Abstract
Interspinous devices have been introduced to provide a minimally invasive surgical alternative for patients with lumbar spinal stenosis or foraminal stenosis. Little is known however, of the effect of interspinous devices on intersegmental range of motion (ROM). The aim of this in vivo study was to investigate the effect of a novel minimally invasive interspinous implant, InSwing®, on sagittal plane ROM of the lumbar spine using an ovine model. Ten adolescent Merino lambs underwent a destabilization procedure at the L1–L2 level simulating a stenotic degenerative spondylolisthesis (as described in our earlier work; Spine 15:571–576, 1990). All animals were placed in a side-lying posture and lateral radiographs were taken in full flexion and extension of the trunk in a standardized manner. Radiographs were repeated following the insertion of an 8-mm InSwing® interspinous device at L1–L2, and again with the implant secured by means of a tension band tightened to 1 N/m around the L1 and L2 spinous processes. ROM was assessed in each of the three conditions and compared using Cobb’s method. A paired t-test compared ROM for each of the experimental conditions (P < 0.05). After instrumentation with the InSwing® interspinous implant, the mean total sagittal ROM (from full extension to full flexion) was reduced by 16% from 6.3° to 5.3 ± 2.7°. The addition of the tension band resulted in a 43% reduction in total sagittal ROM to 3.6 ± 1.9° which approached significance. When looking at flexion only, the addition of the interspinous implant without the tension band did not significantly reduce lumbar flexion, however, a statistically significant 15% reduction in lumbar flexion was observed with the addition of the tension band (P = 0.01). To our knowledge, this is the first in vivo study radiographically showing the advantage of using an interspinous device to stabilize the spine in flexion. These results are important findings particularly for patients with clinical symptoms related to instable degenerative spondylolisthesis.
Keywords: Interspinous implant, Biomechanics, Spondylolisthesis, Lumbar spine, Kinematics
Introduction
Lumbar spinal stenosis (LSS) is characterized by a narrowing of the spinal canal with encroachment of the neural structures from degenerated or hypertrophied osteoligamentous structures. Decreased disc height, bulging of the posterior annulus and buckling of the ligamenta flava are among the most common viscoelastic structures contributing to LSS; while hypertrophic facet joints and laminar thickening are among the major osteogenic contributors to the narrowing of the spinal canal and neuroforamina. It is well established that the diameter of the spinal canal decreases during extension [5] which in turn amplifies stenotic conditions in the presence of degenerative changes [24]. The patterns of sagittal motion are also disturbed during extension in stenotic patients [35].
The incidence and prevalence of LSS is rising with the aging of our populations, representing the most common reason for lumbar spine surgery in persons over 65 years of age [38]. When standardized conservative treatment fails in LSS patients, the standard of care consists of surgical decompression. Of concern in decompressive lumbar spinal surgery is the creation of instability as a consequence of the degenerative nature of LSS [8]. Segmental instability is often considered a cause for low back pain [23] mostly related to degenerative processes [21]. Subsequently, more invasive methods have been developed including rigid stabilization systems with pedicle screw fixation [26]. Some of these involve implants secured to the spine by pedicle screw fixation such as the Graf [9] and Dynesys [33] systems. In spite of encouraging early results of pedicle screw systems for flexible intervertebral stabilization [7, 10], some long-term results were less optimistic [11, 27]. Increased lumbar lordosis, stretching of the Dacron parts, mal-positioning, and/or loosening of pedicle screws have been reported as reasons for failure. Accelerated adjacent segment disc degeneration from abnormal load sharing is also a concern with implantation of rigid systems [19]. As a result, dynamic stabilization systems have been developed to prevent overloading of adjacent spinal segments [28]. It has been proposed that, combined with a tension band, stabilization could also be obtained in flexion, thus avoiding the need for pedicle screw fixation [29]. Little biomechanical data exists to support these notions.
Interspinous implants have been developed to assist in providing dynamic spinal stabilization in order to avoid or supplement LSS decompression. Placing an implant between adjacent spinous processes avoids the capacity decreasing effect of sagittal extension. Interspinous implants are also thought to decrease intra-discal pressure [34], unload the facet joints [39], restore foraminal height [12], and provide improved spinal stability (especially in extension) [13, 36], and offer the advantage of being minimally invasive. Several such implants have been developed, some connecting spinous processes and laminae [18], others placed between two adjacent spinous processes with a spring [17], one with a silicone implant [22], another with a U-shaped device [16], and another called the X-Stop Interspinous Process Distraction System [30, 40]. A different type of implant for non-rigid stabilization of lumbar segments uses polyetheretherketone (PEEK), an interspinous blocker fixed to the spine by two bands looped and tensioned around the adjacent spinous processes, termed the Wallis system [29]. The principle of all these systems consists of inserting the spacer between the spinous processes at the stenotic level in order to increase the intervertebral space, stretch the ligamenta flava and posterior annular fibers, thus enlarging both the central canal and neuroforamina [2, 20]. Little is known however, about how these interspinous implants influence the in vivo range of motion (ROM) of the lumbar spine.
The purpose of this in vivo study was to investigate the effect of a novel less invasive interspinous implant, the InSwing®, on flexion–extension ROM of the lumbar spine in an ovine model with a simulated, induced stenotic degenerative spondylolisthesis. We hypothesized that following insertion of the InSwing® implant and fastening with the tension band, there would be a reduction in both flexion and extension, and an overall concomitant decrease in ROM of the lumbar spine.
Methods
Specimens
Ten adolescent Merino lambs (24–30 kg) were used for the study.
Injury
A destabilization procedure was performed at the level of L1–L2 on both sides, thus simulating an instability resembling stenotic degenerative spondylolisthesis. The surgery consisted of a posterior arthrectomy and partial facetectomy as described previously [1].
Implant
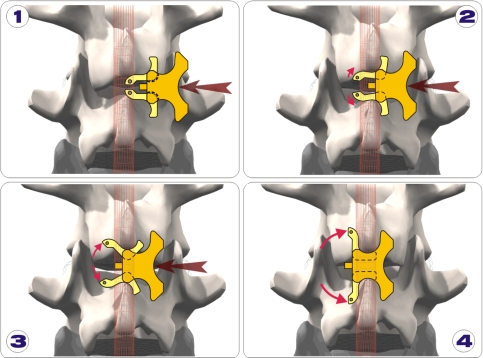
The InSwing® implant is made of PEEK and has self-pivotating (opening) L-shaped wings which allow for unilateral insertion. Once inserted through the interspinous space the wings automatically open on the contralateral side securing the implant between the spinous processes (Fig. 1). After insertion, the longitudinal pressure of the upper and lower ends of the adjacent spinal processes on the base of the L-shaped wings, insure locking in correct position. The instrumentation allows for a unilateral insertion (Fig. 2) by means of mirrored hook-shaped tension band inserters who are passed blindly around the adjoining spinous processes, allowing to stay close to the bone without involving the erector spinae muscle on the other side. A polyamide tension can be passed in eyelets on the implant and around the adjacent spinous processes.
Fig. 1.
a Insertion of the InSwing® interspinous device is accomplished via a unilateral approach. b Following insertion the wings of the device automatically open c on the contralateral side thus securing the implant between the spinous processes. d Following insertion, longitudinal pressure cranially and caudally insure its placement
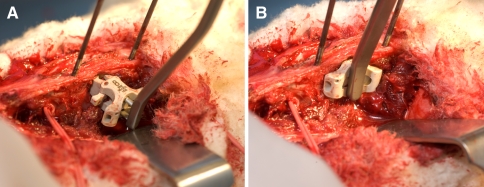
Fig. 2.
Insertion of the 8-mm InSwing® interspinous device demonstrating a the self-pivotating (opening) L-shaped wings allowing for unilateral insertion. b Once inserted through the interspinous space the wings automatically open on the contralateral side securing the implant between the spinous processes
Testing procedure
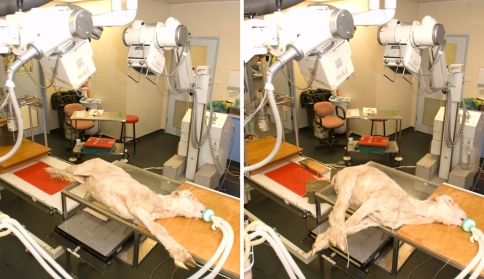
Following general anesthesia, the animal was placed in a side-lying posture and lateral radiographs were taken in full flexion and extension of the trunk. Each radiograph was centered at the level of L1–L2. The flexion position was achieved by securing a rope above the carpus and the tarsus of both forelimbs and hind limbs (Fig. 3). The extension position was achieved by securing a rope to both forelimbs and attaching it to one end of the table, and another rope to the hind limbs and attaching it to the opposite end of the table. The same radiographic protocol was repeated following the insertion of an 8-mm InSwing® interspinous device at L1–L2. This insertion required only a minimal dissection of the paraspinal muscles on the left side. The supraspinous ligament remained intact as did the paraspinal muscles on the contralateral side.
Fig. 3.
Radiographic set-up for imaging of the lumbar spine at full flexion (right) and extension (left) in the sagittal plane
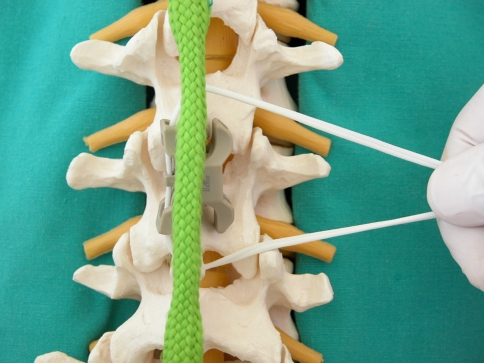
Finally, tension band (Fig. 4) was passed in the implant and around the L1 and L2 spinous processes and tightened to 1 N/m, another new set of flexion–extension radiographs were acquired. The tension was obtained with a proprietary dynamometric band tightening device provided by the implant manufacturer and enforced by securing the band with metal clips.
Fig. 4.
The tension band is looped through pre-fabricated holes in the InSwing® interspinous device and subsequently secured around the adjacent spinous processes of L1 and L2 and then tightened to a tension of 1 N/m and fixed with metal clips
Measurement technique
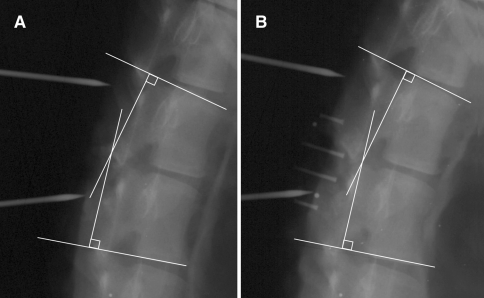
Intersegmental ROM was assessed in each of the conditions and compared using Cobb’s method [1] at the superior endplate of L1 relative to the inferior endplate of L2 (Fig. 5).
Fig. 5.
Sagittal plane radiographs of the ovine lumbar spine demonstrating the Cobb method of lumbar analysis of L1–L2 of the initial condition (a) and with the InSwing® device in place (b)
Statistical analysis
A paired t-test compared ROM for each of the experimental conditions. Statistical analysis was conducted using MATLAB software (The Mathworks, Inc., Natick, MA, USA). Statistical significance was set at P < 0.05.
Ethical approval
The study was approved by the Institutional Animal Ethics Committee.
Results
Table 1 summarizes the Cobb flexion and extension measurements and provides the mean and range for each of the three test conditions. Following the first test condition, the L1–L2 destabilization procedure, the mean total sagittal plane intersegmental ROM was 6.3 ± 2.7°. After instrumentation with the InSwing® interspinous implant, the mean total sagittal plane ROM was reduced by 15.9% to 5.3 ± 2.7°. The addition of the tension band, the third test condition, resulted in a 42.9% reduction in total sagittal plane ROM to 3.6 ± 1.9°, as compared to the initial ROM results following the destabilization procedure. These reductions in total sagittal plane ROM, as a result of the implant itself (P = 0.47) and then the addition of the tension band (P = 0.06), were not statistically significant.
Table 1.
Cobb flexion and extension measurements and calculated intersegmental range of motion (ROM) for all subjects following testing conditions at the L1–L2 intervertebral level
| Subject | Pre-implant | With implant no band | With implant with band | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Extension | Flexion | ROM | Extension | Flexion | ROM | Extension | Flexion | ROM | |
| 1 | 9° | 12° | 3° | 4° | 14° | 10° | 3° | 9° | 3° |
| 2 | 7° | 14° | 7° | 9° | 14° | 5° | 6° | 11° | 5° |
| 3 | 11° | 15° | 4° | 9° | 15° | 6° | 7° | 14° | 7° |
| 4 | 7° | 16° | 9° | 8° | 16° | 8° | 12° | 16° | 4° |
| 5 | 6° | 12° | 6° | 10° | 10° | 0° | 10° | 13° | 3° |
| 6 | 5° | 14° | 9° | 10° | 14° | 4° | 8° | 8° | 0° |
| 7 | 5° | 15° | 10° | 11° | 15° | 4° | 8° | 10° | 2° |
| 8 | 5° | 13° | 8° | 4° | 10° | 6° | 6° | 10° | 4° |
| 9 | 10° | 14° | 4° | 8° | 12° | 4° | 10° | 13° | 3° |
| 10 | 15° | 18° | 3° | 15° | 21° | 6° | 12° | 17° | 5° |
| Mean | 8° | 14.3° | 6.3° | 8.8° | 14.1° | 5.3° | 8.2° | 12.1° | 3.6° |
| Range | (5–15°) | (12–18°) | (3–10°) | (4–15°) | (10–21°) | (0–10°) | (3–12°) | (8–17°) | (0–7°) |
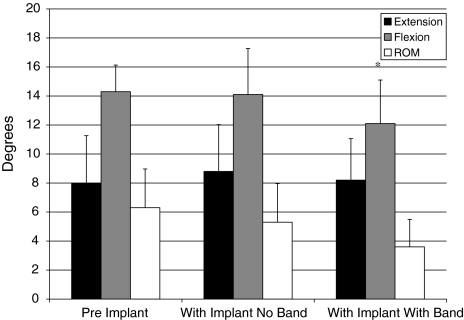
The mean observed lumbar flexion ROM following the destabilization procedure was 14.3 ± 1.8°. The addition of the interspinous implant without the tension band resulted in an insignificant (P = 0.74) 1.4% reduction in lumbar flexion. In contrast, a 15.4% reduction in lumbar flexion ROM was observed when comparing mean results following the destabilization procedure (14.3 ± 1.8°), to readings made after instrumenting with the InSwing® interspinous implant and securing with the tension band (12.1 ± 3.0°). This reduction in lumbar flexion ROM with the addition of the implant and tension band was statistically significant (P = 0.01). Figure 6 summarizes the mean changes in lumbar extension, flexion, and ROM from the initial condition, pre-implant, to those measurements obtained following implantation with the interspinous device, and those with the addition of the tension band to the interspinous device.
Fig. 6.
Mean changes in lumbar extension, flexion, and range of motion (ROM) from the initial pre-implant condition to those measurements obtained following the implantation of the interspinous device and with the addition of the tension band. Error bars represent the standard deviations of the mean. The asterisk denotes a significant difference (P < 0.05)
Discussion
In the quest for addressing the various conditions of the spine, there is a double tendency for moving toward minimal invasive surgery on the one hand, and the adoption of non-fusion technologies on the other. These tendencies have led to the development and progressive clinical adoption and adaptation of several interspinous devices. The InSwing® device is a novel interspinous implant that answers both these concerns. Although there are more and more clinical papers appearing in the literature (none with evidence from randomized controlled trials) there is a lack of in vivo studies comparing changes in ROM before and after implantation of such devices. The sheep is a suitable model for implant testing, keeping in mind that the ROM is smaller compared to humans [14].
The current arsenal of posteriorly implanted non-fusion devices for the lumbar spine can roughly be divided into pedicle-based systems, interspinous devices and others. Some of the implants aim to limit extension, others flexion, and still others aim to limit both extension and flexion. The pedicle-based systems are surgically more aggressive, leading researchers to look for alternate less invasive solutions. The X-Stop, restricting only extension, is the most widely published of these implants [37]. It is still limited however, by a rather invasive surgical technique. Other devices, constraining only in extension, have also been developed (e.g., Coflex®) [36]. In contrast, the DIAM® and Wallis® devices provide the added benefit of a tension band, which has been suggested to offer some control over flexion, although this has yet to be proven. Both DIAM and Wallis devices require quite a bit of surgical exposure and offer limited control over the amount of tension applied to the band.
Alternatively, the InSwing® device used in the current investigation is less invasive (as it is inserted unilaterally) and with the dynamometric band tightening device can provide controlled tensioning of the band. The importance of the tension band is confirmed in our findings showing that the addition of the tension band significantly reduced lumbar flexion ROM providing increased stability to the lumbar spine.
Only a few other studies have investigated interspinous implants secured with tension bands. Floman et al. [6] used the Wallis device after primary disc excision in the hope of reducing recurrent disc herniation. In their non-randomized study, they found the implant to probably be incapable of reducing the incidence of recurrent herniation. In a literature review by Christie et al. [4], the mechanisms of action and effectiveness of interspinous distraction devices were investigated. They [4] report dynamic stabilization as a system that favorably alters the movement and load transmission of a spinal motion segment, without the intention of fusion of the segment. In other words, such a system would restrict motion in the direction or plane that produces pain, or painful motion, but would otherwise allow a full ROM. The authors of that study report that, despite some variation in their proposed indications, interspinous implants share the mechanism of limiting extension of the lumbar spine and, as a result, appear to improve clinical symptoms [4].
Degenerative spondylolisthesis however, often causes segmental instability leading to segmental spinal stenosis resulting from the anterior slip of the cephalad vertebra. In the current study, an appreciable linear decrease in intersegmental ROM was observed following the introduction of the InSwing® interspinous device, which was further accentuated with the addition of the tension band. These findings therefore promote the indication for the use of such implants to increase spinal stability; at least in the sagittal plane. Indeed, we believe that the observed reduction of flexion in this study corresponds with a decrease of anterior slippage in degenerative spondylolisthesis. To which extent a 15% limitation of flexion as observed in the current study would equate to a similar reduction in the human cannot be ascertained from these data. Further in vivo in human studies will assist in understanding the clinical utility of the InSwing®.
In related work, Kim et al. [15] researched the effects of the DIAM, by looking at disc height, 1 year after surgery. The study did not however include an evaluation of the kinematic stabilization effects of the implant. Phillips et al. [25] performed an in vitro study similar to the current study using the DIAM. In their work, these researchers investigated changes in motion of the lumbar spine with the DIAM device, after partial facetectomy and discectomy, in flexion–extension, lateral bending, and axial rotation. Their specimens were tested under the following conditions: (1) intact; (2) after unilateral hemifacetectomy at L4–L5; (3) #2 and discectomy; and (4) #3 with DIAM. Angular motion values at the operated and adjacent segments were assessed. Their findings suggest that insertion of the DIAM device after discectomy restored the angular motion to below the level of the intact segment in flexion–extension [25]. The authors concluded that the DIAM device is effective in stabilizing the unstable segment, reducing the increased segmental flexion–extension, and lateral bending motions observed after discectomy. Their study did not investigate the use of the implant with or without the tension band, nor did it give any indication as to the amount of tension applied on the band.
Study limitations
The destabilization procedure was considered to create what most resembles a degenerative spondylolisthesis. Indeed, a real slip was not observed as there was no time for disk degeneration with slackening of the annulus fibrosus and osteorthritis of the facet joints to develop. The increase in sagital motion, however closely resembled what is clinically observed in degenerative spinal stenosis.
In addition to the use of an ovine model without compressive trunk and muscular loads as would be present in the upright human, there are several limitations to the current investigation. These limitations include the size of the implant used, the choice of tension band tightening to 1 N/m, and the sensitivity of the measurement technique for intersegmental ROM. In the present study we found the 8-mm size implants to best fit our in vivo model. This was possible as the animals used were all in the same weight range, offering nearly identical interspinous spaces. In a human clinical setting however, different implant sizes are available to adapt to individual anatomies. The choice of tension band tightening at 1 N/m was based on earlier biomechanical in vitro studies [32]. Last, although Cobb’s method has been found to be accurate and reliable [31], the method can have significant inter examiner reliability errors (±4.0°, 95% confidence interval) with mean differences of observer measurements for intra-examiner and inter of 1° or less [3].
Notwithstanding the limits of our findings, the current in vivo study clearly shows how the use of an inter-spinal spacer such as the InSwing® device, combined with a tension band, can limit flexion. This finding suggests the device may be particularly attractive for LSS cases due to a degenerative spondylolisthesis. A randomized controlled study of the use of the InSwing® for this indication should provide a conclusive answer as to the perceived benefits of the system.
Conclusions
The interspinous device investigated tended to reduce the total sagittal ROM at the level of the implant, however the results were not significant. The addition of a tension band was found to significantly stabilize the spine in flexion. To our knowledge, this is the first in vivo study radiographically showing the advantage of using an interspinous device (InSwing®), to stabilize the spine in flexion. These results are particularly important in light of the non-fusion devices currently proposed for patients with clinical symptoms of instable degenerative spondylolisthesis.
Acknowledgments
The authors would like to thank the Foundation for the Advancement of Chiropractic Education and Chiropractic Biophysics Non-Profit, Inc. for their support of this study.
References
- 1.Boden SD, Wiesel SW. Lumbosacral segmental motion in normal individuals. Have we been measuring instability properly? Spine. 1990;15:571–576. doi: 10.1097/00007632-199006000-00026. [DOI] [PubMed] [Google Scholar]
- 2.Bono CM, Vaccaro AR. Interspinous process devices in the lumbar spine. J Spinal Disord Tech. 2007;20:255–261. doi: 10.1097/BSD.0b013e3180331352. [DOI] [PubMed] [Google Scholar]
- 3.Cakir B, Richter M, Kafer W, Wieser M, Puhl W, Schmidt R. Evaluation of lumbar spine motion with dynamic X-ray—a reliability analysis. Spine. 2006;31:1258–1264. doi: 10.1097/01.brs.0000217763.80593.50. [DOI] [PubMed] [Google Scholar]
- 4.Christie SD, Song JK, Fessler RG. Dynamic interspinous process technology. Spine. 2005;30:S73–S78. doi: 10.1097/01.brs.0000174532.58468.6c. [DOI] [PubMed] [Google Scholar]
- 5.Dai LY, Xu YK, Zhang WM, Zhou ZH. The effect of flexion–extension motion of the lumbar spine on the capacity of the spinal canal. An experimental study. Spine. 1989;14:523–525. doi: 10.1097/00007632-198905000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Floman Y, Millgram MA, Smorgick Y, Rand N, Ashkenazi E. Failure of the Wallis interspinous implant to lower the incidence of recurrent lumbar disc herniations in patients undergoing primary disc excision. J Spinal Disord Tech. 2007;20:337–341. doi: 10.1097/BSD.0b013e318030a81d. [DOI] [PubMed] [Google Scholar]
- 7.Freudiger S, Dubois G, Lorrain M. Dynamic neutralisation of the lumbar spine confirmed on a new lumbar spine simulator in vitro. Arch Orthop Trauma Surg. 1999;119:127–132. doi: 10.1007/s004020050375. [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara A, Tamai K, An HS, Kurihashi T, Lim TH, Yoshida H, Saotome K. The relationship between disc degeneration, facet joint osteoarthritis, and stability of the degenerative lumbar spine. J Spinal Disord. 2000;13:444–450. doi: 10.1097/00002517-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Graf H. Lumbar instability: surgical treatment without fusion. Rachis. 1992;412:123–137. [Google Scholar]
- 10.Grevitt MP, Gardner AD, Spilsbury J, Shackleford IM, Baskerville R, Pursell LM, Hassaan A, Mulholland RC. The Graf stabilisation system: early results in 50 patients. Eur Spine J. 1995;4:169–175. doi: 10.1007/BF00298241. [DOI] [PubMed] [Google Scholar]
- 11.Grob D, Benini A, Junge A, Mannion AF. Clinical experience with the Dynesys semirigid fixation system for the lumbar spine: surgical and patient-oriented outcome in 50 cases after an average of 2 years. Spine. 2005;30:324–331. doi: 10.1097/01.brs.0000152584.46266.25. [DOI] [PubMed] [Google Scholar]
- 12.Humke T, Grob D, Grauer W, Sandler A, Dvorak J. Foraminal changes with distraction and compression of the L4/5 and L5/S1 segments. Eur Spine J. 1996;5:183–186. doi: 10.1007/BF00395511. [DOI] [PubMed] [Google Scholar]
- 13.Kettler A, Drumm J, Heuer F, Haeussler K, Mack C, Claes L, Wilke HJ. Can a modified interspinous spacer prevent instability in axial rotation and lateral bending? A biomechanical in vitro study resulting in a new idea. Clin Biomech (Bristol, Avon) 2008;23:242–247. doi: 10.1016/j.clinbiomech.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Kettler A, Liakos L, Haegele B, Wilke HJ. Are the spines of calf, pig and sheep suitable models for pre-clinical implant tests? Eur Spine J. 2007;16:2186–2192. doi: 10.1007/s00586-007-0485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KA, McDonald M, Pik JH, Khoueir P, Wang MY. Dynamic intraspinous spacer technology for posterior stabilization: case-control study on the safety, sagittal angulation, and pain outcome at 1-year follow-up evaluation. Neurosurg Focus. 2007;22:E7–E9. [PubMed] [Google Scholar]
- 16.Kong DS, Kim ES, Eoh W. One-year outcome evaluation after interspinous implantation for degenerative spinal stenosis with segmental instability. J Korean Med Sci. 2007;22:330–335. doi: 10.3346/jkms.2007.22.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laudet CG, Elberg JF, Robine D. Comportement biomécanique d’un ressort inter-apophysaire vertébral postérieur analyse expérimentale du comportement discal en compression et en flexion/extension. Rachis. 1993;5:101–107. [Google Scholar]
- 18.Leahy JC, Mathias KJ, Heaton A, Shepherd DE, Hukins DW, Deans WF, Brian MW, Wardlaw D. Design of spinous process hooks for flexible fixation of the lumbar spine. Proc Inst Mech Eng [H] 2000;214:479–487. doi: 10.1243/0954411001535507. [DOI] [PubMed] [Google Scholar]
- 19.Levin DA, Hale JJ, Bendo JA. Adjacent segment degeneration following spinal fusion for degenerative disc disease. Bull NYU Hosp Jt Dis. 2007;65:29–36. [PubMed] [Google Scholar]
- 20.Lindsey DP, Swanson KE, Fuchs P, Hsu KY, Zucherman JF, Yerby SA. The effects of an interspinous implant on the kinematics of the instrumented and adjacent levels in the lumbar spine. Spine. 2003;28:2192–2197. doi: 10.1097/01.BRS.0000084877.88192.8E. [DOI] [PubMed] [Google Scholar]
- 21.Mimura M, Panjabi MM, Oxland TR, Crisco JJ, Yamamoto I, Vasavada A. Disc degeneration affects the multidirectional flexibility of the lumbar spine. Spine. 1994;19:1371–1380. doi: 10.1097/00007632-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Minns RJ, Walsh WK. Preliminary design and experimental studies of a novel soft implant for correcting sagittal plane instability in the lumbar spine. Spine. 1997;22:1819–1825. doi: 10.1097/00007632-199708150-00004. [DOI] [PubMed] [Google Scholar]
- 23.Nachemson A. Recent advances in the treatment of low back pain. Int Orthop. 1985;9:1–10. doi: 10.1007/BF00267031. [DOI] [PubMed] [Google Scholar]
- 24.Penning L, Wilmink JT. Posture-dependent bilateral compression of L4 or L5 nerve roots in facet hypertrophy. A dynamic CT-myelographic study. Spine. 1987;12:488–500. doi: 10.1097/00007632-198706000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Phillips FM, Voronov LI, Gaitanis IN, Carandang G, Havey RM, Patwardhan AG. Biomechanics of posterior dynamic stabilizing device (DIAM) after facetectomy and discectomy. Spine J. 2006;6:714–722. doi: 10.1016/j.spinee.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Resnick DK, Choudhri TF, Dailey AT, Groff MW, Khoo L, Matz PG, Mummaneni P, Watters WC, III, Wang J, Walters BC, Hadley MN. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: fusion in patients with stenosis and spondylolisthesis. J Neurosurg Spine. 2005;2:679–685. doi: 10.3171/spi.2005.2.6.0679. [DOI] [PubMed] [Google Scholar]
- 27.Rigby MC, Selmon GP, Foy MA, Fogg AJ. Graf ligament stabilisation: mid- to long-term follow-up. Eur Spine J. 2001;10:234–236. doi: 10.1007/s005860100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmoelz W, Huber JF, Nydegger T, Dipl I, Claes L, Wilke HJ. Dynamic stabilization of the lumbar spine and its effects on adjacent segments: an in vitro experiment. J Spinal Disord Tech. 2003;16:418–423. doi: 10.1097/00024720-200308000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Senegas J. Mechanical supplementation by non-rigid fixation in degenerative intervertebral lumbar segments: the Wallis system. Eur Spine J. 2002;11(Suppl 2):S164–S169. doi: 10.1007/s00586-002-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siddiqui M, Smith FW, Wardlaw D. One-year results of X Stop interspinous implant for the treatment of lumbar spinal stenosis. Spine. 2007;32:1345–1348. doi: 10.1097/BRS.0b013e31805b7694. [DOI] [PubMed] [Google Scholar]
- 31.Singer KP, Edmondston SJ, Day RE, Breidahl WH. Computer-assisted curvature assessment and Cobb angle determination of the thoracic kyphosis. An in vivo and in vitro comparison. Spine. 1994;19:1381–1384. doi: 10.1097/00007632-199406000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Sterna J, Chopek L, Ciupik A, Dobkiewicz J, Pienazek J, Radek M, Szpalski M (2006) Evaluation of implantation procedure and research of influence of Inter-S dynamic tensioning stabilization system. In: XIII Meeting of the Neuroorthopedic Section of the Polish Society of Neurosurgery, Zakopane, April 27–29, 2006
- 33.Stoll TM, Dubois G, Schwarzenbach O. The dynamic neutralization system for the spine: a multi-center study of a novel non-fusion system. Eur Spine J. 2002;11(Suppl 2):S170–S178. doi: 10.1007/s00586-002-0438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swanson KE, Lindsey DP, Hsu KY, Zucherman JF, Yerby SA. The effects of an interspinous implant on intervertebral disc pressures. Spine. 2003;28:26–32. doi: 10.1097/00007632-200301010-00008. [DOI] [PubMed] [Google Scholar]
- 35.Szpalski M, Michel F, Hayez JP. Determination of trunk motion patterns associated with permanent or transient stenosis of the lumbar spine. Eur Spine J. 1996;5:332–337. doi: 10.1007/BF00304349. [DOI] [PubMed] [Google Scholar]
- 36.Tsai KJ, Murakami H, Lowery GL, Hutton WC. A biomechanical evaluation of an interspinous device (Coflex) used to stabilize the lumbar spine. J Surg Orthop Adv. 2006;15:167–172. [PubMed] [Google Scholar]
- 37.Verhoof OJ, Bron JL, Wapstra FH, Royen BJ. High failure rate of the interspinous distraction device (X-Stop) for the treatment of lumbar spinal stenosis caused by degenerative spondylolisthesis. Eur Spine J. 2008;17:188–192. doi: 10.1007/s00586-007-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Blood E, Hanscom B, Herkowitz H, Cammisa F, Albert T, Boden SD, Hilibrand A, Goldberg H, Berven S, An H. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358:794–810. doi: 10.1056/NEJMoa0707136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiseman CM, Lindsey DP, Fredrick AD, Yerby SA. The effect of an interspinous process implant on facet loading during extension. Spine. 2005;30:903–907. doi: 10.1097/01.brs.0000158876.51771.f8. [DOI] [PubMed] [Google Scholar]
- 40.Zucherman JF, Hsu KY, Hartjen CA, Mehalic TF, Implicito DA, Martin MJ, Johnson DR, Skidmore GA, Vessa PP, Dwyer JW, Puccio ST, Cauthen JC, Ozuna RM. A multicenter, prospective, randomized trial evaluating the X STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: two-year follow-up results. Spine. 2005;30:1351–1358. doi: 10.1097/01.brs.0000166618.42749.d1. [DOI] [PubMed] [Google Scholar]