Abstract
Background
This post-hoc analysis of the HF-ACTION (Heart Failure and A Controlled Trial Investigating Outcomes of Exercise Training) cohort explores the primary and secondary results of the HF-ACTION study by etiology and severity of illness.
Methods
HF-ACTION randomized stable outpatients with reduced left ventricular (LV) function and HF symptoms to either supervised exercise training plus usual care or to usual care alone. The primary outcome was all-cause mortality or all-cause hospitalization; secondary outcomes included all cause mortality, cardiovascular mortality or cardiovascular hospitalization and cardiovascular mortality or HF hospitalization. The interaction between treatment and risk variable, etiology or severity as determined by risk score, NYHA class and duration of cardiopulmonary exercise (CPX) test was examined in a Cox proportional hazards model for all clinical endpoints.
Results
There was no interaction between etiology and treatment for the primary outcome (p=0.73), CV mortality or CV hospitalization (p=0.59), or CV mortality or HF hospitalization (p=0.07). There was a significant interaction between etiology and treatment for the outcome of mortality (p=0.03) but the interaction was no longer significant when adjusted for HF-ACTION adjustment model predictors (p=0.08). There was no significant interaction between treatment effect and severity, except a significant interaction between CPX duration and training was identified for the primary outcome of all-cause mortality or all-cause hospitalization.
Conclusion
Consideration of symptomatic (NYHA class II to IV) HF patients with reduced LV function for participation in an exercise training program should be made independent of the cause of HF or the severity of the symptoms.
Introduction
Heart failure (HF) with reduced left ventricular function affects a large patient population that is characterized by heterogeneity.1 Characteristics such as etiology (ischemic versus non-ischemic), exercise tolerance and severity (i.e. NYHA class) of illness are independent risk factors for outcomes.2–4 Therapies for HF patients will occasionally target certain subgroups of patients based on the mechanism of the therapy and/or the differential risk within the subgroups. For example, the implantable cardioverter defibrillator (ICD) was initially evaluated in subjects with ischemic cardiomyopathy due to the higher risk of ventricular arrhythmias and sudden cardiac death in this subgroup.5 ICD indications did expand to include non-ischemic patients when larger populations were evaluated and the therapy was identified to have a greater impact in NYHA class II patients versus class III patients.6
HF-ACTION (Heart Failure and A Controlled Trial Investigating Outcomes of Exercise Training) was a randomized controlled trial evaluating an exercise training (ET) program plus usual care versus usual care (UC) alone in HF subjects with reduced LV function.7 The HF-ACTION study stratified randomization by etiology based on studies showing a differential effect of ET in patients with HF by etiology and evidence demonstrating the clinical benefit of ET in patients with coronary artery disease.8–10 Subsequent to the initiation of the study, a meta-analysis of exercise training trials enrolling HF patients with reduced LV function identified a non-significant differential response by etiology (Hazard Ratio [HR; 95% CI] 0.54 (0.35 to 0.83) vs. 0.93 (0.52 to 1.68), ischemic vs. non-ischemic, p=0.10 for the interaction).11
HF-ACTION identified a modest benefit of ET on the primary outcome of all-cause mortality or all-cause hospitalization after adjusting for four significant predictors of the outcome.12 One of these independent predictors was exercise duration during the cardiopulmonary exercise (CPX) test, an indicator of HF severity. There was a non-significant difference in the primary outcome for participants based on New York Heart Association class (HR [95% CI], NYHA II: 0.95 [0.83–1.08]; NYHA III/IV: 0.85 [0.73–1.00], p value for interaction=0.27). In addition, there was no difference in hazard ratio between ischemic and non-ischemic etiology (HR [95% CI], ischemic: 0.94 [0.82–1.08]; non ischemic: 0.91 [0.78–1.05], p value for interaction=0.73).
The current post-hoc analysis of the HF-ACTION cohort further explores the primary and secondary results of the HF-ACTION study by etiology and severity of illness. We hypothesized that differences in the primary outcome and key secondary outcomes, particularly disease specific outcomes such as HF hospitalization, would exist between subgroups when stratified by etiology or severity of illness.
Methods
A complete description of the study design and ET protocol have been published previously.7 In brief, HF-ACTION was a multicenter, randomized controlled trial designed to test the long-term safety and efficacy of aerobic ET plus evidence-based medical therapy versus UC with evidence-based medical therapy alone in medically stable outpatients with left ventricular dysfunction (ejection fraction ≤ 35%) and NYHA class II to IV HF. The relevant institutional review boards, research ethics boards, and ethics committees of the participating centers approved the study, and the coordinating center approved the protocol. An independent Data Safety Monitoring Board appointed by the trial sponsor, the National Heart, Lung, and Blood Institute (NHLBI), reviewed the protocol. All participants signed informed consent. The study and this analysis were funded by the NHLBI.
Two thousand three hundred and thirty one patients were randomized into the study between April 2003 and February 2007. Blocked randomization was 1:1 and was stratified by etiology of HF (ischemic vs. non-ischemic). Prior to randomization, all patients consenting to participate in the study were to undergo a symptom-limited CPX test using a modified-Naughton treadmill protocol or a stationary cycle ergometer protocol.7 Echocardiograms were obtained within 30 days prior to randomization and were read by a core lab. A 6-minute walk test and Kansas City Cardiomyopathy Questionnaire data were also obtained.
The primary outcome for this analysis was the primary outcome of the HF-ACTION trial, a composite of all-cause mortality or all-cause hospitalization. Secondary outcomes included all-cause mortality, the composite of cardiovascular mortality or cardiovascular hospitalization, and the composite of cardiovascular mortality or HF hospitalization. Although blinding for patients and investigators was not possible, an endpoints committee blinded to treatment assignment reviewed all deaths and many cardiovascular hospitalizations. After the first adjudicated HF hospitalization, hospitalizations were no longer adjudicated by the endpoints committee and the type of hospitalization was based on the assignment by the site investigator. For patients lost to follow-up, searches of the Social Security Death Index and the National Death Index were performed to assess if patients had died during the follow-up period.
Patients were classified as ischemic or non-ischemic at the time of enrollment. Ischemic etiology was defined as the presence of at least 1 of the 4 following criteria: i) angiographic evidence of ≥ 75% lesion in 1 or more of the 3 major epicardial vessels; ii) history of myocardial infarction; iii) history of revascularization procedure; iv) evidence of a significant perfusion defect in the setting of ischemic symptoms.
HF severity was defined using NYHA classification and a risk score derived from a model for the primary outcome using HF-ACTION baseline data.13 The primary analysis evaluating the training effect by HF severity used predicted risk. Variables identified as significant predictors of the primary outcome were Weber Class (categorized peak VO2), symptom stability as assessed by the Kansas City Cardiomyopathy Questionnaire, blood urea nitrogen, patient from USA vs. non-USA, LVEF, gender, beta blocker dose, mitral regurgitation grade, and ventricular conduction prior to the baseline CPX. Because there were fewer missing data for exercise duration than peak oxygen uptake and because exercise duration during the CPX test was also a strong predictor of the primary outcome, a secondary analysis using CPX duration as a surrogate for disease severity was performed.
This analysis used complete cases. For the severity model using the severity score, patients with missing values (KCCQ symptom stability score n=14, mitral regurgitation n=196; rest ECG ventricular conduction n=60; Weber Class n=56; LVEF n=4; blood urea nitrogen n=303; baseline beta-blocker dose n=20; total n=576) were excluded from the analysis. For the severity model using NYHA class, no patients were excluded; 22 patients were excluded in the severity model using CPX duration. Baseline variables were examined among different risk groups, by NYHA class and by etiology. P-values for continuous variables were obtained using the ANOVA F test; when the assumption of normality was not satisfied, the Kruskal-Wallis test (NP) was used. For categorical variables, the chi-square test was used where appropriate.
In order to determine the impact of treatment (ET vs. UC) on clinical outcomes among different etiology or severity groups, the interaction between treatment and risk variable was examined using a Cox proportional hazards model (unadjusted) for all clinical outcomes. The treatmentetiology interaction was also examined in a model adjusted for the variables in the severity model listed above. For a given outcome, if the interaction was statistically significant, the hazard ratio for the two treatment groups was presented for all risk groups (categorized based on HF severity or etiology). If no interactions in secondary outcome models were statistically significant, only the event rate of the primary outcome was presented by etiology or severity and treatment. Kaplan-Meier event rates at two years were examined by etiology and by severity group.
Results
Of the 2331 patients enrolled in HF-ACTION, 1755 patients were included in the analysis using the HF-ACTION adjustment risk model. The median follow-up was 31.6 months. During this time, 1,195 subjects experienced a death or hospitalization, the primary outcome. Baseline characteristics by etiology are shown in Table I. Subjects with ischemic etiology were older and more likely to be male and white. Patients with ischemic etiology tended to have more severe disease as identified by a higher percentage with NYHA class III/IV, lower peak VO2, CPX duration and 6-minute walk time.
Table I.
Baseline Characteristics According to Ischemic or Non-Ischemic Etiology
| Ischemic | Non-Ischemic | P-Value* | ||
|---|---|---|---|---|
| Age | n | 1197 | 1134 | <0.01 NP |
| Median (Q1, Q3) | 63 (56, 71) | 55 (46, 63) | ||
|
| ||||
| Sex | Female n, % | 209, 17 | 452, 40 | <0.01 |
|
| ||||
| Race | Black or African | <0.01 | ||
| American n, % | 243, 21 | 506, 46 | ||
| White n, % | 867, 73 | 559, 50 | ||
| Other n, % | 75, 6 | 46, 4 | ||
|
| ||||
| NYHA HF class | II n, % | 731, 61 | 746, 66 | 0.02 |
| III/IV n, % | 466, 39 | 388, 34 | ||
|
| ||||
| Peak VO2 (mL/kg/min) | n | 1165 | 1110 | <0.01 NP |
| Median (Q1, Q3) | 14.0 (11.1, 17.2) | 15.1 (12.0, 18.1) | ||
|
| ||||
| CPX duration (min.) | n | 1189 | 1120 | <0.01 NP |
| Median (Q1, Q3) | 9.1 (6.6, 11.7) | 10.0 (7.1, 12.5) | ||
|
| ||||
| Serum creatinine (mg/dL) | n | 1088 | 1003 | <0.01 NP |
| Median (Q1, Q3) | 1.3 (1.0, 1.6) | 1.1 (0.9, 1.4) | ||
|
| ||||
| Region | Canada n, % | 113, 9 | 75, 7 | 0.03 |
| France n, % | 41, 3 | 34, 3 | ||
| USA n, % | 1043, 87 | 1025, 90 | ||
|
| ||||
| LVEF (%) | n | 1196 | 1131 | 0.31 NP |
| Median (Q1, Q3) | 25 (20, 30) | 25 (20, 30) | ||
|
| ||||
| BB dose (mg/day carvedilol equivalent) | n | 1189 | 1122 | <0.01 NP |
| Median (Q1, Q3) | 25 (13, 50) | 50 (19, 50) | ||
|
| ||||
| Mitral regurgitation | Non-Severe/None | 0.03 | ||
| * n, % | 975, 90 | 904, 86 | ||
| Severe † n, % | 114, 10 | 142, 14 | ||
|
| ||||
| Ventricular conduction | Normal n, % | 458, 39 | 521, 47 | < 0.01 |
| LBBB n, % | 158, 14 | 221, 20 | ||
| RBBB n, % | 64, 6 | 21, 2 | ||
| IVCD n, % | 169, 15 | 123, 11 | ||
| Paced n, % | 313, 27 | 223, 20 | ||
|
| ||||
| BMI (kg/m2) | n | 1193 | 1131 | <0.01 NP |
| Median (Q1, Q3) | 29 (26, 33) | 31 (26, 37) | ||
|
| ||||
| Loop [diuretic] dose (mg/day furosemide equivalent) | n | 1184 | 1114 | 0.87 NP |
| Median (Q1, Q3) | 40 (20, 80) | 40 (20, 80) | ||
|
| ||||
| CCS Angina class | No Angina n, % | 919, 77 | 1031, 91 | < 0.01 |
| I n, % | 136, 11 | 64, 6 | ||
| II-IV n, % | 139, 12 | 39, 3 | ||
|
| ||||
| Six (6) min walk distance (m) | n | 1178 | 1102 | <0.01 NP |
| Median (Q1, Q3) | 365 (290, 427) | 380 (305, 442) | ||
|
| ||||
| Kansas City Cardiomyopathy Questionnaire Overall Score | n | 1197 | 1133 | 0.04 NP |
| Median (Q1, Q3) | 69 (52, 84) | 67 (50, 82) | ||
a combination of these original categories: None, Trivial, Mild, Mild to Moderate, and Moderate
a combination of the original categories Severe and Moderate to Severe
NP= non-parametric test
Baseline characteristics by HF severity quartile are presented in Table II, with quartile 1 having the lowest risk. Increasing severity was significantly associated with increasing age, diuretic dosing, and percentage of subjects with NYHA class III/IV symptoms, ischemic etiology, severe mitral regurgitation; and decreasing 6-minute walk distance, peak VO2, CPX duration and KCCQ score. In addition, patients identified as having higher HF severity had a lower prevalence of normal ventricular conduction and a higher prevalence of paced rhythm.
Table II.
Baseline Characteristics by HF Severity Based on Predictive Risk
| Heart Failure Severity Quartile | |||||
|---|---|---|---|---|---|
| Q1 (n=439) | Q2 (n=439) | Q3 (n=439) | Q4 (n=438) | p-value | |
| Age | 54 (46, 61) | 59 (51, 68) | 62 (54, 70) | 64 (55, 74) | <0.01 NP |
|
| |||||
| Sex, Female (%) | 125/439 (28) | 150/439 (34) | 122/439 (28) | 98/438 (22) | <0.01 |
|
| |||||
| Race: | <0.01 | ||||
| Black or African American n, % | 110/429 (26) | 136/431 (32) | 170/434 (39) | 170/433 (39) | |
| White n, % | 295/429 (69) | 274/431 (64) | 247/434 (57) | 243/433 (56) | |
| Other n, % | 24/429 (6) | 21/431 (5) | 17/434 (4) | 20/433 (5) | |
|
| |||||
| BMI (kg/m2) | 30 (26,34) | 30 (26,35) | 30 (26,36) | 30 (26,35) | 0.07 NP |
|
| |||||
| NYHA HF Class III/IV (%) | 78/439 (18) | 109/439 (25) | 189/439 (43) | 253/438 (58) | <0.01 |
|
| |||||
| HF Etiology, Ischemic (%) | 176/439 (40) | 214/439 (49) | 249/439 (57) | 259/438 (59) | <0.01 |
|
| |||||
| LVEF | 28 (23, 33) | 25 (21, 31) | 24 (19, 28) | 22 (18, 27) | <0.01 NP |
|
| |||||
| Mitral Regurgitation Severe (%) | 9/439 (2) | 30/439 (7) | 44/439 (10) | 129/438 (29) | <0.01 |
|
| |||||
| Ventricular Conduction Nl/LBBB/RBBB/IVCD/paced (%) | 285/86/4/28/36 (65/20/1/6/8) | 199/75/17/67/81 (45/17/4/15/18) | 154/72/16/68/129 (35/16/4/15/29) | 106/54/32/76/170 (24/12/7/17/39) | <0.01 |
|
| |||||
| Creatinine (mg/dL) | 1.0 (0.9, 1.2) | 1.1 (0.9, 1.4) | 1.2 (1.0, 1.5) | 1.4 (1.1, 1.8) | <0.01 NP |
|
| |||||
| BB dose (mg/day carvedilol equivalent) | 50 (25, 50) | 38 (16, 50) | 25 (13, 50) | 25 (13, 50) | <0. 01 NP |
|
| |||||
| Loop [diuretic] dose (mg/day furosemide equivalent) | 20 (0,40) | 40 (20, 80) | 40 (20, 80) | 80 (40, 120) | <0. 01 NP |
|
| |||||
| Six (6) min walk distance (m) | 427 (374, 481) | 388 (326, 440) | 341 (274, 404) | 305 (239, 373) | <0.01 |
|
| |||||
| CPX duration (min.) | 13.0 (11.0, 15.2) | 10.3 (8.3, 12.2) | 8.4 (6.2, 10.2) | 7.0 (5.0, 8.8) | <0.01 NP |
|
| |||||
| Peak VO2 (mL/kg/min) | 19.1 (17.2, 22.0) | 15.4 (12.9, 17.8) | 13.2 (11.2, 15.1) | 10.6 (8.9, 13.0) | <0.01 NP |
|
| |||||
| Kansas City Cardiomyopathy Questionnaire Overall Score | 76 (60, 88) | 71 (55, 85) | 66 (50, 82) | 59 (43, 76) | <0.01 NP |
Concerning safety, in the ET group and the UC group, 37 (3.2%) patients and 22 (1.9%) patients, respectively, had at least 1 hospitalization due to an event that occurred during or within 3 hours after exercise – these numbers were not significantly different across the quartiles for the HF severity score. The numbers of patients with events during or within 3 hours of exercise between UC and ET groups are 13 versus 23 and 9 versus 14 for ischemic and non-ischemic patients, respectively. The numbers of patients with events in the UC group versus the ET group, respectively, are 3 vs. 8, 1 vs. 6, 5 vs. 4, and 7 vs. 9 among the HF severity quartiles, when ordered from lowest to highest severity.
Patients with ischemic and non-ischemic etiology randomized to ET had a decrease in the primary outcome versus those randomized to UC, although the decrease in the ischemic subgroup (1.3%) was not as great as the decrease within the non-ischemic subgroup (3.8%) (Table III). For non-ischemic compared to ischemic patients, a similar pattern of a larger decrease in the event rate in the ET group compared to the UC group was seen for the secondary outcomes of CV mortality or CV hospitalization and CV mortality or HF hospitalization. Using the overall Cox model results (all events in the entire follow-up), there was no interaction between etiology and treatment for the primary outcome (p=0.73); CV mortality or CV hospitalization (p=0.59); or CV mortality or HF hospitalization (p=0.07).
Table III.
Primary and Secondary Outcome Kaplan – Meier Rates at Two Year by Treatment and ETIOLOGY (ISCHEMIC/NON-ISCHEMIC) Group
| Ischemic | Non-Ischemic | p-value for interaction between etiology and treatment | |||
|---|---|---|---|---|---|
| unadjusted | adjusted | ||||
| All-cause Mortality or Hospitalization | Overall | 65.0 | 55.6 | 0.73 | 0.71 |
| Usual Care | 65.6 | 57.5 | |||
| Exercise | 64.3 | 53.7 | |||
| All-cause Mortality | Overall | 13.7 | 10.4 | 0.03 | 0.08 |
| Usual Care | 13.4 | 12.2 | |||
| Exercise | 14.1 | 8.5 | |||
| CV Mortality or CV Hospitalization | Overall | 54.8 | 45.3 | 0.59 | 0.64 |
| Usual Care | 55.6 | 47.5 | |||
| Exercise | 54.0 | 43.1 | |||
| CV Mortality or HF Hospitalization | Overall | 29.0 | 24.0 | 0.07 | 0.48 |
| Usual Care | 29.1 | 26.9 | |||
| Exercise | 28.9 | 21.0 | |||
When looking at the secondary outcome of mortality, there was an increase in the two-year event rate for ischemic patients in the ET compared to UC, while a decrease in the event rate was seen for non-ischemic patients randomized to ET versus UC. There was a significant interaction between etiology and treatment for the outcome of mortality (p=0.03). Participants with a non-ischemic etiology of their HF had a 43% reduction in risk of death in the ET group as compared with UC (HR=0.57, 95% CI 0.42, 0.76), while the effect of the treatment in participants with ischemic etiology was not statistically significant (HR=0.89, 95% CI 0.67, 1.17). The difference of the treatment effect between the ischemic and non-ischemic patients was no longer statistically significant when adjusted for HF-ACTION risk model predictors (p=0.08).
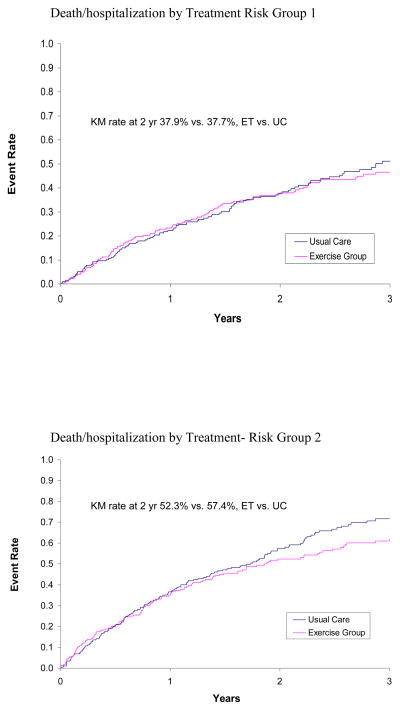
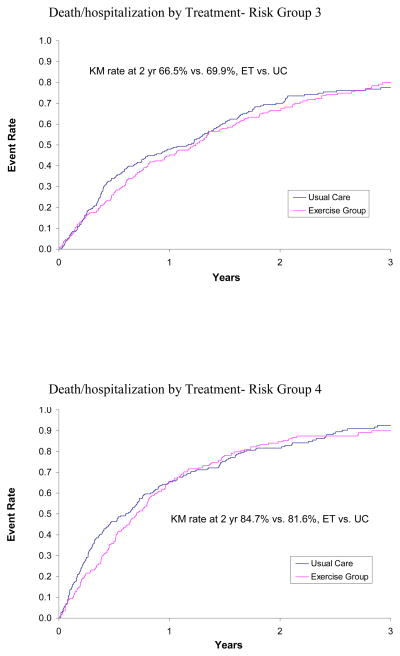
The impact of treatment (ET versus UC) on clinical outcomes by HF severity was evaluated using the three criteria for severity: risk score, NYHA class, and CPX duration. In the primary analysis of subjects by risk score as a continuous variable, the interaction terms that would identify a difference in response to treatment were not statistically significant for the primary outcome or the specified secondary outcomes (Table IVA). The Kaplan Meier rates over 2 years by quartile of risk are provided in Figure 1. The evaluation by NYHA class for the clinical outcomes did not identify a significant interaction that would suggest a differential response (Table IVB).
Table IV.
| A: Impact of treatment on different outcomes by risk | |||
|---|---|---|---|
| Event | Treatment (UC vs. ET) | Risk | Interaction p-values |
| All-cause mortality or all-cause hospitalization (primary end point) | 0.41 | <0.01 | 0.54 |
| Cardiovascular mortality or CV hospitalization | 0.20 | <0.01 | 0.69 |
| Cardiovascular mortality or HF hospitalization | 0.06 | <0.01 | 0.50 |
| All-cause mortality | 0.92 | <0.01 | 0.63 |
| B: Impact of treatment on different outcomes by NYHA class | |||
|---|---|---|---|
| Event | Treatment (UC vs. ET) | NYHA Class (III/IV vs. II) | Interaction |
| All-cause mortality or all-cause hospitalization (primary end point) | 0.42 | <0.01 | 0.29 |
| Cardiovascular mortality or CV hospitalization | 0.33 | <0.01 | 0.52 |
| Cardiovascular mortality or HF hospitalization | 0.32 | <0.01 | 0.37 |
| All-cause mortality | 0.73 | <0.01 | 0.27 |
| C. Impact of treatment on different outcomes by CPX duration | |||
|---|---|---|---|
| Event | Treatment (UC vs. ET) | CPX Duration | Interaction |
| All-cause mortality or all-cause hospitalization (primary end point) | <0.01 | <0.01 | 0.03 |
| Cardiovascular mortality or CV hospitalization | 0.20 | <0.01 | 0.50 |
| Cardiovascular mortality or HF hospitalization | 0.38 | <0.01 | 0.94 |
| All-cause mortality | 0.55 | <0.01 | 0.62 |
Figure 1.
KM curves for Patients in Treatment Arm versus Patients in Usual Care Arm by Risk Quartile
When severity was evaluated as duration on the baseline CPX test, we observed a significant interaction between CPX duration and treatment for the primary outcome (Table IVC). Median exercise duration was 9.6 min (6.9, 12.0). The KM rate for 2 years identified no difference between ET and UC for subjects with CPX duration equal to or greater than the median duration (48.1% vs. 48.2%, ET vs. UC, respectively). For subjects with CPX duration less than the median, there was a reduction in the rate of primary outcome, all-cause mortality or all-cause hospitalization in the ET cohort (70.3% vs. 75.4%, ET vs. UC). The unadjusted hazard ratio comparing ET versus UC for subjects with a CPX duration greater than or equal to 9.6 minutes was 1.02 (0.88–1.19), while the unadjusted hazard ratio for HF-ACTION subjects with a baseline CPX test duration of less than 9.6 minutes was 0.82 (95% CI, 0.72–0.94).
Discussion
Given the large number of patients enrolled in the trial, HF-ACTION provides a unique opportunity to evaluate potential differential effects of training on subgroups. The current study evaluated the safety and efficacy of an ET intervention by two patient characteristics, etiology of HF (ischemic versus non-ischemic) and disease severity, which are commonly used when looking for differential effects of a treatment within a study’s overall cohort. We showed that exercise was safe regardless of etiology of illness or severity of illness. We also showed that the response to ET was, for the most part, not significantly different for HF score, NYHA class or etiology of illness. However, there was a significant interaction between etiology and training for the secondary endpoint of mortality and a significant interaction between CPX duration and training for the primary outcome of all-cause mortality or all-cause hospitalization.
The lack of interaction between etiology and ET for the primary outcome was unexpected. A meta-analysis of exercise-based rehabilitation clinical trials in patients with coronary artery disease identified a significant benefit of ET on all-cause mortality(odds ratio OR 0.80; 95% confidence interval CI: 0.68 to 0.93) and total cardiac mortality (OR 0.74; 95% CI: 0.61 to 0.96).14 The historical use of cardiac rehabilitation in patients with CAD led the HF-ACTION investigators to choose etiology as the only patient characteristic on which to stratify the randomization. However, as shown by this post-hoc analysis of the HF-ACTION data, the modest benefit of ET did not significantly differ by etiology for the primary outcome or two of the secondary outcomes of CV mortality or CV hospitalization and CV mortality or HF hospitalization. Although the current analysis did identify a significant interaction between etiology and training for the secondary outcome of mortality in the unadjusted analysis, this finding should be seen more as confirmation of the ExTraMATCH analysis showing no significant interaction between ET and etiology since the adjusted analysis of the current study did not reach significance and no other significant difference was identified for other endpoints.11
The one exception to the findings on severity was the interaction between CPX duration and ET for the outcome of all-cause mortality or all-cause hospitalization. CPX duration on the baseline test identified a subgroup of subjects defined by performing the test less than the median time of 9.6 minutes who responded more favorably to ET than did subjects with a CPX duration equal to or greater than the median CPX duration. Although these results should be evaluated in light of our other findings concerning severity, they do suggest that exercise duration during CPX testing at baseline may provide a means to identify a group with a higher likelihood of benefiting from participating in a supervised training program. As a direct measurement of physical function or cardiorespiratory reserve, exercise duration measured during a CPX test may be a better method in the clinic setting to identify patients more likely to favorably respond to regular ET. It is important to recognize that the duration is derived from cardiopulmonary tests and not a traditional treadmill test, and there are no data to suggest that these two are equivalent.
Concerning the overall safety of patients with HF participating in a supervised training program, it is reassuring to observe that in patients with a greater severity score and thus more likely to be at risk for adverse events from ET, the trend was a reduction in clinical events and not an increase. The lack of interaction between etiology and training effect also allays concerns about an increase in adverse events. In previous studies, exercise was identified as causing a short-term increased risk of myocardial infarction (MI) and sudden death, possibly due to platelet activation, myocardial ischemia, tachyarrhythmias or coronary artery sheer stress.15–19 Compared to habitual exercisers, patients who initiated exercise after being habitually sedentary were at a 100-fold increased risk for MI and 50-fold increased risk of sudden death.
Limitations
The current study is a post-hoc analysis of a prospective randomized trial and there may be inherent biases created by the inclusion and exclusion criteria used for the study and the type of HF patients that agreed to participate. Although the definitions for ischemia used for the study are standard, there was no evaluation for ischemia performed at the time of enrollment, which may have caused patients to be misclassified in terms of etiology. In a similar fashion, the categorization based on HF severity could have included other means, but the general consistency of the findings for the three definitions used (predictive risk, NYHA class and CPX duration) provides some assurance that our finding of a lack of an interaction between severity and training effect is correct. Additionally, the consistent findings using other definitions of disease severity provide some reassurance of the results using the severity model despite the large number of patients (n=576) excluded from these analyses. Although findings by etiology and risk quartile are presented in the current study, outcomes within subgroups should not be considered conclusive given that the current analysis was not prospective in nature and the size of the cohorts is not adequate for clinical outcomes assessment.
Conclusion
The modest clinical benefit derived from supervised exercise training followed by maintenance home-based exercise that was identified within the overall HF-ACTION cohort was not significantly different when considering either etiology (ischemic vs. non-ischemic) and HF severity at baseline, when the latter was measured by risk model score or NYHA class. The finding that exercise training did show a more favorable effect for the end point of all-cause mortality or all-cause hospitalization when severity of illness was defined by exercise duration less than the median of 9.6 minutes during the baseline CPX test and the trend for a significant interaction between etiology and mortality need to be confirmed given the multiple analyses showing no interaction between severity or etiology and exercise intervention. Exercise training was safe for HF patients with reduced LV function regardless of etiology or severity of symptoms. Consideration of symptomatic (NYHA class II to IV) HF patients with reduced LV function for participation in an exercise training program should be made independent of the cause of HF or the severity of the symptoms.
Acknowledgments
Grant Support: HF-ACTION is funded by the NHLBI; grant numbers 5U01-HL063747, 5U01-HL066461, HL068973, HL068973, HL066501, HL066482, HL064250, HL066494, HL064257, HL066497 HL068980, HL064265, HL066491, HL064264.
Footnotes
The authors report no conflicts of interest.
HF-ACTION is registered: www.clinicaltrials.gov, study number NCT00047437
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Bart BA, Shaw LK, McCants CB, Jr, et al. Clinical determinants of mortality in patients with angiographically diagnosed ischemic or nonischemic cardiomyopathy. J Am Coll Cardiol. 1997;30:1002–8. doi: 10.1016/s0735-1097(97)00235-0. [DOI] [PubMed] [Google Scholar]
- 3.Zugck C, Kruger C, Kell R, et al. Risk stratification in middle-aged patients with congestive heart failure: prospective comparison of the Heart Failure Survival Score (HFSS) and a simplified two-variable model. Eur J Heart Fail. 2001;3:577–85. doi: 10.1016/s1388-9842(01)00167-2. [DOI] [PubMed] [Google Scholar]
- 4.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 6.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 7.Whellan DJ, O’Connor CM, Ousdigian KT, et al. Rationale, design, and baseline characteristics of a Program to Assess and Review Trending INformation and Evaluate CorRelation to Symptoms in Patients with Heart Failure (PARTNERS HF) Am Heart J. 2008;156:833–9. 839, e2. doi: 10.1016/j.ahj.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 8.Keteyian SJ, Brawner CA, Schairer JR, et al. Effects of exercise training on chronotropic incompetence in patients with heart failure. Am Heart J. 1999;138:233–40. doi: 10.1016/s0002-8703(99)70106-7. [DOI] [PubMed] [Google Scholar]
- 9.Thompson PD. The benefits and risks of exercise training in patients with chronic coronary artery disease. JAMA. 1988;259:1537–40. [PubMed] [Google Scholar]
- 10.Laslett L, Paumer L, Amsterdam EA. Exercise training in coronary artery disease. Cardiol Clin. 1987;5:211–25. [PubMed] [Google Scholar]
- 11.Piepoli MF, Davos C, Francis DP, et al. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) BMJ. 2004;328:189. doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connor CM. Determinants of morbidity and mortality in chronic heart failure (CHF) with systolic dysfunction: results of the HF-ACTION predictive model. J Am Coll Cardiol. 2010;55:A28.E270. doi: 10.1016/S0735-1097(10)60271–9. Abstract. [DOI] [Google Scholar]
- 14.Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116:682–92. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Siscovick DS, Weiss NS, Fletcher RH, et al. The incidence of primary cardiac arrest during vigorous exercise. N Engl J Med. 1984;311:874–7. doi: 10.1056/NEJM198410043111402. [DOI] [PubMed] [Google Scholar]
- 16.Mittleman MA, Maclure M, Tofler GH, et al. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of Myocardial Infarction Onset Study Investigators. N Engl J Med. 1993;329:1677–83. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- 17.Willich SN, Lewis M, Lowel H, et al. Physical exertion as a trigger of acute myocardial infarction. Triggers and Mechanisms of Myocardial Infarction Study Group. N Engl J Med. 1993;329:1684–90. doi: 10.1056/NEJM199312023292302. [DOI] [PubMed] [Google Scholar]
- 18.Wallen NH, Goodall AH, Li N, et al. Activation of haemostasis by exercise, mental stress and adrenaline: effects on platelet sensitivity to thrombin and thrombin generation. Clin Sci (Lond) 1999;97:27–35. [PubMed] [Google Scholar]
- 19.Bartsch P. Platelet activation with exercise and risk of cardiac events. Lancet. 1999;354:1747–8. doi: 10.1016/S0140-6736(99)90259-3. [DOI] [PubMed] [Google Scholar]