Abstract
Glucocorticosteroid-induced osteoporosis (GIOP) is the most frequent of all secondary types of osteoporosis. The understanding of the pathophysiology of glucocorticoid (GC) induced bone loss is of crucial importance for appropriate treatment and prevention of debilitating fractures that occur predominantly in the spine. GIOP results from depressed bone formation due to lower activity and higher death rate of osteoblasts on the one hand, and from increased bone resorption due to prolonged lifespan of osteoclasts on the other. In addition, calcium/phosphate metabolism may be disturbed through GC effects on gut, kidney, parathyroid glands and gonads. Therefore, therapeutic agents aim at restoring balanced bone cell activity by directly decreasing apoptosis rate of osteoblasts (e.g., cyclical parathyroid hormone) or by increasing apoptosis rate of osteoclasts (e.g., bisphosphonates). Other therapeutical efforts aim at maintaining/restoring calcium/phosphate homeostasis: improving intestinal calcium absorption (using calcium supplementation, vitamin D and derivates) and avoiding increased urinary calcium loss (using thiazides) prevent or counteract a secondary hyperparathyroidism. Bisphosphonates, particularly the aminobisphosphonates risedronate and alendronate, have been shown to protect patients on GCs from (further) bone loss and to reduce vertebral fracture risk. Calcitonin may be of interest in situations where bisphosphonates are contraindicated or not applicable and in cases where acute pain due to vertebral fracture has to be managed. The intermittent administration of 1-34-parathormone may be an appealing treatment alternative, based on its documented anabolic effects on bone resulting from the reduction of osteoblastic apoptosis. Calcium and vitamin D should be a systematic adjunctive measure to any drug treatment for GIOP. Based on currently available evidence, fluoride, androgens, estrogens (opposed or unopposed) cannot be recommended for the prevention and treatment of GIOP. However, substitution of gonadal hormones may be indicated if GC-induced hypogonadism is present and leads to clinical symptoms. Data using the SERM raloxifene to treat or prevent GIOP are lacking, as are data using the promising bone anabolic agent strontium ranelate. Kyphoplasty performed in appropriately selected osteoporotic patients with painful vertebral fractures is a promising addition to current medical treatment.
Keywords: Glucocorticosteroids, Osteoporosis, Pathophysiology, Bisphosphonates, Parathormone
Introduction
Glucocorticosteroid-induced osteoporosis (GIOP) is the most common type of iatrogenic osteoporosis and also the most frequent of all secondary types of osteoporosis [21, 140]. The side effects on bone are the most predictable and debilitating complication of prolonged administration of systemic corticosteroids. Nevertheless, for many clinical situations there are only limited alternatives to long-term use of glucocorticosteroids (GCs). The thorough understanding of the underlying mechanisms leading to GIOP serves as the basis for developing and applying preventive and therapeutic treatment strategies. The aim of this review is to highlight the most recent findings regarding the pathophysiology of GC-induced bone loss and to document their role with regard to the development and implementation of current or future treatment options.
Pathogenesis of glucocorticoid-induced osteoporosis
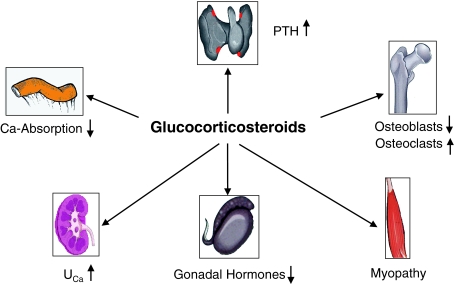
In GIOP, bone loss and the later development of osteoporosis with its well-known crippling consequences of disability and fractures, result from several intricate and interacting mechanisms (Fig. 1).
Fig. 1.
Pathophysiology of glucocorticosteroid-induced osteoporosis. Glucocorticosteroids exert direct deleterious effects on bone leading to decreased formation and increased resorption. In addition, bone loss may be indirectly promoted by reduced gonadal hormone levels and by myopathy as well as by decreased calcium absorption from the gut and increased renal calcium excretion. However, secondary hyperparathyroidism is an inconstant finding
GCs depress bone formation by inhibiting osteoblastogenesis and increasing osteoblast apoptosis [89, 146]. Furthermore, GCs induce the in situ death of isolated segments of bone (osteonecrosis), possibly in relation to an increased osteocyte apoptosis [89, 146] suggesting that GC-induced bone loss may arise from a numerical decrease in bone cells. In addition, GCs may further exacerbate the numerical decrease in osteoblasts by altering the differentiation of stromal cells towards the production of adipocytes rather than osteoblasts, an effect which may be related to the suppression of bone morphogenic protein (BMP)-2 by GCs [90, 91]. Furthermore GCs increase the expression of Notch1 and 2 in osteoblasts, a family of receptors considered to play a negative role in osteoblastic cell differentiation [92], and may suppress IGF-1 secretion and thereby inhibit important stimulatory effects on bone protein synthesis [27, 79]. Finally, GCs induce modifications of the functional characteristics of osteoblasts [76] by directly suppressing their activity and thereby bone matrix synthesis [18, 104].
In the early phase of GC treatment, bone resorption is increased, a recent finding which has been associated with a prolongation of the lifespan of osteoclasts, mediated by a GC-stimulated expression of RANK-ligand [76, 77, 145, 147]. In vitro and animal models have shown that GCs prolong the baseline survival of osteoclasts and antagonize their apoptosis by a glucocorticoid receptor-mediated action [147].
Furthermore, GCs induce a dose-dependent inhibition of calcium (and possibly phosphate) absorption in the gut and a compensatory increase in parathormone secretion [35, 47], an increase in renal calcium elimination [17, 106], a reduction of the sex hormone levels through inhibition of gonadotropin secretion [122] and they directly interfere with estrogen, testosterone and adrenal androgen production [25, 75].
Although the endocrine effects on bone of GC therapy contribute to or may even accelerate the development of GIOP, the core pathogenetic processes affect the bone effector cells. Many of the effects of chronic GC administration on bone can be explained by decreased birth of osteoblast and osteoclast precursors and increased apoptosis of mature osteoblasts and osteocytes, disrupting the fine balance between these processes. It is therefore expected that therapeutic agents that alter the prevalence of apoptosis of osteoblasts and osteoclasts should be able to correct the imbalance in cell numbers that is the basis of the diminished bone mass and increased risk of fractures found in glucocorticoid-induced osteoporosis [144].
Characteristics of GC-induced bone loss
Long-term administration of GCs induces a rapid loss of bone mass of between 5 and 15% annually [36, 105]. Histomorphometric [70] as well as densitometric [126] studies have shown that GC-induced bone loss is most pronounced during the first 3–12 months of therapy, but continues as long as treatment is maintained.
The demineralization is more pronounced in trabecular than in cortical bone compartments [4, 45, 128] and not all regions of the skeleton are affected alike. In a study by Sambrook et al. [126], after 20 weeks of treatment with prednisone (mean daily dose of 7.5 mg) the average loss of bone density in the lumbar spine was 8% in heart transplant patients. In a longitudinal histomorphometric study of LoCascio et al. [69], the treatment with prednisone (10–25 mg) over 5–7 months resulted in a reduction of 27% of the trabecular bone volume in the crista iliaca.
Not all patients treated with GCs are similarly affected [105]. Differences are possibly genetically determined and could be related to variants of the steroid receptor and individual pharmacokinetic differences. For example, it has been shown that the reductase activity of 11 beta-hydroxysteroid dehydrogenase 1, which converts inactive cortisone to active cortisol, is increased in elderly subjects, which could explain the susceptibility of these patients to the debilitating effects of GCs [22]. In addition, the synthesis and activity of this enzyme are GC dependent so that administration of GCs could possibly amplify their own effectiveness [22]. The response of bone formation markers to GCs can be predicted by the urinary measure of this enzyme, a recent finding which may contribute to the identification of individuals at highest risk of developing GIOP [23].
Total bone mineral loss correlates directly with the cumulatively given steroid dose [148]. Although 7.5 mg of prednisone equivalent a day was considered to be the threshold dose for skeletal side effects [123, 124], recently published data have shown that lower doses and even inhaled GCs may induce skeletal side effects [68]. In children under low-dose inhaled steroids even impaired growth has been demonstrated [68, 98]. In adults under high-dose inhaled GCs, a dose-dependent reduction of bone density has been observed [54], and the cumulative dose of inhaled corticosteroids in adult asthmatics was shown to correlate negatively with bone density [149]. Biochemical markers of bone formation and resorption also were significantly suppressed under inhaled corticosteroids, even with a short duration of treatment [84].
Giving GCs every second day, or using pulse therapy, seems to have a favourable effect on growth and on the suppression of the hypothalamus–hypophysis–suprarenal axis but without eliminating entirely the unwanted skeletal side effects [40, 120]. Considering the dose dependence of skeletal side effects of systemic and inhaled corticosteroids, the lowest effective dose should be used.
There are only marginal differences in the extent of bone mineral loss between the different types of corticosteroids. An exception may be the oxazolin derivative deflazacort. Several trials have demonstrated that this synthetic steroid has fewer side effects on bone and mineral metabolism compared to prednisone [35, 67, 70, 81]. However, these studies were based on an assumption that the potency of prednisone relative to deflazacort was 1.2. Recently published studies suggest that the potency of deflazacort has been overestimated in the past. Using a relative potency of 1.4–1.8 there may be no bone-sparing effect of this agent [61].
Bone loss induced by corticosteroid therapy is only partly reversible, as observations after successful treatment of Cushing’s disease [97] or after discontinuing corticosteroid treatment [48, 117] show.
Although not all patients treated with GCs develop GIOP and not all patients with GIOP experience fractures, bone loss remains the most predictable, the most frequent and potentially the most severe complication of GC treatment. The appropriateness of bone protective measures should be evaluated in all patients treated with GCs or in whom such a therapy will be initiated.
Glucocorticosteroid treatment and fractures
Fracture risk increases in a dose-dependent manner in patients treated with oral GCs [71]. In a prospective study of asthmatics under long-term treatment with oral corticosteroids (average duration of therapy 8 years), the cumulative fracture incidence amounted to 40% [4], while other authors reported a fracture incidence as high as 50% [72, 83]. In a large retrospective cohort study, oral treatment with GCs over a mean period of 1.3 years significantly increased the risk of non-vertebral and vertebral fractures by 1.3 and 2.6, respectively. At doses higher than 7.5 mg prednisone or equivalent during an average 0.7 years, the risk for hip and vertebral fractures was increased by 2.3 and 5.2, respectively, while this increase was lower but still significant for lower doses of prednisone (<2.5 mg/day or equivalent) over a mean of 2.8 years, with relative risk (RR) increases for hip and vertebral fractures of 1.2 and 1.6, respectively [137]. These results suggested that the adverse skeletal effects of GCs increased with increasing daily doses [138]. In addition, fracture risk was shown to increase rapidly within the first 3–6 months after initiation of therapy with GCs and to remain increased during the whole duration of treatment [140].
In patients with chronic obstructive pulmonary disease (COPD) treated with inhaled GCs for median 2.7 years, an increase in hip fracture risk was shown in a large population based case-control study [53]. However, COPD is in itself a potential confounding factor associated with an increased risk of osteoporosis due to a variety of disease-related factors, such as poor health, poor nutrition and tobacco consumption [139]. In contrast, a recent systematic review concluded that conventional doses of inhaled GCs for 2–3 years did not increase the risk of vertebral fractures [56]. However, the patients included in the selected trials were at low risk for osteoporosis and were not treated with high doses of inhaled GCs.
In GIOP, vertebral body fractures may occur at higher bone density values than usually observed in post-menopausal osteoporosis [71, 141]. This observation may be at least in part explained by an excessive and rapid thinning of the trabeculae induced by GCs [132], which leads to lower bone strength at identical bone mineral density (BMD) values when compared to post-menopausal osteoporosis. The BMD threshold for the diagnosis of GIOP is therefore generally higher than the T-score threshold of −2.5 standard deviations usually accepted for primary osteoporosis and has been set at −1.0 SD by the American College of Rheumatology [5], while other countries and societies have chosen higher thresholds [32] and/or approaches based on clinical risk [38, 87, 142].
Although the underlying disease, which is the reason for the prescription of GCs, may in itself bear characteristics promoting the development of osteoporosis, GC therapy is an amplifier of the deleterious effects on bone and leads to increased fracture risk.
Treatment of GIOP
Any patient treated with GCs or in whom treatment with GCs will be initiated should be assessed for the presence of modifiable risk factors of osteoporosis, such as alcohol intake, smoking, insufficient physical activity, low dietary calcium intake, and all efforts should be undertaken to correct or eliminate these risk factors. In addition risk factors for fractures and falls, such as advanced age, orthostatic hypotension, treatment with hypnotic drugs, should be identified and appropriately managed. Any other causes of secondary osteoporosis such as hypogonadism, hyperparathyroidism or thyrotoxicosis may potentiate the effects of GCs on bone and contribute to an even more rapid bone loss after the initiation of treatment with GCs (Tables 1, 2, 3).
Table 1.
Pathophysiological rationale for use in the prevention and/or treatment of GIOP
| Therapeutic agents | Rationale for use in GIOP | Proposed mechanism of action |
|---|---|---|
| Calcium, vitamin D and analogues | Decreased calcium absorption in the gut | Counter GC-induced secondary hyperparathyroidism |
| Increased urinary elimination of calcium | ||
| Bisphosphonates | Increased lifespan of osteoclasts | Specific inhibition of bone resorption |
| Decreased lifespan of osteoblasts and osteocytes | Reverse the increased osteoblast and osteocyte apoptosis | |
| Calcitonin | Early increase in bone resorption | Specific inhibitor of osteoclast function |
| Parathormone (intermittent teriparatide) | Increased osteoblast apoptosis | Increases the lifespan of osteoblasts |
| Fluoride | Increased osteoblast apoptosis | Potent osteoblast mitogen |
| Androgens/estrogens (opposed or unopposed) | Decreased sex hormone levels through inhibition of gonadotropin secretion and impairment of androgen production | Increase levels of circulating testosterone/estrogen |
Table 2.
Treatment effects of bone active substances in GIOP
| Therapeutic agents | Effect on BMD | Fracture risk in GIOP patients | ||
|---|---|---|---|---|
| LS | Hip | Vertebral | Non-vertebral | |
| Calcium, vitamin D and analogues | 0 | − | ? | ? |
| Bisphosphonates (alendronate, risedronate) | + | + | − | ? |
| Calcitonin | 0 | − | ? | ? |
| Parathormone (intermittent teriparatide) | + | ? | ? | ? |
| Fluoride | + | 0/− | + ? | + |
| Androgens | + | ? | ? | ? |
| Estrogens (opposed or unopposed) | + | − | ? | ? |
+ increase, 0 maintained, − decreased, ? unknown, LS lumbar spine
Table 3.
Recommendation for use in the prevention and treatment of GIOP by therapeutic class
| Therapeutic agents | Recommendation for use in GIOP |
|---|---|
| Calcium, vitamin D and analogues | Systematic adjunctive measure to any drug treatment, whether dietary intake is sufficient or not |
| Bisphosphonates (alendronate, risedronate) | Gold standard, first line choice |
| Calcitonin | If bisphosphonates are contraindicated or for acute pain management of vertebral fracture |
| Parathormone (intermittent teriparatide) | Data expected, no recommendation possible at this stage |
| Fluoride | No (increased risk for non-vertebral fractures) |
| Androgens | No (paucity of data) |
| Estrogens (opposed or unopposed) | No (paucity of data, trade-off between risks and benefits) |
Based on the pathophysiology of GIOP, several treatment approaches have been proposed to slow down or stop bone loss and decrease subsequent fracture risk. Current treatment options aim at restoring calcium balance and countering secondary hyperparathyroidism (calcium supplementation, vitamin D and derivates, thiazides), at inhibiting bone resorption (bisphosphonates, calcitonin, estrogens), at improving bone formation (fluoride, parathormone) or at exerting anabolic effects on bone (parathormone, androgens).
Calcium, vitamin D and thiazide diuretics
Glucocorticoid treatment results in decreased calcium absorption in the gut and increased urinary calcium elimination leading to a secondary increase in parathormone. Interventions aiming at restoring calcium balance and at countering secondary hyperparathyroidism have therefore been used as an empiric therapy for osteoporosis of various aetiologies for several decades. Calcium and vitamin D supplementation may contribute to increased intestinal calcium absorption and counter the mild secondary hyperparathyroidism resulting from GC induced decreased calcium intestinal absorption and increased calcium renal excretion [46]. Several authors have investigated their effects on negative calcium balance occurring under corticosteroid therapy [9, 46, 134].
In a recent meta-analysis, the efficacy of active vitamin D3 analogues in preserving bone and in decreasing the risk of vertebral fractures was shown to be significantly higher than no treatment, placebo and plain vitamin D3 with or without concomitant calcium [28]. Based on changes in lumbar spine BMD, calcitriol (mean dose 0.6 μg/day), with and without calcium, was significantly more efficacious than calcium in preventing bone loss (−0.2 and −1.3% per year vs. −4.3% per year, P=0.0035) but had no effect at other clinically important sites such as femoral neck and distal radius. Similar results were reported by other authors [125] and with other active vitamin D metabolites such as alfacalcidol [13, 101, 112, 113].
The variability of the outcomes in published clinical studies to date makes it difficult to draw conclusions concerning recommendations for the use of vitamin D and its metabolites in patients under GC treatment, though recent data suggest that active analogues of vitamin D combined with calcium supplements are helpful and cost effective [14]. Calcium or vitamin D alone or in combination has not been proven to stop or prevent GC-induced bone loss and even less so to prevent GIOP-related fractures in prospective randomized trials. In the absence of contraindications, they should however be considered as a mandatory supplementation to the diet and as a systematic adjunctive measure to any drug treatment against GIOP, whether the patient has calcium and/or vitamin D deficiency or not.
In patients treated with GCs, hydrochlorothiazide (25 mg b.i.d.) in combination with sodium restriction was shown to increase intestinal calcium absorption and to lower renal calcium excretion and thus to lower PTH levels [111, 134]. However, the combination with vitamin D may lead to increased risk of hypercalcaemia and to worsening of the GC-induced hypokaliemia. While the effects of thiazide diuretics on bone are poorly documented in clinical trials, they may be considered based on their mechanism of action for patients treated with GCs who need additional antihypertensive treatment [111].
Bisphosphonates
Glucocorticoid treatment is associated with an increased lifespan of the osteoclasts and a decreased lifespan of osteoblasts and osteocytes. Bisphosphonates are specific inhibitors of osteoclast-mediated bone resorption and have been shown in vitro to reverse the increase in osteocyte and osteoblast apoptosis caused by glucocorticoids [96]. Bisphosphonates are the best documented therapeutic class for the prevention [1, 12, 20, 29, 34, 41, 85, 119] and the treatment [2, 16, 37, 51, 95, 107, 110, 121] of GIOP and have shown consistent efficacy in preserving bone mass and/or in preventing fractures in very heterogeneous patient populations of both genders, for variable durations and dosages of GC treatment, for various underlying diseases as reasons for the prescription of GCs and across the individual compounds.
Cyclical administration of etidronate prevents bone loss caused by GC treatment. In post-menopausal women with newly started GC therapy, etidronate given for 2 weeks every 3 months increased lumbar spine BMD over 1 year by 1.4% while a decrease of 5% was shown in the control group treated with calcium alone [85]. In another study in post-menopausal women, the cyclic administration of etidronate in combination with the vitamin D metabolite ergocalciferol increased lumbar spine and femoral neck BMD by 7 and 2.5% respectively, in the first year of GC therapy, while in the second year BMD continued to increase at the femoral neck and remained stable at lumbar spine [29]. Furthermore, in a randomized placebo-controlled trial, 141 men and women recently started on GC therapy were treated for 1 year with either etidronate (400 mg/day) or placebo for 14 days, followed by calcium (500 mg/day) for 76 days: with etidronate bone density increased significantly at lumbar spine and trochanter, but not at the femoral neck [1]. Similar results were reported in another study with 117 patients followed over 1 year [119]. Cyclical etidronate has also been documented for the treatment of GIOP. In a 2-year study in patients treated with GCs for at least 6 months and with low BMD, lumbar spine BMD significantly increased after 6 months of treatment with etidronate and remained stable for the remaining 18 months [95]. These results were confirmed by another study in post-menopausal women with low BMD taking GCs for at least 3 months [37]. In a recently published study over 5 years, including 352 patients who had been taking GCs for at least 1 year for the treatment of asthma, cyclical etidronate caused a significant increase in lumbar spine BMD [16]. Although the results on BMD were consistent across studies, reduction of fracture risk could not be established with etidronate [1, 16].
Intravenous cyclical pamidronate in combination with a calcium supplement was shown to protect patients beginning GC therapy from GC-induced bone loss [11, 12] and to increase BMD in patients with low bone mass treated with GCs [107].
High-dose oral clodronate (2,400 mg/day) was shown to increase BMD at lumbar spine, femoral neck and trochanter in patients on long-term GC therapy for asthma over 12 months of treatment [51]. Intramuscular clodronate (100 mg) administered once weekly to patients with rheumatoid arthritis treated with GCs prevented BMD loss at the lumbar spine, the hip and total body while the patients treated with placebo experienced a continuous loss in bone mass over 4 years. The difference between the two groups was already significant after 12 months of treatment and a significant reduction in the incidence of vertebral fractures could be shown [34].
The effects of alendronate in preventing GIOP were first published in 1997 in a study in 30 patients starting GC therapy for sarcoidosis [41]. Half of the patients received a placebo and half alendronate (5 mg/day). A third group of patients required no GC treatment and served as a control group. In untreated patients BMD remained stable over the duration of the study (−0.6%), while BMD significantly decreased by 4.5% in the placebo group. Alendronate prevented the loss in BMD observed with GCs and increased BMD by 1.0% at 6 months and 0.8% after 1 year. These results were confirmed in a large, multicentre study in which all patients received at least 7.5 mg prednisone/day or its equivalent and in addition a placebo, 2.5, 5 or 10 mg alendronate/day [121]. After 48 weeks, BMD was significantly increased in the lumbar spine, trochanter and femoral neck in the groups receiving 5 or 10 mg alendronate/day, irrespective of the dose or previous duration of GC therapy. These results were confirmed in the 12-month extension that included 208 of the initially enrolled patients. During this extension the incidence of new vertebral fractures was significantly reduced with alendronate versus placebo (6.8% placebo vs. 0.7% pooled alendronate, P<0.05) [2].
The therapeutic effects of risedronate have been examined in two multicenter, randomized, double-blind, placebo-controlled, parallel-group studies of similar design including men and women all receiving calcium supplementation of 500–1,000 mg and most receiving vitamin D supplementation 400 IU daily: a prevention study (n=228) with patients treated for less than 3 months with GCs [20] and a treatment study (n=290) with patients treated with high-dose (prednisone ≥7.5 mg/day or equivalent) oral GCs for at least 6 months prior to enrolment [109]. In the prevention study, lumbar spine BMD was preserved versus baseline in both risedronate (2.5 or 5 mg/day) treatment groups. BMD values at the lumbar spine, the femoral neck and the trochanter were significantly increased versus placebo in the risedronate 5 mg daily treatment group (+3.8±0.8, +4.1±1.0 and +4.6±0.8%, respectively, P<0.001 for all) while no significant differences were observed in the risedronate 2.5 mg daily treatment group or in either group at the radius. A trend toward a decrease in the incidence of vertebral fractures was observed in the 5 mg risedronate group compared with the placebo group (5.7 vs. 17.3%; P=0.072) [20]. In the treatment study, risedronate 5 mg daily significantly increased BMD by an average of 2.9% at the lumbar spine, 1.8% at the femoral neck and 2.4% at the trochanter (P<0.05 for all), whereas BMD was only maintained in the control group. The incidence of vertebral fractures was reduced by 70% in the combined risedronate treatment groups versus placebo (P=0.042). These results were confirmed in the pooled analysis (n=518) of these two studies of similar design [143]. Altogether osteoporosis was present in 35%, osteopenia in 44% and a normal bone density in 21% of the patients. The percentage of patients with pre-existing spinal fractures was 34% (placebo), 28% (risedronate 2.5 mg/day) and 35% (risedronate 5 mg/day). The overall vertebral fracture risk reduction with risedronate 5 mg daily was 70% versus placebo (P=0.01).
Therefore, the bisphosphonates have demonstrated their ability to protect patients treated with GCs from (further) bone loss at all clinically relevant sites including the hip, and have proven their efficacy in reducing fracture risk in the axial skeleton. Based on the heterogeneity of patients included in the different studies, these results may be generalized to the patient population seen in daily medical practice [19].
Calcitonin
Calcitonin inhibits bone resorption through specific receptors located on the osteoclasts and bone resorption is increased under GC therapy, at least during the early phase of treatment.
In a recent systematic review of nine randomized controlled trials in GIOP [24] including 221 patients randomized to calcitonin and 220 to placebo, calcitonin was shown to be more effective than placebo at preserving bone mass at the lumbar spine after 6 and 12 months of therapy, but not at the femoral neck. There was no consistent effect of different dosages (50–100 IU compared to 200–400 IU) on spinal BMD, although the subcutaneous administration route showed a substantially greater effect than the intranasal route. With a relative risk (RR) of 0.71 (95% CI: 0.26–1.89) for vertebral and 0.52 (95% CI: 0.14–1.96) for non-vertebral fractures, the risk of fractures was not significantly different between calcitonin and placebo. In patients with steroid-dependent, chronic obstructive lung disease and associated steroid-induced osteoporosis, calcitonin was shown to reduce back pain significantly versus untreated controls [115, 116].
As calcitonin may preserve spinal but not femoral neck BMD in the first year of GC therapy and as its efficacy for fracture prevention in steroid-induced osteoporosis remains to be established [24], bisphosphonates should be preferred for the treatment and the prevention of GIOP. Calcitonin may be of interest in those patients where bisphosphonates are contraindicated or not applicable and to manage acute pain due to vertebral fracture.
Parathormone
Glucocorticoid therapy induces a secondary hyperparathyroidism as a response to negative calcium balance, and the continuous administration of synthetic human parathyroid hormone 1–34 has been shown to lead to a high bone turnover status in which bone resorption exceeds the capacity of bone formation, resulting in increased cortical porosity and increased bone fragility [136]. The administration of parathormone (PTH) to treat or prevent GIOP may therefore appear to be paradoxical at first sight.
While a sustained elevation of PTH was shown to stimulate bone resorption, an intermittent administration stimulated bone formation [8, 55]. In mice, intermittent administration of PTH increased the lifespan of mature osteoblasts thereby increasing osteoblast number, bone formation rate and bone mass, but did not affect osteoclasts. In contrast, sustained elevation of PTH did not affect osteoblast apoptosis but increased osteoclast number [8, 55]. Therefore, the antiapoptotic effect of PTH on osteoblasts is an appealing therapeutic concept in GC-induced osteoporosis, and may be considered as a causal counter measure against the deleterious effects of GCs on the osteoblasts [55].
The intermittent administration of PTH 1–34 leads to increased bone formation, with increased trabecular bone volume without loss of cortical bone volume [136]. Thus, intermittent and continuous PTH increase bone formation independently of effects on bone resorption, but only intermittent PTH increases bone mass consistently [52]. Such an intermittent administration of PTH 1–34 was shown to have a marked anabolic effect on the skeleton in patients on hormone replacement therapy (HRT), associated with a reduced incidence of vertebral fractures, despite increased bone turnover [65]. These early findings were confirmed by a prospective randomized placebo-controlled fracture endpoint study in 1,637 post-menopausal women with prior vertebral fractures who received a daily subcutaneous dose of recombinant human PTH 1–34 (teriparatide). The risk for new vertebral fractures was significantly reduced by up to 69%, while the risk of new non-vertebral fragility fractures was significantly reduced by up to 54% versus placebo. BMD was increased at lumbar spine and femoral neck but not at the radius [88]. This increase in BMD corresponds to an increase in bone formation known to occur on trabecular, endocortical and periosteal surfaces with intermittent daily teriparatide treatment. Consistently, an increase in vertebral size has been described in 51 post-menopausal women treated chronically with both glucocorticoids and HRT and randomized to either intermittent PTH 1–34 or placebo for 12 months. Since vertebral fracture risk is related to both bone size and bone mass, this increase in vertebral size has been proposed as part of the explanation of the fracture risk reduction associated with this therapy [103]. In estrogen replete post-menopausal osteoporotic women treated with glucocorticoids for inflammatory diseases, bone formation (bone specific alkaline phosphatase and osteocalcin) and bone resorption markers (deoxypyridinoline) were significantly increased as a result of PTH treatment over 24 months (P<0.01 vs. placebo) while the change in resorption lagged behind the increase in formation [62, 63].
Although patients treated with GCs may develop an infraclinical secondary hyperparathyroidism as a compensatory mechanism of their relative hypocalcaemia, the intermittent administration of parathormone may be a conceptually appealing treatment alternative for patients with GIOP, based on its documented anabolic effects on bone resulting from the reduction of osteoblastic apoptosis. However, the evidence of fracture risk reduction in patients treated with GCs is still lacking.
Fluoride
Increased osteoblast apoptosis and resulting decreased osteoblast cell number is one of the key features in the development of GIOP. This observation triggered the idea of trying to stimulate bone formation directly with fluoride, a potent osteoblast mitogen.
Although initial results with natrium fluoride and disodium monofluorophosphate looked promising from the BMD increases reported at the lumbar spine [43, 64, 66, 82, 114], early concerns were unveiled/revealed at the femoral neck level, where at best no effect was observed [64, 66, 114], triggering the question of the antifracture efficacy of fluoride in patients with GIOP. A recent meta-analysis based on a systematic review of the literature [44] including 11 studies with 1,429 post-menopausal women enrolled concluded that although fluoride had an ability to increase BMD at the lumbar spine, it did not result in a reduction in vertebral fractures. In addition, increasing the dose of fluoride increased the risk of non-vertebral fractures and gastrointestinal side effects without any effect on the vertebral fracture rate. The RR for new vertebral fractures was not significantly reduced at 2 years [0.87 (95% CI: 0.51–1.46)] or at 4 years [0.9 (95% CI: 0.71–1.14)], nor was there a decrease in the RR for non-vertebral fractures at 2 years [1.2 (95% CI: 0.68–2.10)]. On the contrary, the latter was significantly increased at 4 years [1.85 (95% CI: 1.36–2.50)], especially for those patients using high dose and non-slow-release fluoride formulations [44]. Thus, the increase in bone density under fluoride therapy is not accompanied by a corresponding decrease in the incidence of vertebral or non-vertebral fractures and even the opposite may apply.
While ongoing research may refine the narrow therapeutic window for the safe and effective use of fluoride, and although the results reported qualify for post-menopausal women and not necessarily for GIOP, prudent prescription should be recommended—particularly since other treatment and prevention alternatives for GIOP, such as the bisphosphonates have demonstrated their safety and efficacy in clinical trials and daily practice.
Gonadal hormone substitution: androgens, estrogens and progestins
GC therapy is known to decrease sex hormone levels through inhibition of gonadotropin secretion and direct impairment of estrogen and androgen production.
Circulating testosterone concentrations are reduced in men treated with glucocorticoids, which might contribute to the loss of bone mass, and low circulating levels of free testosterone in post-menopausal women have been associated with an increased risk for hip and vertebral fractures [26]. Natural and synthetic androgens have been shown to have anabolic effects on bone [127].
In asthmatic men receiving long-term GC treatment, testosterone treatment was shown to increase lumbar spine BMD by 5.0% over 12 months [108]. In women treated with GCs for rheumatic disease, nandrolone decanoate significantly increased forearm bone density by 5.1% over 18 months versus baseline (P<0.01) [3]. However, due to the paucity of published data androgens cannot be recommended for routine use to prevent or treat GIOP.
Estrogens have been shown to inhibit osteoclastic bone resorption, and in post-menopausal women, lower circulating levels of circulating estradiol have been associated with an increased risk of vertebral and non-vertebral fractures [26]. In addition, HRT has been shown to reduce significantly the risk of hip, vertebral and other osteoporotic fractures in post-menopausal women [118]. On the other hand, progesterone binds competitively to the GC receptor of the osteoblast and may act as a GC antagonist [99, 135]. However, published randomized controlled trials of HRT in the prevention or treatment of GIOP are rare.
In a retrospective study of 15 post-menopausal or amenorrheic women aged 34–78 years under long-term high-dose GC treatment, HRT was shown to increase significantly lumbar spine BMD, while the untreated controls were continuously losing bone mass over 12 months [73]. In 200 post-menopausal women with rheumatoid arthritis and 21% of them treated with GC, BMD increased significantly at the lumbar spine with HRT versus calcium 500 mg daily with no significant difference at the femoral neck [49]. The administration of a long-acting progestin (17-alpha-hydroxy-progesterone-kaproat) in patients with steroid dependent asthma led to a considerable increase of the vertebral bone density (+17%), without unwanted side effects on the lipid profile [42].
While HRT was shown to reduce significantly the fracture risk in post-menopausal women in an adequately powered long-term prospective randomized study, the same study documented an increased risk of invasive breast cancer, coronary heart disease, stroke and pulmonary embolism and concluded that in post-menopausal women the overall health risks of HRT exceeded the potential benefits [118]. In post-menopausal women with prior hysterectomy, conjugated equine estrogen significantly increased the risk of stroke and decreased the risk of hip fracture over an average of 6.8 years, with an absolute excess risk of 12 additional strokes and an absolute risk reduction of six fewer hip fractures per 10,000 person-years [6]. Thus, HRT (opposed or unopposed estrogen substitution) should not be recommended for the prevention of osteoporosis in post-menopausal women. Whether a similar risk–benefit ratio would apply to women at increased risk for osteoporosis and osteoporotic fracture due to GC therapy was not established. However, based on currently available evidence HRT cannot be recommended for routine prevention or treatment of GIOP, and each prescription supposes that the risks are weighed against the benefits and adequate patient information about and acceptance of the trade-off has been given.
Tamoxifen, a partial estrogen agonist used as adjuvant endocrine treatment in post-menopausal women with operable breast cancer may inhibit bone resorption, and it has been shown to protect from steroid-induced bone loss over 2 years [33]. Raloxifene, a specific estrogen receptor modulator, lacks any published data for use in GIOP.
Physical medicine and rehabilitation
Fractures are associated with significant performance impairments in physical, functional and psychosocial domains in older women [74] and home exercise programs have not been shown to improve outcomes in post-menopausal women at high risk of fracture [59, 60]. However, controlled trials have shown that musculoskeletal changes related to osteoporosis can be prevented, challenged or reduced with the implementation of adequate patient-tailored rehabilitation programs [129]. The benefits of exercise and physical medicine measures have been reviewed extensively very recently [7, 94] and confirmed their importance in reducing the risk of falls and fractures, in decreasing pain and in improving fitness and overall quality of life in patients with osteoporosis [100]. In osteoporotic or high-risk patients, immobilization should be avoided as much as possible and a regular, albeit moderate physical activity recommended for those with osteoporosis. Specific fall prevention programs may contribute to improve neuromuscular coordination and thereby contribute to reduce the risk and the consequences of falling [93, 133]. Younger people with osteoporosis also need exercise that will preserve or improve bone mass, muscular strength, endurance and cardiovascular fitness [10]. Although braces have neither been shown to lower BMD in adolescents [130, 131] nor to impact back strength in women with osteoporosis [57], conclusive studies are lacking and immobilization is generally not recommended. Physical medicine and rehabilitation measures are independent contributors to bone health [100] and should be systematically evaluated and proposed together with dietary and drug therapy measures to every patient suffering from or at risk for osteoporosis.
Vertebroplasty and surgery for spine deformity
The surgical treatment of spine deformities can be very challenging due to the usually poor bone quality and to the patient’s expectations regarding the improvement of chronic pain, which need to be clarified upfront [39]. For the management of acute pain resulting from vertebral fractures and based on recent clinical results, percutaneous vertebroplasty has become an interesting treatment option to improve functionality and quality of life.
When compared with conservative therapy, percutaneous vertebroplasty results in prompt pain relief and rapid rehabilitation and has become a safe and effective procedure for treating acute osteoporotic vertebral compression fractures [30]. The reported improvements are maintained for at least 6 months and there is no apparent increase in the incidence of fractures post-operatively [80]. In a recently published prospective study including 60 patients with primary osteoporosis and painful vertebral fractures, kyphoplasty was shown to increase vertebral height significantly, to reduce pain and improve mobility [58].
Publications on the prospective clinical outcomes (pain relief, consumption of narcotic analgesics) of vertebroplasty in patients with acute vertebral compression fractures as a complication of GIOP are limited to case reports [31, 78]. However, in a retrospective review of prospective databases including 225 vertebral bodies treated by kyphoplasty in 115 patients (80 with primary osteoporosis and 35 with GIOP), the incidence of post-kyphoplasty vertebral compression fractures was 11.3% in the primary osteoporosis group and 48.6% in the GIOP group (P<0.0001) over an average 11 months of follow-up, suggesting that patients with steroid-induced compression fractures may have an increased incidence of subsequent fractures after a kyphoplasty procedure [50].
Therefore, kyphoplasty performed in appropriately selected osteoporotic patients with painful vertebral fractures is a promising addition to current medical treatment and more research is needed in patients with GIOP in order to understand better the determinants of fracture risk in this specific patient population.
Conclusions
Fractures are the most frequent and debilitating consequence of GIOP. New insights into the pathophysiology of GC-induced osteoporosis have greatly contributed to the understanding of the mechanisms behind the disease and to the development of innovative preventive and therapeutic solutions. Among these, the bisphosphonates risedronate and alendronate have established themselves as the current gold standard for the prevention and treatment of GIOP, based on their documented increase in BMD at all clinically relevant sites and their proven ability to reduce fractures. Calcitonin may be an interesting alternative for patients in whom bisphosphonates cannot be given and/or who are suffering from acute pain after an osteoporotic fracture. Parathormone is a conceptually attractive and promising treatment alternative, although data about fracture risk reduction are still lacking for patients with GIOP.
Interestingly, the main challenge in the prevention and treatment of GIOP may come from the medical community itself, as the main hurdle to treat such a severe disease remains the low awareness of the consequences on bone of GC therapy and the related low intervention rate [15]. Things are, however, evolving rapidly as recent educational programmes have been shown to double the prevalence of preventative therapy for GIOP in hospital patients taking GCs [86].
Acknowledgement
We thank Dr. Philippe Kress for his invaluable contribution to the preparation of the manuscript.
References
- 1.Adachi JD, Bensen WG, Brown J, Hanley D, Hodsman A, Josse R, Kendler DL, Lentle B, Olszynski W, Ste-Marie LG, Tenenhouse A, Chines AA. Intermittent etidronate therapy to prevent corticosteroid-induced osteoporosis. N Engl J Med. 1997;337:382–387. doi: 10.1056/NEJM199708073370603. [DOI] [PubMed] [Google Scholar]
- 2.Adachi JD, Saag KG, Delmas PD, Liberman UA, Emkey RD, Seeman E, Lane NE, Kaufman JM, Poubelle PE, Hawkins F, Correa-Rotter R, Menkes CJ, Rodriguez-Portales JA, Schnitzer TJ, Block JA, Wing J, McIlwain HH, Westhovens R, Brown J, Melo-Gomes JA, Gruber BL, Yanover MJ, Leite MO, Siminoski KG, Nevitt MC, Sharp JT, Malice MP, Dumortier T, Czachur M, Carofano W, Daifotis A. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202–211. doi: 10.1002/1529-0131(200101)44:1<202::AID-ANR27>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 3.Adami S, Fossaluzza V, Rossini M, Bertoldo F, Gatti D, Zamberlan N, Lo Cascio V. The prevention of corticosteroid-induced osteoporosis with nandrolone decanoate. Bone Miner. 1991;15:73–81. doi: 10.1016/0169-6009(91)90111-C. [DOI] [PubMed] [Google Scholar]
- 4.Adinoff AD, Hollister JR. Steroid-induced fractures and bone loss in patients with asthma. N Engl J Med. 1983;309:265–268. doi: 10.1056/NEJM198308043090502. [DOI] [PubMed] [Google Scholar]
- 5.American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. Arthritis Rheum. 2001;44:1496–1503. doi: 10.1002/1529-0131(200107)44:7<1496::AID-ART271>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 7.Bean JF, Vora A, Frontera WR. Benefits of exercise for community-dwelling older adults. Arch Phys Med Rehabil. 2004;85:S31–S42. doi: 10.1016/j.apmr.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, Weinstein RS, O’Brien CA, Manolagas SC, Jilka RL. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003;278:50259–50272. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- 9.Bijlsma JW, Raymakers JA, Mosch C, Hoekstra A, Derksen RH, Baart la Faille H, Duursma SA. Effect of oral calcium and vitamin D on glucocorticoid-induced osteopenia. Clin Exp Rheumatol. 1988;6:113–119. [PubMed] [Google Scholar]
- 10.Borer KT. Physical activity in the prevention and amelioration of osteoporosis in women: interaction of mechanical, hormonal and dietary factors. Sports Med. 2005;35:779–830. doi: 10.2165/00007256-200535090-00004. [DOI] [PubMed] [Google Scholar]
- 11.Boutsen Y, Jamart J, Esselinckx W, Stoffel M, Devogelaer JP. Primary prevention of glucocorticoid-induced osteoporosis with intermittent intravenous pamidronate: a randomized trial. Calcif Tissue Int. 1997;61:266–271. doi: 10.1007/s002239900334. [DOI] [PubMed] [Google Scholar]
- 12.Boutsen Y, Jamart J, Esselinckx W, Devogelaer JP. Primary prevention of glucocorticoid-induced osteoporosis with intravenous pamidronate and calcium: a prospective controlled 1-year study comparing a single infusion, an infusion given once every 3 months, and calcium alone. J Bone Miner Res. 2001;16:104–112. doi: 10.1359/jbmr.2001.16.1.104. [DOI] [PubMed] [Google Scholar]
- 13.Braun JJ, Birkenhager-Frenkel DH, Rietveld AH, Juttmann JR, Visser TJ, Birkenhager JC. Influence of 1 alpha-(OH)D3 administration on bone and bone mineral metabolism in patients on chronic glucocorticoid treatment; a double blind controlled study. Clin Endocrinol (Oxf) 1983;19:265–273. doi: 10.1111/j.1365-2265.1983.tb02989.x. [DOI] [PubMed] [Google Scholar]
- 14.Buckley LM, Hillner BE. A cost effectiveness analysis of calcium and vitamin D supplementation, etidronate, and alendronate in the prevention of vertebral fractures in women treated with glucocorticoids. J Rheumatol. 2003;30:132–138. [PubMed] [Google Scholar]
- 15.Buckley LM, Marquez M, Hudson JO, Downs RW, Vacek P, Small RE, Poses R. Variations in physicians’ judgments about corticosteroid induced osteoporosis by physician specialty. J Rheumatol. 1998;25:2195–2202. [PubMed] [Google Scholar]
- 16.Campbell IA, Douglas JG, Francis RM, Prescott RJ, Reid DM. Five year study of etidronate and/or calcium as prevention and treatment for osteoporosis and fractures in patients with asthma receiving long term oral and/or inhaled glucocorticoids. Thorax. 2004;59:761–768. doi: 10.1136/thx.2003.013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caniggia A, Nuti R, Lore F, Vattimo A. Pathophysiology of the adverse effects of glucoactive corticosteroids on calcium metabolism in man. J Steroid Biochem. 1981;15:153–161. doi: 10.1016/0022-4731(81)90270-3. [DOI] [PubMed] [Google Scholar]
- 18.Chyun YS, Kream BE, Raisz LG. Cortisol decreases bone formation by inhibiting periosteal cell proliferation. Endocrinology. 1984;114:477–480. doi: 10.1210/endo-114-2-477. [DOI] [PubMed] [Google Scholar]
- 19.Cohen D, Adachi JD. The treatment of glucocorticoid-induced osteoporosis. J Steroid Biochem Mol Biol. 2004;88:337–349. doi: 10.1016/j.jsbmb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Cohen S, Levy RM, Keller M, Boling E, Emkey RD, Greenwald M, Zizic TM, Wallach S, Sewell KL, Lukert BP, Axelrod DW, Chines AA. Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 1999;42:2309–2318. doi: 10.1002/1529-0131(199911)42:11<2309::AID-ANR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Compston J. Glucocorticoid-induced osteoporosis. Horm Res. 2003;60(Suppl 3):77–79. doi: 10.1159/000074506. [DOI] [PubMed] [Google Scholar]
- 22.Cooper MS, Rabbitt EH, Goddard PE, Bartlett WA, Hewison M, Stewart PM. Osteoblastic 11beta-hydroxysteroid dehydrogenase type 1 activity increases with age and glucocorticoid exposure. J Bone Miner Res. 2002;17:979–986. doi: 10.1359/jbmr.2002.17.6.979. [DOI] [PubMed] [Google Scholar]
- 23.Cooper MS, Blumsohn A, Goddard PE, Bartlett WA, Shackleton CH, Eastell R, Hewison M, Stewart PM. 11beta-hydroxysteroid dehydrogenase type 1 activity predicts the effects of glucocorticoids on bone. J Clin Endocrinol Metab. 2003;88:3874–3877. doi: 10.1210/jc.2003-022025. [DOI] [PubMed] [Google Scholar]
- 24.Cranney A, Welch V, Adachi JD, Homik J, Shea B, Suarez-Almazor ME, Tugwell P, Wells G (2000) Calcitonin for the treatment and prevention of corticosteroid-induced osteoporosis. Cochrane Database Syst Rev CD001983 [DOI] [PMC free article] [PubMed]
- 25.Crilly R, Cawood M, Marshall DH, Nordin BE. Hormonal status in normal, osteoporotic and corticosteroid-treated postmenopausal women. J R Soc Med. 1978;71:733–736. doi: 10.1177/014107687807101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings SR, Browner WS, Bauer D, Stone K, Ensrud K, Jamal S, Ettinger B. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1998;339:733–738. doi: 10.1056/NEJM199809103391104. [DOI] [PubMed] [Google Scholar]
- 27.Delany AM, Durant D, Canalis E. Glucocorticoid suppression of IGF I transcription in osteoblasts. Mol Endocrinol. 2001;15:1781–1789. doi: 10.1210/me.15.10.1781. [DOI] [PubMed] [Google Scholar]
- 28.Nijs RN, Jacobs JW, Algra A, Lems WF, Bijlsma JW. Prevention and treatment of glucocorticoid-induced osteoporosis with active vitamin D3 analogues: a review with meta-analysis of randomized controlled trials including organ transplantation studies. Osteoporos Int. 2004;15:589–602. doi: 10.1007/s00198-004-1614-5. [DOI] [PubMed] [Google Scholar]
- 29.Diamond T, McGuigan L, Barbagallo S, Bryant C. Cyclical etidronate plus ergocalciferol prevents glucocorticoid-induced bone loss in postmenopausal women. Am J Med. 1995;98:459–463. doi: 10.1016/S0002-9343(99)80345-3. [DOI] [PubMed] [Google Scholar]
- 30.Diamond TH, Champion B, Clark WA. Management of acute osteoporotic vertebral fractures: a nonrandomized trial comparing percutaneous vertebroplasty with conservative therapy. Am J Med. 2003;114:257–265. doi: 10.1016/S0002-9343(02)01524-3. [DOI] [PubMed] [Google Scholar]
- 31.Donovan MA, Khandji AG, Siris E. Multiple adjacent vertebral fractures after kyphoplasty in a patient with steroid-induced osteoporosis. J Bone Miner Res. 2004;19:712–713. doi: 10.1359/JBMR.040207. [DOI] [PubMed] [Google Scholar]
- 32.Eastell R, Reid DM, Compston J, Cooper C, Fogelman I, Francis RM, Hosking DJ, Purdie DW, Ralston SH, Reeve J, Russell RG, Stevenson JC, Torgerson DJ. A UK Consensus Group on management of glucocorticoid-induced osteoporosis: an update. J Intern Med. 1998;244:271–292. doi: 10.1046/j.1365-2796.1998.00408.x. [DOI] [PubMed] [Google Scholar]
- 33.Fentiman IS, Saad Z, Caleffi M, Chaudary MA, Fogelman I. Tamoxifen protects against steroid-induced bone loss. Eur J Cancer. 1992;28:684–685. doi: 10.1016/S0959-8049(05)80125-X. [DOI] [PubMed] [Google Scholar]
- 34.Frediani B, Falsetti P, Baldi F, Acciai C, Filippou G, Marcolongo R. Effects of 4-year treatment with once-weekly clodronate on prevention of corticosteroid-induced bone loss and fractures in patients with arthritis: evaluation with dual-energy X-ray absorptiometry and quantitative ultrasound. Bone. 2003;33:575–581. doi: 10.1016/S8756-3282(03)00208-4. [DOI] [PubMed] [Google Scholar]
- 35.Gennari C. Differential effect of glucocorticoids on calcium absorption and bone mass. Br J Rheumatol. 1993;32(Suppl 2):11–14. doi: 10.1093/rheumatology/32.suppl_2.11. [DOI] [PubMed] [Google Scholar]
- 36.Gennari C, Civitelli R. Glucocorticoid-induced osteoporosis. Clin Rheum Dis. 1986;12:637–654. [PubMed] [Google Scholar]
- 37.Geusens P, Dequeker J, Vanhoof J, Stalmans R, Boonen S, Joly J, Nijs J, Raus J. Cyclical etidronate increases bone density in the spine and hip of postmenopausal women receiving long term corticosteroid treatment. A double blind, randomised placebo controlled study. Ann Rheum Dis. 1998;57:724–727. doi: 10.1136/ard.57.12.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geusens PP, Nijs RN, Lems WF, Laan RF, Struijs A, Staa TP, Bijlsma JW. Prevention of glucocorticoid osteoporosis: a consensus document of the Dutch Society for Rheumatology. Ann Rheum Dis. 2004;63:324–325. doi: 10.1136/ard.2003.008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glassman SD, Alegre GM. Adult spinal deformity in the osteoporotic spine: options and pitfalls. Instr Course Lect. 2003;52:579–588. [PubMed] [Google Scholar]
- 40.Gluck OS, Murphy WA, Hahn TJ, Hahn B. Bone loss in adults receiving alternate day glucocorticoid therapy. A comparison with daily therapy. Arthritis Rheum. 1981;24:892–898. doi: 10.1002/art.1780240705. [DOI] [PubMed] [Google Scholar]
- 41.Gonnelli S, Rottoli P, Cepollaro C, Pondrelli C, Cappiello V, Vagliasindi M, Gennari C. Prevention of corticosteroid-induced osteoporosis with alendronate in sarcoid patients. Calcif Tissue Int. 1997;61:382–385. doi: 10.1007/s002239900352. [DOI] [PubMed] [Google Scholar]
- 42.Grecu EO, Weinshelbaum A, Simmons R. Effective therapy of glucocorticoid-induced osteoporosis with medroxyprogesterone acetate. Calcif Tissue Int. 1990;46:294–299. doi: 10.1007/BF02563818. [DOI] [PubMed] [Google Scholar]
- 43.Greenwald M, Brandli D, Spector S, Silverman S, Golde G. Corticosteroid-induced osteoporosis: effects of a treatment with slow-release sodium fluoride. Osteoporos Int. 1992;2:303–304. doi: 10.1007/BF01623187. [DOI] [PubMed] [Google Scholar]
- 44.Haguenauer D, Welch V, Shea B, Tugwell P, Adachi JD, Wells G. Fluoride for the treatment of postmenopausal osteoporotic fractures: a meta-analysis. Osteoporos Int. 2000;11:727–738. doi: 10.1007/s001980070051. [DOI] [PubMed] [Google Scholar]
- 45.Hahn TJ, Boisseau VC, Avioli LV. Effect of chronic corticosteroid administration on diaphyseal and metaphyseal bone mass. J Clin Endocrinol Metab. 1974;39:274–282. doi: 10.1210/jcem-39-2-274. [DOI] [PubMed] [Google Scholar]
- 46.Hahn TJ, Halstead LR, Teitelbaum SL, Hahn BH. Altered mineral metabolism in glucocorticoid-induced osteopenia. Effect of 25-hydroxyvitamin D administration. J Clin Invest. 1979;64:655–665. doi: 10.1172/JCI109506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hahn TJ, Halstead LR, Baran DT. Effects off short term glucocorticoid administration on intestinal calcium absorption and circulating vitamin D metabolite concentrations in man. J Clin Endocrinol Metab. 1981;52:111–115. doi: 10.1210/jcem-52-1-111. [DOI] [PubMed] [Google Scholar]
- 48.Hall GM, Spector TD, Griffin AJ, Jawad AS, Hall ML, Doyle DV. The effect of rheumatoid arthritis and steroid therapy on bone density in postmenopausal women. Arthritis Rheum. 1993;36:1510–1516. doi: 10.1002/art.1780361105. [DOI] [PubMed] [Google Scholar]
- 49.Hall GM, Daniels M, Doyle DV, Spector TD. Effect of hormone replacement therapy on bone mass in rheumatoid arthritis patients treated with and without steroids. Arthritis Rheum. 1994;37:1499–1505. doi: 10.1002/art.1780371014. [DOI] [PubMed] [Google Scholar]
- 50.Harrop JS, Prpa B, Reinhardt MK, Lieberman I. Primary and secondary osteoporosis’ incidence of subsequent vertebral compression fractures after kyphoplasty. Spine. 2004;29:2120–2125. doi: 10.1097/01.brs.0000141176.63158.8e. [DOI] [PubMed] [Google Scholar]
- 51.Herrala J, Puolijoki H, Liippo K, Raitio M, Impivaara O, Tala E, Nieminen MM. Clodronate is effective in preventing corticosteroid-induced bone loss among asthmatic patients. Bone. 1998;22:577–582. doi: 10.1016/S8756-3282(98)00051-9. [DOI] [PubMed] [Google Scholar]
- 52.Hock JM, Gera I. Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J Bone Miner Res. 1992;7:65–72. doi: 10.1002/jbmr.5650070110. [DOI] [PubMed] [Google Scholar]
- 53.Hubbard RB, Smith CJ, Smeeth L, Harrison TW, Tattersfield AE. Inhaled corticosteroids and hip fracture: a population-based case-control study. Am J Respir Crit Care Med. 2002;166:1563–1566. doi: 10.1164/rccm.200206-606OC. [DOI] [PubMed] [Google Scholar]
- 54.Ip M, Lam K, Yam L, Kung A, Ng M. Decreased bone mineral density in premenopausal asthma patients receiving long-term inhaled steroids. Chest. 1994;105:1722–1727. doi: 10.1378/chest.105.6.1722. [DOI] [PubMed] [Google Scholar]
- 55.Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–446. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones A, Fay JK, Burr M, Stone M, Hood K, Roberts G (2002) Inhaled corticosteroid effects on bone metabolism in asthma and mild chronic obstructive pulmonary disease. Cochrane Database Syst Rev CD003537 [DOI] [PMC free article] [PubMed]
- 57.Kaplan RS, Sinaki M, Hameister MD. Effect of back supports on back strength in patients with osteoporosis: a pilot study. Mayo Clin Proc. 1996;71:235–241. doi: 10.4065/71.3.235. [DOI] [PubMed] [Google Scholar]
- 58.Kasperk C, Hillmeier J, Noldge G, Grafe IA, Dafonseca K, Raupp D, Bardenheuer H, Libicher M, Liegibel UM, Sommer U, Hilscher U, Pyerin W, Vetter M, Meinzer HP, Meeder PJ, Taylor RS, Nawroth P. Treatment of painful vertebral fractures by kyphoplasty in patients with primary osteoporosis: a prospective nonrandomized controlled study. J Bone Miner Res. 2005;20:604–612. doi: 10.1359/JBMR.041203. [DOI] [PubMed] [Google Scholar]
- 59.Kerschan K, Alacamlioglu Y, Kollmitzer J, Wober C, Kaider A, Hartard M, Ghanem AH, Preisinger E. Functional impact of unvarying exercise program in women after menopause. Am J Phys Med Rehabil. 1998;77:326–332. doi: 10.1097/00002060-199807000-00013. [DOI] [PubMed] [Google Scholar]
- 60.Kerschan-Shindl K, Uher E, Kainberger F, Kaider A, Ghanem AH, Preisinger E. Long-term home exercise program: effect in women at high risk of fracture. Arch Phys Med Rehabil. 2000;81:319–323. doi: 10.1016/S0003-9993(00)90078-9. [DOI] [PubMed] [Google Scholar]
- 61.Krogsgaard MR, Thamsborg G, Lund B. Changes in bone mass during low dose corticosteroid treatment in patients with polymyalgia rheumatica: a double blind, prospective comparison between prednisolone and deflazacort. Ann Rheum Dis. 1996;55:143–146. doi: 10.1136/ard.55.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lane NE, Sanchez S, Modin GW, Genant HK, Pierini E, Arnaud CD. Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest. 1998;102:1627–1633. doi: 10.1172/JCI3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lane NE, Sanchez S, Genant HK, Jenkins DK, Arnaud CD. Short-term increases in bone turnover markers predict parathyroid hormone-induced spinal bone mineral density gains in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos Int. 2000;11:434–442. doi: 10.1007/s001980070111. [DOI] [PubMed] [Google Scholar]
- 64.Lems WF, Jacobs WG, Bijlsma JW, Croone A, Haanen HC, Houben HH, Gerrits MI, Rijn HJ. Effect of sodium fluoride on the prevention of corticosteroid-induced osteoporosis. Osteoporos Int. 1997;7:575–582. doi: 10.1007/BF02652565. [DOI] [PubMed] [Google Scholar]
- 65.Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, Dempster D, Cosman F. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350:550–555. doi: 10.1016/S0140-6736(97)02342-8. [DOI] [PubMed] [Google Scholar]
- 66.Lippuner K, Haller B, Casez JP, Montandon A, Jaeger P. Effect of disodium monofluorophosphate, calcium and vitamin D supplementation on bone mineral density in patients chronically treated with glucocorticosteroids: a prospective, randomized, double-blind study. Miner Electrolyte Metab. 1996;22:207–213. [PubMed] [Google Scholar]
- 67.Lippuner K, Casez JP, Horber FF, Jaeger P. Effects of deflazacort versus prednisone on bone mass, body composition, and lipid profile: a randomized, double blind study in kidney transplant patients. J Clin Endocrinol Metab. 1998;83:3795–3802. doi: 10.1210/jc.83.11.3795. [DOI] [PubMed] [Google Scholar]
- 68.Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: a systematic review and meta-analysis. Arch Intern Med. 1999;159:941–955. doi: 10.1001/archinte.159.9.941. [DOI] [PubMed] [Google Scholar]
- 69.LoCascio V, Bonucci E, Imbimbo B, Ballanti P, Adami S, Milani S, Tartarotti D, DellaRocca C. Bone loss in response to long-term glucocorticoid therapy. Bone Miner. 1990;8:39–51. doi: 10.1016/0169-6009(91)90139-Q. [DOI] [PubMed] [Google Scholar]
- 70.LoCascio V, Ballanti P, Milani S, Bertoldo F, LoCascio C, Zanolin EM, Bonucci E. A histomorphometric long-term longitudinal study of trabecular bone loss in glucocorticoid-treated patients: prednisone versus deflazacort. Calcif Tissue Int. 1998;62:199–204. doi: 10.1007/s002239900417. [DOI] [PubMed] [Google Scholar]
- 71.Luengo M, Picado C, Rio L, Guanabens N, Montserrat JM, Setoain J. Vertebral fractures in steroid dependent asthma and involutional osteoporosis: a comparative study. Thorax. 1991;46:803–806. doi: 10.1136/thx.46.11.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis: pathogenesis and management. Ann Intern Med. 1990;112:352–364. doi: 10.7326/0003-4819-112-5-352. [DOI] [PubMed] [Google Scholar]
- 73.Lukert BP, Johnson BE, Robinson RG. Estrogen and progesterone replacement therapy reduces glucocorticoid-induced bone loss. J Bone Miner Res. 1992;7:1063–1069. doi: 10.1002/jbmr.5650070909. [DOI] [PubMed] [Google Scholar]
- 74.Lyles KW, Gold DT, Shipp KM, Pieper CF, Martinez S, Mulhausen PL. Association of osteoporotic vertebral compression fractures with impaired functional status. Am J Med. 1993;94:595–601. doi: 10.1016/0002-9343(93)90210-G. [DOI] [PubMed] [Google Scholar]
- 75.MacAdams MR, White RH, Chipps BE. Reduction of serum testosterone levels during chronic glucocorticoid therapy. Ann Intern Med. 1986;104:648–651. doi: 10.7326/0003-4819-104-5-648. [DOI] [PubMed] [Google Scholar]
- 76.Manolagas SC. Cell number versus cell vigor—what really matters to a regenerating skeleton? Endocrinology. 1999;140:4377–4381. doi: 10.1210/en.140.10.4377. [DOI] [PubMed] [Google Scholar]
- 77.Manolagas SC, Weinstein RS. New developments in the pathogenesis and treatment of steroid-induced osteoporosis. J Bone Miner Res. 1999;14:1061–1066. doi: 10.1359/jbmr.1999.14.7.1061. [DOI] [PubMed] [Google Scholar]
- 78.Mathis JM, Petri M, Naff N. Percutaneous vertebroplasty treatment of steroid-induced osteoporotic compression fractures. Arthritis Rheum. 1998;41:171–175. doi: 10.1002/1529-0131(199801)41:1<171::AID-ART21>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 79.McCarthy TL, Ji C, Chen Y, Kim K, Centrella M. Time- and dose-related interactions between glucocorticoid and cyclic adenosine 3′,5′-monophosphate on CCAAT/enhancer-binding protein-dependent insulin-like growth factor I expression by osteoblasts. Endocrinology. 2000;141:127–137. doi: 10.1210/en.141.1.127. [DOI] [PubMed] [Google Scholar]
- 80.McKiernan F, Faciszewski T, Jensen R. Quality of life following vertebroplasty. J Bone Joint Surg Am. 2004;86-A:2600–2606. doi: 10.2106/00004623-200412000-00003. [DOI] [PubMed] [Google Scholar]
- 81.Messina OD, Barreira JC, Zanchetta JR, Maldonado-Cocco JA, Bogado CE, Sebastian ON, Flores D, Riopedre AM, Redondo G, Lazaro A. Effect of low doses of deflazacort vs prednisone on bone mineral content in premenopausal rheumatoid arthritis. J Rheumatol. 1992;19:1520–1526. [PubMed] [Google Scholar]
- 82.Meys E, Terreaux-Duvert F, Beaume-Six T, Dureau G, Meunier PJ. Bone loss after cardiac transplantation: effects of calcium, calcidiol and monofluorophosphate. Osteoporos Int. 1993;3:322–329. doi: 10.1007/BF01637318. [DOI] [PubMed] [Google Scholar]
- 83.Michel BA, Bloch DA, Fries JF. Predictors of fractures in early rheumatoid arthritis. J Rheumatol. 1991;18:804–808. [PubMed] [Google Scholar]
- 84.Morrison D, Ali NJ, Routledge PA, Capewell S. Bone turnover during short course prednisolone treatment in patients with chronic obstructive airways disease. Thorax. 1992;47:418–420. doi: 10.1136/thx.47.6.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mulder H, Struys A. Intermittent cyclical etidronate in the prevention of corticosteroid-induced bone loss. Br J Rheumatol. 1994;33:348–350. doi: 10.1093/rheumatology/33.4.348. [DOI] [PubMed] [Google Scholar]
- 86.Naunton M, Peterson GM, Jones G, Griffin GM, Bleasel MD. Multifaceted educational program increases prescribing of preventive medication for corticosteroid induced osteoporosis. J Rheumatol. 2004;31:550–556. [PubMed] [Google Scholar]
- 87.Nawata H, Soen S, Takayanagi R, Tanaka I, Takaoka K, Fukunaga M, Matsumoto T, Suzuki Y, Tanaka H, Fujiwara S, Miki T, Sagawa A, Nishizawa Y, Seino Y. Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research (2004) J Bone Miner Metab. 2005;23:105–109. doi: 10.1007/s00774-004-0596-x. [DOI] [PubMed] [Google Scholar]
- 88.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 89.O’Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145:1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- 90.Pereira RM, Delany AM, Canalis E. Cortisol inhibits the differentiation and apoptosis of osteoblasts in culture. Bone. 2001;28:484–490. doi: 10.1016/S8756-3282(01)00422-7. [DOI] [PubMed] [Google Scholar]
- 91.Pereira RC, Delany AM, Canalis E. Effects of cortisol and bone morphogenetic protein-2 on stromal cell differentiation: correlation with CCAAT-enhancer binding protein expression. Bone. 2002;30:685–691. doi: 10.1016/S8756-3282(02)00687-7. [DOI] [PubMed] [Google Scholar]
- 92.Pereira RM, Delany AM, Durant D, Canalis E. Cortisol regulates the expression of Notch in osteoblasts. J Cell Biochem. 2002;85:252–258. doi: 10.1002/jcb.10125. [DOI] [PubMed] [Google Scholar]
- 93.Pfeifer M, Sinaki M, Geusens P, Boonen S, Preisinger E, Minne HW. Musculoskeletal rehabilitation in osteoporosis: a review. J Bone Miner Res. 2004;19:1208–1214. doi: 10.1359/JBMR.040507. [DOI] [PubMed] [Google Scholar]
- 94.Phillips EM, Bodenheimer CF, Roig RL, Cifu DX. Geriatric rehabilitation. 4. Physical medicine and rehabilitation interventions for common age-related disorders and geriatric syndromes. Arch Phys Med Rehabil. 2004;85:S18–S22. doi: 10.1016/j.apmr.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 95.Pitt P, Li F, Todd P, Webber D, Pack S, Moniz C. A double blind placebo controlled study to determine the effects of intermittent cyclical etidronate on bone mineral density in patients on long-term oral corticosteroid treatment. Thorax. 1998;53:351–356. doi: 10.1136/thx.53.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pocock NA, Eisman JA, Dunstan CR, Evans RA, Thomas DH, Huq NL. Recovery from steroid-induced osteoporosis. Ann Intern Med. 1987;107:319–323. doi: 10.7326/0003-4819-107-2-319. [DOI] [PubMed] [Google Scholar]
- 98.Priftis K, Everard ML, Milner AD. Unexpected side-effects of inhaled steroids: a case report. Eur J Pediatr. 1991;150:448–449. doi: 10.1007/BF02093730. [DOI] [PubMed] [Google Scholar]
- 99.Prior JC. Progesterone as a bone-trophic hormone. Endocr Rev. 1990;11:386–398. doi: 10.1210/edrv-11-2-386. [DOI] [PubMed] [Google Scholar]
- 100.Prior JC, Barr SI, Chow R, Faulkner RA. Prevention and management of osteoporosis: consensus statements from the Scientific Advisory Board of the Osteoporosis Society of Canada. 5. Physical activity as therapy for osteoporosis. CMAJ. 1996;155:940–944. [PMC free article] [PubMed] [Google Scholar]
- 101.Reginster JY, Froidmont C, Lecart MP, Sarlet N, Defraigne JO. Alphacalcidol in prevention of glucocorticoid-induced osteoporosis. Calcif Tissue Int. 1999;65:328–331. doi: 10.1007/s002239900706. [DOI] [PubMed] [Google Scholar]
- 102.Reginster JY, Kuntz D, Verdickt W, Wouters M, Guillevin L, Menkes CJ, Nielsen K. Prophylactic use of alfacalcidol in corticosteroid-induced osteoporosis. Osteoporos Int. 1999;9:75–81. doi: 10.1007/s001980050118. [DOI] [PubMed] [Google Scholar]
- 103.Rehman Q, Lang TF, Arnaud CD, Modin GW, Lane NE. Daily treatment with parathyroid hormone is associated with an increase in vertebral cross-sectional area in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos Int. 2003;14:77–81. doi: 10.1007/s00198-002-1312-0. [DOI] [PubMed] [Google Scholar]
- 104.Reid IR. Pathogenesis and treatment of steroid osteoporosis. Clin Endocrinol (Oxf) 1989;30:83–103. doi: 10.1111/j.1365-2265.1989.tb03730.x. [DOI] [PubMed] [Google Scholar]
- 105.Reid IR, Heap SW. Determinants of vertebral mineral density in patients receiving long-term glucocorticoid therapy. Arch Intern Med. 1990;150:2545–2548. doi: 10.1001/archinte.150.12.2545. [DOI] [PubMed] [Google Scholar]
- 106.Reid IR, Ibbertson HK. Evidence for decreased tubular reabsorption of calcium in glucocorticoid-treated asthmatics. Horm Res. 1987;27:200–204. doi: 10.1159/000180820. [DOI] [PubMed] [Google Scholar]
- 107.Reid IR, King AR, Alexander CJ, Ibbertson HK. Prevention of steroid-induced osteoporosis with (3-amino-1-hydroxypropylidene)-1,1-bisphosphonate (APD) Lancet. 1988;1:143–146. doi: 10.1016/S0140-6736(88)92721-3. [DOI] [PubMed] [Google Scholar]
- 108.Reid IR, Wattie DJ, Evans MC, Stapleton JP. Testosterone therapy in glucocorticoid-treated men. Arch Intern Med. 1996;156:1173–1177. doi: 10.1001/archinte.156.11.1173. [DOI] [PubMed] [Google Scholar]
- 109.Reid DM, Hughes RA, Laan RF, Sacco-Gibson NA, Wenderoth DH, Adami S, Eusebio RA, Devogelaer JP. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res. 2000;15:1006–1013. doi: 10.1359/jbmr.2000.15.6.1006. [DOI] [PubMed] [Google Scholar]
- 110.Reid DM, Adami S, Devogelaer JP, Chines AA. Risedronate increases bone density and reduces vertebral fracture risk within one year in men on corticosteroid therapy. Calcif Tissue Int. 2001;69:242–247. doi: 10.1007/s00223-001-1060-8. [DOI] [PubMed] [Google Scholar]
- 111.Rejnmark L, Vestergaard P, Heickendorff L, Andreasen F, Mosekilde L. Effects of thiazide- and loop-diuretics, alone or in combination, on calcitropic hormones and biochemical bone markers: a randomized controlled study. J Intern Med. 2001;250:144–153. doi: 10.1046/j.1365-2796.2001.00868.x. [DOI] [PubMed] [Google Scholar]
- 112.Richy F, Ethgen O, Bruyere O, Reginster JY. Efficacy of alphacalcidol and calcitriol in primary and corticosteroid-induced osteoporosis: a meta-analysis of their effects on bone mineral density and fracture rate. Osteoporos Int. 2004;15:301–310. doi: 10.1007/s00198-003-1570-5. [DOI] [PubMed] [Google Scholar]
- 113.Richy F, Schacht E, Bruyere O, Ethgen O, Gourlay M, Reginster JY. Vitamin D analogs versus native vitamin d in preventing bone loss and osteoporosis-related fractures: a comparative meta-analysis. Calcif Tissue Int. 2005;76(3):176–186. doi: 10.1007/s00223-004-0005-4. [DOI] [PubMed] [Google Scholar]
- 114.Rickers H, Deding A, Christiansen C, Rodbro P, Naestoft J. Corticosteroid-induced osteopenia and vitamin D metabolism. Effect of vitamin D2, calcium phosphate and sodium fluoride administration. Clin Endocrinol (Oxf) 1982;16:409–415. doi: 10.1111/j.1365-2265.1982.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 115.Ringe JD. Glucocorticoid-induced osteoporosis. Clin Rheumatol. 1989;8(Suppl 2):109–115. doi: 10.1007/BF02207244. [DOI] [PubMed] [Google Scholar]
- 116.Ringe JD, Welzel D. Salmon calcitonin in the therapy of corticoid-induced osteoporosis. Eur J Clin Pharmacol. 1987;33:35–39. doi: 10.1007/BF00610377. [DOI] [PubMed] [Google Scholar]
- 117.Rizzato G, Montemurro L. Reversibility of exogenous corticosteroid-induced bone loss. Eur Respir J. 1993;6:116–119. [PubMed] [Google Scholar]
- 118.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 119.Roux C, Oriente P, Laan R, Hughes RA, Ittner J, Goemaere S, Di Munno O, Pouilles JM, Horlait S, Cortet B. Randomized trial of effect of cyclical etidronate in the prevention of corticosteroid-induced bone loss. Ciblos Study Group. J Clin Endocrinol Metab. 1998;83:1128–1133. doi: 10.1210/jc.83.4.1128. [DOI] [PubMed] [Google Scholar]
- 120.Ruegsegger P, Medici TC, Anliker M. Corticosteroid-induced bone loss. A longitudinal study of alternate day therapy in patients with bronchial asthma using quantitative computed tomography. Eur J Clin Pharmacol. 1983;25:615–620. doi: 10.1007/BF00542348. [DOI] [PubMed] [Google Scholar]
- 121.Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, Thamsborg G, Liberman UA, Delmas PD, Malice MP, Czachur M, Daifotis AG. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med. 1998;339:292–299. doi: 10.1056/NEJM199807303390502. [DOI] [PubMed] [Google Scholar]
- 122.Sakakura M, Takebe K, Nakagawa S. Inhibition of luteinizing hormone secretion induced by synthetic LRH by long-term treatment with glucocorticoids in human subjects. J Clin Endocrinol Metab. 1975;40:774–779. doi: 10.1210/jcem-40-5-774. [DOI] [PubMed] [Google Scholar]
- 123.Sambrook P, Nguyen T. Vertebral osteoporosis in rheumatoid arthritis patients: effect of low dose prednisone therapy. Br J Rheumatol. 1992;31:573–574. doi: 10.1093/rheumatology/31.8.573. [DOI] [PubMed] [Google Scholar]
- 124.Sambrook PN, Cohen ML, Eisman JA, Pocock NA, Champion GD, Yeates MG. Effects of low dose corticosteroids on bone mass in rheumatoid arthritis: a longitudinal study. Ann Rheum Dis. 1989;48:535–538. doi: 10.1136/ard.48.7.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sambrook P, Birmingham J, Kelly P, Kempler S, Nguyen T, Pocock N, Eisman J. Prevention of corticosteroid osteoporosis. A comparison of calcium, calcitriol, and calcitonin. N Engl J Med. 1993;328:1747–1752. doi: 10.1056/NEJM199306173282404. [DOI] [PubMed] [Google Scholar]
- 126.Sambrook PN, Kelly PJ, Keogh AM, Macdonald P, Spratt P, Freund J, Eisman JA. Bone loss after heart transplantation: a prospective study; discussion 121. J Heart Lung Transplant. 1994;13:116–120. [PubMed] [Google Scholar]
- 127.Schneider HP. Androgens and antiandrogens. Ann NY Acad Sci. 2003;997:292–306. doi: 10.1196/annals.1290.033. [DOI] [PubMed] [Google Scholar]
- 128.Seeman E, Wahner HW, Offord KP, Kumar R, Johnson WJ, Riggs BL. Differential effects of endocrine dysfunction on the axial and the appendicular skeleton. J Clin Invest. 1982;69:1302–1309. doi: 10.1172/JCI110570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sinaki M. Nonpharmacologic interventions. Exercise, fall prevention, and role of physical medicine. Clin Geriatr Med. 2003;19:337–359. doi: 10.1016/S0749-0690(02)00111-8. [DOI] [PubMed] [Google Scholar]
- 130.Snyder BD, Zaltz I, Breitenbach MA, Kido TH, Myers ER, Emans JB. Does bracing affect bone density in adolescent scoliosis? Spine. 1995;20:1554–1560. doi: 10.1097/00007632-199507150-00002. [DOI] [PubMed] [Google Scholar]
- 131.Snyder BD, Katz DA, Myers ER, Breitenbach MA, Emans JB. Bone density accumulation is not affected by brace treatment of idiopathic scoliosis in adolescent girls. J Pediatr Orthop. 2005;25:423–428. doi: 10.1097/01.bpo.0000158001.23177.8d. [DOI] [PubMed] [Google Scholar]
- 132.Stellon AJ, Webb A, Compston JE. Bone histomorphometry and structure in corticosteroid treated chronic active hepatitis. Gut. 1988;29:378–384. doi: 10.1136/gut.29.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stevens JA, Olson S. Reducing falls and resulting hip fractures among older women. MMWR Recomm Rep. 2000;49:3–12. [PubMed] [Google Scholar]
- 134.Suzuki Y, Ichikawa Y, Saito E, Homma M. Importance of increased urinary calcium excretion in the development of secondary hyperparathyroidism of patients under glucocorticoid therapy. Metabolism. 1983;32:151–156. doi: 10.1016/0026-0495(83)90221-4. [DOI] [PubMed] [Google Scholar]
- 135.Tenenbaum HC, Kamalia N, Sukhu B, Limeback H, McCulloch CA. Probing glucocorticoid-dependent osteogenesis in rat and chick cells in vitro by specific blockade of osteoblastic differentiation with progesterone and RU38486. Anat Rec. 1995;242:200–210. doi: 10.1002/ar.1092420209. [DOI] [PubMed] [Google Scholar]
- 136.Uzawa T, Hori M, Ejiri S, Ozawa H. Comparison of the effects of intermittent and continuous administration of human parathyroid hormone(1–34) on rat bone. Bone. 1995;16:477–484. doi: 10.1016/8756-3282(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 137.Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 138.Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford) 2000;39:1383–1389. doi: 10.1093/rheumatology/39.12.1383. [DOI] [PubMed] [Google Scholar]
- 139.Staa TP, Leufkens HG, Cooper C. Use of inhaled corticosteroids and risk of fractures. J Bone Miner Res. 2001;16:581–588. doi: 10.1359/jbmr.2001.16.3.581. [DOI] [PubMed] [Google Scholar]
- 140.Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777–787. doi: 10.1007/s001980200108. [DOI] [PubMed] [Google Scholar]
- 141.Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum. 2003;48:3224–3229. doi: 10.1002/art.11283. [DOI] [PubMed] [Google Scholar]
- 142.Staa TP, Geusens P, Pols HA, Laet C, Leufkens HG, Cooper C. A simple score for estimating the long-term risk of fracture in patients using oral glucocorticoids. QJM. 2005;98:191–198. doi: 10.1093/qjmed/hci029. [DOI] [PubMed] [Google Scholar]
- 143.Wallach S, Cohen S, Reid DM, Hughes RA, Hosking DJ, Laan RF, Doherty SM, Maricic M, Rosen C, Brown J, Barton I, Chines AA. Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int. 2000;67:277–285. doi: 10.1007/s002230001146. [DOI] [PubMed] [Google Scholar]
- 144.Weinstein RS. Glucocorticoid-induced osteoporosis. Rev Endocr Metab Disord. 2001;2:65–73. doi: 10.1023/A:1010007108155. [DOI] [PubMed] [Google Scholar]
- 145.Weinstein RS, Manolagas SC. Apoptosis and osteoporosis. Am J Med. 2000;108:153–164. doi: 10.1016/S0002-9343(99)00420-9. [DOI] [PubMed] [Google Scholar]
- 146.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Weinstein RS, Chen JR, Powers CC, Stewart SA, Landes RD, Bellido T, Jilka RL, Parfitt AM, Manolagas SC. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J Clin Invest. 2002;109:1041–1048. doi: 10.1172/JCI200214538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wolinsky-Friedland M. Drug-induced metabolic bone disease. Endocrinol Metab Clin North Am. 1995;24:395–420. [PubMed] [Google Scholar]
- 149.Wong CA, Walsh LJ, Smith CJ, Wisniewski AF, Lewis SA, Hubbard R, Cawte S, Green DJ, Pringle M, Tattersfield AE. Inhaled corticosteroid use and bone-mineral density in patients with asthma. Lancet. 2000;355:1399–1403. doi: 10.1016/S0140-6736(00)02138-3. [DOI] [PubMed] [Google Scholar]