Abstract
To evaluate the results of surgical treatment in patients with unlocked full-segmented hemivertebra treated by excision. Twenty-six patients with a mean age of 12.4±1.7 years were included in the study. The mean duration of follow-up was 47.8±21.9 months. Diagnosis of type-IA hemivertebra was established by clinical, radiological, CT, and MRI evaluation. Preoperatively, patients were randomly allocated into two groups. In the first group, patients underwent anterior hemivertebrectomy initially; this was followed by posterior excision of the hemivertebra, posterior instrumentation, and fusion. In the second group, posterior components of the hemivertebra were excised at first, then the hemivertebra body was excised anteriorly, and this was followed by anterior instrumentation and fusion. For both groups, compression was applied to the convex side while distraction was applied to the concave side. Frontal and sagittal plane analysis of radiograms obtained preoperatively, postoperatively, and after a minimum period of 2 years was performed. The balance was analyzed clinically and radiologically by the measurement of the lateral trunk shift (LT) and shift of head (SH). The mean preoperative and postoperative Cobb angles were 45.5°∓11.4° and 16.8°∓7.9°, respectively, and postoperatively, a mean correction rate of 64.4±13.9% was obtained (P=0.00). The mean correction rate was 61.2±13.3% (19.2°∓7.6°) for the last follow-up visit. Sagittal plane analysis demonstrated either conservation of physiological sagittal contours or a normalizing effect following excision of hemivertebra combined with anterior or posterior instrumentation. When postoperative balance values were compared, a statistically significant correction was found in terms of LT and SH values. Although none of the patients had complete balance (SH: 0 mm) or balanced curves (0 mm<SH<15 mm) preoperatively, 20 (76.9%) of the patients had a balanced trunk after surgical intervention. Circumferential fusion could be achieved in all cases. No neurological complication developed, the only complication was delayed wound healing. In view of these data, it is concluded that these techniques can be safely used for this patient group at low thoracic, thoracolumbar, and lumbar levels of vertebral column with high correction rates.
Keywords: Congenital scoliosis, Surgical treatment, Hemivertebra excision
Introduction
Spinal curves related to hemivertebra are not only severe and progressive but also can cause trunk shift and imbalance as well. Those located at the lumbosacral region lead to pelvic obliqueness and unevenness in functional leg length [1]. It is believed that especially unsegmented and cross-sited hemivertebra are not progressive and lead to a balanced deformity. However, it has been demonstrated for fully segmented hemivertebra that the spinal curve increases particularly in growth spurt periods [1, 2].
Many treatment methods have been tried for the deformity related to hemivertebra. The oldest one is the complete excision of hemivertebra. According to Winter [3], the excision of hemivertebra makes complete improvement possible as well as obtaining fusion, which is attempted with traditional methods. Lubicky has suggested that many indications for surgery are relative, except the excision of lumbosacral hemivertebra and that it should be carried out before the development of thoracolumbar compensatory curve and pelvic obliqueness. In addition, he considers the emergence of neurological deficit as a definite indication. Complete excision of fully segmented hemivertebra has been proposed [1].
Hemivertebra excision is usually readily carried out. The most common method is the excision of the hemivertebra body and disk in the form of Y, and subsequent excision of the posterior components, if present [1, 4, 5]. According to Lubicky [1], excision of the posterior components first through posterior approach, and then the excision of anterior hemivertebra body and compression with Zielke or Dwyer operation is the other method. These procedures can be performed at a single session. Of late, it has been common practice to perform them in two steps but on the same operation day [3, 4, 6, 7]. Several recent reports recommend the simultaneous performance of two procedures during the same session as this is more reliable and efficient [4, 8, 9]. Complete excision of hemivertebra from posterior is quite easy and reliable [10, 11].
Holte and colleagues reported the favorable effect of instrumentation on correction, and Hall et al. suggested a lower correction loss and pseudoarthrosis rate for patients undergoing instrumentation [7, 12].
According to Lubicky, although this operation can be carried out at any age, a suitable instrumentation cannot be made in small children in order to increase correction rates and protect fusion area, and the necessity of corrective body casts is a problem for this age group [1]. Ruf and Harms [13, 14] demonstrated a new technique allowing successful hemivertebra excision, notably in small children. Some authors suggest that hemivertebrectomy can be performed at any age, other authors prefer to carry out the intervention during adolescence [11, 15].
In the present study, 26 adolescent patients with unlocked completely segmented vertebrae were enrolled. The results of anterior or posterior instrumentation following complete hemivertebra excision, of two steps in a single session, have been evaluated. In addition, any possible impact of the location of the instrumentation on the outcome has also been investigated.
Patients and methods
Hundred and three patients with congenital scoliosis were operated in our clinic between January 1990 and January 2002. Of these patients, 26 with unlocked complete segmented hemivertebra and at least 2 years of follow-up were included in the study. Their mean age was 12.4±1.7 years (9–15 years) 17 patients were male and 9 were female.
A thorough physical and neurological examination was performed for all patients, and additional systemic and organ anomalies were investigated. Standing anterior–posterior, side and bending radiograms as well as radiograms of anterior–posterior pelvis and other suspicious regions of skeletal system were obtained. By using these radiograms, the angles of the curves were measured by Cobb method. The Cobb angles of the upper and lower secondary curves were also measured in a similar manner. On lateral radiograms, sagittal contours between T2 and T12 and L1–L5 vertebrae were measured by Cobb method. 30°–50° and 40°–60° were considered as normal thoracic physiological kyphosis and lumbar lordosis, respectively [20]. All measurements were made in collaboration with radiologists. In all patients, CT of the deformity site was obtained and magnetic resonance imaging of all vertebrae was carried out. Routine laboratory investigations were made and the patients were consulted with other relevant departments.
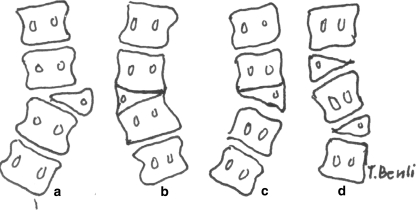
Patients were classified according to the classification of Winter and Lubicky (Fig. 1) [1, 5, 20] and type I-A patients were included. According to Lubicky, the most important indication for the excision of hemivertebra is the presence of curve progression [1]. In the present study, surgical intervention was planned in patients with rapidly progressive curves (as detected during follow-up visits) and neurological and clinical complaints (in order to control progression and obtain correction).
Fig. 1.
Classification of formation deficiency: A unincarcerated fully segmented hemivertebra (Type I-A), B incarcerated segmented hemivertebra (Type I-B), C incarcerated semi-segmented hemivertebra (Type I-C) and D hemimethameric shift (Type I-D) (1)
Patients were randomly allocated into two groups. Twelve patients in the first group underwent anterior hemivertebrectomy (excision of the deformed body) followed by posterior lamina, facet and pedicle excision of the hemivertebra, posterior Texas Scottish Rite Hospital (TSRH) system instrumentation, and posterior fusion, during the same session. The second group of patients underwent posterior excision of the hemivertebra parts first; this was followed by anterior hemivertebra excision, and Cotrel–Dubousset–Hopf (CDH) instrumentation, again during the same session. For both groups, compression was utilized at the convex side and distraction was applied at the concave side.
The same surgical team carried out all operations. Autologous blood transfusion was made in all patients using the ‘cell-saver’ system (Electromedics). Intraoperatively, the autotransfusion unit saved an average of 680±140 cc blood, and a mean of 1.6±1.2 U of saved blood were transfused. None of the patients needed homologous blood transfusion. The hematocrit value was reduced to a mean of 0.7±0.6 mg/dl, with a statistically significant difference (P<0.05). Mean operation time was 2.7±1.3 h. Wake-up test was performed for the first four patients. When available, somatosensory evoke potentials (SSEP) were monitored in nine patients, using Caldwell-Quantum 80 system. For the last 16 patients of this study, SSEP and ‘transcranial cortical magnetic stimulation-motor evoked potentials’ (TkMMEP) were combined for intraoperative neurological monitoring.
Preoperative antibiotic prophylaxis was administered to all patients using 2 g of a first generation cephalosporin or 1 g of sulbactam ampicillin. Antibiotic prophylaxis was continued for 3 days postoperatively, the dose being reduced to 0.5 g/day.
For fusion, ribs excised via anterior approach, tricortical grafts from crista iliaca, and autologous cancellous chip grafts from the posterior iliac spine were used.
Patients were turned to their right and left sides on the first postoperative day, were allowed to sit on day 2 and walk on day 3. In all patients, vitraten mold Boston device was used for 4 months postoperatively.
Balance analysis of patients was carried out radiologically; shoulder asymmetry and distance from the center of gravity was measured clinically by a plumb line swinging from C7 to intergluteal crease. In addition, the subjective complaints of the patients were recorded. Two radiological parameters were analyzed on preoperative, early postoperative and last follow-up radiographs: Lateral trunk shift (LT) and shift of head (SH). LT was measured as the distance from midpoint of apical vertebra of major curve to the mid sacral line (MSL). SH was measured as the distance between the MSL and midpoint of the seventh cervical vertebra. If SH was 0 mm, i.e., if the vertebra is in the midline, then the curve was considered as a “completely balanced” one. If the SH is higher than 0 mm, but lower than 15 mm, since the imbalance was not clinically recognized, it was regarded as a “balanced” curve.
In addition to the routine follow-up visits, at 3rd, 6th and 12th months postoperatively, they were invited for the last visit in January 2004, and the clinical and radiological investigations were repeated. Their frontal and sagittal plane curves were evaluated. A solid fusion mass with consolidation, absence of any clinical complaints or implant deficiency, and a correction loss of 5° or less was considered as complete fusion. Presence of pain, absence of a complete consolidation, and a correction loss over 10° was regarded as the development of pseudoarthrosis. For patients with a correction loss between 5° and 10°, development of a fusion mass was anticipated, and watchful waiting was continued. Additionally, subjective complaints of the patients related to balance, implant failure, and other complications were recorded.
For statistical evaluation, SPSS 9.0 program was used in order to detect the ‘difference between the means of same sample’. A P value <0.05 was considered statistically significant.
Results
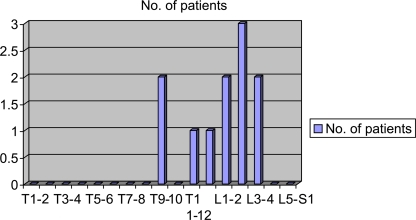
Radiological investigation revealed Type I-A formation deformity and absence of any other skeletal-muscular or systemic congenital deformity in all 26 patients. In addition, MR imaging did not reveal any intraspinal anomaly. Most frequently involved vertebral level was L2–3 (Fig. 2).
Fig. 2.
Distribution of the patients by the level of hemivertebral segments
Frontal plane
The frontal plane analysis of all patients is shown in Tables 1, 2, and 3. Overall, mean preoperative and postoperative Cobb angles of the major curve were 45.5°∓11.4° and 16.8°∓7.9°, respectively, with a statistically significant difference (P=0.00). Overall, 64.4±13.9% of correction was obtained postoperatively, and the final mean correction rate was 61.2±13.3% (19.2°∓7.6°) at the last follow-up visit. The difference between early postoperative and last follow-up visit correction rates was not significant statistically, with a minimal loss of correction (P=0.06).
Table 1.
The clinical and radiological findings of the patients with congenital scoliosis due to unincarcerated fully segmented hemivertebra
| No. | Patients | Age | Sex | Follow-up (months) | Level | Instrumentation | Preoperative Cobb angle (°) | Postoperative Cobb angle (°) | Final Cobb angle (°) | Preoperative shift of head (mm) | Postoperative shift of head (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MG | 11 | F | 71 | T9–10 | Anterior-CDH | 46 | 20 | 24 | 34.0 | 0.0 |
| 2 | CD | 12 | M | 52 | T9–10 | Anterior-CDH | 58 | 22 | 25 | 36.5 | 5.0 |
| 3 | MU | 12 | M | 48 | L1–2 | Anterior-CDH | 40 | 20 | 22 | 22.5 | 0.0 |
| 4 | DA | 13 | M | 36 | L1–2 | Anterior-CDH | 30 | 6 | 10 | 14.0 | 8.0 |
| 5 | SS | 12 | F | 24 | L2–3 | Anterior-CDH | 50 | 20 | 23 | 27.5 | 12.5 |
| 6 | KL | 13 | M | 48 | T12–L1 | Anterior-CDH | 50 | 16 | 16 | 33.0 | 12.5 |
| 7 | TR | 15 | F | 40 | L2–3 | Anterior-CDH | 44 | 20 | 24 | 20.0 | 12.0 |
| 8 | ES | 11 | F | 35 | T9–10 | Anterior-CDH | 36 | 6 | 10 | 20.0 | 5.0 |
| 9 | AB | 12 | M | 30 | T12–L1 | Anterior-CDH | 40 | 20 | 24 | 30.0 | 0.0 |
| 10 | EY | 13 | M | 28 | L3–4 | Anterior-CDH | 45 | 24 | 24 | 25.0 | 15.0 |
| 11 | BB | 14 | M | 24 | L2–3 | Anterior-CDH | 40 | 20 | 22 | 22.5 | 12.5 |
| 12 | RT | 14 | M | 24 | L2–3 | Anterior-CDH | 50 | 20 | 20 | 25.0 | 6.0 |
| 13 | UB | 9 | M | 94 | T9–10 | Posterior-TSRH | 30 | 6 | 10 | 12.0 | 15.0 |
| 14 | TK | 9 | M | 88 | T11–12 | Posterior-TSRH | 36 | 16 | 20 | 20.0 | 0.0 |
| 15 | LL | 11 | F | 84 | T12–l1 | Posterior-TSRH | 40 | 20 | 24 | 18.0 | 8.0 |
| 16 | RG | 12 | M | 72 | L2–3 | Posterior-TSRH | 40 | 20 | 25 | 39.0 | 10.0 |
| 17 | BC | 14 | M | 66 | L2–3 | Posterior-TSRH | 56 | 16 | 20 | 20.0 | 15.0 |
| 18 | AD | 15 | M | 24 | L3–4 | Posterior-TSRH | 50 | 24 | 24 | 20.0 | 8.0 |
| 19 | AS | 13 | F | 24 | L3–4 | Posterior-TSRH | 40 | 14 | 14 | 20.0 | 0.0 |
| 20 | MS | 13 | M | 59 | T7–8 | Posterior-TSRH | 80 | 40 | 40 | 54.0 | 27.0 |
| 21 | BD | 11 | M | 48 | T11–12 | Posterior-TSRH | 55 | 10 | 15 | 40.0 | 20.0 |
| 22 | BN | 12 | F | 42 | T12–L1 | Posterior-TSRH | 66 | 20 | 20 | 42.5 | 27.5 |
| 23 | HS | 13 | F | 36 | L2–3 | Posterior-TSRH | 36 | 8 | 10 | 36.0 | 6.0 |
| 24 | TB | 12 | F | 28 | L2–3 | Posterior-TSRH | 50 | 18 | 20 | 27.5 | 15.0 |
| 25 | EO | 9 | M | 24 | L3–4 | Posterior-TSRH | 30 | 0 | 0 | 27.5 | 12.5 |
| 26 | AK | 14 | M | 24 | L3–4 | Posterior-TSRH | 46 | 10 | 14 | 25.0 | 10.0 |
Table 2.
The frontal plane curve assessment of the patients with both anterior and posterior surgical intervention
| No. of instrumented mobile segment | Preoperative Cobb angle (°) | Postoperative Cobb angle (°) | t | P | Postoperative correction rates (%) | Final Cobb angle (°) | t | P | Final correction rates (%) | t | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anterior instrumentation (n=12) | 1.4±0.5 | 44.1±7.5 | 17.8±5.8 | 16.1 | 0.00 | 60.7±12.1 | 20.3±5.4 | 13.5 | 0.00 | 56.9±12.6 | 1.3 | 0.22 |
| Posterior instrumentation (n=14) | 3.3±1.6 | 46.8±14.1 | 15.9±9.6 | 12.7 | 0.00 | 67.5±15.1 | 18.3±9.3 | 10.9 | 0.00 | 64.8±13.2 | 1.5 | 0.16 |
| t | 1.28 | 1.54 | ||||||||||
| P | >0.05 | >0.05 | ||||||||||
| Total (n=26) | 2.4±1.5 | 45.5±11.4 | 16.8±7.9 | 18.5 | 0.00 | 64.4±13.9 | 19.2±7.6 | 15.9 | 0.00 | 61.2±13.3 | 1.9 | 0.06 |
Table 3.
The sagittal plane contours assessment of the patients with anterior and posterior surgical intervention
| Vertebral level | Preoperative sagittal contour (°) | Postoperative sagittal contour (°) | Final sagittal contour (°) | |
|---|---|---|---|---|
| Anterior instrumentation (n=12) | Thoracic (n=3) | 36.0±5.6 | 37.0±4.2 | 37.0±4.2 |
| Thoracolumbar (n=2) | 10.0±0.0 | 2.5±3.5 | 6.0±2.8 | |
| Lumbar (n=7) | 26.8±8.9 | 37.5±6.1 | 36.7±4.1 | |
| Posterior instrumentation (n=14) | Thoracic (n=2) | 25.0±5.0 | 35.0±5.0 | 33.7±7.1 |
| Thoracolumbar (n=4) | 10.5±1.0 | 0.0±0.0 | 0.5±1.0 | |
| Lumbar (n=8) | 36.4±3.8 | 34.3±7.9 | 36.0±2.8 | |
| Total (n=26) | Thoracic (n=5) | 29.4±7.5 | 35.8±4.3 | 35.0±5.8 |
| Thoracolumbar (n=6) | 10.3±0.8 | 0.8±2.0 | 2.3±3.2 | |
| Lumbar (n=15) | 32.0±8.1 | 35.8±7.0 | 36.3±3.3 |
When anterior and posterior instrumentation groups were compared, there was no statistically significant difference in terms of early postoperative or final correction rates (Table 3). Overall, mean correction loss was 2.5°∓1.9°. For anterior and posterior instrumentation groups, mean correction losses of Cobb angle were 2.5°∓1.7° and 2.4°∓2.1°, respectively (Figs. 3, 4).
Fig. 3.
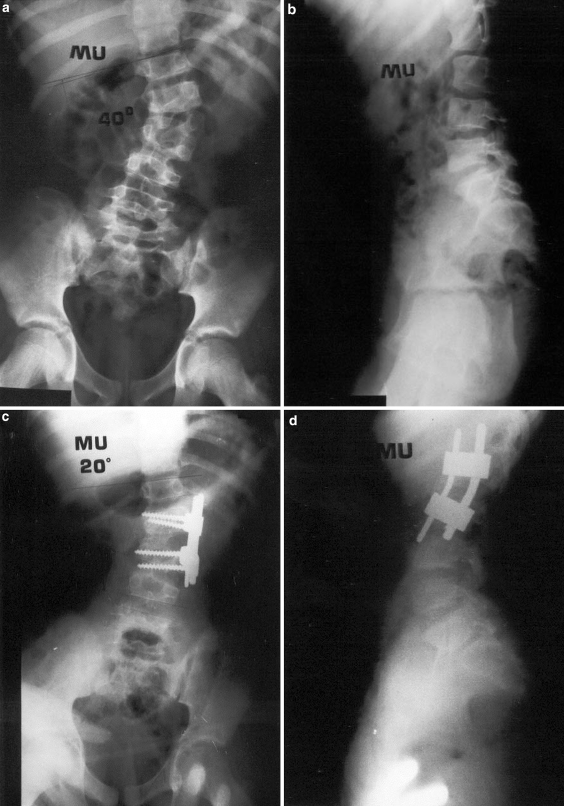
M.U., 12 year-old-male patient with L1–L2 unincarcerated fully segmented hemivertebra. Preoperative anterior–posterior (a) and lateral (b), postoperative anterior–posterior (c), and lateral (d) radiographies are seen. The patient had circumferential fusion and anterior instrumentation following complete hemivertebra excision. The preoperative 40° curve was corrected by 50% postoperatively
Fig. 4.
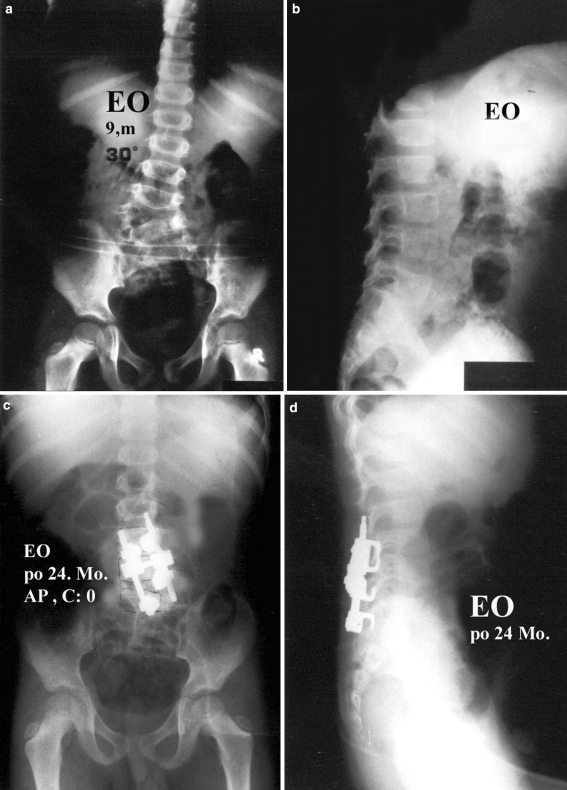
E.O., 9 year-old-male patient with L3–L4 unincarcerated fully segmented hemivertebra. The preoperative anterior–posterior (a), lateral (b), postoperative 24th month anterior–posterior (c), and lateral (d) radiographies are seen. The 30° curve before surgery was improved to 0° after anterior and posterior hemivertebra excision and posterior instrumentation. No correction loss was observed at the 24th month follow-up visit
Sagittal plane
Preoperative, postoperative and last follow-up visit values for sagittal contours at the hemivertebral region are shown in Table 3. Five patients had hemivertebra at the thoracic region. Preoperatively, only three (patient 1, 8, and 13) of these patients had thoracic kyphosis within normal physiological ranges at the thoracic region, postoperatively, all of these patients were improved to this range and thoracic kyphosis values were conserved postoperatively and at the last follow-up visit. In six patients with hemivertebra at thoracolumbar junction, thoracolumbar junction angle was brought from 10.3°∓0.8° to 0.8°∓2.0°, postoperatively. Five of these six patients had angles below 5° and one had 0° angle, which was considered within normal physiological limits. In 15 patients, hemivertebra was at the lumbar region. Of these patients, only four had lumbar lordosis within physiological ranges preoperatively, and six patients did so postoperatively.
In the sagittal plane, although it was expected that anterior compression with anterior instrumentation would exert kyphotic effect and posterior compression would exert lordotic effect, these effects were not pronounced. In patients with hemivertebra at the thoracic region and undergoing anterior instrumentation, it was established that kyphotic effect was favorable for the three patients already having hypokyphosis and thoracic kyphosis angles remained between 30° and 50°, and these values were preserved at the last visit. In seven patients with lumbar hemivertebra who underwent anterior instrumentation, kyphotic effect was minimal, with no important change in lumbar lordosis angles.
Marked improvement observed in two patients is thought to be associated with the derotation of prebent CDH rods. In one of the two patients with hemivertebra at the thoracolumbar junction and anterior instrumentation, postoperatively complete correction was obtained and junction angle was reduced to 5°.
In two patients (patient 13 and 20) with thoracic hemivertebra and posterior instrumentation, lordotic effect was not observed and thoracic kyphosis angles remained within physiological range (30°–50°). In this group, in four patients (Patient 14, 15, 21, and 22) with thoracolumbar hemivertebra, junction angle was lowered to 0°, which is considered within the normal physiological value; and 2° of correction loss was seen in one patient and no correction loss occurred in the rest. In eight patients with lumbar hemivertebra and posterior instrumentation, lordotic effect played a positive role; and in six of these patients, a lumbar lordosis within physiological range was obtained, with minimal correction loss at the last follow-up visit (0°–5°).
Trunk balance analysis
Overall, preoperative LT and SH values were 39.6±18.4 mm and 27.6±9.6 mm, respectively (Table 4). Postoperatively, LT and SH values regressed to 15.6∓9.5 mm and 9.5±7.7 mm, corresponding to the correction rates of 61.4±17.9% and 67.6±21.6%, respectively. The correction rates were all significant. Correction rates obtained for LT values were correlated with those for Cobb values of the curves in the frontal plane. Early postoperative correction rates in LT and SH values were not significantly different than correction rates obtained at the last follow-up visit. At the last visit, correction losses of 2.7±3.4 mm and 1.0±3.5 mm was found for LT and SH values, respectively.
Table 4.
Balance analysis of the patients
| Lateral trunk shift (LT) | Preoperative (mm) | Postoperative (mm) | t | P | Postoperative correction rate (%) | Final (mm) | t | P | Final correction rate (%) | t | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anterior instrumentation (n=12) | 41.7±13.8 | 17.4±9.1 | 12.5 | 0.00 | 60.6±14.4 | 20.9±8.9 | 9.3 | 0.00 | 50.6±13.6 | 3.4 | 0.74 |
| Posterior instrumentation (n=14) | 37.9±21.9 | 14.6±9.9 | 5.8 | 0.00 | 62.0±20.9 | 16.6±11.7 | 6.2 | 0.00 | 57.5±18.5 | 2.4 | 0.32 |
| t | 0.20 | 0.52 | |||||||||
| P | >0.05 | >0.05 | |||||||||
| Total (n=26) | 39.6±18.4 | 15.9±9.5 | 10.4 | 0.00 | 61.4±17.9 | 18.6±10.5 | 10.1 | 0.00 | 54.3±16.5 | 4.03 | 0.13 |
| Shift of head (SH) | Preoperative (mm) | Postoperative (mm) | t | P | Postoperative correction rate (%) | Final (mm) | t | P | Final correction rate (%) | t | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anterior instrumentation (n=12) | 26.0±6.6 | 7.4±5.5 | 8.5 | 0.00 | 70.9±21.3 | 10.0±4.6 | 7.8 | 0.00 | 59.8±18.4 | 3.9 | 0.89 |
| Posterior instrumentation (n=14) | 29.0±11.6 | 11.3±8.9 | 9.9 | 0.00 | 64.6±22.1 | 10.9±8.3 | 7.7 | 0.00 | 64.4±21.2 | 0.1 | 0.93 |
| t | 0.74 | 0.59 | |||||||||
| P | >0.05 | >0.05 | |||||||||
| Total (n=26) | 27.6±9.6 | 9.5±7.7 | 13.2 | 0.00 | 67.6±21.6 | 10.5±6.7 | 10.9 | 0.00 | 62.3±19.8 | 2.4 | 0.23 |
Preoperatively, none of the patients had a completely or clinically balanced curve. Postoperatively, a complete balance was obtained in 23.1% (n=6) and a clinical balance in 53.8% (n=14) of patients. Overall, 21 patients became balanced (76.9%). At the last visit, none of the postoperatively balanced patients lost their balance or developed any imbalance problem.
When balance values are considered, Table 4, for anterior and posterior instrumentation groups preoperative LT and SH values were comparable, and statistically significant correction was obtained in both groups. Additionally, the number of fully balanced and balanced patients was higher in anterior instrumentation group. A completely balanced or balanced curve was observed in 11 patients (91.7% of 12 patients) and 9 patients (64.3% of 14 patients) in the anterior and posterior instrumentation groups, respectively. At the last visit, correction losses were minimal in both groups. The rate of balanced curves did not change at the last visit in both groups.
Instrumented mobile segments
In anterior and posterior instrumentation groups, mean number of instrumented and fused mobile segments were 1.4±0.5 (1–2) and 3.3±1.6 (2–7), respectively, indicating that higher number of segments were conserved in anterior instrumentation group.
Complications
No pseudoarthrosis or neurological deficit occurred. A patient (patient 22) with monoparesis before the operation fully recovered postoperatively. Delay in wound healing occurred only in one patient and was treated medically without any need for surgical intervention.
Discussion
Royle performed the first hemivertebra excision in 1928 [1]. Prior to the development of modern instrumentation systems, excision of hemivertebra was not commonly performed, as it did not provide significant correction, except for fusion effect on curves and ceasing of progression; it also had serious neurological and systemic complications [1, 5, 16, 21]. Recently, Leatherman and Dickson [22] popularized it again. In many subsequent studies, it has been reported that complete excision of hemivertebra at the same session at one or two steps leads to pronounced improvement as well as spontaneous correction [4, 7, 10, 19]. Bradford and Boachie-Adjei reported a 70% correction in Cobb angle. Only 1° of correction loss occurred after anterior–posterior hemivertebra excision simultaneously performed at a single step [4]. King and Lowery [18], in their series with 7 patients, reported a 29.7° of final curve following two-step excision and 18° curve with simultaneous single step intervention. Callahan et al. reported the results of ten patients with 67% correction in curves at 40°, and Shono et al. reported 64% correction [11, 23]. Lazar and Hall improved preoperative curves from 47° to 14° in their series of 11 patients, while Hall and colleagues improved it from 54° to 33° [9, 12].
In the present study, 26 patients with congenital scoliosis, with fully segmented unlocked hemivertebra close to the lumbosacral junction, without development of a compensatory thoracolumbar curve and pelvic obliqueness but showing progression at follow-up visits and/or having neurological complaints such as pain and paresthesia, were considered as candidates for hemivertebra excision. All but four had curves over 40°. In these four patients, although they had curves between 30° and 36°, hemivertebrectomy was planned, since they progressed to this level in a short time span, and had marked cosmetic complaints and pain. Hemivertebrae of 26 patients were completely excised with anterior or posterior approaches during the same session with anterior or posterior fusion. Mean preoperative frontal Cobb angle was 45.5±11.4° and brought to 16.8±7.9° (64.4±13.9%), with a statistically significant difference (P=0.00). A 2.5±1.9° correction loss was found at the last follow-up visit, thus leading to a final correction rate of 61.2±13.3%. Although all patients had a minimum 48 months of follow-up, there are still some patients who have not completed their growth, so we cannot predict whether the final correction rate will be less or not. However, the correction rate obtained at the end of a mean follow-up period of 4 years and the minimal correctional loss still seem satisfactory.
Hemivertebra excision has been most frequently performed at lumbosacral region [1, 3, 5, 24–26]. Holte et al. [7] reported the results of 37 patients: 6 patients had mid-thoracic, 9 thoracolumbar, 7 lumbar, and 17 had lumbosacral hemivertebra. Deviren et al. [8] reported a 59% correction with thoracic and thoracolumbar hemivertebra excision in ten patients, and concluded that these procedures are safe in experienced hands. Of 26 patients included in this study, 5 had hemivertebra at thoracic region, 6 at thoracolumbar junction, 15 at lumbar region; and a neurological deficit was not developed in any of the patients undergoing hemivertebra excision. We conclude that excision can be safely carried out in these regions as well.
Sagittal contours at the hemivertebral region were impaired, particularly when the defect is in thoracolumbar region. In all but one of six patients with hemivertebra at thoracolumbar junction, abnormal junction was completely corrected. Also, sagittal contours were preserved at thoracic and lumbar regions and brought within normal ranges. In all patients, circumferential fusion mass was obtained and no pseudoarthrosis occurred. In view of these findings, it can be concluded that high correction and fusion rates were achieved with hemivertebra excision and anterior–posterior instrumentation, with minimal correction loss at the last follow-up visit.
Shono et al. [11] reported an improvement in lateral body shift from 23 mm to 3 mm. in patients with hemivertebra excision. Likewise, Deviren et al. [8] reported an improvement from 35 to 11 mm. In our study, preoperative mean LT and SH values were 39.6 mm and 27.6 mm, postoperatively regressing to 15.9 mm (61.4%) and 9.5 mm (67.6%), respectively. And the apical vertebra and head were markedly brought to the midline. At the last visit, minimal correction losses were seen, but final correction rates were not significantly different from postoperative ones (P>0.05). While all patients preoperatively had unbalanced curves, postoperatively 76.9% had balanced curves. At the last follow-up visit, this rate was retained with minimal correction loss.
The most widely used method in congenital scoliosis is in-situ fusion [1]. Keiffer has proposed that fusion in children is beneficial in terms of prevention of deformity progression [15]. Hemiepiphysiodesis and/or convex fusion to cease growth at the opposite site are the most widely used methods. There are also successful results reported with the “egg-shell” procedure performed transpedicularly. The ideal age for hemivertebrectomy is also controversial [1]. Kleemme et al. published the results of excision in six patients after a mean follow-up period of 34 months, and reported a 70° final correction in patients followed for 3 months after anterior–posterior complete excision. They concluded that this technique could be safely carried out in small children as well [19]. As well as other methods, some authors proposed that hemivertebrectomy could be performed at every age during childhood period. According to their point of view, hemivertebral excision can be performed more easily in small children. Moreover, they suggest that the defect caused with hemivertebrectomy is not a disadvantage, as it is rather small. However, it is unpredictable how the fusion will affect the deformity until the cessation of the growth. The most important problems for the children operated older than this age are the filling of the space created after excision and the correction of the curve. This can be done by correctional cast braces. Filling and closing of the space by compression is easier with instrumentation applications [1, 5, 10]. On the other hand, instrumentation in children brings about the problems of finding suitable pediatric instruments, subcutaneous protuberance of the instruments, difficulty in wound closure, wound healing problems, and infection. Shono et al. reported that single step posterior hemivertebra excision could be performed more safely in the adolescents [11]. Lubicky claims that hemivertebrectomy can be performed at any age. Lubicky [1] has reported that it is more difficult to close the space produced by hemivertebra excision, especially around the end of adolescence and in adulthood. In our study, hemivertebrectomy results of preadolescent–adolescent patients and other age groups were not compared. So, it is not possible to make a clear suggestion; no such comparative study exists in the literature. Our results suggest that: [20] hemivertebrectomy can be easily performed at adolescent ages and the space formed after the excision can be easily closed by instrumentation [27]; surgical procedure is neurologically safe and high correction rates can be obtained at frontal plane [6]; it is possible to obtain physiological sagittal contours and body balance in most of the patients. Therefore, we suggest that hemivertebra excision is a convenient, successful, and safe method, particularly in untreated or neglected congenital scoliosis cases due to type I-A hemivertebra.
There are few literature data on the use of anterior instrumentation following hemivertebrectomy [1, 5], and to the best of our knowledge there is no study comparing the results of anterior instrumentation with those of posterior instrumentation. In this study, the results obtained from 12 patients undergoing anterior instrumentation after hemivertebra excision were compared with those obtained from cases undergoing posterior instrumentation. For the anterior and posterior instrumentation groups, correction rates were similar (60.7±12.1% and 67.5±15.1%). For both groups, minimal correction losses were observed at the last follow-up visit.
When sagittal contours are considered, anterior instrumentation exerted favorable kyphotic effects on the thoracic region. Posterior instrumentation had positive lordotic effect at the lumbar region. Thoracolumbar junction angle was completely corrected with posterior instrumentation. In patients undergoing anterior instrumentation, we assumed that prebent CDH rods (a double rod system) could prevent kyphotic effect at the thoracic region. When TSRH instrumentation is used, there is a lordotic effect at the thoracal region of patients with normal kyphosis and a kyphotic effect at the lumbar region of the patients with normal lordosis because of the compression at the convex side, but these side effects are eliminated by giving physiological contours to the rods and placing the rods with cantilever maneuver and in mild distraction at the concave side.
Lateral trunk shift and head shift was significantly improved with minimal correction loss at the last follow-up visit in both groups. When groups were compared with respect to the number of mobile segments instrumented, it was seen that mean 1.4±0.6 segments were incorporated in fusion area in anterior instrumentation and 3.0±1.8 segments in posterior instrumentation, with the superiority of anterior instrumentation preserving more mobile segments.
No neurological deficit has been reported in many patients undergoing hemivertebra excision [4, 9, 11, 13, 19]. This technique is safe in this respect even in thoracic and thoracolumbar regions [8, 23]. Holte et al. [7] reported the development of radiculopathy in seven patients, pseudoarthrosis in three, wound infection in three, and the need to extend fusion area in six patients in their study including 37 patients. King and Lowery [18] reported one case of root paresis in their series of seven patients. In the present study, no neurological deficit occurred; only in one patient delayed wound healing was observed.
Conclusion
Complete hemivertebra excision carried out during the same session at two steps is quite safe for low thoracic, thoracolumbar and lumbar regions of vertebral column. High correction and fusion rates can be achieved with anterior and posterior instrumentation. Minimal correction loss is observed at follow-up visits. Sagittal contours may be brought within normal physiological ranges. Lateral body shift is considerably corrected and head is shifted to midline in the majority of the patients. At the last follow-up visit, the number of balanced patients was preserved, and no additional imbalance or decompensation problem was observed. Frontal plane, sagittal plane, and balance value correction rates were similar for anterior and posterior instrumentation groups. Therefore, the surgeon should determine the site of instrumentation by his or her experience. Preservation of higher number of mobile segments is an advantage of anterior instrumentation. We conclude that complete hemivertebra excision along with anterior or posterior instrumentation is an efficacious and safe method in adolescent patients with hemivertebra.
References
- 1.Lubicky JP. Congenital scoliosis. In: Bridwell K, DeWald RL, editors. The textbook of spinal surgery. 2. Philadelphia: Lippincott-Raven Publishers; 1997. pp. 345–364. [Google Scholar]
- 2.McMaster MJ, Singh H. Natural history of congenital kyphosis and kyphoscoliosis. A study of one hundred and twelve patients. J Bone Joint Surg. 1999;81-A(10):1367–1383. doi: 10.2106/00004623-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Winter R. Congenital Scoliosis: the role of anterior and posterior fusion. J Turk Spine Surg. 1994;5(3):81. [Google Scholar]
- 4.Bradford DS, Boachie–Adjei O. One stage anterior and posterior hemivertebral resection and arthrodesis for congenital scoliosis. J Bone Joint Surg. 1990;72-A:536–540. [PubMed] [Google Scholar]
- 5.Winter RB. Congenital scoliosis. Orthop Clin North Am. 1988;19:395–408. [PubMed] [Google Scholar]
- 6.Bergoin M, Bollini G, Taibi L, Cohen G. Excision of hemivertebrae in children with congenital scoliosis (Abstract) Ital J Orthop Traumatol. 1986;12(2):179–184. [PubMed] [Google Scholar]
- 7.Holte DC, Winter RB, Lonstein JE, Denis F. Excision of hemivertebrae and wedge resection in the treatment of congenital scoliosis. J Bone Joint Surg. 1995;77-A:159. doi: 10.2106/00004623-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Deviren V, Bevren S, Smith JA, Emami A, Hu SS, Bradford DS. Excision of hemivertebrae in the management of congenital scoliosis involving the thoracic and thoracolumbar spine. J Bone Joint Surg. 2001;83-B:496–500. doi: 10.1302/0301-620X.83B4.11699. [DOI] [PubMed] [Google Scholar]
- 9.Lazar RD, Hall JE. Simultaneous anterior and posterior hemivertebra excision. Clin Orthop. 1999;364:76–84. doi: 10.1097/00003086-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura H, Matsuda H, Konishi S, Yamano Y. Single-stage excision of hemivertebrae via the posterior approach alone for congenital spine deformity: follow-up period longer than ten years. Spine. 2002;27(1):110–115. doi: 10.1097/00007632-200201010-00026. [DOI] [PubMed] [Google Scholar]
- 11.Shono Y, Abumi K, Kaneda K. One-stage posterior hemivertebra resection and correction using segmental posterior instrumentation. Spine. 2001;26:752–757. doi: 10.1097/00007632-200104010-00011. [DOI] [PubMed] [Google Scholar]
- 12.Hall JE, Herndon WA, Levine CR. Surgical treatment of congenital scoliosis with or without Harrington instrumentation. J Bone Joint Surg. 1981;63-A:608–619. [PubMed] [Google Scholar]
- 13.Ruf M, Harms J. Hemivertebra resection by a posterior approach innovative operative technique and first results. Spine. 2002;27(10):116–1123. doi: 10.1097/00007632-200205150-00020. [DOI] [PubMed] [Google Scholar]
- 14.Ruf M, Harms J. Posterior hemivertebra resection with transpedicular instrumentation: early correction in children aged 1 to 6 years. Spine. 2003;28(18):2132–2138. doi: 10.1097/01.BRS.0000084627.57308.4A. [DOI] [PubMed] [Google Scholar]
- 15.Kieffer J, Dubousset J. Combined anterior and posterior convex epiphysiodesis for progressive congenital scoliosis in children aged < or =5 years. Eur Spine J. 1994;3:120–125. doi: 10.1007/BF02221453. [DOI] [PubMed] [Google Scholar]
- 16.Jashwhich D, Ali RM, Patel TC, Green DW. Congenital scoliosis. Curr Opin Pediatr. 2001;12:61–66. doi: 10.1097/00008480-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Kesling KL, Lonstein JEA, Denis F, Perra JH, Schwender JD, Transfeldt EE, Winter RB. Crankshaft phenomenon after posterior spinal arthrodesis for congenital scoliosis: a review of 54 patients. Spine. 2003;28(3):267–271. doi: 10.1097/00007632-200302010-00012. [DOI] [PubMed] [Google Scholar]
- 18.King JD, Lowery GL. Results of lumbar hemivertebral excision for congenital scoliosis. Spine. 1991;16:778–782. doi: 10.1097/00007632-199107000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Klemme WR, Polly DW, Urchowski JR. Hemivertebral excision for congenital scoliosis in very young children. J Pediatr Orthop. 2001;21(6):761–764. doi: 10.1097/00004694-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Bernhard M. Normal spinal anatomy: normal sagittal plane alignment. In: Bridwell KH, DeWald RL, editors. The textbook of spinal surgery. Philadelphia: Lippincott-Raven Publishers; 1997. pp. 188–189. [Google Scholar]
- 21.Compere EL. Excision of hemivertebrae for correction of congenital scoliosis. J Bone Joint Surg. 1932;14-A:555–560. [Google Scholar]
- 22.Leatherman KD, Dickson RA. Two stage correction surgery for congenital deformities of the spine. J Bone Joint Surg. 1979;61-B(3):324–328. doi: 10.1302/0301-620X.61B3.479255. [DOI] [PubMed] [Google Scholar]
- 23.Callahan BC, Georgopoulus G, Ellert RE. Hemivertebral excision for congenital scoliosis. J Pediatr Orthop. 1997;17:96–99. doi: 10.1097/00004694-199701000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Winter RB. Convex anterior and posterior hemiarthrodesis and hemiepiphysiodesis in young children with progressive congenital scoliosis. J Pediatr Orthop. 1981;1:361–366. doi: 10.1097/01241398-198112000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Winter RB, Moe JH, Lonstein JE. Posterior spinal arthrodesis for congenital scoliosis. An analysis of the cases of two hundred and ninety patients five to nineteen years old. J Bone Joint Surg. 1984;66-A:1188–1197. [PubMed] [Google Scholar]
- 26.Winter RB, Lonstein JE, Denis F, Sta-Ana la Rosa H. Convex growth arrest for progressive congenital scoliosis due to hemivertebrae. J Pediatr Orthop. 1988;8:633–638. doi: 10.1097/01241398-198811000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Benli IT, Tüzüner M, Akalin S, Kis M, Aydin E, Tandogan R. Spinal imbalance and decompensation problems in patients treated with Cotrel-Dubousset instrumentation. Eur Spine J. 1996;5:380–386. doi: 10.1007/BF00301965. [DOI] [PubMed] [Google Scholar]