Abstract
The management of patients with subaxial cervical injuries lacks consensus, particularly in regard to the decision which surgical approach or combination of approaches to use and which approach yields the best clinical outcome in the distinct injury. The trauma literature is replete with reports of surgical techniques, complications and gross outcome assessment in heterogeneous samples. However, data on functional and clinical outcome using validated outcome measures are scanty. Therefore, the authors performed a study on plated anterior cervical decompression and fusion for unstable subaxial injuries with focus on clinical outcome. For the purpose of a strongly homogenous subgroup of patients with subaxial injuries without spinal cord injuries, robust criteria were applied that were fulfilled by 28 patients out of an original series of 131 subaxial injuries. Twenty-six patients subjected to 1- and 2-level fusions without having spinal cord injury could be surveyed after a mean of 5.5 years (range 16–128 months). The cervical spine injury severity score averaged 9.6. Cross-sectional outcome assessment included validated outcome measures (Neck pain disability index, Cervical Spine Outcome Questionnaire, SF-36), the investigation of construct failure and successful surgical outcome were defined by strict criteria, the reconstruction and maintenance of local and total cervical lordosis, adjacent-segment degeneration and intervertebral motion, and the fusion-rate using an interobserver assessment. Self-rated clinical outcome was excellent or good in 81% of patients and moderate or poor in 19% that corresponded to the results of the validated outcome measures. Results of the NPDI averaged 12.4 ± 12.7% (0–40). With the SF-36 mean physical and mental component summary scores were 47.0 ± 9.8 (18.2–59.3) and 52.2 ± 12.4 (14.6–75.3), respectively. Using merely non-constrained plates, construct failure was observed in 31% of cases and loss of local lordosis, expressed as a mean injury angle of 14°, postoperative angle of −5.5° and follow-up angle of −1°, was significant. However, total cervical lordosis was within the limits of normalcy (−24.3° ± 13.3) and fusion-rate was 88.5%. The progression of adjacent-level degeneration was shown to be significantly influenced by a decreased plate-to-disc-distance. Adjacent-level intervertebral motion was not altered due to the adjacent fusion, but reduced in the presence of advanced adjacent-level degeneration. Patients were more likely to maintain a high satisfaction level if they succeeded to maintain segmental lordosis (<0°), had a solid fusion, an increased plate-to-disc distance, and if they were judged to have a successful surgical outcome that included the absence of construct failure and reconstruction of lordosis within ±1 SD of normalcy. Using validated outcome vehicles the interdependencies between radiographical, functional and clinical outcome parameters could be substantiated with statistically significant correlations. The use of validated outcome vehicles in a subgroup of patients with plated anterior cervical fusions for subaxial injuries is recommended. With future studies, it enables objective comparison of surgical techniques and related radiographical, functional and clinical outcome.
Electronic supplementary material
The online version of this article (doi:10.1007/s00586-008-0879-3) contains supplementary material, which is available to authorized users.
Keywords: Cervical spine, Injury, Subaxial, Outcome, Instrumentation
Introduction
The principles in the treatment of unstable cervical spine injuries are reduction and stabilization of the injured segment until restoration of native stability, maintenance of cervical lordosis and decompression where indicated. Methods of treatment range from non-operative to combined anterior and posterior surgical fusion. Patients with posttraumatic instability are at increased risk of chronic pain states as well as neurologic injury [6]. Hence, owing to refinements in spinal instrumentations internal fixation is frequently employed and increasingly the halo thoracic vest (HTV) is considered obsolete [34]. There is however, debate on the indications for anterior, posterior, or combined surgery [33].
Anterior cervical decompression, fusion and plating (ACDFP) has gained popularity as the standard procedure [10]. The anterior approach is a non-traumatic and it provides the ability for decompression, reduction of dislocated facet joints, interbody grafting with reconstruction and maintenance of lordosis [3, 21, 33, 48, 58, 77]. ACDFP also reduces the need to sacrifice adjacent motion segments in cases of facet joint injury which precludes single-level posterior fixation [81]. In contrast, posterior approaches may be injurious to adjacent levels; this has been postulated to cause late deformity [68, 81]. Additionally there have also been concerns regarding the rate of wound infection, the inability to address a disrupted disc prior to reduction, and the ability of stand-alone posterior stabilization to prevent segmental kyphosis despite including more segments into the fusion mass [29, 42, 54, 60, 64, 75, 83]. However, there have been also both theoretical and practical concerns regarding the use of anterior plating alone in severe instabilities [32].
When determining the effectiveness of different treatment modalities the use of validated measurement tools to assess relevant clinical outcome is vital [33]. The appropriate method of fixation for a given injury pattern is not defined and despite a better understanding of spinal stability, knowledge of the impact of each technique on the functional recovery of patients remains limited [5]. Owing to heterogeneous characteristics of most samples published, only a few with detailed reporting of clinical results are amenable for data pooling [33, 34, 56, 64]. For now much of our detailed information in subaxial injury refers to spinal cord injuries (SCIs) and mechanical behaviour of instrumented constructs [29], but not the clinical outcome [34]. Neurologic recovery is probably an inappropriate outcome when assessing the merits of each technique used in cohorts composed of neurologically injured and intact patients [33]. Results of homogenous samples with long-term outcomes that are not flawed by the sequelae of neurological injury are in desirable [57].
The objective of the current study was to investigate a homogenous subgroup of patients with subaxial injuries without SCI subjected to ACDFP. Homogenous sample characteristics should be achieved due to strict in- and exclusion criteria. The authors designed a study on a retrospective case series with cross-sectional outcome assessment. Specifically, to assess the mid- to long-term impact of radiographic construct changes, reconstruction and maintenance of sagittal balance, as well as adjacent-segment degeneration and motion on clinical outcome.
Materials and methods
Patient sample
We performed a medical charts review and recorded all patients that had been treated for cervical spine injuries between 1996 and 2006 at the authors’ institution. A database-generated retrospective cohort analysis was conducted for all subaxial injuries treated surgically, resulting in 131 cases. For the purpose of investigating a homogenous consecutive series of patients, inclusion criteria were as follows: (a) disco-ligamentous injury at C3 to T1, (b) surgical treatment by means of ACDFP with 1- or 2-level fusion, (c) interval injury to index treatment ≤10 days, (d) understanding of the author’s language, (e) full-set of injury and postoperative radiographs, CT or MRI-scans if available, (f) minimum 1-year follow-up, (g) muscles strength of grade ≥1/5 according to the MRC (Royal Medical Research Council of Great Britain strength grading scale) in case of a nerve root involvement.
Patients were excluded if they met the following criteria: (a) SCI (ASIA A–D) (b) significant shoulder-girdle injury necessitating surgery, (c) peripheral nerve injuries, (d) prohibitive medical comorbidity and end-stage diseases, (e) drug or alcohol withdrawal, (f) non-contiguous cervical injury or injury at C1–2, (g) polytrauma, (h) congenital cervical deformity, (i) injuries from neoplastic disease, infections or lesions associated with ankylosing spondylitis and DISH, (j) isolated traumatic disc protrusion, (k) psychiatric illness necessitating medical treatment, (l) documented osteoporosis, (m) postoperative tracheostomy, (n) grade II° or III° skull-brain trauma, (o) age <16 or >70 on admission, (p) failed prior non-surgical treatment, (q) prior medical or surgical treatment for degenerative cervical disease or trauma, (r) workers’ compensation claims, and (s) multilevel surgery (>2-levels).
Treatment
During the study period patients with cervical spine trauma had plain radiographs and most had CT-scans to delineate or exclude fracture. In the absence of frank instability, MRI-scanning or flexion–extension views were performed to reveal discoligamentous injury and dynamic instability, respectively. Surgery was indicated if there was evidence of a discoligamentous instability or a vertebral body (VB) wedging of ≥10° including burst VB. Closed manual or traction reduction was applied in cases with dislocations or gross sagittal displacement. Preoperatively cervical alignment and immobilization were maintained with a cervical orthosis or by axial traction. Patients were operated by means of ACDFP using tricortical iliac crest autografts and a right-sided cervical anterolateral approach. Postoperatively, patients were placed into a semi-rigid collar for 4–6 weeks. Follow-up controls were scheduled at 3 weekly intervals for the first 3 months, then again at 6 months and finally once a year.
Radiographic analysis
Injury cervical spine radiographs, intraoperative and postoperative radiographs at the day of surgery were taken in supine position. The injury radiographs were available as hard copy prints or stored digitally. All hard-copy prints were digitalized using a film digitizer (Sierra Plus, Vidar Systems Corp., Herndon/USA). At final follow-up, sitting anterior–posterior (AP) and lateral (lat) radiographs were performed with the lateral in neutral posture, flexion and extension. The radiographs were taken on a digital X-ray system (Vertix 3D-III unit, Siemens, Erlangen/Germany) and analyzed using a commercial software that allows for measurements with 0.1 mm increments and enhancing of vertebral levels at the cervicothoracic junction (Escape Medical Viewer V3, Escape Thessaloniki/Greece). In some cases it was not possible to adequately visualize the caudal cervical spine [79]. Hence, if the landmarks necessary for distinct measurements were poorly discernable, these were excluded from statistical analysis.
Analysis of sagittal alignment
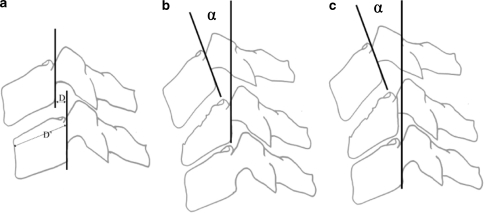
Sagittal plane assessment on injury radiographs was performed as recommended by the Spine Trauma Study Group [13]: The segmental rotation angle (SRA) was defined by the intersecting VB tangent lines at each injured level resembling the Harrison posterior tangent method [46] (Fig. 1). With the Harrison method the intra- and interobserver reliability was shown to be high [73] and a 2° error of measurement was to be expected [46]. Hence, sample size was calculated on the basis of a 2° effect change regarding geometrical measurements. In general, kyphosis was indicated by a positive and lordosis by a negative value. The anterior shear and intervertebral translation, respectively, was the amount of translation calculated as percentage of upper VB translation (in millimetres) to upper VB depth of the adjacent-caudal vertebra. In two-level injuries, e.g., with tear-drop fractures, the major translational displacement and the angulation between upper and lower end-vertebra was taken for calculation (Fig. 1). Anterior shear was indicated by a positive and posterior shear by a negative value. The percentage translation and SRA were measured on all injury radiographs and CT-scans available. The image displaying the most severe abnormality was selected for evaluation and statistical analysis.
Fig. 1.
Measurement of the segmental rotation angle (SRA) in a 1-level injury/fusion, b 2-level injury/fusion and c intervertebral translation is calculated as percentage of upper VB translation (D, in mm) to upper VB depth of the adjacent-caudal vertebra (D′ in mm, parallel to caudal endplate)
Classification
Each patient’s injury was analyzed via a combination of radiographic films, CT-scans with multiplanar reconstructions, and/or MRI-scans. The morphological configuration of the injury type was assessed using the AO-classification according to Magerl et al. [70]. To assess injury severity and describe injury pattern we applied the cervical spine injury severity score (CSISS) [71] with the assistance of its author. Fractures of the lateral mass C3–T1 were further stratified using the classification of Kotani et al. [62] (Fig. 2). Facet fractures were differentiated as follows: F1, superior facet fracture unilateral; F2, superior facet fracture bilateral; F3, inferior facet fracture unilateral; F4, inferior facet fracture bilateral.
Fig. 2.
Type 1 (left) lateral ass fracture-separation; Type 2 (middle) lateral mass burst fracture that can involve one or both facets; Type 3 (right) lateral mass split-fracture, that can involve one or both facets. Differentiation of lateral mass fractures according to Kotani et al. as a modifier to the classification of disco-ligamentous unstable subaxial cervical injuries
Analysis of absolute and segmental rotation angle
At follow-up, the SRA was assessed for all levels C2–T1 using the Harrison method [45]. In neutral position, the segmental rotation angles are expressed as the SRA, and the segmental range of motion (ROM) calculated from the SRA on flexion–extension films is expressed as the rSRA for each level. The rSRA was plotted against the physiological standards provided by Reitman et al. [80] for C2–7 and Frobin et al. [38] for C1–2. With the rSRA C1–2 to C6–7 significant differences compared to the physiological standard were assumed with motion exceeding ±2 standard deviations (SD) of normalcy [12] and described as hyper- or hypo-mobile. To assess atlantoaxial alignment in sagittal plane, the C1–2 angle was measured on follow-up neutral and flexion–extension radiographs. It was defined as the angle subtended by a line drawn parallel to the inferior aspects of C1 and a line connecting the inferior aspect of the axis vertebra and the lamina of C2 (Fig. 3). Similarly for the SRA, the cervical lordosis C2–7 was expressed as the absolute rotation angle C2–7 (ARA C2–7) using the Harrison method (Fig. 3). Differences on flexion–extension films and ROM were calculated and expressed as the rARA C2–7.
Fig. 3.
The sagittal tangent method for measurement of the absolute rotation angle C2–7 (ARA C2–C7) [73] and the measurement for the C1–2 angle
Assessment of construct geometry
Besides the SRA and translation measurements, construct geometry was assessed on postoperative and follow-up lateral radiographs determining the distances between endplates (construct height) by drawing a line tangential to the most extreme point of the VB in the rostral and caudal direction along the endplates (for superior and inferior endplates, respectively). Measurements were then made between the endplates at the anterior and posterior aspects of the VBs. Changes in construct height were expressed as the ratio of the posterior (a, Fig. 4) divided by the anterior (b, Fig. 4) height. At final follow-up, the plate-to-disc-distance (PDD) was assessed recording the distance between the superior and inferior endplates of the fused VBs and the ends of the plate (Fig. 4 ‘d’ and ‘e’). A plate impinging on the adjacent cephalad or caudal level was defined as having a distance ‘d’/‘e’ ≤1 mm. Definition of radiographic failure of the construct included a change in translation of >15% (≈3.5 mm) of VB depth, a change of the SRA >5° or gross ‘descriptive failure’ such as plate/graft dislodgement [75]. We recorded a descriptive instrumentation failure referring to the presence of screw loosening on AP or lat radiographs by inspecting for displacement of screw heads beyond the most anterior aspect of the plate, a gap between the plate and underlying VB and lucency around the screws.
Fig. 4.

Assessment of construct height. Distance ‘a’ and ‘b’ connect the upper and lower most edges of the vertebral bodies posteriorly and anteriorly, respectively. With the measurements the ratio a:b is calculated to assess changes in construct height during the radiographic course. Distance ‘d’ and ‘e’ resemble the distances between the upper and lower ends of the plate to the respective cervical disc spaces (PDD)
Adjacent-segment degeneration
The grade of adjacent-segment degeneration on injury and final follow-up lat-radiographs was assessed using the modified grading system of Kellgren (Table 1) which was shown to have good to excellent inter-examiner agreement [19, 97]. With that classification anterior spurring within the anterior longitudinal ligament (ALL) at adjacent disc spaces was assigned Grade 1 or Grade 2 according to the extension of ossification. Calculating the differences between the ASD on injury and follow-up, the increase of ASD or the new onset of any ASD was calculated and expressed as the progression of ASD.
Table 1.
Classification of adjacent-segment degeneration according to Kellgren et al. [19]
| Grade | Definition |
|---|---|
| 0 | Absence of degeneration in the disc (no ossification of the ALL) |
| 1 | Minimal anterior osteophytosis (or ossification of the ALL) |
| 2 | Definite anterior osteophytosis, possible narrowing of the disc space, some sclerosis of the vertebral plates |
| 3 | Moderate narrowing of the disc space, definite sclerosis of the vertebral plates, osteophytosis |
| 4 | Severe narrowing of the disc space, sclerosis of the vertebral plates, multiple large osteophytes |
ALL anterior longitudinal ligament
Assessment of fusion
For the assessment of fusion at the cephalad and the caudal graft-host junctions, interobserver rating was performed including the author and a senior radiologist (R.F.) using the classification of Vavruch et al. [91] that differentiates four types according to the presence or lack of bridging bone anterior and/or through the disc space (Table 2). The treatment was classified as resulting in pseudoarthrosis if a ‘2B’ healing was observed at any level subjected to surgery. Fusion grade was also assessed using the Bridwell et al. [14] classification (Table 2). Accordingly, Grade 4 was assigned as definitely no solid fusion achieved. If, based on both classifications, the two observers would disagree on the definitive presence of fusion, the restriction of intervertebral motion in flexion–extension at the fusion levels was taken into account. Hence, if the rSRA was >3° the level was defined as not fused.
Table 2.
Classification of fusion grades according to Bridwell et al. [14] and fusion type according to Vavruch et al. [91]
| Grade | Definition |
|---|---|
| Fusion grades | |
| I | Fused with remodeling and trabeculae |
| II | Graft intact, not fully remodeled and incorporated, no luciencies |
| III | Graft intact with definite lucency at the top or the bottom of the graft |
| IV | Graft definitely not fused with graft resorption and collapse |
| Type | Definition |
|---|---|
| Fusion types | |
| 1A | Bridging bone anterior and through the disc space |
| 1B | Bridging bone anterior but not through the disc space |
| 2A | Bridging bone not anterior but through the disc space |
| 2B | No bridging bone at all |
Clinical outcome analysis
Functional outcome
At follow-up all patients were subjected to clinical examination. Maximum active ROM in flexion, extension, and rotation was assessed with a goniometer (5° increments). Results of ROM were calculated for age- and gender-related percentage restriction as compared to data of normals published by Castro et al. [15]. It was expressed as the corrected range of motion (cROM).
The current study design allowed the inclusion of patients with nerve root involvement by terms of radiculopathy and weakness at the time of injury. The study sample included only patients without SCI. If radiculopathy or weakness was present at follow-up, muscle strength was evaluated during active movement against resistance. Muscular strength was graded from 0 (no contraction) to 5 (normal) according to the MRC.
Clinical outcome
The outcomes vehicles included the 36-item short-form health survey (SF-36-v2), the long-term Cervical Spine Outcome Questionnaire (CSOQ) [9] and the neck pain disability index (NPDI) [94]. The SF-36 is a commonly used generic health-related quality of life (HRQOL) questionnaire that consists of 36 items and assesses 8 health dimensions. A physical and mental component score (PCS and MCS) can be derived. The PCS ranges from 73 (high level of functioning) to 8 (low level of functioning), and the MCS ranges from 74 (excellent state of mental health) to 10 (poor state of mental health). This questionnaire has been found reliable, valid, and responsive when considering cervical pathology [33]. The CSOQ consists of six composite scores addressing severity of neck and arm pain, functional disability, psychological distress, physiological symptoms and healthcare utilization. Each composite score can yield a total of 0 or 100 points resembling excellent or poor outcome. The CSOQ has shown high test–retest reliability and good validity [9]. The participants’ satisfaction according to three distinct answers ticked within the CSOQ is separately reported. Cervical spine disability was quantified by the NPDI [94], which is a combined score including functional disability as well as pain and cognitive skills. The NPDI questionnaire concerns ten areas, and for each area the patient selects one of six statements on an ordinal scale of 0–5, giving a total of 50 points as the worst outcome. It is expressed as a percentage ranging between 0 and 100%. The areas are pain severity, personal care, lifting, reading, headache, concentration capacity, work, driving, sleep and leisure time. The NPDI is reported to be valid, reliable and sensitive [93]. The subjective perception of overall outcome was rated by the patients at follow-up as excellent, good, moderate, or poor. The incidence of dysphagia was evaluated with the grading system of Bazaz et al. [8] that defines four grades: none, mild, moderate, or severe dysphagia based on subjective symptoms calculated by distinct combinations of existing difficulties with liquid and solid nutrition.
We defined ‘successful surgical outcome’ using robust criteria: Anatomic restoration of the injured levels within ±1 SD of normalcy [43], no need for revision surgery, absence of a radiographic construct failure, presence of a fusion, recovery from radiculopathy and loss of muscle strength defined as a MRC of ≥4, and no neurological deterioration. If there was intermitting paraesthesia without pain in a cervical root level distribution, this was not judged fulfilling the criteria of a radiculopathy.
Work status
The work status was recorded at injury and follow-up as well as the time until return to work. It was further delineated using the Denis work scale (DWS, Table 3).
Table 3.
Work scale according to Denis [24]
| Grade | Criteria |
|---|---|
| W1 | Return to previous employment (heavy labor) or physically demanding activities |
| W2 | Able to return to previous employment (sedentary) or return to heavy labor with lifting restrictions |
| W3 | Unable to return to previous employment but working full-time at a new job |
| W4 | Unable to return to full-time work |
| W5 | No work, completely disabled |
Donor site morbidity
Patients were asked to grade their pain if worst during the last 30 days at the side of iliac crest harvest on a VAS with 10 delineating ‘worst pain’ and 0 ‘no pain’.
Complications
Any medical or surgical complication related to the procedure during the clinical course to follow-up was recorded.
Statistical analysis
Statistical analyses included along with descriptive statistics, parametric methods (independent two-sided Student’s t test, Pearson’s correlation coefficient) as well as non-parametric tests (Mann–Whitney U test, Spearman’s correlations coefficient). The interobserver reliability was calculated using Cohen’s weighted kappa and interpretation of strength of agreement was done according to the criteria of Landis and Koch [65]. A P value less than 5% indicated a statistical significant result. All analyses were done using Statistica 6.1 (StatSoft, Tulsa, OK, USA) and StatXact (Cytel Software Corp., Cambridge, MA, USA).
Results
Patient sample and demographics
Of the patients, 28 met the criteria, 1 was lost to follow-up and 1 was shown as unable to complete clinical outcome questionnaires. Hence, the final sample comprised 26 patients and follow-up rate was 92.9%. There were 5 female and 21 male patients. Mean age at injury was 42.4 ± 18.7 years (range 16–70 years) and 47.7 ± 19.7 years (range 18–80 years) at follow-up. The length of follow-up was 67 ± 37 months on average (median: 60 months, 95%CI: 52–82 months; range 16–128 months). Mean hospital stay was 9.2 ± 5 days (range 5–22 days). Out of 26 patients, 8 (30.8%) were smokers at time of injury. Except for minor skull trauma such as lacerations and wounds, 6 patients (23.1%) had concomitant peripheral skeletal injuries. At follow-up, no patient complained about disability or pain derived from the shoulder-girdle or peripheral injuries. The main injury mechanism was a motor vehicle or bicycle accident in 10 patients (38.5%), 5 (19.2%) were skiing related, 5 a fall from height, 3 (11.5%) diving into shallow water and 3 due to a direct impact. Patients’ main characteristics and injury pattern are summarized in Table 4 and in Table 11 (Table 11, Electronic supplementary material).
Table 4.
Main characteristics of patients with cervical spine injuries C3 to T1 undergoing ACDFP
Surgical treatment
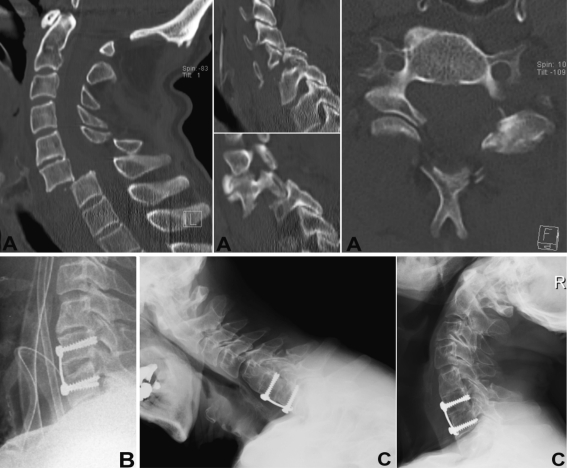
Eight patients (30.8%) were transferred to the authors’ institution but none had delayed diagnosis. Mean interval injury-to-surgery was 1.9 ± 2.6 days (range 0–10 days); 21 patients (88.5%) were immobilized in a semi-rigid cervical collar until surgery, 5 patients were placed in axial traction. Prior to surgery, four out of eight patients with unilateral dislocated facets were subjected to successful manual reduction and four patients had axial traction for gross realignment and open reduction [77] was performed at time of anterior surgery. One patient underwent corpectomy of C5, whereas the others had discectomy, intervertebral strut grafting and plating. Constrained (CS-) plates were used in 3 patients (11.5%, CSLP, Synthes, Paoli, PA, USA) and non-constrained (NC-) plates in 23 patients (88.5%, H-Plate, Leibinger). Bicortical screw anchorage was performed in 22 patients (84.6%) and unicortical in 4 patients (15.4%). A 1-level ACDFP was performed in 23 patients (88.5%) and 2-level fusion in 3 (11.5%). Fusion-levels included C4–5 during 5 patients (19.2%), C5–6 in 9 (34.6%), C6–7 in 9 (34.6%), C4–6 twice (7.7%), and each once (3.8%) C7–T1 and C6–T1. Results of surgical treatment are given with Figs. 5, 6, and 7.
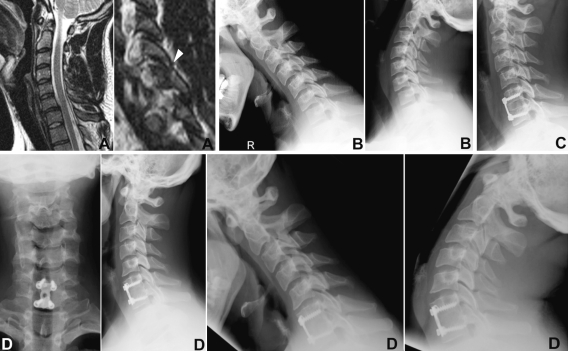
Fig. 5.
Case 5. 35-year-old patient. Injury MRI (a) and flexion–extension films (b) revealed disco-ligamentous injury at C6–7 w/superior facet fracture of C7 (arrow head). Patient was subjected to ACDFP (c). At 5.5 years follow-up (d) he showed excellent self-rated outcome with solid fusion, favourable sagittal ROM and resolved radiculopathia of C6
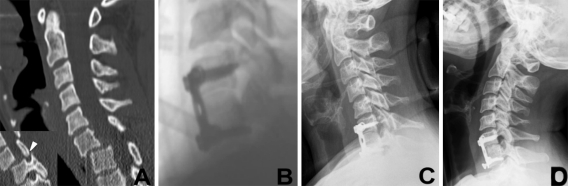
Fig. 6.
Case 4. 44-year-old patient suffered unilateral facet dislocation C6–7 (a, arrow head) and underwent ACDFP C6–7 (b). Three months follow-up (c) showed screw-loosening with significant loss of lordosis at fusion level. At 1.5 years follow-up patient showed moderate clinical outcome w/construct failure, increasing kyphosis and pseudoarthrosis at the fusion level (d)
Fig. 7.
Case 6. 70-year-old patient suffered an AO type C 2.1.4 injury at C6–7 w/superior facet fracture of C7 left, dislocated facet of C6 right and laminar fracture of C6 (a). Following closed reduction the patient was subjected to ACDFP (b). At 3 years follow-up he showed good clinical self-rated outcome and significant recovery from motor loss of C6 nerve root distributed muscles. Note progression of ASD at C4–5 (c) with almost autofusion of C4–5
Radiographic results
Analysis of sagittal alignment in neutral position
CT-scans and MRI-images were available in 21 (80.7%) and in 4 (15.4%) patients, respectively. Mean injury SRA at the involved levels was 8.2° ± 10.7 (range −9° to 33.7°) and translation was 16.8% ± 23.8 (range −32.5 to 71.5%). Postoperatively, mean SRA was −6.2° ± 4.9 (range −16° to 0°) and translation was 8.1% ± 6.9 (range −5.9 to 26%). At final follow-up, mean SRA was −1.1° ± 4.3 (range −10.6° to 7.2°) and translation was 7.7% ± 10.6 (range −5.2 to 40.3%). At follow-up, the mean loss of the SRA was 5.1° ± 5.6 (range −3.3° to 23.2°), and 7.7% ± 8.1 (range 0.2 to 34.1%) for translation. Overall, 11 of 25 patients (44%) had a kyphotic SRA at fusion level (SRA > 0°). Follow-up SRA for all levels is listed in Table 5. The difference between injury and postoperative SRA (8.2° vs. −6.2°) revealed a mean correction of 14° and was statistically significant (P < 0.000001). It remained significant until follow-up (P < 0.0001), but the loss of lordosis was also significant (P = 0.009). In patients that had received a CS-plate, the change of the SRA was significantly less than if treated with a NC-plate (P = 0.004). Also, patients that had suffered unilateral facet dislocation showed significantly decreased SRA at follow-up (P = .01). Concerning anterior shear, the difference between injury and postoperative translation was significant (P = 0.03) with no significant changes to follow-up.
Table 5.
Results of segmental (SRA) and absolute rotation angles (ARA) C2–T1 (in degree)
| ARA C2–7 | SRA C1–2 | SRA C2–3 | SRA C3–4 | SRA C4–5 | SRA C5–6 | SRA C6–7 | SRA C7–T1 | |
|---|---|---|---|---|---|---|---|---|
| Neutral position | ||||||||
| Harrison [45] | −34.1 ± 9.2 | – | −7.6 ± 3.9 | −6.4 ± 3.3 | −7.2 ± 3.9 | −6.3 ± 3.7 | −6.7 ± 4.2 | – |
| Kuntz [63]a | −17 ± 14 | −29 ± 7c | – | – | – | – | – | – |
| Current study: all levels | −24.5 ± 13.3 (−45.4 to 2.8) |
−20.2 ± 7.5 (−6.8 to −34.3) |
−5.4 ± 7.5 (−20.4 to 10.1) |
−4.8 ± 8.7 (−21.6 to 8.2) |
−5.8 ± 5.5 (−17 to 10.3) |
−3.3 ± 4.5 (−14.7 to 4.0) |
−2.3 ± 5.1 (−9.2 to 11.4) |
−4.8 ± 5.2 (−12.8 to −6) |
| Current study: fused levels | – | – | – | – | −3.1 ± 4.2 (−10.6 to 2.4) |
−1.1 ± 3-8 (−7.5 to 4.0) |
−0.5 ± 4.7 (−9 to 7.2) |
−0.3 |
| rARA C2–7 | rSRA C1–2 | rSRA C2–3 | rSRA C3–4 | rSRA C4–5 | rSRA C5–6 | rSRA C6–7 | rSRA C7–T1 | |
|---|---|---|---|---|---|---|---|---|
| ROM flexion–extension | ||||||||
| Frobin [38] female subjects | – | 10.9 ± 4.9 | 8.4 ± 3.4 | 15.2 ± 4.7 | 17.0 ± 5.5 | 17.9 ± 6.6 | 11.4 ± 6.8 | – |
| Frobin [38] male subjects | – | 11.6 ± 4.6 | 8.0 ± 3.1 | 11.6 ± 3.6 | 14.4 ± 4.6 | 12.2 ± 5.19 | 9.8 ± 5.7 | – |
| Reitman [30, 80] | – | – | 9.9 ± 3.7 | 15.2 ± 3.2 | 16.9 ± 3.8 | 15.8 ± 4.2 | 13.5 ± 5.2 | – |
| Current study: non-fused levels | 52.5 ± 16.5 (18.4–82.8) |
11.0 ± 5.3 (5.2–21.3) |
9.8 ± 5.7 (0.1–19.4) |
13.3 ± 6.2 (6.8–21.8) |
14.4 ± 7.7 (0.5–25.9) |
13.2 ± 8.8 (6.5–27.5) |
12.3 ± 6.2 (4.7–19.2) |
9.9 ± 3.7 (2.7–16.9)b |
Comparison with normalized data from literature
aReview of literature
b42.3% of C7–T1 levels not assessable on lat radiographs
cDifferent technique applied compared to current study
Fracture morphology and severity
The injury levels included C4–5 in 5 patients (19.2%), C5–6 in 9 (34.6%), C6–7 in 9 (34.6%), C4–6 twice (7.7%), and once (3.8%) at C7–T1 and C6–T1. Hence, 77% of patients had injury at C5–T1. One patient showed a contiguous superior left-sided facet fracture of T1 with a fracture dislocation of C6–7. Eight patients (30.8%) had associated facet fractures, 4 (25.4%) had fractures of the lateral mass, and 5 (19.2%) had associated laminar fractures. The study encountered 8 patients (30.8%) that had unilateral facet dislocations with or without facet or lateral mass fractures. According to the AO-Magerl classification, 15 patients (57.7%) had Type B injuries, 9 (36.6%) had Type C, and 2 (7.7%) had Type A. Of note, facet dislocations are encountered within Type C injuries. The CSISS was 9.6 ± 4.7 (range 2–20) on average and was correlative with the injury SRA (r = 0.58, P = 0.002), injury translation (r = 0.51, P = 0.008) and the AO type (r = 0.56, P = 0.003). The follow-up SRA at the fusion level was significantly related to the CSISS (r = 0.45, P = 0.02) and to the presence of a facet dislocation (P = 0.01).
Analysis of segmental and absolute sagittal rotation
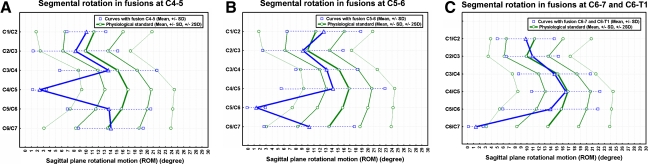
The rSRA of the fused and mobile levels C2 to T1 as well as the C1–2 angles are summarized in Table 5, plotted against data of normals and visualized in Fig. 8. Statistical analysis revealed no significant differences between the rSRA at the adjacent-cephalad and -caudal levels (Table 6). A hypomobility was present at C2–3 level in 2 patients (7.7%), at C3–4 in 7 (26.9%), at C4–5 in 3 (11.5%), at C5–6 in 4 (15.4%), and at C6–7 in 1 (3.8%). Hypermobility was present in 3 patients at the C2–3 level (11.5%), and each once (3.8%) at the C3–4, C4–5, and C5–6 level. At all, 12 of 23 patients (52.2%) were found to have a deviation greater ±2 SD from normalcy at any of the adjacent-levels.
Fig. 8.
Sagittal segmental rotations C1–C7 reconstructed as curves according to the rSRA of C1–7. Results are plotted against the physiological standard [38] ±1 and ±2 SD. Curves are separately plotted for fusions at the C4–5 level (a), C5–6 level (b), C6–7 and C6–T1 level (c)
Table 6.
Results of intervertebral ROM (in degree) at follow-up according to the rSRA at adjacent levels to the fusion
| Fourth cephalad level | Third cephalad level | Second cephalad level | First cephalad level | Fusion level | First caudal level | Second caudal level | Third caudal level | |
|---|---|---|---|---|---|---|---|---|
| N (levels)a | 11 | 19 | 26 | 26 | 26 | 19 | 12 | 1 |
| Mean | 11.1 | 12.5 | 12.9 | 13.9 | 1.8 | 11.2 | 11.8 | 13.6 |
| SD | 4.6 | 6.7 | 6.4 | 7.5 | 1.4 | 6.5 | 4.2 | – |
| Min | 5.7 | 6.8 | 0.1 | −6.5 | 0.0 | 2.7 | 7.6 | 13.6 |
| Max | 19.4 | 21.3 | 21.2 | 27.5 | 4.6 | 22.2 | 19.2 | 13.6 |
aNumber of levels assessable
The ARA C2–7 in neutral position was −24.5° ± 13.3 (range −45.4 to 2.8), 10° ± 7.2 (range −3.4° to −26.5°) in flexion and −42.5° ± 14.7 (range −5.3° to −71°) in extension. Statistical analysis revealed a significant inverse correlation between the ARA C2–7 and the C1–2 angle (r = −0.44, P = 0.025) and the SRA at C7–T1 (r = −0.54, P = 0.03). The rARA C2–7 averaged 52.5° ± 16.5 (range 18.4°–82.8°) and significantly decreased with age (r = −0.39, P = 0.048) as did the rSRA of the first adjacent-cephalad and -caudal level (r = −0.54, P = 0.005; r = −0.57, P = 0.01). In contrast, the rARA C2–7 increased with higher rSRA at the first adjacent-cephalad (r = 0.42, P = 0.03) and first adjacent-caudal level (r = 0.56, P = 0.01). Age had a stronger influence on the rSRA at the first adjacent-levels than on the total neck motion in terms of the rARA C2–7 (r = −0.54 and −0.57 vs. r = −0.39). If the rSRA were plotted against the incidence of ASD, a significant inverse correlation existed between the ASD and the rSRA at the first adjacent-caudal level (r = −0.52, P = 0.02). Similar weighted correlations did not exist for the first adjacent-cephalad level (P = 0.3). Patients that had suffered higher CSISS had significantly reduced rARA C2–7 (r = −0.45, P = 0.02).
Fusion rate
According to the interobserver rating and assessment of fusion, fusion rate was 88.5%. Patients having a non-union had significantly increased loss of local lordosis (P = 0.005) and a decreased NPDI as compared to those who achieved fusion (P = 0.006). The mean rSRA for the fusion levels was 1.6° ± 1.3 (range 0°–4.6°). Only once the rSRA at fusion level had to be taken into account for final definition of fusion because both observers rated a Type 2A according to the Vavruch but a Grade 4 according to the Bridwell classification. The rSRA was 3.1° and the case was classified as non-fused. In another case with C7–T1 fusion the level could not be reliably assessed; 6 years radiographs already delineated a solid fusion and there were no signs of instrument failure or motion exceeding 3°. Therefore, successful fusion was assigned. The results of the interobserver assessment are listed in Table 7. The kappa values for the Vavruch classification regarding the cephalad and caudal graft-endplate junctions were calculated as 0.64 and 0.5 resembling substantial and moderate agreement, respectively. The kappa value for the Bridwell classification was 0.33, resembling fair agreement. All patients that had not succeeded to fusion had AO Type C injuries and the difference was statistically significant (P = 0.04).
Table 7.
Results of interobserver assessment of fusion
| Type | Vavruch classification | Grade | Bridwell classification | ||||
|---|---|---|---|---|---|---|---|
| Observer 1 | Observer 2 | Observer 1 | Observer 2 | Observer 1 | Observer 2 | ||
| Cephalad (N = 28) | Caudal (n = 28) | Graft (n = 28) | |||||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||
| 1A | 19 (67.9) | 13 (46.4) | 20 (71.4) | 14 (50) | I | 20 (71.4) | 12 (42.8) |
| 1B | 6 (21.4) | 10 (35.7) | 5 (17.9) | 9 (32.1) | II | 4 (14.2) | 5 (17.9) |
| 2A | 1 (3.6) | 3 (10.7) | 2 (7.1) | 4 (14.2) | III | 2 (7.1) | 9 (32.1) |
| 2B | 2 (7.1) | 2 (7.1) | 1 (3.6) | 1 (3.6) | IV | 2 (7.1) | 2 (7.1) |
| SD | 0.9 | 1.0 | |||||
| Range | 1–4 | 1–4 | |||||
Assessment of cephalad and caudal graft-endplate junctions (n = 56) according to Vavruch et al. and assessment of graft fusion according to Bridwell et al. (n = 28 levels)
Construct geometry
Postoperatively, the ratio calculated for construct height was 1.03 ± 0.08 on average (range 0.9–1.16) and 1.01 ± 0.06 (range 0.92–1.12) at follow-up. The change of construct height was significant (P = 0.03). The cephalad PDD averaged 2.5 mm ± 2.0 (range −1.3 to 6.9 mm) and the caudal PDD averaged 4.0 mm ± 2.4 (range 0–8.5 mm). 14 patients (53.8%) had a cephalad PDD <3 mm and 5 (19.2%) had a caudal PDD <3 mm, the measurement was not applicable caudally in 3 individuals. Patients with a caudal PDD <3 mm had significantly decreased first adjacent-caudal rSRA (P = 0.02). A total of 5 plates (19.2%) impinged on the cephalad and 3 (26.7%) on the caudal disc space (24 assessable adjacent-caudal levels).
A total of 106 screws were placed in 26 individuals. The rate of screw-loosening was 21.7%, occurring in 12 patients (46.2%) with NC-plates applied and including two screw breakages in a case with a solid fusion. The incidence of radiographic construct failure was 30.8% (8 patients). The incidence of construct failure was significantly higher in surgeries at the C6–7 level (P = 0.01) as compared to the more cephalad levels and in patients that postoperatively showed lower correction of the injury kyphosis and translation (P = 0.01; P = 0.02). Taking into account the segmental lordosis at fusion level compared to that of a physiological standard [45], 11 patients (42.3%) had a successful surgical outcome. The incidence of having a successful surgical outcome was significantly increased in patients that had AO Type A or B injuries as compared to Type C injuries (P = 0.03).
Adjacent-segment degeneration
The results of grading patients’ severity of ASD on injury and follow-up radiographs are summarized in Table 8. Statistical analysis for second adjacent-caudal levels could not be performed because of too small a number of levels. The patients’ injury ASD at the first adjacent-cephalad level showed a significant correlation with ASD at the first adjacent-caudal level (r = 0.47, P = 0.02). At time of injury the difference between ASD at the first adjacent-cephalad and first adjacent-caudal level was not significant, whereas the difference between ASD at the second and first adjacent-cephalad reached significance (P = 0.045). On follow-up radiographs, 17 patients (65.4%) showed some presence of ASD at the first adjacent-cephalad level and in 8 out of 17 patients (47.1%) there was a progression of an already existing ASD. In contrast, a new onset ASD was evident in 7 patients (41.2%); 2 patients had no changes of ASD (11.7%). The first adjacent-caudal level was discernable on injury and follow-up radiographs in 22 patients, 14 (63.6%) showed presence of ASD. In 4 of 14 patients (28.6%) there was a progression of an already existing degenerative process, whereas new onset ASD was evident in 7 patients (50%); 3 patients (21.4%) had no change in ASD; 14 patients (53.8%) were found to have new onset ASD at the first adjacent levels merely resembling ossifications of the ALL (Type 1 and 2 according to the Kellgren classification). Concerning the progression of ASD between injury and follow-up, both the increase of ASD at the first adjacent-cephalad and first adjacent-caudal level was significant (P = 0.0003 and P = 0.003) with the difference between both adjacent levels not reaching significance. But, the difference of the progression of ASD between the second adjacent-cephalad and first adjacent-cephalad levels was significant (P = 0.0009).
Table 8.
Results of the assessment of adjacent-segment degeneration according to the classification of Kellgren [19]
| Second cephalad level | First cephalad level | First caudal levela | Second caudal levelb | |
|---|---|---|---|---|
| Injury | 0.19 ± 0.63 (0–3) | 0.65 ± 0.98 (0–3) | 0.67 ± 1.1 (0–4) | 1.15 ± 1.4 (0–4) |
| Follow-up | 0.23 ± 0.65 (0–4) | 1.46 ± 1.42 (0–4) | 1.22 ± 1.31 (0–4) | 1.17 ± 1.75 (0–4) |
| Progression | 0.04 ± 0.2 (0–1) | 0.81 ± 0.85 (0–3) | 0.61 ± 0.72 (0–2) | 0.27 ± 0.65 (0–2) |
a7.7 and 11.5% of fusions did not have a cervical first adjacent-caudal level and could not be assessed on injury or follow-up radiographs, respectively
b15.4 and 53.8% of fusions did not have second adjacent-caudal levels and could not be assessed on injury or follow-up radiographs, respectively
If we calculated for the influence of patients’ age, statistical analysis revealed a significant correlation between age and the progression (or new onset) of ASD at the first adjacent-cephalad level (r = 0.41, P = 0.04), and between age and the grade of follow-up ASD at first adjacent-cephalad (r = 0.63, P = 0.0005) and first adjacent-caudal level (r = 0.65, P = 0.0009). The progression of the ASD at the first adjacent-cephalad and first adjacent-caudal level was also significantly correlated to the cephalad and caudal PDD, respectively (r = −0.49, P = 0.01 and r = −0.45, P = 0.03). The correlation was stronger for the PDD than for age if calculated for the progression of ASD. The progression of ASD at the first adjacent-cephalad level was significantly linked to a PDD of <3 mm (P = 0.049).
Outcome analysis
Functional outcome
Clinically, total ROM for flexion–extension was 100.8° ± 25.1 (range 55°–145°) and 117.7° ± 25.4 (range 60°–160°) for rotation. ROM showed an inverse correlation to increasing age (r = −0.54, P = 0.004; r = −0.44, P = 0.02). The cROM for flexion–extension was reduced to 79.1% ± 17.5 (range 47–127.9%) and to 81.8% ± 16.4 (range 16.4–113.6) for rotation. The radiographically assessed ROM in flexion–extension (rARA C2–7) was correlative with the cROM (r = −0.39, P = 0.049).
With increased ASD at the first adjacent-cephalad level at follow-up we noticed a significantly reduced clinical ROM in flexion–extension (r = −0.61, P = 0.001), but, controlling for age and gender, the cROM in flexion–extension just yielded significance (r = −0.39, P = 0.05). The same was true for the first adjacent-caudal level and for clinical ROM in flexion–extension (P = 0.01, r = −0.52) not yielding significance if calculated for the cROM (P = 0.4, r = −0.2).
Neurological injury
On admission, 26 patients (96.2%) had ASIA E neurological status. One patient had signs of spinal shock that resolved immediately after manual axial realignment of the spine during emergency treatment; 13 patients (50%) had nerve root involvement with 6 showing isolated radiculopathy (46.2%). The remaining 7 patients (53.8%) showed slight to severe reduction of muscle strength in terms of the MRC (2–4). At follow-up, radiculopathy resolved in all patients with only 1 reporting on intermittent paraesthesias related to the C7 nerve root. All patients with preoperative loss of muscle strength recovered to at least an MRC of 4. No patient deteriorated neurologically.
Clinical outcome
The outcome measures and questionnaires utilized showed good consistency when assessing differences in clinical outcome. The NDPI showed high correlation with all CSOQ-composite scores (r = 0.6–0.85, P = 0.01 to <0.0001) and the SF-36 composite scores (PCS: r = −0.57, P = 0.002; MCS: r = −0.54, P = 0.004). The same interdependence existed between the SF-36 PCS and the CSOQ-composite scores (r = −0.42 to −0.7, P = 0.03 to <0.0001). Assessment of subjective-rated global outcomes showed strong correlation with the NPDI (r = 0.74, P < 0.0001), the CSOQ-composite scores (r = 0.57–0.74, P = 0.02 to <0.0001) and the SF-36 (PCS: r = 0.7 P = 0.0001; MCS: r = −0.49, P = 0.01). Subjective-rated global outcome was excellent in 14 patients (53.8%), good in 7 (26.9%), and moderate in 5 (19.2%). No patient noted a poor outcome. According to the validated outcome measures applied, overall results were favorable (Tables 9, 10). Concerning detailed questions within the CSOQ (Q38, 40, 41), 22 patients (84.6%) ticked that they would further recommend the procedure they received, while 4 (15.4%) ticked ‘with some reservations’ (Q38); 12 patients (46.2%) judged their results much better than expected, while 7 (26.9%) judged them ‘somewhat better’, 4 the ‘same’ as expected, 1 ‘somewhat worse’ and 1 ‘much worse’ than expected (Q40); 16 patients (61.5%) judged they would be ‘extremely satisfied’ if the neck condition would remain the same as at follow-up, 4 (15.4%) judged ‘moderately satisfied’, 4 ‘neither satisfied nor dissatisfied’ and 2 (7.7%) judged ‘moderately dissatisfied’ (Q41). Notably, with the latter query (Q41) patients were more likely to judge ‘extremely satisfied’ if there was no construct failure (P = 0.01), a presence of fusion (P = 0.03), if the SRA was <0° and thus lordotic (P = 0.05), and if duration of follow-up increased (r = −0.5, P = 0.009). Statistical analysis revealed that patients with AO Type C had reduced outcome in terms of increased CSOQ-psychologic distress score (P = 0.013) and decreased SF-36 MCS (P = 0.02) compared to patients suffering AO Type A or B injuries. With both the CSOQ-psychologic distress score and the SF-36 MCS patients showed significantly better results with increasing duration of follow-up (r = −0.46, P = 0.02; r = 0.4, P = 0.04). There was a strong inverse correlation between the cephalad PDD and the NPDI (r = −0.61, P = 0.001), the CSOQ-psychologic distress score (r = −0.6, P = 0.02), the CSOQ-physical symptoms score (r = −0.63, P = 0.001), the CSOQ-health care utilization score (r = −0.63, P = 0.001), the SF-36 PCS (r = 0.57, P = 0.003), the SF-36 MCS (r = 0.55, P = 0.006) and the self-rated patients’ outcome (r = −0.62, P = 0.001).
Table 9.
Results of the SF-36-v2 (transformed scores, median)
| SF-36 subscales | Current study (n = 26) |
|---|---|
| Physical functioning | 77.3 |
| Social functioning | 88.5 |
| Role physical | 72.4 |
| Role-emotional | 85.3 |
| Mental health | 77.1 |
| Vitality | 63.2 |
| Bodily pain | 72.3 |
| General health | 67.9 |
Table 10.
Clinical outcome following treatment of subaxial injuries assessed with the NDPI, the CSOQ, and the SF-36 component summary scores
| Study | FU (mo) | N | NPDI (%) | CSOQ | SF-36 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Composite neck pain severity score | Composite shoulder/arm pain severity score | Functional disability score | Psychological distress score | Physical symptoms score | Healthcare utilization score | Physical component summary score | Mental component summary score | ||||
| Current, ACDFP | 62 | 26 | 12.4 ± 12.7 (0–40) | 15.6 ± 14.0 (0–40) | 15.0 ± 13.5 (0–33.3) | 8.1 ± 12.2 (0–28.6) | 19.0 ± 17.8 (0–50) | 26.6 ± 21.5 (0–66.7) | 9.2 ± 15.2 (0–40) | 47.0 ± 9.8 (18.2–59.3) | 52.2 ± 12.4 (14.6–75.3) |
| Fisher [33] HTV | 24 | 30 | – | 20.7 ± 17.7 | 25.8 ± 18.7 | 19.1 ± 24.1 | 30.1 ± 20.5 | 33.6 ± 22.6 | – | 43.6 ± 9.5 | 52.4 ± 9.3 |
| Fisher [30, 33, 80] ACP | 24 | – | 23.8 ± 23.5 | 21.5 ± 23.1 | 28.2 ± 23.9 | 32.9 ± 24.1 | 45.1 ± 20.9 | – | 32.6 ± 13.3 | 53.3 ± 14.3 | |
| Kandiziora [56] Cagea | 24 | 52 | 20 | – | – | – | – | – | – | – | – |
| Kandiziora [56] ICGa | 24 | 19 | – | – | – | – | – | – | – | ||
| Kwon [64] ACDFPb | 12 | 20 | – | – | – | – | – | – | – | 46.8 ± 3.1 | 52.3 ± 2.2 |
| Kwon [64] Posterior instrumented fusionb | 12 | 22 | – | – | – | – | – | – | – | 46.9 ± 2.0 | 49.3 ± 2.3 |
Comparison to literature
HTV Halo thoracic vest, 67% w/neurology; ACP anterior corpectomy and plating, 95% w/neurology; ICG iliac crest autograft
aACDFP for both groups
bWith anterior and posterior treatment the NASS cervical spine questionnaire was 85.8 ± 8 and 81.7 ± 3.5 for pain scores, respectively
Patients that succeeded to fusion showed significantly increased outcome in terms of the CSOQ-neck pain severity score (P = 0.02), CSOQ-shoulder/arm pain severity score (P = 0.04), CSOQ-functional disability score (P = 0.005), CSOQ-psychological distress score (P = 0.05), CSOQ-health care utilization score (P = 0.04), SF-36 MCS (P = 0.01) and the NPDI (P = 0.006). These patients also had the higher cROM in flexion–extension (P = 0.01).
The NPDI (r = −0.41, P = 0.04), CSOQ-neck pain severity score (r = −0.46, P = 0.02), the CSOQ-shoulder/arm pain severity score (r = −0.56, P = 0.003), the CSOQ-functional disability score (r = −0.57, P = 0.003), and the CSOQ-physical symptoms score (r = −0.47, P = 0.02) showed an inverse correlation with the cROM for axial rotation. The same was with the CSOQ-physical symptoms score and cROM for flexion–rotation (r = −0.55, P = 0.003) and the rARA C2–7 (r = −0.54, P = 0.004).
Work status
Seven patients (26.9%) were already retired at time of injury unrelated to prior injury or neck pain related issues. At follow-up, out of 19 patients who were occupied at time of injury 16 had W1 according to the DWS (84.2%), 1 had W3, and 2 had W4. 3 patients (15.8%) were not able to resume previous full-time employment or unable to work, but 84% went back to their previous work with a mean time out of work of 13.7 ± 9.7 weeks (range 3–38 weeks).
Donor site morbidity
The amount of iliac crest bone harvested was small performing only 1- to 2-level fusions and the VAS-pain-score yielded a mean of 0.5 ± 1.2 (range 0–4).
Complications
Five patients (19.2%) sustained a surgical complication: 2 patients (7.7%) showed postoperative dysphagia that resolved until 3 months follow-up and 1 (3.8%) had a postoperative wound haematoma requiring surgical drainage. Further course was uneventful. In another patient (3.8%) postoperative CT-scan displayed a bicortical screw fixation that deemed too long and was changed for a shorter one. One patient (3.8%) showed a postoperative right-sided paresis of the hypoglossal nerve that resolved incompletely. There were no cervical infections. Two revision surgeries (7.7%) were performed. There were no complications related to the iliac crest graft harvesting. With the dysphagia grading system of Bazaz et al. [8] mean score was 0.3 ± 0.5 (range 0–1) for liquids and 0.5 ± 0.7 (range 0–2) for solid nutrition. Patients noticed higher disability scores for liquids and solids with increasing ASD at the first adjacent-cephalad level (r = 0.46, P = 0.02; r = 0.41, P = 0.04).
Discussion
In regard to the trauma literature, conclusions that might be drawn from several previous studies suffer on heterogeneous samples [3, 7, 33, 40, 44, 55, 57, 59, 62, 67, 77, 82, 83, 89, 95], and direct comparisons of results after different treatments remain difficult [29, 81]. But the accurate collection of qualitative outcome data is essential because it reflects what is relevant to the patient and society [33]. In this context, the current study is unique reporting on a homogenous subgroup of patients without spinal cord injury with mid- to long-term follow-up. A salient feature is the inclusion of validated patient-oriented outcome measures as part of the follow-up assessment. The detailed reporting of findings will enable data pooling with upcoming studies.
Reconstruction and maintenance of sagittal alignment
In the treatment of subaxial cervical injuries several authors reported that the restoration of cervical lordosis using ACDFP was not a major problem and that the reconstruction did not encounter a significant loss in the long-term [3, 56, 77]. However, the incidence of radiographic failure and loss of alignment relates to its definition. The series of Aebi [3] and Reindl [77] defined construct failure as >10° kyphosis. Johnson [54] used a change in translation of >3.5 mm and angulation >11° which was present in 13% of patients as compared to 31% in the current series using >5° as cut-off. In two previous series the SRA in 1- and 2-level plated fusions averaged +3.5° [33] and −3.6° [54], while Kwon [64] reported a mean SRA of −8.8° after 1-level ACDF and 4.7° after using lateral mass fixation. When compared to normalcy 50% treated posteriorly and 16.7% treated anteriorly had local kyphosis. We noticed a significant loss of lordosis of 5° with a mean SRA of −1° at follow-up, which is still higher as compared to Kwon’s posterior group. However, one has to consider that in the current study and in previous reports using ACDFP [33, 54] the wide range of the SRA (+20° to −20°) indicates that several patients actually had a kyphotic segmental posture. This fact deserves attention as the literature serves evidence that reconstruction and maintenance of a sagittal balance and a lordotic cervical spine has impact on the patients’ outcomes [5, 33, 60, 76, 88, 95]. Given the potential risks that can go with a cervical kyphotic deformity (CKD) [60, 88] as well as the young age of the patients with subaxial trauma [3, 11, 21, 40, 55, 82, 85] the goal of maintaining a lordotic posture across the injury segments may not be trivial. Maintaining sagittal balance avoids an escalating cycle of abnormal forces that may lead to progressive CKD, compromise of the adjacent motion segments and potential neurologic symptoms. Anterior shifting of the axis puts increased biomechanical stress on the posterior neck stabilizers that can lead to fatigue of the surrounding soft-tissues and pain. As kyphosis increases, so does the flexion movement, thereby increasing the likelihood of progressive kyphosis. In contrast, lordosis allows for physiological load distribution towards the posterior elements, unloading of the anteriorly situated construct and further decompressing of the spinal canal by posteriorly shifting the spinal cord [60, 88]. The clinical relevance of kyphosis, however, is not fully understood, and particularly not addressed sufficiently in the trauma population [29]. In a study of Fisher et al. [33] local kyphosis did not affect outcome in terms of the SF-36 score perhaps because the cohort was small and heterogeneous with respect to neurologic status which might have outweighed any sequelae from kyphosis. Follow-up was 55 months and the potential biomechanical detrimental sequelae of kyphosis may manifest years later [33].
In a study by Ohara et al. [73] and Nojiri et al. [72] the C1–2 and Oc–C2 angle showed a significant inverse relation with the C2–7 angle suggesting that alignment changes of the upper and lower cervical spine are interrelated. We have corroborated these findings in a trauma population observing interdependence between the C1–2 and C7–T1 angles and the lordosis C2–7. If lordosis at C2–7 was reduced, e.g., due to a lack of lordosis at the fusion level, there was a compensatory lordotic adjustment at the C1–2 and C7–T1 levels yielding for the maintenance of a global cervical lordosis.
Anterior, posterior or combined surgery?
Anterior cervical decompression, fusion and plating for unstable subaxial injuries is increasingly recognized as the standard [10, 21, 29, 33]. The majority of dislocations are amenable for closed reduction [77, 78, 89] or reduction can be achieved intraoperatively [29, 77] as it was in the current study. ACDFP became popular as satisfactory clinical outcomes, low complication but high fusion rates were reported [3, 11, 56, 64, 77]. Nevertheless, although ACDFP was suggested to confer excellent immediate stability making any posterior element instability clinically insignificant [85], early changes in construct geometry and mechanical failures occur and promote a debate regarding the use of anterior, posterior or combined stabilization for patients with marked instability [29]. Using ACDFP, the incidence of loss of alignment has been reported as 13–19% [47, 54], the need for supplemental posterior stabilization as 10–21% [20, 92] and revision rates average of 6.5% [10, 11, 20, 40, 41, 47, 51, 55, 59, 77, 82, 85, 95]. Early plate designs relying on NC-plate-screw interfaces were suggested to be plagued by increased mechanical failure, screw loosening and pseudoarthrosis [10, 11, 30, 40, 69, 77, 82, 85] most notably in multilevel constructs [60]. Bicortical screw anchorage has shown no significant enhancement of screw pull-out resistance [87], additionally we observed screw-loosening in 46% of patients that had bicortically anchored NC-plates.
Statistical analysis revealed a significant loss of the postoperative SRA and 31% of patients were judged to have construct failure (Fig. 6). The risk for failure was significantly increased in surgeries at and near to the cervicothoracic junction, at the C6–7 level as compared to the more cephalad which possibly reflects that the caudal levels are more difficult to be instrumented as well as increasingly biomechanically challenged mirroring prior experience [54]. In addition, risk for failure increased in AO Type B and C injuries and with higher CSISS, respectively, as well as with comminution of the facets or lateral mass. Currently, the authors use CS-plates, as they confer increased construct rigidity [60]. Despite this Johnson et al. [54] using CS-plates reported a 13% (n = 11) failure rate with associated pseudoarthrosis in 9 patients with 1-level flexion-distraction injuries. Data that concur with other series reporting loss of alignment in about one-fifth of cases using either CS- or semi-constrained plates [21, 47, 57].
Anterior cervical decompression, fusion and plating has been shown to be less effective at providing stabilization than posterior fixation when the posterior elements were incompetent [2, 27]. In contrast Lifeso et al. [68] who reported on compressive-extension injuries stage-1 observed that the use of rigid posterior lateral mass constructs resulted in late kyphotic deformity but there was a 100% fusion-rate with no failure if ACDFP was applied. In a series of 29 subaxial instabilities treated with posterior lateral mass plating, Pateder et al. [75] observed a mean change of lordosis of only 2° in 28 of 29 patients (range 0°–6°). However, 1–5 levels had to be included into the fusion mass to yield sufficient construct rigidity and good radiographic outcome. Biomechanically, cervical pedicle screw constructs confer the highest construct rigidity for posterior-only treatment but clinical trauma series remain sparse [1, 62].
Mirroring the reported failures, the average rate of combined anterior–posterior surgeries as the index procedure for subaxial injuries is 11% [3, 21, 51, 52, 55, 57, 59, 89, 95] and the evidence of redo surgeries after ACDFP warrants concerns that distinct injury patterns are not amenable for anterior-only surgery. Those injury patterns demanding combined surgery are not stratified yet [29]. The undoubted increase in construct stability with 360° fusions [4, 18, 58] has to be weighed against higher potential for surgical morbidity and prolonged surgical time. Several authors recommend 360° surgery in the presence of injuries to both anterior and posterior columns, in ‘severe’ fracture-dislocations or in cases with ‘gross’ instability, poor bone quality and increased rigidity of the spine, such as in ankylosing spondylitis and DISH [7, 21, 31, 44, 47, 48, 55, 81, 95]. In Johnson’s series [54] radiographic failure rate with 1-level ACDFP was strongly correlated with facet fractures combined with superior end-plate compression fractures of the lower vertebra suggesting a 360° procedure. Harrington et al. [44] showed that employing a 360° approach for patients with bilateral facet damage yielded no construct failures. In contrast, Dvorak et al. [30] demonstrated that for a 1-level posterior element injury fracture-dislocation model, BMD was the single most important factor for stabilization achieved with ACDFP and the current authors could not find a significant correlation between construct failure and the presence of posterior element insufficiency that we assessed by distinct classifications.
Nevertheless, although the current sample had no bilateral facet injuries, we and several colleagues observed satisfactory clinical outcomes [3, 10, 56, 82] stressing that ACDFP is successful in most disco-ligamentous injuries with and without posterior elements involved. In selected cases posterior stabilization may be required. The questions that remain however are: how much stability is implied by each fracture pattern, if this instability demands 360° fusion and if a slight loss of correction is clinically significant. These questions can only be answered using a prospective or metanalysis approach based on long-term data of homogenous samples akin to this current. The authors observed increased construct failures and non-unions in patients that had AO Type C fractures which warrants further research regarding to anterior-only or 360°-surgeries in these injuries.
Adjacent-segment degeneration
It has been postulated that cervical fusion alters the biomechanical conditions at adjacent segments resulting in increased loading, intradiscal pressure and shear, and excessive movement which in turn might lead to accelerated ASD [16, 17, 50, 61, 79, 96]. Long-term follow-up studies in ACDFP established that 10–25% of patients develop ASD at a mean of 10 years after the index surgery with some requiring reoperation [50]. ASD has also been suggested to reflect the natural progression of underlying degenerative disease [50]. Theoretically, the assessment of ASD in a trauma population is an ideal estimate as these young patients frequently have no symptomatic cervical disc disease. In two series of Blauth with ACDFP for subaxial injuries in 79 [11] and 87 patients [10], asymptomatic adjacent-level anterior ossifications were seen in 47.3 and 51.8%, respectively. Goffin et al. [41] reported on adjacent-level degenerative changes in 60% of patients that had ACDFP, again without causing symptoms. Significant factors related to ASD were multilevel fusion, lower cervical segment fusion and Frankel A–C patients. ASD included the development of anterior osteophytes and ossification of the ALL; within the disc space the authors observed no signs of advanced degeneration. Goffin related ASD to excessive extension of plates up or down into adjacent healthy discs and the extensive surgical dissection and damage to the ALL. Primary or secondary impingement of a plate has been observed [60] but is seldom reported as a technical failure [3, 28, 60, 85]. It contributes to ASD causing anterior bony thorns [28, 55] as it was observed in the current study. Therefore, DuBoys et al. [28] and Ripa et al. [82] recommended that plates should be at least 3 mm away of the adjacent disc as micromotions can lead to implant loosening or anterior ossifications that were seen in 77 and 42% of adjacent-cephalad and -caudal levels, respectively, in a study of Park et al. [74]. The latter observed that adjacent-level ossifications were more likely to develop and progress if the PDD was <5 mm. In this context, using a cut-off of 3 mm the current study could substantiate that the PDD should be at least 3 mm. The authors observed that the progression of ASD at the first adjacent-levels significantly correlated with the PDD which calls for caution during surgical exposure and instrumentation [28].
The analysis of ASD had limitations due to the nature of a retrospective analysis of patients aging 18–80 years at follow-up and a follow-up of 16–128 months (but with a median of 60 months). In addition, owing to a small sample the current study could not confirm or refute the assumption that fusion fosters ASD in a trauma population. The grades of ASD both at the first adjacent-cephalad and -caudal level were strongly related to the patients age and those first-adjacent levels that encountered higher grades of follow-up ASD also had the higher grades of injury ASD. Our results rather reflect an increase of pre-existing degenerative changes (Fig. 7). The progression of the ASD and new onset ASD in terms of anterior ossifications that were seen in about 50% of patients were pronounced in patients with a decreased PDD. Patients that had increased ASD at the first adjacent-cephalad level were likely to have higher disability grades according to the dysphagia grading of Bazaz et al. [8]. In addition, although we did not observe a significant correlation between the progression or new onset of ASD and the clinical outcomes, strong correlations existed between the cephalad PDD and the NPDI and the CSOQ-composite scores. The ASD at least of the first adjacent-cephalad level seems to be related to a decreased PDD, which in turn can affect clinical outcome. Although limitations existed, the authors used the possibility assessing the ASD in a trauma population to add insight to the course of the adjacent segment in plated anterior fusions for cervical injuries and otherwise ‘healthy’ patients.
Morphological injury pattern
For collaborative purposes, the detailed description of injury pattern is important. Therefore, the authors applied the AO-Magerl classification delineating injury morphology, a separated description of injury to the facets and lateral mass as subtleties of injury pattern, and the CSISS [71] that gives an objective score on the severity of injury. Statistical analysis revealed that patients with AO Type C injuries had reduced clinical outcomes in terms of the CSOQ-psychologic distress score and the SF-36 MCS. In addition, patients with increased CSISS had a significantly reduced rARA C2–7, which might reflect late sequelae from occult injury to adjacent-level soft-tissue surroundings. The loss of lordosis at the fusion–block was found to be significantly related to the CSISS and the presence of facet dislocations, and the incidence of having a ‘successful surgical outcome’ was significantly increased in patients that had AO Type A or B injuries compared to Type C injuries. Our findings reflect that increased trauma to the injury levels increase the biomechanical challenges to yield a successful surgical and clinical outcome and predispose affected levels to loss of alignment.
Fusion rate
In the trauma setting most surgeons use tricortical iliac crest grafts [3, 10, 11, 21, 40, 41, 47, 52, 55, 59, 77, 82, 85, 89, 95] and the time to union is reported with 3–4 months [3, 21, 82, 95] reflecting observations in the current series. Stulik et al. [89] reported a fusion-rate of 99% in 68 patients after ACDFP and Kandiziora et al. [56] reported a fusion rate of 96% in 56 patients using either a TMC or iliac crest graft with ACDFP. Anecdotically, fusion rates are high in the trauma population using ACDFP and the authors found the reported rate averaging 99% (Table 11, Electronic suppl material). In contrast, a metaanalysis on 1-level ACDFP in a degenerative population [37] revealed a fusion-rate of 92%. Various criteria have been used for the assessment of fusion in ACDFP for injuries C3–T1 [47, 90, 95] but assessing a solid fusion remains difficult [36] and should be done carefully. The fusion rate in the current series was 88.5% but the definition of fusion included the use of two classifications and blinded interobserver assessment. We did not find a significant linkage between any of the radiographic parameters and the presence of fusion, but patients with non-union had significant decreased outcome in terms of the NPDI. The use of the classifications is worthy as it allows for comparative research on different techniques and instrumentations. So, e.g., with observer 1 and 2 the rate of Type IV fusions, that is a definitive non-union, was 7% using NC-plates which is comparable to the rate of Type IV fusions reported by Johnson et al. [54] who used CS-plates.
Functional outcome
In a laboratory setting [25], a significant compensatory adjacent-level increase in ROM for extension was observed after a 1-level C6–7 fusion. In flexion, adjacent-level ROM also increased but did not reach significance. Findings concurred with those of DiAngelo et al. [25] and Dmitriev et al. [26] observing increased ROM at the motion segments adjacent to 2- and 3-level in vitro fusions. However, the biomechanical laboratory findings do not echo clinical reality: in a motion analysis of Kolstad et al. [61] adjacent-motion to a 1-level ACDF did not exhibit a significant change between the preoperative and 12 months follow-up state. Likewise, Reitman et al. [79] investigating the intervertebral motion adjacent to 1- and 2-level fusions observed that the presence of fusion had no effect on the adjacent-level ROM. The results concur with the current data derived from a trauma population revealing no significant difference between the ROM of the adjacent levels. The rSRA were measured with a conventional X-ray technique and were less than those found in normals using sophisticated techniques (Tables 5, 6, Fig. 8) [80]. Only some adjacent-levels exceeded the ranges of ±2 SD off normalcy reflecting hyper- or hypomobility. In the individual case, sagittal plane rotational motion depends on how well the patients perform [61] which might have contributed to the smaller rSRA in the current series. In addition, radiographically assessed ROM in flexion–extension (rARA C2–7) was smaller than that assessed clinically. Hence, the intervertebral motions measured have to be put into perspective, but still allow us to capture inter-level differences of motion pattern adjacent to the fused levels. We observed a significant correlation between the rSRA at the first adjacent-cephalad and first adjacent-caudal level and the rARA C2–7 with the correlation being stronger than for age. So, increased ROM at the first adjacent levels was mirrored by an increased ability of the cervical spine for total sagittal ROM. Coincidently, we observed a significant inverse correlation between the ASD at the first adjacent-caudal level and it’s rSRA as well as the age of patients. One might conclude that in the presence of increased ASD and increased age the sagittal adjacent-level ROM and thus the total sagittal ROM of the cervical spine decreases. In our study inadequate PDD has been shown to contribute to ASD highlighting the importance of this technical aspect of surgery. This concurs with the observations by Ripa et al. [82] who noticed that patients with advanced anterior bony bridging at the first adjacent-disc levels, as a result of small PDD, had near or complete autofusion on flexion–extension films.
In a study of Hilbrand et al. [49] the postoperative clinical ROM in flexion for 1- to 4-level ACDFP averaged 50°–64° and axial rotation showed a mean of 63°–72°. The authors demonstrated that cervical fusion of up to four levels did not reduce cervical ROM significantly [49]. Rather, the clinically assessed cervical ROM decreases with age [15, 23] and was reflected by a strong inverse relationship between the absolute ROM and age in the current sample. Therefore, the authors expressed total ROM for flexion–extension and axial rotation as the cROM [15] and, in contrast to previous studies assessing only absolute ROM of the cervical spine after ACDF for subaxial injuries [3, 89], we observed that patients with increased cROM as well as rARA C2–7 were likely to have better clinical outcomes (SF-36, NDPI, CSOQ).
Clinical outcome
Good and excellent results for ACDF in populations with degenerative disorders were found in 62–82% [39], but reliable outcomes based on validated measures are scanty for a trauma population: In a series of Reindl et al. [77] with ACDFP in 41 patients outcome was assessed using a VAS. Pain averaged 3 of 10. Concerning number of levels fused, associated polytrauma (32%), and neurology (29% Frankel A–C), the sample is too heterogeneous to compare outcome variables. Assigning pain as the outcome measure, Blauth et al. [10] reported on ACDFP in two groups of 57 and 87 patients with a mean of 12 years and 3–10 years follow-up, SCI included. They observed 30 and 55% showed pain in resting position, 67 and 61% work-load dependant pain. 81 and 79% achieved work status, 12 and 8% were unable to work because of injury sequela and 7 and 12% were retired or unemployed, respectively. The return to work rate is an important estimate in the assessment of spinal injuries [34] and in the current study 84% of patients went back to previous employment after a mean of 14 weeks. As one of the first trauma studies using validated outcomes vehicles, Fisher et al. [33] applied the SF-36 and CSOQ. Questionnaires were completed by 67% of patients treated either with the HTV or anterior corpectomy and plating (ACP) for flexion tear drop fractures. 67 and 95% had neurological deficit, respectively. Owing to the benefits of using validated queries Fisher’s results can be plotted against the current (Table 10). Given the severity of injuries and the rate of neurologically injured in their series, outcome was reduced following both HVT and ACP compared to our sample. Kandiziora et al. [56] investigated the efficacy of interbody cages compared to iliac crest autografts in 52 ACDFP for subaxial injuries. The evaluation of outcome included assessment of neck pain and arm pain using a VAS, the NPDI, and the cervical spine functional score [53, 91]. Usage of the NPDI allows plotting Kandiziora’s results against ours (Table 10). In terms of the NDPI the current sample had a superior outcome. Their sample included SCIs (11% Frankel A–D) and patients having workers compensation claims (25%) that may explain their decreased outcomes into perspective. In another study of the Vancouver group on unstable unilateral facet injuries [64] 20 neurologically intact patients received ACDF and 22 posterior fusion using wires or lateral mass plates. Outcome vehicles included 12 months SF-36 and the NASS questionnaires. Although not statistically significant, clinical outcome measures were in favor of the anterior group. Working on a homogenous sample the outcome in terms of the SF-36 paralleled that of the current study (Table 10). We observed excellent or good outcomes in 81% of patients.
Statistical analysis revealed that several technical and injury-related variables had impact on clinical outcome. Patients were significantly more likely to maintain a high satisfaction level if they succeeded to maintain segmental lordosis (<0°), had a solid fusion, an increased PDD, and if they were judged to have a ‘successful’ surgical outcome. Except for the presence of a fusion, we could not identify the failure of maintaining local and total lordosis and the presence of construct failure to be single predictors of better clinical outcomes in terms of the validated measures (NPDI, CSOQ, SF-36). The latter might be referred to Type 1 statistical error and a small sample, respectively. Nevertheless, the current study offers reliable long-term data (mean 5.5 years) from a homogenous sample of 26 patients with subaxial trauma. The study stresses that the usage of validated outcome measures applied on a homogenous sample is an important concern which will allow sound comparisons of upcoming samples. Assessment of pooled, hard clinical outcome data are deemed necessary to identify, e.g., which radiographic changes in construct geometry and alignment are “failures” and which are asymptomatic radiographic findings [54].
Complications
The current study largely reflected the rate of common complications observed with plated ACDFP [21, 42, 66, 77]. We had no neurologic deficits, cervical or iliac crest infections, RLN palsy or occult instabilities [21, 59, 64, 75, 77, 82]. The incidence of soft tissue haematoma following ACDF was reported to be 5.6 with 2.4% requiring revision [35]. We had one haematoma to be revised. Ripa et al. [82] noted 1 excessive length of a screw indicating redo surgery, which was also the case in 1 of our patients owing to the fact that bicortical screw fixation was deemed indicated. According to his grading scale, Bazaz et al. [8] observed the rate of mild dysphagia with 13%, moderate 0.4%, and severe 0.4% after ACDF at 2 years follow-up. We noticed 7 patients (26.9%) having mild difficulties with liquids and 8 patients (30.7%) with solid nutrition. Three patients (15.8%) had moderate difficulties with swallowing solids. The incidence of dysphagia was not significantly related to construct failures. High rates of dysphagia however warrant the use of CS-plates that show at least the ability to decrease symptomatic screw back-outs that we had seen in 46% of patients.
Graft donor site pain and morbidity is a liberally used criticism of using autograft [86]. Silber et al. [86] noticed serious impairments in functional activities of daily living in about 10% of patients due to the iliac crest site and mean VAS was 3.8 at 4 years follow-up. In contrast, Shamsaldin et al. [84] demonstrated that after harvesting bone from the iliac crest for 1-level ACDF, 92% had no persisting pain after 1 year. Likewise, in a series on thoracolumbar fractures Delawi et al. [22] reported donor site pain in 14%, but ‘pain’ on a VAS averaged 1.6 only [22]. Our findings concur with that of Delawi and Shamsaldin with the patients’ iliac crest related pain averaging only 0.5 on a VAS. Pain derived from iliac crest is a negligible concern in bone harvesting for 1- to 2-level ACDFP.
Conclusion
The authors demonstrated that the use of validated outcome vehicles enables a representative comparison of alike cases and offers objective insight into the mid- long-term outcome of subaxial injuries. With the application of hard in- and exclusion criteria a homogenous group of patients without SCI subjected to ACDFP for unstable subaxial injuries was selected for detailed outcome analysis. The current study assessed variables that are of interest in the outcome research of cervical spine injuries without SCI. Based on the current study and aware of the limitations of a retrospective outcome analysis, the authors conclude that there is little evidence of accelerated ASD following subaxial plating for 1- to 2-level trauma, that plate positioning in relation to PDD is an important technical consideration affecting outcome, and that reconstruction and maintenance of a lordotic balanced spine fosters construct strength, fusion rate and increases the rate of good clinical outcomes. Analysis of radiographic and clinical failures in sight of a critical review of literature depicted that patients with disco-ligamentous injury with increased injury severity (AO Type C), bilateral dislocations and facet fractures, reduced bone quality, and surgery at the CTJ benefit by a 360° surgical approach. Prospective studies and, in particular, meta-analysis with data pooling of studies such as the current offering detailed information of mid- to long-term outcomes will shed more light on the ideal surgical technique for each distinct injury pattern.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Characteristics of patients with ACDFP for subaxial injuries. Current results and comparison to literature* (DOC 103 kb)
References
- 1.Abumi K, Itoh H, Taneichi H, Kaneda K. Transpedicular screw fixation for traumatic lesions of the middle and lower cervical spine: description of the techniques and preliminary report. J Spinal Disord. 1994;7:19–28. doi: 10.1097/00002517-199407010-00003. [DOI] [PubMed] [Google Scholar]
- 2.Adams MS, Crawford NR, Chamberlain RH, et al. Biomechanical comparison of anterior cervical plating and combined anterior-lateral mass plating. Spine J. 2001;1:166–170. doi: 10.1016/S1529-9430(01)00049-3. [DOI] [PubMed] [Google Scholar]
- 3.Aebi M, Zuber K, Marchesi D. Treatment of cervical spine injuries with anterior plating. Indications, techniques, and results. Spine. 1991;16(suppl 3):S38–S45. doi: 10.1097/00007632-199103001-00008. [DOI] [PubMed] [Google Scholar]
- 4.Allyson I, Zambrano I, Tataria J, Ameerally A, Agulnick M, Goodwin JSL, et al. Biomechanical evaluation of surgical constructs for stabilization of cervical teardrop frctures. Spine J. 2006;6:514–523. doi: 10.1016/j.spinee.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 5.American Association if Neurological Surgeons Treatment of subaxial cervical spinal injuries. Neurosurgery. 2002;50(suppl 3):S156–S165. doi: 10.1097/00006123-200203001-00024. [DOI] [PubMed] [Google Scholar]
- 6.American Association of Neurological Surgeons Treatment of subaxial cervical spinal injuries. Neurosurgery. 2002;50(suppl 3):S156–S165. doi: 10.1097/00006123-200203001-00024. [DOI] [PubMed] [Google Scholar]
- 7.Arnold PM, Bryniarski M, McMahon JK. Posterior stabilization of subaxial cervical spine trauma: indications and techniques. Injury. 2005;36:S-B36–S-B43. doi: 10.1016/j.injury.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine. 2002;27:2453–2458. doi: 10.1097/00007632-200211150-00007. [DOI] [PubMed] [Google Scholar]
- 9.BenDebba M, Heller J, Ducker TB, Eisinger JM (2002) Cervical spine outcomes questionnaire. Its development and psychometric properties. Spine 27:2116–2124. doi:10.1097/00007632-200210010-00007 [DOI] [PubMed]
- 10.Blauth M, Schmidt U, Bastia L, Knop C, Tscherne H. Anterior interbody fusion for cervical spine injuries—indications, implants, technique and results. Zentralbl Chir. 1998;123:919–929. [PubMed] [Google Scholar]
- 11.Blauth M, Schmidt U, Dienst M, Knop C, Lobenhoffer P, Tscherne H. Long-term outcome of 57 patients after ventral interbody spondylodesis of the lower cervical spine. Unfallch. 1996;99:925–939. doi: 10.1007/s001130050076. [DOI] [PubMed] [Google Scholar]
- 12.Bogduk N, Mercer S. Biomechanics of the cervical spine. I: normal kinematics. Clin Biomech (Bristol, Avon) 2000;15:633–648. doi: 10.1016/S0268-0033(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 13.Bono CM, Vaccaro AR, Fehlings M, Fisher C, Dvorak M, Ludwig S, Harrop J (2006) Measurement techniques for lower cervical spine injuries. Spine 31:603–609. doi:10.1097/01.brs.0000201273.39058.dd [DOI] [PubMed]
- 14.Bridwell KH, Lenke LG, McEnerey KW, et al. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine. 1995;20:1410–1418. doi: 10.1097/00007632-199520120-00014. [DOI] [PubMed] [Google Scholar]
- 15.Castro WH, Sautmann A, Schilgen M, Sautmann M. Noninvasive three-dimensional analysis of cervical spine motion in normal subjects in relation to age and sex. An experimental examination. Spine. 2000;25:443–449. doi: 10.1097/00007632-200002150-00009. [DOI] [PubMed] [Google Scholar]
- 16.Chang U-K, Kim DH, Lee MC, Willenberg R, Kim S–H, Lim J (2007) Changes in adjacent-level disc pressure and facet joint force after cervical arthroplasty compared with cervical discectomy and fusion. J Neurosurg Spine 7:33–39 [DOI] [PubMed]
- 17.Chang U-K, Kim DH, Lee MC, Willenberg R, Se-Hoon K, Lim J. Range of motion change after cervical arthroplasty with Prodisc-C and prestige artificial discs compared with anterior cervical discectomy and fusion. J Neurosurg Spine. 2007;7:40–46. doi: 10.3171/SPI-07/07/040. [DOI] [PubMed] [Google Scholar]
- 18.Coe JD, Warden KE, Sutterlin CE, III, McAfee PC. Biomechanical evaluation of cervical spinal stabilization methods in a human cadaveric model. Spine. 1989;14:1122–1131. doi: 10.1097/00007632-198910000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Cote P, Cassidy JD, Yong-Hing Sibley J, Loewy J. Apophysial joint degeneration, disc degeneration, and sagittal curve of the cervical spine: can they be measured reliably on radiographs? Spine. 1995;22:859–864. doi: 10.1097/00007632-199704150-00007. [DOI] [PubMed] [Google Scholar]
- 20.Daentzer D, Boeker D-K. Operative stabilization of traumatic instabilities of the lower cervical spine. Experience with an angle unstable anterior plate-screw system in 95 patients. Unfallch. 2004;107:175–180. doi: 10.1007/s00113-003-0722-6. [DOI] [PubMed] [Google Scholar]
- 21.Daentzer D, Böker DK. Operative stabilization of traumatic instabilities of the lower cervical spine. Experience with an angle unstable anterior plate-screw-system. Unfallch. 2004;107:175–180. doi: 10.1007/s00113-003-0722-6. [DOI] [PubMed] [Google Scholar]
- 22.Delawi D, Dhert WJ, Castelein RM, Verbout AJ, Oner FC. The incidence of donor site pain after bone graft harvesting from the posterior iliac crest may be overestimated: a study on spine fracture patients. Spine. 2007;32:1865–1868. doi: 10.1097/BRS.0b013e318107674e. [DOI] [PubMed] [Google Scholar]
- 23.Demaille-Wlodyka S, Chiquet C, Lavaste J-F, Skalli W, Revel M, Poiraudaeau S (2007) Cervical range of motion and cephalic kinesthesis—ultrasonographic analysis by age and sex. Spine 32:E254–E261. doi:10.1097/01.brs.0000259919.82461.57 [DOI] [PubMed]
- 24.Denis F. Spinal instability as defined by the three-column spine concept in acute spinal trauma. Clin Orthop Relat Res. 1983;189:65–76. [PubMed] [Google Scholar]
- 25.DiAngelo DJ, Foley KT. An improved biomechanical testing protocol for evaluating spinal arthroplasty and motion preservation devices in a multilevel human cadaveric model. Neurosurg Focus. 2004;17:E4. [PubMed] [Google Scholar]
- 26.Dmitriev AE, Kuklo TR, Lehman RA, Jr, Rosner MK. Stabilizing potential of anterior, posterior and circumferential fixation for multilevel cervical arthrodesis. Spine. 2007;32:E188–E196. doi: 10.1097/01.brs.0000257577.70576.07. [DOI] [PubMed] [Google Scholar]
- 27.Do Koh Y, Lim TH, Won You J, Eck J, An HS. A biomechanical comparison of modern anterior and posterior plate fixation of the cervical spine. Spine. 2001;26:15–21. doi: 10.1097/00007632-200101010-00005. [DOI] [PubMed] [Google Scholar]
- 28.DuBois CM, Bolt PM, Todd AG, Gupta P, Wtzel FT, Phillips FM. Static versus dynamic plating for multilevel anterior cervical discectomy and fusion. Spine J. 2007;7:188–193. doi: 10.1016/j.spinee.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Dvorak MF, Fisher GF, Fehlings MG, Rampersaud YR, Öner FC, Arabi B, et al. The surgical approach to subaxial cervical spine injuries. An evidence-based algorithm based on the SLIC classification system. Spine. 2007;32:2620–2629. doi: 10.1097/BRS.0b013e318158ce16. [DOI] [PubMed] [Google Scholar]
- 30.Dvorak MF, Pitzen T, Zhu Q, Gordon JD, Fisher CG, Oxland TR. Anterior cervical plate fixation: a biomechanical study to evaluate the effects of plate design, endplate preparation, and bone density. Spine. 2005;30:294–301. doi: 10.1097/01.brs.0000152154.57171.92. [DOI] [PubMed] [Google Scholar]
- 31.Einsiedel T, Schmelz A, Arand M, Wilke HJ, Gebhard F, Hartwig E, et al. Injuries of the cervical spine in patients with ankylosing spondylitis: experience at two trauma centers. J Neurosurg Spine. 2006;5:33–45. doi: 10.3171/spi.2006.5.1.33. [DOI] [PubMed] [Google Scholar]
- 32.Elsaghir H, Bohm H. Anterior versus posterior cervical plating in cervical corpectomy. Arch Orthop Trauma Surg. 2000;120:549–554. doi: 10.1007/s004020000153. [DOI] [PubMed] [Google Scholar]
- 33.Fisher CG, Dvorak MFS, Leith J, Wing PC. Comparison of outcomes for unstable lower cervical flexion teardrop fractures managed with halo thoracic vest versus anterior corpectomy and plating. Spine. 2002;27:160–166. doi: 10.1097/00007632-200201150-00008. [DOI] [PubMed] [Google Scholar]
- 34.Fisher CG, Noonan VK, Dvorak MF. Changing face of spine trauma care in North America. Spine. 2006;31(suppl):S2–S8. doi: 10.1097/01.brs.0000217948.02567.3a. [DOI] [PubMed] [Google Scholar]
- 35.Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine. 2007;32:2310–2317. doi: 10.1097/BRS.0b013e318154c57e. [DOI] [PubMed] [Google Scholar]
- 36.Fountas KN, Kapsalaki EZ, Smith BE, Nikolakakos LG, Richardson CH, Smisson HF, Robinson JS, Parish DC (2006) Interobservation variation in determining fusion rates in anterior cervical discectomy and fusion procedures. Eur Spine J 16:39–45 [DOI] [PMC free article] [PubMed]
- 37.Fraser JF, Härtl R. Anterior approaches to fusion of the cervical spine: a metaanalysis of fusion rates. J Neurosurg Spine. 2007;6:298–303. doi: 10.3171/spi.2007.6.4.2. [DOI] [PubMed] [Google Scholar]
- 38.Frobin W, Leivseth G, Biggemann M, Brinckmann P. Sagittal plane segmental motion of the cervical spine. A new precision measurement protocol and normal motion data of healthy adults. Clin Biomech (Bristol, Avon) 2002;17:21–31. doi: 10.1016/S0268-0033(01)00105-X. [DOI] [PubMed] [Google Scholar]
- 39.Garvey TA, Transfeldt EE, Malcolm JR, Kos P. Outcome of anterior cervical discectomy and fusion as perceived by patients treated for dominant axial mechanical cervical spine pain. Spine. 2002;27:1887–1895. doi: 10.1097/00007632-200209010-00015. [DOI] [PubMed] [Google Scholar]
- 40.Goffin J, Plets C, Berg R. Anterior cervical fusion and osteosynthetic stabilization according to Caspar: a prospective study of 41 patients with fractures and/or dislocations of the cervical spine. Neurosurgery. 1989;25:865–871. doi: 10.1097/00006123-198912000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Goffin J, Loon J, Calenbergh F, plets C. Long-term results after anterior cervical fusion and osteosynthetic stabilization for fractures and/or dislocations of the cervical spine. J Spinal Disord. 1995;8:500–508. doi: 10.1097/00002517-199512000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Graham JJ. Complications of cervical spine surgery. A five-year report on a survey of the membership of the Cervical Spine Research Society by the Morbidity and Mortality Committee. Spine. 1989;14:1046–1050. doi: 10.1097/00007632-198910000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Hardacker JW, Shuford RF, Capicotto PN, Pryor PW. Radiographic standing cervical segmental alignment in adult volunteers without neck symptoms. Spine. 1997;22:1472–1480. doi: 10.1097/00007632-199707010-00009. [DOI] [PubMed] [Google Scholar]
- 44.Harrington JF, Park MCP. Single level arthrodesis as treatment for midcervical fracture subluxation. A chohort study. J Spinal Disord Tech. 2007;20:42–48. doi: 10.1097/01.bsd.0000211255.05626.b0. [DOI] [PubMed] [Google Scholar]
- 45.Harrison DD, Janik TJ, Troyanovich SJ, Holland B. Comparisons of Lordotic cervical spine curvatures to a theoretical model of the static sagittal cervical spine. Spine. 1996;21:667–675. doi: 10.1097/00007632-199603150-00002. [DOI] [PubMed] [Google Scholar]
- 46.Harrison DE, Harrison DD, Cailliet R, Troyanovic SJ, Janik TJ, Holland B. Cobb method or Harrison posterior tangent method. Which to choose for lateral cervical radiographic analysis. Spine. 2000;25:2072–2078. doi: 10.1097/00007632-200008150-00011. [DOI] [PubMed] [Google Scholar]
- 47.Henriques T, Olerud C, Bergman A, Jonsson H., Jr Distractive flexion injuries of the subaxial cervical spine treated with anterior plate alone. J Spinal Disord Tech. 2004;17:1–7. doi: 10.1097/00024720-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Henrqies T, Olerud C, Bergman A, Jonsson H., Jr Distractive flexion injuries of the subaxial cervical spine treated with anterior plate alone. J Spinal Disord Tech. 2004;17:1–7. doi: 10.1097/00024720-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Hilibrand AS, Balasubramanian K, Eichenbaum M, Thinnes JH, Daffner S, Berta S, et al. The effect of anterior cervical fusion on neck motion. Spine. 2006;31:1688–1692. doi: 10.1097/01.brs.0000224165.66444.71. [DOI] [PubMed] [Google Scholar]
- 50.Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion. Spine J. 2004;4:190S–194S. doi: 10.1016/j.spinee.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Hofmeister H, Bühren V. Therapeutical concepts for injuries of the lower cervical vertebral column. Orthopade. 1999;28:401–413. doi: 10.1007/s001320050365. [DOI] [PubMed] [Google Scholar]
- 52.Hofmeister M, Potulski M, Späth K, Jaschke H, Bühren V. Clinical results of ventral fixation of injuries of the cervical vertebral column. Osteosyn Intern. 1998;6:112–120. [Google Scholar]
- 53.Javid D, Hedlund R, Vavruch L, Leszniewski W. Is the efficacy of the Cloward procedure overestimated? Technique of evaluation affects the outcome. Eur Spine J. 2001;10:222–227. doi: 10.1007/s005860100261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson MG, Fisher CG, Boyd M, Pitzen T, Oxland TR, Dvorak MF. The radiographic failure of single segment anterior cervical plate fixation in traumatic cervical flexion distraction injuries. Spine. 2004;29:2815–2820. doi: 10.1097/01.brs.0000151088.80797.bd. [DOI] [PubMed] [Google Scholar]
- 55.Kalff R, Kocks W, Grote W, Schmit-Neuerburg KP. Operative spondylodesis in injuries of the lower cervical spine. Neurosurg Rev. 1993;16:211–220. doi: 10.1007/BF00304331. [DOI] [PubMed] [Google Scholar]
- 56.Kandiziora F, Pflugmacher R, Scholz M, Schnake K, Putzier M, Kodadadyan-Klostermann C, Haas NP (2005) Treatment of traumatic cervical spine instability with interbody fusion cages: a prospective controlled study with a 2-year follow-up. Injury 36:S-B27–S-B35 [DOI] [PubMed]
- 57.Khoo LT, Benae JL, Gravori T. Anterior plating for cervical traumatic fractures: an analysis of graft height and segmental lordosis preservation. Int J Spin Surg. 2005;1:1–11. [Google Scholar]
- 58.Kim SM, Lim TJ, Paterno J, Park J, Kim DH. A biomechanical comparison of three surgical approaches in bilateral subaxial cervical facet dislocation. J Neurosurger (Spine 1) 2004;1:108–115. doi: 10.3171/spi.2004.1.1.0108. [DOI] [PubMed] [Google Scholar]
- 59.Kocis J, Wendsche P, Visna P, Muzik V, Pasa L. Injuries to the lower cervical spine. Acta Chir Orthop Traumatol Cech. 2004;71:366–372. [PubMed] [Google Scholar]
- 60.Koller H, Hempfing A, Ferraris L, Maier O, Hitzl W (2007) 4- and 5-level anterior fusions of the cervical spine: review of literature and clinical results. Eur Spine J 2055–2071 [DOI] [PMC free article] [PubMed]
- 61.Kolstad F, Nygaard OP, Leivseth G. Segmental motion adjacent to anterior cervical arthrodesis. Spine. 2007;32:512–517. doi: 10.1097/01.brs.0000256448.04035.bb. [DOI] [PubMed] [Google Scholar]
- 62.Kotani Y, Abumi K, Ito M, Minami A. Cervical spine injuries associated with lateral mass and facet joint fractures: new classification and surgical treatment with pedicle fixation. Eur Spine J. 2005;14:69–77. doi: 10.1007/s00586-004-0793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuntz C, Levin LS, Ondra SL, Shaffrey CI, morgan CJ. Neutral upright sagittal spinal alignment from the occiput to the pelvis in asymptomatic adults: a review and resynthesis of the literature. J Neurosurg Spine. 2007;6:104–112. doi: 10.3171/spi.2007.6.2.104. [DOI] [PubMed] [Google Scholar]
- 64.Kwon BK, fisher CG, Boyd MC, Cobb J, Jebson VN, Wing P, Dvorak MF. A prospective randomized controlled trial of anterior compared with posterior stabilization for unilateral facet injuries of the cervical spine. J Neurosurg Spine. 2007;7:1–12. doi: 10.3171/SPI-07/07/001. [DOI] [PubMed] [Google Scholar]
- 65.Landis JR, Koch GG. The measurements of observer agreement for categorial data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 66.Lee MJ, Bazaz R, Furey CG, Yoo J. Risk factors for dysphagia after anterior cervical spine surgery: a two-year prospective cohort study. Spine J. 2007;7:141–147. doi: 10.1016/j.spinee.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 67.Lemons VR, Wagner FC., Jr Stabilization of subaxial cervical spine injuries. Surg Neurol. 1993;39:511–518. doi: 10.1016/0090-3019(93)90038-3. [DOI] [PubMed] [Google Scholar]
- 68.Lifeso RM, Colucci MA. Anterior fusion for rotationally unstable cervical spine fractures. Spine. 2000;25:2028–2034. doi: 10.1097/00007632-200008150-00005. [DOI] [PubMed] [Google Scholar]
- 69.Lowery GL, McDonoug RF. The significance of hardware failure in anterior cervical plate fixation. Patients with 2- to 7-year follow-up. Spine. 1998;23:181–186. doi: 10.1097/00007632-199801150-00006. [DOI] [PubMed] [Google Scholar]
- 70.Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S (1994) A comprehensive classification of thoracic and lumbar injuries. Eur Spine J 3:184–201. doi:10.1007/BF02221591 [DOI] [PubMed]
- 71.Moore TA, Vaccaro A, Anderson PA. Classification of lower cervical spine injuries. Spine. 2006;31:S37–S43. doi: 10.1097/01.brs.0000217942.93428.f7. [DOI] [PubMed] [Google Scholar]
- 72.Nojiri K, Matsumoto M, Chiba K, Maruiwa H, Nakamura M, Nishizawa T, Toyama Y. Relationship between alignment of upper and lower cervical spine in asymptomatic individuals. J Neurosurg (Spine 1) 2003;99:80–81. doi: 10.3171/spi.2003.99.1.0080. [DOI] [PubMed] [Google Scholar]
- 73.Ohara A, Miyamoto K, Naganawa T, Matsumoto K, Shimizu K. Reliabilities of an correlations among five standard methods of assessing the sagittal alignment of the cervical spine. Spine. 2006;31:2585–2591. doi: 10.1097/01.brs.0000240656.79060.18. [DOI] [PubMed] [Google Scholar]
- 74.Park JB, Watthanaaphisit T, Riew KD (2007) Timing of development of adjacent-level ossification after anterior cervical arthrodesis with plates. Spine J 7:633–636 [DOI] [PubMed]
- 75.Pateder DB, Carbone JJ. Lateral mass screw fixation for cervical spine trauma: associated complications and efficacy in maintaining alignment. Spine J. 2006;6:40–43. doi: 10.1016/j.spinee.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 76.Rabin D, Picket GE, Bisnaire L, Duggal N (2007) The kinematics of anterior cervical discectomy and fusion versus artificial cervical disc: a pilot study. Neurosurgery 61:100–104 (comment: p105, Benzel EC) [DOI] [PubMed]
- 77.Reindl R, Quellet J, Harvey EJ, Berry G, Arlet V. Anterior reduction for cervical spine dislocation. Spine. 2006;31:648–652. doi: 10.1097/01.brs.0000202811.03476.a0. [DOI] [PubMed] [Google Scholar]
- 78.Reinhold M, Blauth M, Rosiek R, Knop C. Reduction of traumatic dislocations and facet fractures. Unfallch. 2006;109:1064–1072. doi: 10.1007/s00113-006-1188-0. [DOI] [PubMed] [Google Scholar]
- 79.Reitman CA, Hipp JA, Nguyen L, Esses SI. Changes in segmental intervertebral motion adjacent to cervical arthrodesis: a prospective study. Spine. 2004;29:E221–E226. doi: 10.1097/00007632-200406010-00022. [DOI] [PubMed] [Google Scholar]
- 80.Reitman CA, Mauro KM, Nguyen L, Ziegler JM, Hipp JA. Intervertebral motion between flexion and extension in asymptomatic individuals. Spine. 2004;29:2832–2843. doi: 10.1097/01.brs.0000147740.69525.58. [DOI] [PubMed] [Google Scholar]
- 81.Resnick DK, Trost GR. Use of ventral plates for cervical arthrodesis. Neurosurgery. 2007;60(suppl 1):S1–S112. doi: 10.1227/01.NEU.0000215350.95209.CA. [DOI] [PubMed] [Google Scholar]
- 82.Ripa DR, Kowall MG, Meyer PR, Rusin J. Series of ninety-two traumatic cervical spine injuries stabilized with anterior ASIF plate fusion technique. Spine. 1990;16(suppl 3):S46–S55. doi: 10.1097/00007632-199103001-00009. [DOI] [PubMed] [Google Scholar]
- 83.Roy-Camille R, Saillant G, Laville C, Benazet JP. Treatment of lower cervical spinal injuries. Spine. 1992;17:S442–S446. doi: 10.1097/00007632-199210001-00017. [DOI] [PubMed] [Google Scholar]
- 84.Shamsaldin M, Mouchaty H, Desogus N, Costagliola C, Di Lorenzo N. Evaluation of donor site pain after anterior iliac crest harvesting for cervical fusion: a prospective study on 50 patients. Acta Neurochir (Wien) 2006;148:1071–1074. doi: 10.1007/s00701-006-0864-8. [DOI] [PubMed] [Google Scholar]
- 85.Shoung HM, Lee LS. Anterior metal plate fixation in the treatment of unstable lower cervical spine injuries. Acta Neurochir (Wien) 1989;98:55–59. doi: 10.1007/BF01407177. [DOI] [PubMed] [Google Scholar]
- 86.Silber JS, Anderson DG, Daffner SD, Brislin BT, Leland JM, Hilibrand AS, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28:134–139. doi: 10.1097/00007632-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 87.Spivak JM, Chen D, Kummer FJ. The effect of locking fixation screws on the stability of anterior cervical plating. Spine. 1999;24:334–338. doi: 10.1097/00007632-199902150-00005. [DOI] [PubMed] [Google Scholar]
- 88.Steinmetz MP, Kager CD, Benzel EC. Ventral correction of postsurgical cervical kyphosis. J Neurosurg (Spine 1) 2002;97:1–7. doi: 10.3171/spi.2003.98.1.0001. [DOI] [PubMed] [Google Scholar]
- 89.Stulik J, Krbec M, Vyskocil T (2003) Injuries of the lower cervical vertebrae—the monocortical stabilization technique. Acta Chir Orthop Traumatol Cech 70:226–232 [PubMed]
- 90.Tuli SK, Chen P, Eichler M, Woodard EJ. Reliability of radiologic assessment of fusion: cervical fibular allograft model. Spine. 2004;29:856–860. doi: 10.1097/00007632-200404150-00007. [DOI] [PubMed] [Google Scholar]
- 91.Vavruch L, Hedlund R, Javid D, Leszniewski W, Shalabi A. A prospective randomized comparison between the cloward procedure and a carbon fiber cage in the cervical spine. A clinical and radiologic study. Spine. 2002;27:1694–1701. doi: 10.1097/00007632-200208150-00003. [DOI] [PubMed] [Google Scholar]
- 92.Vecsei V, Fuchs M, Gäbler CH. Indications for treatment of severe unstable injuries of the cervical spine by combined anterior and posterior internal fixation. Osteos Intern. 1998;6:121–128. [Google Scholar]
- 93.Venon H, Mior S. The neck disability index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14:409–419. [PubMed] [Google Scholar]
- 94.Vernon H, Mior S. The neck disability index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14:409–419. [PubMed] [Google Scholar]
- 95.Vesel M, Straus I, Mawed Al, Dobravec M, Jug M. Angular stable plates in lower cervical spine fractures. Osteo Trauma Care. 2006;14:22–27. doi: 10.1055/s-2006-921368. [DOI] [Google Scholar]
- 96.Weinhoffer SL, Guyer RD, Herbert M, Griffith SL. Intradiscal pressure measurements above instrumented fusion. A cadaveric study. Spine. 1995;20:526–531. doi: 10.1097/00007632-199503010-00004. [DOI] [PubMed] [Google Scholar]
- 97.White AP, Biswas D, Smart LR, Haims A, Grauer JN. Utility of flexion–extension radiographs in evaluating the degenerative cervical spine. Spine. 2007;32:975–979. doi: 10.1097/01.brs.0000261409.45251.a2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Characteristics of patients with ACDFP for subaxial injuries. Current results and comparison to literature* (DOC 103 kb)