Abstract
The current gold standard in lumbar fusion consists of transpedicular fixation in combination with an interbody interponate of autologous bone from iliac crest. Because of the limited availability of autologous bone as well as the still relevant donor site morbidity after iliac crest grafting the need exists for alternative grafts with a comparable outcome. Forty patients with degenerative spinal disease were treated with a monosegmental spondylodesis (ventrally, 1 PEEK-cage; dorsally, a screw and rod system), and randomly placed in two groups. In group 1, autogenous iliac crest cancellous bone was used as a cage filling. In group 2 the cages were filled with an allogenic cancellous bone graft. Following 3, 6, 9 and 12 months, the clinical outcome was determined on the basis of: the Oswestry Low Back Pain Disability Questionnaire; patient satisfaction; patient willingness to undergo the operation again; and a visual analog scale for pain. The radiological outcome was based on both fusion rate (radiographs, computed tomography), and on the bone mineral density of the grafts. After 6 months, the X-rays of the patients in group 2 had a significantly lower rate of fusion. Aside from this, there were no further significant differences. After 12 months, radiological results showed a similar fusion rate in both groups. Donor site complications consisted of five patients with hematoma, and three patients with persistent pain in group 1. No implant complications were observed. If a bone bank is available for support and accepting the low risk of possible transmission of infectious diseases, freeze–dried allogenic cancellous bone can be used for monosegmental spondylodeses. The results demonstrated an equivalent clinical outcome, as well as similar fusion rates following a 12-month period. This is in despite of a delayed consolidation process.
Keywords: Circumferential lumbar fusion, Allogenic cancellous bone, Autologous bone, PEEK-cage, Bone grafts
Introduction
The current gold standard for lumbar segmental spondylodesis is posterolateral stabilization by means of internal fixation, and ventral intercorporal interposition of an autogenous corticocancellous iliac crest graft [16, 23, 26]. In the absence of clinical differences, cages augmented with autogenous cancellous bone show similar mechanical characteristics to structural bone grafts [6, 18, 40]. Fusion rates of up to 100% have been achieved with these methods according to the literature [28, 35, 40].
With around 300,000 spinal fusion operations currently performed in the USA every year, and a complication rate of up to 30% when obtaining autogenous iliac crest grafts, there is clearly a need for alternatives [1, 2, 11, 13, 19]. Each alternative must be judged against the biological characteristics of the previous standard, based on factors like osteoinductivity, osteoconductivity and osteogenic potential [23, 26].
Allogenic cancellous bone is used successfully in the field of cervical spondylodesis, particularly for monosegmental treatment [9, 39]. Biologically, allogenic cancellous bone appears to be inferior in terms of a lack of osteoinductivity and osteogenic potential. There is also a markable risk of interindividual infections [8, 26]. The advantages of an allograft lie both in its availability, and in the prevention of a second operation coupled with the associated complication rate. Another advantage, is the long shelf life of the graft [29]. In order to avoid the transmission of infectious agents, allografts should be subjected to procedures for the inactivation of microorganisms.
Up until now, the literature has not yielded a prospective study comparing the use of allogenic cancellous bone with the use of autogenous cancellous bone in ventrally interposed polyetheretherketone cages (PEEK-cages) in human circumferential lumbar spondylodesis. The aim of the present study was to compare the application of allogenic cancellous bone in monosegmental lumbar spondylodesis with the use of autogenic cancellous bone from iliac crest in PEEK cages. Hypothesis of the current study was superiority in fusion of the autogenic cancellous bone. The hypothesis’ target parameters were both the osseous segmental consolidation and the clinical outcome over the chronological course of 1 year.
Materials and methods
Study design
Patients, who presented persistent lumbosacral, and/or, pseudoradicular complaints after an unsuccessful conservative therapy, covering a period of at least 6 months, were enrolled in this prospective, randomized, non-blind study. Randomization was performed through the computerized employing Randlist Software (DataInf GmbH, Tuebingen, Germany).
At the same time, osteochondrosis in Modic stage ≥2 with a residual disc height of less than seven millimeters, resulting from idiopathic intervertebral disc degeneration of segments L4/5 or L5/S1, had to be detectable in MRI [25]. The presence of an isthmic spondylolisthesis up until Grade I according to Meyerding, without the need of decompression in the degenerated segment, did not lead to exclusion.
Patients were excluded from the study if they had degeneration of adjacent segments. This was verified in every case by MRI and by discography in the level above and/ or below the degenerated one.
Further exclusion criteria included: additional degenerative findings; spinal deformities or destructive processes; previous operations on the lumbar spine; patients on long-term medication with corticoids or nonsteroidal anti-inflammatory drugs (NSAID), with pain chronification ≥ stage II according to Gerbershagen [14]; patients with osteoporosis, kidney and liver diseases, malignant tumors, a BMI > 30 kg/m²; pregnancy; and chronic nicotine, alcohol or drug abuse.
The conduct of this study was approved by the local ethics committee under decision No. 2015/Si. 280.
Patients and groups
Forty-four patients (22 men, 22 women), who treated surgically with a monosegmental ventrodorsal spondylodesis between September 2003 and July 2004, were included in this study and randomly allocated to two groups. The patients’ average age at the time of surgery was 45.5 (26–62) years.
Group 1—autogenous iliac crest cancellous bone (control group)
The 22 patients of the control group all received autogenous iliac crest cancellous bone. In 5 of the 22 patients, an isthmic spondylolisthesis up to Grade I was present. The autogenous cancellous bone was removed directly before the ventral main surgery and was obtained from the right iliac bone over a separate oblique approach, dorsolateral to the spina iliaca anterior superior. After deperiostation of about 2 cm², a trephine (inner diameter: 6.4 mm, outer diameter 8.0 mm, Aesculap, Tuttlingen, Germany) was used for cortical fenestration of the outer rim of the iliac crest. Cancellous bone, 2–3 cm3, was harvested with a spoon and was sterilely stored on ice until implantation. The iliac crest window was covered with both the cortical bone from the trephine and with bone wax. A drain was placed before closing the wound and was subsequently left for 2 days following surgery.
Group 2—allogenic cancellous bone (study group)
The 22 patients of the study group were treated with a block of freeze–dried, human allogenic cancellous bone (tissue bank, Charité, Institute for Transfusion Medicine, Berlin, Germany). The allogenic graft was rehydrated under sterile conditions in an isotonic saline solution for at least 30 min before application. In 6 of the 22 patients, an isthmic spondylolisthesis, up until grade I was observed.
Human allogenic cancellous bone [shelf-life at room temperature (15–25°C): 5 years] is licensed as a medical product, in accordance with section 105 AMG (German Medicines Law) (license No. 3004134.00.00). Potential bone tissue donors underwent clinical examination for a variety of infectious diseases; evident at time of death. These included: viral hepatitis; tuberculosis; syphilis; septicemia; systemic viral disease and mycoses. Positive donors, including those with malignancies; those treated previously with human growth factor preparations; and dura mater transplant recipients, were all excluded. Every bone tissue donor was tested for the hepatitis B virus surface antigen (HBsAg); for antibodies against HIV-1/2; HBc and HCV; for the HIV/HBV/HCV/HAV genome (PCR); and Treponema pallidum, ante or post mortem. Following the mechanical preparation and defatting with a chloroform/methanol mixture, a sterilization procedure was performed under constant agitation (laboratory shaker THYS 2, MLW, Leipzig, Germany), and at a low pressure (200 mbar). This was also done at room temperature employing a desiccator for 4 h. Lipid free transplants were covered with PES solution (v/v, 1/7.5) [PES: 2% peracetic acid, 96% ethanol, aqua ad iniectabilia (ratio v/v/v 2/1/1)] [30]. Lyophilization (lyophilizator TG 5.4 Vakutec, Heidenau, Germany) was carried out for 24 h under standard conditions (product: start −26°C, end +34°C; condenser: start −65°C, end −75°C; pressure: start 0.85 ATM, end 0.37 ATM). The residual moisture of the tissues was between 1 and 6%.
Surgical method
Circumferential spondylodesis was conducted in a single session in all cases. To begin with, anterior, and then posterior surgery was performed. After cleaning out the intervertebral disc and removing the cartilaginous endplate via a pararectal retroperitoneal approach, a Visios-PEEK-cage (Synthes GmbH, Solothurn, Switzerland) with defined dimensions 11 × 24 × 30 mm (height × depth × breadth) was press fit inserted into the intervertebral space after distraction in each group. In group 1 the cages were filled with an autogenous iliac crest cancellous bone. In group 2 they were filled with a standardized block of freeze–dried allogenic cancellous bone, which had been cut to the shape of the cage interior before sterilization. No further material was inserted around the cages in either group, because the cage covered the majority of the vertebra’s endplates. The cage dimensions and height have been kept constant in every patient to reach comparability of the fusion process, which is thought to be correlated to the amount of the graft and the distance to get fused.
Dorsal spondylodesis was conducted using a monoaxial angle-stabilizing screw and rod system (Colorado IITM, Medtronic, Memphis, USA). The autogenous corticocancellous material, obtained through the decortication and partial resection of the facet joints in the fusion area, was placed dorsally between the laminae and the facet joints, in each case.
Every patient was operated on by the same surgeon. Also, each of the patients were then mobilized without an orthesis, and given physiotherapy from the first postoperative day onwards.
Data collection
Each of the patients were given clinical and radiological examinations; preoperatively; postoperatively; and then 3, 6, 9 and 12 months subsequently.
During the perioperative period, the mean duration of surgery and intraoperative blood loss, as well as the length of the patients’ hospital stay were all recorded. Implant and non-implant related complications, ascertained both during and after the operation, were monitored up until follow-up.
The Oswestry Low Back Pain Disability Questionnaire Version 2.0 according to Fairbank [12] was used to assess subjective functional impairment. In addition, pain quantity was estimated by using a visual analog scale (VAS), with a scale graduation of 0–100 mm (0 mm—minimal pain; 100 mm—maximal pain). During the follow-up appointments, the patients were additionally asked about their degree of satisfaction with the operation, and also their willingness to undergo the operation again under the same conditions.
The qualitative radiographic evaluation of fusion was based on the criteria for vertebral body fusion using intersomatic cages suggested by McAfee et al. [24] and Ray [32]. This was on the basis of both plain and extension–flexion radiographs. Each of the following criteria had to be fulfilled for a positive decision:
Lack of any visible motion using Hutter’s method, or less than 2° of intersegmental change, as seen on flexion and extension radiographs in Simmons method [17, 34].
Lack of a dark halo around the implant material.
Lack of loss of disc space height of more than 1 mm, indicating a resistance to collapse of the cancellous vertebral bone.
Lack of visible fracture of the device, graft, or vertebrae.
Lack of substantial sclerotic changes in the recipient bone bed or the graft.
Visible bridging bone around the PEEK fusion cage as seen on anterior–posterior or lateral radiographs.
In addition, qualitative fusion was determined by thin-layer CT scans (layer thickness—1 mm) using sagittal and coronary plane reconstructions. According to the protocol of Williams et al. [36], fusion was defined by fulfilling each of the following criteria:
Lack of any lucency at the implant material margins.
Lack of any visible fracture of the device, graft, or vertebrae.
Lack of any cystic changes within the endplates adjacent to the implant.
Lack of any linear defects (fracture) through intervertebral new bone within, or adjacent to the PEEK-cage parallel to the endplates.
Lack of a high subsidence level of the cages or dislocation.
A bridging bone external to the cage.
The radiographs and CT-reconstructions were evaluated independently and blinded by both a radiologist specialized in spinal imaging, and an orthopedic surgeon. A second independent orthopedic surgeon was used to adjudicate conflicting fusion findings.
For evaluation of biological remodeling processes of the grafts used, the bone density of the bone grafts within the cages was determined in Hounsfield units at the measuring time points. This was based on the thin-section-CT data. The measurement was performed employing a standardized grid at a defined area within the PEEK cages. For comparison, the absolute mean values of the measuring area within the cages were calculated for both groups and compared. In addition, the respective change in bone density was determined in relation to the previous measuring time point.
Statistical analysis
The data from this study was analyzed through the use of the SPSS 14.0 statistics software program (SPSS Inc. Chicago, USA). A t test was used for normally distributed, continuous variables. The Wilcoxon-test or the Mann–Whitney U test was applied for continuous variables which were not normally distributed. Categorical variables were analyzed with Fisher’s exact test, or with a χ²-test. If needed, a Bonferroni correction was performed. The interobserver variability was tested using κ-statistics. The significance level for all the statistical tests was P = 0.05.
Results
Two patients from group 2; and two patients from group 1 failed to attend every follow-up and were therefore excluded. Up until the time of exclusion from the study, the course of the study had been free of complications in any of these patients.
Perioperative data
In group 1, segment L4/5 was treated in 4 patients (20.0%), and segment L5/S1 in 16 patients (80.0%). In group 2, the operation was conducted at the level L4/5 in 3 patients (15%) and at the level L5/S1 in 17 patients (85%).
The mean duration of surgery was significantly higher in the control group (P < 0.0001), whereby the average time taken to remove the iliac crest cancellous bone was 19 min in this group.
The mean surgery related blood loss and the average length of the patients’ hospital stay were not significantly different between the two groups (Table 1).
Table 1.
Demographic patient and intraoperative data
| Variable | Group 1 (autologous bone) | Group 2 (allogenic bone) |
|---|---|---|
| Number of patients (operated)a | 20 (22) | 20 (22) |
| Age (years) (Min.–Max.) | 45.4 (26–62) | 45.5 (34–62) |
| Gender (male/female) | 11/9 | 10/10 |
| Mean blood loss (ml) | 212 | 181 |
| Mean OP-duration (min)b | 137 | 119 |
| Mean hospitalization (days) | 7 | 7 |
aFour patients got lost during follow-up and were excluded
bTime from cut to suture, without the time for turning around the patient
In the control group, a hematoma occurred postoperatively in five patients (25.0%). In only two of these patients the hematoma was visible in the skin maybe because of an insufficient closure of the fascia. Because of a swelling and or pain in the first 2 weeks after operation a sonography was performed in three other patients, observing a deep subfascial hematoma.
One patient in the control group experienced pain at the site of removal up until the third month, and another up until the ninth month postoperatively. A third patient experienced pain up until the time of the final follow-up. Revision surgery was not necessary in either of the two groups.
Follow-up examinations
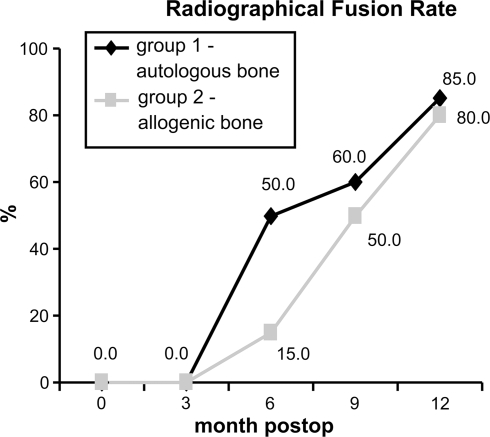
The radiological fusion rate did not show any significant differences between the two groups at any time throughout the examination; other than at the 6-month follow-up. Three months postoperatively, fusion was not observed in any patients, of either group. After 6 months, ten patients in group 1 (50.0%), and three patients (15.0%) in group 2, had undergone fusion (P = 0.041). Twelve of the 20 patients (60.0%) in group 1 showed fusion after 9 months, compared to that of 10 patients (50.0%) in group 2. After 12 months, 17 patients (85.0%) in group 1, and 16 patients (80.0%) in group 2, showed fusion in surgical area (κ = 0.90) (Fig. 1).
Fig. 1.
Radiographical fusion rate between the groups over time
The evaluation of the fusion rate through computed tomography was not able to show any significant differences between the two groups during follow-up. The computed tomographic fusion rates obtained after 3 months were 5.0% for group 2 (n = 1), versus 10.0% for group 1 (n = 2). At 6 months postoperatively, the rate increased in group 2 to 15.0% (three patients), and in group 1, to 35.0% (seven patients). At the 9-month control, the number of fusions in group 2 reached 45.0% (nine patients), while in group 1, the percentage rose to 55.0% (11 patients). At the end of the examination series (12 months), both patient groups had achieved a comparable rate of ventral fusions; group 2 had reached 65.0% (n = 13), versus group 1 at 70.0% (n = 14) (κ = 0.86) (Figs. 2, 3a, b).
Fig. 2.
Fusion rate in computed tomography between the groups over time
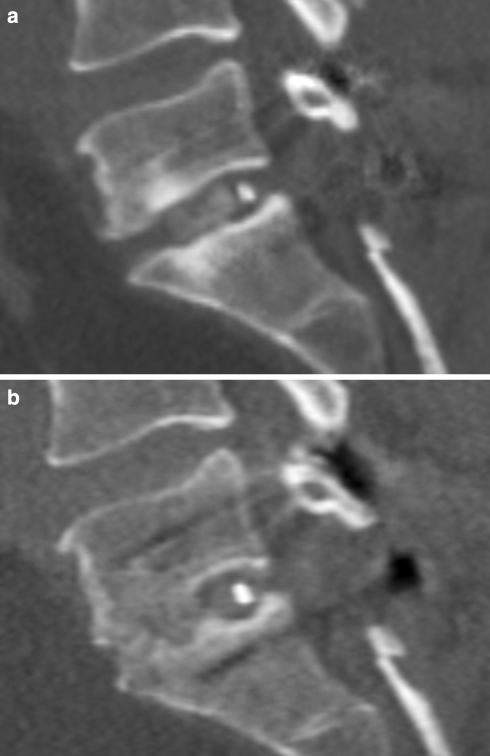
Fig. 3.
Two dimensional reconstructions (computed tomography) of a patient with fusion a at 3-month follow-up. Cage interposition at level L5/S1; no bridging bone but bone graft inside the cage; b at 12-month follow-up. Compared to the 3-month follow-up presenting increasing bone mass bridging the segment inside and anterior to the cage
In quantitative computed tomographic determination of the mean absolute values for bone density at the measuring area, a significant difference between the groups was only found at the immediate, postoperative time point. Group 2 showed a lower density here (P = 0.007).
The difference in mean absolute values of the measuring area up until the next examination time point was positive in the control group overall, but was negative in the intervals 0–3 and 6–9 months. In contrast to this, the measuring area showed positive bone density differences in the study group, except for the examination intervals 3–6 and 9–12 months (negative here). Only in group 2 was the bone density difference in the time interval 0–3 months statistically significant (P = 0.034) (Fig. 4).
Fig. 4.
Mean bone mineral density inside the cage (CT) between the groups over time
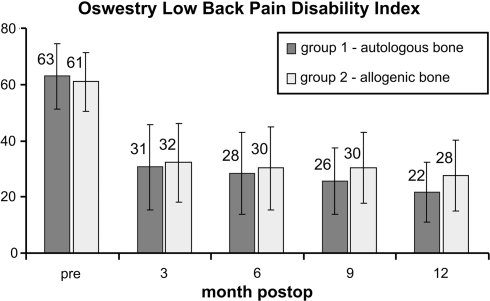
Based on the data, the Oswestry score did not reveal any significant difference between the two surgery groups at any of the postoperative follow-up time points. In both groups, a significant improvement compared to that of their preoperative condition was shown at all time points (P < 0.0001 in each case) (Fig. 5).
Fig. 5.
Oswestry low back pain disability index between the groups over time. Whiskers indicate a single standard deviation
The visual analog scale showed comparable analog values in both groups preoperatively (67.90 mm group 1 vs. 65.40 mm group 2). At each time point, a significant improvement on the preoperative value could be determined in both groups (P < 0.0001 between all time points in both groups). Based on the data, there was no significant difference between the two groups at any of the time points. After 12 months, group 1 showed a mean analog value of 22.05 mm and group 2 of 22.80 mm (Table 2).
Table 2.
Visual analog scale
| Time | Group 1 (mm) (autologous bone) | Group 2 (mm) (allogenic bone) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| preop | 67.9 | 12.3 | 65.4 | 8.3 |
| 3 month postop | 31.0 | 12.4 | 29.9 | 12.4 |
| 6 month postop | 29.9 | 15.5 | 29.8 | 15.7 |
| 9 month postop | 26.4 | 9.2 | 27.1 | 9.9 |
| 12 month postop | 22.0 | 9.3 | 22.8 | 10.5 |
SD standard deviation
Both the patients’ subjective satisfaction with the surgical outcome, and their response to the hypothetical question concerning their willingness to undergo the operation again, did not differ between the two groups at any time point (Table 3).
Table 3.
Patient’s satisfaction
| Time | Variable | Group 1 (n = 20) (autologous bone) (%) | Group 2 (n = 20) (allogenic bone) (%) |
|---|---|---|---|
| 3-month postop | Excellent/good | 85 | 90 |
| Operate again | 80 | 85 | |
| 6-month postop | Excellent/good | 85 | 75 |
| Operate again | 80 | 80 | |
| 9-month postop | Excellent/good | 80 | 75 |
| Operate again | 80 | 75 | |
| 12-month postop | Excellent/good | 75 | 80 |
| Operate again | 75 | 80 |
Discussion
The present study is the first in conducting a prospective, randomized comparison of the clinical application of freeze–dried allogenic cancellous bone with autogenous iliac crest cancellous bone; within ventrally intervertebrally interposed PEEK cages in segmental lumbar spondylodesis. The clinical outcomes of both procedures are comparable. The radiological fusion rates imply a chronologically delayed bone consolidation in the study group, but do not show any differences after a 1-year period. Nevertheless, non-significant results must be interpreted carefully because of the number of patients included into this study. Differences between the groups may also arise after a longer follow-up period.
Following 12 months, the fusion rate achieved in both groups corresponds, both radiologically and in the CT, to the fusion rates described in the literature for the same surgical method and a similarly restrictive evaluation method [33]. It does not show a superiority of one of the implants over the other. The lower fusion rate, shown radiologically in the study group at the 6-month follow-up, implies a chronological delay of spondylodesis. The chronological course of the fusion rate in CT supports this hypothesis, but cannot be verified with significant results. The different course of consolidation is possibly attributable to a lower biological competence of allogenic cancellous bone; taking into account the osteoinductivity and osteogenic potential [26].
The radiological evaluation of fusion rates must be viewed critically. Beside surgical exploration, it currently represents the only way of obtaining an objective assessment of fusion rates. It also fluctuates, depending on the method used [33]. In this study, rather restrictive parameters were chosen for the evaluation of fusion. Nevertheless, it must be taken into account, that the evaluation of radiological procedures is subject to a predictive value of no more than 70% [7, 10]. Surprisingly, we were able to observe bone bridging around the cage in most of the cases despite not placing graft at this position (see Fig. 3b). Comparable bridging was described in previous studies after implantation of lumbar disc prosthesis [31]. Maybe, this observation is attributable to the hematoma resulting from preparation of the endplates which serves as a primary callus in a bone defect.
The lower bone density, determined directly postoperatively in the study group, is probably attributable to the reduced proportion of organic tissue in the allogenic graft.
A remodeling of autogenous iliac crest grafts within approximately 10–12 weeks after surgery has been described in the literature [2]. A remodeling of the autogenous graft would also explain the drop in mean bone density in the examination interval between 0 and 3 months postoperatively.
In contrast, a significant increase in bone density occurred in the study group in the same time interval. This circumstance may possibly be explained by a colonization of the allogenic grafts with recruited osteoblasts and a consecutive increase in biomass.
Overall, the graphic presentation of bone density over time in the control group shows a W-shaped profile compared to an M-shaped profile in the study group. This situation also implies a chronological shift of remodeling processes in the allogenic graft to approximately 3 months later.
During the evaluation of clinical parameters, the evident lack of any detectable differences between the two groups leads to the conclusion that the two procedures are comparable. The later incidence of osseous consolidation in the study group did not lead to any complications that may have influenced the clinical data. Twelve months postoperatively, the improvements seen in both the Oswestry Low Back Pain Disability Questionnaire, and in the visual analog scale, were consistent with the data given in the literature [15, 27].
In the control group, hematomas of the graft removal site were observed in five patients (25.0%). Persistent pain, lasting up to a 12-month period, was also ascertained in three patients (15.0%). The spectrum of complications described in the literature ranges from chronic pain; pelvic fractures; hematomas; vascular and nerve injuries; to superficial and deep infections [1, 2, 13, 19]. In addition, the removal of autogenous iliac crest grafts results in a prolonged surgical duration. This was demonstrated in this study, and must be taken into account when comparing the costs of the grafts [2].
Until now, conducted animal experiments have shown a superiority of autogenous over allogenic cancellous bone [4, 38]. To counterpoint this, the position of studies on humans appears to be contradictory. Despite the lower biological competence when compared to autogenous cancellous bone, good results could be achieved with allogenic grafts in cervical spondylodeses [9, 39]. Kozak et al. [20] also used allogenic cancellous bone in femoral ring allografts on the lumbar spine, and retrospectively demonstrated a fusion rate of 97%. The research group surrounding Wimmer retrospectively compared the use of non-structural autografts from the iliac crest to non-structural allografts from the femoral head. Similarly to the present study, they did not find any significant difference in the mean pseudoarthrosis rate between the groups—after a 2- to 4-year interval [37]. By contrast, Brantigan et al. [5] described a superiority of autogenous cancellous bone in PEEK cages when compared to ventrally interposed allogenic cancellous bone blocks without cages; 2 years after lumbar spondylodesis by means of posterolateral interbody fusion, and internal fixation. However, two differing surgical methods were evaluated here.
Beside osteoconductivity, the requirements placed on a graft include biocompatibility, mechanical–physical primary stability and degradability, on which remodeling and thus the resulting bone stability decisively depends [3]. The synthetic intervertebral cages used in this study were able to compensate for possible structural deficits of cancellous bone grafts mechanically [6, 18, 21]. In studies without a cage the delayed fusion of the allogenic graft group could possibly lead to subsidence, micromotion and deterioration of the clinical result.
During sterilization and preparation, the osteoconductivity of allogenic cancellous bone drops when compared to that of autogenous cancellous bone [22, 29]. Whether the reduced osteoconductivity or the lack of both, osteoinductivity and osteogenic potential of allogenic grafts ultimately leads to a delayed radiological spondylodesis, remains a matter for future research work [26].
When compared with autogenous iliac crest cancellous bone, freeze–dried allogenic cancellous bone offers the advantages of sufficient availability, and the absence of any morbidity on removal. Compared to grafts like tricalcium phosphate it supports real bone ultrastructure for cellular ingrowths and seeding. A further benefit is the decrease in surgery duration, enabled by the avoidance of surgical graft recovery. Disadvantages arise from the possible risk of interindividual infections in the absence of inactivation procedures, and also in the costs of manufacturing the grafts [8].
The results of our study show that freeze–dried allogenic cancellous bone can be used for monosegmental spondylodeses. When compared with autologous cancellous bone, similar clinical results are attained. Additionally, despite a delayed consolidation process detected radiologically, comparable fusion rates following a 12-month period are achieved.
References
- 1.Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA (1996) Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res 300–309. doi:10.1097/00003086-199608000-00037 [DOI] [PubMed]
- 2.Boden SD. Overview of the biology of lumbar spine fusion and principles for selecting a bone graft substitute. Spine. 2002;27:S26–S31. doi: 10.1097/00007632-200208151-00007. [DOI] [PubMed] [Google Scholar]
- 3.Boden SD, Schimandle JH. Biologic enhancement of spinal fusion. Spine. 1995;20:113S–123S. doi: 10.1097/00007632-199512151-00007. [DOI] [PubMed] [Google Scholar]
- 4.Brantigan JW, McAfee PC, Cunningham BW, Wang H, Orbegoso CM. Interbody lumbar fusion using a carbon fiber cage implant versus allograft bone An investigational study in the Spanish goat. Spine. 1994;19:1437–1444. doi: 10.1097/00007632-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Brantigan JW, Steffee AD. A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine. 1993;18:2106–2107. doi: 10.1097/00007632-199310001-00030. [DOI] [PubMed] [Google Scholar]
- 6.Brantigan JW, Steffee AD, Geiger JM. A carbon fiber implant to aid interbody lumbar fusion mechanical testing. Spine. 1991;16:S277–S282. doi: 10.1097/00007632-199106001-00020. [DOI] [PubMed] [Google Scholar]
- 7.Brodsky AE, Kovalsky ES, Khalil MA. Correlation of radiologic assessment of lumbar spine fusions with surgical exploration. Spine. 1991;16:S261–S265. doi: 10.1097/00007632-199106001-00017. [DOI] [PubMed] [Google Scholar]
- 8.Buck BE, Malinin TI, Brown MD. Bone transplantation and human immunodeficiency virus. An estimate of risk of acquired immunodeficiency syndrome (AIDS) Clin Orthop Relat Res. 1989;240:129–136. [PubMed] [Google Scholar]
- 9.Buttermann GR, Glazer PA, Bradford DS (1996) The use of bone allografts in the spine. Clin Orthop Relat Res 75–85. doi:10.1097/00003086-199603000-00010 [DOI] [PubMed]
- 10.Dawson EG, Clader TJ, Bassett LW. A comparison of different methods used to diagnose pseudarthrosis following posterior spinal fusion for scoliosis. J Bone Joint Surg Am. 1985;67:1153–1159. [PubMed] [Google Scholar]
- 11.Deyo RA, Nachemson A, Mirza SK. Spinal-fusion surgery—the case for restraint. N Engl J Med. 2004;350:722–726. doi: 10.1056/NEJMsb031771. [DOI] [PubMed] [Google Scholar]
- 12.Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry Low Back Pain Disability Questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 13.Fernyhough JC, Schimandle JJ, Weigel MC, Edwards CC, Levine AM. Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine. 1992;17:1474–1480. doi: 10.1097/00007632-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Gerbershagen HU, Lindena G, Korb J, Kramer S. Health-related quality of life in patients with chronic pain. Schmerz. 2002;16:271–284. doi: 10.1007/s00482-002-0164-z. [DOI] [PubMed] [Google Scholar]
- 15.Glassman S, Gornet MF, Branch C, Polly D, Jr, Peloza J, Schwender JD, Carreon L. MOS short form 37 and Oswestry Disability Index outcomes in lumbar fusion: a multicenter experience. Spine J. 2006;6:21–26. doi: 10.1016/j.spinee.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Hahnel H, Muschik M, Zippel H, Gutsche H. Lumbar segmental spondylodesis-isolated ventral or combined dorsoventral? A comparison of results. Z Orthop Ihre Grenzgeb. 1991;129:197–203. doi: 10.1055/s-2008-1040183. [DOI] [PubMed] [Google Scholar]
- 17.Hutter CG. Posterior intervertebral body fusion. A 25-year study. Clin Orthop Relat Res. 1983;179:86–96. doi: 10.1097/00003086-198310000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Janssen ME, Nguyen C, Beckham R, Larson A. Biological cages. Eur Spine J. 2000;9(Suppl 1):S102–S109. doi: 10.1007/PL00008315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz JN. Lumbar spinal fusion Surgical rates, costs, and complications. Spine. 1995;20:78S–83S. doi: 10.1097/00007632-199512151-00002. [DOI] [PubMed] [Google Scholar]
- 20.Kozak JA, Heilman AE, O’Brien JP. Anterior lumbar fusion options. Technique and graft materials. Clin Orthop Relat Res. 1994;300:45–51. [PubMed] [Google Scholar]
- 21.Lund T, Oxland TR, Jost B, Cripton P, Grassmann S, Etter C, Nolte LP. Interbody cage stabilisation in the lumbar spine: biomechanical evaluation of cage design, posterior instrumentation and bone density. J Bone Joint Surg Br. 1998;80:351–359. doi: 10.1302/0301-620X.80B2.7693. [DOI] [PubMed] [Google Scholar]
- 22.Malanin TI. Bone Grafts and Bone Substitutes. In: Habal MB, Reddi AH, editors. Acquisition and banking of bone allografts. Philadelphia: WB Saunders; 1992. [Google Scholar]
- 23.Marchesi DG. Spinal fusions: bone and bone substitutes. Eur Spine J. 2000;9:372–378. doi: 10.1007/s005860000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAfee PC, Boden SD, Brantigan JW, Fraser RD, Kuslich SD, Oxland TR, Panjabi MM, Ray CD, Zdeblick TA. Symposium: a critical discrepancy-a criteria of successful arthrodesis following interbody spinal fusions. Spine. 2001;26:320–334. doi: 10.1097/00007632-200102010-00020. [DOI] [PubMed] [Google Scholar]
- 25.Modic MT, Masaryk TJ, Ross JS, Carter JR. Imaging of degenerative disk disease. Radiology. 1988;168:177–186. doi: 10.1148/radiology.168.1.3289089. [DOI] [PubMed] [Google Scholar]
- 26.Muschler G, Lane J, Dawson E. The biology of spinal fusion. In: Cotler J, Cotler H, editors. Spinal fusion, science and technique. Heidelberg: Springer; 1990. [Google Scholar]
- 27.Niemeyer T, Bovingloh AS, Halm H, Liljenqvist U. Results after anterior-posterior lumbar spinal fusion: 2–5 years follow-up. Int Orthop. 2004;28:298–302. doi: 10.1007/s00264-004-0577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavlov PW, Meijers H, Limbeek J, Jacobs WC, Lemmens JA, Obradov-Rajic M, Kleuver M. Good outcome and restoration of lordosis after anterior lumbar interbody fusion with additional posterior fixation. Spine. 2004;29:1893–1899. doi: 10.1097/01.brs.0000137067.68630.70. [DOI] [PubMed] [Google Scholar]
- 29.Pelker RR, Friedlaender GE. Biomechanical aspects of bone autografts and allografts. Orthop Clin North Am. 1987;18:235–239. [PubMed] [Google Scholar]
- 30.Pruss A, Baumann B, Seibold M, Kao M, Tintelnot K, Versen R, Radtke H, Dörner T, Pauli G, Göbel UB. Validation of the sterilization procedure of allogeneic avital bone transplants using peracetic acid-ethanol. Biologicals. 2001;29:59–66. doi: 10.1006/biol.2001.0286. [DOI] [PubMed] [Google Scholar]
- 31.Putzier M, Funk JF, Schneider SV, Gross C, Tohtz SW, Khodadadyan-Klostermann C, Perka C, Kandziora F. Charite total disc replacement–clinical and radiographical results after an average follow-up of 17 years. Eur Spine J. 2006;15:183–195. doi: 10.1007/s00586-005-1022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ray CD. Threaded titanium cages for lumbar interbody fusions. Spine. 1997;22:667–679. doi: 10.1097/00007632-199703150-00019. [DOI] [PubMed] [Google Scholar]
- 33.Santos ER, Goss DG, Morcom RK, Fraser RD. Radiologic assessment of interbody fusion using carbon fiber cages. Spine. 2003;28:997–1001. doi: 10.1097/00007632-200305150-00007. [DOI] [PubMed] [Google Scholar]
- 34.Simmons JW. Posterior lumbar interbody fusion with posterior elements as chip grafts. Clin Orthop Relat Res. 1985;193:85–89. [PubMed] [Google Scholar]
- 35.Videbaek TS, Christensen FB, Soegaard R, Hansen ES, Hoy K, Helmig P, Niedermann B, Eiskjoer SP, Bunger CE. Circumferential fusion improves outcome in comparison with instrumented posterolateral fusion: long-term results of a randomized clinical trial. Spine. 2006;31:2875–2880. doi: 10.1097/01.brs.0000247793.99827.b7. [DOI] [PubMed] [Google Scholar]
- 36.Williams AL, Gornet MF, Burkus JK. CT evaluation of lumbar interbody fusion: current concepts. AJNR Am J Neuroradiol. 2005;26:2057–2066. [PMC free article] [PubMed] [Google Scholar]
- 37.Wimmer C, Krismer M, Gluch H, Ogon M, Stockl B (1999) Autogenic versus allogenic bone grafts in anterior lumbar interbody fusion. Clin Orthop Relat Res 122–126. doi:10.1097/00003086-199903000-00015 [DOI] [PubMed]
- 38.Xue Q, Li H, Zou X, Bunger M, Egund N, Lind M, Christensen FB, Bunger C. Healing properties of allograft from alendronate-treated animal in lumbar spine interbody cage fusion. Eur Spine J. 2005;14:222–226. doi: 10.1007/s00586-004-0771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zdeblick TA, Ducker TB. The use of freeze–dried allograft bone for anterior cervical fusions. Spine. 1991;16:726–729. doi: 10.1097/00007632-199107000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Zelle B, Konig F, Enderle A, Bertagnoli R, Dorner J. Circumferential fusion of the lumbar and lumbosacral spine using a carbon fiber ALIF cage implant versus autogenous bone graft: a comparative study. J Spinal Disord Tech. 2002;15:376–379. doi: 10.1097/00024720-200210000-00005. [DOI] [PubMed] [Google Scholar]