Abstract
Low back pain and pelvic pain (LBPP) is common during pregnancy and up to 40% of women still have symptoms half a year after delivery. The aim of the study was to investigate determinants and the prevalence of persistent LBPP after pregnancy in a Swedish cohort. In a previous study 891 women had responded to a questionnaire on risk factors and prevalence of LBPP during pregnancy. Altogether 72% (n=639) of the women had reported LBPP during pregnancy. These respondents were sent a second questionnaire at approximately 6 months after delivery. The response rate was 72.6% (n=464). Independent t-test and Pearson’s chi-squared test were used to test the difference between the two groups. In response to the questionnaire, 43.1% of the women reported persistent LBPP 6 months after delivery. Women with persistent LBPP after pregnancy had had significantly earlier onset of pain during pregnancy, higher maternal age, higher body mass index (BMI), and assessed a higher level of pain due to LBPP during pregnancy and after pregnancy, and included a higher proportion of women with joint hyper-mobility. In summary, recurrent or continuous LBPP is prevalent after pregnancy. BMI as well as hyper-mobility are prominent determinants of persistent LBPP after pregnancy. Level and onset of pain during pregnancy were strong predictors of persistent LBPP.
Keywords: Low back pain, Pelvic pain, Pregnancy, Determinants, Long-term outcome
Background
Low back pain and pelvic pain (LBPP) affects more than half of pregnant women [2, 7, 9, 19]. The condition interferes with most activities of daily living [8, 11]. Women who have previously experienced pelvic pain during pregnancy experience a relapse of 85% during a subsequent pregnancy [11].
In the literature the reported prevalence of persistent LBPP postpartum has varied ranging from 5 to 43% half a year after delivery [1, 10, 18, 20, 26]. Longer follow-up has shown that 5% of all pregnant women, or 20% of all women with back pain during pregnancy, have pain 3 years later [17]. A decline in the prevalence of back pain principally occurs during the first 6 months after delivery [21], and high pain intensity in pregnancy indicates a poor prognosis [20]. In retrospective studies 10–25% of women with chronic back pain reported that their first event of back pain occurred during pregnancy [3, 4, 24]. A history of back pain, younger age, and greater weight have been found to be predisposing factors for the condition after childbirth [6]. Decrease in bone density during pregnancy is not associated with back pain or pelvic pain during pregnancy [5]. Postpartum back pain has been associated with considerably perceived disabilities in movement-related activities [16]. A recent review discusses terminology for and clinical presentation and prevalence for pregnancy-related pelvic girdle pain during pregnancy and post partum [27].
Results from the present cohort concerning prevalence and risk factors of LBPP during pregnancy have previously been reported [14]. A high prevalence of LBPP (72%) was found among the respondents, which most probably is an overestimation of the true prevalence, however, the majority of women experience LBPP during pregnancy [14]. Parity, body mass index (BMI), history of hyper-mobility, as well as amenorrhea and previous LBPP were factors influencing the risk of development of LBPP [14]. The current study is an extension of the primary study [12–14].
Aim
The aim of this study was to investigate determinants and prevalence of persistent LBPP approximately half a year after pregnancy in women who have experienced LBPP during pregnancy.
Subjects and methods
In a previous study [14] all women who delivered in the Departments of Obstetrics and Gynecology at Umeå University Hospital (UUH) and the Sunderby Hospital (SH) in the counties of Västerbotten and Norrbotten in northern Sweden, were invited to complete a questionnaire (questionnaire 1=Q1) on their obstetric and gynecological history, actual pregnancy and delivery. The first date of inclusion (i.e. the date of delivery) was 1 January 2002 and the last date was, 30 April 2002 at both departments. The women received verbal and written information of the aims of the study from a midwife on duty at the department within usually 24 h of the delivery. Women who agreed to participate received a questionnaire with a unique number. The questionnaire was usually collected before the women were discharged from hospital; women who had not completed the questionnaire by then were given a postage-prepaid envelope.
The participant’s identification number was recorded and each participant was given a unique questionnaire number. The identification number of the women who declined participation was likewise recorded, for analysis of missing data. For inclusion in the study, the women had to have reached a gestational age of at least 23 weeks, ending in a live birth or stillbirth. The study used a cross-sectional design. During the period from 1 January 2002 to 30 April 2002 the total number of women delivered at the UUH and SH was 1,114, with 516 (46.3%) delivered at UUH and 598 women (53.7%) at the SH.
Another inclusion criterion was competence in the Swedish language, which decreased the number of eligible women to 1,071. Non-respondents were women who either did not receive a questionnaire or did not complete the questionnaire they were given. The net sample consisted of 891 respondents (Q1) and the response rate was therefore 83.2% (891/1,071). Place of delivery did not influence risk in the logistic regression analyses. Detailed information on the sample has been presented in a previous paper [14].
Women reporting LBPP (n=639) during pregnancy (Q1) were followed up with a second questionnaire (Q2) at approximately 6 months after delivery. The questionnaire included 39 questions on different issues such as LBPP after pregnancy, use of medical services, family situation, perceived health, sick leave, sexual life, physical activities, oral contraception and breast-feeding. One or, if required, several reminders were sent to the subjects if no response on first Q2. The women filled in the date of completion of the questionnaire, however, 17 dates were evidently incorrect and therefore, the date of the postal reception was noted as date of completion of the questionnaire. By mistake, some questionnaires were sent to the respondents before 5.5 months post-delivery.
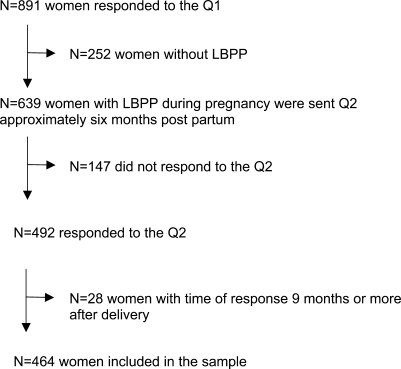
The sample is presented in Fig. 1. Altogether 77.0% (492/639) of eligible subjects responded to the Q2. Twenty-eight women were excluded because they completed the questionnaire 9 months or more after date of delivery. The net sample included 464 (72.6%) women who responded to the Q2.
Fig. 1.
The sample
Ethics
The study was approved by the Ethics Committee at the Umeå University (Dnr. 01–335) and each woman gave her informed oral consent (Q1).
Definitions
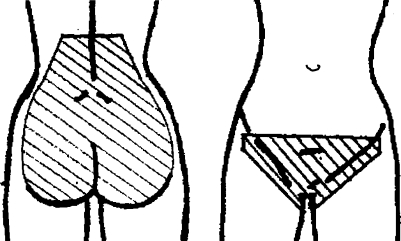
Low back pain or pelvic pain during pregnancy (LBPP) in the previous study (Q1) was defined as ‘recurrent or continuous pain for more than 1 week from the lumbal spine or pelvis’ during recent pregnancy. A woman was considered to have LBPP during pregnancy if she positively answered the specific question on localization of pain, which included marking the affected area on a drawing included in the questionnaire (Fig. 2) [14].
Fig. 2.
Localization of low back pain and pelvic pain
The respondents were requested to estimate their experience of the highest level of pain due to LBPP during pregnancy (both Q1 and Q2) on a 10-cm visual analogue scale (VAS), with the end-points of ‘no pain’ (0 cm) and ‘worst thinkable pain’ (10 cm).
Actual low back pain and pelvic pain (LBPP) after pregnancy in the present study (Q2) was defined as a positive response on the question whether the subject had actual low back pain or pelvic pain. The response alternatives to this question were ‘yes, recurrent pain’, ‘yes, continuous pain’ and ‘no pain’. Fourteen women gave a time point at which LBPP had ceased; however, they also declared that they had since had recurrent pain. These subjects were allocated to the ‘no pain’ group.
Experience of highest level of pain due to LBPP after pregnancy. The respondents in the present study (Q2) were requested to estimate the highest level of pain due to LBPP after delivery and also, to specify the highest level of pain due to LBPP after delivery experienced during the previous week.
Persistent LBPP after pregnancy included women with both ‘recurrent pain’ and ‘continuous pain’ defined as LBPP after pregnancy.
Time remission of LBPP after pregnancy. The respondents declared at which time after pregnancy the symptoms of pain had ceased. The time was given in months and weeks after the delivery.
All women were requested to score their total experience of the delivery (in both the Q1 and the Q2) on a VAS with end-points at 0 and 10 cm, where 0 denoted ‘very bad’ and 10, ‘very good’.
Parity. Number of births
Pre-pregnancy weight (Q1) was defined as reported weight prior to the pregnancy.
End-pregnancy weight (Q1) was defined as reported weight prior to the delivery.
Actual weight was defined as weight at the time of completing the Q2.
Body mass index (BMI) was defined as weight (kg)/height2 (m2).
Statistics
Mean values and standard deviations (SD) (mostly not presented) were calculated for parametric data. Independent-samples t-test and Pearson’s chi-squared test were used to test the difference between two groups for parametric and categorical data, respectively. To evaluate response consistency in the questionnaire, the intra-class correlation coefficient (single measure; consistency definition) was calculated for a subgroup of respondents (n=20) who completed a second, identical questionnaire (Q2). Odds ratios (OR) and their corresponding 95% confidence intervals (CI) were calculated by using logistic regression in univariate and multivariate analyses. The population attributable proportion (PAP = p(RR − 1)/[1 + p(RR − 1)], p = the proportion of people exposed in the population), which is the proportion of cases in the population that should not have occurred, had the incidence of the outcome among those who were exposed been the same as for those who were unexposed, was calculated when appropriate.
Results
Different background and outcome factors are presented in Table 1. Altogether 200 (43.1%) respondents reported recurrent or continuous LBPP 6 months after delivery (Table 1). Parity, number of pregnancies, gestational age, birth weight, and maternal height did not differ between women experiencing LBPP after pregnancy in relation to women with remission of LBPP after pregnancy (Tables 1 and 2).
Table 1.
Background and outcome factors
| Variable | All subjects (%) | No paina (%) | Recurrent painb (%) | Continuous painc (%) | P-value | Non-respondents (%) | |
|---|---|---|---|---|---|---|---|
| a v. b + c | n | p-valued | |||||
| Number of subjectse (%) | 464 (100.0) | 264 (56.9) | 168 (36.2) | 32 (6.9) | 175 (100.0) | ||
| Educational level, n (%) (Q1) | n=463 | n=264 | n=167 | n=32 | n=175 | ||
| Up to university level | 245 (52.9) | 142 (53.8) | 87 (52.1) | 16 (50.0) | 0.665 | 115 (65.7) | 0.004 |
| University education | 218 (47.1) | 122 (46.2) | 80 (47.9) | 16 (50.0) | 60 (34.3) | ||
| Mean age at delivery, years (Q1) | 30.2 | 29.6 | 30.8 | 31.8 | 0.001 | 29.6 | 0.168 |
| Mean age at Q2, yrs | 30.7 | 30.1 | 31.3 | 32.3 | 0.002 | ||
| Time of responsef, months | 6.1 | 6.1 | 6.1 | 6.2 | 0.075 | ||
| Parity, mean n (Q1) | 1.9 | 1.8 | 1.9 | 1.8 | 0.181 | 2.2 | 0.001 |
| No. of pregnancies (Q1) |
n=456 2.4 |
n=259 2.3 |
n=166 2.5 |
n=31 2.2 |
0.128 |
n=170 2.8 |
0.001 |
| Gestational age, days (Q1) |
n=464 278.1 |
n=264 277.9 |
n=168 278.0 |
n=32 279.9 |
0.762 |
n=174 276.6 |
0.233 |
| Birth weight, g (Q1) | 3 597 | 3 567 | 3 663 | 3 497 | 0.256 | 3 511 | 0.134 |
| Mode of delivery (Q1), n (%) | |||||||
| Vaginal delivery | 342 (73.5) | 201 (76.1) | 120 (71.4) | 20 (62.5) | 0.010 | 120 (68.6) | 0.540 |
| Vacuum extraction | 34 (7.3) | 17 (6.3) | 13 (7.7) | 4 (12.5) | 12 (6.9) | ||
| Elective CS | 44 (9.5) | 15 (5.7) | 23 (13.7) | 6 (18.8) | 21 (12.0) | ||
| Emergency CS | 42 (9.0) | 28 (9.6) | 12 (6.2) | 2 (6.2) | 22 (12.6) | ||
| Forceps | 3 (0.6) | 3 (1.1) | – | – | – | ||
| Total experience of deliveryg (Q1) |
n=453 7.9 |
n=258 7.9 |
n=164 8.0 |
n=31 7.6 |
0.786 |
n=171 7.8 |
0.742 |
| Total experience of deliveryg (Q2) |
n=458 7.7 |
n=258 7.7 |
n=168 7.8 |
n=32 7.4 |
0.843 | ||
| Pearson’s correlation coefficient of Q1 and Q2 | r2=0.743 | r2=0.798 | r2=0.607 | r2=0.872 | |||
| Hyper-mobilityh (Q1) | n=458 | n=261 | n=166 | n=31 | n=170 | ||
| Yes | 83 (18.1) | 41 (15.7) | 33 (19.9) | 9 (29.0) | 0.123 | 38 (22.4) | 0.232 |
| No | 375 (81.9) | 220 (84.3) | 133 (80.1) | 22 (71.0) | 132 (77.6) | ||
| Hyper-mobilityi (Q1) | n=458 | n=261 | n=166 | n=31 | n=170 | ||
| Yes | 111 (24.2) | 54 (20.7) | 46 (27.7) | 11 (35.5) | 0.042 | 47 (27.6) | 0.381 |
| No | 347 (75.8) | 207 (79.3) | 120 (72.3) | 20 (64.5) | 123 (72.4) | ||
Difference between groups analyzed by t-test for parametric data and Pearson’s chi-squared test for categorical data, and Pearson’s correlation coefficient where applicable
LBPP low back pain and pelvic pain; Q1 first questionnaire; Q2 second questionnaire
a‘No pain’ denotes respondents reporting remission of LBPP after pregnancy
b‘Recurrent pain’ denotes respondents reporting recurrent LBPP after pregnancy
c‘Continuous pain’ denotes respondents reporting continuous LBPP after pregnancy
dStatistical testing non-respondents versus respondents in last column
eNumber of subjects: total subject number and number for each subject category, which is also the number for each variable unless otherwise indicated (in italics)
fTime of response: mean number of months, i.e., the time from delivery until the date given in the Q2 or the date of reception of the Q2
gTotal experience of delivery scored on a 10-cm Visual Analogue Scale with possible scores of 0-10, where 0 denotes ‘very bad’ and 10 denotes ‘very good’
hHyper-mobility = women reported diagnosed as having hyper-mobility
iHyper-mobility = women reported diagnosed as having hyper-mobility and/or a perception of hyper-mobility
Table 2.
Weight, body mass index (BMI) and perception of weight as determinants of LBPP after pregnancy
| Variable | All Subjects (%)d | No paina (%)d | Recurrent painb (%)d | Continuous painc (%)d | P-value | Non-respondents | |
|---|---|---|---|---|---|---|---|
| a v. b + c | n | p-valuee | |||||
| Weight at Q2, kg |
n=449 69.2 |
n=258 67.6 |
n=159 71.5 |
n=32 71.0 |
0.002 | ||
| Height |
n=462 166.3 |
n=264 165.9 |
n=66 166.7 |
n=32 167.6 |
0.078 |
n=172 166.5 |
0.686 |
| Pre-pregnancy BMI (Q1) |
n=456 24.5 |
n=260 24.0 |
n=164 25.4 |
n=32 24.8 |
0.001 |
n=167 24.6 |
0.777 |
| End-pregnancy BMI (Q1) |
n=455 30.0 |
n=260 29.4 |
n=164 30.9 |
n=31 30.3 |
0.001 |
n=164 30.4 |
0.442 |
| BMI at Q2 |
n=447 25.0 |
n=258 24.6 |
n=157 25.7 |
n=32 25.3 |
0.011 | ||
| Pearson’s correlation coefficient of pre-pregnancy BMI and BMI at Q2 |
n=444 r2=0.918 |
n=255 r2=0.920 |
n=157 r2=0.916 |
n=32 r2=0.902 |
|||
| Pearson’s correlation coefficient of end-pregnancy BMI and BMI at Q2 |
n=443 r2=0.904 |
n=255 r2=0.896 |
n=157 r2=0.907 |
n=31 r2=0.922 |
|||
| Satisfied with pre-pregnancy weight (Q1) | n=462 | n=264 | n=166 | n=32 | n=171 | ||
| Yes | 244 (52.8) | 147 (55.7) | 76 (45.8) | 21 (65.6) | 0.154 | 89 (52.0) | 0.864 |
| No | 218 (47.2) | 117 (44.3) | 90 (54.2) | 11 (34.4) | 82 (48.0) | ||
| Perceived problems with actual or previous overweight (Q1) | n=457 | n=261 | n=164 | n=32 | n=172 | ||
| Yes | 131 (28.7) | 66 (25.3) | 56 (34.1) | 9 (28.2) | 0.065 | 65 (37.8) | 0.028 |
| No | 326 (71.3) | 195 (74.7) | 108 (65.6) | 23 (71.9) | 107 (62.2) | ||
Difference between groups analyzed by t-test for parametric data and Pearson’s chi-squared test for categorical data, and Pearson’s correlation coefficient where applicable
LBPP low back pain and pelvic pain; Q1 first questionnaire; Q2 second questionnaire
a‘No pain’ denotes respondents reporting remission of LBPP after pregnancy
b‘Recurrent pain’ denotes respondents reporting recurrent LBPP after pregnancy
c‘Continuous pain’ denotes respondents reporting continuous LBPP after pregnancy
dNumber of subjects: in italics
eStatistical testing non-respondents vs respondents in last column
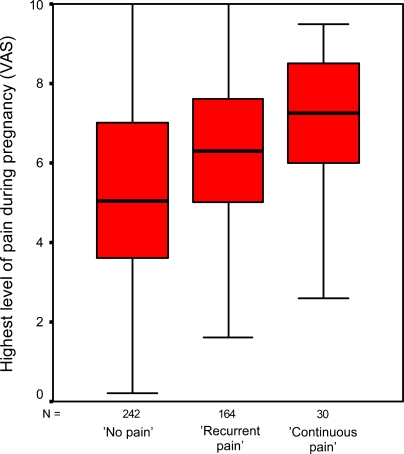
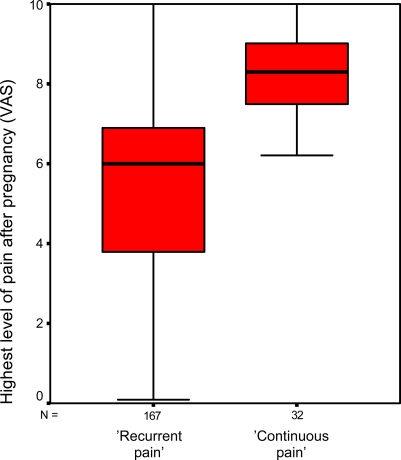
Debut of pain and level of pain during and after pregnancy as determinants of LBPP after pregnancy are presented in Table 3. Women reporting continuous pain had an almost significantly higher level of pain during pregnancy than did women reporting recurrent pain (7.0 vs. 6.2, Q1, P=0.058). This relation was highly significant for assessment of pain during the whole period after delivery (7.8 vs. 5.5, Q2, P=0.001) and assessment of pain during the previous week (6.6 vs. 4.2, Q2, P=0.001). Women with persistent LBPP had an earlier onset of pain during pregnancy than did women with remission of LBPP (Table 3), however, there was no statistically significant difference between women with recurrent and women with continuous LBPP after pregnancy (P=0.536). Level of pain during pregnancy was a strong predictor of risk of persistent LBPP after pregnancy in univariate and multivariate logistic regression analyses (Table 4). Experienced highest level of pain due to LBPP during pregnancy is shown in a box-plot diagram for the different groups (Fig. 3) as well as the highest level of pain experienced after delivery (whole period) for women with recurrent or continuous LBPP after pregnancy (Fig. 4).
Table 3.
Pain as determinant of LBPP after pregnancy
| Variable | All subjectsd | No paina, d | Recurrent painb, d | Continuous painc, d | P-value | Non-respondents | |
|---|---|---|---|---|---|---|---|
| a v. b + c | n | p-valuee | |||||
| Onset of LBPP during pregnancy, weeks (Q1) | (n=407) 22.3 | (n=225) 23.2 | (n=154) 21.2 | (n=28) 20.2 | 0.004 | (n=144) 21.6 | 0.369 |
| Assessment of painf during pregnancy (Q1) | (n=436) 5.7 | (n=242) 5.2 | (n=164) 6.2 | (n=30) 7.0 | <0.001 | (n=157) 6.1 | 0.049 |
| Assessment of painf after pregnancy whole period (Q2) | (n=434) 3.6 | (n=235) 1.8 | (n=167) 5.5 | (n=32) 7.8 | <0.001 | – | |
| Pearson’s correlation coefficient of Q1 and Q2 | r2=0.386 | r2=0.184 | r2=0.432 | r2=0.612 | |||
| Assessment of painf during the previous week (Q2) | (n=199) 4.6 | – | (n=167) 4.2 | (n=32) 6.6 |
b v.c <0.001 |
– | |
Difference between groups analyzed by t-test for parametric data and Pearson’s chi-squared test for categorical data, and Pearson’s correlation coefficient where applicable
LBPP low back pain and pelvic pain; Q1 first questionnaire; Q2 second questionnaire
a‘No pain’ denotes respondents reporting remission of LBPP after pregnancy
b‘Recurrent pain’ denotes respondents reporting recurrent LBPP after pregnancy
c‘Continuous pain’ denotes respondents reporting continuous LBPP after pregnancy
dNumber of subjects: in italics
eStatistical testing non-respondents versus respondents in last column
fAssessment of pain, i.e., of highest level of LBPP, scored on a Visual Analogue Scale, where end-point 0 denotes ‘no pain’ and 10 denotes ‘worst thinkable pain’
Table 4.
Odds ratios (OR) and their 95% confidence intervals (CI) for recurrent or continuous pain 6 months after delivery in relation to maximum level of pain during pregnancy due to LBPP
| No paina No. (%) | Recurrentb or continuous painc | ||
|---|---|---|---|
| Maximum level of pain during pregnancy | No. | Percent | |
| 0–2 | 25 (10.3) | 6 | 3.1 |
| >2–4 | 49 (20.2) | 23 | 11.9 |
| >4–6 | 75 (31.0) | 54 | 27.8 |
| >6–8 | 72 (29.8) | 76 | 39.1 |
| >8–10 | 21 (8.7) | 35 | 18.0 |
| Variable | Crude OR (n=436) | CI 95% | |
|---|---|---|---|
| 0–2 | 1.00 | – | |
| >2–4 | 1.96 | 0.70–5.43 | |
| >4–6 | 3.00 | 1.15–7.82 | |
| >6–8 | 4.40 | 1.71–11.35 | |
| >8–10 | 6.94 | 2.44–19.70 | |
| Adjusted for BMI | OR (n=419) | CI 95% | |
|---|---|---|---|
| 0–2 | 1.00 | – | |
| >2–4 | 1.61 | 0.57–4.57 | |
| >4–6 | 2.80 | 1.06–7.36 | |
| >6–8 | 3.90 | 1.50–10.15 | |
| >8–10 | 6.39 | 2.24–18.25 | |
| Adjusted for maternal age | OR (n=436) | CI 95% | |
|---|---|---|---|
| 0–2 | 1.00 | – | |
| >2–4 | 1.73 | 0.61–4.87 | |
| >4–6 | 2.60 | 0.98–6.88 | |
| >6–8 | 4.25 | 1.63–11.10 | |
| >8–10 | 7.37 | 2.56–21.19 | |
| Adjusted for parity | OR (n=436) | CI 95% | |
|---|---|---|---|
| 0–2 | 1.00 | – | |
| >2–4 | 1.94 | 0.69–5.39 | |
| >4–6 | 2.91 | 1.11–7.61 | |
| >6–8 | 4.31 | 1.66–11.15 | |
| >8–10 | 6.72 | 2.36–19.11 | |
| Adjusted for BMI, parity, maternal age | OR (n=419) | CI 95% | |
|---|---|---|---|
| 0–2 | 1.00 | – | |
| >2–4 | 1.41 | 0.49–4.09 | |
| >4–6 | 2.39 | 0.89–6.40 | |
| >6–8 | 3.79 | 1.43–10.01 | |
| >8–10 | 6.71 | 2.30–19.54 | |
LBPP low back pain and pelvic pain
a‘No pain’ denotes respondents reporting remission of LBPP after pregnancy
b‘Recurrent pain’ denotes respondents reporting recurrent LBPP after pregnancy
c‘Continuous pain’ denotes respondents reporting continuous LBPP after pregnancy
Fig. 3.
Highest level of pain during pregnancy
Fig. 4.
Highest level of pain after pregnancy
Pre-pregnancy BMI, end-pregnancy BMI as well as BMI at 6 months after delivery were highly inter-correlated and were significantly increased in women with recurrent or continuous LBPP after pregnancy (Table 2). When categorizing BMI at 6 months after delivery the risk of persistent LBPP was almost significant for women with BMI≥25 in relation to women with BMI<25 (n=447; crude OR [COR], 1.42; 95% CI, 0.97–2.07) and the corresponding PAP was 15.8%. When adjusting for parity (n=447; OR, 1.41; 95% CI, 0.96–2.06) and hyper-mobility (n=441; OR, 1.43; 95% CI, 0.97–2.10) the risk was similar.
A total of 83 (18.1%) women reported that they had been diagnosed as having hyper-mobile joints (Table 1). The proportion of women reporting diagnosis of having hyper-mobility and/or perceived hyper-mobility was significantly increased among women with persistent LBPP after pregnancy (Table 1). The risk of persistent LBPP was increased if reported hyper-mobility (n=458; crude OR [COR], 1.56; 95% CI, 1.01–2.40) and the corresponding PAP was 11.9%. The risk estimate was only slightly corrected when adjusting for BMI (n=441; OR, 1.58; 95% CI, 1.02–2.46) and maternal age (n=458; OR, 1.66; 95% CI, 1.07–2.59).
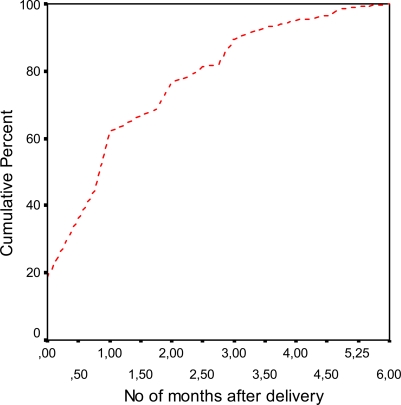
Assessment of ‘total experience of the delivery’ was measured both in the Q1 and in the Q2 and was highly correlated in the different groups (Table 1). Time at remission of LBPP after pregnancy is shown in Fig. 5 with a mean value of 1.36 months (median value=1.00 month; SD=1.35; n=229).
Fig. 5.
Time point at remission of LBPP after pregnancy
Validity of the data, and non-participant data
The validity of the data in Q1 has been extensively discussed in previous publications [12–14]. The sample of eligible women at delivery was 1,071 women and the participation rate in the Q1 was 83.2%. The non-respondents were of the same age, had the same number of pregnancies and births and were delivered by the same methods (Q1) as the respondents [14]. Pre-term births were more frequent among non-respondents (Q1) [14]. Consistency of the responses on different issues was evaluated. Cohen’s kappa was calculated investigating the first and second set of answers (Q1) and there was total agreement on questions on the women’s birth year, date of delivery, birth weight, method of delivery, and educational level [14].
The number of eligible subjects in the Q2 was 639 women reporting LBPP during pregnancy. The response rate was 72.6% (464/639), and the proportion of non-respondents was therefore 27.4%. There were no statistically significant differences (t-test or Pearson’s chi-squared test where appropriate) between respondents and non-respondents with regard to maternal age, maternal height, gestational age, birth weight, mode of delivery, onset of pain due to LBPP during pregnancy, total experience of the delivery, hyper-mobility, and pre-pregnancy or end-pregnancy BMI (Tables 1 and 2). Non-respondents had significantly more pregnancies and deliveries, and had had a higher level of pain due to LBPP during pregnancy (Q1), this group also included a lower proportion of university-educated women than the respondents (Table 1).
For evaluation of the consistency of the responses in the questionnaire (Q2) some respondents were asked to fill in the questionnaire a second time. Women were asked (by telephone) to redo the questionnaire and if they reacted positively to the request, a second questionnaire was sent by post within approximately 2–3 weeks of collection of the primary Q2. Twenty women completed the Q2 a second time. The intra-class correlation coefficient (single measure, consistency definition) was 0.90 (95% CI 0.75–0.96; n=20) for total experience of the delivery (Q2); 0.83 (95% CI 0.61–0.94; n=19) for experience of highest level of pain due to LBPP after pregnancy; 0.64 (95% CI 0.14–0.88; n=12) for experience of highest level of pain due to LBPP after pregnancy the previous week; and 0.99 (95% CI 0.98–1.00; n=18) for actual weight.
Discussion
In the current study LBPP was prevalent at 6 months after delivery. A small percentage of women (6.9%) experienced continuous LBPP while the majority of women with LBPP after pregnancy (36.2%) suffered from recurrent LBPP. The level of persistent LBPP after pregnancy was similar to that reported in other studies [18, 20, 26, 27]. In most respects but not all, women with recurrent and continuous LBPP had similar characteristics.
The etiology of LBPP during pregnancy and persistent LBPP after pregnancy is still obscure [27]. The life-time incidence of low back pain among Swedish women has been estimated to 66% [24] and several Swedish studies show a high prevalence of LBPP during pregnancy [9, 14, 19]. Maternal ethnicity has not been shown to influence prevalence of LBPP during pregnancy [27]. Remission of LBPP after pregnancy principally occurs during the first half year after delivery [18, 21] and it has been estimated that 5% of all women post partum suffer from persistent lumbopelvic pain requiring medical help [27]. The main risk factors for persistent back pain after pregnancy are the presence of back pain before and during pregnancy, and engaging in physically heavy work [18, 22, 25]. However, as previously pointed out, back pain in women post-partum is not a unitary concept [15]. The respondents were divided into three groups of level of pain at 6 months post partum related to LBPP after pregnancy: ‘no pain’ (i.e. remission of LBPP), ‘recurrent pain’ and ‘continuous pain’. The objective was to create a tool, which would reflect the severity of the condition and thus make possible an assessment of grade of risk.
One of the main findings in this study is the importance of BMI as a determinant of persistent LBPP after pregnancy. Women with persistent LBPP after pregnancy had significantly higher weight (Q2), and higher pre-pregnancy and end-pregnancy BMI (Q1) as well as higher BMI at 6 months after delivery (Q2) than did women with remission of LBPP after pregnancy. Greater weight has previously been associated with back pain 1–2 months post-partum [6]. Among women with recurrent LBPP the proportion of women reporting problems with actual or previous overweight in the Q1 was significantly increased, which was almost the case with women with continuous LBPP. In a current publication [12] perceived actual or previous overweight was seen to be a significant risk factor for LBPP during pregnancy. When categorizing BMI and calculating OR in logistic regression analyses the estimates were almost significant which would have probably been the case given the material had been larger. The calculated PAP reached almost 16% supporting weight as a prominent determinant for persistent LBPP after pregnancy. Since weight is strongly related to nutritional intake and physical activities, lifestyle changes resulting in decreased weight may probably reduce the prevalence of LBPP both during and after pregnancy.
A proportion of 24.2% of the respondents were diagnosed as having hyper-mobile joints and/or perceived themselves to be hyper-mobile. Women with persistent LBPP after pregnancy included a significantly higher proportion of women with these characteristics (P=0.042). PAP, which denotes the proportion of cases that would not have occurred if the factor under investigation would not have exerted any influence on the condition, was almost 12%, thus confirming hyper-mobility as a significant factor contributing to persistent LBPP after pregnancy. Changes in muscle activity and motor coordination have previously been observed in women with LBPP [27]. Persistent lumbar pain as well as pelvic pain 3 years after pregnancy has been concluded to probably be caused by insufficiency in the large pelvic and dorsal muscles [17]. The findings in the current study indicates that strain on the joints, ligaments and muscles due to increased weight and existing hyper-mobility may impede or delay remission of LBPP after pregnancy.
As mentioned in the Introduction, results from this cohort (Q1) have previously been published [12–14]. Parity, a major determinant of LBPP during pregnancy [14] was not a determinant of LBPP after pregnancy, since there was no significant difference in parity between women with remission of LBPP, and women with persistent LBPP after pregnancy. A review confirms this finding [27].
Associations between post-term deliveries, heavier newborns and risk of pelvic pain and pelvic joint instability have been found previously [23]; however, in the current study there was no association with gestational age or birth weight, and these findings are consistent with the findings of others [25]. Maternal age at delivery was significantly increased among women with persistent LBPP after pregnancy, which has been previously reported [25]. Onset of pain during pregnancy was significantly earlier in women with persistent LBPP, which also confirms previous results [25].
Methodological considerations
Pelvic pain is the most important clinical problem among spine disorders during pregnancy. The aim of the current project was mainly to study pelvic pain during and after pregnancy, however, lumbal pain symptoms could not be excluded since the referral pain sites for lumbal pain interfere with the anatomical location of the pelvic joints and the study was based on self-reported data extracted from questionnaires and not from clinical examinations. However, since lumbal pain is most commonly stable whether pelvic pain increases [19] the determinants and outcomes studied are mostly related to pelvic pain during pregnancy.
Evaluation of data is previously presented in the paper. The prevalence of persistent LBPP (recurrent or continuous) may have been adjusted if the non-respondents had in fact responded to the Q2. Assuming that the non-respondents had all been categorized into the ‘no pain’ group the prevalence of persistent LBPP would have been 31.3%. However, in analysis of respondents there was a positive association between assessment of highest level of pain during pregnancy and risk of recurrent pain and continuous pain after pregnancy. The non-respondents had a significantly higher level of assessed pain during pregnancy, which indicates that the true prevalence of persistent LBPP may have been higher than the calculated prevalence (43.1%). The educational level was lower among the non-respondents; however, in the previous paper educational level only expressed a moderate effect on risks [14].
The response time of the questionnaire was around 6 months on average for all respondents and there were no significant differences between groups. Recall bias is an important issue to consider when conducting retrospective data collection. In the current study the recall period covered 6 months with probable vital events during all this period. However, assessment of ‘total experience of the delivery’ and ‘highest level of pain due to LBPP during pregnancy’ in the Q1 and Q2 was highly correlated indicating that experience and assessment of delivery and pain were fairly constant among the respondents during the whole study period.
A 5% significance level (95% CI) was applied in all tests. Having done 25 statistical calculations between study groups, we are aware of the risk that at least one of them might incorrectly have been significant, the so-called mass-significance problem.
Conclusions
In summary, recurrent or continuous LBPP is prevalent after pregnancy. BMI 6 months after pregnancy is highly correlated to pre-pregnancy and end-pregnancy BMI. Due to the PAPs, BMI as well as hyper-mobility are prominent determinants for persistent LBPP after pregnancy, and pre-pregnancy reduction of overweight would probably reduce the prevalence of LBPP during and after pregnancy. Level and onset of LBPP during pregnancy were strong predictors for persistent LBPP. Parity, gestational age, and birth weight did not influence the risk of persistent LBPP after pregnancy.
Acknowledgments
The author is grateful to Anna Pohjanen, gynecologist at the Department of Obstetrics and Gynecology, Sunderby Hospital for good collaboration on data collection; and the project assistant, Anita Nilsson, for the excellent assistance. The author would like to thank all women participants for sharing their obstetric history and experiences during pregnancy and after delivery. This study was supported by grants from the County Council of Västerbotten, the Joint Committee of the Northern Sweden Health Care Region, and the Medical Faculty of Umeå University.
Glossary
- Q1
The first questionnaire after delivery
- Q2
The second questionnaire at approximately 6 months after delivery
- SD
Standard deviation
- VAS
Visual analogue scale
References
- 1.Albert H, Godskesen M, Westergaard J. Prognosis in four syndromes of pregnancy-related pelvic pain. Acta Obstet Gynecol Scand. 2001;80:505–510. doi: 10.1034/j.1600-0412.2001.080006505.x. [DOI] [PubMed] [Google Scholar]
- 2.Berg G, Hammar M, Moller-Nielsen J, Linden U, Thorblad J. Low back pain during pregnancy. Obstet Gynecol. 1988;71:71–75. [PubMed] [Google Scholar]
- 3.Biering-Sorensen F. Low back trouble in a general population of 30-, 40-, 50-, and 60-year-old men and women. Study design, representativeness and basic results. Dan Med Bull. 1982;29:289–299. [PubMed] [Google Scholar]
- 4.Biering-Sorensen F. A prospective study of low back pain in a general population. I. Occurrence, recurrence and aetiology. Scand J Rehabil Med. 1983;15:71–79. [PubMed] [Google Scholar]
- 5.Bjorklund K, Naessen T, Nordstrom ML, Bergstrom S. Pregnancy-related back and pelvic pain and changes in bone density. Acta Obstet Gynecol Scand. 1999;78:681–685. doi: 10.1034/j.1600-0412.1999.780804.x. [DOI] [PubMed] [Google Scholar]
- 6.Breen TW, Ransil BJ, Groves PA, Oriol NE. Factors associated with back pain after childbirth. Anesthesiology. 1994;81:29–34. doi: 10.1097/00000542-199409001-00028. [DOI] [PubMed] [Google Scholar]
- 7.Fast A, Shapiro D, Ducommun EJ, Friedmann LW, Bouklas T, Floman Y. Low-back pain in pregnancy. Spine. 1987;12:368–371. doi: 10.1097/00007632-198705000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Hansen A, Jensen DV, Wormslev M, Minck H, Johansen S, Larsen EC, Wilken-Jensen C, Davidsen M, Hansen TM. [Pregnancy associated pelvic pain. II: Symptoms and clinical findings] Ugeskr Laeger. 2000;162:4813–4817. [PubMed] [Google Scholar]
- 9.Kristiansson P, Svardsudd K, Schoultz B. Back pain during pregnancy: a prospective study. Spine. 1996;21:702–709. doi: 10.1097/00007632-199603150-00008. [DOI] [PubMed] [Google Scholar]
- 10.Larsen EC, Wilken-Jensen C, Hansen A, Jensen DV, Johansen S, Minck H, Wormslev M, Davidsen M, Hansen TM. Symptom-giving pelvic girdle relaxation in pregnancy. I: Prevalence and risk factors. Acta Obstet Gynecol Scand. 1999;78:105–110. doi: 10.1034/j.1600-0412.1999.780206.x. [DOI] [PubMed] [Google Scholar]
- 11.Mens JM, Vleeming A, Stoeckart R, Stam HJ, Snijders CJ. Understanding peripartum pelvic pain. Implications of a patient survey (discussion 1369–1370) Spine. 1996;21:1363–1369. doi: 10.1097/00007632-199606010-00017. [DOI] [PubMed] [Google Scholar]
- 12.Mogren I (2006) Perceived health, sick leave, psychosocial situation, and sexual life in women with low back pain and pelvic pain during pregnancy. Acta Obstet Gynecol Scand (in press) [DOI] [PubMed]
- 13.Mogren I. Previous physical activity decreases the risk of low back pain and pelvic pain during pregnancy. Scand J Public Health. 2005;33:300–306. doi: 10.1177/140349480503300410. [DOI] [PubMed] [Google Scholar]
- 14.Mogren IM, Pohjanen AI. Low back pain and pelvic pain during pregnancy: prevalence and risk factors. Spine. 2005;30:983–991. doi: 10.1097/01.brs.0000158957.42198.8e. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson-Wikmar L, Harms-Ringdahl K, Pilo C, Pahlback M. Back pain in women post-partum is not a unitary concept. Physiother Res Int. 1999;4:201–213. doi: 10.1002/pri.166. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson-Wikmar L, Pilo C, Pahlback M, Harms-Ringdahl K. Perceived pain and self-estimated activity limitations in women with back pain post-partum. Physiother Res Int. 2003;8:23–35. doi: 10.1002/pri.269. [DOI] [PubMed] [Google Scholar]
- 17.Noren L, Ostgaard S, Johansson G, Ostgaard HC. Lumbar back and posterior pelvic pain during pregnancy: a 3-year follow-up. Eur Spine J. 2002;11:267–271. doi: 10.1007/s00586-001-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostgaard HC, Andersson GB. Postpartum low-back pain. Spine. 1992;17:53–55. doi: 10.1097/00007632-199201000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Ostgaard HC, Andersson GB, Karlsson K. Prevalence of back pain in pregnancy. Spine. 1991;16:549–552. doi: 10.1097/00007632-199105000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Ostgaard HC, Roos-Hansson E, Zetherstrom G. Regression of back and posterior pelvic pain after pregnancy. Spine. 1996;21:2777–2780. doi: 10.1097/00007632-199612010-00013. [DOI] [PubMed] [Google Scholar]
- 21.Ostgaard HC, Zetherstrom G, Roos-Hansson E. Back pain in relation to pregnancy: a 6-year follow-up. Spine. 1997;22:2945–2950. doi: 10.1097/00007632-199712150-00018. [DOI] [PubMed] [Google Scholar]
- 22.Russell R, Dundas R, Reynolds F. Long term backache after childbirth: prospective search for causative factors. BMJ. 1996;312:1384–1388. doi: 10.1136/bmj.312.7043.1384a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saugstad LF. Is persistent pelvic pain and pelvic joint instability associated with early menarche and with oral contraceptives? Eur J Obstet Gynecol Reprod Biol. 1991;41:203–206. doi: 10.1016/0028-2243(91)90025-G. [DOI] [PubMed] [Google Scholar]
- 24.Svensson HO, Andersson GB, Hagstad A, Jansson PO. The relationship of low-back pain to pregnancy and gynecologic factors. Spine. 1990;15:371–375. doi: 10.1097/00007632-199005000-00006. [DOI] [PubMed] [Google Scholar]
- 25.To WW, Wong MW. Factors associated with back pain symptoms in pregnancy and the persistence of pain 2 years after pregnancy. Acta Obstet Gynecol Scand. 2003;82:1086–1091. doi: 10.1046/j.1600-0412.2003.00235.x. [DOI] [PubMed] [Google Scholar]
- 26.Turgut F, Turgut M, Cetinsahin M. A prospective study of persistent back pain after pregnancy. Eur J Obstet Gynecol Reprod Biol. 1998;80:45–48. doi: 10.1016/S0301-2115(98)00080-3. [DOI] [PubMed] [Google Scholar]
- 27.Wu WH, Meijer OG, Uegaki K, Mens JM, Dieen JH, Wuisman PI, Ostgaard HC. Pregnancy-related pelvic girdle pain (PPP). I: Terminology, clinical presentation, and prevalence. Eur Spine J. 2004; : 575–589. doi: 10.1007/s00586-003-0615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]