.48T = ATCC 10128 = IMI 040041 = MUCL 31201 = NRRL 1045 | Tuber (Solanum tuberosum), Connecticut, USA | JN680302 | JN899374 |
| Penicillium sabulosum | CBS 261.87T = FRR 2743 | Spoiled pasteurized fruit juice, New South Wales, Sydney, Australia | JN899294 | |
| Penicillium samsonii | CBS 137.84T = CECT 2772 = IMI 282404 = IMI 327872 | Fruit, damaged by insect, Valladolid, Spain | JN680273 | JN899369 |
| Penicillium shearii | CBS 290.48T = ATCC 10410 = IMI 039739 = IMI 039739iv = NRRL 715 | Soil, Tela, Honduras | JN121631 | |
| Penicillium siamense | CBS 475.88T = IMI 323204 | Forest soil, Lampang, Thurn District, Ban Daen Tham, Thailand | JN899385 | |
| Penicillium simplicissimum | CBS 372.48NT = ATCC 10495 = IMI 039816 | Flannel bag, Cape, South Africa | JN121662 | |
| Penicillium stipitatum | CBS 375.48T = ATCC 10500 = NRRL 1006 = IMI 39805 | Rotting wood, Louisiana, USA | JN680303 | JN899348 |
| Penicillium stolkiae | CBS 315.67T = IMI 136210 = ATCC 18546 | Peaty forest soil, Eastern Transvaal, South-Africa | JN680295 | |
| Penicillium tardum | CBS 258.37T = NRRL 2116 | Unknown source | JN899293 | |
| CBS 378.48T = ATCC 10503 = IMI 040034 = NRRL 1073 | Dead twig, France | JN899297 | ||
| Penicillium tularense | CBS 430.69T = ATCC 22056 = IMI 148394 | Soil, under Pinus ponderosa and Quercus kelloggii, Tulare Co., Pine Flat, California, USA | JN121681 | |
| Penicillium variabile | CBS 385.48NT = ATCC 10508= IMI 040040 = NRRL 1048 | Cocos fibre, Johannesburg, South Africa | JN680304 | JN899343 |
| Penicillium varians | CBS 386.48T = ATCC 10509 = IMI 040586 = NRRL 2096 | Cotton yarn, UK | JN680305 | JN899368 |
| Penicillium verruculosum | CBS 388.48NT = ATCC 10513= DSM 2263= IMI 040039 = NRRL 1050 | Soil, Texas, USA | JN899367 | |
| Penicillium victoriae | CBS 274.36T = IMI 058412 = MUCL 9651 | Dried leaf, Tobaheide, Sumatra | JN680289 | JN899393 |
| Penicillium viridicatum | CBS 390.48NT = ATCC 10515= IBT 23041 = IMI 039758 = IMI 039758ii = NRRL 963 | Air, District of Columbia, Washington D.C., USA | JN121668 | |
| Phialosimplex caninus | CBS 128032T = UAMH 10337 | Bone marrow aspirate ex canine, San Antonio, Texas, USA | JN121587 | |
| Phialosimplex chlamydosporus | CBS 109945T = FMR 7371 = IMI 387422 | Disseminated infection in a dog | JN121566 | |
| Phialosimplex sclerotialis | CBS 366.77T = IAM 14794 | Fodder of ray-grass and lucerne, France | JN121661 | |
| Rasamsonia eburnea | CBS 100538T = IBT 17519 | Soil, Taipei, Taiwan | JN680325 | |
| Rasamsonia argillacea | CBS 101.69T = IMI 156096 = IBT 31199 | Mine tip with a very high surface temperature; Staffordshire, UK | JN121556 | |
| Rasamsonia byssochlamydoides | CBS 413.71T = IBT 11604 | Dry soil under Douglas fir, Oregon, USA | JN121675 | |
| Rasamsonia emersonii | CBS 393.64T = DTO 48I1 = IBT 21695 = ATCC 16479 = IMI 116815 = IMI 116815ii | Compost, Italy | JN121670 | |
| Sagenoma viride | CBS 114.72T ATCC 22467 = NRRL 5575 | Soil, Australia | JN121571 | |
| Sagenomella bohemica | CBS 545.86T = CCF 2330 = IAM 14789 | Peloids for balneological purposes, Frantiskovy Lázne Spa, West Bohemia, Czech Republic | JN121699 | JN899400 |
| Sagenomella diversispora | CBS 398.69 | Forest soil under Populus tremuloides, Petawawa, Ontario, Canada | JN121673 | |
| CBS 399.69 = MUCL 15012 | Forest soil under Thuja occidentalis, Aberfoyle, Ontario, Canada | JN121674 | ||
| Sagenomella griseoviridis | CBS 426.67T = ATCC 18505 = IMI 113160 | Unknown source | JN121677 | |
| Sagenomella humicola | CBS 427.67T = ATCC 18506 = IMI 113166 | Forest soil under Thuja occidentalis, Ontario, Canada | JN121678 | |
| Sagenomella striatispora | CBS 429.67T = ATCC 18510 = IMI 113163 | Soil, Guelph, Ontario, Canada | JN121679 | |
| Sagenomella verticillata | CBS 415.78A | Gymnosperm forest soil, Sweden | JN680307 | |
| Sclerocleista ornata | CBS 124.53NT = ATCC 16921 = IMI 055295 = MUCL 15643 = NRRL 2256 | Soil in oak forest, Dane Co., Madison, Wisconsin, USA | JN121581 | |
| Talaromyces assiutensis | CBS 118440 | Soil, Fes, Morocco | JN899320 | |
| CBS 147.78T | Soil, amended with crushed buffalo hoofs and incubated for 5 months at 35 oC, Egypt | JN680275 | JN899323 | |
| Talaromyces austrocalifornicus | CBS 644.95T = IBT 17522 | Soil, campus Univ. South California, Los Angelos, USA | JN680316 | JN899357 |
| Talaromyces bacillisporus | CBS 296.48T = ATCC 10126 = IMI 040045 = NRRL 1025 | Begonia leaf, New York City, New York, USA | JN121634 | JN899329 |
| Talaromyces barcinensis | CBS 649.95T = IBT 17518 | Soil, Barcelona, Spain | JN680318 | JN899349 |
| Talaromyces brevicompactus | CBS 102661T = AS 3.4676 | Moulded vegetables, Prov. Sechuan, Wolong, China | JN680326 | |
| Talaromyces convolutus | CBS 100537T = IBT 14989 | Soil, Kathmandu, Nepal | JN121553 | JN899330 |
| Talaromyces cyanescens | CBS 114900 = FMR 8388 | Tortosa, Catalina, Spain | JN899391 | |
| Talaromyces derxii | CBS 412.89T = NHL 2981 | Cultivated soil, Okayama Prefecture, Kurashiki City, Higashitomii, Japan | JN680306 | JN899327 |
| CBS 413.89T = NHL 2982 | Cultivated soil, Okayama Prefecture, Kurashiki City, Higashitomii, Japan | JN899299 | JN899326 | |
| Talaromyces emodensis | CBS 100536T = IBT 14990 | Soil, Kathmandu, Nepal | JN121552 | JN899337 |
| Talaromyces flavus | CBS 310.38NT = IMI 197477 = NRRL 2098 | Unknown substrate, New Zealand | JN121639 | JN899360 |
| Talaromyces galapagensis | CBS 751.74T = IFO 31796 | Shaded soil under Maytenus obovata, Isla Santa Cruz, Galapagos Islands, Ecuador | JN680321 | JN899358 |
| Talaromyces gossypii | CBS 645.80T = FRR 1966 = IMI 198365 | Gossypium, India | JN680317 | JN899334 |
| Talaromyces helicus var. boninensis | CBS 650.95T = IBT 17516 | Lawn soil, Kominato, Chichijima, Ogasawara-mura, Tokyo-to, Japan | JN680319 | JN899356 |
| Talaromyces helicus var. helicus | CBS 335.48T = ATCC 10451 = DSM 3705 = IMI 040593 = NRRL 2106 | Soil, Sweden | JN680300 | JN899359 |
| Talaromyces helicus var. major | CBS 652.66T = IMI 100914 | Swamp soil, near Attenborough, Nottingham, UK | JN680320 | JN899335 |
| Talaromyces indigoticus | CBS 100534T = IBT 17590 | Soil, Nagasaki-ken, Minamikushiyama-mura, Japan | JN680323 | JN899331 |
| Talaromyces intermedius | CBS 152.65T = BDUN 267 = IFO 31752= IMI100874 | Alluvial pasture and swamp soil, Attenborough, Nottingham, England | JN680276 | JN899332 |
| Talaromyces leycettanus | CBS 398.68T = ATCC 22469 = IMI 178525 | Coal spoil tip soil, Leycett, Staffordshire, England, UK | JN121672 | |
| Talaromyces luteus | CBS 348.51NT = IMI 089305 | Soil, UK | JN121656 | |
| Talaromyces macrosporus | CBS 317.63T = FRR 404 = IMI 197478 | Apple juice, Stellenbosch, South Africa | JN680296 | JN899333 |
| Talaromyces mimosinus | CBS 659.80T = FRR 1875 = IMI 223991 | Soil from creek bank, Nattai River, New South Wales, Australia | JN899302 | JN899338 |
| Talaromyces muroii | CBS 756.96T = PF 1153 | Soil, Hualien County, Chingpu, Taiwan | JN680322 | JN899351 |
| Talaromyces ocotl | CBS 102855T | Heat-treated soil from forest of Pinus hartwegii, Veracruz, Mexico | JN680327 | |
| Talaromyces ohiensis | CBS 127.64T | Soil treated with cyanamide, Germany | JN680272 | JN899355 |
| Talaromyces purpureus | CBS 475.71T = ATCC 24069 = ATCC 52513 = FRR 1731 = IMI 181546 | Soil, near Esterel, France | JN121687 | JN899328 |
| Talaromyces subinflatus | CBS 652.95T = IBT 17520 | Copse soil, Hahajima, Ogasawara-mura, Tokyo-to, Japan | JN899301 | JN899397 |
| Talaromyces tardifaciens | CBS 250.94T | Unknown source | JN680283 | JN599361 |
| Talaromyces thermophilus | CBS 236.58T = ATCC 10518 = IMI 048593 = NRRL 2155 | Parthenium argentatum, decaying plant; California, USA | JN121611 | |
| Talaromyces trachyspermus | CBS 373.48T = ATCC 10497 = IMI 040043 = NRRL 1028 | Unknown source, USA | JN121664 | JN899354 |
| Talaromyces ucrainicus | CBS 162.67T = ATCC 22344 = FRR 3462 | Unknown source | JN680277 | JN899394 |
| Talaromyces udagawae | CBS 579.72T = FRR 1727 = IMI 197482 | Soil, Misugimura, Japan | JN680310 | JN899350 |
| Talaromyces unicus | CBS 100535T = CCRC 32703 = IBT 18385 | Soil, Chiayi County, Funlu, Taiwan | JN680324 | JN899336 |
| Talaromyces wortmanii | CBS 391.48T = ATCC 10517 = IMI 040047 = NRRL 1017 | Unknown source | JN121669 | JN899352 |
| Thermoascus aurantiacus | CBS 396.78 | Sawdust, in lumber yard, Toronto, Ontario, Canada | JN121671 | |
| CBS 891.70 = IMI 173037 | Wood, Firenze, Italy | JN121719 | ||
| Thermoascus crustaceus | CBS 181.67T = ATCC 16462 = IMI 126333 | Parthenium argentatum, decaying plant; Salinas, California, USA | JN121591 | |
| Thermoascus thermophilus | CBS 528.71NT = IMI 123298 = NRRL 5208 | Wood and bark of Pinus, Sweden | JN121697 | |
| Thermomyces lanuginosus | CBS 218.34 = MUCL 8338 | Fruit shell of Theobroma cacao | JN121599 | |
| CBS 224.63 = MUCL 8337 | Mushroom compost; Gossau-Zürich Switzerland | JN121602 | ||
| CBS 288.54 = MUCL 8340 | Stomach of bovine foetus, Netherlands | JN680291 | ||
| Trichocoma paradoxa | CBS 103.73 | Unknown source, Japan | JN121558 | |
| CBS 247.57 = MUCL 39666 = IBT 31159 | Unknown source, Hachijô, Japan | JN121617 | ||
| CBS 788.83 | Rotting stump of cut down tree, Myojoji Temple near Hakui Noto Park, Ishikawa Pref., Japan | JN121718 | JN899398 | |
| Warcupiella spinulosa | CBS 512.65NT = ATCC 16919 = IMI 075885 = NRRL 4376 | Jungle soil, Berakas-Muara, Brunei | JN121692 | |
Morphology and physiology
Cultures were grown for 7 d on Czapek agar, Czapek yeast autolysate agar (CYA), oatmeal agar (OA) and/or malt extract agar (MEA) plates at 25 °C or, if required, another temperature. Medium compositions follow Samson et al. (2010). Cultures were grown for up to 3 wk for ascomata production.
Extrolite analysis
Nearly all species described in the genera Penicillium sensu lato (including those formerly classified in Eupenicillium), Penicillium subgenus Biverticillium, Talaromyces, Aspergillus and its many associated teleomorphic genera, and Paecilomyces (including those formerly or still classified in the associated teleomorph genus Byssochlamys) were analysed qualitatively for their profiles of secondary metabolites as determined by HPLC with diode array detection. Many strains of each species were examined, whenever available, but in some cases only the ex-type culture was available. Cultures were inoculated on the media CYA, MEA (Blakeslee formula, using Difco malt extract), YES agar (Samson et al. 2010, Difco yeast extract) and OA. All cultures were analysed chemically using three agar plugs from a 7 d old culture grown at 25 °C (Smedsgaard 1997). Different methods were used for HPLC analysis, but the methods were essentially based on Frisvad & Thrane (1987, 1993). Since 1997, the method for Nielsen & Smedsgaard (2003) was used and after 2010 the UPLC method of Nielsen et al. (2011) was applied. Metabolites were identified via their diode-array based UV-VIS spectra and in some cases by their mass spectra, and by comparison to authenticated standards (Nielsen et al. 2011).
For the extrolites analyses, the biosynthetic families of the sampled genera were compared using UPGMA cluster analysis (NTSYS version 2.11). All metabolites were classified according to biosynthetic families; for example the viridicatin biosynthetic family consists of cyclopenol, cyclopenin, cyclopeptin, dehydrocyclopeptin, viridicatin, viridicatol and 3-methoxyviridicatin (Turner & Aldridge 1983). This family was scored as one character in the cluster analysis. The exometabolites were also combined into biosynthetic families and tabulated as such. For example, many species of Talaromyces and Penicillium subgenus Biverticillium produce the azaphilones mitorubrin, mitorubrinal, mitorubrinol, mitorubrinol acetate, mitorubrinic acid, funicone, deoxyfunicone, actofunicone, 3-O-methylfunicone, kasanosin A and B, diazaphilonic acid, and wortmin; they are here collectively called the mitorubrins, while the related metabolites vermistatins and penicidones are called vermistatins (see Šturdíková et al. 2000, Nicoletti et al. 2009, Osmanova et al. 2010). Some chlorinated azaphilones such as helicusins (Yoshida et al. 1995) and luteusins (Fujimoto et al. 1990, Yoshida et al. 1996a, b) are epimers of the sclerotiorins from P. sclerotiorum, and are treated as two families, albeit closely related to the mitorubrins.
DNA extraction, amplification and sequencing
Isolates used for molecular studies were grown on MEA for 7–14 d at the required temperature prior to DNA extraction. DNA was extracted from the cells using the UltraClean™ Microbial DNA Kit (MoBio Laboratories), following the protocols of the manufacturer. A part of the RPB1 gene was amplified to study the phylogenetic relationships among Penicillium and other related genera. This fragment was amplified using the primer pair RPB1-F1843 5'-ATTTYGAYGGTGAYGARATGAAC-3' and RPB1-R3096 5'-GRACRGTDCCRTCATAYTTRACC-3' (Houbraken & Samson 2011). Primer RPB1-F1843 corresponds with position 1490–1512 of GenBank no. XM_002146871 (P. marneffei, ATCC 18224) and RPB1-R3096 corresponds with position 2610–2633. An addition primer, RPB1-R2623 5'-GCRTTGTTSARATCCTTMARRCTC-3' was occasionally used as an internal primer for sequencing (Houbraken & Samson 2011). The ITS regions were sequenced to study the relationship among Talaromyces and the related biverticillate anamorphic species. Fragments containing the ITS region were amplified using primers V9G (de Hoog & Gerrits van den Ende 1998) and LS266 (Masclaux et al. 1995). Sequencing reactions were performed with the Big Dye Terminator Cycle Sequencing Ready Reaction Kit v. 3.1 (Applied Biosystems) and carried out for both strands to ensure consistency of the consensus sequence.
Data analyses
For the DNA sequence analyses, alignments were performed using the software Muscle as implemented in the MEGA5 programme (Tamura et al. 2011). The RAxML (randomised accelerated maximum likelihood) software (v. 7.2.8, Stamatakis et al. 2008) was used for the Maximum Likelihood (ML) analysis. The robustness of trees in the ML analyses was evaluated by 100 bootstrap replications. The phylogram based on RPB1 sequences is rooted with Coccidioides immitis (strain RS; full genome strain), and Trichocoma paradoxa (CBS 788.83) is used as an outgroup in the ITS analysis.
RESULTS
Phylogenetic generic delimitation of Talaromyces and biverticillate anamorphic species
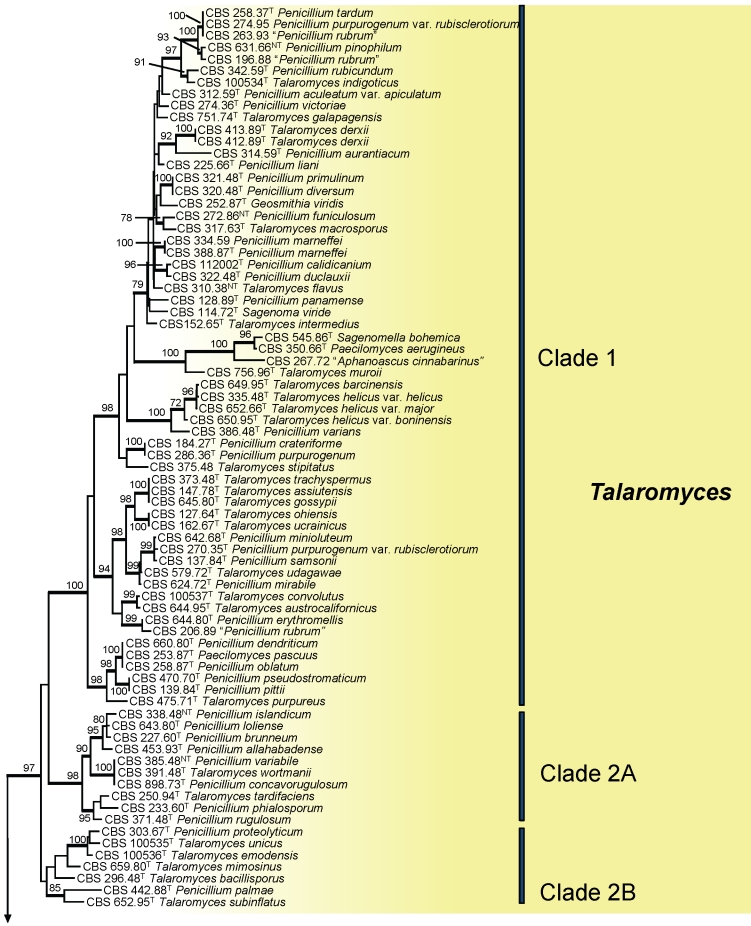
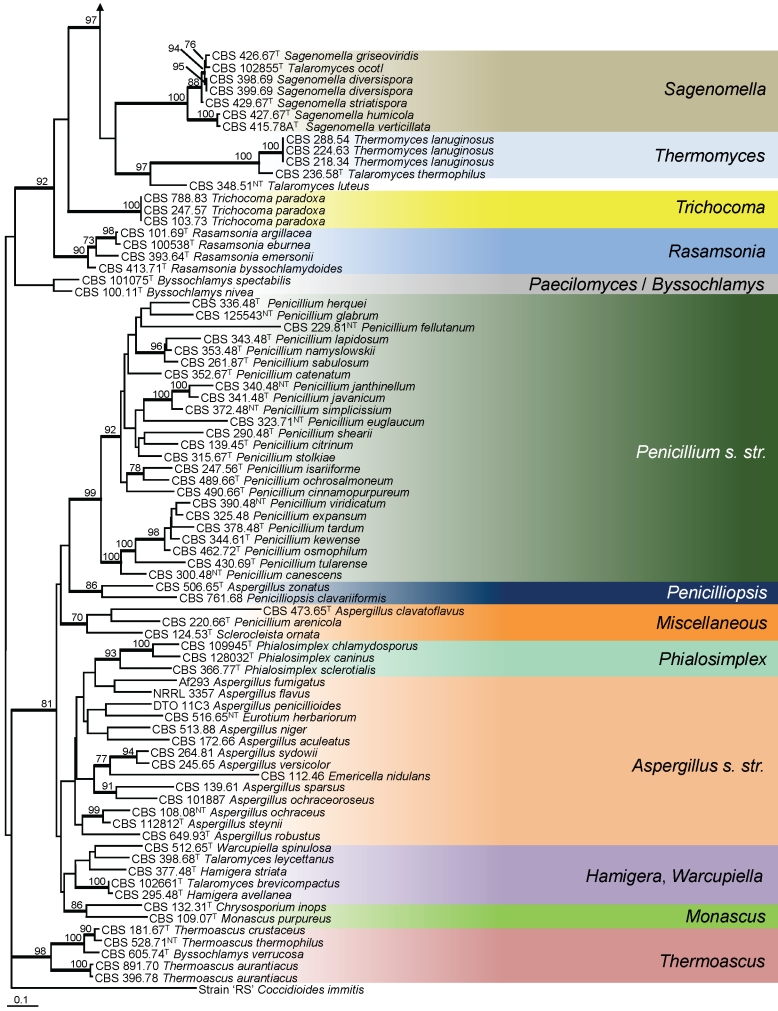
The phylogenetic relationships of Talaromyces and species of Penicillium subgenus Biverticillium among other related genera were studied using partial RPB1 sequences. One-hundred fifty-six strains were included in this analysis. The length of the alignment was 496 characters (exon data only, no introns observed) and 323 of those characters were variable. The proportion of gaps and completely undetermined characters in the alignment was 0.60 %. Figure 1 shows that members of the subgenus Biverticillium and Talaromyces are accommodated in a well-supported (97 % bs), monophyletic clade (= Talaromyces s. str.) and that species of the Penicillium subgenera Aspergilloides, Furcatum and Penicillium form an independent, well-supported clade (Penicillium s. str.). The majority of described Talaromyces species belong to Talaromyces s. str., but some species are dispersed in other clades, including Talaromyces ocotl, T. luteus, T. thermophilus, T. eburneus, T. emersonii, T. byssochlamydoides, T. spectabilis, T. brevicompactus, T. striatus and T. leycettanus. Talaromyces ocotl is in a well-supported clade with the type species of Sagenomella, S. diversispora, and other Sagenomella species. The former T. emersonii, T. eburneus and T. byssochlamydoides form a clade recently recognised and described as the genus Rasamsonia (Houbraken et al. 2011). Talaromyces thermophilus is also excluded from Talaromyces s. str. and is closely related to the type species of Thermomyces, Therm. lanuginosus. Basal to Therm. lanuginosus and T. thermophilus is Talaromyces luteus. This species is on a separate branch and no other closely related species were found in our analysis. The uniqueness of the species is supported by the production of large amounts of the prenylated diketopiperaziners talathermophilins A and B, not found in any other species (Chu et al. 2010). The phylogenetic position of T. leycettanus is not convincingly defined. This species is positioned near Warcupiella spinulosa and Hamigera striata (= Talaromyces striatus), but bootstrap support is lacking. Talaromyces brevistipitatus occurs on a well-supported branch with H. avellanea. Comparison of ITS and calmodulin sequences shows that this species is closely related to NRRL 2108, an undescribed, phylogenetically distinct Hamigera species (ITS 100 % bs, calmodulin 99 % bs) (Peterson et al. 2010). The majority of members of subgenus Biverticillium sensu Pitt (1980) are phylogenetically placed within Talaromyces s. str., with P. isariiforme as the only exception. This species belongs to Penicillium s. str. and is closely related to P. ochrosalmoneum. This relationship was also confirmed by extrolite data (see below).
Fig. 1.
. Best-scoring Maximum Likelihood tree calculated using RAxML, based on partial RPB1 sequences showing the relationships among members of Talaromyces and Penicillium subgenus Biverticillium and related genera. The bootstrap support percentages of the maximum likelihood (ML) analysis are presented at the nodes. Bootstrap support values less than 70 % are not shown and branches with bootstrap support values > 70 % are thickened. The bar indicates the number of substitutions per site. The tree is rooted with Coccidioides immitis (strain RS).
Figure 1 indicates that the following species phylogenetically belong in Talaromyces: Aphanoascus cinnabarinus (CBS 267.72), Sagenomella bohemica (CBS 545.86T), Paecilomyces aerugineus (CBS 350.66T), Geosmithia viridis (CBS 252.87T) and Sagenoma viride (CBS 114.72T). The former three strains are on a well-supported sister clade basal to Talaromyces muroii CBS 756.96.
Species delimitation and synonymies within Talaromyces
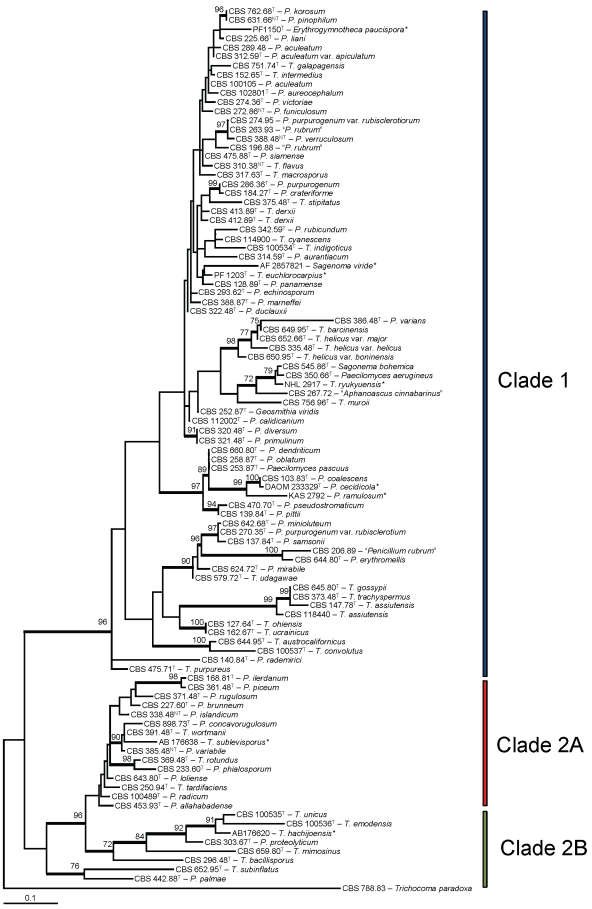
The ITS analysis (Fig. 2) was used in this study to provide a preliminary circumscription of the species belonging to the Talaromyces clade. Ninety-seven strains were included in the ITS analysis. The used primer pair V9G and LS266 also amplifies a part of the 18S and 28S rDNA; however, for analysis, only the span including the ITS regions and 5.8S rDNA was used. The length of the alignment was 483 characters and 221 characters were variable.
Fig. 2.
. Best-scoring Maximum Likelihood tree calculated using MEGA 5.0 based on ITS sequences showing the relationship among members of the Talaromyces and members of Penicillium subgenus Biverticillium. The bootstrap support percentages of the maximum likelihood (ML) analysis are presented at the nodes. Bootstrap support values less than 70 % are not shown and branches with bootstrap support values > 75 % are thickened. The bar indicates the number of substitutions per site. The tree is rooted with Trichocoma paradoxa (CBS 788.83). T. = Talaromyces; P. = Penicillium. Strains indicated with * are ITS sequencing obtained from GenBank.
Most bootstrap support values in the ITS analysis are low, less than 70 %. Only a few branches are supported with values higher than 70 %. The majority of Talaromyces species are on a branch with 96 % bootstrap support (clade 1, Fig. 2). This clade is also present in the RPB1 analysis (100 % bs). Another large clade was present in the ITS phylogram and this clade is supported with 96 % boostrap (clade 2). This clade can be divided in two subclades (2A and 2B), both present in the RPB1 analysis; however, the relationship among these subclades is not supported statistically. Talaromyces dendriticus, T. oblatus, and Paecilomyces pascuus are in the same lineage and the former two species share the same ITS sequence. Talaromyces assiutensis and T. gossypii also have similar ITS sequences and are phenotypically similar (Frisvad et al. 1990a).
Extrolite analysis
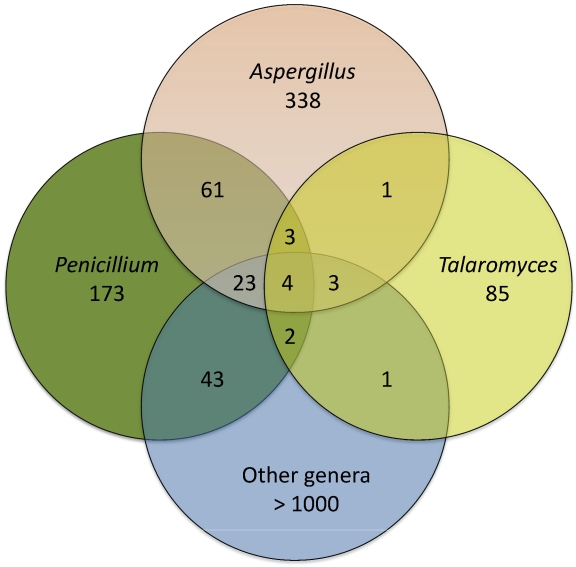
In general, Talaromyces species produce many biosynthetic families of polyketides and meroterpenoids, but rather few families of nonribosomal peptides and terpenes. By examining HPLC-DAD results from all described species of Penicillium, Aspergillus and their teleomorphs, and by searching the literature for families of exometabolites produced by these fungi, it is obvious that Talaromyces species have unique and specific extrolites (Table 2). Figure 3 shows the common exometabolite families in Talaromyces/Biverticillium, Penicillium, Aspergillus and other genera. Aspergillus and Penicillium share 91 biosynthetic families, but shares more of these with other fungal genera than with Talaromyces. A few exometabolites are shared among Talaromyces, Penicillium and Aspergillus including alternariols, asperphenamate, botryodiploidin, dehydrocarolic acid, emodins, geodins, gregatins, herqueinone, 3-hydroxyphtalic acid, italinic acid, lichexanthones, mellein, monordens, pinselin, rugulosuvines, rugulovasines, secalonic acids and zeorins. Most of these metabolites have relatively simple structures, and many occur in other genera less related phylogenetically to any of the penicilloid and aspergilloid genera. Considering the large number of shared exometabolite biosynthetic families in common between Penicillium and Aspergillus, Talaromyces is clearly different, which corresponds with all other data for these genera.
Table 2.
Secondary metabolite (exometabolite) biosynthetic families known from Talaromyces and Penicillium subgenus Biverticillium. (P) means also found in Penicillium and its teleomorphic state Eupenicillium, (A) means also found in species of Aspergillus. (Others) means also found in other fungi outside Penicillium, Aspergillus, Talaromyces and related genera.
| Secondary metabolite (exometabolite) biosynthetic families | ||
|---|---|---|
| AF-110 | 5-Hydroxymethylfurfural | Purpurogenones |
| Alternariols * (P and others) | Hydromethylmaltol | Rasfonin |
| Anthglutin | 4-Hydroxy-4,5-dicarboxy pentadecanoic acid (T. spiculisporus) | Rubratoxins |
| Apiculides (incl. NG-011's * (others)) | 7-Hydroxy-2,5-dimethylchromane | Rugulosins (& flavoskyrin) * (others) |
| AS-186-G | 3-Hydroxymethyl-6,8-dimethoxycoumarin | Rugulotrosins |
| Asperphenamates & asperglaucid * (A, P) | 3-Hydroxyphthalic acid * (P) | Rugulosuvine * (P) |
| Atrovenetinon methyl acetal (P. verruculosum) | Islandic acids | Rugulovasines * (P) |
| Epi-Austdiols (7-epiaustdiol & 8-O-methylepiaustdiol) (the stereoisomer austdiol found in Aspergillus) | (+)-Isocitric acid + Decylcitric acid (T. spiculisporus) | Secalonic acids * (A, P, others) |
| Austins * (A, P) | Italinic acids * (P) | Speciferone* (others) |
| BE-24811 | Juglones | Spiculisporic acids (= minioluteic acids) |
| BE-31405's | Lichexanthone * (others) | SQ 30957 |
| Berkeleyamides | Luteusins | Stemphyperylenole |
| Botryodipoidin * (P & others) | Maculosin * (others) | Stipitatic acids |
| Chrodinanine A | Mellein * (A) | Talaperoxides |
| Cordyanhydrides | Methyl-4-carboxy-5-hydroxyphthalaldehydrate | Talaroconvolutins |
| Cyclochlorotines & islanditoxin | 3-Methyl-6-hydroxy-8-methoxy-3,4-dihydroisocoumarins | Talaroderxine |
| Dehydrocarolic acids * (A, P) | Miniolutelides, berkeleydione, berkeleytriones, berkeleyacetals, dhilirolides | Talaroflavones |
| Diethylphthalate (Artefact?) | Mitorubrins & kasanosins & funicones | Talaromycins |
| 5,6-Dihydro-3,5-dihydroxy-6-hydroxymethyl-2H-pyran-2-one | Monascins & monascorubramin | Talarotoxins |
| 4,6-Dihydroxy-5-methylphthalide | Monordens * (A, others) | TAN-931 |
| (2E,2E’,7S,7’E)-4,9-Dioxo-7-(4’,9’-dioxo-2’,7’decadienoyloxy)-2-decanoic acid | NG-061 | Thailandolides |
| Diversonols | NK-374200 | Trachyspermic acids |
| Duclauxins | OF-4949's | Trachyspic acid |
| Emodins * (A, P, others) | Penicilliopsin * (others) | Triacetic lactone |
| Erythroskyrins | Penisimplicins | (-)-2,3,4-Trihydroxy-butanamide |
| Flavomannin | Penisimplicissins | Vermicellins |
| Funiculosic acids | Penitrinic acid & penitricins | Vermiculins |
| Funiculosin | Pevalic acid | Vermilutins |
| Geodins * (A, P) | PF-1092A | Vermistatins & penicidones |
| Glauconic acids | Pinselic acid | Vertoskyrin |
| Gregatins and penicilliols * (A, P) | Pinselin * (A, others) | Wortmannilactones |
| Helicusins | Purpactins (= penicillides = vermixocins) | Wortmannins * (others) |
| Herqueinones* (P) | Purpuride | Xanthoradones |
| Zeorins * (A, others) | ||
Fig. 3.
. Common exometabolite families in Talaromyces/Biverticillium, Penicillium, Aspergillus and other genera.
Among the few extrolites shared by Penicillum, Aspergillus and Talaromyces are the ergochromes, secalonic acid D & F. These anthraquinone derived metabolites are found in P. isariiforme, P. chrysogenum, Aspergillus aculeatinus, P. dendriticum and P. pseudostromaticum (Samson et al. 1989, Frisvad & Samson 2004, Houbraken et al. 2011). It is also possible that there are optical antipodes of these compounds produced in these genera, as was found in Aspergillus versicolor ((+) versicolamide)) and A. sclerotiorum ((-)-versicolamide) (Williams 2011). If this is so, it may indicate that the extrolites of Talaromyces and Penicillium / Aspergillus may also differ in stereochemical aspects. Another example of shared yet different extrolites is the azaphilones, which are common in species of Talaromyces and related biverticillate anamorphic species (Frisvad et al. 1990a, Nicoletti et al. 2009, Osmanova et al. 2010), but could not be found in Aspergillus and Penicillium sensu stricto. When similar compounds were found in Talaromyces, stereoisomers of the compounds were found in Aspergillus and Penicillium. For example, while sclerotiorins occur in P. sclerotiorum, the epimers are found in Talaromyces helicus and T. luteus (Yoshida et al. 1995, 1996a, b). Austdiol was isolated from Aspergillus pseudoustus (Vleggaar et al. 1974, Samson et al. 2011), but 7-epi-austdiol from a Talaromyces species (Liu et al. 2010).
Misidentifications of strains can make these comparisons difficult, but the overwhelming majority of extrolites found in Talaromyces are not found in Aspergillus or Penicillium. Although vermistatins, penisimplisins, penisimplicissins were reported from Penicillium simplicissimum (Komai et al. 2005), the producing strain was misidentified and actually represents a species of Talaromyces. The opposite has also happened, and metabolites attributed to a species of subgenus Biverticillium are later found to be produced by species of Penicillium sensu stricto. Penicillium verruculosum was reported to produce verruculogen, hence the name (Cole et al. 1972, Cole & Kirksey 1973), but the strain was later reidentified as P. brasilianum (Frisvad 1989).
Penicillium isariiforme (Samson et al. 1989) and P. ochrosalmoneum (Wicklow & Cole 1984) both produce large amounts of citreoviridin, supporting their close relationship indicated by the phylogenetic analyses, as noted above (Fig. 1).
DISCUSSION
The symmetrical, biverticillate penicillus was used as a defining character by Wehmer (1914), and Thom (1915a, b). Wehmer (1914) proposed to call this group the Verticillata, while Thom (1915a) referred to it as the Penicillium luteum-purpurogenum group. Biourge (1923) was the first who named this group as the subgenus Biverticillium, but included species such as P. citrinum (as P. aurifluum), P. atramentosum etc., which are no longer regarded as members of this subgenus (Houbraken et al. 2010). The characteristic lanceolate or acerose phialides was used as a more definitive morphological character of subgenus Biverticillium and related Talaromyces anamorphs (Raper & Thom 1949), because biverticillate branched conidiophores with flask-shaped phialides are mainly found in unrelated species such as P. citrinum. Although the lanceolate phialides occur in most species of subgenus Biverticillium, some species, e.g. P. rugulosum, have phialides that are not slender and have an apical portion tapering into a long acuminate point.
Thom (1930) treated some of the Penicillia in his Biverticillate-Symmetrica group and distinguished four sections: Ascogena, Coremigena, Luteo-virida (Funiculosa and Luteo-purpurogena) and Miscellanea. Later, Raper & Thom (1949) subdivided the group into the P. luteum series, P. duclauxii series, P. funiculosum series, P. purpurogenum series, P. rugulosum series and P. herquei series. This grouping is inconsistent with our phylogenetic analysis of the biverticillate group. The classification proposed by Pitt (1980) is more in concordance with the phylogenetic and taxonomic treatment proposed here, although he included a few species in Penicillium subgenus Biverticillium, namely P. isariiforme, P. clavigerum and P. vulpinum (as P. claviforme) that are now classified in Penicillium sensu stricto. The same conclusion was shown by the early molecular results of LoBuglio & Taylor (1993), and subsequently supported by the physiological, morphological and extrolite characters reviewed in the Introduction, and generated during this study.
In general, Penicillium sensu stricto and Aspergillus share many more features with each other than they do with Talaromyces. This includes micro- and macro-morphology, good growth on low water activity media, and the many shared exometabolite families. Talaromyces produces a series of metabolites that are apparently unique to this genus (J.C. Frisvad unpubl. data). The characteristic yellow and red colony and mycelial colours in Talaromyces are often caused by accumulation of mitorubrins and other azaphilones and unique anthraquinones and mitorubrins that are not found in Aspergillus and Penicillium. Some azaphilones are found in Penicillium sclerotiorum and Penicillium hirayamae, but only their optical antipodes are found in Talaromyces.
Penicillium and Talaromyces species excluded from the revised Talaromyces genus
Figure 1 shows that a number of species described in the genus should be excluded from Talaromyces s. str. Phylogenetically, T. ocotl CBS 102855T belongs to Sagenomella, as also suggested using phenotypic characters (Heredia et al. 2001). The anamorph of this species was not formally named, described only as Sagenomella sp., and thus the new combination Sagenomella ocotl is proposed in the taxonomy section below.
Our analysis confirms the distinctiveness of the recently described genus Rasamsonia erected for thermotolerant or thermophilic species with distinctly rough-walled conidiphore stipes, olive-brown conidia, and ascomata, if present, with a scanty hyphal covering. Talaromyces eburneus, T. emersonii, T. byssochlamydoides were assigned to this genus, together with the anamorphic species originally described as Geosmithia argillacea and G. cylindrospora (Houbraken et al. 2011,2011).
Talaromyces thermophilus is the only member of Talaromyces section Thermophila (Stolk & Samson 1972). LoBuglio et al. (1993) already noted that this species is the most divergent Talaromyces species, occupying a basal position to the major Talaromyces clade. Houbraken et al. (2011,2011) showed that this species is closely related to Thermomyces lanuginosus and our partial RPB1 sequence data confirm this relationship (Fig. 1). We did not examine type material of Talaromyces thermocitrinus (as ‘thermocitrinum’) and the conclusion of Mouchacca (2007), who tentatively placed this species in synonymy with T. thermophilus, is not followed here. Talaromyces luteus is further basal to T. thermophilus and Therm. lanuginosus and this species might represent a distinct genus. For the present, T. thermophilus and T. luteus will be retained in Talaromyces. More research is needed to confirm whether the assignment of these species to Thermomyces is warranted.
Udagawa & Suzuki (1994) described Talaromyces spectabilis with a Paecilomyces anamorph. Houbraken et al. (2008) transferred this species to Byssochlamys and showed that it is the teleomorph of Paec. variotii. In a single name system, Paec. variotii is the oldest genus and species name for this taxon, and thus the correct name for the holomorph.
Talaromyces brevicompactus, T. striatus (= Hamigera striata) and T. leycettanus are distant from Talaromyces s. str. and phylogenetically more closely related to Penicillium s. str. and Aspergillus. Figure 1 shows that H. striata and T. leycettanus are closely related. Further phylogenetic support for this relationship was presented in the studies of Ogawa & Sugiyama (2000) and Houbraken & Samson (2011). These two species are phylogenetically distant from Talaromyces s. str. and more closely related to Hamigera. Peterson et al. (2010) delimited Hamigera phylogenetically but stated that T. leycettanus and H. striata do not belong to this genus, and followed Benjamin's (1955) placement of H. striata in Talaromyces. In this study, we retain H. striata and T. leycettanus in Hamigera and Talaromyces, respectively. A thorough study on Hamigera and related genera is needed to clarify the correct placement of these species. Kong (1999) described Talaromyces brevicompactus, stating that this species is closely related to Hamigera avellanea (as Talaromyces avellaneus). The anamorph of this species was described in Merimbla, thus confirming the relationship with Hamigera. Sequence comparisons of this species showed that it is similar to NRRL 2108, a phylogenetically undescribed Hamigera species (J. Houbraken, unpubl. data, Peterson et al. 2010). We wait with combining this species in Hamigera until a more data and strains become available.
Species described in other genera but phylogenetically within Talaromyces
Phylogenetic analysis shows that “Aphanoascus cinnabarinus”, Sagenomella bohemica, Paecilomyces aerugineus, Geosmithia viridis and Sagenoma viride belong to Talaromyces. The genus Sagenoma is typified with S. viride, and therefore this genus can be considered as a synonym of Talaromyces. Our data support the conclusions of von Arx (1987), who correctly transferred this species in Talaromyces, and this is reflected in the taxonomy section below.
Houbraken & Samson (2011) discussed the confusion over Aphanoascus cinnabarinus, which has persisted since the description of the genus Aphanoascus by Zukal (1890). Most authors follow Apinis (1968) and consider the genus Aphanoascus to be typified by A. fulvescens Zukal. In addition, the neotypification of A. cinnabarinus by Udagawa & Takada (1973) was incorrect, because their neotype strain had a Paecilomyces anamorph, whereas Zukal's original description and illustrations clearly showed a Chrysosporium-like anamorph (Stolk & Samson 1983). Based on morphological features, Stolk & Samson (1983) indicated that Chromocleista cinnabarina (as A. cinnabarinus sensu Udagawa & Takada) belongs to the Eurotiales and suggested that this species is intermediate between Thermoascus and Talaromyces. Our phylogenetic study, and that of Houbraken & Samson (2011), clarified that C. cinnabarina belongs to Talaromyces s. str. The taxonomic position of Chromocleista cinnabarina (as A. cinnabarinus sensu Udagawa & Takada) will be discussed in a forthcoming paper. Paecilomyces aerugineus was proposed by Samson (1974) for Spicaria silvatica Oudemans sensu Apinis. This species resembles the anamorph of A. cinnabarinus sensu Udagawa & Takada and a more detailed study is necessary to clarify this relationship.
TAXONOMY
Penicillium itself has a long list of generic synonyms (see Seifert et al. 2011) that must be considered for the species formerly included in subgenus Biverticillium. These synonyms of Penicillium are discussed in the Appendix to this paper. As it turns out, none of these are appropriate for subgenus Biverticillium, leaving the comparatively young Talaromyces as the oldest well-known generic name as the new home for the anamorphic species of subgenus Biverticillium.
Yaguchi et al. (1994a) introduced Erythrogymnotheca for the single species E. paucispora. No specimens of E. paucispora were studied; however, examination of the available ITS data on GenBank and the original description shows that this species belongs in Talaromyces. As a consequence, Erythrogymnotheca is synonymised with Talaromyces. Comparison of an ITS sequence of E. paucispora (AB176603) shows that it is related to P. korosum, P. pinophilum and P. liani in Talaromyces (Fig. 2). The original description suggests that Talaromyces and Erythrogymnotheca differ in ascus characteristics and ascospore morphology. However, these genera also share characters. The ascomatal initials of E. paucispora approximate those of Talaromyces flavus and other species of Talaromyces. Furthermore, E. paucispora produces a loose hyphaI yellow- or red-pigmented ascomata similar to those of other Talaromyces species and the main ubiquinone systems are Q-10 and Q-10 (H2), also indicating a relationship with Talaromyces (Paterson 1998, Yaguchi et al. 1994a).
Matsushima (2001) described Paratalaromyces from soil collected in Taiwan, distinguishing it by a distinct textura epidermoidea layer in the ascomatal wall, and the presence of spinulose marginal hyphae. We have not seen the type but the description of Paratalaromyces lenticularis is similar to that of Talaromyces unicus (Tzean et al. 1992). We consider the genus a synonym here.
Visagie & Seifert (unpubl. data) report on the generic name Lasioderma Mont., typified by L. flavo-virens Durieu & Mont., which is conspecific with Penicillium aureocephalum Munt.-Cvetk., Hoyo & Gómez-Bolea. The name Lasioderma is widely used as an insect genus, and a formal proposal for the conservation of Talaromyces against this older name is being prepared.
Talaromyces C.R. Benj., Mycologia 47: 681. 1955.
= Penicillium Link subgenus Biverticillium Dierckx apud Biourge Cellule 33: 31. 1923.
= Penicillium subg. Biverticillata-Symmetrica Thom, The Penicillia: 158. 1930.
= Sagenoma Stolk & G.F. Orr, Mycologia 66: 676. 1974.
= Erythrogymnotheca Yaguchi & Udagawa, Mycoscience 35: 219. 1994.
= Paratalaromyces Matsush., Matsush. Mycol. Mem. 10: 111 (2003) [2001].
Ascomata cleistothecial, usually with a distinctly hyphal exterior wall, often yellow, occasionally white, creamish, pinkish or reddish. Asci 8-spored, globose to ellipsoidal, ascus initials sometimes with morphologically distinguishable gametangia, mature asci produced in chains. Ascospores one-celled, rarely smooth-walled, but often with surface ornamentation and wings, hyaline to yellow, in strains producing abundant red pigment occasionally red. Conidiophores comprising smooth or rough-walled elements, with long hyaline stipes, generally terminating in a single whorl of 3–10 metulae, appearing symmetrical in face view (in some species with a single subterminal lateral branch that afterwards repeats the branching pattern of the main axis, but then with the whole conidiophore appearing asymmetrical), each metula with a terminal whorl of phialides. Conidiogenous cells phialidic, aculeate or acerose, rarely ampulliform, periclinal thickening usually visible in the conidiogenous aperture, with or without a cylindrical collarette. Conidia aseptate, green in mass, in basipetal connected chains, usually ellipsoidal to fusiform.
Type species: Talaromyces vermiculatus (P.A. Dang.) C.R. Benj., Mycologia 47: 684. 1955.
The name Talaromyces was introduced by Benjamin (1955), and the type species is T. vermiculatus (P.A. Dang.) C.R. Benj. One of the authors (RAS) personally visited several herbaria in Paris to locate holotype or other original material of Penicillium vermiculatum P.A. Dang. Dangeard (1907) described and illustrated both the anamorph and teleomorph under this name, but his material could not be located. To repair the shortcoming of the typification of Talaromyces, the lectotype for P. vermiculatum is here designated as Plate XVIII in Dangeard (1907, available at the Biodiversity Heritage Library, www.biodiversitylibrary.org). It was selected from among the plates XVI—XX because it includes the most detailed drawings of the anamorph, but also includes elements of the teleomorph. Herb. IMI 197477 is here designated as the epitype of Penicillium vermiculatum P.A. Dang. This specimen, which is also the holotype of Penicillium dangeardii J. Pitt, the seldom-used name for the anamorph of T. flavus, is derived from the equivalent cultures CBS 310.38, IMI 19447, and NRRL 2098. The latter strain was considered typical of P. vermiculatum by Raper & Thom (1949), the last major treatment to use this Penicillium name as a distinct species.
List of species
The following list includes previously accepted species of Talaromyces and proposals to transfer the species of Penicillium subgenus Biverticillium to Talaromyces.
Our phylogenetic studies demonstrate that several taxa represent complexes of morphologically cryptic phylogenetic species, requiring further study. For example, we analysed members of the Penicillium purpurogenum complex (including P. purpurogenum, P. rubrum, P. crateriforme, P. sanguineum) and found that several species group could be distinguished by sequencing certain genes (N. Yilmaz, unpubl. data) and had distinct macromorphological features and unique extrolite profiles. The full phylogenetic diversity of the P. purpurogenum species complex requires more investigation, and a more detailed account will be published elsewhere.
ACCEPTED SPECIES IN TALAROMYCES
Talaromyces aculeatus (Raper & Fennell) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560639.
Basionym: Penicillium aculeatum Raper & Fennell, Mycologia 40: 535. 1948.
Talaromyces albobiverticillius (H.-M. Hsieh, Y.-M. Ju & S.-Y. Hsieh) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560683.
Basionym: Penicillium albobiverticillium H.-M. Hsieh, Y.-M. Ju & S.-Y. Hsieh, Fung. Sci. 25: 26. 2010.
Talaromyces allahabadensis (B.S. Mehrotra & D. Kumar) Samson, Yilmaz & Frisvad, comb. nov. MycoBank MB560640.
Basionym: Penicillium allahabadense B.S. Mehrotra & D. Kumar, Canad. J. Bot. 40: 1399. 1962.
Talaromyces apiculatus Samson, Yilmaz & Frisvad, sp. nov. MycoBank MB560641.
= Penicillium aculeatum var. apiculatum Abe, S., 1956, J. Gen. Appl. Microbiol., Tokyo 2: 124. 1956 (nom. inval., Art. 36).
Penicillio aculeato simile, sed conidiis apiculatis distinguitur.
Typus: Japan from soil (CBS H-20755 – Holotype, culture ex-type CBS 312.59)
Note: Species similar to Penicillium aculeatum but differing by apiculate conidia.
Talaromyces assiutensis Samson & Abdel-Fattah, Persoonia 9: 501. 1978.
Anamorphic synonym: Penicillium assiutense Samson & Abdel Fattah (simultaneously published, identical holotype).
Talaromyces aurantiacus (J.H. Mill., Giddens & A.A. Foster) Samson, Yilmaz, & Frisvad, comb. nov. MycoBank MB560642.
Basionym: Penicillium aurantiacum J.H. Mill., Giddens & A.A. Foster, Mycologia 49: 797. 1957.
Talaromyces austrocalifornicus Yaguchi & Udagawa Trans. Mycol. Soc. Japan 34: 245. 1993.
Anamorphic synonym: Penicillium austrocalifornicum Yaguchi & Udagawa (simultaneously published, identical holotype).
Talaromyces bacillisporus (Swift) C. R. Benj., Mycologia 47: 682. 1955.
≡ Penicillium bacillisporum Swift, Bull. Torrey Bot. Club 59: 221, 1932.
Talaromyces boninensis (Yaguchi & Udagawa) Samson, Yilmaz, & Frisvad, comb. nov. MycoBank MB560643.
Basionym: Talaromyces helicus var. boninensis Yaguchi & Udagawa, Transactions Mycological Society Japan 33: 511. 1992.
Talaromyces brunneus (Udagawa) Samson, Yilmaz & Frisvad, comb. nov. MycoBank MB560644.
Basionym: Penicillium brunneum Udagawa, J. Agric. Sci. (Tokyo) Nogyo Daigaku 5: 16. 1959.
Talaromyces calidicanius (J.L. Chen) Samson, Yilmaz & Frisvad, comb. nov. MycoBank MB560645.
Basionym: Penicillium calidicanium J.L. Chen, Mycologia 94(5): 870. 2002.
Talaromyces cecidicola (Seifert, Hoekstra & Frisvad) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560646.
Basionym: Penicillium cecidicola Seifert, Hoekstra & Frisvad, Stud. Mycol. 50: 520. 2004.
Talaromyces coalescens (Quintan.) Samson, Yilmaz & Frisvad, comb. nov. MycoBank MB560647.
Basionym: Penicillium coalescens Quintan., Mycopathol. 84: 115. 1984.
Talaromyces convolutus Udawaga, Mycotaxon 48: 141. 1993.
Anamorphic synonym: Penicillium convolutum Udagawa (simultaneously published, identical holotype).
Talaromyces dendriticus (Pitt) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560648.
Basionym: Penicillium dendriticum Pitt, The Genus Penicillium: 413. 1980.
Talaromyces derxii Takada & Udagawa, Mycotaxon 31: 418. 1988.
Anamorphic synonym: Penicillium derxii Takata & Udagawa (simultaneously published, identical holotype).
Talaromyces diversus (Raper & Fennell) Samson, Yilmaz & Frisvad, comb. nov. MycoBank MB560649.
Basionym: Penicillium diversum Raper & Fennell, Mycologia 40: 539. 1948.
Talaromyces duclauxii (Delacr.) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560650.
Basionym: Penicillium duclauxii Delacr., Bull. Soc. Mycol. France 7: 107. 1891.
Talaromyces echinosporus (Nehira) Samson, Yilmaz & Frisvad, comb. nov. MycoBank MB560651.
Basionym: Penicillium echinosporum Nehira, J. Ferment. Technol., Osaka 11: 861. 1933.
Note: Penicillium asperosporum G. Smith, Trans. Brit. Mycol. Soc. 48: 275. 1965. (= Penicillium echinosporum G. Sm., Trans. Brit. Mycol. Soc. 45: 387. 1962, non Nehira in J. Ferment. Technol. 11: 849. 1933) belongs in Penicillium section Aspergilloides (Houbraken & Samson 2011).
Talaromyces emodensis Udagawa, Mycotaxon 48: 146. 1993. Anamorphic synonym: Penicillium emodense Udagawa (simultaneously published, identical holotype).
Talaromyces erythromellis (A.D. Hocking) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560652.
Basionym: Penicillium erythromellis A.D. Hocking apud Pitt, The Genus Penicillium: 459. 1980.
Talaromyces euchlorocarpius Yaguchi, Someya & Udagawa, Mycoscience 40: 133. 1999.
Anamorphic synonym: Penicillium euchlorocarpium Yaguchi, Someya & Udagawa (simultaneously published, identical holotype).
Note: We have not seen the type, but the description and the ITS sequences available in GenBank (AB176617) show that this is a distinct species of Talaromyces.
Talaromyces flavo-virens (Durieu & Mont.) Visagie, Llimona & Seifert, ined.
Note: A manuscript on this species and its relationship to Penicillium aureocephalum Munt.-Cvetk., Hoyo & Gómez-Bolea is being prepared for publication in Mycotaxon.
Talaromyces flavus (Klöcker) Stolk & Samson, Stud. Mycol. 2: 10. 1972.
Anamorphic synonym: Penicillium dangeardii Pitt, The Genus Penicillium: 472. 1980.
Talaromyces funiculosus (Thom) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560653.
Basionym: Penicillium funiculosum Thom, Bull. Bur. Anim. Ind. U.S. Dep. Agric. 118: 69. 1910.
Talaromyces galapagensis Samson & Mahoney, Trans. Brit. Mycol. Soc. 69: 158. 1977.
Anamorphic synonym: Penicillium galapagense Samson & Mahoney (simultaneously published, identical holotype).
Talaromyces hachijoensis Yaguchi, Someya & Udagawa, Mycoscience 37: 157. 1996.
Note: We have not seen the type but the description and the ITS sequences available in GenBank (AB176620) show that this is a distinct species of Talaromyces. It is unusual in the genus for its apparent lack of an anamorph.
Talaromyces helicus (Raper & Fennell) C.R. Benj., Mycologia 47: 684. 1955.
≡ Penicillium helicum Raper & Fennell, Mycologia 40: 515. 1948.
Talaromyces indigoticus Takada & Udagawa, Mycotaxon 46: 129. 1993.
Anamorphic synonym: Penicillium indigoticum Takada & Udagawa (simultaneously published, identical holotype).
Talaromyces intermedius (Apinis) Stolk & Samson, Stud. Mycol. 2: 21. 1972.
Anamorphic synonym: Penicillium intermedium Stolk & Samson, Stud. Mycol. 2: 21. 1972.
Talaromyces islandicus (Sopp) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560654.
Basionym: Penicillium islandicum Sopp, Skr. Vidensk.-Selsk. Christiania, Math.-Naturvidensk. Kl. 11: 161. 1912.
Talaromyces loliensis (Pitt) Samson, Yilmaz & Frisvad, comb. nov. MycoBank MB560655.
Basionym: Penicillium loliense Pitt, The Genus Penicillium: 450. 1980
Talaromyces macrosporus (Stolk & Samson) Frisvad, Samson & Stolk, Ant. van Leeuwenhoek 57: 186. 1990.
Anamorphic synonym: Penicillium macrosporum Frisvad, Filt., Samson & Stolk. nom. illegit. Art. 53 (non P. macrosporum Berk. & Broome 1882).
Talaromyces marneffei (Segretain, Capponi & Sureau) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560656.
Basionym: Penicillium marneffei Segretain, Capponi & Sureau apud Segretain, Bull. Soc. Mycol. France 75: 416. 1959 [1960].
Talaromyces mimosinus A.D. Hocking apud Pitt, The Genus Penicillium: 507. 1980.
Anamorphic synonym: Penicillium mimosinum A. D. Hocking (simultaneously published, identical holotype).
Talaromyces minioluteus (Dierckx) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560657.
Basionym: Penicillium minioluteum Dierckx, Ann. Soc. Sci. Bruxelles 25: 87. 1901.
Talaromyces muroii Yaguchi, Someya & Udagawa, Mycoscience 35: 252. 1994.
Note: This species is unusual in Talaromyces because of its lack of a known anamorph.
Talaromyces palmae (Samson, Stolk & Frisvad) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560658.
Basionym: Penicillium palmae Samson, Stolk & Frisvad, Stud. Mycol. 31: 135. 1989.
Talaromyces panamensis (Samson, Stolk & Frisvad) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560659.
Basionym: Penicillium panamense Samson, Stolk & Frisvad, Stud. Mycol. 31: 136. 1989.
Talaromyces paucisporus (Yaguchi, Someya & Udagawa) Samson & Houbraken, comb.nov. MycoBank MB560684.
Basionym: Erythrogymnotheca paucispora Yaguchi, Someya & Udagawa, Mycoscience 35: 219. 1994.
Talaromyces phialosporus (Udagawa) Samson, Yilmaz & Frisvad, comb. nov. MycoBank MB560660.
Basionym: Penicillium phialosporum Udagawa, J. Agric. Sci. (Tokyo) Nogyo Daigaku 5: 11. 1959.
Talaromyces piceus (Raper & Fennell) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560661.
Basionym: Penicillium piceum Raper & Fennell, Mycologia 40: 533. 1948.
Talaromyces pinophilus (Hedgcock) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560662.
Basionym: Penicillium pinophilum Hedgcock apud Thom, Bull. Bur. Anim. Ind. US Dept. Agric. 118: 37. 1910.
Talaromyces pittii (Quintan.) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560663.
Basionym: Penicillium pittii Quintan., Mycopathol. 91: 69. 1985.
Talaromyces primulinus (Pitt) Samson, Yilmaz & Frisvad, comb. nov. MycoBank MB560664.
Basionym: Penicillium primulinum Pitt, The Genus Penicillium: 455. 1980.
Talaromyces proteolyticus (Kamyschko) Samson, Yilmaz & Frisvad, comb. nov. MycoBank MB560665.
Basionym: Penicillium proteolyticum Kamyschko, Not. Syst. Crypt. Inst. Bot. Acad. Sci. USSR 14: 228. 1961.
Talaromyces pseudostromaticus (Hodges, G.M. Warner, Rogerson) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560666.
Basionym: Penicillium pseudostromaticum Hodges, G.M. Warner & Rogerson, Mycologia 62: 1106. 1970.
Talaromyces purpureus (E. Müll. & Pacha-Aue) Stolk & Samson, Stud. Mycol. 2: 57. 1972.
Anamorphic synonym: Penicillium purpureum Stolk & Samson, Stud. Mycol. 2: 57. 1972.
Talaromyces purpurogenus (Stoll) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560667.
Basionym: Penicillium purpurogenum Stoll, Beitr. Charakt. Penicillium-Arten: 32. 1904.
Talaromyces rademirici (Quintan.) Samson, Yilmaz & Frisvad, comb. nov. MycoBank MB560668.
Basionym: Penicillium rademirici Quintan., Mycopathol. 91: 69. 1985.
Talaromyces radicus (A.D. Hocking & Whitelaw) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560669.
Basionym: Penicillium radicum A.D. Hocking & Whitelaw, Mycol. Res. 102: 802. 1998.
Talaromyces ramulosus (Visagie & K. Jacobs) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560670.
Basionym: Penicillium ramulosum Visagie & K. Jacobs, Mycologia 101: 890. 2009.
Talaromyces rotundus (Raper & Fennell) C.R. Benj., Mycologia 47: 683. 1955.
≡ Penicillium rotundum Raper & Fennell, Mycologia 40: 518. 1948.
Talaromyces ryukyuensis (S. Ueda & Udagawa) Arx, Persoonia 13: 282. 1987.
≡ Sagenoma ryukyuense S. Ueda & Udagawa, Mycotaxon 20: 499. 1984.
Note: We have not seen the type but the description and the ITS sequences available in GenBank (AB176628) show that this is a distinct species of Talaromyces.
Talaromyces rubicundus (J.H. Mill., Giddens & A.A. Foster) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560671.
Basionym: Penicillium rubicundum J.H. Mill., Giddens & A.A. Foster, Mycologia 49: 797. 1957.
Talaromyces rugulosus (Thom) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560672.
Basionym: Penicillium rugulosum Thom, Bull. Bur. Anim. Ind. US Dept. Agric. 118: 60. 1910.
Talaromyces sabulosus (Pitt & A.D. Hocking) Samson, Yilmaz & Frisvad, comb. nov. MycoBank MB560673.
Basionym: Penicillium sabulosum Pitt & A. D. Hocking, Mycologia 77: 818. 1985.
Talaromyces siamensis (Manoch & C. Ramírez) Samson, Yilmaz & Frisvad, comb. nov. MycoBank MB560674.
Basionym: Penicillium siamense Manoch & C. Ramírez, Mycopathol. 101: 32. 1988.
Talaromyces stipitatus (Thom) C.R. Benj., Mycologia 47: 684. 1955.
≡ Penicillium stipitatum Thom, Mycologia 27: 138. 1935.
Talaromyces sublevisporus (Yaguchi & Udagawa) Samson, Yilmaz & Frisvad, comb. et stat. nov. MycoBank MB560675.
Basionym: Talaromyces wortmannii var. sublevisporus Yaguchi & Udagawa, Mycoscience 35: 63. 1994.
Note: We have not examined the ex-type of this species but from the ITS data (GenBank AB176638), this seems to be a separate species.
Talaromyces tardifaciens Udagawa, Mycotaxon 48: 150. 1993.
Anamorphic synonym: Penicillium tardifaciens Udagawa (simultaneously published, identical holotype).
Talaromyces trachyspermus (Shear) Stolk & Samson, Stud. Mycol. 2: 32. 1972.
Anamorphic synonym: Penicillium spiculisporum Leman, Mycologia 12: 268. 1920.
Talaromyces ucrainicus Udagawa, in Stolk & Samson, Stud. Mycol. 2: 34. 1972.
Anamorphic synonym: Penicillium ucrainicum Panasenko, Mycologia 56: 59. 1964.
Talaromyces udagawae Stolk & Samson, Stud. Mycol. 2: 36. 1972.
Anamorphic synonym: Penicillium udagawae Stolk & Samson (simultaneously published, identical holotype).
Talaromyces unicus Tzean, J.L. Chen & Shiu, Mycologia 84: 739. 1992.
Anamorphic synonym: Penicillium unicum Tzean, J.L. Chen & Shiu (simultaneously published, identical holotype).
Talaromyces variabilis (Sopp) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560676.
Basionym: Penicillium variabile Sopp, Skr. Vidensk.-Selsk. Christiania, Math.-Naturvidensk. Kl. 11: 169. 1912.
Talaromyces varians (G. Sm.) Samson, Yilmaz & Frisvad, comb. nov. MycoBank MB560677.
Basionym: Penicillium varians G. Sm., Trans. Brit. Mycol. Soc. 18: 89. 1933.
Talaromyces verruculosus (Peyronel) Samson, Yilmaz, Frisvad & Seifert, comb. nov. MycoBank MB560678.
Basionym: Penicillium verruculosum Peyronel, Germi Atmosf. Fung. Micel.: 22. 1913.
Talaromyces viridis (Stolk & G.F. Orr) von Arx, Persoonia 13: 2821. 1987.
≡ Sagenoma viride Stolk & G.F. Orr, Mycologia 66: 677. 1974.
Talaromyces viridulus Samson, Yilmaz & Frisvad, nom. nov. MycoBank MB560679.
Basionym: Geosmithia viridis Pitt & A.D. Hocking, Mycologia 77: 822. 1985 = P. viride (Pitt & A.D. Hocking) Frisvad, Samson & Stolk, Persoonia 14: 229. 1990, nom. illegit. Art. 53 (non Fres. 1851 nec Rivera 1873 nec Sopp 1912 nec (Matr.) Biourge 1923). Non Talaromyces viridis (Stolk & G.F. Orr) Arx.
Talaromyces wortmannii (Klöcker) C.R. Benjamin, Mycologia 47: 683. 1955.
≡ Penicillium wortmannii Klöcker, Compt-Rend. Trav. Carlsberg Lab. 6: 100. 1903.
EXCLUDED SPECIES AND TAXA, WHICH NEED FURTHER TAXONOMIC STUDY
Penicillium concavorugulosum S. Abe, J. Gen. Appl. Microbiol, Tokyo 2: 127. 1956 (nom. inval. Art. 36).
Note: This species was invalidly described, but our ITS data (Fig. 2) show that it is related to T. wortmanii. Further study is required but extrolite data indicate that this species is unique (J.C. Frisvad, unpubl. data).
Penicillium crateriforme J.C. Gilman & E.V. Abbott, Iowa State Coll. J. Sc. 1: 293. 1927.
Note: Our ITS data (Fig. 2) show that this species is a synonym of P. purpurogenum.
Penicillium ilerdanum C. Ramírez, A.T. Martínez & Berer., Mycopathol. 72: 32. 1980.
Note: Frisvad et al. (1990b) considered this species synononymous with Penicillium piceum Raper & Fennell, which is confirmed by our ITS data (Fig. 2).
Penicillium isariiforme Stolk & J.A. Mey., Trans. Brit. Mycol. Soc. 40: 187. 1957.
Note: According to Houbraken & Samson (2011), this species, included in subgenus Biverticillium by Pitt (1980), is correctly classified in Penicillium sensu lato.
Penicillium korosum J.N. Rai, Wadhwani & J.P. Tewari, Ant. van Leeuwenhoek 35: 430. 1969.
Note: This species requires further investigation, but our ITS sequence (Fig. 2) indicates that it is similar to P. pinophilum.
Penicillium krugeri C. Ramírez, Mycopathol. 110: 23. 1990.
Note: We have been unable to examine authentic material, and the correct classification of this species is uncertain.
Penicillium lignorum Stolk, Ant. van Leeuwenhoek 35: 264. 1969.
Note: A preliminary phylogenetic analysis indicates that this species does not belong to Talaromyces and might represent a new genus (J. Houbraken, unpubl. data).
Penicillium mirabile Beliakova & Milko, Mikol. Fitopatol. 6: 145. 1972.
Note: The ex-type culture is in poor condition and although our ITS data (Fig. 2) indicate that is a distinct species, it should be further investigated.
Penicillium oblatum Pitt & A.D. Hocking, Mycologia 77: 810. 1985.
Note: In our ITS phylogeny (Fig. 2), this species is close to Paecilomyces pascuus and Penicillium dendriticum and needs further study.
Penicillium pascuum (Pitt & A.D. Hocking) Frisvad, Samson & Stolk, Persoonia 14: 229. 1990.
≡ Paecilomyces pascuus Pitt & A. D. Hocking, Mycologia 77: 822. 1985.
Note: See on the position of this species under P. oblatum above.
Penicillium rubrum Stoll, Beitr. Charakt. Penicillium-Arten: 35. 1904.
Note: Although the name is well-known, the taxonomic position of the taxon remains doubtful because no type material has been located. A possible solution would be lectotypification from Stoll's illustrations, followed by epitypification to become a usable name.
Penicillium purpurogenum var. rubrisclerotium Thom, Mycologia 7: 137. 1915.
Note: Our ITS data (Fig. 2) indicate that this species is synonymous with P. minioluteum.
Penicillium samsonii Quintan., Mycopathol. 91: 69. 1985.
= Talaromyces minioluteus (Dierckx) Samson, Yilmaz, Frisvad & Seifert (see above).
Penicillium tardum Thom, The Penicillia: 485. 1930.
Note: Raper & Thom (1949) pointed out that there is confusion about the type culture and the status of this species will be subject of further studies.
Penicillium victoriae Szilv., Archiv. Hydrobiol. 14, Suppl. 6: 535. 1936.
= Penicillium janthinellum Biourge, Cellule 33: 258. 1923 (Pitt, 1980).
Note: Pitt (1980) synonymised this species under Penicillium janthinellum, but our studies showed that it clearly belongs in Talaromyces. Because there is only one strain, the exact identity of this fungus requires further study.
Talaromyces barcinensis Yaguchi & Udagawa, Trans. Mycol. Soc. Japan 34: 15. 1993.
Anamorphic synonym: Penicillium barcinense Yaguchi & Udagawa (simultaneously published, identical holotype).
Note: Our ITS sequence data show that this species is close to Talaromyces helicus and further study should determine its correct taxonomic position.
Talaromyces brevicompactus Kong, Mycosystema 18: 9. 1999.
Anamorphic synonym: Merimbla brevicompacta Kong, Mycosystema 18: 9. 1999 (simultaneously published, identical holotype).
Note: Fig. 1 shows that this species belongs in Hamigera. Comparison of partial β-tubulin and calmodulin sequences of the ex-type strain of T. brevicompactus with recent published data shows that this species represents a distinct species (J. Houbraken, unpubl. data). The new combination in Hamigera will be made elsewhere.
Talaromyces byssochlamydoides Stolk & Samson, Stud. Mycol. 2: 45. 1972.
Anamorphic synonym: Paecilomyces byssochlamydoides Stolk & Samson (simultaneously published, same holotype).
= Rasamsonia byssochlamydoides (Stolk & Samson) Houbraken & Frisvad, Ant. van Leeuwenhoek, in press.
Talaromyces eburneus Yaguchi, Someya & Udagawa, Mycoscience 35: 249. 1994.
Anamorphic synonym: Geosmithia eburnea Yaguchi, Someya & Udagawa (simultaneously described, holotype identical)
≡ Rasamsonia eburnea (Yaguchi, Someya & Udagawa) Houbraken & Frisvad, Ant. van Leeuwenhoek, in press.
Talaromyces emersonii Stolk, Ant. van Leeuwenhoek 31: 262. 1965.
Anamorphic synonym: Penicillium emersonii Stolk (simultaneously described, holotype identical), Ant. van Leeuwenhoek 31: 262. 1965.
= Rasamsonia emersonii (Stolk) Houbraken & Frisvad, Ant. van Leeuwenhoek, in press.
Talaromyces gossypii Pitt, The Genus Penicillium: 500. 1980
= Talaromyces assiutensis, Samson & Abdel-Fattah, Persoonia 9: 501. 1978 (fide Frisvad et al. 1990a).
Talaromyces lagunensis Udagawa, Uchiy. & Kamiya, Mycoscience 35: 403. 1994.
Anamorphic synonym: Penicillium lagunense Udagawa, Uchiy. & Kamiya (simultaneously published, identical holotype).
Note: We have been unable to examine authentic material, and the correct classification of this species is uncertain.
Talaromyces leycettanus H.C. Evans & Stolk, Trans. Brit. Mycol. Soc. 56: 45. 1971.
Anamorphic synonym: Penicillium leycettanus H.C. Evans & Stolk (simultaneously published, identical holotype).
≡ Paecilomyces leycettanus (H.C. Evans & Stolk) Stolk, Samson & H.C. Evans, Persoonia 6: 342. 1971.
Note: Houbraken & Samson (2011) showed that this species is phylogenetically unrelated to Talaromyces and close to Hamigera. Its taxonomic position requires further investigation.
Talaromyces luteus (Zukal) C.R. Benj., Mycologia 47: 681. 1955.
≡ Penicillium luteum Zukal, Sitzungsber Kaiserl. Akad. Wiss. Math-Naturwiss. C1., Abt. 1, 98: 561. 1890.
Note: Although the phenotype of this species resembles species of Talaromyces, our molecular analysis shows that it is phylogenetically unique and basal to T. thermophilus.
Talaromyces malagensis (Thüm.) Stalpers & Samson 1984, in Stalpers, Stud. Mycol. 24: 69. 1984.
Note: Stolk & Samson (1972) considered Sporotrichum malagense a dubious synonym of T. udagawae, based on their failure to find ascospores and conidia in the type material (herb. W). Later, Stalpers (1984) studied material preserved in herb. BR which is authentic and labelled as “type”. It agrees with Thümen's original diagnosis and contains both fertile Talaromyces cleistothecia and a sporulating biverticillate anamorph. Therefore, the new combination to Talaromyces was proposed. The species resembles T. udagawae or T. luteus, but in the absence of a living culture we cannot determine its precise taxonomic identity.
Talaromyces ocotl Bills & Heredia, Mycologia 90: 533. 1998.
Note: Figure 1 shows that this species belongs to Sagenomella and the new combination is proposed here:
Sagenomella ocotl (Bills & Heredia) Samson, Houbraken & Frisvad, comb. nov. MycoBank MB560681.
Basionym: Talaromyces ocotl Bills & Heredia, Mycologia 93: 533. 1998.
Talaromyces ohiensis Pitt, The Genus Penicillium: 502. 1980.
Anamorphic synonym: Penicillium ohiense L. H. Huang & J. A. Schmitt, Ohio J. Sci. 75: 78. 1975.
Note: Pitt (1980) considered this species to be related to T. luteus, but our ITS data clearly show that is synonymous with T. ucrainicus.
Talaromyces panasenkoi Pitt, The Genus Penicillium: 482. 1980.
Anamorphic synonym: Penicillium panasenkoi Pitt (simultaneously published, identical holotype).
Note: Pitt (1980) proposed T. panasenkoi as a new species for the invalidly published P. ucraininum Panasenko; however, Stolk & Samson (1972) had already proposed Talaromyces ucrainicus Udagawa for this taxon. Talaromyces panasenkoi Pitt is therefore a synonym of T. ucrainicus.
Talaromyces retardatus Udagawa, Kamiya & Kaori Osada, Trans. Mycol. Soc. Japan 34: 9. 1993.
Anamorphic synonym: Penicillium retardatum Udagawa, Kamiya & Kaori Osada (simultaneously published, identical holotype).
Note: No strain was available for examination and the status of this species is thus unknown.
Talaromyces spectabilis Udagawa & Suzuki, Mycotaxon 50: 82. 1994.
= Byssochlamys spectabilis (Udagawa & Suzuki) Houbraken & Samson, Appl. Environ. Microbiol. 74: 1618. 2008.
= Paecilomyces variotii Bainier Bull. Soc. mycol. Fr. 23: 27. 1907.
Note: The oldest generic and species name for this species is P. variotii, which becomes the correct name for the holomorph.
Talaromyces striatus (Raper & Fennell) C.R. Benj., Mycologia 47: 682. 1955.
= Hamigera striata (Raper & Fennell) Stolk & Samson, Persoonia 6: 347. 1971.
Talaromyces thermocitrinus Subrahm. & Gopalkr., Ind. Bot. Reporter 35: 35. 1984 (as ‘T. thermocitrinum’).
Note: We have not seen the type, but judging from the substrate (dust on books), and the mention of yellow cleistothecia, it is possible that this is an Eurotium species, a typical contaminant of books and other material in archives. However, its reported thermophily is different from known species of the mesophilic genus Eurotium.
Talaromyces thermophilus Stolk, Ant. van Leeuwenhoek 31: 268. 1965.
Basionym: Penicillium dupontii Griffon & Maubl., Bull. Trimmest. Soc. mycol. Fr. 27: 73. 1911.
Note: Figure 1 shows that this species is related to Thermomyces lanuginosus, and should be transferred to Thermomyces (Houbraken et al. 2011,2011, Houbraken & Samson 2011).
APPENDIX: OTHER POSSIBLE GENERIC NAMES
As noted above in the Taxonomy section, in order to adopt Talaromyces as the generic name for the former Penicillium subgenus Biverticillium, older genera considered synonyms of Penicillium sensu lato had to be considered. These are treated below.
Aspergillopsis Sopp, Vid.-Selsk. Skr. I. Math.-naturv. Kl. 11: 201. 1912. (Taf. xx, Fig. 149, Taf. xxiii, Fig. 31).
Type species: A. fumosus Sopp 1912.
Note: This generic name is illegitimate (Art. 53), being a later homonym of Aspergillopsis Speg. 1910. Pitt (1980) considered Sopp's genus a tentative synonym of Merimbla Pitt.
Citromyces Wehmer, Ber. dt. Bot. Ges. 11: 338. 1893.
Type species: C. pfefferianus Wehmer 1893 = Penicillium glabrum (Wehmer) Westling 1911, fide Pitt 1980.
Note: Wehmer's genus was considered a synonym of Penicillium by many authors, including Raper & Thom (1949) and Pitt (1980), with C. pfefferianus considered a probable synonym of P. glabrum (subgenus Aspergillioides) by Pitt (1980). Therefore, the genus remains a synonym of Penicillium sensu stricto.
Coremium Link: Fr., Mag. Ges. naturf. Freunde, Berlin 3: 19. 1809: Fries, Syst. mycol. 1: xlviii, 1821.
Type species: C. glaucum Link 1809.
Note: This genus was described in the same publication as Penicillium. Raper & Thom (1949) and Seifert & Samson (1985) both considered the type species to be a synonym of the type species of Penicillium, P. expansum Link 1809. Therefore, Coremium remains a synonym of Penicillium sensu stricto.
Eladia G. Sm., Trans. Br. mycol. Soc. 44: 47. 1961.
Type species: Eladia saccula (Dale) G. Sm. 1961 = Penicillium sacculum Dale 1926.
Note: This genus was considered a synonym of Penicillium by Stolk & Samson (1985), but was considered distinct by Pitt (1980), and von Arx (1981). In the multigene phylogenetic study by Houbraken & Samson (2011), Eladia is clearly included in Penicillium sensu stricto and that synonymy is accepted here.
Floccaria Grev., Scott. Crypt. Fl., Vol. 6, Pl. 301. 1828.
Type species: F. glauca Grev. 1828.
Note: There is no known extant type according to Seifert & Samson (1985), who searched for it in K and E. The illustration shows a synnematous fungus that could well be P. expansum, but there are no microscopic details. Therefore, this name can be discounted as a possible generic name for the species formerly ascribed to subgenus Biverticillium.
Geosmithia Pitt, Can. J. Bot. 57: 2021. 1980.
Type species: Geosmithia lavendula (Raper & Fennell) Pitt 1980 = Penicillium lavendulum Raper & Fennell 1948.
Note: Although von Arx (1981) considered Geosmithia a synonym of Penicillium, it is polyphyletic as presently circumscribed. Using SSU sequences, Ogawa et al. (1997) showed that G. lavendula, and a second common species G. putterilli, belong to the Bionectriaceae, Hypocreales. Similar results were obtained using ITS sequences by Kolařík et al. (2004), using LSU sequences by Schroers et al. (2005) and then multigene phylogenies by Kolařík & Kirkendall (2010). Despite this, some anamorphs attributed to Geosmithia have been described recently in Talaromyces (e.g. Yaguchi et al. 2005). Because the type species is not associated with the same order as Penicillium, Geosmithia need not be considered as a possible home for species of subgenus Biverticillium, but neither should it be considered a synonym of Penicillium.
Hormodendrum Bonord., Handbuch allg. Mykol.: 76. 1851.
Type species: Amphitrichum olivaceum Corda 1837 = Hormodendrum olivaceum (Corda) Bonord. 1851, lectotype selected by Clements & Shear 1931.
Note: Hormodendron has variously been treated as a synonym of Penicillium by von Arx (1974) and de Hoog & Hermanides-Nijhoff (1977) but more often as a synonym of Cladosporium Link, following the study of the type specimen by Hughes (1958). There is no reason to consider this name further as a synonym of Penicillium or as a possible receptacle for the species of subgenus Biverticillium.
Merimbla Pitt, Can. J. Bot. 57: 2394. 1980.
Type species: M. ingelheimensis (F.H. Beyma) Pitt 1980 = Penicillium ingelheimense F.H. Beyma 1942.
Note: Merimbla was considered a possible synonym of Penicillium by von Arx (1981), but this has not generally been accepted. Merimbla ingelheimensis was considered the anamorph of Hamigera avellanea by Stolk & Samson (1971), but is now known to be a closely related but phylogenetically distinct species (Peterson et al. 2010). The Hamigera clade is phylogenetically distinct from subgenus Biverticillium in the multigene analyses of Peterson et al. (2010) and Houbraken & Samson (2011). In a single name system, we consider Merimbla a synonym of the older genus Hamigera.
Monilia Fr., Syst. mycol. 3: 409. 1832.
Type species: M. caespitosa (L.: Fr.) Fr. 1832 / Mucor caespitosus L. 1753.
Note: Donk (1963) suggested that M. caespitosa might be a species of Penicillium based on the protologue. However, this generic name was formally rejected to conserve usage of Monilia Bonorden for the well-known genus of fruit pathogens. Therefore, it is unavailable as a possible generic name for species included in subgenus Biverticillium.
Moniliger Letell., Fig. Champ., Pl. 668. 1839. Figs 3, 4.
Type species: not designated, two original species.
Note: According to Seifert et al. (2011), Letellier included two species, with illustrations clearly representing Aspergillus. The synonymy of Moniliger with Penicillium proposed by Kirk et al. (2008) thus seems unlikely, and the genus is better listed as a synonym of Aspergillus.
Penicillium Link: Fr., Mag. Ges. naturf. Freunde, Berlin 3: 16. 1809.: Fries, Syst. mycol. 3: 406. 1832.
Type species: P. expansum Link 1809, fide Thom 1910.
Note: With this revision, and that of Houbraken & Samson (2011), Penicillium is now used exclusively for the nominal Clade including P. expansum, and species in the now synonymous genus Eupenicillium F. Ludw. 1892 (Houbraken & Samson 2011).
Pritzeliella Henn., Hedwigia Beibl. 42: 88. 1903.
Type species: P. caerulea Henn. 1903.
Note: Clements & Shear (1931) suggested that Pritzeliella should be considered a synonym of Penicillium without further commenting on the identity of its type species. Seifert & Samson (1985) examined the holotype of P. caerulea and considered it a synonym of Penicillium coprophilum (subgenus Penicillium). Its status as a synonym of Penicillium sensu stricto thus remains unchanged.
Rhodocephalus Corda, Ic. Fung. 1: 21. 1837 (Tab. vi, Fig. 282).
Type species: R. candidus Corda 1837 = Penicillium leucocephalum Rabenh. 1844.
Note: Corda (1837) illustrated and described his species as having aseptate stipes, a branched, asymmetrical penicillate head, with long chains of ameroconidia. Rabenhorst (1844) renamed the species in Penicillium, changing the epithet, a conclusion followed by Lindau (1907). Thom (1930) and Raper & Thom (1949) disagreed, stating that the illustration in the protologue has branched conidial chains that would exclude the fungus from Penicillium. This a debatable conclusion, because the chains are simply overlapping in the illustration and there is no clear indication of branching. Pitt (1980) evidently did not examine the protologue when he suggested a synonymy with Aspergillus candidus. Hughes (1958) did not report on the type, and according to Holubová (in litt. to Seifert, 1991), there is no material of Rhodocephalus in the Corda herbarium (PRM). The asymmetrical conidiophores illustrated by Corda discount this as a possible genus for species of subgenus Biverticillium, but its exact identity is unknown.
Torulomyces Delitsch, Systematik der Schimmelpilze: 91. 1943 (Taf. 30, Figs 232–235).
Type species: T. lagena Delitsch 1943 = Monocillium lagena (Delitsch) Hashmi, W.B. Kendr. & Morgan-Jones 1972 = Penicillium lagena (Delitsch) Stolk & Samson 1983.
Note: Torulomyces was included as a synonym of Penicillium sensu stricto in the phylogenetic study of Houbraken & Samson (2011).
Yunnania H.-Z. Kong, Mycotaxon 69: 320. 1998.
Type species: Y. penicillata H.-Z. Kong 1998.
Note: Houbraken & Samson (2011) sequenced the ITS of authentic cultures of Y. penicillata, showing a relationship with the Microascales, suggesting a synonymy with Scopulariopsis or Scedosporium might be appropriate.
Acknowledgments
We are appreciative of discussions with Dave Malloch, and contributions by Ellen Hoekstra in the early years of this project. Cobus Visagie (South Africa) and Xavier Llimona (Spain) allowed us to access unpublished data on P. aureocephalum and the genus Lasioderma. We are also grateful for nomenclatural advice received from Scott Redhead and Uwe Braun. We also thank Sung-Yuan Hsieh for providing the ITS sequence and the culture of Penicillium albobiverticillium.
REFERENCES
- Apinis AE. (1968). Relationship of certain keratinophilic Plectascales. Mycopathologia et Mycologia Applicata 35: 97–104 [Google Scholar]
- Arx JA von. (1974). The genera of fungi sporulating in pure culture, 2nd ed. J. Cramer, Veduz: [Google Scholar]
- Arx JA von. (1981). The genera of fungi sporulating in pure culture, 3rd ed. J. Cramer, Veduz: [Google Scholar]
- Arx JA von. (1987). A re-evaluation of the Eurotiales. Persoonia 13: 273–300 [Google Scholar]
- Benjamin CR. (1955). Ascocarps of Aspergillus and Penicillium. Mycologia 47: 669–687 [Google Scholar]
- Berbee ML, Yoshimura A, Sugiyama J, Taylor JW. (1995). Is Penicillium monophyletic? An evaluation of phylogeny in the family Trichocomaceae from 18S, 5.8S and ITS ribosomal DNA sequence data. Mycologia 87: 210–222 [Google Scholar]
- Biourge P. (1923). Les moisissures du groupe Penicillium Link. Cellule 33: 7–331 [Google Scholar]
- Chu YS, Niu NM, Wang YL, Guo JP, Pan WZ, Huang XW, Zhang KQ. (2010). Isolation of putative biosynthetic intermediates of prenylated indole alkaloids from a thermophilic fungus Talaromyces thermophilus. Organic Letters 12: 4356–4359 [DOI] [PubMed] [Google Scholar]
- Clements FC, Shear CL. (1931). The genera of Fungi. H. W. Wilson, New York: [Google Scholar]
- Cole RJ, Kirksey JW. (1973). The mycotoxin verruculogen: a 6-O-methylindole. Journal of Agricultural and Food Chemistry 21: 927–929 [DOI] [PubMed] [Google Scholar]
- Cole RJ, Kirksey JW, Moore JH, Blankenship BR, Diener UL, Davis ND. (1972). Tremorgenic toxin from Penicillium verruculosum. Applied Microbiology 24: 248–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda ACJ. (1837). Icones fungorum hucusque cognitorum 1: 1–32 [Google Scholar]
- Dangeard P-A. (1907). L’origine du périthèce chez les Ascomycètes. Le Botaniste, 10e ser.: 1–385 [Google Scholar]
- Donk MA. (1963). Proposals for conservation of some of fungi. Monilia Bon. (Deuteromycetes). I. Taxon 12: 266–271 [Google Scholar]
- Emmons CW. (1935). The ascocarps in species of Penicillium. Mycologia 27: 128–150 [Google Scholar]
- Frisvad JC. (1989). The connection between the penicillia and aspergilli and mycotoxins with special emphasis on misidentified isolates. Archives of Environmental Contamination and Toxicology 18: 452–467 [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Filtenborg O, Samson RA, Stolk AC. (1990a). Chemotaxonomy of the genus Talaromyces. Antonie van Leeuwenhoek 57:179–189 [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Samson RA, Stolk AC. (1990b). Chemotaxonomy of Eupenicillium javanicum and related species. pp. 445–453, in Samson RA, Pitt JI. (eds). Modern Concepts in Penicillium and Aspergillus Classification. Plenum Press, New York, USA: [Google Scholar]
- Frisvad JC, Samson RA. (2004). Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of the food and air-borne terverticillate Penicillia and their mycotoxins. Studies in Mycology 49: 1–173 [Google Scholar]
- Frisvad JC, Thrane U. (1987). Standardised high-performance liquid chromatography of 182 mycotoxins and other fungal metabolites based on alkylphenone retention indices and UV-VIS spectra (diode array detection). Journal of Chromatography 404: 195–214 [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Thrane U. (1993). Liquid column chromatography of mycotoxins. In: Betina, V. (ed.): Chromatography of mycotoxins: techniques and applications. Journal of Chromatography Library 54 Elsevier, Amsterdam: pp. 253–372 [Google Scholar]
- Fujimoto H, Matsudo T, Yamaguchi A, Yamazaki M. (1990). Two new fungal azaphilones from Talaromyces luteus, with monoamine oxidase inhibitory effect. Heterocycles 30: 607–616 [Google Scholar]
- Hawksworth DL, Crous PW, Redhead SA, Reynolds DR, Samson RA, Seifert KA, Taylor JW, Wingfield MJ, et al. (2011). The Amsterdam Declaration on Fungal Nomenclature. IMA Fungus 2: 105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galagan JE, Calvo SE, Cuomo C, Ma LJ, Wortman JR, Batzoglou S, Lee SI, et al. (2005). Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438: 1105–1115 [DOI] [PubMed] [Google Scholar]
- Heredia G, Reyes M, Arias RM, Bills GF. (2001). Talaromyces ocotl sp. nov. and observations on T. rotundus from conifer forest soils of Veracruz State, Mexico. Mycologia 93: 528–540 [Google Scholar]
- Hong SB, Cho HS, Shin HD, Frisvad JC, Samson RA. (2006). Novel Neosartorya species isolated from soil in Korea. International Journal of Systematic and Evolutionary Microbiology 56: 477–486 [DOI] [PubMed] [Google Scholar]
- Hoog GS de, Hermanides-Nijhoff E. (1977). Survey of black yeasts and allied hyphomycetes. Studies in Mycology 15: 178–221 [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG. (1998). Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41: 183–189 [DOI] [PubMed] [Google Scholar]
- Houbraken J, Samson RA. (2011). Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Studies in Mycology 70: 1–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbraken J, Frisvad JC, Samson RA. (2010). Taxonomy of Penicillium citrinum and related species. Fungal Diversity 44: 117–133 [Google Scholar]
- Houbraken J, Frisvad JC, Samson RA. (2011). Fleming’s penicillin producing strain is not Penicillium chrysogenum but P. rubens. IMA Fungus 2: 87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbraken J, Spierenburg H, Frisvad JC. (2011). Rasamsonia, a new genus comprising thermotolerant and thermophilic Talaromyces and Geosmithia species. Antonie van Leeuwenhoek, in press. DOI 10.1007/s10482-011-9647-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbraken J, Varga J, Rico-Munoz E, Johnson S, Samson RA. (2008). Sexual reproduction as the cause of heat resistance in the food spoilage fungus Byssochlamys spectabilis (anamorph Paecilomyces variotii). Applied and Environmental Microbiology 74: 1613–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SJ. (1958). Revisiones hyphomycetum aliquot cum appendice de nominibus rejiciendis. Canadian Journal of Botany 36: 727–836 [Google Scholar]
- Kakinuma N, Iwai H, Takahashi S, Hamano K, Yanagisawa T, Nagai K, Tanaka K, Suzuki K, Kirikae F, Kirikae T, Nakagawa A. (2000). Quinolactacions A, B, and C: novel quinolone compounds from Penicillium sp. EPF-6 I. Taxonomy, production, isolation and biological properties. Journal of Antibiotics 53: 1247–1251 [DOI] [PubMed] [Google Scholar]
- Kim WG, Song NK, Yoo ID. (2001). Quinolactacins A1 and A2, new acetylcholinesterase inhibitors from Penicillium citrinum. Journal of Antibiotics 54: 831–835 [DOI] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Stalpers JA. (2008). Dictionary of the Fungi, 10th edn. CAB International, Wallingford, UK: [Google Scholar]
- Kolařík M, Kubátová A, Pažoutová S, Šrůtka P. (2004). Morphological and molecular characterisation of Geosmithia putterillii, G. pallida comb. nov. and G. flava sp. nov., associated with subcorticolous insects. Mycological Research 108: 1053–1069 [PubMed] [Google Scholar]
- Kolařík M, Kirkendall LR. (2010). Evidence for a new lineage of primary ambrosia fungi in Geosmithia Pitt (Ascomycota: Hypocreales). Fungal Biology 114: 676–689 [DOI] [PubMed] [Google Scholar]
- Komai S, Hosoe T, Itabashi T, Nozawa K, Yaguchi T, Fuklushima K, Kawai K. (2005). New vermistatin derivatives isolated from Penicillium simplicissimum. Heterocycles 65: 2771–2776 [Google Scholar]
- Kong H-Z. (1999). A new species of Talaromyces. Mycosystema 18: 9–11 [Google Scholar]
- Kuraishi H, Aoki M, Itoh M, Katayama Y, Sugiyama J, Pitt JI. (1991). Distribution of ubiquinones in Penicillium and related genera. Mycological Research 95: 705–711 [Google Scholar]
- Leal JL, Bernabé M. (1998). Taxonomic applications of polysaccharides. pp. 153-181 in Frisvad JC, Bridge PD, Arora DK. (eds) Chemical Fungal Taxonomy. Marcel Dekker, New York, USA: [Google Scholar]
- Lindau G. (1907). Rabenhorst’s Kryptogamen-Flora, Pilze - Fungi imperfecti, 2nd ed., 1(9): 1–112 [Google Scholar]
- Liu F, Cai X-L, Yang H, Xia X-K, Guo Z-Y, Yuan J, Li M-F, She Z-G, Lin Y-C. (2010). The bioactive metabolites of the mangrove endophytic fungus Talaromyces sp. ZH-154 isolated from Kandelia candel (L.) Druce. Planta Medica 76: 185–189 [DOI] [PubMed] [Google Scholar]
- LoBuglio KF, Pitt JI, Taylor JW. (1993). Phylogenetic analysis of two ribosomal DNA regions indicates multiple independent losses of a sexual Talaromyces state among asexual Penicillium species in subgenus Biverticillium. Mycologia 85: 592–604 [Google Scholar]
- LoBuglio KF, Taylor JW. (1993). Molecular phylogeny of Talaromyces and Penicillium species in subgenus Biverticillium. In: The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematic (Reynolds DR, Taylor JW, eds), C.A.B., International, Surrey: 115–119 [Google Scholar]
- López-Villavicencio M, Aguileta G, Giraud T, de Vienne DM, Lacoste S, Couloux A, Dupont J. (2010). Sex in Penicillium: combined phylogenetic and experimental approaches. Fungal Genetics and Biology 47: 693–706 [DOI] [PubMed] [Google Scholar]
- Malloch D. (1985). The Trichocomaceae: relationships with other Ascomycetes. In: Advances in Penicillium and Aspergillus systematics (Samson RA, Pitt JI, eds) Plenum Press, New York: 365–382 [Google Scholar]
- Masclaux F, Guého H, Hoog GS de, Christen R. (1995). Phylogenetic relationships of human-pathogenic Cladosporium (Xylohypha) species inferred from partial LSU rRNA sequences. Journal of Medical and Veterinary Mycology 33: 327–338 [DOI] [PubMed] [Google Scholar]
- Matsushima T. (2001). Paratalaromyces gen. nov. Matsushima Mycological memoirs 10: 111–115 [Google Scholar]
- Mouchacca J. (2007). Heat-tolerant fungi and applied research: On the taxonomic position of some overlooked thermophilic fungi. Cryptogamie 28: 215–223 [Google Scholar]
- Nicoletti R, Manzo E, Ciavatta ML. (2009). Occurrence and bioactivities of funicone-related compounds. International Journal of Molecular Science 10: 1430–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen KF, Smedsgaard J. (2003). Fungal metabolite screening: database of 474 mycotoxins and fungal metabolites for dereplication by standardised liquid chromatography-UV-mass spectrometry methodology. Journal of Chromatography A 1002: 111–136 [DOI] [PubMed] [Google Scholar]
- Nielsen KF, Månsson M, Rank C, Frisvad JC, Larsen TO. (2011). Dereplication of microbial natural products by LC-DAD-TOFMS. Journal of Natural Products. DOI:10.1021/np200254t [DOI] [PubMed] [Google Scholar]
- Nierman WC, Pain A, Anderson MJ, Wortman JR, Kim HS, Arroyo J, Berriman M, et al. (2005). Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438: 1151–1156 [DOI] [PubMed] [Google Scholar]
- Norvell LL. (2011). Fungal nomenclature. 1. Melbourne approves a new code. Mycotaxon 116: 481–490 [Google Scholar]
- Notermans SHW, Cousin MA, de Ruiter GA, Rombouts FM. (1998). Fungal immunotaxonomy. pp. 121–152 in Frisvad JC, Bridge PD, Arora DK. (eds) Chemical Fungal Taxonomy. Marcel Dekker, New York, USA: [Google Scholar]
- Ogawa H, Yoshimura A, Sugiyama J. (1997). Polyphyletic origins of species of the anamorphic genus Geosmithia and the relationships of the cleistothecial genera: Evidence from 18S, 5S and 28S rDNA sequence analyses. Mycologia 89: 756–771 [Google Scholar]
- Ogawa H, Sugiyama J. (2000). Evolutionary relationships of the cleistothecial genera with Penicillium, Geosmithia, Merimbla and Sarophorum anamorphs as inferred from 18S rDNA sequence divergence. In: Integration of modern taxonomic methods for Penicillium and Aspergillus classification (Samson RA, Pitt JI, eds) Plenum Press, New York: 149–161 [Google Scholar]
- Osmanova N, Schultze W, Ayoub N. (2010). Azaphilones: a class of fungal metabolites with diverse biological activities. Phytochemical Reviews 9: 315–342 [Google Scholar]
- Paterson R. (1998). Chemotaxonomy of fungi by unsaponifiable lipids. In: Chemical Fungal Taxonomy (Frisvad JC, Bridge PD, Arora DK, eds) Marcel Dekker, New York: 183–218 [Google Scholar]
- Pel HJ, de Winde JH, Archer DB, Dyer PS, Hofmann G, Schaap PJ, Turner G, et al. (2007). Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nature Biotechnology 25: 221–231 [DOI] [PubMed] [Google Scholar]
- Peterson SW. (2000). Phylogenetic analysis of Penicillium species based on ITS and LSU-rDNA nucleotide sequences. In: Integration of modern taxonomic methods for Penicillium and Aspergillus classification (Samson RA, Pitt JI, eds) Plenum Press, New York: 163–178 [Google Scholar]
- Peterson SW, Jurjevic Z, Bills GF, Stchigel AM, Guarro J, Vega FE. (2010). Genus Hamigera, six new species and multilocus DNA sequence based phylogeny. Mycologia 102: 847–864 [DOI] [PubMed] [Google Scholar]
- Pitt JI. (1978). Geosmithia gen. nov. for Penicillium lavendulum and related species. Canadian Journal of Botany 57: 2021–2030 [Google Scholar]
- Pitt JI. (1980). The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press, London: [Google Scholar]
- Pitt JI, Samson RA. (1993). Species names in current use in the Trichocomaceae (Fungi, Eurotiales). Koeltz Scientific Books, Königstein: [Google Scholar]
- Pitt JI, Samson RA, Frisvad JC. (2000). List of accepted species and their synonyms in the family Trichocomaceae. In: Integration of modern taxonomic methods for Penicillium and Aspergillus classification (Samson RA, Pitt JI, eds) Plenum Press, New York: 9–49 [Google Scholar]
- Proksa B. (2010). Talaromyces flavus and its metabolites. Chemical Papers 64: 696–714 [Google Scholar]
- Rabenhorst L. (1844). Deutschlands Kryptogamenflora, 2nd ed., vol. 1: 1–614 [Google Scholar]
- Ramírez C. (1982). Manual and atlas of the Penicillia. Amsterdam: Elsevier Biomedical Press; [Google Scholar]
- Raper KB, Fennell DI. (1965). The genus Aspergillus. Baltimore: Williams & Wilkins, Baltimore, MD: [Google Scholar]
- Raper KB, Thom C. (1949). Manual of the Penicillia, Williams & Wilkins, Baltimore, MD: [Google Scholar]
- Samson RA, Houbraken J, Thrane U, Frisvad JC, Andersen B. (2010). Food and Indoor Fungi. CBS laboratory manual Series 2 CBS-KNAW Fungal Biodiversity Centre, Utrecht, NL: [Google Scholar]
- Samson RA. (1974). Paecilomyces and some allied hyphomycetes. Studies in Mycology 6: 1–119 [Google Scholar]
- Samson RA, Seifert KA. (1985). The ascomycete genus Penicilliopsis and its anamorphs. In: Advances in Penicillium and Aspergillus systematic (Samson RA, Pitt JI, eds) Plenum Press, New York: 397–426 [Google Scholar]
- Samson RA, Varga J, Meijer M, Frisvad JC. (2011). New taxa in Aspergillus section Usti. Studies in Mycology 69: 81–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson RA, Stolk AC, Frisvad JC. (1989). Two new synnematous species of Penicillium. Studies in Mycology 31: 133–143 [Google Scholar]
- Schroers HJ, Geldenhuis MM, Wingfield MJ, Schoeman MH, Yen YF, Shen WC, Wingfield BD. (2005). Classification of the guava wilt fungus Myxosporium psidii, the palm pathogen Gliocladium vermoesenii and the persimmon wilt fungus Acremonium diospyri in Nalanthamala. Mycologia 97: 375–395 [DOI] [PubMed] [Google Scholar]
- Seifert KA, Samson RA. (1985). The genus Coremium and the synnematous penicillia. In: Advances in Penicillium and Aspergillus Systematics. (Samson R.A., Pitt J.I, eds). Plenum Publishers, New York: pp. 143–154 [Google Scholar]
- Seifert KA, Morgan-Jones G, Gams W, Kendrick B. (2011). The genera of Hyphomycetes. CBS-KNAW Fungal Biodiversity Centre, Utrecht: [Google Scholar]
- Sharpton TJ, Stajich JE, Rounsley SD, Gardner MJ, Wortman JR, Jordar VS, Maiti R, et al. (2009). Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Research 19: 1722–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedsgaard J. (1997). Micro-scale extraction procedure for standardised screening of fungal metabolite production in cultures. Journal of Chromatography A 760: 264–270 [DOI] [PubMed] [Google Scholar]
- Stalpers JA. (1984). A revision of the genus Sporotrichum. Studies in Mycology 24: 1–105 [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. (2008). A rapid bootstrap algorithm for the RAxML Web-Servers. Systematic Biology 75: 758–771 [DOI] [PubMed] [Google Scholar]
- Stolk AC. (1969). Four new species of Penicillium. Antonie van Leeuwenhoek 35: 261–274 [DOI] [PubMed] [Google Scholar]
- Stolk AC, Samson RA. (1971). Studies on Talaromyces and related genera I. Hamigera gen. nov. and Byssochlamys. Persoonia 6: 341–357 [Google Scholar]
- Stolk AC, Samson RA. (1972). The genus Talaromyces – studies on Talaromyces and related genera II. Studies in Mycology 2: 1–65 [Google Scholar]
- Stolk AC, Samson RA. (1983). The ascomycete genus Eupenicillium and related Penicillium anamorphs. Studies in Mycology 23: 1–149 [Google Scholar]
- Stolk AC, Samson RA. (1985). A new taxonomic scheme for Penicillium anamorphs. In: Advances in Penicillium and Aspergillus systematic (Samson RA, Pitt JI, eds) Plenum Press, New York: 163–192 [Google Scholar]
- Šturdíková M, Slugeň D, Lešová K, Rosenberg M. (2000). Microbial production of coloured azaphilone metabolites. Chemicke Listy 94: 105–110 [Google Scholar]
- Sugiyama J. (1998). Relatedness, phylogeny, and evolution of the fungi. Mycoscience 39: 487–511 [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. (2011). MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution. Doi: 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom C. (1915a). The Penicillium luteum-purpurogenum group. Mycologia 7: 134–142 [Google Scholar]
- Thom C. (1915b). The Penicillium Group-Verticillatae of Wehmer. Science 41: 172 [Google Scholar]
- Thom C. (1930). The Penicillia. Williams & Wilkins, Baltimore: 1–644 [Google Scholar]
- Turner WB, Aldridge DC. (1983). Fungal metabolites II. Academic Press, London: [Google Scholar]
- Tzean SS, Chien JL, Shiu SH. (1992). Talaromyces unicus sp. nov. from Taiwan. Mycologia 84: 739–749 [Google Scholar]
- Udagawa S, Suzuki S. (1994). Talaromyces spectabilis, a new species of food-borne ascomycetes. Mycotaxon 50: 81–88 [Google Scholar]
- van Reenen-Hoekstra ES, Frisvad JC, Samson RA, Stolk AC. (1990). The Penicillium funiculosum complex – well defined species and problematic taxa. In: Samson R.A., Pitt J.I. (eds.): Modern concepts in Penicillium and Aspergillus classification. Plenum Press, New York: pp. 173–191 [Google Scholar]
- Vleggaar R, Steyn PS, Nagel DW. (1974). Constitution and absolute configuration of austdiol, the main toxic metabolite from Aspergillus ustus. Journal of the Chemical Society Perkin Transactions 1 1974: 45–49 [DOI] [PubMed] [Google Scholar]
- Wang L, Zhuang W-Y. (2007). Phylogenetic analyses of penicillia based on partial calmodulin gene sequences. BioSystems 88: 113–112 [DOI] [PubMed] [Google Scholar]
- Wehmer C. (1914). Coremium silvaticum n. sp. nebst Bemerkungen zur Systematik der Gattung Penicillium. Berichte deutsche Botanische Gesellschaft 32: 373–384 [Google Scholar]
- Wicklow DT, Cole RJ. (1984). Citreoviridin in standing corn infested by Eupenicillium ochraosalmoneum. Mycologia 76: 959–961 [Google Scholar]
- Williams RW. (2011). Natural products synthesis: enabling tools to penetrate nature’s secrets of biogenesis and biomechanisms. Journal of Organic Chemistry 76: 4221–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi T, Someya A, Udagawa S. (1994a). Erythrogymnotheca, a new genus of Eurotiales. Mycoscience 35: 219–222 [Google Scholar]
- Yaguchi T, Someya A, Udagawa S. (1994b). Two new species of Talaromyces from Taiwan and Japan. Mycoscience 35: 249–255 [Google Scholar]
- Yaguchi T, Udagawa S-I, Nishimura K. (2005). Geosmithia argillacea is the anamorph of Talaromyces eburneus as a heat resistant fungus. Cryptogamie, Mycologie 26: 133–141 [Google Scholar]
- Yoshida E, Fujimoto H, Baba M, Yamazaki M. (1995). Four new chlorinated azaphilones, helicusins A – D, closely related to 7-epi-sclerotiorin, from an ascomycetous fungus, Talaromyces helicus. Chemical and Pharmaceutical Bulletin 43: 1307–1310 [Google Scholar]
- Yoshida E, Fujinoto H, Yamazaki M. (1996a). Isolation of three new azaphilones, luteosin C, D, and E, from an ascomycete, Talaromyces luteus. Chemical and Pharmaceutical Bulletin 44: 284–287 [DOI] [PubMed] [Google Scholar]
- Yoshida E, Fujinoto H, Yamazaki M. (1996b). Revised stereostructures of luteosins C and D. Chemical and Pharmaceutical Bulletin 44: 1775 [DOI] [PubMed] [Google Scholar]