Abstract
Frailty and delirium, though seemingly distinct syndromes, both result in significant negative health outcomes in older patients. Frailty and delirium may be different clinical expressions of a shared vulnerability to stress in older patients and future research will determine whether this vulnerability is age-related, pathological, genetic, or environmental or, most likely, a combination of all of these factors. This paper explores the clinical overlap of frailty and delirium, describes possible pathophysiological mechanisms linking the two, and proposes research opportunities to further our knowledge of the interrelationships between these important geriatric syndromes.
Frailty, a diminished ability to compensate to stressors, is generally viewed as a chronic condition, while delirium is an acute change in attention and cognition. However, there is a developing literature on transitions in frailty status around acute events, as well as on delirium as a chronic, persistent condition. If frailty predisposes a patient to delirium and delirium delays recovery from a stressor, then both syndromes may contribute to a downward spiral of declining function, increasing risk, and negative outcomes. Additionally, frailty and delirium may have shared pathophysiology, such as inflammation, atherosclerosis, and chronic nutritional deficiencies, which will require further investigation.
The fields of frailty and delirium are rapidly evolving and future research may help to better define the interrelationship of these common and morbid geriatric syndromes. Because of the heterogeneous pathophysiology and presentation associated with frailty and delirium, typical of all geriatric syndromes, multicomponent prevention and treatment strategies are most likely to be effective, and should be developed and tested.
Keywords: Frailty, Delirium, Aged, Research
INTRODUCTION
Frailty and delirium are key geriatric syndromes that can impact independent functioning. Geriatric syndromes are defined by a constellation of signs and symptoms to describe the heterogeneous response of the older patient to physiological and metabolic challenges, rather than the classic textbook presentation of a disease.1, 2 This presentation results from the complex interaction of age, physiology, integrated control mechanisms, and pathology. Homeostasis is the regulation of an organ system to maintain a constant internal environment. The age-related decline in physiologic function in nearly every organ system results in a constricted range over which homeostasis can be maintained, which is termed presbyhomeostenosis.3 Additionally, the reduction in responsiveness of integrated physiologic regulatory systems4 limits the ability to recruit other organ systems to assist with the compensation to a stressor. Age-related pathology or systemic disease can further reduce homeostatic capacity and lead to symptomatic decompensation. This combination of decreased physiological reserve and system regulation reduces the capacity to adapt to stressors with age. Clinically, these stressors present heterogeneously (in the organ system made most vulnerable by homeostenotic processes) as geriatric syndromes such as falls, incontinence, delirium, or frailty.5
Frailty and delirium appear to be distinct clinical phenotypes. However, in practice, both syndromes can manifest in response to a stressor in vulnerable elders. Either frailty or delirium may predominate but the interactions between these syndromes and ensuing long-term consequences have not yet been identified. We propose that future research should focus on the time period before and after the stressor to elucidate mechanisms that precipitate functional decline in older patients.
This article proposes that frailty and delirium are different representations of decompensation to stress in vulnerable older patients that commonly co-present and potentiate risk with unmeasured consequences and thus, need further examination in longitudinal studies.
FRAILTY AND DELIRIUM DEFINED
Table 1 compares the definitions, timing, and criteria of frailty and delirium. Frailty is a state of increased vulnerability to stress related to diminished homeostatic capacity across multiple physiologic systems.4 Frailty can be characterized by sarcopenia, reduced energy expenditure, and weight loss, and occurs commonly in the presence of chronic conditions.6, 7 Of the several proposed working definitions of frailty, the most adopted criteria are those of Fried et al that are derived from the Cardiovascular Health Study and include: weight loss, poor grip strength, slow walking speed, exhaustion, and low physical activity.7 The frail state was defined by presence of three or more of these criteria while one or two criteria characterize pre-frailty (an intermediate state between robustness and frailty).
Table 1.
Comparison of Frailty and Delirium: Definition, Time Course, and Features
| Frailty | Delirium | |
|---|---|---|
| Definition | Vulnerability in multiple physiologic systems | Acute change in attention and cognition |
| Time Course | Chronic | Acute with fluctuation |
| Acute change with stressors | Reversible in most cases | |
| Potentially reversible | Persistence in some cases | |
| Features | Sarcopenia* | Inattention |
| Reduced energy expenditure* | Thought disorders | |
| Nutritional Deficiency* | Altered consciousness | |
| Weight loss | ||
| Decreased physical activity |
Note: these frailty features differ from the CHS phenotype criteria in the text- frailty may involve more features than defined in the CHS criteria.
Delirium is defined as an acute change in cognition and specifically, attention (DSM-IV-TR®).8 Key features of delirium include acute onset over hours to days and fluctuation over the course of a day, which help to distinguish it from other cognitive deficits. In addition, thought disturbances, perceptual disturbances, and fluctuations in consciousness may occur. The Confusion Assessment Method is the most widely used diagnostic algorithm for delirium and requires the presence of a) an acute onset and fluctuating course and b) inattention, as well as, either c) disorganized thinking or d) altered level of consciousness.9 Analogous to pre-frailty, “subsyndromal delirium” refers to a state in which some features of delirium are present, but not enough to support the full syndrome.10
Time course: “Chronic” frailty vs. “acute” delirium
The course of frailty is generally considered to be chronic with progressive decline. On the other hand, delirium commonly occurs acutely in response to a stressor such as hospitalization and classically is said to resolve quickly.11 Thus, the time course of frailty and delirium are seemingly mutually exclusive; however, there is ongoing work examining the dynamic nature of both frailty and delirium.12-14 Considerable fluctuation has been described in the severity of frailty,14 especially around acute health events. Recovery is typically slow and incomplete following such an event. Meanwhile, delirium can persist for months, often at a subsyndromal level.13 A recent systematic review found that up to 20% of hospitalized patients had persistent delirium at 6 months.15 Thus, despite the classic representation that frailty is chronic and delirium is an acute process, they may co-occur with increased potential for negative outcomes.
Is frailty exclusively a physical state and delirium a purely cognitive syndrome?
Frailty has been operationalized as a disorder of physical function,7 but several groups have proposed including cognitive impairment as a frailty criterion.16, 17 Delirium is an acute disorder of cognitive function and may be a cognitive manifestation of frailty, where the brain is unable to compensate in the setting of acute systemic stressors. However, delirium is often associated with a decline in physical as well as cognitive functioning. The current frailty criteria, focused on physical function, are necessary for definitional purposes and have made a strong case for using predominantly physical elements. However, the functional approach to the geriatric patient makes it difficult to isolate physical from cognitive performance. The contribution of cognitive function to frailty is not well understood, but could be important and warrants further investigation.18
RESEARCH DIRECTIONS
Because frailty and delirium may be linked in a downward spiral of increasing risk and negative outcomes, there is a need for further investigation of the common mechanisms of risk, pathophysiology, and recovery. Additionally, long-term studies are necessary to determine if the negative outcomes of frailty and delirium can be prevented or treated. Table 2 highlights questions for research to better elucidate the potential reciprocal relationship between frailty and delirium. Below, we summarize the evidence available in the major research areas to provide a framework for continued study of the interrelationships of frailty and delirium.
Table 2.
Future research directions to define the interrelationship of frailty and delirium
| Research Area | Questions for Future Research |
|---|---|
| Risk Factors | Is frailty is an independent risk factor for delirium? |
| Determine the role/impact/effect of cognitive functioning in frailty. | |
| How does comorbidity, both medical and neurological, interact with frailty and delirium? | |
| Pathophysiology | What are the common pathophysiological mechanisms of frailty and delirium? |
| Are there genetic predispositions? | |
| Do frailty and delirium share biomarkers (e.g. cytokines)? | |
| Recovery from Stressor | Does delayed or incomplete recovery from a stressor signify pre-stressor frailty? |
| Does delirium trigger a transition from a pre-frail state to frailty? | |
| Can delirium be used as a model for a stressor to examine the subsequent frailty course? | |
| Long-term Outcomes | Is there anything unique about delirium as a “precipitant” of frailty that would modify the known relationship between frailty and adverse functional outcomes? |
| Does delirium cause long-term cognitive and functional decline? | |
| Does recovery from frailty describe a resilience that protects against negative long-term outcomes? | |
| Prevention | Does preventing delirium prevent subsequent frailty? |
| Does prevention or treatment of frailty reduce delirium risk? | |
| Treatments | Are there common prevention or treatment interventions that can be used for frailty and delirium? |
| Will multidisciplinary programs impact on the course of frailty or delirium? | |
| System | Are there systemic measures that can capture delirium and frailty in existing records to define the scope of the problem? |
| Will incentives to prevent delirium have an impact on subsequent frailty? | |
| What are the cost implications of superimposed delirium and frailty to the patient, institution, physician, system, and society? |
Risk Factors
Frailty as a risk factor for delirium
At present, limited direct evidence is available examining frailty as a risk factor for delirium. In a small study of older non-cardiac surgical patients, preoperative frailty score independently predicts postoperative delirium.19 Because function and frailty are intuitively linked, additional indirect evidence also supports a possible frailty-delirium association. Robinson et al found that pre-existing functional impairment was independently associated with postoperative delirium.20 In a study of non-cardiac surgery patients, the Specific Activity Scale, a marker of preoperative functional capacity, was identified as an independent risk factor for delirium.21 Inclusion of baseline frailty assessment in studies of acute delirium would improve the understanding of frailty as a risk factor for delirium.
Delirium as a risk factor for frailty
Following an acute stressor, recovery is reliant on patient, disease, and environmental factors. Foremost among the patient factors is intact cognitive function to complete the disease treatments and recovery protocol. Delirium has been associated with incident long-term cognitive impairment22 and may accelerate existing cognitive decline and thus impede the recovery process.23 Patients with persistent delirium have been shown to be less likely to regain ADL function.24 Thus, the persistent or residual effects of delirium may retard both physical and cognitive recovery, ultimately resulting in new or increasing frailty and/or long-term disability and institutionalization.
Pathophysiology
Geriatric syndromes are typified by nonlinear relationships between multiple complex contributors.1 Therefore, addressing potential mechanisms for these syndromes is challenging. However, current evidence suggests common factors, described below, may link frailty and delirium mechanistically. This work needs further refinement.
Inflammation
Inflammation is a systemic reaction to injury or infection, which acts as a defensive and restorative mechanism. Cytokines are proteins secreted by cells of the immune system, which mediate local and systemic inflammation thru intercellular communication. Cytokines can be grouped into pro-inflammatory (CRP, IL-6, IL-1, TNF-α, etc) or anti-inflammatory (IL-10, IL-4, etc) cytokines.25 With age, there is an increased amount of circulating inflammatory cytokines and this is heightened in age-associated conditions such as atherosclerosis,26 cognitive impairment,27 and frailty.28 When primed by increased baseline levels, the pro-inflammatory response to stressors is more pronounced.25 Preliminary studies have found that both delirium and frailty are associated with increased levels of peripheral inflammatory cytokines. Serum levels of Il-6 and Il-8 are elevated in hip fracture patients who experience delirium.29 Similarly, frailty has been associated with increased levels of inflammatory cytokines in steady state.30, 31 Future work could examine baseline inflammation, the acute inflammatory response to a stressor and cytokine levels during the subacute recovery phase as a common pathophysiologic pathway of frailty and delirium.
Atherosclerosis
Atherosclerosis is the leading cause of death and the most common systemic pathology in older patients. While the clinical sequelae of large vessel atherosclerosis are well described, small vessel disease is associated with many geriatric syndromes, including falls, urinary incontinence and depression.32 Since frailty and delirium are also geriatric syndromes, and there is strong evidence for common risk factors for geriatric syndromes, small vessel disease likely also contributes to frailty and delirium.1 Consistent with this, frailty has been associated with overt cardiac disease and atherosclerosis burden, as well as subclinical markers of cardiovascular disease.33, 34 Furthermore, arterial stiffness is a risk marker for thigh sarcopenia which may contribute to reduced activity and slow walking speed- key components of frailty.35
The incidence of delirium is nearly doubled in patients undergoing cardiac and vascular surgeries, relative to elective orthopedic or abdominal surgery.36 Additionally, atherosclerosis risk factors and atherosclerosis burden are risk factors for delirium. 11, 33, 37 In the brain, increased small vessel disease (i.e. leukoaraiosis, which describes white matter changes on brain imaging) is related to declining performance on measures of attention and executive functions.38 Because delirium is primarily a disorder of attention, chronic deficits in attention or executive function may predispose patients to develop delirium in the face of a stressor.39 However, the effects of small vessel disease may generalize beyond cognitive function. The Leukoaraiosis and Disability in the Elderly Study (LADIS) demonstrated a two-fold higher risk of transitioning to disability or death after 3 years in the group with severe white matter changes versus the mild group.40 Atherosclerotic risk and, in particular, radiological evidence of cerebrovascular disease burden should be incorporated into future studies examining the relationship of delirium and frailty.
Genetic link
Inheritance of the Apolipoprotein E4 allele (ApoE4) confers a greater risk for cardiovascular disease41, Alzheimer's disease,42 and according to a recent meta analysis, delirium.43 The link between APOe4 and frailty status is less well defined; while the presence of Apoe4 allele was associated with increased mortality in the Canadian Study of Health and Aging, but there was no significant association between apoE4 and frailty (or apoE4 and delirium).44 The work with ApoE4 lays an important foundation for future research to include genotyping of participants and to consider Genome-wide Association Studies to determine candidate genetic risk factors underlying frailty and delirium.
Nutritional Deficiency
Malnutrition is prevalent in the older population, particularly in the setting of chronic disease and in institutionalized elders.45 Reductions in caloric intake may lead to sarcopenia and thus, to frailty. As noted above, “shrinking” is a conspicuous feature of the frailty phenotype.7 The stressors of acute-on-chronic disease, such as exacerbations of chronic obstructive pulmonary disease and congestive heart failure, increase susceptibility to accelerated weight loss by muscle protein catabolism, inactivity and counter-regulatory hormone surges.46 This combination of multiple physiological insults can breach the threshold of frailty and result in physical and functional decline and ultimately loss of independence.47
Particular interest has been invested in micronutrient deficiency relative to risk of negative health outcomes in older patients. Low levels of micronutrients (folate, and vitamins C, D, and E) have shown a cross-sectional association with frailty status,48 and low vitamin E level was significantly associated with subsequent decline in physical performance in an older cohort.49 Vitamin D is widely known to contribute to bone health and deficiency in Vitamin D leads to myopathy and can contribute to increased falls.50 Recent work analyzing data from NHANES III shows a 3.7- to 4-fold increase in the odds of frailty if hypovitaminosis D is present.51 While there is no clear link between vitamin D deficiency and delirium, there is a body of evidence suggesting lifelong supplementation may be neuroprotective.52
Malnutrition may be a significant predisposing risk factor for development of delirium.11, 53 Conversely, the occurrence of delirium in a vulnerable older patient can stress at-risk fat and protein stores and potentiate sarcopenia. Nutritional requirements in the delirious intensive care patient are routinely addressed thru tube or parenteral feeding regimes, though these regimens often fail to meet the patient's caloric needs. Delirious patients outside of the ICU setting are particularly vulnerable to underfeeding and weight loss, none more so than in the nursing home setting.54 Loss of independence with self-feeding, poor quality food, and polypharmacy are documented risk factors for undernutrition in the institutionalized older adult. Therefore, it behooves geriatricians to comprehensively screen for and treat malnutrition across care settings to prevent delirium, new-onset or worsening frailty, and functional decline.
Further work will more clearly define the relationship of malnutrition (at macro- and micronutrient level) with both frailty and delirium.
Recovery from Stressors
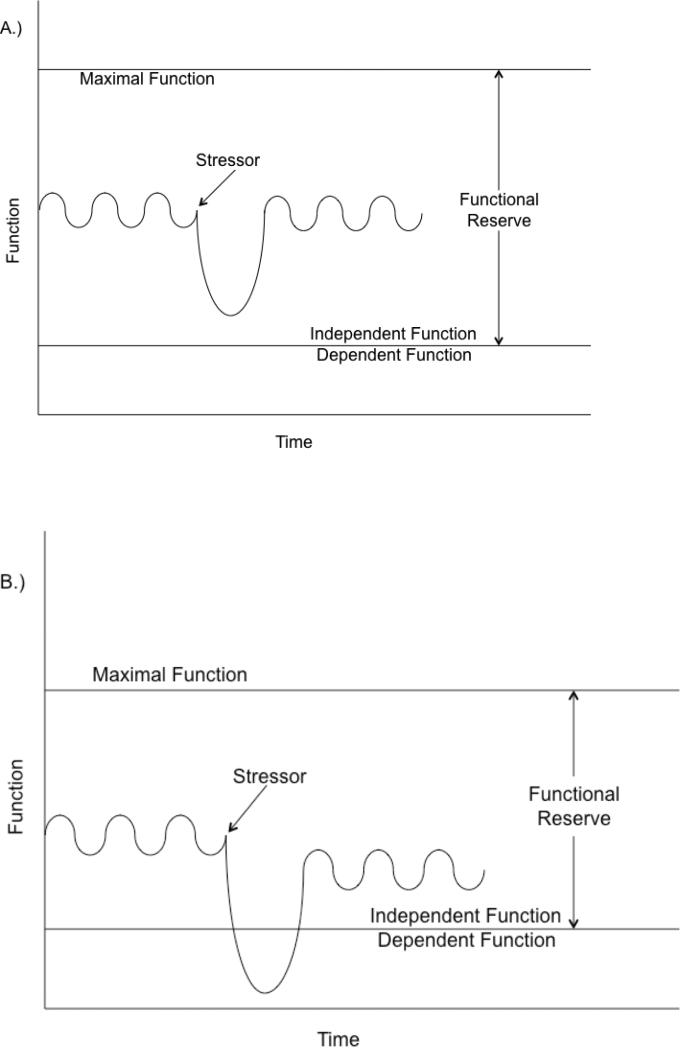
By definition, frail patients have a baseline vulnerability to stressors. Thus, when a frail patient is exposed to a stressor, there is often decompensation in function. (Figure 1) Clinically, this is frequently seen when patients become acutely ill or undergo surgery and are unable to return to function at baseline levels. Delirium may be a presentation of this decompensation to the inciting stressor.
Figure 1. Functional decline after a stressor.
In patient A, the stressor results in a decline in function which does not cross the threshold of independent function. The patient is likely non-frail, because the functional level returns to the baseline, indicating that the patient was able to fully compensate to the stressor. In patient B, a similar stressor causes a decline in function which transiently results in dependence. While the patient subsequently recovers independence, it is at a lower level of functioning than prior to the stressor. The patient is likely frail because she does not return to her baseline level of function, indicating that she was unable to fully compensate to the stressor. This patient has a constricted functional reserve compared with the patient above.
Note: Day-to-day functional variation is depicted by a sine wave.
Logically, the amount of decompensation should be related to the “sum” of the magnitude of the stressor (e.g. sepsis is a larger stressor than a viral upper respiratory tract infection) and the baseline level of vulnerability (i.e. more frail patients are more susceptible to decompensation). However, age-related loss of complexity in multiple integrated physiologic systems makes this process less predictable.4 Provided that the stressor is amenable to treatment and the patient survives, a period of recovery follows where function improves. After resolution of the stressor, the patient's course of recovery may help to define their pre-stressor frailty state. Incomplete recovery suggests that indeed the patient was unable to compensate to stressors and was frail. Complete recovery would suggest that the patient was able to compensate to stressor and therefore, was more robust. As a result, studies examining the impact of a uniform stressor, such as elective surgery or an acute medical condition, on long term functional status, could provide insight into pre-stressor frailty status. This would allow further validation of the frailty phenotype as a predictor of post-acute recovery while providing an individualized marker of physiologic reserve. Standardized identification of prevalent frailty will be necessary for any future frailty or delirium interventional trials.
Long-term outcomes
Frailty and delirium, independently, predict mortality, functional decline, and disability in the older patient.7, 55 In the Women's Health and Aging Study, baseline frailty was associated with increased mortality, severe disability, and nursing home placement.56 Similarly the mortality of delirium is comparable to that of an acute MI or sepsis11 and several studies link incident delirium with subsequent functional decline and institutionalization.11, 55, 57 While most of these delirium studies have focused on short-term and intermediate term outcomes, the long-term outcomes have shown similar effect. 57 However, significant study attrition, often related to the high mortality and morbidity of delirium, reduces statistical power to examine long-term outcomes after delirium. Currently, no studies have directly examined the long-term outcomes of concomitant frailty and delirium. We acknowledge that common adverse outcomes do not necessitate a common pathway, so future work should concentrate on the potentially shared pathophysiological contributors explored above.
Prevention and Treatment
The multifactorial nature of frailty and delirium as geriatric syndromes suggest that a single strategy for treatment and prevention will not work in all patients; instead, multicomponent prevention and treatment strategies will need to be tested. Delirium has the strongest evidence for multicomponent prevention, with up to 40% of delirium episodes being preventable.11 Complex multidimensional initiatives such as the Hospital Elder Life Program (HELP) program, which targeted interventions at sensory, mobility, sleep, fluid balance, nutritional and orientation levels, reduce incident delirium in a controlled setting.58 At present, there is less evidence supporting the benefit of delirium treatment strategies compared to prevention; these studies are challenging in that they require effective identification and treatment of the many possible underlying causes of delirium. Nonetheless, there have been some successes, mainly outside the U.S.59, 60 Given the broad spectrum of frailty criteria, a multifactorial prevention and treatment approach with targeted interventions in multiple domains (e.g. mobility and balance programs, falls prevention, nutritional assessment and supplementation, strength training, cognitive stimulus, exploring social reserves and amenities, treating occult depression) will likely be needed. Rigorous research is necessary so that evidence-based frailty and delirium intervention programs become integral components of the care pathway for vulnerable older adults.
Future directions
This paper has outlined common links between delirium and frailty. While frailty is prototypically a chronic condition, delirium is an acute condition and both syndromes represent significant sources of morbidity and mortality for older patients with considerable societal cost. These syndromes are multifactorial with risk factors and potentially causative mechanisms (e.g. inflammation, atherosclerosis, and poor nutrition) that overlap. Unfortunately, there are limited data in many of these biological mechanisms. We hope that this manuscript serves as a springboard for increased discussion and investigation around these mechanisms. Longitudinally, frailty may be a risk factor for delirium and delirium may precipitate or hasten frailty. Specific attention should also focus on transitions in frailty states (e.g. robust to pre-frail to frail and vice-versa) in elders who develop delirium when exposed to acute stressors, and in the post-delirium recovery period to determine the potential for reversibility of frailty. Finally, such studies could better explore the joint contribution of these geriatric syndromes to poor outcomes and begin the process of developing mutual prevention and treatment strategies.
ACKNOWLEDGMENTS
Dr. Rudolph is supported by a VA Rehabilitation Research Career Development Award. Dr. Quinlan is a VA Fellow at the VA Boston Healthcare System GRECC. Dr. Gill (K24AG021507) and Dr. Marcantonio (K24AG035075) are supported by Midcareer Investigator Awards in Patient-Oriented Research from the National Institute on Aging. Drs. Marcantonio and Inouye are supported by a grant from the NIA (P01AG031720). Dr. Inouye is supported by a grant from the Alzheimer's Association (Grant No. IIRG-08-88738) and by the Milton and Shirley F. Levy Family Chair.
Footnotes
Author Contributions:
Concept and design: Quinlan, Marcantonio, Inouye, Gill, Kamholz, Rudolph
Data acquisition: N/A
Analysis and interpretation: N/A
Preparation of manuscript: Quinlan, Marcantonio, Inouye, Gill, Kamholz, Rudolph
Conflict of Interest:
The authors maintained independence in the completion of this work.
REFERENCES
- 1.Inouye SK, Studenski S, Tinetti ME, et al. Kuchel GA. Geriatric syndromes: Clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarrett PG, Rockwood K, Carver D, et al. Cosway S. Illness presentation in elderly patients. Arch Intern Med. 1995;155:1060–1064. [PubMed] [Google Scholar]
- 3.Taffet G. Physiology of Aging. In: Cassel C, editor. Geriatric medicine: an evidence-based approach. 4th edN. Springer-Verlag; New York: 2003. pp. 27–35. [Google Scholar]
- 4.Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: Implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64:1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Resnick NM, Marcantonio ER. How should clinical care of the aged differ? Lancet. 1997;350:1157–1158. doi: 10.1016/S0140-6736(05)63817-2. [DOI] [PubMed] [Google Scholar]
- 6.Bortz WM., 2nd A conceptual framework of frailty: A review. J Gerontol A Biol Sci Med Sci. 2002;57:M283–288. doi: 10.1093/gerona/57.5.m283. [DOI] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 8.Diagnostic and Statistical Manual of Mental Disorders. 4th edition. American Psychiatric Association; Washington DC: 2000. Text Revision. [Google Scholar]
- 9.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 10.Cole M, McCusker J, Dendukuri N, et al. The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc. 2003;51:754–760. doi: 10.1046/j.1365-2389.2003.51255.x. [DOI] [PubMed] [Google Scholar]
- 11.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 12.Lang PO, Michel JP, Zekry D. Frailty syndrome: A transitional state in a dynamic process. Gerontology. 2009;55:539–549. doi: 10.1159/000211949. [DOI] [PubMed] [Google Scholar]
- 13.Kiely DK, Marcantonio ER, Inouye SK, et al. Persistent delirium predicts greater mortality. J Am Geriatr Soc. 2009;57:55–61. doi: 10.1111/j.1532-5415.2008.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill TM, Gahbauer EA, Allore HG, et al. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 15.Cole MG, Ciampi A, Belzile E, et al. Persistent delirium in older hospital patients: A systematic review of frequency and prognosis. Age Ageing. 2009;38:19–26. doi: 10.1093/ageing/afn253. [DOI] [PubMed] [Google Scholar]
- 16.Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008;56:2211–2116. doi: 10.1111/j.1532-5415.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avila-Funes JA, Amieva H, Barberger-Gateau P, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc. 2009;57:453–461. doi: 10.1111/j.1532-5415.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 18.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 19.Leung JM, Tsai TL, Sands LP. Brief report: preoperative frailty in older surgical patients is associated with early postoperative delirium. Anesth Analg. 2011;112:1199–1201. doi: 10.1213/ANE.0b013e31820c7c06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson TN, Raeburn CD, Tran ZV, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249:173–178. doi: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]
- 21.Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271:134–139. [PubMed] [Google Scholar]
- 22.McCusker J, Cole M, Dendukuri N, et al. Delirium in older medical inpatients and subsequent cognitive and functional status: A prospective study. CMAJ. 2001;165:575–583. [PMC free article] [PubMed] [Google Scholar]
- 23.Fong TG, Jones RN, Shi P, et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72:1570–1575. doi: 10.1212/WNL.0b013e3181a4129a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiely DK, Jones RN, Bergmann MA, et al. Association between delirium resolution and functional recovery among newly admitted postacute facility patients. J Gerontol A Biol Sci Med Sci. 2006;61:204–208. doi: 10.1093/gerona/61.2.204. [DOI] [PubMed] [Google Scholar]
- 25.Giunta S. Exploring the complex relations between inflammation and aging (inflamm-aging): Anti-inflamm-aging remodelling of inflamm- aging, from robustness to frailty. Inflamm Res. 2008;57:558–563. doi: 10.1007/s00011-008-7243-2. [DOI] [PubMed] [Google Scholar]
- 26.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 27.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition--the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- 28.Leng SX, Xue QL, Tian J, et al. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 29.van Munster BC, Korevaar JC, Zwinderman AH, et al. Time-course of cytokines during delirium in elderly patients with hip fractures. J Am Geriatr Soc. 2008;56:1704–1709. doi: 10.1111/j.1532-5415.2008.01851.x. [DOI] [PubMed] [Google Scholar]
- 30.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: Results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 31.Kanapuru B, Ershler WB. Inflammation, coagulation, and the pathway to frailty. Am J Med. 2009;122:605–613. doi: 10.1016/j.amjmed.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: Is there a link? J Gerontol A Biol Sci Med Sci. 2004;59:818–826. doi: 10.1093/gerona/59.8.m818. [DOI] [PubMed] [Google Scholar]
- 33.Klein BE, Klein R, Knudtson MD, et al. Frailty, morbidity and survival. Arch Gerontol Geriatr. 2005;41:141–149. doi: 10.1016/j.archger.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 35.Ochi M, Kohara K, Tabara Y, et al. Arterial stiffness is associated with low thigh muscle mass in middle-aged to elderly men. Atherosclerosis. 2010;212:327–332. doi: 10.1016/j.atherosclerosis.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Rudolph JL, Marcantoni ER. Caring for the postoperative patient with delirium. The Hospitalist. 2004;8:20–25. [Google Scholar]
- 37.Rudolph JL, Babikian VL, Birjiniuk V, et al. Atherosclerosis is associated with delirium after coronary artery bypass graft surgery. J Am Geriatr Soc. 2005;53:462–466. doi: 10.1111/j.1532-5415.2005.53165.x. [DOI] [PubMed] [Google Scholar]
- 38.Jokinen H, Kalska H, Ylikoski R, et al. MRI-defined subcortical ischemic vascular disease: Baseline clinical and neuropsychological findings. The LADIS Study. Cerebrovasc Dis. 2009;27:336–344. doi: 10.1159/000202010. [DOI] [PubMed] [Google Scholar]
- 39.Rudolph JL, Jones RN, Grande LJ, et al. Impaired executive function is associated with delirium after coronary artery bypass graft surgery. J Am Geriatr Soc. 2006;54:937–941. doi: 10.1111/J.1532-5415.2006.00735.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inzitari D, Pracucci G, Poggesi A, et al. Changes in white matter as determinant of global functional decline in older independent outpatients: Three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ. 2009;339:b2477. doi: 10.1136/bmj.b2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minihane AM, Jofre-Monseny L, Olano-Martin E, et al. ApoE genotype, cardiovascular risk and responsiveness to dietary fat manipulation. Proc Nutr Soc. 2007;66:183–197. doi: 10.1017/S0029665107005435. [DOI] [PubMed] [Google Scholar]
- 42.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: A causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc Natl Acad Sci U S A. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Munster BC, Korevaar JC, Zwinderman AH, et al. The association between delirium and the apolipoprotein E epsilon 4 allele: New study results and a meta-analysis. Am J Geriatr Psychiatry. 2009;17:856–862. doi: 10.1097/JGP.0b013e3181ab8c84. [DOI] [PubMed] [Google Scholar]
- 44.Rockwood K, Nassar B, Mitnitski A. Apolipoprotein E-polymorphism, frailty and mortality in older adults. J Cell Mol Med. 2008;12:2754–2761. doi: 10.1111/j.1582-4934.2008.00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lesourd B. Nutrition: A major factor influencing immunity in the elderly. J Nutr Health Aging. 2004;8:28–37. [PubMed] [Google Scholar]
- 46.Paddon-Jones D. Interplay of stress and physical inactivity on muscle loss: Nutritional countermeasures. J Nutr. 2006;136:2123–2126. doi: 10.1093/jn/136.8.2123. [DOI] [PubMed] [Google Scholar]
- 47.Formiga F, Lopez-Soto A, Masanes F, et al. Influence of acute exacerbation of chronic obstructive pulmonary disease or congestive heart failure on functional decline after hospitalization in nonagenarian patients. Eur J Intern Med. 2005;16:24–28. doi: 10.1016/j.ejim.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Bartali B, Frongillo EA, Bandinelli S, et al. Low nutrient intake is an essential component of frailty in older persons. J Gerontol A Biol Sci Med Sci. 2006;61:589–593. doi: 10.1093/gerona/61.6.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartali B, Frongillo EA, Guralnik JM, et al. Serum micronutrient concentrations and decline in physical function among older persons. JAMA. 2008;299:308–315. doi: 10.1001/jama.299.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: A meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilhelm-Leen ER, Hall YN, Deboer IH, et al. Vitamin D deficiency and frailty in older Americans. J Intern Med. 2010;268:171–180. doi: 10.1111/j.1365-2796.2010.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Annweiler C, Schott AM, Berrut G, et al. Vitamin D and ageing: neurological issues. Neuropsychobiology. 2010;62:139–150. doi: 10.1159/000318570. [DOI] [PubMed] [Google Scholar]
- 53.Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119:229–236. doi: 10.1161/CIRCULATIONAHA.108.795260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Culp KR, Cacchione PZ. Nutritional status and delirium in long-term care elderly individuals. Appl Nurs Res. 2008;21:66–74. doi: 10.1016/j.apnr.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murray AM, Levkoff SE, Wetle TT, et al. Acute delirium and functional decline in the hospitalized elderly patient. J Gerontol. 1993;48:M181–186. doi: 10.1093/geronj/48.5.m181. [DOI] [PubMed] [Google Scholar]
- 56.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 57.Marcantonio ER, Flacker JM, Michaels M, et al. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618–624. doi: 10.1111/j.1532-5415.2000.tb04718.x. [DOI] [PubMed] [Google Scholar]
- 58.Inouye SK, Bogardus ST, Jr, Baker DI, et al. The Hospital Elder Life Program: A model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program. J Am Geriatr Soc. 2000;48:1697–1706. doi: 10.1111/j.1532-5415.2000.tb03885.x. [DOI] [PubMed] [Google Scholar]
- 59.Pitkala KH, Laurila JV, Strandberg TE, et al. Multicomponent geriatric intervention for elderly inpatients with delirium: A randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2006;61:176–181. doi: 10.1093/gerona/61.2.176. [DOI] [PubMed] [Google Scholar]
- 60.Pitkala KH, Laurila JV, Strandberg TE, et al. Multicomponent geriatric intervention for elderly inpatients with delirium: Effects on costs and health-related quality of life. J Gerontol A Biol Sci Med Sci. 2008;63:56–61. doi: 10.1093/gerona/63.1.56. [DOI] [PubMed] [Google Scholar]