Abstract
Chronic graft-versus-host disease (cGVHD) is a major cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation. Currently, no reliable biomarkers are available to predict the onset or progression of cGVHD. Therefore, in this study, we collected peripheral blood mononuclear cells from four patients with cGVHD and four ones with non-GVHD after hematopoietic stem cell transplantation and employed Affymetrix GeneChip Human U133 Plus 2.0 microarrays to screen the genes differentially expressed in cGVHD versus non-GVHD groups, with the aim to identify potential clinical biomarkers to predict cGVHD risk or progression. Microarray analysis demonstrated that the expression of 3180 genes changed significantly in cGVHD versus non-GVHD, with 879 genes upregulated and 2301 genes downregulated. Among them we chose CD28 and PI3K as candidates for further verification. Flow cytometry and quantitative real-time polymerase chain reaction analysis confirmed the significant upregulation of CD28 and PI3K in samples from patients with cGVHD compared with patients with non-GVHD, respectively. In conclusion, our study suggested that the upregulation of CD28 and PI3K contributed to the onset and progression of cGVHD and provided evidence that CD28 and PI3K may serve as promising biomarkers for cGVHD.
Introduction
Chronic graft-versus-host disease (cGVHD) occurs in >50% of long-term survivors after allogeneic hematopoietic stem cell transplantation (HSCT) and is associated with significant morbidity and mortality (Flowers et al., 2008). With greater availability and use of human leukocyte antigen (HLA)-matched unrelated donors, the incidence of cGVHD is rising rapidly (Seaton et al., 2003). Several risk factors for cGVHD after HSCT have been proposed, including prior acute GVHD, older age of the patients, the use of donor lymphocyte infusions, and the use of unrelated or HLA-mismatched donors (Poloni et al., 2011). However, none of them can indicate the onset and progression of cGVHD with high correlation before the disease is evident clinically. Therefore, it is urgent to search for novel clinical biomarkers to allow for accurate diagnosis and monitor of cGVHD.
Microarray analysis can provide a global view on hematologic diseases with its promise capacity for profiling expression patterns of thousands of genes in a single experiment. Several studies have investigated the gene expression profiles occurring in acute GVHD (Takahashi et al., 2008) or acute myeloid leukemia (Wilson et al., 2006; Bonadies et al., 2011), whereas only few studies have addressed the global gene expression in cGVHD condition. Therefore, in this study, we performed gene expression profiling analysis by employing microarrays to screen the genes differentially expressed in patients with cGVHD versus non-GVHD, with the aim to identify potential clinical markers to predict cGVHD risk or progression. Our results demonstrated that CD28 and PI3K were significantly upregulated in patients with cGVHD compared with patients with non-GVHD, suggesting that these two molecules may serve as good markers for evaluating cGVHD status.
Patients and Methods
Patients
Between April 2005 and December 2008, four patients with cGVHD and four patients with non-GVHD, all of whom received allogeneic HSCT at Guangdong General Hospital, were enrolled in this study (Table 1). The diagnosis and global assessment of cGVHD were based on the National Institutes of Health consensus criteria (Figueroa et al., 2005). Four patients with non-GVHD, who did not suffer from cGVHD after allogeneic HSCT for 2 years follow-up, were designated as study controls. This study was approved by the Ethics Committee of Guangdong General Hospital and informed consent was obtained for all patients.
Table 1.
Patient Characteristics
| |
Non-GVHD |
cGVHD |
||||||
|---|---|---|---|---|---|---|---|---|
| Patient No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Age (years) | 36 | 34 | 31 | 44 | 22 | 33 | 27 | 50 |
| Sex | F | M | M | M | F | M | M | M |
| Diagnosis | AML | AML | AML | AML | AML | AML | AML | AML |
| Donor | Sibling | Sibling | Sibling | Sibling | Sibling | Sibling | Sibling | Sibling |
| HLA disparity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prophylaxis GVHD | …CSA/sMTX/MM F… | …CSA/sMTX/MM F… | ||||||
| Grade of cGVHD | Severe | Severe | Severe | Severe | Severe | Severe | Severe | Severe |
F, female; M, male; AML, acute myeloid leukemia; CSA, cyclosporine; sMTX, short-term MTX; MMF, mycophenolate mofetil; cGVHD, chronic graft-versus-host disease.
RNA isolation, amplification, and hybridization
For each of the four patients with cGVHD and four patients with non-GVHD, 4 mL of venous blood was collected. Total RNA from nucleated blood cells was isolated following the lysis of erythrocytes and removal of cell debris. In brief, immediately after sample collection, leukocytes were concentrated by centrifugation, and Buffer EL (Qiagen) was added to lyse erythrocytes. Cells were washed in Buffer EL to eliminate the abundance of globin species from mRNA pool. RNA was then purified with RNeasy Mini kit (Qiagen) (Buzzeo et al., 2008). The quality and quantity of purified total RNA were assessed by Agilent 2100 Bioanalyzer RNA 6000 NanoChip (Agilent). Only samples with an A260/A280 between 1.7 and 2.2 were used for following experiments. About 5.0 μg total RNA was used to generate cDNA, which was then hybridized onto Affymetrix U133 Plus 2.0 GeneChip oligonucleotide arrays (Affymetrix) according to the manufacturer's instructions.
Microarray statistical analysis
The microarray data were normalized by using robust multiarray average (Irizarry et al., 2003). Using the resulting normalized intensities, an empirical Bayes method was applied (Kendziorski et al., 2003). The lognormal-normal model was employed to estimate the expression of each gene. Genes were ranked according to this value and the results were further filtered according to the magnitude of change in the expression; only genes that were at least twofold up- or downregulated were considered. To evaluate the differences in the variability of gene expression, an F test for equal variances of the cGVHD and non-GVHD control groups was performed for each gene. To account for multiple testing issues, q-values were calculated (Storey and Tibshirani, 2003) to provide a local expected false discovery rate for a list of genes with the most significant difference in the variance. All computations were performed using R language and environment.
Flow cytometry
Blood cells isolated from 20 patients with cGVHD and 20 patients with non-GVHD were subjected to flow cytometry analysis for the detection of CD28 in CD4+ and CD8+ T cells subpopulation on a FACScan (Becton Dickinson) equipped with a 15 mW air-cooled argon laser tuned at 488 nm. The cells were prepared for the measurement according to the protocols provided with the assays or antibodies; data acquisition and analysis were performed by the CellQuest (Becton Dickinson) software.
Quantitative real-time polymerase chain reaction
To validate the results of the microarray experiment, quantitative real-time polymerase chain reaction (qRT-PCR) was performed on 60 individual patient samples that had not been used in the microarray experiment. Total RNA was extracted with Trizol kit (Invitrogen) and 5.0 μg total RNA was used in the first-strand cDNA synthesis with random hexamers and Superscript II Reverse Transcriptase (Invitrogen) according to the manufacturer's instructions. Quantitative detection of PI3K expression in cDNA from peripheral blood mononuclear cells (PBMCs) was performed using TaqMan real-time PCR. To precisely determine the copy number of PI3K, a duplex vector including a fragment of the PI3K and ABL genes was constructed and used as a reference. Based on the DNA concentration, measured by spectrophotometry and confirmed by quantitative gel electrophoresis, standard dilutions of the vector from 107 to 101 copies were prepared. Briefly, PCR was performed in a 25 μL total volume containing 2 μL cDNA, 25 pmol of each primer (PI3K-f and PI3K-b for PI3K gene amplification; ABL-f and ABL-b for ABL gene amplification), 10 nmol dNTPs, 1.5 U AmpliTaq Gold (Applied Biosystems), 5 pmol 6FAM-TAMRA probe, and PCR buffer containing 4.5 mM MgCl2. After an initial denaturation at 95°C for 5 min, 40 cycles consisting of 95°C for 15 s and 64°C for 1 min were performed. Primers and probes for PI3K and ABL gene amplification were synthesized by Invitrogen and are listed in Table 2.
Table 2.
Sequences of Primers and Probes for Quantitative Real-Time Polymerase Chain Reaction
| Primers/probes | Sequence | Purpose |
|---|---|---|
| PI3K-f | 5′-CGGCTTTTTCAACCCTTTTTAAA | Forward primer |
| PI3K-b | 5′-CATGCCGATAGCAAAACCAAT | Reverse primer |
| PI3K-p | 5′-FAM-CAGTAGGCAACCGTGAAGAAAAGATCCTCA-TAMRA | Probe |
| ABL-f | 5′-GATGTAGTTGCTTGGGACCCA | Forward primer |
| ABL-b | 5′-TGGAGATAACACTCTAAGCATAACTAAAGGT | Reverse primer |
| ABL-p | 5′-FAM-CCATTTTTGGTTTGGGCTTCACACCATT-TAMRA | Probe |
Results
Differential gene expression patterns in cGVHD versus non-GVHD control
Microarray analysis demonstrated that 3180 genes showed significant changes in expression between the cGVHD and non-GVHD groups, with 879 genes upregulated and 2301 genes downregulated (Fig. 1). A representative list of these genes was compiled, with focus on the immune-related genes which show the greatest magnitude fold change in expression (Table 3).
FIG. 1.
Heat map of the genes differentially expressed in cGVHD versus non-GVHD patients. The red, green, black, and gray colors represent the upregulation, downregulation, no change, and no expression, respectively. cGVHD, chronic graft-versus-host disease. Color images available online at www.liebertonline.com/dna
Table 3.
Representative Genes Differentially Expressed in Chronic Graft-Versus-Host Disease Versus Nonchronic Graft-Versus-Host Disease
| Probe ID | Gene symbol | Gene name | FCa |
|---|---|---|---|
| 221658_s_at | IL21R | Interleukin 21 receptor | 7.13 |
| 209823_x_at | HLA-DQB1 | Major histocompatibility complex, class II, DQ beta 1 | 6.34 |
| 206295_at | IL18 | Interleukin 18 (interferon-gamma-inducing factor) | 5.88 |
| 211861_x_at | CD28 | CD28 molecule | 5.07 |
| 211410_x_at | KIR2DL5A | Killer cell immunoglobulin-like receptor, two domains, long cytoplasmic tail, 5A | 4.53 |
| 205125_at | PLCD1 | Phospholipase C, delta 1 | 3.93 |
| 216876_s_at | IL17A | Interleukin 17A | 3.87 |
| 205945_at | IL6R | Interleukin 6 receptor | 3.7 |
| 216482_x_at | ZNF79 | Zinc finger protein 79 | 3.64 |
| 209929_s_at | IKBKG | Inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase gamma | 3.33 |
| 209828_s_at | IL16 | Interleukin 16 (lymphocyte chemoattractant factor) | 3.27 |
| 208375_at | IFNA1 | Interferon, alpha 1 | 3.26 |
| 216133_at | TRD@ | T cell receptor delta locus | 3.25 |
| 208991_at | STAT3 | Signal transducer and activator of transcription 3 (acute-phase response factor) | 3.22 |
| 200799_at | HSPA1A | Heat shock 70 kDa protein 1A | 3.22 |
| 204891_s_at | LCK | Lymphocyte-specific protein tyrosine kinase | 3.08 |
| 203879_at | PIK3CD | Phosphoinositide-3-kinase, catalytic, delta polypeptide | 2.99 |
| 204683_at | ICAM2 | Intercellular adhesion molecule 2 | 2.87 |
| 211372_s_at | IL1R2 | Interleukin 1 receptor, type II | 2.87 |
| 201648_at | JAK1 | Janus kinase 1 | 2.79 |
| 216355_at | PCDHB17 | Protocadherin beta 17 pseudogene | 2.60 |
| 211799_x_at | HLA-C | Major histocompatibility complex, class I, C | 2.58 |
| 201208_s_at | TNFAIP1 | Tumor necrosis factor, alpha-induced protein 1 (endothelial) | 2.45 |
| 204777_s_at | MAL | mal, T-cell differentiation protein | 2.23 |
| 205456_at | CD3E | CD3e molecule, epsilon (CD3-TCR complex) | 2.09 |
| 217478_s_at | HLA-DMA | Major histocompatibility complex, class II, DM alpha | 2.06 |
| 210031_at | CD247 | CD247 molecule | 2.05 |
| 203373_at | SOCS2 | Suppressor of cytokine signaling 2 | −2.08 |
| 210354_at | IFNG | Interferon, gamma | −2.38 |
| 203953_s_at | CLDN3 | Claudin 3 | −2.50 |
| 208474_at | CLDN6 | Claudin 6 | −2.63 |
| 208375_at | IFNA1 | Interferon, alpha 1 | −3.23 |
| 207533_at | CCL1 | Chemokine (C-C motif) ligand 1 | −3.23 |
| 220034_at | IRAK3 | Interleukin-1 receptor-associated kinase 3 | −3.23 |
| 207901_at | IL12B | Interleukin 12B (natural killer cell stimulatory factor 2, cytotoxic lymphocyte maturation factor 2, | −3.33 |
| 220813_at | CYSLTR2 | Cysteinyl leukotriene receptor 2 | −3.57 |
| 204932_at | TNFRSF11B | Tumor necrosis factor receptor superfamily, member 11b | −3.95 |
| 221319_at | PCDHB8 | Protocadherin beta 8 | −4.35 |
| 221399_at | EDA2R | Ectodysplasin A2 receptor | −4.76 |
| 211876_x_at | PCDHGA12 | Protocadherin gamma subfamily A, 12 | −9.09 |
Log2-fold change in signal intensity calculated as [2log2(avg cGVHD–avg non-GVHD)]. FC>0, upregulated; FC<0, downregulated.
FC, fold change; TCR, T-cell receptor.
Upregulation of CD28 in T-cell subpopulation from patients with cGVHD
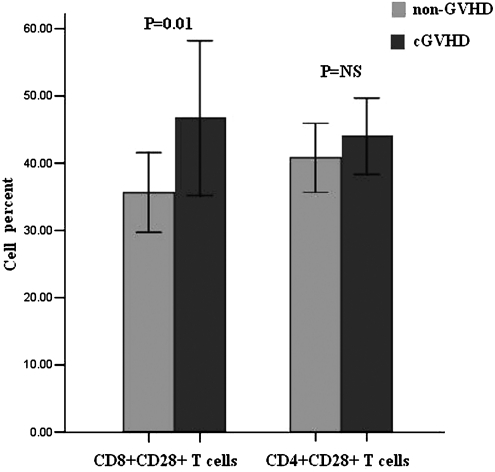
As CD28 showed more than fivefold increase of expression between cGVHD and non-GVHD (Table 3), we performed flow cytometry to detect CD28 expression level in both CD4+ and CD8+ T-cell subpopulation. The portion of CD8+CD28+ T cells was higher in cGVHD than in non-GVHD patients (46.71%±24.61% vs. 35.61%±12.67%, p=0.03; Fig. 2), but no significant difference in the portion of CD4+CD28+ T cells was found between cGVHD and non-GVHD patients (44.07%±12.07% vs. 40.85%±11.05%, p=0.568; Fig. 2).
FIG. 2.
Percentages of CD8+CD28+ and CD4+CD28+ T cells in cGVHD and non-GVHD patients detected by flow cytometry. NS, not significant.
Upregulation of PI3K in PBMCs from patients with cGVHD
PI3K showed about threefold increase of expression in patients with cGVHD (Table 3). To confirm the upregulation of PI3K in cGVHD, we performed qRT-PCR to examine the level of PI3K mRNA in the PBMCs derived from 40 patients with cGVHD, including 20 low-grade cGVHD (grade I or II) and 20 high-grade cGVHD (grade III or IV), and 20 non-GVHD controls. The results demonstrated that PI3K mRNA level was significantly higher in samples from patients with cGVHD than from those with non-GVHD. Further, we found a statistically significant difference in PI3K mRNA level between low-grade cGVHD and high-grade cGVHD samples. Data were analyzed using a one-way analysis of variance (F2,57=18.70, p=0.00) followed by the Dunnett test. PI3K mRNA level of low-grade cGVHD samples showed significant difference from that of non-GVHD (p=0.01) and also from that of high-grade cGVHD (p=0.01) (Fig. 3).
FIG. 3.
Validation of the upregulation of PI3K mRNA expression in peripheral blood mononuclear cells from cGVHD patients by quantitative real-time polymerase chain reaction. The bar graph indicated the quantitative real-time polymerase chain reaction analysis of PI3K transcript level in peripheral blood mononuclear cells from non-GVHD, low-grade cGVHD, and high-grade cGVHD.
Discussion
Recently, genome-wide expression profiling analysis has been applied to the studies of many diseases, leading to significant advances in our understanding of their pathogenesis (Wagner et al., 2004; Borovecki et al., 2005; Takata et al., 2005). In this study, we screened genes differentially expressed in PBMCs between patients with cGVHD and with non-GVHD by employing Affymetrix GeneChip Human U133 Plus 2.0 microarrays, which could reveal more accurate and comprehensive gene expression patterns than long cDNA microarrays could (Ljubimova et al., 2001). The comparison of gene expression profiles between cGVHD and non-GVHD samples demonstrated that interleukin 21 receptor (IL21R), major histocompatibility complex class II DQ alpha 1 (HLA-DQB1), killer cell immunoglobulin-like receptor two domains long cytoplasmic tail 5A (KIR2DL5A), and interleukin 6 receptor (IL6R) were significantly upregulated, whereas protocadherin gamma subfamily A 12 (PCDHGA12), ectodysplasin A2 receptor (EDA2R), and interleukin 12B were downregulated. In accordance with the known pathological features of cGVHD, these differentially expressed genes included a large number of inflammatory cytokines such as IL-6, IL-l7, and TNF-α, which have been proposed to play important roles in the development of cGVHD (Tanaka et al., 1995).
In the present study, we performed microarray analysis to demonstrate that cGVHD has some unique molecular signatures and identifies networks of genes or pathways that may be of significance in the pathogenesis of cGVHD. Interestingly, the microarray results indicated that the expression of CD28 and PI3K was increased in patients with cGVHD. Although gene microarray results are generally accurate and reproducible, many authors agree that these data must be verified by subsequent mRNA analysis (Cao et al., 2002). On the basis of a recent report that treatment of refractory cGVHD with mesenchymal stem cells led to the downregulation of CD28 (Weng et al., 2010), we assumed that CD28 and its downstream effector PI3K play important roles in the pathogenesis of cGVHD. Therefore, we further validated the upregulation of CD28 and PI3K in cGVHD by flow cytometry and qRT-PCR analysis.
CD28, a costimulatory molecule, is present on the cell membrane and can be detected highly sensitively by flow cytometry. Based on flow cytometric analysis, the proportion of CD28+ T cells was higher in patients with cGVHD than non-GVHD, which is consistent with the microarray data. Moreover, we validated by flow cytometric analysis that CD28 on the CD8+ T cells, but not the CD4+ T cell subpopulation, increased with extended course of cGVHD. This is partly due to the fact that T-lymphocyte subsets of CD8+ cells serve as active cytolytic T lymphocytes (Parkman, 1993) and can participate in transplantation immunity and rejection reaction (Gilliam et al., 1996; Rojas et al., 2005). CD28 expressed on CD8+ T cells in patients with cGVHD thus regulates the differentiation after antigen stimulation into long-term survival memory T cells, implicating that these cells participate in cGVHD.
The previous studies reported that cGVHD starts with the expansion of mature donor T cells that recognize minor or major histocompatibility antigens of the recipient (Perez-Simon et al., 2006), and efficient T-cell activation requires antigen recognition and costimulation (Wulfing et al., 2002). CD28 is one of the best-understood costimulatory molecules and is expressed predominantly on T cells. It can deliver a costimulatory signal, which in conjunction with T cell receptor signal leads to T cell activation. CD28 propagates intracellular signals to promote T cell proliferation and enhance the expression of cytokine/chemokine, thus playing an important role in the pathogenesis of multiple myeloma (Bahlis et al., 2007). In accordance with these reports, our results show that the proportion of CD8+CD28+ T cells was higher in patients with cGVHD, indicating that the increased proliferation of CD8+ T cells accelerates cGVHD, which may be initiated by interaction between CD28 and its ligands (Yu et al., 1998). These results suggest that inhibiting CD28 costimulation by blocking CD28 ligands or using monovalent anti-CD28 reagents before transplantation could inhibit the expansion of CD8+ T cells and decrease the incidence of cGVHD.
More significantly, we found that the expression of PI3K was correlated with the occurrence and grade of cGVHD. We confirmed by qRT-PCR that PI3K mRNA level was increased in patients with cGVHD. In this study, a high level of PI3K mRNA was detected in the PBMCs but not in T cells, because the study was retrospective and the blood samples of enrolled patients were from our specimen bank in which single T cells had not been separated. Thus, we need further select venous blood from patients with cGVHD to confirm that the expression of PI3K is upregulated in specific T cells from these patients.
Statistical analysis revealed different expression levels of PI3K between grade I or II and grade III or IV cGVHD patients. We could not compare all the grades of patients with cGVHD, because blood from patients in all grades was not available. Moreover, the grade is a subjective judgment of cGVHD severity (Sullivan et al., 1991). Therefore, we only compared low grade (grade I or II) and high grade (grade III or IV) to evaluate dynamic expression of PI3K in different courses of cGVHD. Accumulating evidence has highlighted the critical role of the PI3K signaling pathway in the development, activation, and homeostasis of T cells (Koyasu, 2003; Okkenhaug and Vanhaesebroeck, 2003). In response to a variety of stimuli including alloantigens or autoantigens, which can differ between donor and recipient, PI3K signaling modulates the expression of survival and mitogenic factors in T cells (Harriague and Bismuth, 2002). The expansion of these activated T cells at the early phase of cGVHD can subsequently induce damage to target organs directly or indirectly (Perez-Simon et al., 2006). In addition, LY294002, a PI3K inhibitor, inhibited the activation of primary T cells (Crooks et al., 1995). Based on these data, we propose that PI3K, as a switch of T cell activation, plays an important role in cGVHD. By investigating multiple-grade points throughout cGVHD development, rather than comparing PI3K expression in patients who suffered from cGVHD to that in non-GVHD controls, we can begin to establish a functional correlation between PI3K expression and prospective development of cGVHD. In this aspect, PI3K may serve as a novel biomarker to predict the incidence and development of cGVHD.
As the T cell responses induced by CD28-mediated cosignaling overlap the reported functions of PI3K in lymphocyte activation (Appleman et al., 2002), it is conceivable that CD28-PI3K may be the critical signaling molecule in T cell costimulation in cGVHD (Harada et al., 2001). CD28 can directly bind to PI3K by a well-characterized YMNM binding motif in its cytoplasmic domain (Cai et al., 1995; Okkenhaug et al., 2001), and PI3K signaling pathway is considered to be important for CD28-mediated costimulation (Pages et al., 1994). Previous studies reported that dual inhibition of CD28 and PI3K may achieve more potent alleviation of pathological immune responses than that achieved with either inhibitor alone, suggesting that CD28 stimulation through the PI3K pathway allows efficient T cell activation (Garcon et al., 2008). Although the relevant PI3K catalytic isoforms and the contribution of CD28 to PI3K activity in T cells have not been determined unequivocally, some reports indicated that CD28 and PI3K isoform (p110) act in parallel and complementary pathways to activate T cells (Okkenhaug et al., 2007; Garcon et al., 2008), whereas other studies suggested that it is only through p85 that PI3K binds to CD28, contributing to T-cell activation, survival, and division (Alcazar et al., 2009). We will distinguish the subtypes of PI3K in activated T cells of cGVHD in a further study.
In summary, given the rising incidence of cGVHD and poor response of many patients to conventional immunosuppressive treatments, it is extremely urgent to develop sensitive biomarkers for the early diagnosis and therapy of cGVHD (Deeg et al., 1998). Our data establish a correlation between CD28/PI3K expression and the incidence and severity of cGVHD. These findings suggest that anti-CD28 reagents or PI3K inhibitors could potentially reduce the risk of cGVHD. Moreover, increased CD8+CD28+ T cells and PI3K expression may predict the incidence and severity of cGVHD, which will facilitate the treatment of cGVHD. Further studies are necessary to elucidate the detailed mechanism by which specific CD8+CD28+ T cell subpopulations and PI3K pathway contribute to cGVHD following allogeneic HSCT. Longitudinal follow-up of transplantation patients will be required to establish whether CD28/PI3K downregulation is correlated with the alleviation of cGVHD and a better clinical outcome.
Acknowledgments
This work was supported by the National Science Foundation of China (Grant Nos. 30571771, 30972790, and 81070445), The Natural Science Foundation of Guangdong Province (Grant Nos. 10151008004000024 and 06020896), Science and Technology Planning Project of Guangdong Province (Grant Nos. 2006B36005003 and 2009A030200009), and Science and Technology Planning Project of Guangzhou (Grant No. 2008A1-E4011-4).
Disclosure Statement
No competing financial interests exist.
References
- Alcazar I. Cortes I. Zaballos A. Hernandez C. Fruman D.A. Barber D.F., et al. p85beta phosphoinositide 3-kinase regulates CD28 coreceptor function. Blood. 2009;113:3198–3208. doi: 10.1182/blood-2008-04-152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleman L.J. van Puijenbroek A.A. Shu K.M. Nadler L.M. Boussiotis V.A. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J Immunol. 2002;168:2729–2736. doi: 10.4049/jimmunol.168.6.2729. [DOI] [PubMed] [Google Scholar]
- Bahlis N.J. King A.M. Kolonias D. Carlson L.M. Liu H.Y. Hussein M.A., et al. CD28-mediated regulation of multiple myeloma cell proliferation and survival. Blood. 2007;109:5002–5010. doi: 10.1182/blood-2006-03-012542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadies N. Foster S.D. Chan W.I. Kvinlaug B.T. Spensberger D. Dawson M.A., et al. Genome-wide analysis of transcriptional reprogramming in mouse models of acute myeloid leukaemia. PLoS One. 2011;6:e16330. doi: 10.1371/journal.pone.0016330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovecki F. Lovrecic L. Zhou J. Jeong H. Then F. Rosas H.D., et al. Genome-wide expression profiling of human blood reveals biomarkers for Huntington's disease. Proc Natl Acad Sci USA. 2005;102:11023–11028. doi: 10.1073/pnas.0504921102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzeo M.P. Yang J. Casella G. Reddy V. A preliminary gene expression profile of acute graft-versus-host disease. Cell Transplant. 2008;17:489–494. doi: 10.3727/096368908785096042. [DOI] [PubMed] [Google Scholar]
- Cai Y.C. Cefai D. Schneider H. Raab M. Nabavi N. Rudd C.E. Selective CD28pYMNM mutations implicate phosphatidylinositol 3-kinase in CD86-CD28-mediated costimulation. Immunity. 1995;3:417–426. doi: 10.1016/1074-7613(95)90171-x. [DOI] [PubMed] [Google Scholar]
- Cao Z. Wu H.K. Bruce A. Wollenberg K. Panjwani N. Detection of differentially expressed genes in healing mouse corneas, using cDNA microarrays. Invest Ophthalmol Vis Sci. 2002;43:2897–2904. [PubMed] [Google Scholar]
- Crooks M.E. Littman D.R. Carter R.H. Fearon D.T. Weiss A. Stein P.H. CD28-mediated costimulation in the absence of phosphatidylinositol 3-kinase association and activation. Mol Cell Biol. 1995;15:6820–6828. doi: 10.1128/mcb.15.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg H.J. Leisenring W. Storb R. Nims J. Flowers M.E. Witherspoon R.P., et al. Long-term outcome after marrow transplantation for severe aplastic anemia. Blood. 1998;91:3637–3645. [PubMed] [Google Scholar]
- Figueroa J.P. Rose J.C. Massmann G.A. Zhang J. Acuna G. Alterations in fetal kidney development and elevations in arterial blood pressure in young adult sheep after clinical doses of antenatal glucocorticoids. Pediatr Res. 2005;58:510–515. doi: 10.1203/01.PDR.0000179410.57947.88. [DOI] [PubMed] [Google Scholar]
- Flowers M.E. Apperley J.F. van Besien K. Elmaagacli A. Grigg A. Reddy V., et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood. 2008;112:2667–2674. doi: 10.1182/blood-2008-03-141481. [DOI] [PubMed] [Google Scholar]
- Garcon F. Patton D.T. Emery J.L. Hirsch E. Rottapel R. Sasaki T., et al. CD28 provides T-cell costimulation and enhances PI3K activity at the immune synapse independently of its capacity to interact with the p85/p110 heterodimer. Blood. 2008;111:1464–1471. doi: 10.1182/blood-2007-08-108050. [DOI] [PubMed] [Google Scholar]
- Gilliam A.C. Whitaker-Menezes D. Korngold R. Murphy G.F. Apoptosis is the predominant form of epithelial target cell injury in acute experimental graft-versus-host disease. J Invest Dermatol. 1996;107:377–383. doi: 10.1111/1523-1747.ep12363361. [DOI] [PubMed] [Google Scholar]
- Harada Y. Tanabe E. Watanabe R. Weiss B.D. Matsumoto A. Ariga H., et al. Novel role of phosphatidylinositol 3-kinase in CD28-mediated costimulation. J Biol Chem. 2001;276:9003–9008. doi: 10.1074/jbc.M005051200. [DOI] [PubMed] [Google Scholar]
- Harriague J. Bismuth G. Imaging antigen-induced PI3K activation in T cells. Nat Immunol. 2002;3:1090–1096. doi: 10.1038/ni847. [DOI] [PubMed] [Google Scholar]
- Irizarry R.A. Hobbs B. Collin F. Beazer-Barclay Y.D. Antonellis K.J. Scherf U., et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- Kendziorski C.M. Newton M.A. Lan H. Gould M.N. On parametric empirical Bayes methods for comparing multiple groups using replicated gene expression profiles. Stat Med. 2003;22:3899–3914. doi: 10.1002/sim.1548. [DOI] [PubMed] [Google Scholar]
- Koyasu S. The role of PI3K in immune cells. Nat Immunol. 2003;4:313–319. doi: 10.1038/ni0403-313. [DOI] [PubMed] [Google Scholar]
- Ljubimova J.Y. Lakhter A.J. Loksh A. Yong W.H. Riedinger M.S. Miner J.H., et al. Overexpression of alpha4 chain-containing laminins in human glial tumors identified by gene microarray analysis. Cancer Res. 2001;61:5601–5610. [PubMed] [Google Scholar]
- Okkenhaug K. Ali K. Vanhaesebroeck B. Antigen receptor signalling: a distinctive role for the p110delta isoform of PI3K. Trends Immunol. 2007;28:80–87. doi: 10.1016/j.it.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkenhaug K. Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3:317–330. doi: 10.1038/nri1056. [DOI] [PubMed] [Google Scholar]
- Okkenhaug K. Wu L. Garza K.M. La Rose J. Khoo W. Odermatt B., et al. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat Immunol. 2001;2:325–332. doi: 10.1038/86327. [DOI] [PubMed] [Google Scholar]
- Pages F. Ragueneau M. Rottapel R. Truneh A. Nunes J. Imbert J., et al. Binding of phosphatidylinositol-3-OH kinase to CD28 is required for T-cell signalling. Nature. 1994;369:327–329. doi: 10.1038/369327a0. [DOI] [PubMed] [Google Scholar]
- Parkman R. Is chronic graft versus host disease an autoimmune disease? Curr Opin Immunol. 1993;5:800–803. doi: 10.1016/0952-7915(93)90140-n. [DOI] [PubMed] [Google Scholar]
- Perez-Simon J.A. Sanchez-Abarca I. Diez-Campelo M. Caballero D. San Miguel J. Chronic graft-versus-host disease: pathogenesis and clinical management. Drugs. 2006;66:1041–1057. doi: 10.2165/00003495-200666080-00002. [DOI] [PubMed] [Google Scholar]
- Poloni A. Sartini D. Emanuelli M. Trappolini S. Mancini S. Pozzi V., et al. Gene expression profile of cytokines in patients with chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation with reduced conditioning. Cytokine. 2011;53:376–383. doi: 10.1016/j.cyto.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Rojas B. Cuhna R. Zafirakis P. Ramirez J.M. Lizan-garciia M. Zhao T., et al. Cell populations and adhesion molecules expression in conjunctiva before and after bone marrow transplantation. Exp Eye Res. 2005;81:313–325. doi: 10.1016/j.exer.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Seaton E.D. Szydlo R.M. Kanfer E. Apperley J.F. Russell-Jones R. Influence of extracorporeal photopheresis on clinical and laboratory parameters in chronic graft-versus-host disease and analysis of predictors of response. Blood. 2003;102:1217–1223. doi: 10.1182/blood-2002-11-3351. [DOI] [PubMed] [Google Scholar]
- Storey J.D. Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K.M. Agura E. Anasetti C. Appelbaum F. Badger C. Bearman S., et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–259. [PubMed] [Google Scholar]
- Takahashi N. Sato N. Takahashi S. Tojo A. Gene-expression profiles of peripheral blood mononuclear cell subpopulations in acute graft-vs-host disease following cord blood transplantation. Exp Hematol. 2008;36:1760–1770. doi: 10.1016/j.exphem.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Takata R. Katagiri T. Kanehira M. Tsunoda T. Shuin T. Miki T., et al. Predicting response to methotrexate, vinblastine, doxorubicin, and cisplatin neoadjuvant chemotherapy for bladder cancers through genome-wide gene expression profiling. Clin Cancer Res. 2005;11:2625–2636. doi: 10.1158/1078-0432.CCR-04-1988. [DOI] [PubMed] [Google Scholar]
- Tanaka J. Imamura M. Kasai M. Zhu X. Kobayashi S. Hashino S., et al. Cytokine receptor gene expression in peripheral blood mononuclear cells during graft-versus-host disease after allogeneic bone marrow transplantation. Leuk Lymphoma. 1995;19:281–287. doi: 10.3109/10428199509107899. [DOI] [PubMed] [Google Scholar]
- Wagner R.A. Tabibiazar R. Powers J. Bernstein D. Quertermous T. Genome-wide expression profiling of a cardiac pressure overload model identifies major metabolic and signaling pathway responses. J Mol Cell Cardiol. 2004;37:1159–1170. doi: 10.1016/j.yjmcc.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Weng J.Y. Du X. Geng S.X. Peng Y.W. Wang Z. Lu Z.S., et al. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant. 2010;45:1732–1740. doi: 10.1038/bmt.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C.S. Davidson G.S. Martin S.B. Andries E. Potter J. Harvey R., et al. Gene expression profiling of adult acute myeloid leukemia identifies novel biologic clusters for risk classification and outcome prediction. Blood. 2006;108:685–696. doi: 10.1182/blood-2004-12-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulfing C. Sumen C. Sjaastad M.D. Wu L.C. Dustin M.L. Davis M.M. Costimulation and endogenous MHC ligands contribute to T cell recognition. Nat Immunol. 2002;3:42–47. doi: 10.1038/ni741. [DOI] [PubMed] [Google Scholar]
- Yu X.Z. Martin P.J. Anasetti C. Role of CD28 in acute graft-versus-host disease. Blood. 1998;92:2963–2970. [PubMed] [Google Scholar]