Abstract
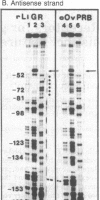
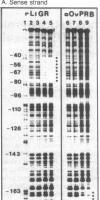
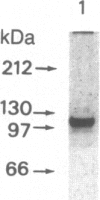
The chicken lysozyme gene can be induced in oviduct cells by four classes of steroid hormones, including glucocorticosteroids and progestins. The glucocorticosteroid receptor of rat liver and the progesterone receptor of rabbit uterus both bind, although with different relative affinities, to two sites in the promoter region of the chicken lysozyme gene located, respectively, between 50 and 80 and between 160 and 200 base pairs upstream of the transcription start point. Now we show that the purified progesterone binding unit of the chicken oviduct progesterone receptor (Mr 110,000, or so-called B subunit) generates a DNase I protection pattern ("footprint") in the promoter-distal site that is longer than the footprint generated by the glucocorticosteroid receptor. Methylation protection studies within the promoter-distal binding site identify four contact points for the chicken progesterone receptor and three contact points for the glucocorticosteroid receptor, of which only one is shared by both receptors. Computer graphics models allow one to envisage a different interaction of each receptor with the B form of the DNA double helix.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bash P. A., Pattabiraman N., Huang C., Ferrin T. E., Langridge R. Van der waals surfaces in molecular modeling: implementation with real-time computer graphics. Science. 1983 Dec 23;222(4630):1325–1327. doi: 10.1126/science.222.4630.1325. [DOI] [PubMed] [Google Scholar]
- Catelli M. G., Binart N., Elkik F., Baulieu E. E. Effect of tamoxifen on oestradiol and progesterone-induced synthesis of ovalbumin and conalbumin in chick oviduct. Eur J Biochem. 1980;107(1):165–182. doi: 10.1111/j.1432-1033.1980.tb04637.x. [DOI] [PubMed] [Google Scholar]
- Geisse S., Scheidereit C., Westphal H. M., Hynes N. E., Groner B., Beato M. Glucocorticoid receptors recognize DNA sequences in and around murine mammary tumour virus DNA. EMBO J. 1982;1(12):1613–1619. doi: 10.1002/j.1460-2075.1982.tb01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronemeyer H., Govindan M. V., Chambon P. Immunological similarity between the chick oviduct progesterone receptor forms A and B. J Biol Chem. 1985 Jun 10;260(11):6916–6925. [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Langridge R., Ferrin T. E., Kuntz I. D., Connolly M. L. Real-time color graphics in studies of molecular interactions. Science. 1981 Feb 13;211(4483):661–666. doi: 10.1126/science.7455704. [DOI] [PubMed] [Google Scholar]
- Logeat F., Pamphile R., Loosfelt H., Jolivet A., Fournier A., Milgrom E. One-step immunoaffinity purification of active progesterone receptor. Further evidence in favor of the existence of a single steroid binding subunit. Biochemistry. 1985 Feb 12;24(4):1029–1035. doi: 10.1021/bi00325a034. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Pennequin P., Schimke R. T. Induction of ovalbumin mRNA sequences by estrogen and progesterone in chick oviduct as measured by hybridization to complementary DNA. J Biol Chem. 1975 Oct 25;250(20):8105–8110. [PubMed] [Google Scholar]
- Ogata R. T., Gilbert W. An amino-terminal fragment of lac repressor binds specifically to lac operator. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5851–5854. doi: 10.1073/pnas.75.12.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkawitz R., Schütz G., von der Ahe D., Beato M. Sequences in the promoter region of the chicken lysozyme gene required for steroid regulation and receptor binding. Cell. 1984 Jun;37(2):503–510. doi: 10.1016/0092-8674(84)90380-5. [DOI] [PubMed] [Google Scholar]
- Renoir J. M., Mester J., Buchou T., Catelli M. G., Tuohimaa P., Binart N., Joab I., Radanyi C., Baulieu E. E. Purification by affinity chromatography and immunological characterization of a 110kDa component of the chick oviduct progesterone receptor. Biochem J. 1984 Feb 1;217(3):685–692. doi: 10.1042/bj2170685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Beato M. Contacts between hormone receptor and DNA double helix within a glucocorticoid regulatory element of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1984 May;81(10):3029–3033. doi: 10.1073/pnas.81.10.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Geisse S., Westphal H. M., Beato M. The glucocorticoid receptor binds to defined nucleotide sequences near the promoter of mouse mammary tumour virus. Nature. 1983 Aug 25;304(5928):749–752. doi: 10.1038/304749a0. [DOI] [PubMed] [Google Scholar]
- Schrader W. T., Birnbaumer M. E., Hughes M. R., Weigel N. L., Grody W. W., O'Malley B. W. Studies on the structure and function of the chicken progesterone receptor. Recent Prog Horm Res. 1981;37:583–633. doi: 10.1016/b978-0-12-571137-1.50017-7. [DOI] [PubMed] [Google Scholar]
- Tuohimaa P., Renoir J. M., Radanyi C., Mester J., Joab I., Buchou T., Baulieu E. E. Antibodies against highly purified B-subunit of the chick oviduct progesterone receptor. Biochem Biophys Res Commun. 1984 Mar 15;119(2):433–439. doi: 10.1016/s0006-291x(84)80267-3. [DOI] [PubMed] [Google Scholar]
- Westphal H. M., Fleischmann G., Beato M. Photoaffinity labeling of steroid binding proteins with unmodified ligands. Eur J Biochem. 1981 Sep;119(1):101–106. doi: 10.1111/j.1432-1033.1981.tb05582.x. [DOI] [PubMed] [Google Scholar]
- von der Ahe D., Janich S., Scheidereit C., Renkawitz R., Schütz G., Beato M. Glucocorticoid and progesterone receptors bind to the same sites in two hormonally regulated promoters. Nature. 1985 Feb 21;313(6004):706–709. doi: 10.1038/313706a0. [DOI] [PubMed] [Google Scholar]