Synopsis
Behavioral treatment should be the first line of intervention for overweight and obese individuals. This paper provides an overview of the structure and principles of behavioral weight loss treatment. The short- and long-term effectiveness of this approach is reviewed. Strategies for improving weight loss maintenance are described, including prolonging contact between patients and providers (either in the clinic or via Internet or telephone), facilitating high amounts of physical activity, and combining lifestyle modification with pharmacotherapy. Finally, innovative programs that can be used to disseminate behavioral approaches beyond traditional academic settings are discussed.
Keywords: obesity, weight loss, dieting, lifestyle, behavioral
Weight loss treatment is recommended for adults with a body mass index (BMI) of 30 kg/m2 or higher, as well as those with BMI of 25 kg/m2 or higher who have weight-related comorbidities.1 Behavioral treatment should be the first line of intervention for overweight and obese individuals.1 This paper first provides an overview of the structure and principles of behavioral weight loss treatment. Second, the short- and long-term effectiveness of this approach is reviewed. Third, strategies for improving weight loss maintenance are described. Finally, dissemination of behavioral treatment is addressed.
Structures and principles of behavioral treatment
The lifestyle modification interventions delivered in the Diabetes Prevention Program and Look AHEAD research studies are exemplars of behavioral treatment programs.2, 3 The intervention materials from both of these programs are in the public domain and may be used for educational or research purposes. Behavioral weight loss treatment also has been described in detail in other publications.4–6 The structure and three key components of this approach– goal setting, self monitoring, and stimulus control – will be reviewed here. Additional information about dietary and physical activity recommendations in behavioral programs can be found in Part IV of this volume.
Structure of treatment
Behavioral treatment is usually provided on a weekly basis for an initial period of 4 to 6 months. Programs that are focused on building weight loss maintenance skills may continue treatment after this period with bi-weekly sessions. Treatment is often provided in groups of 10 to 15 participants. Group therapy may be more effective than individual treatment. A randomized controlled trial found that group treatment induced a significantly larger initial weight loss than individual care.7 Group treatment is cost-effective, and group sessions provide a combination of empathy, social support, and healthy competition.8 Each session is typically scheduled to last 60 or 90 minutes. Group leaders are professionals with degrees in nutrition, psychology, or a related field. Sessions begin with private measurement of weight. Once the group convenes, each patient provides a brief report on his or her success in meeting behavioral goals. A new weight management skill is taught in each session according to a structured curriculum. Examples of skills taught include making healthy selections when eating in restaurants, using portion control, and obtaining social support for behavior changes.
Goal setting
Behavioral treatment specifies objective goals that can be easily measured. This allows for clear assessment of progress. Each patient has a target for average daily calorie intake, weekly minutes of physical activity, and number of days for which food records will be completed. Each week, patients share with the group how successful they were in meeting these goals. Patients often report that they appreciate the accountability that results from this check-in. Amount of weight change is typically not shared with the group. However, group leaders typically advise patients to expect 0.5 to 1.0 kg per week of weight loss, with an ultimate goal of losing 10% of initial body weight. Patients also may set goals for additional, specific behaviors that are expected to produce or maintain weight loss. When patients target a particular behavior to be changed, they are encouraged to operationalize the goal and carefully consider factors such as how, when, and where the behavior will be completed.
Self-monitoring
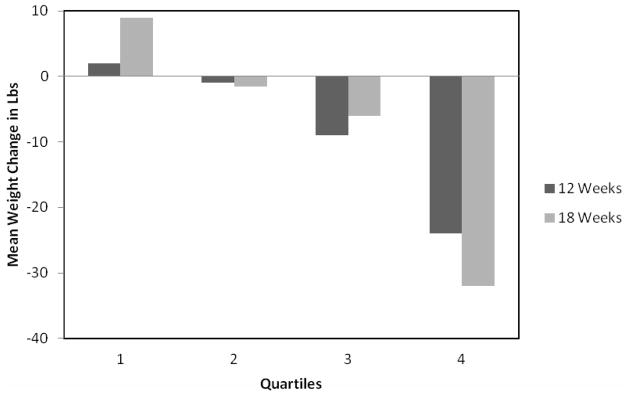
The systematic recording of target behaviors is a cornerstone of behavioral treatment. Self-monitoring provides regular feedback about whether target behaviors are improving, deteriorating, or being maintained. As shown in Figure 1, self-monitoring is strongly associated with weight loss success. Patients who monitor their eating and weight most consistently have the largest weight losses.9–12 Throughout treatment, patients keep a weekly record of all food and beverages consumed and calculate their daily calorie intake, and, in some programs, fat intake. An example of a food record is shown in Figure 2. In the early phase of treatment, this food record is a critical tool for identifying eating patterns that can be modified in order to reduce calorie intake. Periodically, patients may be asked to monitor particular factors associated with eating behaviors, such as hunger level, mood, or place of eating. Patients also record minutes of physical activity or use a pedometer to track daily number of steps.
Figure 1.
Mean weight change per quartile of monitoring index (i.e., frequency of recording weight and eating within a behavioral treatment program). Higher quartiles indicate more often frequently engaging in self-monitoring behaviors. Figure reproduced from: Baker RC, Kirschenbaum DS. Self-monitoring may be necessary for successful weight control. Behav Ther 1993;24:377–394.
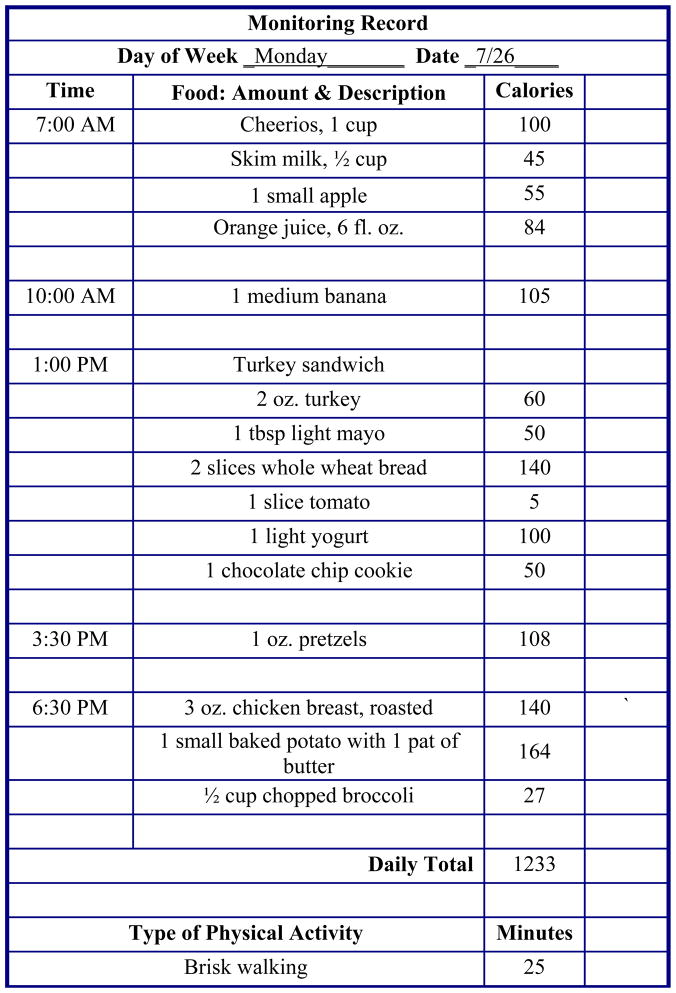
Figure 2.
An example of a self-monitoring record. Participants record the times, amounts, and calories of foods consumed and the physical activity they engage in. The extra column can be used to monitor additional contextual information (e.g., places, feelings). Reprinted from: Butryn ML, Clark VL, Coletta MC. Behavioral Approaches to the Treatment of Obesity. In: Akabas SR, Lederman SA, Moore BJ, editors. Understanding obesity: biological, psychological and cultural influences. New York: Wiley; in press.
Stimulus control
Stimulus control principles are used to change the internal and external cues associated with targeted eating and activity behaviors.13 Patients are taught to change their immediate environment (e.g., in the home and workplace) so that it facilitates, rather than hinders, behavior change. Reducing exposure to particularly tempting high-calorie foods should reduce consumption of such foods. If, for example, a patient would like to have one serving of ice cream per week but finds it difficult to control intake of this particular food, it may be unnecessarily challenging to have a large container of ice cream in the freezer at all times. Instead, a patient may wish to buy a single serving of ice cream once per week. Increasing the availability and visibility of healthy food (e.g., by placing a large bowl of fruit slices in the front of the refrigerator) should facilitate desirable eating behaviors. Physical activity also can be promoted by, for example, placing sneakers and appropriate clothing next to the bed if a patient plans to go for a walk immediately upon waking in the morning.
Effectiveness of behavioral treatment
Participants treated with a comprehensive behavioral approach lose approximately 8–10 kg, equal to 8–10% of initial weight.14 Approximately 80% of patients who begin treatment complete it.14 Thus, lifestyle modification yields favorable results as judged by the criteria for success (i.e., a 5% to 10% reduction in initial weight) proposed by the National Institutes of Health.1 Weight loss has more than doubled over the past 30 years as treatment duration has increased three-fold.14 Although several new components, including cognitive restructuring, have been added to the behavioral approach since 1974, the most parsimonious explanation for the larger weight losses is the longer duration of treatment. The rate of weight loss has remained constant at about 0.4 to 0.5 kg per week.
Weight loss often reaches its peak at approximately 6 months, and then weight regain often begins in the absence of weight maintenance therapy. As shown in Figure 3, a meta-analysis of behavioral treatment programs that provided treatment for a range of 13 to 52 sessions found that at 1 year, 28% of individuals had a weight loss of ≥10% of baseline weight, 26% had a weight loss of 5–9.9%, and 38% had a weight loss of ≤4.9%.15 Patients, on average, regain one-third of lost weight within 1 year of treatment ending, and while rate of weight regain may slow after that, nearly one-half of participants return to their original weight within 5 years.5, 14, 16, 17
Figure 3.
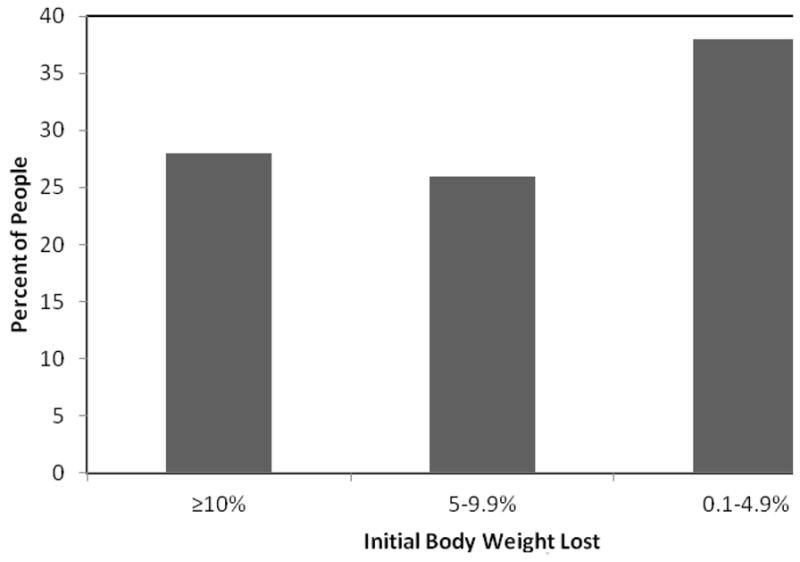
Mean percent of initial body weight lost at 1 year following involvement in a behavioral weight loss program. Data adapted from: Christian JG, Tsai AG, Bessesen DH. Interpreting weight losses from lifestyle modification trials: Using categorical data. Int J Obes 2010;34:207–209.
Individuals who initially succeed at weight loss in a behavioral treatment program are likely to find that their efforts are eventually challenged by profound environmental influences (e.g., large portion sizes, labor saving devices) that remain highly influential after a weight loss program has ended.18, 19 Metabolic responses to weight loss, biological preferences for palatable foods, and conservation of energy also make weight loss maintenance challenging.20 In the context of these biological factors and the obesogenic environment, weight loss maintenance may require long-term vigilance with regard to eating behavior and physical activity.21–24
Improving weight loss maintenance
In the following section, methods of improving weight loss maintenance in behavioral treatment are addressed. Strategies that are highlighted include facilitating long-term patient-provider contact in person or via the Internet or telephone; promoting higher levels of physical activity; and combining behavioral interventions with medication.
Long-term patient-provider contact
Frequent, long-term patient-provider contact, following initial weight loss, is perhaps the most successful method of preventing weight regain. Such contact, typically provided in group sessions, appears to provide patients the support and motivation needed to continue to practice weight control behaviors which include regularly monitoring body weight, food intake, and physical activity.25 Several studies conducted in the 1980s first documented the benefits of this approach.26–28 Perri et al, for example, found that individuals who attended every-other-week group maintenance sessions for the year following weight reduction maintained 13.0 kg of their 13.2-kg end-of-treatment weight loss, whereas those who did not receive such therapy maintained only 5.7 kg of a 10.8-kg loss.28 In reviewing 13 studies on this topic, Perri and Corsica found that patients who received long-term treatment, which averaged 41 sessions over 54 weeks, maintained 10.3 kg of their initial 10.7-kg weight loss.16 More recently, Wing and colleagues29 showed that monthly, on-site group counseling was more effective in preventing weight regain over 18 months of intervention than was an education-control group or an Internet-based intervention. Participants in the three groups regained 2.5, 4.9, and 4.7 kg, respectively, of an initial loss of approximately 19 kg. Participants in all three groups who monitored their weight weekly or more frequently were the most successful in maintaining their lost weight.29
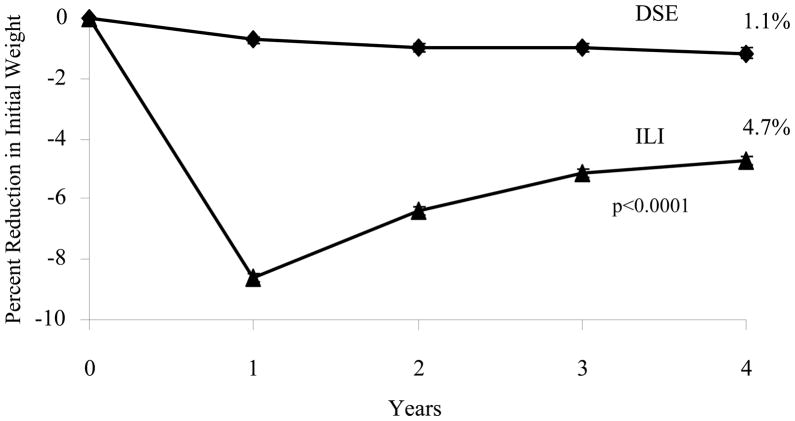
The Look AHEAD study is assessing the long-term health consequences of intentional weight loss in overweight/obese patients with type 2 diabetes over up to 13.5 years of treatment with an intensive lifestyle intervention.30 The study is providing long-term behavioral counseling, based on its benefits described above. Participants in the intensive lifestyle intervention (ILI) were provided three group and one individual treatment session for the first 6 months, followed by two group and one individual session during months 7 to 12. 3 At the end of the first year, ILI participants lost a mean of 8.6% of initial weight, compared with a significantly smaller 0.7% for participants in an education-control group, referred to as Diabetes Support and Education (DSE).32, 33 From years 2 to 4, ILI participants were provided one on-site meeting (of 20–25 minutes) per month with their lifestyle counselor, as well as with an additional monthly contact by telephone (5 to 15 minutes) or e-mail, in modeling the twice monthly contact provided by Perri et al in their series of studies.26–28 Participants were also offered (but not required to attend) monthly open-group sessions, at which they could weigh-in and receive new diaries with which to monitor their weight, food intake, and physical activity. They also were invited (but not required) to attend two or three group refresher courses per year, each of which offered 6 to 10 weeks of treatment with which to either attempt to lose more weight or to reverse weight regain.3
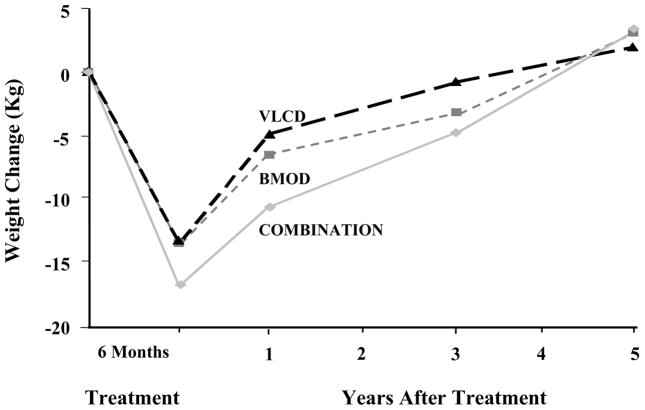
As shown in Figure 4, at the end of year 4, ILI participants had a mean weight loss of 4.7%, compared with a significantly smaller 1.0% for those in DSE.34 This is among the largest weight losses achieved with a lifestyle intervention at 4 years, slightly larger than the loss obtained in the DPP at the same time.35 Look AHEAD’s study design prevents investigators from evaluating the benefits of the twice monthly treatment contacts (or the other intervention components) in maintaining the weight losses achieved at the end of the first year. A third treatment arm, consisting of ILI participants who received no further behavioral treatment after the first year, would be necessary to test the efficacy of the weight loss maintenance therapy provided. However, the long-term patient-provider contact did appear to slow the rate of regain that is usually observed following the end of behavioral weight loss interventions. Figure 5 presents results of an early lifestyle intervention by Wadden et al.36 It shows that after 6 months of weight loss, achieved with different dietary interventions, participants regained to their baseline weight over 4 to 5 years of follow-up.
Figure 4.
Percent change in weight for participants in the intensive lifestyle intervention (ILI) and diabetes support and education (DSE) groups of the Look AHEAD (Action for Health in Diabetes) trial during 4 years of follow-up. Reprinted from: Look AHEAD Research Group, Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med 2010;170:1566–75.
Figure 5.
Weight changes during five years following treatment by very low calorie diet (VLCD), behavior therapy (BMOD), or a combination of VLCD and BMOD. Reprinted from: Wadden TA, Sternberg JA, Letizia KA, et al. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: a five-year perspective. Int J Obes 1989;13 Suppl 2:39–46.
Current evidence suggests that individuals with refractory obesity should receive patient-provider support indefinitely to facilitate the maintenance of lost weight. The need for such care is revealed by findings that participants regain lost weight when maintenance therapy is terminated.16
Long-term contact with Internet and telephone
Using Internet and telephone also may facilitate extended contact with the treatment team and promote weight loss maintenance. These interventions have the potential to reach large numbers of adults and to improve cost-effectiveness of intervention. Patients may be especially interested in one of these modes of treatment delivery if they wish to have more flexibility in the time at which intervention resources are utilized; if arrangements for transportation and child care are difficult to arrange; or if they prefer treatment with a certain amount of anonymity.
More than a dozen RCTs have examined use of the Internet to deliver behavioral treatment to obese adults.37 Short-term studies of these programs typically found that they were more effective than minimal treatment, but less effective than face-to-face treatment.38–42 In general, effective technology-based programs tailored information and feedback from therapists and provided structured instruction in diet, physical activity, and behavioral strategies.37 Feedback features, such as progress charts, and social support features, such as Web chats with other participants, have been associated with greater success.43 Systemic reviews have indicated that higher usage of website features is associated with larger weight losses.44
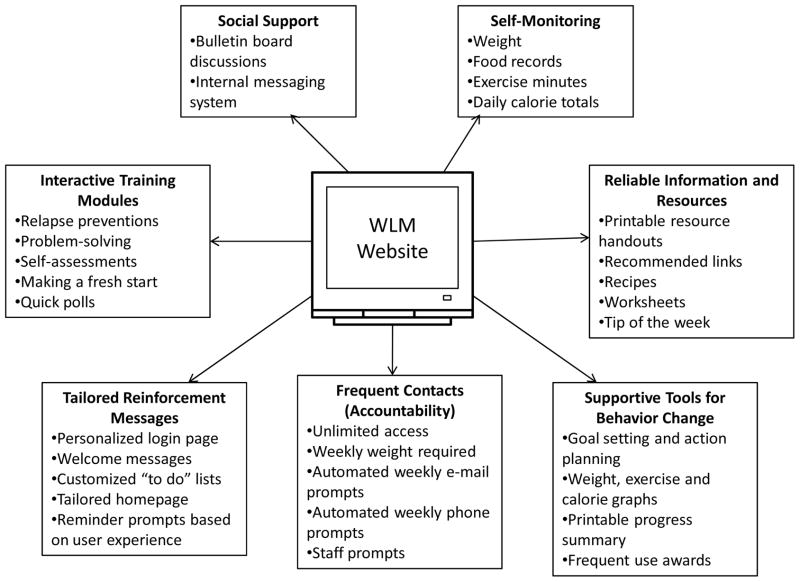
Several studies have examined use of the Internet specifically for weight loss maintenance, with mixed results. The components of one such program, designed specifically for weight loss maintenance, are shown in Figure 6. In each of the studies reviewed here, participants were randomly assigned to a weight loss maintenance intervention after initial weight loss, which was typically 7–10 kg. In two such studies, personal contact was more effective for weight loss maintenance than Internet treatment, consistent with many results in the weight loss literature. Harvey-Berino et al45 compared the effectiveness of 12-month maintenance programs delivered over the Internet or face-to-face (with minimal or frequent contact). At end of treatment, sustained weight loss was poorer in the Internet condition than in minimal and frequent contact face-to-face conditions (5.7 kg, 10.4 kg, and 10.4 kg, respectively). Svetkey et al46 randomly assigned participants to one of three weight loss maintenance conditions: 1) an interactive Internet-based intervention that encouraged participants to monitor and submit their weight, food intake, and physical activity; 2) monthly telephone counseling, with in-person visits every fourth month; or 3) an education control group. After 30 months of maintenance treatment, weight regain in the Internet condition (5.2 kg) was significantly higher than in the personal-contact condition (4.0 kg), and not significantly different from the self-directed group (5.5 kg).
Figure 6.
Key interactive website features. Reproduced from: Funk KL, Stevens VJ, Bauck A, et al. Development and implementation of a tailored self-assessment tool in an internet-based weight loss maintenance program. Clinical Practice & Epidemiology in Mental Health 2011; 7: 67–73.
In two other studies, Internet and face-to-face weight loss maintenance treatment were found to be equally effective. Harvey-Berino et al47 conducted another comparison of a 12-month program delivered over the Internet or face-to-face (with minimal or frequent contact). Weight loss after 1 year of maintenance treatment did not significantly differ between groups (7.6 kg, 5.5 kg, and 5.1 kg, for the Internet, minimal face-to-face contact, and frequent face-to-face contact conditions, respectively). Wing et al29 compared a monthly Internet treatment with monthly face-to-face treatment and a quarterly newsletter control condition. After 18 months of maintenance intervention, the amount of weight gain was not significantly different in the Internet (4.7 kg) and face-to-face (2.5 kg) conditions. Weight gain was significantly less in both of these conditions than in the control group (4.9 kg).
It appears that frequent telephone contacts can help maintain weight loss in adults. Perri and colleagues48 recently demonstrated that twice monthly counseling, delivered in 15–20 minute individual telephone sessions, was as effective as on-site group counseling (delivered on the same schedule in 60-minute sessions) in maintaining an initial weight loss of approximately 10 kg.26 (The initial weight loss was achieved during a 6-month run-in program that provided group lifestyle modification.) Participants in both intervention groups regained only 1.2 kg during the year of counseling, compared with a significantly greater 3.7 kg for participants in an education-control group. As described earlier, Svetkey and colleagues46 similarly demonstrated the benefits of brief, individual monthly telephone counseling sessions (of 5–15 min) in preventing weight regain. When scheduling telephone calls, the same therapist optimally should contact the patient on each occasion. A study in which patients were contacted by staff members unknown to them failed to produce weight maintenance results superior to those of a no-contact group.49
Effective programs delivered via Internet or telephone should be developed as an option for adults who do not have access to face-to-face treatment, and evaluation of these programs as a method of extending treatment contact should continue.
Promoting high levels of physical activity
High levels of physical activity may facilitate weight loss maintenance. A systematic review of observational and prospective evidence found that individuals who engaged in physical activity experienced less weight regain than those individuals who did not, and those individuals who engaged in the highest levels of physical activity experienced less regain than those who engaged in less physical activity.50 A minimum of 30 to 60 minutes of activity is typically associated with a weight loss maintenance benefit. The adults enrolled in the National Weight Control Registry (NWCR), who all lost at least 13.6 kg and maintained that weight loss for at least 1 year, have reported engaging in very high levels of physical activity – the equivalent of walking 28 miles/week.51 Assessment of NWCR participants with accelerometers confirmed that they engaged in an average of 290 minutes per week of sustained (i.e., bouts of 10 minutes or more), moderate-to-vigorous physical activity.52 Prospective analysis of maintenance of intentional weight loss in the Nurses’ Health Study II found that, compared to women who remained sedentary, women who engaged in 30 or more minutes per day of physical activity were more likely to limit their weight regain to 30% or less.53
Among the limited number of studies that have used experimental designs to examine the relationship between physical activity level and weight loss maintenance, the pattern of results has been less clear.21, 28, 54–57 In several studies, experimental condition did not produce differences in weight loss maintenance. However, across groups, participants who reported exercising the most typically experienced the greatest weight loss maintenance. For example, Wadden et al55 found that a 1-year program of supervised exercise training did not results in better weight loss maintenance than a program of diet alone, but the participants who reported exercising regularly during follow-up had less weight regain than those who did not report exercising. Maintenance of physical activity appeared challenging for these participants. Of those who originally received on-site exercise training, only 50% reported exercising regularly during the last 4 months of the 1-year follow-up.
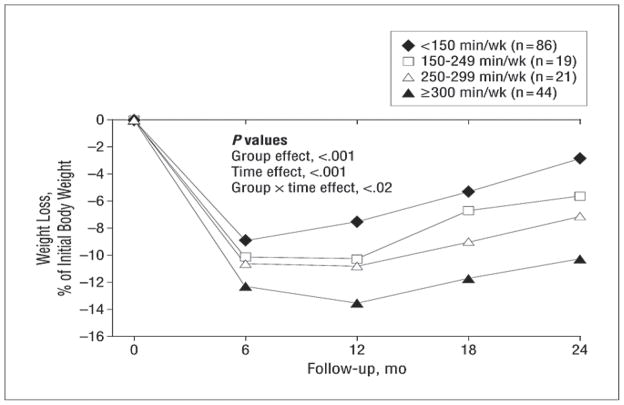
Jakicic et al58 randomly assigned women to behavioral weight loss intervention groups that varied according to targeted physical activity energy expenditure (1000 vs. 2000 kcal per week) and intensity (moderate vs. vigorous). (All participants also reduced calorie intake.) Weight loss at 2 years did not differ between conditions. Participants generally did not sustain the prescribed differences in physical activity, which may have contributed to the lack of differences in weight loss among group. As shown in Figure 7, level of physical activity was associated with weight loss maintenance across treatment conditions. A high level of physical activity was needed to achieve weight loss maintenance: individuals who maintained a weight loss of 10% or more had increased their physical activity since baseline by, on average, 275 min/wk.
Figure 7.
Percentage weight loss by minutes per week of physical activity. Reprinted from: Jakicic JM, Marcus BH, Lang W, et al. Effect of Exercise on 24-Month Weight Loss Maintenance in Overweight Women. Arch Intern Med 2008;168:1550–1559.
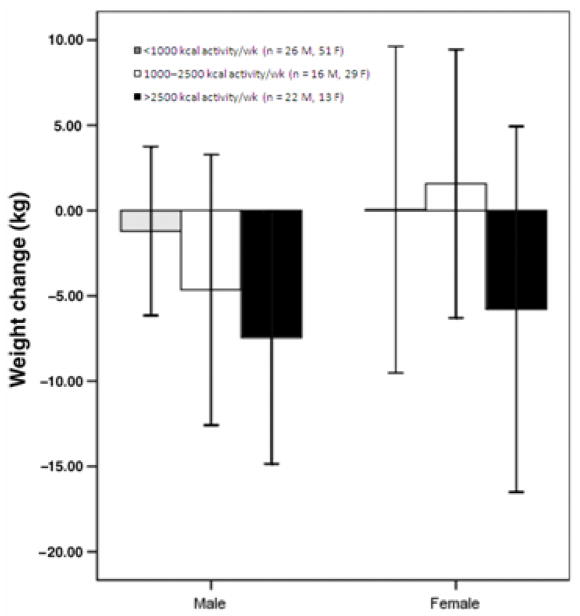
Jeffery et al 54, 55 conducted a similar study that yielded comparable results. Participants who were randomly assigned to complete a high level of physical activity (equivalent to approximately 75 minutes per day of brisk walking) experienced significantly greater weight loss maintenance at 18 months than those told to complete a lower level of physical activity. However, most participants were unable to sustain this high level of physical activity after 18 months, so group differences were no longer apparent by 30 months. As shown in Figure 8, the subset of participants who were able to sustain a high level of physical activity (i.e., expending at least 2500 kcal per week with exercise) maintained significantly larger weight losses at 30 months than those participants who engaged in a lower level of physical activity.
Figure 8.
Weight changes by physical activity level at 30 months. Main effect of sex, P = 0.04; main effect of exercise group, P = 0.003; sex x exercise group interaction, P = 0.29 (NS). Error bars are ± 1 SD. Reprinted from: Tate DF, Jeffery RW, Sherwood NE, et al. Long-term weight losses associated with prescription of higher physical activity goals. Are higher levels of physical activity protective against weight regain? Am J Clin Nutr 2007;85: 954–959.
Based on the available evidence, The American College of Sports Medicine59 concluded that adults who wish to minimized weight regain should engage in the equivalent of 60 minutes per day of brisk walking.23, 60–62 Additional experimental research must be conducted to learn more about how physical activity can promote weight loss maintenance. Given the limitations of the available research, there is a particular need for studies that have sufficient statistical power, long periods of follow-up, and objective assessment of physical activity. Behavioral therapy also may need to be refined or supplemented with strategies that promote long-term adherence to prescribed levels of physical activity.
Combining behavioral and pharmacologic approaches
The long-term use of weight loss medications could provide another option for improving the maintenance of lost weight.63, 64 This approach recognizes that obesity is a chronic disorder that requires long-term pharmacologic treatment, in the same manner that type 2 diabetes or hypertension requires chronic pharmacologic intervention to achieve optimal control of these conditions. The long-term prescription of weight loss medications represents a marked change in their use, which previously was limited to only 6 to 12 weeks.64
Adding weight loss medication to comprehensive behavioral treatment significantly improves the induction of weight loss, as demonstrated by several studies.11, 65 The combination of medication plus lifestyle modification is also more effective than lifestyle modification alone (i.e., with placebo) in maintaining weight loss, originally achieved with diet and exercise alone. Hill et al,66 for example, found that in patients who had lost an average of 10 kg during a diet run-in period, those who were assigned to receive orlistat (a lipase inhibitor) plus lifestyle modification regained only 32% of their weight loss in the following year, compared with a significantly greater gain of 56% in those treated by placebo plus lifestyle intervention. Orlistat-treated patients also maintained significantly greater reductions in LDL cholesterol.
Several factors currently limit pharmacotherapy’s use to improve the maintenance of lost weight, the most critical of which is that only one medication – orlistat – is approved by the U.S. Food and Drug Administration (FDA) for long-term administration. And this medication is only modestly effective, producing but a 3 to 4 kg greater weight loss than placebo.63 Sibutramine, a serotonin-norepinephrine re-uptake inhibitor, facilitated the maintenance of a 10 kg weight loss at 2 years.67 However, the drug was removed from the market in November 2010 because of findings that it increased cardiovascular morbidity and mortality in patients who had a prior history of CVD disease.68 Rimonabant, a cannabinoid inverse agonist, was removed from the market in Europe (and never approved in the U.S.) because of its association with symptoms of depression and suicidal ideation.69,70 This appropriately led other manufacturers to discontinue development of their cannabinoid agents, despite success in maintaining lost weight.71 In 2010, the FDA declined to approve any of the three weight loss medications that it reviewed. These included lorcaserin, a selective serotonin 2C receptor agonist,72 the combination of phentermine and topirimate,73 and the combination of bupropion and naltrexone.74 The sponsors of these three medications are still seeking approval by FDA. The next weight loss medication to be reviewed by FDA is likely to be liraglutide, a GLP-1 inhibitor, which is approved by the FDA for the treatment of type 2 diabetes.75
The long-term treatment of obesity with the combination of lifestyle modification and pharmacotherapy remains of interest to investigators; however, numerous factors have limited progress in this area. These include the general lack of reimbursement for weight loss medications (requiring payment out of pocket), as well as patients’ unrealistic treatment expectations, which can cause them to stop taking medication when they stop losing weight.76 Thus, advances are required in the medications themselves, in the FDA’s perceptions of weight loss agents, in health care payers reimbursement policies, and in patients’ weight loss expectations before pharmacological therapy can significantly improve the long-term management of obesity.
Dissemination of behavioral treatment
Intensive behavioral treatment, as provided in the Diabetes Prevention Program (DPP)34 and Look AHEAD3 are successful but can be time-consuming, costly, and unavailable to most overweight or obese individuals. Such programs typically are offered in academic medical centers and, as a result, are rarely accessible to rural populations. Intensive lifestyle interventions also utilize registered dietitians, psychologists and other professionals who have expertise in the behavioral treatment of obesity. Yet, there is not a sufficient number of these providers to treat all of the individuals who need lifestyle modification. Thus, investigators are currently exploring ways to adapt these approaches to reach a larger proportion of overweight and obese individuals.
Primary care practice
Several trials have evaluated the feasibility of providing lifestyle modification in a primary care-based setting. Ashley et al77 randomly assigned overweight women to a dietitian-led intervention, a dietitian-led intervention with meal replacements, or a primary care office intervention with meal replacements. Participants in the primary care group attended brief every-other-week visits with their primary care physician or nurse, following the LEARN manual.4 After 1 year of treatment, completers in the primary care group lost 4.3% of initial weight vs. 4.1% in the dietitian-led group. However, participants in the dietitian-led group that incorporated meal replacements lost 9.1%, significantly more than both of the other groups. Another study randomly assigned physicians to provide a tailored weight-loss intervention or standard treatment to 144 African-American female patients.78,79 The tailored intervention consisted of 6 monthly, brief counseling sessions with the physician. At month 6, the tailored group lost significantly more weight than the standard group (−2.0 kg vs. +0.2 kg), but there was no difference between groups at the 12- or 18-month follow-up assessments. In a third study, participants were randomly assigned to receive quarterly visits with their primary care physician (PCP), along with weight loss materials, or these visits plus eight additional PCP visits with a medical assistant trained to deliver brief weight loss counseling.80 The participants who received the additional counseling, compared with those who did not, lost significantly more weight at 6 months (4.4 kg vs. 0.9 kg) but regained their weight between month 6 and month 12 (i.e., after counseling visits were discontinued).
Results of these and other studies indicate that while primary care settings offer an option for disseminating behavioral weight loss treatment, the benefits generally are modest.81 There are several barriers to implementing lifestyle interventions in primary care, including a lack of physician time, training, and reimbursement. These factors explain physicians’ reluctance to add an intensive weight management to an already busy practice.
Community settings
Community centers and workplaces offer additional venues for weight management. Ackermann et al successfully adapted the DPP to be delivered by YMCA staff in a group format.82 Participants randomly assigned to the treatment group received 16 sessions modeled on the original DPP protocol. At 6 months, participants in the intervention lost 6.0% of initial weight, compared with a loss of 2.0% in control participants who received advice only. This significant difference was still present at 12 months. Cost per participant in the study was estimated to be $275–$325, which was substantially lower than the estimated cost of $1400 per participant during the first year of the original DPP.83 Another study similarly adapted components of the DPP to be delivered to residents of rural communities.48 All of the obese women in the study first participated in a 6-month weight-loss intervention through their local Cooperative Extension Service (CES) office, and were then randomized to receive telephone counseling, face-to-face counseling, or newsletters twice monthly for an additional year. Counseling during both the induction and maintenance phases was conducted by trained CES staff. Women lost an average of 10.0 kg after the 6-month weight loss program. Thereafter, those in the telephone and face-to-face groups regained less weight (1.2 kg in both) than the newsletter group (3.7 kg) during the extended treatment phase. Both of these studies demonstrate that community organizations may be viable options for adapting weight loss interventions.
Worksites also may provide a suitable setting for weight management programs, given the amount of time people spend at work, as well as employers’ potential motivation to improve the health of their employees. Workplace programs can include multiple components, such as changes to the work environment, social support and/or competitions, and healthy messaging throughout the worksite.84 A recent systematic review of worksite programs found a net loss of 1.3 kg after 6–12 months of intervention.84 Inclusion of structured sessions and behavioral counseling appeared to improve programs’ efficacy. The costs of such interventions are usually lower than the intensive lifestyle interventions provided in academic settings, and may prove cost-effective for employers by reducing medical expenditures and increasing employee productivity.
Commercial weight loss programs
Commercial weight loss programs offer behavioral weight management to the general public. These programs are diverse and may include behavioral and dietary counseling (in-person or via telephone or the Internet), prepackaged meals, and/or group support. The three largest commercial providers are Weight Watchers, Jenny Craig, and Nutrisystem. Weight Watchers offers a point-system dietary plan in combination with group meetings or a Web-based monitoring program. Jenny Craig customers choose from in-person or telephone-delivered individual counseling, in addition to pre-packaged meals. Nutrisystem also offers a pre-packaged (home-delivered) meal plan. The company does not provide personal counseling but online self-monitoring tools are available on the Website.
Randomized controlled trials (RCTs) of these three major commercial interventions have revealed that they produce greater weight loss than control groups.85–89 A large trial that randomized participants to Weight Watchers or to self-help found that the Weight Watchers group lost significantly more weight than the self-help group at both 1 year (4.3 kg vs. 1.3 kg) and 2 years (2.9 kg vs. 0.2 kg).86 A recent RCT sponsored by Jenny Craig randomly assigned women to usual care (i.e., two in-person counseling sessions and monthly contacts), face-to-face counseling at a Jenny Craig center; or telephone-based Jenny Craig counseling.88 Women in the face-to-face group lost a mean of 10.1 kg at 1 year and maintained an average weight loss of 7.4 kg at 2 years. Those in the telephone-based group lost a mean of 8.5 kg at 1 year and sustained a loss of 6.2 kg at 2 years. Participants in the usual care group lost 2.4 kg at 1 year and maintained a loss of 2.0 kg at 2 years. Both counseling groups were superior to usual care but did not differ significantly from each other. One trial has tested Nutrisystem. At 3 months, participants who were prescribed the Nutrisystem diet lost significantly more than those in a diabetes support and education group (7.1% vs.0.4%).89 Results of all these trials are generally encouraging. However, we note that the trials typically provided participants with additional on-site support and free products, which are not offered to regular consumers. Moreover, access to commercial weight loss programs may be limited by the costs involved.
Technology-based interventions
As discussed earlier in this chapter, investigators are increasingly studying methods to provide effective behavioral weight loss programs via the Internet. One example of a more wide-scale Internet-based intervention is Shape Up Rhode Island, a statewide team-based competition that encouraged weight loss and increased physical activity.90 The 16-week program consisted of online self-monitoring, free community events and workshops, and free pedometers. The 2007 campaign enrolled 4,717 adults. The 3,311 participants who completed ≥ 12 weeks lost an average of 3.2 kg. Additional studies are underway to test the efficacy of smartphones, social networking, and Websites for weight loss, especially in young adults.91 As these types of technology become more accessible and popular, there are more possibilities for interventions with broad public health impact.
Conclusions
Weight losses achieved in primary care and community settings are usually modest, compared to the results from academic-based intensive lifestyle interventions such as the DPP34 and Look AHEAD.3 However, innovative programs potentially can provide weight loss to a larger number of obese and overweight individuals and at a lower cost. Thus, such programs are likely to have a more positive net effect on our nation’s weight and health than are traditional, academically-based programs, with their limited reach.
Summary
This review has shown that behavioral treatment is effective in inducing a 10% weight loss, which is sufficient to significantly improve health. Weight loss maintenance is challenging for most patients. Long-term outcomes have the potential to be improved through various methods, including prolonging contact between patients and providers (either in the clinic or via Internet or telephone), facilitating high amounts of physical activity, or combining lifestyle modification with pharmacotherapy. Innovative programs also are being developed to disseminate behavioral approaches beyond traditional academic settings.
Acknowledgments
The authors thank Stephanie Kerrigan and Caroline Moran for their assistance in manuscript preparation. Preparation of this review was supported, in part, by grant K24-DK065018 to Dr. Wadden.
Footnotes
Disclosures: The authors have no financial disclosures or conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.NHLBI. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: Executive summary. expert panel on the identification, evaluation, and treatment of overweight in adults. Am J Clin Nutr. 1998;68:899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Prevention Program (DPP) Research Group. The diabetes prevention program (DPP): Description of lifestyle intervention. Diabetes care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Look AHEAD Research Group. The look AHEAD study: A description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brownell KD. The LEARN Program for Weight Management. Dallas: American Health Publishing Company; 2000. [Google Scholar]
- 5.Wing RR. Behavioral weight control. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. New York: The Guilford Press; 2002. pp. 301–316. [Google Scholar]
- 6.Wadden TA, Butryn ML. Behavioral treatment of obesity. Endocrinol Metab Clin North Am. 2003;32:981–1003. doi: 10.1016/s0889-8529(03)00072-0. [DOI] [PubMed] [Google Scholar]
- 7.Renjilian DA, Perri MG, Nezu AM, et al. Individual versus group therapy for obesity: Effects of matching participants to their treatment preferences. J Consult Clin Psychol. 2001;69:717–721. [PubMed] [Google Scholar]
- 8.Wadden TA, Foster GD. Behavioral treatment of obesity. Med Clin North Am. 2000;84:441–61. vii. doi: 10.1016/s0025-7125(05)70230-3. [DOI] [PubMed] [Google Scholar]
- 9.Butryn ML, Phelan S, Hill JO, et al. Consistent self-monitoring of weight: A key component of successful weight loss maintenance. Obesity. 2007;15:3091–3096. doi: 10.1038/oby.2007.368. [DOI] [PubMed] [Google Scholar]
- 10.Boutelle KN, Kirschenbaum DS. Further support for consistent self-monitoring as a vital component of successful weight control. Obes Res. 1998;6:219–224. doi: 10.1002/j.1550-8528.1998.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 11.Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353:2111–2120. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 12.Baker RC, Kirschenbaum DS. Self-monitoring may be necessary for successful weight control. Behav Ther. 1993;24:377–394. [Google Scholar]
- 13.Foster GD. Clinical implications for the treatment of obesity. Obesity. 2006;14:182S–185S. doi: 10.1038/oby.2006.303. [DOI] [PubMed] [Google Scholar]
- 14.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132:2226–2238. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 15.Christian JG, Tsai AG, Bessesen DH. Interpreting weight losses from lifestyle modification trials: Using categorical data. Int J Obes. 2010;34:207–209. doi: 10.1038/ijo.2009.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perri MG, Corsica JA. Improving the maintenance of weight lost in behavioral treatment of obesity. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. New York: Guilford; 2002. pp. 357–379. [Google Scholar]
- 17.Curioni CC, Lourenço PM. Long-term weight loss after diet and exercise: A systematic review. Int J Obes. 2005;29:1168–1174. doi: 10.1038/sj.ijo.0803015. [DOI] [PubMed] [Google Scholar]
- 18.Lowe MR. Self-regulation of energy intake in the prevention and treatment of obesity: Is it feasible? Obes Res. 2003;11 (Suppl):44S–59S. doi: 10.1038/oby.2003.223. [DOI] [PubMed] [Google Scholar]
- 19.Drewnowski A, Rolls BJ. How to modify the food environment. J Nutr. 2005;135:898–899. doi: 10.1093/jn/135.4.898. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum M, Hirsch J, Gallagher DA, et al. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr. 2008;88:906–912. doi: 10.1093/ajcn/88.4.906. [DOI] [PubMed] [Google Scholar]
- 21.Jakicic JM, Marcus BH, Gallagher KI, et al. Effect of exercise duration and intensity on weight loss in overweight, sedentary women: A randomized trial. JAMA. 2003;290:1323–1330. doi: 10.1001/jama.290.10.1323. [DOI] [PubMed] [Google Scholar]
- 22.Catenacci VA, Ogden LG, Stuht J, et al. Physical activity patterns in the national weight control registry. Obesity. 2008;16:153–161. doi: 10.1038/oby.2007.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tate DF, Jeffery RW, Sherwood NE, et al. Long-term weight losses associated with prescription of higher physical activity goals. are higher levels of physical activity protective against weight regain? Am J Clin Nutr. 2007;85:954–959. doi: 10.1093/ajcn/85.4.954. [DOI] [PubMed] [Google Scholar]
- 24.Jeffery RW, Wing RR, Sherwood NE, et al. Physical activity and weight loss: Does prescribing higher physical activity goals improve outcome? Am J Clin Nutr. 2003;78:684–689. doi: 10.1093/ajcn/78.4.684. [DOI] [PubMed] [Google Scholar]
- 25.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–341. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 26.Perri MG, Shapiro RM, Ludwig WW, et al. Maintenance strategies for the treatment of obesity: An evaluation of relapse prevention training and posttreatment contact by mail and telephone. J Consult Clin Psychol. 1984;52:404–413. doi: 10.1037//0022-006x.52.3.404. [DOI] [PubMed] [Google Scholar]
- 27.Perri MG, McAdoo WG, McAllister DA, et al. Enhancing the efficacy of behavior therapy for obesity: Effects of aerobic exercise and a multicomponent maintenance program. J Consult Clin Psychol. 1986;54:670–675. doi: 10.1037//0022-006x.54.5.670. [DOI] [PubMed] [Google Scholar]
- 28.Perri MG, McAllister DA, Gange JJ, et al. Effects of four maintenance programs on the long-term management of obesity. J Consult Clin Psychol. 1988;56:529–534. doi: 10.1037//0022-006x.56.4.529. [DOI] [PubMed] [Google Scholar]
- 29.Wing RR, Tate DF, Gorin AA, et al. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355:1563–71. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 30.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD (action for health in diabetes): Design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 31.Look AHEAD Research Group. A description of the lifetyle intervention and the evidence supporting it. Obesity. 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: One-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wadden TA, West DS, Neiberg RH, et al. One-year weight losses in the look AHEAD study: Factors associated with success. Obesity. 2009;17:713–722. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The Look AHEAD Research Group. Long term effects of lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes: Four year results of the look AHEAD trial. Arch Intern Med. 2010;170:1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DPP DPPRG-Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wadden TA, Sternberg JA, Letizia KA, et al. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: A five-year perspective. Int J Obes. 1989;13:39–46. [PubMed] [Google Scholar]
- 37.Saperstein SL, Atkinson NL, Gold RS. The impact of internet use for weight loss. Obes Rev. 2007;8:459–465. doi: 10.1111/j.1467-789X.2007.00374.x. [DOI] [PubMed] [Google Scholar]
- 38.Tate DF, Wing RR, Winett RA. Using internet technology to deliver a behavioral weight loss program. JAMA. 2001;285:1172–1177. doi: 10.1001/jama.285.9.1172. [DOI] [PubMed] [Google Scholar]
- 39.Tate DF, Jackvony EH, Wing RR. Effects of internet behavioral counseling on weight loss in adults at risk for type 2 diabetes: A randomized trial. JAMA. 2003;289:1833–1836. doi: 10.1001/jama.289.14.1833. [DOI] [PubMed] [Google Scholar]
- 40.Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human e-mail counseling, computer-automated tailored counseling, and no counseling in an internet weight loss program. Arch Intern Med. 2006;166:1620–1625. doi: 10.1001/archinte.166.15.1620. [DOI] [PubMed] [Google Scholar]
- 41.Harvey-Berino J, West D, Krukowski R, et al. Internet delivered behavioral obesity treatment. Prev Med. 2010 doi: 10.1016/j.ypmed.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Digenio AG, Mancuso JP, Gerber RA, Dvorak RV. Comparison of methods for delivering a lifestyle modification program for obese patients. Ann Intern Med. 2009;150:255–262. doi: 10.7326/0003-4819-150-4-200902170-00006. [DOI] [PubMed] [Google Scholar]
- 43.Krukowski RA, Harvey-Berino J, Ashikaga T, et al. Internet-based weight control: The relationship between web features and weight loss. Telemed J E Health. 2008;14:775–782. doi: 10.1089/tmj.2007.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neve M, Morgan PJ, Jones PR, et al. Effectiveness of web-based interventions in achieving weight loss and weight loss maintenance in overweight and obese adults: A systematic review with meta-analysis. Obes Rev. 2010;11:306–321. doi: 10.1111/j.1467-789X.2009.00646.x. [DOI] [PubMed] [Google Scholar]
- 45.Harvey-Berino J, Pintauro S, Buzzell P, et al. Does using the internet facilitate the maintenance of weight loss. Int J Obes Relat Metab Disord. 2002;26:1260. doi: 10.1038/sj.ijo.0802051. [DOI] [PubMed] [Google Scholar]
- 46.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: The weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 47.Harvey-Berino J, Pintauro S, Buzzell P, et al. Effect of internet support on the long-term maintenance of weight loss. Obes Res. 2004;12:320–329. doi: 10.1038/oby.2004.40. [DOI] [PubMed] [Google Scholar]
- 48.Perri MG, Limacher MC, Durning PE, et al. Extended-care programs for weight management in rural communities: The treatment of obesity in underserved rural settings (TOURS) randomized trial. Arch Intern Med. 2008;168:2347–2354. doi: 10.1001/archinte.168.21.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wing RR, Jeffery RW, Hellerstedt WL, et al. Effect of frequent phone contacts and optional food provision on maintenance of weight loss. Ann Behav Med. 1996;18:172–176. doi: 10.1007/BF02883394. [DOI] [PubMed] [Google Scholar]
- 50.Fogelholm M, Kukkonen-Harjula K. Does physical activity prevent weight gain: A systematic review. Obes Rev. 2000;1:95–111. doi: 10.1046/j.1467-789x.2000.00016.x. [DOI] [PubMed] [Google Scholar]
- 51.Klem ML, Wing RR, McGuire MT, et al. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr. 1997;66:239–246. doi: 10.1093/ajcn/66.2.239. [DOI] [PubMed] [Google Scholar]
- 52.Catenacci VA, Grunwald GK, Ingebrigtsen JP, et al. Physical activity patterns using accelerometry in the national weight control registry. Obesity. 2010 doi: 10.1038/oby.2010.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mekary RA, Feskanich D, Hu FB, et al. Physical activity in relation to long-term weight maintenance after intentional weight loss in premenopausal women. Obesity. 2010;18:167–174. doi: 10.1038/oby.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weinstock RS, Dai H, Wadden TA. Diet and exercise in the treatment of obesity: Effects of 3 interventions on insulin resistance. Arch Intern Med. 1998;158:2477–2483. doi: 10.1001/archinte.158.22.2477. [DOI] [PubMed] [Google Scholar]
- 55.Wadden TA, Vogt RA, Foster GD, et al. Exercise and the maintenance of weight loss: 1- year follow-up of a controlled clinical trial. J Consult Clin Psychol. 1998;66:429–433. doi: 10.1037//0022-006x.66.2.429. [DOI] [PubMed] [Google Scholar]
- 56.Wing RR, Venditti E, Jakicic JM, et al. Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care. 1998;21:350–359. doi: 10.2337/diacare.21.3.350. [DOI] [PubMed] [Google Scholar]
- 57.Leermakers EA, Perri MG, Shigaki CL, et al. Effects of exercise-focused versus weight- focused maintenance programs on the management of obesity. Addict Behav. 1999;24:219–227. doi: 10.1016/s0306-4603(98)00090-2. [DOI] [PubMed] [Google Scholar]
- 58.Jakicic JM, Marcus BH, Lang W, et al. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med. 2008;168:1550–1559. doi: 10.1001/archinte.168.14.1550. discussion 1559–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Donnelly J, Blair SN, Jakicic JM, et al. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:450–471. doi: 10.1249/MSS.0b013e3181949333. Special Communications: Position Stand. [DOI] [PubMed] [Google Scholar]
- 60.Ewbank PP, Darga LL, Lucas CP. Physical activity as a predictor of weight maintenance in previously obese subjects. Obes Res. 1995;3:257–263. doi: 10.1002/j.1550-8528.1995.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 61.Jakicic JM, Winters C, Lang W, et al. Effects of intermittent exercise and the use of home exercise equipment on adherence, weight loss, and fitness in overweight women: A randomized controlled trial. JAMA. 1999;282:1554–1560. doi: 10.1001/jama.282.16.1554. [DOI] [PubMed] [Google Scholar]
- 62.Schoeller DA, Shay K, Kushner RF. How much physical activity is needed to minimize weight gain in previously obese women? Am J Clin Nutr. 1997;66:551–556. doi: 10.1093/ajcn/66.3.551. [DOI] [PubMed] [Google Scholar]
- 63.Bray GA. Drug treatment of the overweight patient. Gastroenterology. 2007;132:2239–2252. doi: 10.1053/j.gastro.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 64.Yanovski SZ, Yanovski JA. Obesity. N Engl J Med. 2002;346:591–602. doi: 10.1056/NEJMra012586. [DOI] [PubMed] [Google Scholar]
- 65.Phelan S, Wadden TA. Combining behavioral and pharmacological treatments for obesity. Obes Res. 2002;10:560–574. doi: 10.1038/oby.2002.77. [DOI] [PubMed] [Google Scholar]
- 66.Hill JO, Hauptman J, Anderson JW, et al. Orlistat, a lipase inhibitor, for weight maintenance after conventional dieting: A 1-y study. Am J Clin Nutr. 1999;69:1108–1116. doi: 10.1093/ajcn/69.6.1108. [DOI] [PubMed] [Google Scholar]
- 67.James WP, Astrup A, Finer N, et al. Effect of sibutramine on weight maintenance after weight loss: A randomised trial. The Lancet. 2000;356:2119–2125. doi: 10.1016/s0140-6736(00)03491-7. [DOI] [PubMed] [Google Scholar]
- 68.James WP, Caterson ID, Coutinho W, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Eng J Med. 2010;363:905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 69.Pi-Sunyer FX, Aronne LJ, Heshmati HM, et al. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–75. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- 70.Jones D. End of the line for cannabinoid receptor 1 as an anti-obesity target? Nat Rev Drug Discov. 2008;7:961–2. doi: 10.1038/nrd2775. [DOI] [PubMed] [Google Scholar]
- 71.Wadden TA, Fujioka K, Toubro S, et al. A randomized trial of lifestyle modification and taranabant for maintaining weight loss achieved with a low-calorie diet. Obesity. 2010;18:2301–10. doi: 10.1038/oby.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–56. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 73.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341–52. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 74.Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity. 2011;19:110–20. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Astrup A, Rössner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–16. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 76.Wadden TA, Womble LG, Sarwer DB, et al. Great expectations: I’m going to lose 25% of my weight no matter what you say. J Consult Clin Psychol. 2003;71:1084–1089. doi: 10.1037/0022-006X.71.6.1084. [DOI] [PubMed] [Google Scholar]
- 77.Ashley JM, St Jeor ST, Schrage JP, et al. Weight control in the physician's office. Arch Intern Med. 2001;161:1599–604. doi: 10.1001/archinte.161.13.1599. [DOI] [PubMed] [Google Scholar]
- 78.Martin PD, Rhode PC, Dutton GR, et al. A primary care weight management intervention for low-income African-American women. Obesity. 2006;14:1412–20. doi: 10.1038/oby.2006.160. [DOI] [PubMed] [Google Scholar]
- 79.Martin PD, Dutton GR, Rhode PC, et al. Weight loss maintenance following a primary care intervention for low-income minority women. Obesity. 2008;16:2462–7. doi: 10.1038/oby.2008.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsai AG, Wadden TA, Rogers MA, et al. A primary care intervention for weight loss: results of a randomized controlled pilot study. Obesity. 2010;18:1614–8. doi: 10.1038/oby.2009.457. [DOI] [PubMed] [Google Scholar]
- 81.Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. J Gen Intern Med. 2009;24:1073–9. doi: 10.1007/s11606-009-1042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ackermann RT, Finch EA, Brizendine E, et al. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med. 2008;35:357–63. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ackermann RT, Marrero DG. Adapting the Diabetes Prevention Program lifestyle intervention for delivery in the community: the YMCA model. Diabetes Educ. 2007;33:69, 74–5, 77–8. doi: 10.1177/0145721706297743. [DOI] [PubMed] [Google Scholar]
- 84.Anderson LM, Quinn TA, Glanz K, et al. The effectiveness of worksite nutrition and physical activity interventions for controlling employee overweight and obesity: a systematic review. Am J Prev Med. 2009;37:340–57. doi: 10.1016/j.amepre.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 85.Heshka S, Greenway F, Anderson JW, et al. Self-help weight loss versus a structured commercial program after 26 weeks: a randomized controlled study. Am J Med. 2000;109:282–7. doi: 10.1016/s0002-9343(00)00494-0. [DOI] [PubMed] [Google Scholar]
- 86.Heshka S, Anderson JW, Atkinson RL, et al. Weight loss with self-help compared with a structured commercial program: a randomized trial. JAMA. 2003;289:1792–8. doi: 10.1001/jama.289.14.1792. [DOI] [PubMed] [Google Scholar]
- 87.Rock CL, Pakiz B, Flatt SW, Quintana EL. Randomized trial of a multifaceted commercial weight loss program. Obesity. 2007;15:939–49. doi: 10.1038/oby.2007.614. [DOI] [PubMed] [Google Scholar]
- 88.Rock CL, Flatt SW, Sherwood NE, et al. Effect of a free prepared meal and incentivized weight loss program on weight loss and weight loss maintenance in obese and overweight women: a randomized controlled trial. JAMA. 2010;304:1803–10. doi: 10.1001/jama.2010.1503. [DOI] [PubMed] [Google Scholar]
- 89.Foster GD, Borradaile KE, Vander Veur SS, et al. The effects of a commercially available weight loss program among obese patients with type 2 diabetes: a randomized study. Postgrad Med. 2009;121:113–8. doi: 10.3810/pgm.2009.09.2046. [DOI] [PubMed] [Google Scholar]
- 90.Wing RR, Pinto AM, Crane MM, et al. A statewide intervention reduces BMI in adults: Shape Up Rhode Island results. Obesity. 2007;17:991–5. doi: 10.1038/oby.2008.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.National Heart, Lung, and Blood Institute Press Release. [Accessed May 9, 2011];Trials use technology to help young adults achieve healthy weights. 2010 November 29; http://public.nhlbi.nih.gov/newsroom/home/GetPressRelease.aspx?id=2744.