Abstract
A cross-sectional study to investigate regional cerebral blood flow (rCBF) in patients with chronic whiplash syndrome and chronic neck pain patients without previous history of trauma along with a healthy control group. Chronic neck pain is a common disorder and a history of cervical spine injury including whiplash trauma constitute a risk factor for persistent neck pain. The aetiology of the late whiplash syndrome is unknown with no specific diagnostic criteria based on imaging, physiological, or psychological examination. Earlier studies indicate a parieto-occipital hypoperfusion but it is unclear if the hypoperfusion represents a response to chronic pain. The rCBF was monitored in 45 patients with chronic neck pain: 27 cases with chronic whiplash syndrome and 18 age and gender matched cases with non-traumatic chronic neck pain. The rCBF was estimated with single-photon emission computed tomography (SPECT) using technetium-99m hexamethylpropylene amine oxime (HMPAO). The non-traumatic patients displayed rCBF changes in comparison with the whiplash group and the healthy control group. These changes included rCBF decreases in a right temporal region close to hippocampus, and increased rCBF in left insula. The whiplash group displayed no significant differences in rCBF in comparison with the healthy controls. The present study suggests different pain mechanisms in patients with chronic neck pain of non-traumatic origin compared to those with chronic neck pain due to a whiplash trauma.
Keywords: Whiplash, Chronic neck pain, 99mTc-HMPAO rCBF-SPECT, Brain imaging, Regional cerebral blood flow
Introduction
Chronic neck pain is a common disorder in the Western world [1, 6]. According to a recent study, nearly one-fifth of the population in northern Sweden reported chronic neck pain, defined as continuous neck pain of more than 6 months duration [15]. Several factors such as female gender, number of children, self-assessed health (age-matched general health evaluated on a four point scale), psychological status, educational level, mental stress, and musculoskeletal pain in general have been associated with the development of chronic neck pain [7, 8, 40]. Cross-sectional and longitudinal population studies have shown that a history of cervical spine injury including whiplash trauma constitute a risk factor for persistent neck pain [7, 8, 24]. About one-third of the patients with a whiplash injury develop chronic symptoms, often leading to a long-term sick leave/disability with consequences for the individual and the society, i.e. late whiplash syndrome [3, 37].
The aetiology of the late whiplash syndrome is unknown and several theories have been proposed varying from musculoskeletal lesions to psychological problems. There are, however, no specific diagnostic criteria based on imaging, physiological, or psychological examination. No organic lesions have been verified and reports of the psychological influence to the syndrome are contradictory [35, 36]. The diagnosis of chronic whiplash syndrome remains clinical until new means of diagnostics has been developed. Generalised hypersensitivity and central nervous pain mechanisms have been the focus of interest lately [2, 9, 38]. Earlier studies indicate a parieto-occipital hypoperfusion but it is unclear if the hypoperfusion represents a response to chronic pain [14, 28–31]. It has been suggested that these blood flow changes may be caused by activation of nociceptive afferent nerves from the cervical spine [29].
Studies comparing rCBF at rest in chronic pain patients to normal controls [11, 18, 21, 25, 26, 41] have reported reductions or asymmetric changes in thalamus and reductions in frontal, temporal, parietal, occipital regions. These reports are not conclusive since knowledge about cerebral pain mechanisms is mainly based on experimental studies. However, the rCBF response in acute experimental noxious stimuli are well evaluated and rCBF changes have been registered in secondary somatosensory cortex (SII), the insular regions, the anterior cingulate cortex, and with slightly less consistency the contralateral thalamus as well as the primary somatosensory cortex (SI) [33]. Changes in these regions are proposed to reflect the sensory, cognitive and affective dimensions of pain [10, 11, 33].
Chronic whiplash syndrome which can be considered as a subgroup of chronic neck pain in general has been continuously debated. Our hypothesis was that chronic neck pain patients have a similar rCBF pattern irrespective of traumatic or non-traumatic origin.
The aim of the present study was to investigate rCBF in patients with chronic symptoms after whiplash injury and in patients with chronic neck pain without a history of neck injury and compare the rCBF findings with healthy subjects at rest. The study was approved by the ethical and radiation protection committee at Umeå University.
Materials and methods
The present study includes 45 patients with chronic neck pain referred to Norrland’s University Hospital at Umea in Sweden in the period 1997–2001. All patients gave written informed consent to participate in the study. The inclusion criteria were disabling chronic neck pain that resulted in full or halftime sick leave, or change in profession. All patients underwent a clinical investigation to exclude infection, rheumatoid arthritis, tumour or metastases as a source of pain. Furthermore, patients with a history of head injury, loss of consciousness, fractures of the cervical spine, serious psychiatric disorders and other severe disorders of the central nervous system or drug abuse were excluded.
Twenty-seven of the patients (18 women, 9 men) had a chronic whiplash syndrome. The mean age was 41 years (range 26–65) and the average pain duration was 7.1 years (range 3–20) (Table 1).
Table 1.
Subject characteristics in whiplash, non-traumatic and the healthy subjects
| Healthy controls | Non-traumatic | Whiplash | t test* | |
|---|---|---|---|---|
| Number of subjects | 15 | 18 | 27 | |
| Male | 8 | 5 | 9 | |
| Female | 7 | 13 | 18 | |
| Mean age | ||||
| Years | 55.5±4.4 | 44.0±9.6 | 40.9±11.6 | 0.338 NS |
| Range | 50–61.0 | 29–61.2 | 26–65.4 | |
| Pain duration | ||||
| Years | 8.5±3.2 | 7.1±5.0 | 0.248 NS | |
| Range | 3–15.0 | 3–20.0 | ||
| Duration of education (years) | 10.3 | 11.0 | 0.138 NS | |
| VAS (1–10) | 5.5 | 5.3 | 0.921 NS | |
| Sick leave (%) | 77 | 66 | 0.432 NS | |
VAS visual analogue scale
* Independent sample t test between patient groups
Eighteen of the patients (13 women, 5 men) had a non-traumatic chronic neck pain with a mean age of 44 years (range 29–62). The average pain duration was 8.5 years (range 3–15) (Table 1). The non-traumatic patients were selected from a patient medical chart database at the orthopaedic department based on the inclusion and exclusion criteria above. Sixty-one patients fulfilled these criteria and 26 of these matched the whiplash patients regarding age, sex, symptom duration, degree of pain, and educational level (Table 1). These patients were asked to join the study and 18 agreed to participate. The diagnosis was a degenerative disorder without myelopathy or cervical nerve root affliction that would not benefit from surgical treatment.
Fifteen healthy subjects (8 men, 7 women) were included from a large prospective research project in the community of Umeå, Betula [27] as the healthy control group. The inclusion criteria were good subjective and objective health. The mean age of the control group was 55 (range 50–61) years. The Radiation Committee of Umeå University approved this group to be recruited among healthy individuals older than 50 years.
Subjective complaints
The subjective complaints in the 45 patients were collected from the medical charts and summarised in Table 2. Additional information was achieved by interviewing the patients. Data from each group were compared by Mann–Whitney U test. Pain intensity was scored by a visual analogue scale (VAS) 1–10.
Table 2.
Subjective complaints in the whiplash and non-traumatic patient group
| Complaints | Patient group | Percentage of group | Mann–Whitney U |
|---|---|---|---|
| Neckpain | Whiplash | 100 | 1.000 NS |
| Non-traumatic | 100 | ||
| Headache | Whiplash | 96 | 0.001*** |
| Non-traumatic | 56 | ||
| Cervico-brachioalgia | Whiplash | 85 | 0.723 NS |
| Non-traumatic | 89 | ||
| Neck stiffness | Whiplash | 69 | 0.859 NS |
| Non-traumatic | 67 | ||
| Backpain | Whiplash | 59 | 0.202 NS |
| Non-traumatic | 78 | ||
| Sleep disturbances | Whiplash | 41 | 0.335 NS |
| Non-traumatic | 56 | ||
| Poor concentration | Whiplash | 54 | 0.014* |
| Non-traumatic | 17 | ||
| Forgetfulness | Whiplash | 88 | 0.001** |
| Non-traumatic | 39 | ||
| Fatigue | Whiplash | 37 | 0.901 NS |
| Non-traumatic | 39 |
NS non-significant
Mann–Whitney U non-parametric test ***P<0.001, **P<0.01, *P<0.05
Image acquisition and processing
The rCBF-SPECT studies were performed at the department of Diagnostic Radiology and Nuclear Medicine, at Norrland’s University Hospital, in Umeå. A three-headed gamma camera, Neurocam (General Electric, Milwaukee, WI, US) equipped with low energy high resolution (LEHR) collimators, was used. The spatial resolution of the system (FWHM) at a 100 mm distance from the camera was 6.9 mm. The SPECT studies were performed in a 360° stepwise rotation, with 90 s acquisition in 64 equal angles. The images were acquired in a 128×128 pixel matrix. All datasets were reconstructed into transaxial images using the filtered back projection technique. A 2D Hanning filter with a cut-off frequency of 0.9 cm−1 was used for pre-processing. The pixel size in reconstructed images was 2×2 mm. Attenuation correction was performed according to the Chang algorithm [4]. The outline was automatically defined at 10% of maximum count with an ellipse in each slice. The attenuation coefficient was set to the standard value of 0.12 cm−1 for this system. The image data was exported as Interfile data from the GE Genie station (General Electric, Milwaukee, WI, US) and converted to ANALYZE format before further analysis.
The radiotracer 99mTc-Hexamethylparaamnooxime, (HMPAO) was prepared on the day of examination by eluting 99mTcO4- from a 99Mo/99mTc generator just before adding it to a freeze-dried kit (Ceretec™, Nycomed Amersham plc, Buckinghampshire, UK) according to the instructions of the manufacturer.
Each patient received 1,000 MBq 99mTc-HMPAO i.v., as in clinical routine. The healthy controls received a reduced activity of 600 MBq, according to the local radiation protection committee. A 30 min rest with eyes closed in a dimly lit room preceded the administration of radiotracer. Following injection, a 10–40 min rest was undertaken before examination with the scintillation camera. In order to obtain a robust scanning geometry an individually formed thermoplastic face holder [22] was used together with carbon fibre instrumentation connected to the examination bed. The thermoplastic face holder did not significantly affect the attenuation. The uptake of 99mTc-HMPAO at rCBF-SPECT reflects the brain function, which is related to the blood flow. The uptake in the brain occurs mainly 1–2 min following the injection and is influenced by the emotional state at the time of injection.
Statistical parametric mapping 99 (SPM99 Welcome Department of Cognitive Neurology, University College, London) was used to estimate differences in rCBF between the groups. Calculations were performed with Matlab 6.0 (Mathworks Inv., Sherborn, MA, USA). The rCBF-SPECT studies were registered to the SPM SPECT template using a non-linear anatomical standardisation with 12 affined parameters, and bi-linear interpolation. For the statistical analysis, the registered image data were smoothed with a 12 mm 3D Gaussian filter kernel. The confounding effect of varying global activity was removed by proportional scaling to a global value of 50 ml/min per 100 g. A grey matter threshold of 0.8 was used. A compare-populations design (two sample t test) using one scan per subject condition was used to evaluate differences in rCBF between the three groups. The evaluation design in SPM-contrasts was defined to investigate regions of increased or decreased radiotracer uptake in all three groups compared to each other. An SPM-cluster was considered significant at a P value below 0.05 corrected for multiple non-independent comparisons. Peaks consisting of more than 10 voxels (k=10) with an uncorrected P value of 0.001 is reported and related to previous findings.
To determine the anatomical localisation of the SPM data, a conversion of MNI coordinates to the Talairach brain atlas was done using a non-linear function described at CBU Imaging web site (http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html). All SPM results are described in Talairach coordinates. The SPM99 add on MNI Space utility (MSU) developed in the PET Lab of Institute of the Human Brain (http://www.ihb.spb.ru/~pet_lab/MSU/MSUMain.html) was used to analyze the anatomical extension of the significant clusters in terms of anatomical region labels used by the Talairach Daemon (http://www.ric.uthscsa.edu/ric_resources/resources.html). SPM cluster extension is described in terms of hemispheres, brain lobes, gyri and lobules, as well as nuclei. The SPM data were also compared to MRI-templates in SPM in order to visualise the anatomical localisations.
Results
Subjective complaints
The clinical symptoms are summarised in Table 2. All patients had complaints from the head, neck and back region. A higher number of complaints such as headache, forgetfulness and concentration problems were registered in the whiplash patient group compared to the non-traumatic pain patient group.
SPM-contrasts to detect altered blood flow
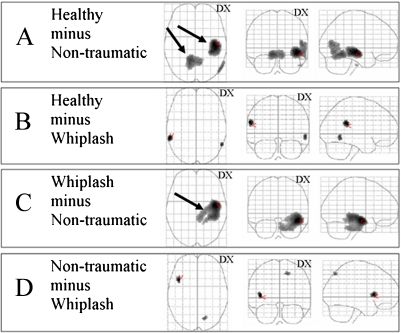
Non-traumatic patients compared to healthy controls: The contrast to detect a relatively lower rCBF in non-traumatic pain patients compared to healthy controls (healthy minus non-traumatic) showed two significant clusters in the right hemisphere and right cerebellum (Fig. 1a). The major cluster with reduced rCBF in the right hemisphere (11 ml, 1,323 voxels) mainly involved the temporal lobe and the limbic lobe. This cluster included one-fourth of the caudate tail and approximately one-fifth of hippocampus and a part of the parahippocampal gyrus. The minor cluster (7 ml, 894 voxels) was located at the centre of the cerebellum, and was distributed around two-thirds in the left lobe involving mainly the anterior lobe. The cluster size and Talairach coordinates are described in Table 3 and the anatomical localisations of the clusters in Table 4. A non-significant peak with a lower rCBF was detected in the right temporooccipital cortex (P=0.001 uncorrected), (Fig. 1a, Table 3). The contrast to detect relatively higher rCBF in non-traumatic pain patients compared to healthy controls (non-traumatic minus healthy) showed no significant cluster.
Fig. 1.
Maximum intensity projections (MIP) of regions with differences in rCBF between the non-traumatic, whiplash and healthy groups. a Healthy minus non-traumatic, b healthy minus whiplash, c whiplash minus non-traumatic, d non-traumatic minus whiplash. Significant (P<0.05 corrected) clusters are marked with black arrows. The contrasts whiplash minus healthy and non-traumatic minus healthy did not show any significant clusters
Table 3.
Cerebral regions with differences in rCBF between the non-traumatic, whiplash and healthy groups showing size, Talairach coordinate, anatomical location
| Cluster P (corrected) | Cluster Size (voxels) | t value | Voxel P (uncorrected) | Talairach coordinates x,y,z (mm) | |||
|---|---|---|---|---|---|---|---|
| Healthy minus non-traumatic | |||||||
| R. temporal lobe | 0.000* | 1,323 | 4.57 | 0.000 | 46 | −20 | −7 |
| R. middle temporal gyrus BA 37 | 0.112 | 269 | 3.90 | 0.000 | 55 | −66 | 9 |
| L. cerebellum culmen | 0.002* | 894 | 3.95 | 0.000 | −10 | −51 | −8 |
| Healthy minus whiplash | |||||||
| L. superior temporal gyrus | 0.485 | 97 | 3.82 | 0.000 | −57 | −38 | 20 |
| R. middle temporal gyrus | 0.785 | 37 | 3.65 | 0.000 | 57 | −55 | −2 |
| Whiplash minus non-traumatic | |||||||
| R. temporal lobe | 0.000* | 2,700 | 4.75 | 0.000 | 46 | −12 | −11 |
| Non-traumatic minus whiplash | |||||||
| R. parietal lobe precuneus BA7 | 0.861 | 23 | 3.42 | 0.001 | 18 | −67 | 49 |
| L. insula, BA 13 | 0.584 | 75 | 3.62 | 0.000 | −40 | 16 | 3 |
Height threshold, P<=0.001 uncorrected, k=10; voxel size = 2×2×2 mm
*Significant clusters (P<0.05 corrected)
Table 4.
An anatomical description of regions involved in significant clusters (P<0.05 corrected), two in the contrast ‘healthy minus non-traumatic’ and one in the contrast ‘whiplash minus non-traumatic’ estimated by MSU (MNI Space utility)
| Brain region | Healthy minus non-traumatic | Whiplash minus non-traumatic | |||||
|---|---|---|---|---|---|---|---|
| Percentage of cluster | Percentage of brain region | Percentage of cluster | Percentage of brain region | Percentage of cluster | Percentage of brain region | ||
| Hemispheres | Left cerebrum | 0.6 | 0 | ||||
| Right cerebrum | 100 | 1.4 | 82.3 | 2.3 | |||
| Left cerebellum | 71.5 | 5.5 | |||||
| Right cerebellum | 27.5 | 2.1 | 3.2 | 0.7 | |||
| Right brainstem | 9.7 | 11.1 | |||||
| Lobes | Limbic lobe (R) | 6.1 | 0.9 | 0.5 | 0.1 | 21.6 | 6.9 |
| Pons (R) | 9.4 | 24.9 | |||||
| Temporal lobe (R) | 66.7 | 6 | 47.7 | 8.8 | |||
| Gyri and lobules | Insula (L) | ||||||
| Insula (R) | 5.5 | 3.4 | 2.9 | 3.7 | |||
| Parahippocampal gyrus (R) | 5.8 | 3.8 | 0.1 | 0.1 | 20.2 | 27 | |
| Middle temporal gyrus (R) | 11.4 | 3 | 4.9 | 2.6 | |||
| Superior temporal gyrus (R) | 6.7 | 1.7 | 3.7 | 1.9 | |||
| Nuclei | Hippocampus (R) | 1.9 | 20.5 | 2.5 | 54.9 | ||
| Caudate tail (R) | 1.1 | 27.3 | 0.5 | 25.5 | |||
MSU estimates the brain regions part of the cluster (first column) and the reverse relation, cluster part of different brain regions (second column). The regions are described in terms of four Talairach daemon atlas levels dividing the brain into hemispheres, brain lobes, gyri/lobules and nuclei
Whiplash patients compared to healthy controls: the contrast to detect relatively lower rCBF in whiplash patients compared to healthy controls (healthy minus whiplash) did not reveal any significant clusters. However, two non-significant small regional differences, one in the right temporal region and one in the left temporoparietal region (Fig. 1b) were detected at an uncorrected voxel level of P=0.001. The Talairach coordinates are shown in Table 3. The reversed contrast to detect an increased rCBF in whiplash compared to healthy controls did not reveal any significant difference.
Whiplash compared to non-traumatic patients: The contrast to detect relatively higher rCBF in whiplash compared to non-traumatic pain patients (whiplash minus non-traumatic) showed one significant extensive cluster including 22 ml, 2,700 voxels, mainly located in the right hemisphere extending into the right side of the brainstem. Half of the cluster was located in the right temporal lobe, one-fifth in the limbic lobe, and one tenth in pons. The cluster involved half of hippocampus and a quarter of the parahippocampal gyrus in which the global maxima was located (Table 3, Fig. 1c). The anatomical localisation of the significant cluster is presented in Table 4. The contrast to detect a relatively higher rCBF in non-traumatic pain patients compared to whiplash patients (non-traumatic minus whiplash) showed a non-significant small region in the parietal lobe involving precuneus. In addition, there was also an rCBF difference in the anterior part of the left insula (uncorrected P=0.001), (Fig. 1d, Table 3).
Discussion
Our hypothesis of a similar rCBF pattern in chronic neck pain patients irrespective of a traumatic or non-traumatic origin was not supported by our results. Although the whiplash group and the non-traumatic group were comparable concerning clinical parameters of importance for the development of chronic pain, there was a considerable difference in rCBF between the groups. No significant changes were detected in the whiplash group while the non-traumatic group showed a significant altered rCBF pattern compared to healthy controls.
The rCBF pattern in patients with non-traumatic chronic neck pain with reductions in parts of the parahippocampal gyrus close to hippocampus may reflect a suppression of brain systems subserving episodic memory and emotional response to a known aversive stimulus [16, 32]. The affected temporal regions normally participate in the cognitive processing of pain and memory, and it might be that the rCBF changes seen could either reflect an anxiety related cognitive processing of pain [5, 23, 34, 39] or could be a coping strategy for handling a known painful situation [32] in the non-traumatic pain group. Unilateral chronic pain has shown contralateral rCBF decrease in thalamus [13, 17, 20]. This decrease could not be seen on the chosen significance level. An explanation to this disagreement could be the fact that the chronic neck pain in the patient groups was without lateralisation. The significant decrease of rCBF in parts of cerebellum in the non-traumatic neck pain group is supported by studies showing motor-related areas. i.e. the striatum, cerebellum and supplementary motor area, being associated with pain [11, 33]. The pronounced rCBF reductions in the medial temporal lobe support a continuous pain processing at rest in the non-traumatic group. These rCBF changes were not seen in the whiplash group. The finding of a reduced rCBF in the left temporoparietal region, mainly located in somatosensory area in the whiplash group compared to the healthy group (Fig. 1b) is interesting, even though not significant, since it is in concordance with previous studies showing changes in these regions [29]. This reduction might support afferent pain input with absent pain processing. The reason for the unexpected ‘normal findings’ in the whiplash group is not known. The peaks with a higher rCBF in pain related areas as left insular region and right precuneus in non-traumatic patients compared to whiplash patients further indicate a difference in pain mechanism between the groups.
The fact that patients with chronic neck pain have a heterogeneous aetiology presents a problem when selecting patients. However, all chronic pain patients have been extensively examined including an MRI investigation. In addition, all patients have achieved symptom-diagnosis like chronic neck pain secondary to degenerative disorders or chronic whiplash syndrome. Both groups included a few patients who had radicular symptoms without neurological deficiencies. There were no significant differences in any of the investigated clinical parameters except a history of whiplash trauma.
The included patients represent a common chronic disorder in the Western society. These patients are often difficult to treat since they usually lack an organic diagnosis. Hence, we consider it important to perform cerebral investigations in a variety of chronic pain patients in order to explore differences in central expression of pain. Our findings may enhance the knowledge of central pain processing in chronic pain patients.
Although the whiplash patients express the same degree of pain and disability as the other chronic patients, rCBF changes reflecting cerebral pain processing was not seen in comparison to the healthy controls. The similarity of whiplash and healthy controls as well as the differences between traumatic and non-traumatic patients is unexpected and has to be validated and further investigated.
Due to the small number of included patients and the fact that there were no significant differences between pain groups in terms of gender distribution, we abstained from evaluating the gender differences in this study. However, in previous studies differences between male and female subjects in the mean rCBF and a lateralisation of brain activity has been observed [12, 19].
This study provides support for a continuous cerebral pain processing in non-traumatic chronic neck pain patients. However, support for a continuous cerebral pain processing could not be found in the whiplash patients. Therefore, the present study suggests different pain mechanisms in patients with chronic neck pain of non-traumatic origin compared to neck pain after whiplash injury. Thus, the present study could not diminish the controversy concerning chronic neck pain in chronic whiplash patients. Further studies to investigate differences in brain function/blood flow patterns at different pain models/mechanisms are, therefore, needed.
Acknowledgements
Financial support was provided by Stiftelsen Länsförsäkringsbolagens Forskningsfond, The National Societies for Aiding Traffic Accident Victims and Polio Victims, Borgerskapets Research Foundation in Umeå, foundations of Lion, KK (kunskap och kompetens) and the Medical Faculty, Umeå University. We are grateful to the Betula project and Professor Lars-Göran Nilsson for providing control data supported by the Swedish Council for Research in the Humanistic and Social Sciences.
References
- 1.Andersson HI, Ejlertsson G, Leden I, Rosenberg C. Chronic pain in a geographically defined general population: studies of differences in age, gender, social class, and pain localization. Clin J Pain. 1993;9:174–182. doi: 10.1097/00002508-199309000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Banic B, Petersen-Felix S, Andersen OK, Radanov BP, Villiger PM, Arendt-Nielsen L, et al. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain. 2004;107:7–15. doi: 10.1016/j.pain.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Barnsley L, Lord S, Bogduk N. Whiplash injury. Pain. 1994;58:283–307. doi: 10.1016/0304-3959(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 4.Chang LT. A method for attenuation correction in radionuclide computed tomography. IEEE Trans Nucl-Sci. 1978;25:638–643. [Google Scholar]
- 5.Cloninger CR. A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatr Dev. 1986;4:167–226. [PubMed] [Google Scholar]
- 6.Cote P, Cassidy JD, Carroll L. The Saskatchewan Health and Back Pain Survey. The prevalence of neck pain and related disability in Saskatchewan adults. Spine. 1998;23:1689–1698. doi: 10.1097/00007632-199808010-00015. [DOI] [PubMed] [Google Scholar]
- 7.Cote P, Cassidy JD, Carroll L. The factors associated with neck pain and its related disability in the Saskatchewan population. Spine. 2000;25:1109–1117. doi: 10.1097/00007632-200005010-00012. [DOI] [PubMed] [Google Scholar]
- 8.Croft PR, Lewis M, Papageorgiou AC, Thomas E, Jayson MI, Macfarlane GJ, et al. Risk factors for neck pain: a longitudinal study in the general population. Pain. 2001;93:317–325. doi: 10.1016/S0304-3959(01)00334-7. [DOI] [PubMed] [Google Scholar]
- 9.Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Giani C, Zbinden AM, Radanov BP. Central hypersensitivity in chronic pain after whiplash injury. Clin J Pain. 2001;17:306–315. doi: 10.1097/00002508-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Davis KD, Taub E, Duffner F, Lozano AM, Tasker RR, Houle S, et al. Activation of the anterior cingulate cortex by thalamic stimulation in patients with chronic pain: a positron emission tomography study. J Neurosurg. 2000;92:64–69. doi: 10.3171/jns.2000.92.1.0064. [DOI] [PubMed] [Google Scholar]
- 11.Derbyshire SW, Jones AK, Creed F, Starz T, Meltzer CC, Townsend DW, et al. Cerebral responses to noxious thermal stimulation in chronic low back pain patients and normal controls. Neuroimage. 2002;16:158–168. doi: 10.1006/nimg.2002.1066. [DOI] [PubMed] [Google Scholar]
- 12.Derbyshire SW, Nichols TE, Firestone L, Townsend DW, Jones AK. Gender differences in patterns of cerebral activation during equal experience of painful laser stimulation. J Pain. 2002;3:401–411. doi: 10.1054/jpai.2002.126788. [DOI] [PubMed] [Google Scholar]
- 13.Di Piero V, Ferracuti S, Sabatini U, Tombari D, Di Legge S, Pantano P, et al. Diazepam effects on the cerebral responses to tonic pain: a SPET study. Psychopharmacology (Berl) 2001;158:252–258. doi: 10.1007/s002130100843. [DOI] [PubMed] [Google Scholar]
- 14.Freitag P, Greenlee MW, Wachter K, Ettlin TM, Radue EW. fMRI response during visual motion stimulation in patients with late whiplash syndrome. Neurorehabil Neural Repair. 2001;15:31–37. doi: 10.1177/154596830101500105. [DOI] [PubMed] [Google Scholar]
- 15.Guez M, Hildingsson C, Nilsson M, Toolanen G. The prevalence of neck pain: a population-based study from northern Sweden. Acta Orthop Scand. 2002;73:455–459. doi: 10.1080/00016470216329. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh JC, Meyerson BA, Ingvar M. PET study on central processing of pain in trigeminal neuropathy. Eur J Pain. 1999;3:51–65. doi: 10.1016/S1090-3801(99)90188-X. [DOI] [PubMed] [Google Scholar]
- 17.Iadarola MJ, Max MB, Berman KF, Byas-Smith MG, Coghill RC, Gracely RH, et al. Unilateral decrease in thalamic activity observed with positron emission tomography in patients with chronic neuropathic pain. Pain. 1995;63:55–64. doi: 10.1016/0304-3959(95)00015-K. [DOI] [PubMed] [Google Scholar]
- 18.Jones AK, Derbyshire SW. Reduced cortical responses to noxious heat in patients with rheumatoid arthritis. Ann Rheum Dis. 1997;56:601–607. doi: 10.1136/ard.56.10.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kastrup A, Li TQ, Glover GH, Kruger G, Moseley ME. Gender differences in cerebral blood flow and oxygenation response during focal physiologic neural activity. J Cereb Blood Flow Metab. 1999;19:1066–1071. doi: 10.1097/00004647-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Kupers RC, Gybels JM, Gjedde A. Positron emission tomography study of a chronic pain patient successfully treated with somatosensory thalamic stimulation. Pain. 2000;87:295–302. doi: 10.1016/S0304-3959(00)00295-5. [DOI] [PubMed] [Google Scholar]
- 21.Kwiatek R, Barnden L, Tedman R, Jarrett R, Chew J, Rowe C, et al. Regional cerebral blood flow in fibromyalgia: single-photon-emission computed tomography evidence of reduction in the pontine tegmentum and thalami. Arthritis Rheum. 2000;43:2823–2833. doi: 10.1002/1529-0131(200012)43:12<2823::AID-ANR24>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 22.Larsson A, Johansson L, Sundstrom T, Ahlstrom KR. A method for attenuation and scatter correction of brain SPECT based on computed tomography images. Nucl Med Commun. 2003;24:411–420. doi: 10.1097/00006231-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. Differential limbic-cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol Psychiatry. 2000;48:30–42. doi: 10.1016/S0006-3223(00)00874-X. [DOI] [PubMed] [Google Scholar]
- 24.Marshall PD, O’Connor M, Hodgkinson JP. The perceived relationship between neck symptoms and precedent injury. Injury. 1995;26:17–19. doi: 10.1016/0020-1383(95)90546-A. [DOI] [PubMed] [Google Scholar]
- 25.Nakabeppu Y, Nakajo M, Gushiken T, Tsuchimochi S, Tani A, Kanmura Y. Decreased perfusion of the bilateral thalami in patients with chronic pain detected by Tc-99m-ECD SPECT with statistical parametric mapping. Ann Nucl Med. 2001;15:459–463. doi: 10.1007/BF02988354. [DOI] [PubMed] [Google Scholar]
- 26.Newberg AB, Lariccia PJ, Lee BY, Farrar JT, Lee L, Alavi A. Cerebral blood flow effects of pain and acupuncture: a preliminary single-photon emission computed tomography imaging study. J Neuroimaging. 2005;15:43–49. doi: 10.1177/1051228404271005. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson L-G, Bäckman L, Erngrund K, Nyberg L, Adolfsson R, Bucht G, et al. The Betula prospective cohort study: memory, health, and aging. Aging Neuropsychol Cogn. 1997;4:1–32. doi: 10.1080/13825589708256633. [DOI] [Google Scholar]
- 28.Otte A, Ettlin T, Fierz L, Mueller-Brand J. Parieto-occipital hypoperfusion in late whiplash syndrome: first quantitative SPET study using technetium-99m bicisate (ECD) Eur J Nucl Med. 1996;23:72–74. doi: 10.1007/BF01736993. [DOI] [PubMed] [Google Scholar]
- 29.Otte A, Ettlin TM, Nitzsche EU, Wachter K, Hoegerle S, Simon GH, et al. PET and SPECT in whiplash syndrome: a new approach to a forgotten brain? J Neurol Neurosurg Psychiatry. 1997;63:368–372. doi: 10.1136/jnnp.63.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otte A, Goetze M, Mueller-Brand J. Statistical parametric mapping in whiplash brain: is it only a contusion mechanism? Eur J Nucl Med. 1998;25:306–307. [PubMed] [Google Scholar]
- 31.Otte A, Mueller-Brand J, Fierz L. Brain SPECT findings in late whiplash syndrome. Lancet. 1995;345:1513. doi: 10.1016/S0140-6736(95)91075-1. [DOI] [PubMed] [Google Scholar]
- 32.Petrovic P, Ingvar M, Stone-Elander S, Petersson KM, Hansson P. A PET activation study of dynamic mechanical allodynia in patients with mononeuropathy. Pain. 1999;83:459–470. doi: 10.1016/S0304-3959(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 33.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/S0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 34.Reiman EM, Raichle ME, Butler FK, Herscovitch P, Robins E. A focal brain abnormality in panic disorder, a severe form of anxiety. Nature. 1984;310:683–685. doi: 10.1038/310683a0. [DOI] [PubMed] [Google Scholar]
- 35.Richter M, Ferrari R, Otte D, Kuensebeck HW, Blauth M, Krettek C. Correlation of clinical findings, collision parameters, and psychological factors in the outcome of whiplash associated disorders. J Neurol Neurosurg Psychiatry. 2004;75:758–764. doi: 10.1136/jnnp.2003.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriquez AA, Barr KP, Burns SP. Whiplash: pathophysiology, diagnosis, treatment, and prognosis. Muscle Nerve. 2004;29:768–781. doi: 10.1002/mus.20060. [DOI] [PubMed] [Google Scholar]
- 37.Spitzer WO, Skovron ML, Salmi LR, Cassidy JD, Duranceau J, Suissa S, et al. Scientific monograph of the Quebec task force on whiplash-associated disorders: redefining “whiplash” and its management. Spine. 1995;20:1S–73S. [PubMed] [Google Scholar]
- 38.Sterling M, Jull G, Vicenzino B, Kenardy J. Sensory hypersensitivity occurs soon after whiplash injury and is associated with poor recovery. Pain. 2003;104:509–517. doi: 10.1016/S0304-3959(03)00078-2. [DOI] [PubMed] [Google Scholar]
- 39.Sugiura M, Kawashima R, Nakagawa M, Okada K, Sato T, Goto R, et al. Correlation between human personality and neural activity in cerebral cortex. Neuroimage. 2000;11:541–546. doi: 10.1006/nimg.2000.0564. [DOI] [PubMed] [Google Scholar]
- 40.Viikari-Juntura E, Martikainen R, Luukkonen R, Mutanen P, Takala EP, Riihimaki H. Longitudinal study on work related and individual risk factors affecting radiating neck pain. Occup Environ Med. 2001;58:345–352. doi: 10.1136/oem.58.5.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wik G, Fischer H, Bragee B, Kristianson M, Fredrikson M. Retrosplenial cortical activation in the fibromyalgia syndrome. Neuroreport. 2003;14:619–621. doi: 10.1097/00001756-200303240-00019. [DOI] [PubMed] [Google Scholar]