Abstract
In this retrospective cohort study, two surgical methods of conventional open-door laminoplasty and deep extensor muscle-preserving laminoplasty were allocated for the treatment of cervical myelopathy, and were specifically compared in terms of axial pain, cervical spine function, and quality of life (QOL) with a minimum follow-up period of 2 years. Eighty-four patients were divided into two groups and received either a conventional open-door laminoplasty (CL group) or laminoplasty using a deep extensor muscle-preserving approach (MP group). The latter approach was performed by preserving multifidus and semispinalis cervicis attachments followed by open-door laminoplasty and re-suture of the bisected spinous processes at each decompression level. The average follow-up period was 38 months (25–53 months). The preoperative and follow-up evaluations included the original Japanese Orthopaedic Association (JOA) score, the new tentative JOA score including cervical spine function and QOL, and the visual analogue scale (VAS) of axial pain. Radiological analyses included cervical lordosis and flexion–extension range of motion (flex–ext ROM) (C2–7), and deep extensor muscle areas on MR axial images. The JOA recovery rates were statistically equivalent between two groups. The MP group demonstrated a statistically superior cervical spine function (84% vs 63%) and QOL (61% vs 45%) when compared to the CL group at final follow-up (P < 0.05). The average VAS scores at final follow-up were 2.3 and 4.9 in MP and CL groups (P < 0.05). The cervical lordosis and flex–ext ROM were statistically equivalent. The percent deep muscle area on MRI demonstrated a significant atrophy in CL group compared to that in MP group (56% vs 88%; P < 0.01). Laminoplasty employing the deep extensor muscle-preserving approach appeared to be effective in reducing the axial pain and deep muscle atrophy as well as improving cervical spine function and QOL when compared to conventional open-door laminoplasty.
Keywords: Laminoplasty, Cervical myelopathy, Muscle preservation
Introduction
Cervical laminoplasty has become a common surgical treatment for cervical myelopathy, providing significant neurologic improvement [3, 6, 9–11]. According to a representative meta-analysis by Ratliff and Cooper comprising 71 clinical series from a total of 2,580 patients, an overall neurologic recovery rate of 55% in average (20–81%) was reported and several different surgical laminoplasty techniques demonstrated statistically equivalent results [10]. A decrease in cervical range of motion (ROM) was also reported with an average decrease in range of 50% (17–80%) and there were no significant differences between different laminoplasty surgical techniques [10].
Persistent neck and shoulder girdle pain is a notable postoperative complication of posterior cervical spine surgery [5, 8, 10, 19]. The overall incidence of this axial pain varied markedly and ranged from 6 to 60% [5, 8, 10, 19]. Wada et al. reported the long-term comparative outcome of anterior corpectomy and fusion vs conventional hemi-open laminoplasty, demonstrating a 40% incidence of axial pain in laminoplasty over a 10-year follow-up [19]. Hosono et al. reported a higher incidence of 60% in 72 patients who were treated using a modified hemi-open type laminoplasty for cervical spondylotic myelopathy (CSM) [5]. Several authors have clinically evaluated the incidence of axial pain, radiologic alignment, ROM, and neurologic outcome in laminoplasty patients [1, 5, 8, 16, 17, 19–21], however, the relationship between axial pain and other parameters is still unclear. Importantly, there has not been any quantitative investigation into whether axial pain influences actual cervical spine function during daily living and quality of life (QOL) with patient-based subjective outcome measures.
The new laminoplasty concept of maintaining deep extensor muscle was developed and clinically applied by Shiraishi in 2002 [14]. This surgical technique included the preservation of multifidus and semispinalis muscular attachments to spinous processes using an interlaminar approach with specially designed self-retaining retractors [14]. The expanded use of this technique includes skip laminectomy and spinal canal enlargement followed by re-suturing the bisected spinous processes with muscular attachment [12, 13, 15]. In comparison between skip laminectomy and conventional open-door laminoplasty, Shiraishi et al. reported a reduction in axial pain, and the preservation of lordosis and deep extensor muscle [12]. However, even with the increased popularity of this technique, the clinical data regarding axial pain reduction, cervical mobility, curvature preservation, cervical spine function, and QOL have been extremely scarce.
The objective of this study was to compare two surgical groups; firstly, conventional open-door laminoplasty and subsequently deep extensor muscle-preserving laminoplasty approaches to treat cervical myelopathy, specifically assessing axial pain, cervical spine function, and QOL with minimum follow-up period of 2 years.
Materials and methods
Patient demographics
Between August 2000 and December 2005, 84 patients received a cervical laminoplasty either with conventional open-door laminoplasty (CL group; 42 points) or laminoplasty using a deep extensor muscle-preserving approach (MP group; 42 points). The latter method was performed according to Shiraishi preserving multifidus and semispinalis cervicis muscles described later in the surgical procedure section [14]. Equal numbers of patients were allocated into two surgical groups by operation date: 2000–2002 for CL group and 2003–2005 for MP group. The average follow-up periods were 43 (25–53) and 32 (26–41) months in the retrospective CL and MP groups. There were 64 males and 20 females, and the average age at surgery was 62 years old (34–87 years). The preoperative diagnoses were CSM in 51 points, and ossification of posterior longitudinal ligament (C-OPLL) in 33 points. There were no patients with apparent segmental instability and kyphosis. Most cases were accompanied with the developmental narrow spinal canal, and the decompressive laminae were distributed from C2 to T1. The number of decompressed lamina was 5.4 ± 0.8 in CL group, and 3.7 ± 1 in MP group. The number of patients in which C7 was included in the decompression area was 28 in CL group and 10 in MP group. Typical decompression patterns were C3 to C6 or C7 in the CL group and C4–6 in MP group.
Surgical procedures
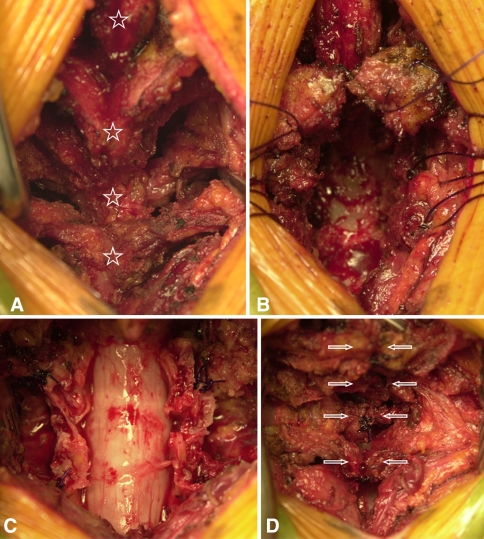
The surgical method of conventional open-door laminoplasty performed in CL group was described below. The lamina was cut on the midline using a surgical burr, and the lateral gutters were created bilaterally at the medial border of the facet joint. Then, the right and left halves of lamina were lifted and bilaterally opened leaving the sutures joining the ligamentum flavum to lateral muscles. No bone graft was performed at the lateral gutters. The laminoplasty in MP group utilized a deep extensor muscle-preserving approach in addition to previously described open-door laminoplasty. After incision of the nuchal ligament to expose the spinous processes along the midline, consecutive laminae were exposed while preserving the multifidus and semispinalis muscles (Fig. 1a) [14]. The spinous processes were vertically divided using a surgical burr or osteotome with the preservation of extensor muscle attachments, and were transversely osteotomized at the base of spinous processes. The stay sutures were placed through the bone and extensor muscles were laterally retracted (Fig. 1b). The open-door laminoplasty was performed using the method described in CL group (Fig. 1c). Finally, extensor muscles reconstruction was performed with tight suturing of the bisected spinous processes (Fig. 1d). In both CL and MP groups, the self-retaining retractors were used to an equal degree during the decompression procedure. The average operation time was about 75 min in the CL group with the additional muscle-preserving procedure taking extra 5–10 min. Postoperatively, the patients did not apply the collar and mobilized the neck freely. The isometric cervical muscle exercise was started on day 3 after surgery.
Fig. 1.
a–d Surgical procedure of deep extensor muscle-preserving laminoplasty. (Bottom of figure signifies the cephalad direction of the patient). a Exposure of the spinous processes in the midline, the consecutive laminae were exposed while preserving the multifidus and semispinalis cervicis muscle. The each star signifies a spinous process. b The spinous processes were vertically bisected using a surgical burr or osteotome with the preservation of extensor muscle attachments. The stay sutures were placed through the bone for the later closure. c Open-door laminoplasty was followed by the ligamentum flavum suture to the lateral paraverbral muscles. d Reconstruction of extensor muscles with tight sutures to the bisected spinous processes (arrows)
Quantitative analyses of clinical outcome
The preoperative and final follow-up clinical outcome was evaluated by the Japanese Orthopaedic Association score (JOA score) [7], and the recovery rate was calculated using Hirabayashi’s method [4]. The degree of axial neck pain was evaluated with a visual analogue scale (VAS; 0–10) at preoperative, 3, 6, 12, 24 months and final follow-up point. The overall mean preoperative VAS score was 3.5 ± 3.2 and there was no significant difference between two groups (P > 0.05). The additional VAS analysis was performed with or without the inclusion of C7 in the decompression range. The cervical spine and spinal cord function, the QOL were evaluated with the new tentative JOA score consisting of a total of 24 distinct items [2]. This consisted of self response-questionnaires and the quantification expressed as the percent of the total score (Tables 1, 2). Radiographically, cervical lordosis and flexion–extension ROM (flex–ext ROM) was measured from C2 to C7 preoperatively and at final follow-up. Atrophy of the deep extensor muscles was quantified using MR T1 axial images. At C4/5 and C5/6 levels, the muscle area of multifidus, semispinalis, and longissimus were measured as the group using Scion image (Scion Corp., MD, USA) with two image slices at each level. The average muscle group area at follow-up was divided by the preoperative value and was expressed as the percent to preoperative value.
Table 1.
The cervical spine and spinal cord function questionnaire of new tentative JOA score (JOACMEQ) [2]
| 1. Can you fasten the front buttons of your blouse or shirt using both hands? (1) I can do it without difficulty (2) I can do it if I spend time (3) I cannot do it (0) I am not sure |
8. Do you have a feeling of residual urine even after voiding of urine (urination)? (1) I rarely have such a feeling (2) I sometimes have such a feeling, and sometimes not (3) I usually have such a feeling (0) I am not sure |
| 2. Can you eat a meal using a spoon or a fork with your right hand? (1) I can do it without difficulty (2) I can do it if I spend time (3) I cannot do it (0) I am not sure |
9. Can you void urine immediately in the toilet? (1) I almost always can do it immediately (2) I sometimes can do it immediately, and sometimes not (3) I usually cannot do it immediately (0) I am not sure |
| 3. Can you raise your right arm? (1) I can raise it straight upward (2) I can raise it upward when flexed a little (3) I can raise it halfway (up to shoulder level) (4) I cannot raise it |
10. While in the sitting position, can you look up at the ceiling by drawing your head directly backward? (1) I can do it without difficulty. (2) I can do it with some effort (3) I cannot do it |
| 4. Can you walk on a flat surface? (1) I can do it without difficulty (2) I can do it slowly (3) I can do it with support (of a handrail, a stick, or a walker) (4) I can do it only slowly even with support (5) I cannot do it (0) I am not sure |
11. Can you drink a glass of water in one gulp? (1) I can do it without difficulty (2) I can do it with some effort (3) I cannot do it (0) I am not sure |
| 5. Can you stand on your right leg without the support of your hand? (1) I can do it for more than 10 s (2) I can do it for less than 10 s (3) I can hardly do it (0) I am not sure |
12. Can you see your feet when you walk down the stairs? (1) I can do it without difficulty (2) I can do it with some effort (3) I cannot do it (0) I am not sure |
| 6. Do you have urinary incontinence? (1) No (2) I have it when I sneeze or strain myself (3) I have it when I do not release urine over a period of more than 2 h (4) Frequently (5) Always |
13. While in the sitting position, can you turn your head toward the person who is seated behind you and speak to him/her while looking him/her in the face? (1) I cannot do it (2) I can do it with some effort (3) I can do it without difficulty |
| 7. How often do you go to the bathroom (to void urine) at night? | |
| (1) Hardly ever | |
| (2) Once or twice | |
| (3) Three times or more |
This study utilized the tentative version of JOACMEQ, however, the final version of JOACMEQ was established [2]
Table 2.
Quality of life questionnaire of new tentative JOA score (JOACMEQ) [2]
| 1. What is your present health condition? (1) Excellent. (2) Very good. (3) Good. (4) Not very good. (5) Poor. |
The following are questions about your feelings during the last month (circle the item number of each question that best applies) |
| The following are ordinary daily activities. Please indicate if you have difficulty doing them because of your poor health condition and, if so, how difficult you think it is to do them. Circle the item number that most applies | 7. Were you discouraged and depressed? (1) Always (2) Almost always (3) Sometimes (4) Rarely (5) Not at all |
| 2. Climbing the stairs to one floor above (1) I have great difficulty (2) I have some difficulty (3) I do not have any difficulty |
8. Were you exhausted? (1) Always (2) Almost always (3) Sometimes (4) Rarely (5) Not at all |
| 3. Bending forward, kneeling, and stooping (1) I have great difficulty (2) I have some difficulty (3) I do not have any difficulty |
9. Did you feel pleasant? (1) Always (2) Almost always (3) Sometimes (4) Rarely (5) Not at all |
| 4. Walking a kilometer (1) I have great difficulty (2) I have some difficulty (3) I do not have any difficulty |
Circle the item number of each of the following topics that best applies to your condition |
| When you engaged in your work or daily activities (including housework) during the last month, did you have any of the problems listed below because of your physical condition? (Circle the item number in each topic that best applies.) | 10. I am in decent health (1) Completely yes (2) Almost yes (3) I am not sure (4) I hardly think so (5) I do not think so |
| 5. I could not do my work or daily activities as well as I expected (1) Always (2) Almost always (3) Sometimes (4) Rarely (5) I was able to do my work or daily activities as well as I expected |
11. I feel my health will get worse (1) Completely yes (2) Almost yes (3) I am not sure (4) I hardly think so (5) I do not think so |
| 6. How severely was your work (including housework) hindered during the last month because of the pain? (1) Not at all (2) A little (3) Slightly (4) Fairly (5) Greatly |
This study utilized the tentative version of JOACMEQ, however, the final version of JOACMEQ was established [2]
Statistical analysis
Each parameter was compared using an unpaired Student’s t-test between preoperative and final follow-up data. The time-related change in cervical spine and spinal cord function, QOL, and VAS in the MP group was compared postoperatively over 6 months and at the final follow-up point.
Results
The postoperative follow-up periods were 32 (26–41) and 43 (25–53) months in the MP and CL groups, respectively. The preoperative and final follow-up JOA score in the MP group was 9.1 ± 3.5 and 14.6 ± 1.6 (average ± STD), respectively. The preoperative and final follow-up JOA score in the CL group was 8.6 ± 2.9 and 13.8 ± 2.4 (average ± STD), respectively. The recovery rate of JOA score was 69 ± 15% and 65 ± 20% in MP and CL groups respectively, which was statistically insignificant (P > 0.05).
The cervical spine and spinal cord function were evaluated across 13 distinct categories using the new tentative JOA scores, demonstrating respective values of 84.7 ± 13.9% and 72.5 ± 18.1% in MP and CL groups at final follow-up. This was statistically higher in the MP group (P < 0.05). The cervical spine function comprising four items included on the respective new tentative JOA score was 83.5 ± 19.0% and 62.5 ± 22.8% in the MP and CL groups, that was statistically higher in MP group (P < 0.05). The QOL scores comprising 11 items in the new tentative JOA score were 61.0 ± 20.5% and 44.9 ± 17.5% in MP and CL group at final follow-up, respectively. The MP group QOL was statistically higher than that of the CL group at the significance level of P = 0.05. The average VAS scores of axial neck pain were 2.3 ± 2.3 and 4.9 ± 2.6 in the MP and CL group at final follow-up, respectively. The axial pain was statistically greater in the CL group at the significant level of P = 0.05. Regarding the inclusion of C7 in the decompression range, the VAS scores at final follow-up were 2.0 ± 2.1 and 2.7 ± 2.3 with or without C7 in MP group, respectively. There was no significant difference between these two sets of values. Similarly, the VAS scores in the MP group were 4.7 ± 2.6 and 5.1 ± 2.8 with or without C7, respectively, which was not significantly different. There were no complications and re-operations required in either MP and CL group.
Radiographically, the cervical lordosis measured from C2 to C7 on lateral radiographs was 13 ± 9° and 16 ± 9° in the MP and CL groups at final follow-up, respectively, which was statistically insignificant (P > 0.05). The comparison of lordosis angles between preoperative and final follow-up was not significant in both the MP and CL groups. The flex–ext ROM, which was normalized to the preoperative level (% to preop), was 98 ± 42% and 77 ± 40% at final follow-up, which was statistically insignificant (P > 0.05).
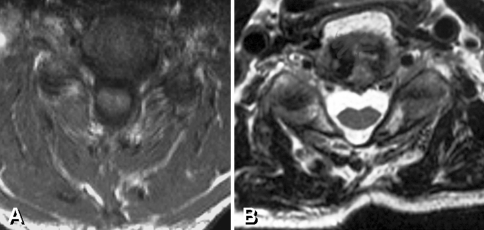
The normalized deep extensor muscle area (%follow-up/preop) was 88 ± 15% and 56 ± 20% in the MP and CL groups at final follow-up, respectively, which was statistically significant at P = 0.01 level. This meant that the MP group showed less extensor muscle atrophy than that in CL group (Fig. 2).
Fig. 2.
a, b Representative axial MR images of deep extensor muscle after muscle-preserving laminoplasty and conventional open-door laminoplasty. a Deep extensor muscles are preserved in muscle-preserving laminoplasty. b Significant deep extensor muscle atrophy was demonstrated in conventional laminoplasty with a flat configuration of neck surface
The time-related change of parameters in the MP group is shown in Table 3. The spinal cord and cervical spine function was 87.0 ± 10.4% and 84.7 ± 13.9% at 6 months postoperative and final follow-up (over 2 years), respectively. There was no significant difference between these two time points. The cervical spine function was 87.5 ± 13.6% and 83.5 ± 19.0% at 6 months postoperative and final follow-up (over 2 years), respectively, which were not significantly different between these two time points. The QOL scores were 62.2 ± 12.5% and 61.0 ± 20.5% at postoperative 6 months and final follow-up (over 2 years), respectively, which were not significantly different between these two time points. The VAS scores were 2.3 ± 2.0 and 2.4 ± 2.3 at postoperative 6 months and final follow-up (over 2 years) respectively and were not significantly different between these two time points. Overall, the time-related changes were not demonstrated in any clinical parameters.
Table 3.
Time-related change of clinical parameters in MP group
| Postoperative, 6 months | Final follow-up, >2 years | Statistical value | |
|---|---|---|---|
| Cord and C-function (%) | 87.0 ± 10.4 | 84.7 ± 13.9 | NS |
| C-function (%) | 87.5 ± 13.6 | 83.5 ± 19.0 | NS |
| QOL (%) | 62.2 ± 12.5 | 61.0 ± 20.5 | NS |
| VAS | 2.3 ± 2.0 | 2.4 ± 2.4 | NS |
Cord and C-function spinal cord and cervical spine function, C-function cervical spine function, QOL quality of life, NS no significant difference
Representative case presentations
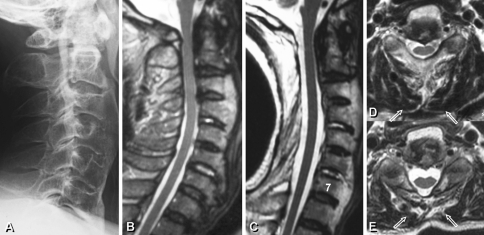
Case 1
A 67-year-old male presented with severe cervical myelopathy due to ossification of the posterior longitudinal ligament (Fig. 3). Conventional laminoplasty from C1 to C7 was performed. Four years and 2 months postoperatively, the cervical myelopathy had improved with the JOA score recovery of 7.5–13 points. However, the VAS score of axial neck pain significantly increased from 0 to 5 at final follow-up. The significant deep extensor muscle atrophy was demonstrated on an MRI with 40% reduction in muscle area at final follow-up.
Fig. 3.
a–e A cervical OPLL patient received conventional open-door laminoplasty from C1 to C7. Note significant deep extensor muscle atrophy (box arrows): preoperative and follow-up comparison (d and e, respectively)
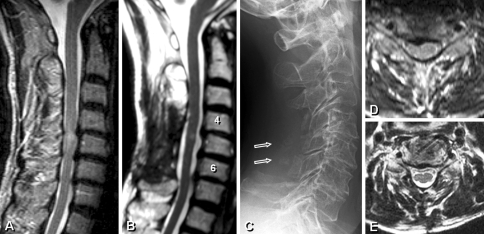
Case 2
A 72-year-old male with CSM received the muscle-preserving laminoplasty from C4 to C6 (Fig. 4). After 3 years postoperatively, the JOA score improved from 8.5 to 14.5 and the VAS score of axial neck pain decreased from 6 to 0. An axial MR scan demonstrated the preservation of deep muscle volume of approximately 91%.
Fig. 4.
a–e Cervical spondylotic myelopathy patient received the deep extensor muscle-preserving laminoplasty from C4 to C6. Box arrows signify the reattached spinous processes with deep extensor muscles. Note reductions in deep extensor muscle at follow-up (e) compared to preoperative image (d)
Discussion
The present study demonstrated that laminoplasty utilizing the deep extensor muscle-preserving approach provided significant axial pain reduction, better cervical spine function, QOL, and prevention of deep muscle atrophy, when compared to conventional open-door laminoplasty with minimum follow-up period of 2 years. This surgical approach was originally developed by Shiraishi [14], and we modified this technique for conventional open-door laminoplasty.
Axial pain has been a topic of contention in terms of its definition, anatomical origin, and whether it influences patients’ cervical spine function, active daily living, and the long-term QOL. In this study, axial pain was defined as the neck and/or shoulder girdle pain, which worsened after a long-period of sitting or standing. Different anatomical origins of such pain arose from: trapezius muscle, nuchal ligament, especially its attachment to C7, spinous processes of the lower cervical vertebrae, and deep extensor muscles, etc. Hosono et al. compared C3–7 and C3–6 laminoplasty using a hemi-open approach, demonstrating significant decrease in axial pain when excluding C7 from the decompressive area [5]. Yukawa et al. prospectively compared open-door laminoplasty (C3–6) with skip laminectomy of C4 and C6, demonstrating no significant difference in axial pain between two groups [21]. In turn, Takeuchi et al. emphasized the preservation of semispinalis cervicis attached to C2 spinous processes when comparing conventional C3–7 laminoplasty with C4–7 laminoplasty and C3 laminectomy [17]. In the results of this study, the latter group demonstrated significantly increased axial pain-free patients [17]. The present study did not control the decompression range with an average reduction of 1.7 laminae in the MP group; therefore, the clinical outcome was influenced by both the decrease in surgical invasiveness and muscle preservation. However, our analysis regarding with or without the inclusion of C7 in the decompression range demonstrated no statistical differences in terms of VAS of axial pain both in CL and MP groups. A quarter of MP group patients had no axial pain at all, whereas the majority of patients in the CL group noted some axial pain.
Cervical spine function and QOL have not been evaluated from the patients’ standpoint in several reports regarding recent laminoplasty techniques. The questionnaire utilized in this study was the tentative version of the new JOA score including daily activity questions regarding cervical motion and spinal cord function, and some parts of SF-36 [2]. Although many reports have only utilized VAS for pain evaluation, the reliability of VAS is often questionable, and the individual variation is large. The tentative JOA score utilized in this study demonstrated highly significant differences between the two groups especially in cervical spine function. Several QOL questionnaires such as SF-36 are influenced by patients’ socioeconomic situation during the long-term evaluation; therefore, the interpretation of the data requires the great care.
In terms of cervical flex–ext ROM, there was the relatively larger ROM in the MP group, which was 98% compared to the preoperative value when compared to 77% in the CL group. Meta-analysis by Ratliff and Cooper demonstrated that the mean decrease in ROM after conventional laminoplasty was 50% (17–80%) [10], while recent reports of conventional hemi-open laminoplasty showed a 30–36% decrease in ROM [1, 16]. Using the preservation of semispinalis cervicis muscle attached to C2, Takeuchi et al. demonstrated a 19% ROM reduction when compared to 47% when the muscle attachment was sacrificed [18]. In turn, Yukawa et al. utilized the muscle-preserving C4 and C6 skip laminectomy, resulting in no significant ROM difference compared to C3–6 open-door laminoplasty [21]. Although CSM and OPLL are not comparable even in historical reports, our MP group may suggest a positive effect in preserving cervical mobility.
In our present study, the preservation of deep extensor muscle area was clearly demonstrated in the MP group. Clinically, this was also apparent in the appearance of the neck in patients after years. After conventional laminoplasty, the posterior neck configuration tends to be flat; however, the soft round shape of the neck is often preserved after muscle-preserving laminoplasty. Shiraishi et al. reported a 13% atrophy in the deep extensor muscle after C4 and C6 skip laminectomy vs 60% atrophy in conventional open-door laminoplasty [12]. In this surgical procedure, bisected spinous processes with extensor muscles were re-sutured at C4 and C6. Our data demonstrate a 12% rate of atrophy in our muscle-preserving laminoplasty, which is consistent with Shiraishi’s report.
Controversy lies in the long-term effects of a decreased range of decompression on the neurological outcome and the clinical complication rate. In the surgical decision of decompression range, we rigorously distinguish CSM and cervical OPLL. In OPLL patients, a preventive wider range of decompression was considered, when compared to CSM.
Even under this limitation in the combined background of both CSM and OPLL patients, no deterioration in spinal cord function or re-operation due to neurological problems were demonstrated during the average 32-month follow-up periods. However, further longer follow-up is necessary together with particular evaluation of CSM patients for the future study.
Conclusion
The two surgical cohorts of conventional open-door laminoplasty and laminoplasty with deep extensor muscle preservation were compared with minimum follow-up period of 2 years. Laminoplasty employing the deep extensor muscle-preserving approach appears effective in reducing axial pain and deep muscle atrophy as well as improving cervical spine function and the QOL when compared to conventional open-door laminoplasty.
References
- 1.Chiba K, Ogawa Y, Ishii K. Long-term results of expansive open-door laminoplasty for cervical myelopathy—average 14-year follow-up study. Spine. 2006;31:2998–3005. doi: 10.1097/01.brs.0000250307.78987.6b. [DOI] [PubMed] [Google Scholar]
- 2.Fukui M, Chiba K, Kawakami M. An outcome measure for patients with cervical myelopathy: Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire (JOACMEQ): Part 1. J Orthop Sci. 2007;12:227–240. doi: 10.1007/s00776-007-1118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirabayashi K, Tomita Y, Chiba K. Expansive laminoplasty for myelopathy in ossification of the longitudinal ligament. Clin Orthop Relat Res. 1999;359:35–48. doi: 10.1097/00003086-199902000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Hirabayashi K, Toyama Y. Choice of surgical procedure for cervical ossification of the posterior longitudinal ligaments. In: Yonenobu K, Sakou T, Ono K, editors. ossification of the posterior longitudinal ligament. Tokyo: Springer; 1997. pp. 135–142. [Google Scholar]
- 5.Hosono N, Sakaura H, Mukai Y. The source of axial pain after cervical laminoplasty—C7 is more crucial than deep extensor muscles. Spine. 2007;32:2985–2988. doi: 10.1097/BRS.0b013e31815cda83. [DOI] [PubMed] [Google Scholar]
- 6.Itoh T, Tsuji H. Technical improvements and results of laminoplasty for compressive myelopathy in the cervical spine. Spine. 1985;10:729–736. doi: 10.1097/00007632-198510000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Japanese Orthopaedic Association Scoring system for cervical myelopathy. Nippon Seikeigeka Gakkai Zasshi. 1994;68:490–503. [Google Scholar]
- 8.Kawaguchi Y, Kanamori M, Ishihara H. Minimum 10-year followup after en bloc cervical laminoplasty. Clin Orthop Relat Res. 2003;411:129–139. doi: 10.1097/01.blo.0000069889.31220.62. [DOI] [PubMed] [Google Scholar]
- 9.Kawai S, Sunago K, Doi K. Cervical laminoplasty (Hatori’s method). Procedure and follow-up results. Spine. 1988;13:1245–1250. doi: 10.1097/00007632-198811000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Ratliff JK, Cooper PR. Cervical laminoplasty: a critical review. J Neurosurg Spine. 2003;98(Suppl 3):230–238. doi: 10.3171/spi.2003.98.3.0230. [DOI] [PubMed] [Google Scholar]
- 11.Seichi A, Takeshita K, Ohnishi I. Long-term results of double-door laminoplasty for cervical stenotic myelopathy. Spine. 2001;26:479–487. doi: 10.1097/00007632-200103010-00010. [DOI] [PubMed] [Google Scholar]
- 12.Shiraishi T, Fukuda K, Yato Y. Results of skip laminectomy—minimum 2-year follow-up study compared with open-door laminoplasty. Spine. 2003;28:2667–2672. doi: 10.1097/01.BRS.0000103340.78418.B2. [DOI] [PubMed] [Google Scholar]
- 13.Shiraishi T, Yato Y. New double-door laminoplasty procedure for the axis to preserve all muscular attachments to the spinous process. Technical note. Neurosurg Focus. 2002;12:E9. doi: 10.3171/foc.2002.12.1.10. [DOI] [PubMed] [Google Scholar]
- 14.Shiraishi T. A new technique for exposure of the cervical spine laminae. Technical note. J Neurosurg (Spine 1) 2002;96:122–126. doi: 10.3171/spi.2002.96.1.0122. [DOI] [PubMed] [Google Scholar]
- 15.Shiraishi T. Skip laminectomy—a new treatment for cervical spondylotic myelopathy, preserving bilateral muscular attachments to the spinous processes: a preliminary report. Spine J. 2002;2:108–115. doi: 10.1016/S1529-9430(01)00118-8. [DOI] [PubMed] [Google Scholar]
- 16.Suk KS, Kim TK, Lee JH. Sagittal alignment of the cervical spine after the laminoplasty. Spine. 2007;32:E656–E660. doi: 10.1097/BRS.0b013e318158c573. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi K, Yokoyama T, Aburakawa S. Axial symptoms after cervical laminoplasty with C3 laminectomy compared with conventional C3–7 laminoplasty. A modified laminoplasty preserving the semispinalis cervicis inserted into axis. Spine. 2005;30:2544–2549. doi: 10.1097/01.brs.0000186332.66490.ba. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi K, Yokoyama T, Ono A. Cervical range of motion and alignment after laminoplasty preserving or reattaching the semispinalis cervicis inserted into axis. J Spinal Disord Tech. 2007;20:571–576. doi: 10.1097/BSD.0b013e318046363a. [DOI] [PubMed] [Google Scholar]
- 19.Wada E, Suzuki S, Kanazawa A. Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy. A long-term follow-up study over 10 years. Spine. 2001;26:1443–1448. doi: 10.1097/00007632-200107010-00011. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida M, Tamaki T, Kawakami M. Does reconstruction of posterior ligamentous complex with extensor musculature decrease axial symptoms after cervical laminoplasty? Spine. 2002;27:1414–1418. doi: 10.1097/00007632-200207010-00008. [DOI] [PubMed] [Google Scholar]
- 21.Yukawa Y, Kato F, Ito K. Laminoplasty and skip laminectomy for cervical compressive myelopathy. Range of motion, postoperative neck pain, and surgical outcomes in a randomized prospective study. Spine. 2007;32:1980–1985. doi: 10.1097/BRS.0b013e318133fbce. [DOI] [PubMed] [Google Scholar]