Abstract
The goal of surgical treatment for degenerative lumbar spinal stenosis (LSS) is to effectively relieve the neural structures by various decompressive techniques. Microendoscopic decompressive laminotomy (MEDL) is an attractive option because of its minimally invasive nature. The aim of prospective study was to investigate the effectiveness of MEDL by evaluating the clinical outcomes with patient-oriented scoring systems. Sixty consecutive patients receiving MEDL between December 2005 and April 2007 were enrolled. The indications of surgery were moderate to severe stenosis, persistent neurological symptoms, and failure of conservative treatment. The patients with mechanical back pain, more than grade I spondylolisthesis, or radiographic signs of instability were not included. A total of 53 patients (36 women and 17 men, mean age 62.0) were included. Forty-five patients (84.9%) were satisfied with the treatment result after a follow-up period of 15.7 months (12–24). The clinical outcomes were evaluated with the Oswestry disability index (ODI) and the Japanese Orthopedic Association (JOA) score. Of the 50 patients providing sufficient data for analysis, the ODI improved from 64.3 ± 20.0 to 16.7 ± 20.0. The JOA score improved from 9.4 ± 6.1 to 24.2 ± 6.0. The improvement rate was 73.9 ± 30.7% and 40 patients (80%) had good or excellent results. There were 11 surgical complications: dural tear in 5, wrong level operation in 2, and transient neuralgia in 4 patients. No wound-related complication was noted. Although the prevalence of pre-operative comorbidities was very high (69.8%), there was no serious medical complication. There was no post-operative instability at the operated segment as evaluated with dynamic radiographs at final follow-up. We concluded that MEDL is a safe and very effective minimally invasive technique for degenerative LSS. With an appropriate patient selection, the risk of post-operative instability is minimal.
Keywords: Microendoscopic decompressive laminotomy, Minimally invasive surgical procedures, Spinal stenosis, Treatment outcomes
Introduction
Degenerative lumbar spinal stenosis (LSS) is the most common indication for lumbar spine surgery in adults over the age of 65 [32]. Although some studies claimed improvement with conservative treatment, several comparative studies showed better outcome favoring surgical treatment for patients with moderate to severe stenosis [1, 3–5, 15, 20, 32].
The goal of surgery for LSS is to relieve the neurologic symptoms by decompressing the stenosis. Classically, decompressive procedures involved extensive dissection of paraspinal muscles and removal of the posterior elements including the lamina, spinous processes, interspinous ligaments, and sometimes the facet joints [33]. Concerns for post-operative instability have led to the recommendations for concomitant fusion with or without instrumentation [18, 26]. Although some studies showed improved outcomes with successful fusions, the indication, cost-benefit, and associated comorbidities of spinal fusion have always been the issue of debate [8]. The fusion rate has improved significantly with advanced fusion technology such as new internal fixation devices, interbody fusion cages, bone grafts substitutes, and biologic enhancement. However, a Cochrane review suggested that the clinical improvement is probably marginal [11]. It is also disappointed that the re-operation rate has not decreased by introduction of these fusion technologies [21]. A meta-analysis concluded that the least invasive surgical procedure had the highest rate of success and the fewest complications [22].
Microendoscopic decompressive laminotomy (MEDL) was developed in 2002 for the treatment of LSS. It has gradually replaced the classic decompressive procedure and become the standard one in many countries. By using a tubular retractor with the incorporated fibro-optic endoscopic system, this minimally invasive decompressive technique usually involves less blood loss, less muscle dissection, and less injury to the stabilizing structures. Sufficient decompression of the stenosis was demonstrated by a cadaver study and some preliminary clinical studies [12, 16, 25].
The goal of this study is to evaluate the clinical outcomes of MEDL using objective evaluation tools, and to evaluate the possibility to avoid post-operative instability after the decompressive surgery.
Materials and methods
Totally 60 consecutive patients undergoing MEDL for LSS between December 2005 and April 2007 were enrolled in this prospective study. The selection criteria were as follows: (1) neurogenic claudication or radicular leg pain with associated neurologic signs referring to the LSS syndrome; (2) moderate to severe spinal canal stenosis shown on cross-sectional imaging such as MRI or CT scan, and (3) failure of conservative treatment for at least 3 months. The patients who had either mechanical low back pain or segmental instability were not included. The mechanical lower back pain was defined as pain that was induced by posture change, or that prevented the patient from sitting or standing for more than 30 min. Patients were considered to have segmental instability if they had isthmic spondylolisthesis, degenerative spondylolisthesis with more than 4 mm of translation or intervertebral angle reversal on dynamic radiographs [29].
The patients were positioned prone on the Relton–Hall frame after general anesthesia. Precise localization was achieved with frequent fluoroscopic checks. The disc level and interlaminar space were identified with PA view and the skin was marked. A spinal needle was inserted and localization was rechecked with lateral view. Serial tubal dilators were inserted through the small skin incision and paraspinal muscles to create the endoscopic tunnel. The working channel was then inserted along the dilators and docked onto the lamina. The endoscopic (METRx, Medtronics, Minneapolis, MN) and camera systems were mounted on the working channel via a connecting ring (Fig. 1). The localization was reconfirmed with lateral view of fluoroscopy before the decompressive procedure. Decompression was done under the 20-degree endoscope and the tubular retraction system. For patients with unilateral neurological symptoms, we performed unilateral laminotomy and foraminotomy. For patients with bilateral neurological symptoms, we performed unilateral laminotomy for bilateral decompression (ULBD) as described by Guiot et al. [12] to decompress the central canal and bilateral lateral recesses. The degenerative ligamentum flava were completely excised. Decompression of the ipsilateral lateral recess was achieved by medial facetectomy. In order to preserve integrity of the facet joint as much as possible, we used specially designed instruments such as curved high-speed pneumatic burrs with diamond heads, curved Kerrison punches, and curved narrow osteotomes to undercut the facet joint. Then we tilted the retractor tube to decompress the central canal and contralateral lateral recess. This process was performed by gently pressing down the dura sac, excising the ligamentum flavum with straight and curved Kerrison punches, and undercutting the lamina with straight narrow osteotomes (Fig. 2). The adequacy of decompression was determined by observing pulsation of the dura sac and probing the traversing nerve roots to make sure their mobility, as well as final fluoroscopic recheck to confirmed the extent of decompression.
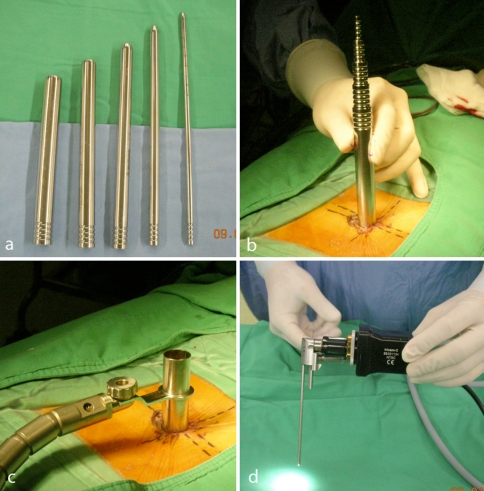
Fig. 1.
a The serial dilators used to create the endoscopic portal. b The assembly of the serial dilators. c The setup of the working channel, which was mounted rigidly on the surgical table. d The endoscope and attached camera. Note the 20-degree offset of the light source
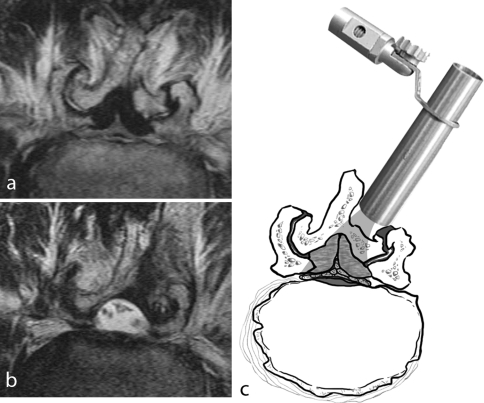
Fig. 2.
a Pre-operative axial T2-weighted MRI image showed severe stenosis due to degeneration of the facet joints and the ligamentum flava. b Post-operative axial T2-weighted MRI image showed adequate decompression with preservation of the facet joints and the spinous process. c Schematic demonstration for the unilateral laminotomy for bilateral decompression under the endoscope
When performing multi-level decompression, the skin incision was centered at the midpoint between selected intervertebral disc levels. The skin incision was mobilized one level above or below after releasing the underlying connective tissues. Thus multi-level decompression was done through one skin incision and multiple separate muscular portals for each level. For the patients with small stature, up to three-level decompression could be done through a single surgical incision.
All the patients had routine AP, lateral, dynamic lateral radiographs pre-operatively, 6 months after the surgery, and at final follow-up. Post-operative instability was defined as progression of listhesis or scoliosis on dynamic radiographs.
Every patient had MR images of the lumbar spine before operation. The severity of stenosis was classified according to the cross sectional area of the dura sac at the axial plane on T1-weighted MR images—severe stenosis for less than 76 mm2, moderate stenosis for between 76 and 100 mm2, and mild stenosis for more than 100 mm2 [29]. When MRI was contraindicated (two patients), CT-myelography was used instead.
The clinical outcomes were evaluated using Oswestry disability index (ODI) for the overall disability and the Japanese Orthopedic Association (JOA) score for the clinical symptoms and signs [9, 14]. The patients were evaluated before operation, at 6 months, and at final follow-up. The range of ODI was 100 to 0 with a lower index corresponding to a better result. Significant improvement was defined as more than 15 points of improvement after the treatment. The range of JOA score was −3 to 29 with a higher score corresponding to a better result. The improvement rate based on JOA score was calculated as follows: (pre-operative score − post-operative score)/(29 − pre-operative score) × 100%. The clinical results were classified into four grades by the improvement rate: excellent (more than 75%), good (between 51 and 75%), fair (between 26 and 50%), and poor (less than 26%). The success of treatment was defined as more than 25% improvement rate in JOA score [14]. At final follow-up, we inquired each patient if he or she was satisfied with the treatment results.
The data about the pre-operative comorbidities, intra-operative, peri-operative, and post-operative complications were retrieved from medical chart review. This investigation has been approved and monitored by the Research Ethics Review Committee of the author’s hospital.
Results
Of the 60 patients included in the study, follow-up was complete in 53 patients (88.3%, 36 women and 17 men). The mean follow-up period was 15.7 months (range 12–24). Seven patients were lost to follow up; two of them died of irrelevant diseases. The mean age at surgery was 62.0 (range 36–86). The symptomatology varied and usually mixed: low back pain in 27 patients, radicular leg pain in 38 patients, neurogenic intermittent claudication in 21 patients, sensory disturbance in 44 patients, motor weakness in 8 patients, and urinary dysfunction in 19 patients.
Thirty-seven patients had degenerative LSS, 13 patients had coexisted degenerative spondylolisthesis, and 4 patients had coexisted degenerative scoliosis. Thirty-eight patients (71.7%) had severe stenosis and 15 patients (28.3%) had moderate stenosis, based on the measurements of MR images.
Totally 78 levels were decompressed in 53 patients. The most frequently involved level was L4-5 (47 patients), followed by L3-4 (16 patients), L5-S (10 patients), L2-3 (4 patients), and L1-2 (1 patient). Thirty-one patients (58.5%) had one-level, 19 (35.8%) had two-level, and 3 (5.7%) had three-level decompression, all through a single surgical wound (Table 1).
Table 1.
Demographic data and clinical characteristics
| Demographic data | |
|---|---|
| Patients included | 60 |
| Patients completed follow-up | 53 |
| Mean age (years) | 62.0 (36–86) |
| Follow-up (months) | 15.7 (12–24) |
| Clinical variables | No. patients |
|---|---|
| Gender | |
| Male | 17 |
| Female | 36 |
| Diagnosis | |
| LSS | 37 |
| LSS + spondylolisthesis | 12 |
| LSS + scoliosis | 3 |
| Spondylolisthesis + scoliosis | 1 |
| Severity of stenosis | |
| Severe | 38 (71.7%) |
| Moderate | 15 (28.3%) |
| Level of decompression | |
| Total levels | 78 |
| L1-2 | 1 (1.3%) |
| L2-3 | 4 (22.2%) |
| L3-4 | 16 (20.5%) |
| L4-5 | 47 (60.3%) |
| L5-S | 10 (12.8%) |
| Singe-level surgery | 31 (58.5%) |
| Two-level surgery | 19 (35.8%) |
| Three-level surgery | 3 (5.7%) |
The mean operation time was 126.7 ± 38.3 min and the estimated blood loss was 104.5 ± 126.2 mL for one level of decompression.
Pre-operative comorbidities were very common in patients with degenerative LSS. The prevalence was as high as 69.8%. Of the 53 patients who completed follow-up, 16 had cardiovascular diseases, 1 had cerebrovascular disease, 2 had pulmonary diseases, 1 had renal insufficiency, 14 had diabetes mellitus requiring treatment, 4 had major depressive disorder, and 2 had arthropathy with disturbance of walking ability. In spite of the very high prevalence of pre-operative comorbidities, there was no major medical complication except that two patients had urinary tract infection requiring short-term oral antibiotics.
Totally 11 surgery-related complications were identified. They were dural tear in five, wrong level operation in two, and transient neuralgia in four patients. There was no surgical wounds related complication.
There was no progression of pre-existed spondylolisthesis or scoliosis. However, post-operative instability with progressive segmental scoliosis developed in one patient who did not have pre-operative spondylolisthesis or scoliosis. Follow-up MRI showed the decompressive procedure involved excessive invasion to the facet joint complex.
Fifty of 53 patients provided sufficient data in functional evaluation. The improvement was very significant as evaluated with either ODI or JOA scoring system. The ODI improved from 64.3 ± 20.0 before surgery to 16.7 ± 20.0 at final follow-up. The average improvement was 47.6 ± 27.5. Forty-three patients (86.0%) got significant improvement. The JOA score improved from 9.4 ± 6.1 before surgery to 24.2 ± 6.0 at final follow-up. The mean improvement rate was 73.9 ± 30.7%. Forty patients (80%) had good or excellent results. Success of treatment was achieved in 45 patients (90%). Of the 53 patients who completed follow-up, 45 patients (84.9%) stated they were satisfied with the treatment result (Table 2). As for the seven patients lost to follow up and failed to complete the functional evaluation, most of them reported significant or complete relief of pre-operative symptoms in the immediate post-operative period. Only one patient stated moderate improvement.
Table 2.
Overall results of functional evaluations
| ODI (n = 50) | |
| Pre-operative | 64.3 ± 20.0 |
| Final follow-up | 16.7 ± 20.0 |
| Improvement | 47.6 ± 27.5 |
| Significant improvement (n = 43) | 86.0% |
| JOA score (n = 50) | |
| Pre-operative | 9.4 ± 6.1 |
| Final follow-up | 24.2 ± 6.0 |
| Improvement rate (%) | 73.9 ± 30.7 |
| Poor (n = 5) | 10% |
| Fair (n = 5) | 10% |
| Good (n = 9) | 18% |
| Excellent (n = 31) | 62% |
Discussion
Lumbar spinal stenosis is a slowly progressive disease complicating the natural degenerative process. Surgical decompression is necessary for patients who failed conservative treatment. The optimal timing of surgical intervention is very difficult to determine because most patients present with fluctuating symptoms for several years. Since delay of surgery seems not to compromise the treatment results [1], a period of conservative treatment is always needed before the doctor suggests surgical treatment [20].
Classical laminectomy could effectively decompress the stenosis and relieve the neurological symptoms. However, the extensive soft tissue dissection and bony destruction might destabilize the spinal column [18, 26, 33]. In the last decade, several less invasive techniques including multiple laminotomy, chimney sublaminar decompression, unilateral laminotomy for bilateral decompression have evolved to overcome such problems [6, 7, 10, 17, 19, 24, 28]. Most of these techniques emphasized limited decompression in order to preserve the integrity of the posterior stabilizing structures, especially the facet joints. The ideal situation is to maintain the spinal stability while allowing sufficient decompression, and thus to prevent the morbidities of spinal fusion. Basically, these techniques still involved a large surgical incision or extensive soft tissue dissection.
The concept of minimally invasiveness has been applied in many surgical fields with great success. The most recognized advantage was minimal soft tissue injury by introducing the advanced endoscopic and fibro-optic video systems. Clinically, the patients had less blood loss, less wound pain, and sooner post-operative recovery. MEDL was a minimally invasive spine surgery adopting the same concept. The preliminary clinical results were very encouraging [2, 12, 16, 25]. Besides, this approach is also a good option for patients with multi-level stenosis. Through a small skin incision (about 16–18 mm), the surgeon can performed two-level or even three-level decompression for the patients with short stature, thus further minimize the surgical incision.
The MEDL is very effective in relieving the neurological symptoms and improving patients’ quality of life. The improvement in JOA score was significant. By the original definition of this scoring system, the success rate of MEDL was as high as 90%; good or excellent results were obtained in 80% patients. In a historical meta-analysis, the success rate was 64% only [30]. As compared with a similar study for traditional open laminectomy, in which only 56.7% patients obtained good or excellent results, the results of our series were much better [13]. The high ODI (64.3) before operation reflected the severity of disability in our series. It decreased to 16.7 after MEDL, and 86.0% patients obtained significant improvement. Forty-five of 53 patients (84.9%) were satisfied with the treatment results.
The post-operative instability is a major concern for any decompressive procedures. The risk is higher in patients with coexisted spondylolisthesis and concomitant fusion was often suggested [31]. With MEDL, the bony destruction was limited at the interlaminar window and most of the facet joints were preserved (Fig. 2). In our series, 16 of 53 patients had either low-grade degenerative spondylolisthesis or scoliosis, but there was no aggravation of low back pain or post-operative instability developed in these patients. Our study confirmed that MEDL is a good surgical option to decompress the stenosis while preserving the intrinsic stability.
The definition of instability is very controversial. Most authors define instability by the dynamic lateral radiographs. We propose that mechanical low back is also a strong indicator for obscure instability, even if there is no gross evidence on the dynamic radiographs. That is the reason why we exclude such patients. In contrast, the lumbar spine with low-grade spondylolisthesis is supposed to be stable and we consider such patients good candidates for MEDL.
MEDL may be a good option for the elderly patients. They tend to have more severe stenosis, more levels of involvement, and more pre-operative comorbidities. It is concerned that the lengthy surgical procedure may increase the peri-operative complications and complications caused by prolonged general anesthesia. In our series, 71.7% of our patient had severe stenosis, 41.5% received multiple level surgeries, and 69.8% had pre-operative comorbidities. However, no serious medical complications occurred after MEDL. Therefore, considering the expected benefits in relieving the neurological symptoms and disability, the minimal invasive nature of the procedure, and quick recovery after the surgery, MEDL has become our choice for the surgical treatment to the elderly patients with LSS [27].
The major limitations of MEDL come from its learning curve [23]. The limited working space, distorted visual field, unrevealed 3-D anatomical landmarks outside the tubular retractor, and unfamiliar tactile sensation, all contribute to the steep learning curve. The 20-degree endoscope is very important because it extends the visual field beyond the narrow tubular retractor. Although specially designed curved instruments, such as the high-speed burrs, curved Kerrison punches, and curved osteotomes are not necessary to ensure sufficient decompression, these instruments can be very helpful in accessing structure beyond the tube and preventing unnecessary injury to the facet joint [14].
The most common encountered surgery-related complication was dural tear, which occurred in 5 of 60 patients (8.3%). The incidence was comparable with and even lower than the reported incidence of 18% in most series of open laminectomy for LSS [30]. However, we thought the dural tears were associated with the learning curve [23], the approach method [24], and the severity of stenosis, because most of them occurred in the early cases, unilateral laminotomy for bilateral decompression, and severe stenosis. Using the diamond burr instead of the cutting burr, 2 mm Kerrison punch instead of 4 mm, preserving the ligamentum flavum as a protective barrier of dura matter till completion of the bony procedure, gentle manipulation of the neural tissues, accumulated experiences and good surgical skills of the surgeon, all might contribute to lower the risk of such complication.
Because of minimal invasiveness, localization is critical and must be precise. Two cases of wrong level surgeries were encountered in our series. There are several ways to minimize such avoidable complication: The image studies should be carefully reviewed because the patient might have personal anomalies such as sacral lumbarization or fused L5/S segments. The fluoroscope must be available and stay in the operating theater all the time through the whole procedure. Localization with both PA and lateral fluoroscopy should be performed when marking the skin, during setup of the endoscopic system, before starting the decompressive procedure, and after the decompression was completed. When there is any doubt about localization, do not hesitate to recheck it with the fluoroscope.
There are some limitations of our study. First, there is no control group for comparison. Second, the follow-up is not long enough to conclude the long-term benefits. Because the initial benefits of surgical decompression might deteriorate over time [4, 20], further longer term studies should pay more attention on duration of symptoms relief, the risk of post-operative instability and re-stenosis, and the incidence of re-operation. Third, because we did not include those patients with higher-grade spondylolisthesis or patients with mechanical low back pain, the conclusion that MEDL can preserve the pre-operative stability can only be applied on carefully selected patients.
Acknowledgments
This study was supported by the grant FEMH-96-C-039 from the Far-Eastern Memorial Hospital.
References
- 1.Amundsen T, Weber H, Nordal HJ, Magnaes B, Abdelnoor M, Lilleas F. Lumbar spinal stenosis: conservative or surgical management? A prospective 10-year study. Spine. 2000;25:1424–1435. doi: 10.1097/00007632-200006010-00016. [DOI] [PubMed] [Google Scholar]
- 2.Asgarzadie F, Khoo LT. Minimally invasive operative management for lumbar spinal stenosis: overview of early and long-term outcomes. Orthop Clin North Am. 2007;38:387–399. doi: 10.1016/j.ocl.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Athiviraham A, Yen D. Is spinal stenosis better treated surgically or nonsurgically? Clin Orthop Relat Res. 2007;458:90–93. doi: 10.1097/BLO.0b013e31803799a9. [DOI] [PubMed] [Google Scholar]
- 4.Atlas SJ, Deyo RA, Keller RB, Chapin AM, Patrick DL, Long JM, Singer DE. The Maine Lumbar Spine Study, Part III. 1-year outcomes of surgical and nonsurgical management of lumbar spinal stenosis. Spine. 1996;21:1787–1794. doi: 10.1097/00007632-199608010-00012. [DOI] [PubMed] [Google Scholar]
- 5.Atlas SJ, Keller RB, Robson D, Deyo RA, Singer DE. Surgical and nonsurgical management of lumbar spinal stenosis: four-year outcomes from the maine lumbar spine study. Spine. 2000;25:556–562. doi: 10.1097/00007632-200003010-00005. [DOI] [PubMed] [Google Scholar]
- 6.Cavusoglu H, Kaya RA, Turkmenoglu ON, Tuncer C, Colak I, Aydin Y. Midterm outcome after unilateral approach for bilateral decompression of lumbar spinal stenosis: 5-year prospective study. Eur Spine J. 2007;16:2133–2142. doi: 10.1007/s00586-007-0471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa F, Sassi M, Cardia A, Ortolina A, Santis A, Luccarell G, Fornari M. Degenerative lumbar spinal stenosis: analysis of results in a series of 374 patients treated with unilateral laminotomy for bilateral microdecompression. J Neurosurg. 2007;7:579–586. doi: 10.3171/SPI-07/12/579. [DOI] [PubMed] [Google Scholar]
- 8.Deyo RA, Ciol MA, Cherkin DC, Loeser JD, Bigos SJ. Lumbar spinal fusion. A cohort study of complications, reoperations, and resource use in the Medicare population. Spine. 1993;18:1463–1470. doi: 10.1097/00007632-199318110-00010. [DOI] [PubMed] [Google Scholar]
- 9.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25:2940–2952. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 10.Fu YS, Zeng BF, Xu JG. Long-term outcomes of two different decompressive techniques for lumbar spinal stenosis. Spine. 2008;33:514–518. doi: 10.1097/BRS.0b013e3181657dde. [DOI] [PubMed] [Google Scholar]
- 11.Gibson JN, Waddell G. Surgery for degenerative lumbar spondylosis: updated Cochrane Review. Spine. 2005;30:2312–2320. doi: 10.1097/01.brs.0000182315.88558.9c. [DOI] [PubMed] [Google Scholar]
- 12.Guiot BH, Khoo LT, Fessler RG. A minimally invasive technique for decompression of the lumbar spine. Spine. 2002;27:432–438. doi: 10.1097/00007632-200202150-00021. [DOI] [PubMed] [Google Scholar]
- 13.Iguchi T, Kurihara A, Nakayama J, Sato K, Kurosaka M, Yamasaki K. Minimum 10-year outcome of decompressive laminectomy for degenerative lumbar spinal stenosis. Spine. 2000;25:1754–1759. doi: 10.1097/00007632-200007150-00003. [DOI] [PubMed] [Google Scholar]
- 14.Ikuta K, Arima J, Tanaka T, Oga M, Nakano S, Sasaki K, Goshi K, Yo M, Fukagawa S. Short-term results of microendoscopic posterior decompression for lumbar spinal stenosis. Technical note. J Neurosurg. 2005;2:624–633. doi: 10.3171/spi.2005.2.5.0624. [DOI] [PubMed] [Google Scholar]
- 15.Jolles BM, Porchet F, Theumann N. Surgical treatment of lumbar spinal stenosis. Five-year follow-up. J Bone Joint Surg. 2001;83:949–953. doi: 10.1302/0301-620X.83B7.11722. [DOI] [PubMed] [Google Scholar]
- 16.Khoo LT, Fessler RG. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery. 2002;51:S146–S154. [PubMed] [Google Scholar]
- 17.Kleeman TJ, Hiscoe AC, Berg EE. Patient outcomes after minimally destabilizing lumbar stenosis decompression: the “Port-Hole” technique. Spine. 2000;25:865–870. doi: 10.1097/00007632-200004010-00016. [DOI] [PubMed] [Google Scholar]
- 18.Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004;29:726–733. doi: 10.1097/01.BRS.0000119398.22620.92. [DOI] [PubMed] [Google Scholar]
- 19.Lin SM, Tseng SH, Yang JC, Tu CC. Chimney sublaminar decompression for degenerative lumbar spinal stenosis. J Neurosurg. 2006;4:359–364. doi: 10.3171/spi.2006.4.5.359. [DOI] [PubMed] [Google Scholar]
- 20.Malmivaara A, Slatis P, Heliovaara M, Sainio P, Kinnunen H, Kankare J, Dalin-Hirvonen N, Seitsalo S, Herno A, Kortekangas P, Niinimaki T, Ronty H, Tallroth K, Turunen V, Knekt P, Harkanen T, Hurri H. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine. 2007;32:1–8. doi: 10.1097/01.brs.0000251014.81875.6d. [DOI] [PubMed] [Google Scholar]
- 21.Martin BI, Mirza SK, Comstock BA, Gray DT, Kreuter W, Deyo RA. Are lumbar spine reoperation rates falling with greater use of fusion surgery and new surgical technology? Spine. 2007;32:2119–2126. doi: 10.1097/BRS.0b013e318145a56a. [DOI] [PubMed] [Google Scholar]
- 22.Niggemeyer O, Strauss JM, Schulitz KP. Comparison of surgical procedures for degenerative lumbar spinal stenosis: a meta-analysis of the literature from 1975 to 1995. Eur Spine J. 1997;6:423–429. doi: 10.1007/BF01834073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowitzke AM. Assessment of the learning curve for lumbar microendoscopic discectomy. Neurosurgery. 2005;56:755–762. doi: 10.1227/01.NEU.0000156470.79032.7B. [DOI] [PubMed] [Google Scholar]
- 24.Oertel MF, Ryang YM, Korinth MC, Gilsbach JM, Rohde V. Long-term results of microsurgical treatment of lumbar spinal stenosis by unilateral laminotomy for bilateral decompression. Neurosurgery. 2006;59:1264–1269. doi: 10.1227/01.NEU.0000245616.32226.58. [DOI] [PubMed] [Google Scholar]
- 25.Palmer S, Turner R, Palmer R. Bilateral decompression of lumbar spinal stenosis involving a unilateral approach with microscope and tubular retractor system. J Neurosurg. 2002;97:213–217. doi: 10.3171/spi.2002.97.2.0213. [DOI] [PubMed] [Google Scholar]
- 26.Postacchini F, Cinotti G. Bone regrowth after surgical decompression for lumbar spinal stenosis. The Journal of Bone and Joint Surgery. 1992;74:862–869. doi: 10.1302/0301-620X.74B6.1447247. [DOI] [PubMed] [Google Scholar]
- 27.Rosen DS, O’Toole JE, Eichholz KM, Hrubes M, Huo D, Sandhu FA, Fessler RG. Minimally invasive lumbar spinal decompression in the elderly: outcomes of 50 patients aged 75 years and older. Neurosurgery. 2007;60:503–509. doi: 10.1227/01.NEU.0000255332.87909.58. [DOI] [PubMed] [Google Scholar]
- 28.Thome C, Zevgaridis D, Leheta O, Bazner H, Pockler-Schoniger C, Wohrle J, Schmiedek P. Outcome after less-invasive decompression of lumbar spinal stenosis: a randomized comparison of unilateral laminotomy, bilateral laminotomy, and laminectomy. J Neurosurg. 2005;3:129–141. doi: 10.3171/spi.2005.3.2.0129. [DOI] [PubMed] [Google Scholar]
- 29.Truumees E. Spinal stenosis: pathophysiology, clinical and radiologic classification. Instr Course Lect. 2005;54:287–302. [PubMed] [Google Scholar]
- 30.Turner JA, Ersek M, Herron L, Deyo R. Surgery for lumbar spinal stenosis. Attempted meta-analysis of the literature. Spine. 1992;17:1–8. doi: 10.1097/00007632-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Vibert BT, Sliva CD, Herkowitz HN. Treatment of instability and spondylolisthesis: surgical versus nonsurgical treatment. Clin Orthop Relat Res. 2006;443:222–227. doi: 10.1097/01.blo.0000200233.99436.ea. [DOI] [PubMed] [Google Scholar]
- 32.Weinstein JN, Lurie JD, Tosteson TD, Hanscom B, Tosteson AN, Blood EA, Birkmeyer NJ, Hilibrand AS, Herkowitz H, Cammisa FP, Albert TJ, Emery SE, Lenke LG, Abdu WA, Longley M, Errico TJ, Hu SS. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356:2257–2270. doi: 10.1056/NEJMoa070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiltse LL, Kirkaldy-Willis WH, McIvor GW. The treatment of spinal stenosis. Clin Orthop Relat Res. 1976;115:83–91. [PubMed] [Google Scholar]