Abstract
Major intermediates of chromosome condensation in erythroleukemia K562 cells are presented. Interphase chromatin structures became visible after reversal of permeabilization. Large-scale chromatin structures and the development of individual interphase chromosomes were observed by fluorescence microscopy. In the linear arrangement the following major intermediates of K562 chromatin condensation could be distinguished: (1) the most decondensed chromatin veil, (2) chromatin ribbon, (3) chromatin funnel, a new intermediate regarded as the earliest visible form of interphase chromosomes, (4) chromatin body, (5) 300 nm chromatin fiber, (6) u, v, or s forms of chromosomes, and (7) linear chromosomes. The observations made in nuclei of K562 cells conform to the model of helical coil chromosome condensation.
Introduction
Different models of mitotic chromosome structure that have been suggested depending on experimental approaches included radial loop (Paulson and Laemmli, 1977; Marsden and Laemmli, 1979; Adolph, 1980), successive hierarchical folding, combined radial loop/helical folding (Rattner and Liin, 1985; Boy de la Tour and Laemmli, 1988), and axial glue mechanisms (Kireeva et al., 2004). Less attention has been paid to the large-scale structure of the interphase chromosome that was deduced from extrapolations from mitotic chromosome models. The scarcity of morphological evidence regarding chromatin folding in interphase is due to the low spatial resolution of light microscopy, nonspecific staining of DNA, lack of observing three-dimensional chromatin structures, stickiness of the nuclear material, and the formation of artifacts upon environmental changes. Imaging techniques failed to show decondensed interphase chromosomes; consequently, there is little solid information concerning the architecture of interphase chromosomes (Lemke et al., 2002). Studies of interphase chromosome structure indicate that the use of fluorescence in situ hybridization on fixed cells potentially damages structure. A new methodology involves photoactivation of labeled histone H3 at mitosis to observe individual and specific human chromosomes in living interphase cells (Müller et al., 2010).
To overcome technical difficulties, we use reversibly permeabilized cells that allow one to (1) open the nucleus during the interphase, (2) isolate the intermediates of the chromatin condensation process, (3) observe individual chromosomal structures by fluorescent microscopy, and (4) analyze the temporal order of early chromosomal forms in a cell cycle-dependent manner (Banfalvi, 2008). Here the major forms of chromatin condensation of human erythroleukemia cells are presented and a new intermediate, which we named “chromatin funnel,” is observed for the first time.
Methods
Isolation of chromatin and chromosome structures
Human erythroleukemia K562 cells were grown in RPMI-1640 medium supplemented with 10% fetal bovine serum. Reversible permeabilization was originally developed for the reversible permeabilization of murine lymphocytes (Banfalvi et al., 1984) and was adapted to human K562 cells. Briefly, 0.5 mL of hypotonic buffer (9 mM HEPES, pH 7.8, 5.8 mM dithiotreitol, 4.5% dextran T-150, 1 mM EGTA, and 4.5 mM MgCl2) was added to 106 cells. Dextran T-150 served as a molecular coat to prevent cellular disruption. A short pulse of permeabilization was carried out for 1 min at 0°C. For the reversal of permeabilization, 20 mL RPMI 1640 medium containing 10% fetal bovine serum was added, and the cells were incubated at 37°C and 5% CO2 for 2 h. Large-scale chromatin structures of human erythroleukemia K562 cells were isolated after reversible permeabilization, stained with DAPI, and observed by fluorescent microscopy as described earlier in other mammalian cells (Nagy et al., 2004; Banfalvi, 2006; Banfalvi et al., 2007).
Results
Linear arrangement and major intermediates of chromatin condensation
Chromatin structures were isolated from reversibly permeabilized cells and after DAPI staining observed by fluorescent microscopy. In the process of cell division, the distribution of the genetic material may form a small nucleus (micronucleus), visible under microscope. The micronucleus is regarded as an erratic nucleus formed during chromosome condensation.
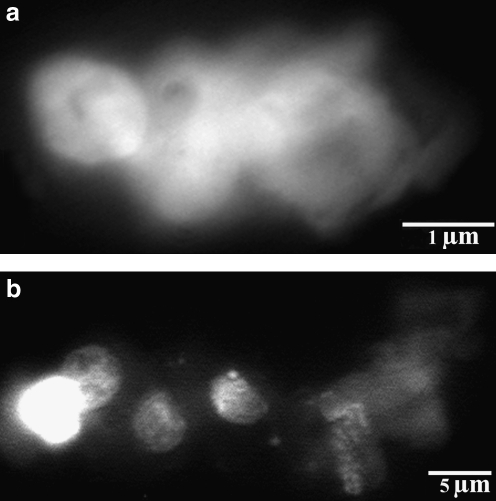
The polarization of decondensed chromatin at one end is similar to micronucleus formation (Fig. 1a). Indeed, we have observed many times in different mammalian cells that during regular chromatin condensation the interphase chromatin turns around itself forming the head portion resembling or being the micronucleus. Such events were seen during early S phase before chromatin structures developed into linearly arranged, distinguishable chromosomes. We regard the opening of the nucleus in the early S interphase, seen as a micronucleus, as a regularly occurring structure in the early steps of chromatin condensation (Banfalvi, unpublished results).
FIG. 1.
Intermediates of large-scale chromatin condensation. (a) Chromatin ribbon formation from chromatin veil. (b) Supercoiling of chromatin ribbon generating chromatin bodies.
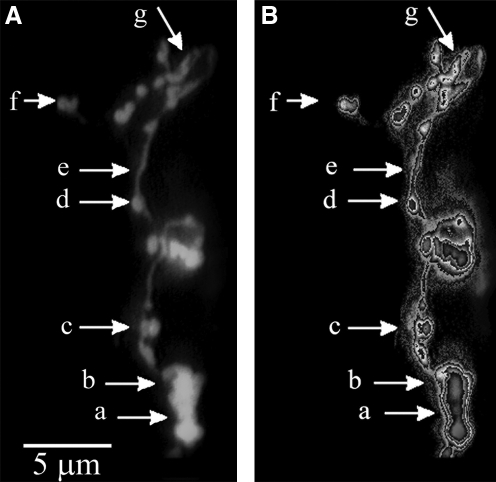
Continuous chromatin coiling generates the formation of chromatin ribbon followed by the appearance of the much tighter chromatin bodies (Fig. 1b) through chromatin funnels, similar to tying a scarf. Major intermediates of chromosome formation are summarized in Figure 2. The estimated size of intermediates is as follows: (1) the decondensed chromatin veil at the bottom of Figure 2A is 1.2–2×5 μm, (2) the chromatin ribbon 0.5–0.7 μm in diameter, (3) the chromatin funnel ∼1 μm, (4) chromatin bodies (round or oval chromosomal forms) 0.7–0.9 μm in diameter, (5) the so-called 300 nm chromatin fiber 250–320 nm in diameter, (6) bent, semicircular (s, v, and u shaped) chromosomes of 0.6–0.8 μm, and (7) 1–1.2 μm long linear chromosomes. The structures of Figure 2A have been subjected to chromatin image analysis (Fig. 2B). The application of this software highlighted the chromatin density levels providing a 3D intensity histogram of intermediates of chromatin condensation. The DAPI fluorescence intensity histogram represents the amount of bound fluorescent dye per image pixel. As the fluorescent signal is proportional to the amount of DNA, the intensity histogram reflects chromatin compactness. Due to the high affinity of DAPI binding to the A-T rich sequences of DNA, such regions of the fibers could be slightly brighter in fluorescence.
FIG. 2.
Intermediates from chromatin veil to linear chromosomes. (A) Intermediates are marked by arrows: (a) decondensed chromatin veil, (b) chromatin ribbon, (c) chromatin funnel (precursor of chromatin body), (d) chromatin body (round or oval chromosomal forms), (e) 300 nm fiber, (f) bent chromosomal forms (s, v, or u shaped) and, (g) linear chromosome. (B) The same intermediates were subjected to chromatin image analysis. Different colors indicate compaction levels from the lowest to the highest condensation. Colors and gradients of the look-up table were tuned to represent typical stages of chromatin compactness. The outer blue gradient corresponds to the DAPI background, green represents the lowest condensation of chromatin, and the inner yellow and red gradients reflect the highest compaction (see also illustration and Supplementary Movies; Supplementary Data available online at www.liebertonline.com/dna).
Discussion
The structure of the interphase chromatin and early forms of chromosomes are not known as the nucleus could be opened only at the end of prophase of mitosis when the membrane surrounding the nucleus disappeared. Attempts to open the nucleus by permeabilization failed due to the stickiness of the nuclear material (Heslop-Harrison et al., 1988). Our approach of opening the nucleus through a short pulse of reversible permeabilization maintained the viability of cells, and allowed to open the nucleus at any time during the cell cycle. As a result, major intermediates of chromatin structures have been described in mammalian and Drosophila cells (Banfalvi et al., 2006, 2007; Banfalvi, 2009). Synchronization of cells (Banfalvi, 2008, 2011) combined with reversible permeabilization made it possible to observe the temporal order of intermediates of chromatin condensation. In this short communication, one new intermediate of chromatin condensation has been revealed, which we named the chromatin funnel (Fig. 2A-c, B-c), which is regarded as the predecessor of chromatin body and the earliest visible interphase chromosome. Figure 2 shows a rare occasion where different intermediates are distinguishable and the continuity of their linear arrangement is not disrupted. The existence of chromatin funnel, which is regarded as the earliest interphase chromosomal form, has been seen in the enlarged nuclei of rat myelocytic leukemia cells (Trencsenyi et al., unpublished results).
The importance of the linear arrangement of chromosomes is that it will lead to the determination of the linear order of chromosomes, their temporal arrangement at different stages of the cell cycle, and the temporal order of chromosome replication. The medical significance of intermediates with different compactness is that individual chromosomes can be analyzed at different stages of development, leading to the early diagnosis of chromosome aberrations not detectable in condensed chromosomes.
Supplementary Material
Acknowledgment
This research was supported by the OTKA grant T042762 (G.B.).
References
- Adolph K.W. Organization of chromosomes in HeLa cells: isolation of histone-depleted nuclei and nuclear scaffolds. J Cell Sci. 1980;42:291–304. doi: 10.1242/jcs.42.1.291. [DOI] [PubMed] [Google Scholar]
- Banfalvi G. Condensation of interphase chromosomes in nuclei of synchronized CHO cells. DNA Cell Biol. 2006;25:641–645. doi: 10.1089/dna.2006.25.641. [DOI] [PubMed] [Google Scholar]
- Banfalvi G. Cell cycle synchronization of animal cells and nuclei by centrifugal elutriation. Nat Protocols. 2008;3:663–673. doi: 10.1038/nprot.2008.34. [DOI] [PubMed] [Google Scholar]
- Banfalvi G. Apoptotic Chromatin Changes. Springer Science+Business Media B.V., Springer Dordrecht, Heidelberg; London, New York: 2009. pp. 125–202. [Google Scholar]
- Banfalvi G. In: Synchronization of mammalian cells and nuclei by centrifugal elutriation. In Cell Cycle Synchronization: Methods and Protocols. Banfalvi G., editor. Humana Press, Springer Science + Business Media; New York: 2011. pp. 25–45. [Google Scholar]
- Banfalvi G. Nagy G. Gacsi M. Roszer T. Basnakian A.G. Common pathway of chromosome condensation in mammalian cells. DNA Cell Biol. 2006;25:295–301. doi: 10.1089/dna.2006.25.295. [DOI] [PubMed] [Google Scholar]
- Banfalvi G. Sooki-Toth A. Sarkar N. Csuzi S. Antoni F. Nascent DNA chains synthesized in recersibly permeable cells of mouse thymocytes. Eur J Biochem. 1984;139:553–559. doi: 10.1111/j.1432-1033.1984.tb08041.x. [DOI] [PubMed] [Google Scholar]
- Banfalvi G. Ujvarosi K. Trencsenyi G. Somogyi C. Nagy G. Basnakian A.G. Cell culture density dependent toxicity and chromatin changes upon cadmium treatment in murine pre-B-cells. Apoptosis. 2007;12:1219–1228. doi: 10.1007/s10495-006-0045-5. [DOI] [PubMed] [Google Scholar]
- Boy De La Tour E. Laemmli U.K. The metaphase scaffold is helically folded: sister chromatids have predominately opposite helical handedness. Cell. 1988;55:937–944. doi: 10.1016/0092-8674(88)90239-5. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J.S. Huelskamp M. Wendroth S. Atkinson M.D. Leicht A.R. Benett M.D. Chromatin and centromeric structures in interphase nuclei. In: Brandham P.E., editor. Kew Chromosome Conference III. Allan & Unwin; London: 1988. pp. 209–217. [Google Scholar]
- Kireeva N. Lakonishok M. Kireev I. Hirano T. Belmont A.S. Visualization of early chromosome condensation: a hierarchical folding, axial glue model of chromosome structure. J Cell Biol. 2004;166:775–785. doi: 10.1083/jcb.200406049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke J. Claussen J. Michel S. Chudoba I. Muhlig P. Westermann M. Sperling K. Rubtsov N. Grummt U.W. Ullmann P. Kromeyer-Hauschild K. Liehr T. Claussen U. The DNA-based structure of human chromosome 5 in interphase. Am J Hum Genet. 2002;71:1051–1059. doi: 10.1086/344286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden M.P.F. Laemmli U.K. Metaphase chromosome structure: evidence for a radial-loop model. Cell. 1979;17:849–858. doi: 10.1016/0092-8674(79)90325-8. [DOI] [PubMed] [Google Scholar]
- Müller I. Boyle S. Singer R.H. Bickmore W.A. Chubb J.R. Stable morphology, but dynamic internal reorganisation, of interphase human chromosomes in living cells. PLoS ONE. 2010;5:e11560. doi: 10.1371/journal.pone.0011560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy G. Gacsi M. Rehak M. Basnakian A.G. Klaisz M. Banfalvi G. Gamma irradiation-induced apoptosis in murine pre-B cells prevents the condensation of fibrillar chromatin in early S phase. Apoptosis. 2004;9:765–776. doi: 10.1023/B:APPT.0000045790.86905.00. [DOI] [PubMed] [Google Scholar]
- Paulson J.R. Laemmli U.K. The structure of histone depleted chromosomes. Cell. 1977;12:817–828. doi: 10.1016/0092-8674(77)90280-x. [DOI] [PubMed] [Google Scholar]
- Rattner J.B. Lin C.C. Radial-loops and helical coils coexist in metaphase chromosomes. Cell. 1985;42:291–296. doi: 10.1016/s0092-8674(85)80124-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.