Abstract
Osteoclasts (OCs) are responsible for bone resorption in inflammatory joint diseases. Monocyte chemotactic protein-1 (MCP-1) has been shown to induce differentiation of monocytes to OC precursors, but nothing is known about the underlying mechanisms. Here, we elucidate how MCPIP, induced by MCP-1, mediates this differentiation. Knockdown of MCPIP abolished MCP-1-mediated expression of OC markers, tartrate-resistant acid phosphatase, and serine protease cathepsin K. Expression of MCPIP induced p47PHOX and its membrane translocation, reactive oxygen species formation, and induction of endoplasmic reticulum (ER) stress chaperones, up-regulation of autophagy marker, Beclin-1, and lipidation of LC3, and induction of OC markers. Inhibition of oxidative stress attenuated ER stress and autophagy, and suppressed expression of OC markers. Inhibition of ER stress by a specific inhibitor or by knockdown of IRE1 blocked autophagy and induction of OC markers. ER stress inducers, tunicamycin and thapsigargin, induced expression of OC markers. Autophagy inhibition by 3′-methyladenine, LY294002, wortmannin or by knockdown of Beclin-1 or Atg 7 inhibited MCPIP-induced expression of OC markers. These results strongly suggest that MCP-1-induced differentiation of OC precursor cells is mediated via MCPIP-induced oxidative stress that causes ER stress leading to autophagy, revealing a novel mechanistic insight into the role of MCP-1 in OCs differentiation.
Keywords: MCP-1, MCPIP, osteoclast differentiation, reactive oxygen species, ER stress
Introduction
There is strong evidence that osteoclast (OC) is the principal cell type responsible for bone resorption in inflammatory joint diseases (Harris, 1990; Sakiyama et al., 2001; Sato and Takayanagi, 2006; Mundy, 2007). Rheumatoid arthritis (RA) is characterized by the presence of inflammatory synovitis accompanied by the destruction of the joint cartilage and bone (Harris, 1990; Mundy, 2007; Sugimura and Li, 2010). OCs are bone-resorbing cells that differentiate from hematopoietic precursors of the monocyte/macrophage lineage (Sakiyama et al., 2001; Boyce et al., 2007). OCs are multinuclear giant cells that stain positive for tartrate-resistant acid phosphatase (TRAP) and serine protease cathepsin K (CTSK) (Kiviranta et al., 2001; Boyce et al., 2007).
Monocyte chemotactic protein-1 (MCP-1), a CC chemokine commonly found at the site of tooth eruption, RA bone degradation, and bacterially induced bone loss (Wise et al., 2002), is known to induce differentiation of monocytes into TRAP and CTSK-positive precursors of OCs. MCP-1 is expressed by mature OCs and its expression is regulated by nuclear factor-κB (NF-κB) (Kim et al., 2005). Several reports showed that MCP-1 is induced by NF-κB ligand RANKL and promotes OC fusion into multinuclear TRAP-positive cells without bone-resorption activity (Kim et al., 2006a,b), which might be called OC precursors. Recently, it has been reported that MCP-1 plays an important role in regulating OC differentiation in an autocrine/paracrine manner under stimulation by RANKL (Miyamoto et al., 2009). How MCP-1 mediates OC differentiation remains unclear.
The cellular effect of MCP-1 is mediated by the CCR2, a G-protein-coupled receptor that is induced by the receptor activator of RANKL (Gerszten et al., 2001; Kim et al., 2005). The signaling process initiated by MCP-1 binding to CCR2 leads to changes in gene expression. Recently, it was found that this MCP-1 binding leads to the induction of a novel zinc-finger protein called MCPIP in human peripheral blood monocytes (Zhou et al., 2006). The biological functions of MCPIP remain poorly understood. Recent results suggest that MCPIP mediates several biological processes initiated by MCP-1. These included cardiomyocyte death (Younce and Kolattukudy, 2010; Younce et al., 2010), adipogenesis (Younce et al., 2009), angiogenesis (Niu et al., 2008), and glial differentiation of neuroprogenitor cells (Vrotsos et al., 2009). These findings suggest that MCPIP is an important mediator of MCP-1-induced processes. Therefore, we postulated that the role of MCP-1 in OC differentiation might be mediated via MCPIP. Here we report that MCP-1 induces differentiation of monocytic cells into OC precursors via MCPIP. We present evidence that strongly suggests that MCPIP mediates differentiation of OC precursors via induction of oxidative stress that causes endoplasmic reticulum (ER) stress that leads to autophagy involved in osteoclastogenesis. Our findings implicate that MCPIP is a novel factor involved in OC precursor differentiation, and thus it may be a new target for diagnosis and treatment of osteoporosis-related disease.
Results
MCP-1 induces OC-related gene expression via MCPIP in human bone marrow monocytes
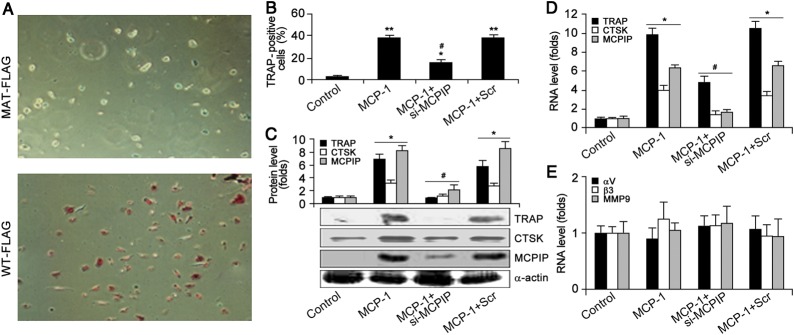
It has been demonstrated that MCP-1 induces TRAP-positive OC precursor formation from human peripheral blood mononuclear cells (Kim et al., 2006a). Here, we found that 50 ng/ml MCP-1 induced TRAP-positive OC precursor cell formation from human bone marrow mononuclear cells (BMCs) (Figure 1B). Immunoblotting (Figure 1C) and real-time polymerase chain reaction (PCR) (Figure 1D) showed that MCP-1 treatment induced expression of OC markers TRAP and CTSK. However, MCP-1 did not affect the expression of the OC functional markers αV integrin, β3 integrin, and MMP9 (Figure 1E). We also found that 50 ng/ml MCP-1 induced up-regulation of MCPIP protein and mRNA levels, which can be suppressed by treatment with MCPIP small interfering RNA (siRNA) (Figure 1C and D). Compared with FLAG-tagged empty vector (MAT-FLAG), expression of FLAG-tagged MCPIP (WT-FLAG) induced TRAP-positive OC precursor cell formation (Figure 1A). MCPIP siRNA also significantly inhibited the formation of OC precursor cells that expressed TRAP and CTSK but showed no effects on expression of αV integrin, β3 integrin, and MMP9 (Figure 1C–E). These results suggest that induction of the TRAP-positive OC precursor cells by MCP-1 treatment of BMCs was mediated via MCPIP.
Figure 1.
MCP-1 induces OC precursor differentiation which was mediated by MCPIP. (A and B) BMCs were transfected with FLAG-tagged MCPIP (WT-MCPIP) or FLAG-tagged empty vector (MAT-FLAG). TRAP-expressing cells were stained and viewed under the Nikon microscope (A). BMNCs treated with MCP-1 alone or with MCPIP siRNA or Scr siRNA. TRAP-expressing cells were stained and TRAP-positive cell proportion was measured (B). At least three fields (∼500 cells), were chosen. *P< 0.05 and **P< 0.01 versus control; #P< 0.05 versus Scr. (C) Immunoblotting showing the expression of TRAP, CTSK, and MCPIP induced by MCP-1. Data were mean ± SD (n = 3). *P< 0.05 versus control; #P< 0.05 versus Scr. (D) Real-time PCR showing the transcription of TRAP, CTSK, and MCPIP induced by MCP-1. *P< 0.05 versus control; #P< 0.05 versus Scr. (E) Real-time PCR showing the transcription of αV integrin, β3 integrin, and MMP9.
Forced expression of MCPIP induces differentiation of monocytes into OC precursors
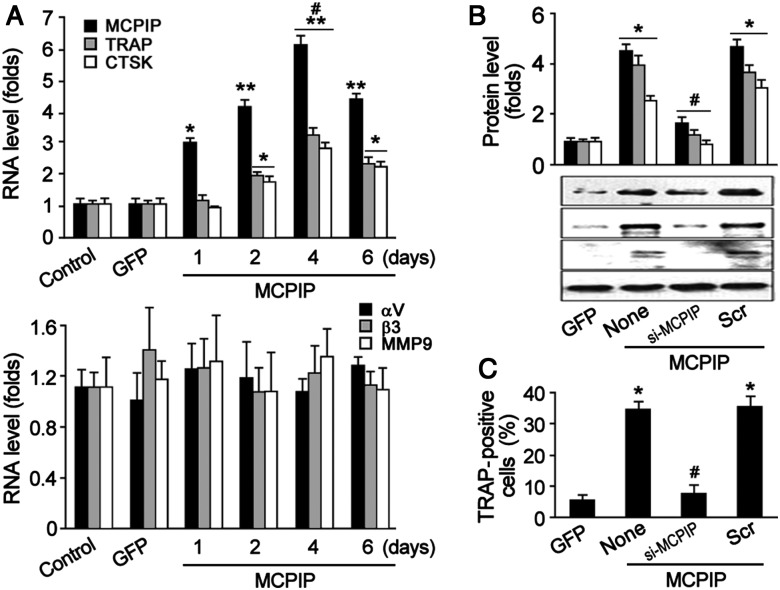
If MCP-1-induced differentiation of OC precursors is mediated by MCPIP, forced expression of MCPIP in monocytes might be expected to induce the formation of TRAP-positive OC precursor cells without MCP-1. To test this possibility, BMCs were transfected with MCPIP-GFP expression vector (Figure 2); increased expression of MCPIP was found 24 h after transfection at mRNA levels as measured by real-time PCR and its expression reached the peak at 4 days after transfection (Figure 2A, upper panel). The expression of TRAP and CTSK was induced at 2 days after MCPIP transfection and reached the peak at 4 days. However, the expression of αV integrin, β3 integrin, and MMP9 showed no significant changes after MCPIP transfection (Figure 2A, bottom panel). Immunoblot analysis showed that MCPIP overexpression induced expression of TRAP and CTSK (Figure 2B), and TRAP staining showed that MCPIP expression significantly elevated formation of TRAP-positive cells (Figure 2C). When MCPIP siRNA was transfected into BMCs for 24 h prior to MCPIP transfection, MCPIP expression was knocked down and the expression of OC-related genes TRAP and CTSK also were down-regulated (Figure 2B). Moreover, the percentage of TRAP-positive cells was lowered by treatment with MCPIP siRNA (Figure 2C). These results suggest that MCPIP transfection causes induction of OC-related genes and formation of TRAP-positive OC precursor cells.
Figure 2.
MCPIP overexpression induces OC-related marker TRAP and CTSK expression. (A) Real-time PCR showing the transcription of TRAP, CTSK, and MCPIP (upper panel) and αV integrin, β3 integrin, and MMP9 (lower panel) induced by MCPIP transfection. *P< 0.05 and **P< 0.01 versus control or GFP; #P< 0.05 versus day 2 or 6. (B) Immunoblotting showing the expression of TRAP, CTSK, and MCPIP induced by MCPIP transfection. Data were mean ± SD (n = 3). *P< 0.01 versus GFP; #P< 0.05 versus MCPIP or Scr. (C) Percentage of TRAP-positive cells. *P< 0.01 versus GFP; #P< 0.01 versus none or Scr.
MCPIP-induced reactive oxygen species production is involved in OC precursor differentiation
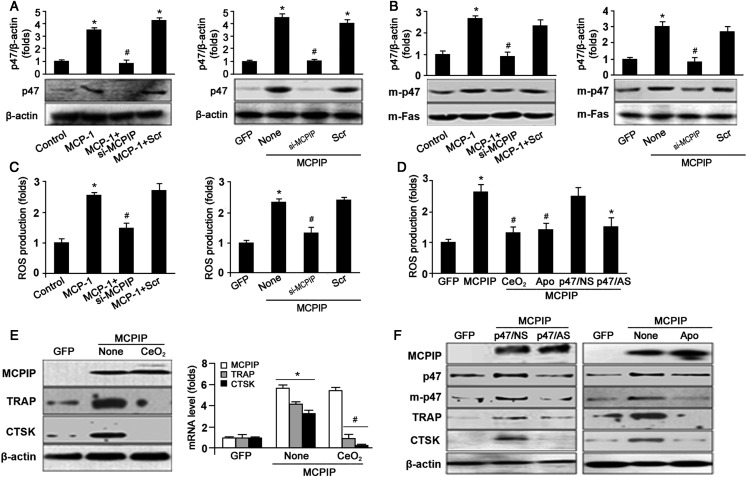
Reactive oxygen species (ROS) derived from NADPH oxidase have been suggested to regulate OC differentiation and prolong the survival of OC precursors (Yamasaki et al., 2009). p47PHOX, a regulatory subunit of NADPH oxidase, has been implicated in ROS generation (Decoursey and Ligeti, 2005). We tested whether forced expression of MCPIP could induce expression and activation of NADPH oxidase by translocation of p47PHOX into the membrane and produce ROS. Dihydrorhodamine 123 (DHR123) staining revealed ROS production by MCPIP transfected cells but not MAT-FLAG controls (Supplementary Figure S1). Immunoblot analysis showed that MCP-1 treatment and MCPIP expression increased the expression of p47PHOX (Figure 3A) and its translocation from cytoplasm into the membrane (Figure 3B). ROS production caused by MCP-1 treatment and MCPIP expression was assessed by DHR123 staining and results showed that MCP-1 treatment and MCPIP transfection remarkably increased ROS generation. Moreover, the effects of MCP-1 treatment or forced MCPIP expression on the expression and translocation of p47PHOX and ROS generation were inhibited by MCPIP siRNA (Figure 3A–C).
Figure 3.
ROS production involvement in MCPIP/MCP-1-induced OC precursor differentiation. (A) Western blot showed that MCP-1 treatment or MCPIP transfection induces p47PHOX expression. *P< 0.05 versus control or GFP; #P< 0.05 versus Scr. (B) Immunoblotting showed that MCP-1 or MCPIP overexpression induces an increase in cytoplasmic membrane-associated p47PHOX. *P< 0.05 versus control or GFP; #P< 0.05 versus Scr. (C) ROS production induced by MCP-1 or MCPIP transfection, or siRNA was detected by using DHR123. *P< 0.05 versus control or GFP; #P< 0.05 versus Scr. (D) MCPIP-induced ROS production was inhibited by ROS, NAD(P)H oxidase inhibitors, and p47PHOX knockdown. *P< 0.05 versus GFP, #P< 0.05 versus MCPIP only. *P< 0.05 compared with p47PHOX non-specific (NS) oligonucleotides transfection cells. (E) The effect of CeO2 on MCPIP, TRAP, and CTSK expression induced by MCPIP transfection. Data were mean ± SD (n = 3). *P< 0.05 versus GFP; #P< 0.05 versus MCPIP only. (F) The effect of p47PHOX AS and apocynin on the expression of related proteins. m-p47, membrane located p47PHOX; p47, total p47PHOX; p47/NS, non-specific RNA; p47/AS, antisense RNA. Apo, apocynin.
To understand whether ROS production is involved in MCPIP-mediated formation of TRAP-positive OC precursor cells, BMCs were treated with CeO2 nanoparticles, an inhibitor of ROS (Tsai et al., 2007) prior to MCPIP transfection. We found that MCPIP-induced ROS production was significantly inhibited by CeO2 (Figure 3D). Immunoblot and real-time PCR analysis showed that CeO2 inhibited MCPIP-induced TRAP and CTSK expression both at protein and mRNA levels (Figure 3E and Supplementary Figure S2A). We further tested the effect of apocynin, an inhibitor of NADPH oxidase on MCPIP-induced induction of OC-related genes TRAP and CTSK. We found that apocynin suppressed MCPIP-induced ROS production (Figure 3D) and expression of TRAP and CTSK (Figure 3F and Supplementary Figure S2E). Moreover, apocynin inhibited MCPIP-induced expression and membrane translocation of p47PHOX (Figure 3F and Supplementary Figure S2D). Furthermore, knock-down of p47PHOX by its specific antisense oligonucleotides (p47/AS) also decreased ROS production, expression and translocation of p47PHOX, and expression of OC-related genes TRAP and CTSK (Figure 3F and Supplementary Figure S2B and C). These results suggest that MCPIP causes ROS production by up-regulating p47PHOX expression and its membrane translocation, and that ROS generation is involved in MCP-1-induced OC precursor differentiation.
MCPIP-induced ROS production causes an ER stress response that is involved in OC precursor differentiation
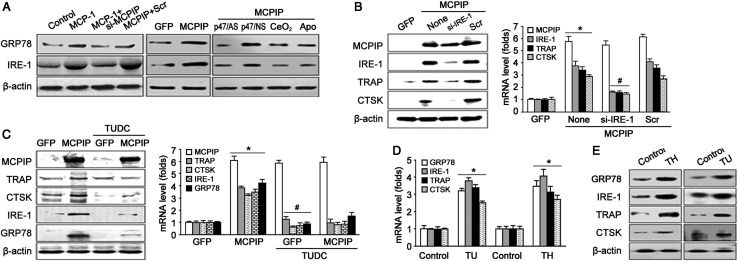
It has been reported that oxidative stress can induce ER stress (Xue et al., 2005; Malhotra et al., 2008). Therefore, we tested whether MCP-1 treatment induces ER stress in BMCs. Immunoblot analysis showed that MCP-1 treatment induced expression of the ER stress markers 78 kDa glucose regulated protein (GRP78) and inositol-requiring enzyme-1 (IRE-1) (Figure 4A, left panel, and Supplementary Figure S3A). This induction was inhibited by MCPIP siRNA (Figure 4A, left panel, and Supplementary Figure S3A), indicating that MCP-1 induced ER stress via MCPIP. We also found that forced expression of MCPIP induced ER stress with up-regulation of GRP78 and IRE-1 as indicated by immunoblot (Figure 4A and Supplementary Figure S3B). To test whether MCPIP induces ER stress through oxidative stress, ROS production was suppressed by CeO2, apocynin, and p47/AS, and then immunoblotting was performed. Results showed that MCPIP-induced expression of GRP78 and IRE-1 was attenuated by CeO2, apocynin, and p47/AS (Figure 4A and Supplementary Figure S3B). These results suggest that MCP-1 mediated ER stress via MCPIP-induced ROS production.
Figure 4.
MCPIP induces ER stress via ROS production which involve in OC precursor differentiation. (A) Immunoblotting shown that MCP-1 or MCPIP transfection induces the expression of GRP78 and IRE-1 (ER stress markers) by inducing ROS production. (B) Blockage of ER stress by IRE-1 siRNA abolished MCPIP-induced mRNA and protein expression of TRAP and CTSK. *P< 0.05 versus GFP, #P< 0.05 versus Scr. (C) Blockage of ER stress by TUDC abolished MCPIP-induced mRNA and protein expression of TRAP and CTSK. *P< 0.05 versus GFP, #P< 0.05 versus MCPIP alone. (D) Real-time PCR showing the expression of TRAP, CTSK, GRP78, and IRE-1 by ER stress inducer. *P< 0.05 versus control. (E) Immunoblotting showing the expression of TRAP, CTSK, GRP78, and IRE-1 by ER stress inducer. TU, tunicamycin; TH, thapsigargin.
To understand whether ER stress is involved in MCPIP-induced expression of OC-related genes TRAP and CTSK, MCPIP-induced ER stress was inhibited by IRE-1 siRNA (Figure 4B and Supplementary Figure S3C) and the ER stress-specific inhibitor tauroursodeoxycholate (TUDC) (Figure 4C and Supplementary Figure S3D). Immunoblot and real-time PCR analysis showed that MCPIP-induced expression of TRAP and CTSK was significantly inhibited by IRE-1 siRNA and by TUDC at both protein and mRNA levels but did not affect the expression of MCPIP (Figure 4B and C).
If ER stress is critically important for OC differentiation, ER stress inducers might induce differentiation of OC precursors without other inducers. In fact, two ER stress inducers, tunicamycin (TU) and thapsigargin (TH), induced expression of GRP78, IRE-1, TRAP, and CTSK at both protein and mRNA levels (Figure 4D and E and Supplementary Figure S3E). This result suggests that induction of ER stress alone could induce OC precursor differentiation. These results strongly support the conclusion that MCPIP-induced ER stress is involved in MCP-1-mediated OC precursor differentiation.
MCPIP-induced oxidative and ER stress leads to autophagy involved in OC precursor differentiation
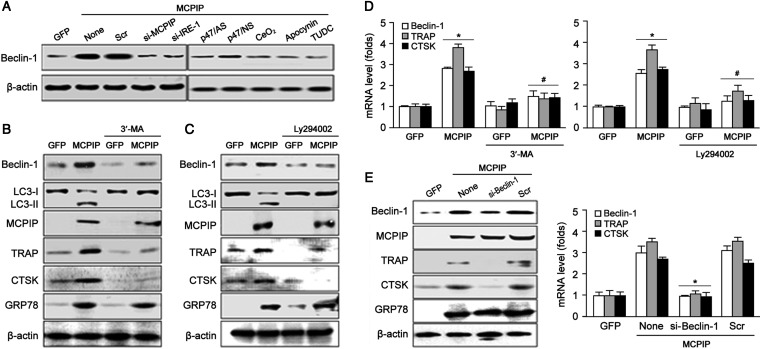
Increased expression of Beclin-1 is a commonly used marker of autophagy (Wang, 2008). Autophagy has been implicated in differentiation in some cellular contexts (Baerga et al., 2009; Singh et al., 2009). However, it is unclear whether autophagy is involved in OC differentiation. Here, we found that forced expression of MCPIP increased expression of Beclin-1; this effect was suppressed by MCPIP siRNA, but not by non-specific scramble (Scr) siRNA (Figure 5A, left panel and Supplementary Figure S4A). This result revealed that MCPIP induced autophagy in BMCs during differentiation into OC precursor cells.
Figure 5.
MCPIP induces autophagy via ROS production and ER stress which involve in OC precursor differentiation. (A) Immunoblotting shown that MCPIP induces autophagy characterized with the marker Beclin-1 expression and ROS/ER stress inhibitor inhibited MCPIP-induced expression of Beclin-1. (B and C) Immunoblotting shown that autophagy blocker 3′-MA and LY294002 blocked MCPIP-induced OC-related gene TRAP and CTSK expression but not GRP78. (D) Real-time PCR shown that autophagy blocker 3′-MA and LY294002 blocked MCPIP-induced expression of TRAP and CTSK but not GRP78. *P< 0.05 versus GFP, #P< 0.05 versus MCPIP alone. (E) Blocking autophagy by Beclin-1 siRNA inhibited MCPIP-induced mRNA and protein expression of TRAP and CTSK but not GRP78. *P< 0.05 versus MCPIP alone.
ER stress is known to induce autophagy. To test whether MCPIP-induced ROS production and ER stress are involved directly in MCPIP-mediated autophagy, MCPIP-expressing cells were treated with CeO2 nanoparticles that can trap free radicals, NADPH inhibitor apocynin, p47/AS, ER stress inhibitor TUDC, or IRE-1 siRNA. Immunoblot analysis showed that MCPIP-induced expression of Beclin-1 was inhibited significantly by inhibition of oxidative stress and ER stress and knockdown of genes involved in these stresses (Figure 5A, right panel, and Supplementary Figure S4A). These results suggested that MCPIP-mediated autophagy was caused by oxidative stress and ER stress during differentiation of BMCs into OC precursor cells.
Recently, it has been shown that the PI3K inhibitors LY294002 and 3′-methyladenine (MA) stop the macroautophagic pathway at the sequestration step in rat hepatocytes (Blommaart et al., 1997; Petiot et al., 2000). In order to investigate whether autophagy is involved in MCPIP-mediated OC precursor differentiation, we tested the effect of LY294002 and 3′-MA on expression of autophagy marker Beclin-1, lipidation of LC3 and expression of OC-related genes, TRAP and CTSK. Real-time PCR analysis showed that 3′-MA and LY294002 significantly inhibited MCPIP-induced expression of Beclin-1, TRAP, and CTSK (Figure 5D). Immunoblot assay demonstrated that 3′-MA and LY294002 inhibited the expression of Beclin-1, TRAP, and CTSK and lipidation of LC-3 (Figure 5B and C and Supplementary Figure S4B and C). However, they showed no effect on expression of GRP78 induced by MCPIP revealing that inhibition of autophagy does not affect ER stress that we propose cause autophagy (Figure 5B and C and Supplementary Figure S4B and C). Furthermore, upon inhibition of autophagy by knockdown of Beclin-1 with specific siRNA, MCPIP-induced expression of OC-related markers TRAP and CTSK was markedly suppressed, but scrambled siRNA showed little effects (Figure 5E and Supplementary Figure S4D). The chemical inhibitors of autophagy and knockdown of Beclin-1 did not affect MCPIP-induced expression of GRP78, a marker of ER stress, which futher leads to autophagy. These results strongly suggested that OC precursor cell differentiation induced by MCPIP expression is mediated via induction of ROS production that causes ER stress, which further leads to autophagy.
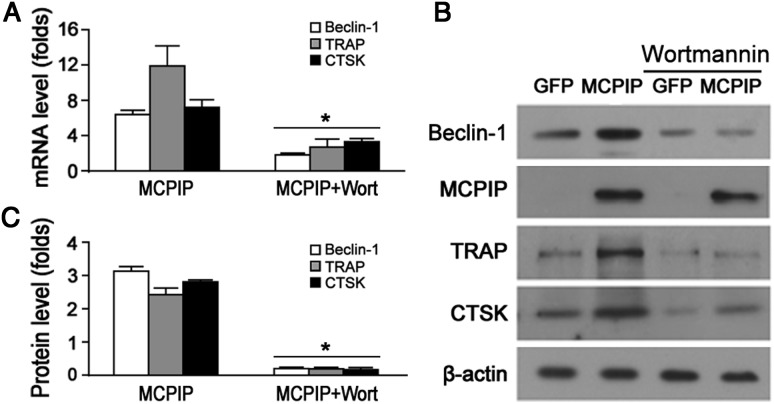
To further comfirm the involvement of autophagy in OC precursor differentiation, we tested a selective inhibitor of PI3K, wortmannin (Blommaart et al., 2007), on MCPIP-induced differentiation. Wortmannin severely inhibited MCPIP-induced OC precursor differentiation as indicated by the expression of OC markers at mRNA level by quantitative real-time PCR (qRT-PCR) and protein level by immunoblot analysis (Figure 6A–C); inhibition of autophagy was reflected by changes in the Beclin-1 levels. In support of the involvement of autophagy in OC precursor differentiation, knockdown of Atg7 by specific siRNA severely inhibited expression of the OC markers TRAP and CTSK both at mRNA and protein level as measured by qRT-PCR and immunoblot analysis, respectively (Supplementary Figure S5B and C), whereas scrambled siRNA did not significantly affect induction of these proteins.
Figure 6.
MCPIP-induced OC precursor differentiation inhibited by PI3K inhibitor, wortmannin. (A) qRT-PCR showing induction of Beclin-1 and TRAP and CTSK at the transcript level by MCPIP transfection, and the inhibition effect of wortmannin on MCPIP. *P< 0.05 versus MCPIP alone. (B) Immunoblotting show that MCPIP induced Beclin-1, and TRAP and CTSK, and that their induction was suppressed by wortmannin. (C) The intensities of immunoblots were measured and normalized to β-actin of the corresponding group. *P< 0.05 compared with MCPIP alone.
Discussion
In this study, we demonstrate that the role of MCP-1 in differentiation of human bone marrow monocytes to OC precursors is mediated via the induction of MCPIP. MCP-1 induces the differentiation of monocytic cells into TRAP and CTSK-positive cells that do not express other OC functional markers such as αV integrin, β3 integrin, and MMP9 and do not exhibit bone resorption (Kim et al., 2006b). The differentiation into functional OCs requires RANKL in addition to MCP-1. Thus, the MCP-1 induced differentiation yields what might be considered osteoclastogenic cells or OC precursors.
It has been demonstrated that MCPIP mediates MCP-1-induced adipogenesis (Younce et al., 2009), glial differentiation of neuroprogenitor cells (Vrotsos et al., 2009), and angiogenesis (Niu et al., 2008). Here, we found that forced expression of MCPIP resulted in high expression NADPH oxidase subunit p47PHOX and an increased level of membrane-associated p47PHOX, causing ROS production. We postulated that this oxidative stress causes ER stress that leads to autophagy involved in OC differentiation. The involvement of this sequence of processes in OC precursor differentiation was strongly supported by our finding that inhibition of p47PHOX expression, NADPH oxidase activity, ROS production, ER stress, or autophagy by chemical inhibitors or by gene knockdown markedly suppressed MCPIP-induced expression of OC-related genes, TRAP and CTSK.
ROS are associated with multiple cellular functions such as cell proliferation, differentiation, and apoptosis (Wolf, 2005). Many reports revealed that high level of intracellular ROS also contributes to angiogenesis (Xia et al., 2007), epithelial–mesenchymal transition (Zhang et al., 2009), survival, and differentiation of OCs (Steinbeck et al., 1998; Yamasaki et al., 2009). The present results demonstrate that MCP-1 treatment and forced expression of MCPIP induce ROS generation during MCPIP-induced OC precursor differentiation. It has been reported that CeO2 nanoparticles function as a free radical scavenger (Niu et al., 2007; Tsai et al., 2007; Younce and Kolattukudy, 2010). Here, we found that CeO2 inhibits the MCP-1- or MCPIP-induced ROS production and expression of OC-related genes.
NADPH oxidase is considered the most important source of ROS by respiratory burst in monocyte/macrophage system (Decoursey and Ligeti, 2005; Bedard and Krause, 2007). NAD(P)H oxidase is a multiple subunit enzyme complex. Assembly of transmembrane subunits and cytosolic subunits of enzyme complex is the first important step for its activation. In this step, p47PHOX is the most important component which is phosphorylated, translocated from cytoplasm to the membrane to interact with gp91phox (Decoursey and Ligeti, 2005; Bedard and Krause 2007; Leto et al., 2009). In the present study, we found that MCP-1 treatment or forced expression of MCPIP resulted in expression of p47PHOX and increased the membrane-associated p47PHOX level, and knockdown of MCPIP in MCP-1-treated cells decreased the expression and translocation of p47PHOX. Moreover, NADPH oxidase activity inhibitor apocynin and knockdown of p47PHOX with antisense oligonucleotides inhibited membrane translocation of p47PHOX, ROS production, and expression of OC-related genes CTSK and TRAP. Thus, MCPIP increases ROS production and induces expression of OC-related genes by increasing expression and translocation of p47PHOX. As a zinc-finger protein with a nuclear localization sequence (Zhou et al., 2006) and RNase activity (Matsushita et al., 2009; Skalniak et al., 2009), MCPIP might serve as a novel regulator for several genes at the transcriptional and post-transcriptional level because of its DNA and RNA binding property. So MCPIP might regulate directly the expression of p47PHOX as a transcriptional factor. Secondly, it has been reported that MCPIP activates MAPK signal pathway (Younce and Kolattukudy, 2010). Activation of MAPK may be an important reason for the MCPIP-induced expression and translocation of p47PHOX oxidase.
ER stress results from the accumulation of misfolded proteins which leads to the induction of the unfolded protein response (UPR) (Malhotra and Kaufman, 2007). ROS production is known to cause proteins to aggregate and misfold. Here, we demonstrate that MCP-1 treatment and forced expression of MCPIP induced ER stress via generation of ROS in the monocytes during induction of OC differentiation. The important role of ER stress in monocyte differentiation into OC precursors was demonstrated by the findings that inhibition of ER stress inhibited differentiation and known ER stress inducers caused differentiation. ER stress inhibitor TUDC and knockdown of IRE-1 showed that inhibition of ER stress leads to inhibition of MCPIP-induced OC precursor differentiation. Here, we also demonstrated that thapsgargin and tunicamycin that are known important inducers of UPR/ER stress induce differentiation of monocytes into OC precursors without MCP-1 or any other inducers. Thapsgargin, an inhibitor of ER-specific Ca-ATPase, has previously been shown to induce OC differentiation from RAW264.7 macrophage cells and mouse bone marrow cells (Takami et al., 1997; Yip et al., 2005).
Autophagy is generally thought of as a survival mechanism, although its dysregulation has been linked to non-apoptotic cell death (Wang, 2008; Glick et al., 2010). Since differentiation involves disappearance of one set of proteins and appearance of a new set of proteins, a self-digestion process such as autophagy could be involved in this process. In fact, autophagy has been reported to be an important event for differentiation of the chronic myelogenous leukemia K562 cells (Colosetti et al., 2009), adipocytes (Malhotra and Kaufman, 2007; Singh et al., 2009; Goldman et al., 2010), paneth cells (Stappenbeck, 2010), and neuronal differentiation (Zeng and Zhou, 2008). Beclin-1 is a critical component in the class III PI3K complex (PI3KC3) that induces the formation of autophagosomes in mammalian systems (Wang, 2008). It has been demonstrated that Beclin-1 bridges autophagy and differentiation, and the process of autophagy and differentiation requires up-regulation of Beclin-1 (Wang, 2008). Inhibition of differentiation by PI3K inhibitors and knockdown of Beclin-1 and Atg7 strongly suggest that MCPIP induced OC precursor differentiation via autophagy. Emerging data now indicate that ER stress is a potent inducer of autophagy (Sakaki and Kaufman, 2008). Here, our results revealed that MCPIP induces OC precursor differentiation via autophagy which depends on MCPIP-induced ROS production and ER stress. It has been reported that ER stress can activate p38 MAPK signal pathway to induce autophagy (Kim et al., 2008). Moreover, ER stress activates phosphorylation of protein kinase-like ERK (PERK), an ER-localized transmembrane protein, and induction of IRE-1 is necessary for radiation-induced autophagy in mouse embryonic fibroblasts (Kim et al., 2010). Activation of NF-κB and MAPK signal pathway is necessary for OC differentiation (Huang et al., 2006). However, it was reported that MCPIP inhibits activation of NF-κB induced by IL-1β (Skalniak et al., 2009) and lipopolysaccharide stimulation (Liang et al., 2008) while over-expression of MCPIP can cause activation of JNK and p38 (Younce and Kolattukudy, 2010). Thus, activation of p38 instead of NF-κB signaling is probably involved in MCPIP-mediated differentiation of monocytes into OC precursors. The overall pathway involved in the MCP-1/MCPIP-mediated OC precursor differentiation is shown in Supplementary Scheme S1.
Inflammatory bone erosion is involved in many pathological conditions (Lu et al., 2007; Ha et al., 2010). Our findings provide a new insight into the mechanism by which MCP-1 induces differentiation of monocytic cells into TRAP- and CTSK-expressing cells that can proceed to differentiate into functional OCs in the presence of RANKL, and suggest that MCPIP may be a novel target for therapy of inflammatory bone erosion.
Materials and methods
Reagents and antibodies
Human BMCs were from Stemcell Technologies. α-MEM, FBS, HBSS, trypsin, recombinant human M-CSF (300-25), human MCP-1, and Trizol reagent were purchased from Invitrogen. Anti-β-actin, CTSK monoclonal antibodies, CeO2 nanoparticles, apocynin, TUDC, 3′-MA, and LY294002 were from Sigma-Aldrich. Anti-TRAP, p47PHOX, Fas, IRE-1, GRP78, Beclin-1, LC3 polyclonal antibodies, goat anti-rabbit and mouse secondary antibodies, and specific siRNA for IRE-1 and Beclin-1 were purchased from Santa Cruz Biotechnology. Specific siRNA for MCPIP and negative control siRNA were obtained from Ambion. Anti-MCPIP polyclonal antibody was prepared as indicated before (Zhou et al., 2006; Younce and Kolattukudy, 2010).
Cell culture and treatment
BMCs were cultured in α-MEM supplemented with 10% FBS containing 30 ng/ml M-CSF, 100 U/ml penicillin, and 100 µg/ml streptomycin in 5% CO2 at 37°C. OC precursor cells were induced after 3-day culture as Ha et al. (2010). At this point, cells were treated with 50 ng/ml MCP-1, inhibitors, or gene transfection. For inhibitor treatment, CeO2 (10 μM), apocynin (100 μg/ml), TUDC (100 μM), 3′-MA (50 μM), and LY294002 (20 μM) were added 6 h before gene transfection.
Gene transfection and siRNA knockdown
OC precursor cells were transfected with 1 μg GFP or MCPIP-GFP eukaryotic expression plasmids for gene-gain-function assay by using Fugene 6. For gene silencing, chemically synthesized siRNA duplex (100 nM) targeting MCPIP, IRE-1, or Beclin-1 was transfected into OC precursor cells using DharmaFECT (Dharmacon) for 24 h prior to transfection with MCPIP-GFP or GFP plasmid. A scrambled siRNA was used as a negative control. For knockdown expression of p47PHOX, specific antisense (AS) (5′-CCAGCAGGGCGATGTGACGGATGAA-3′) and sense (5′-ATGGGGGACACCTTCATCCGTCAC-3′) oligonucleotides were designed and synthesized by phosphorothioate modification by Integrated DNA Technologies. The oligonucleotides were transfected into OC precursor cells using Lipofectamine 2000 for 24 h before MCPIP–GFP plasmid transfection.
TRAP staining
Three days after MCP-1 treatment or 4 days after MCPIP transfection, cells were fixed for histological staining for TRAP as described previously (Kim et al., 2006a). Briefly, following fixation, cells were stained with freshly prepared TRAP staining solution (naphthol AS-MX phosphate, fast red violet LB salt, and potassium sodium tartrate). On each coverslip, totally at least 500 cells were examined and the TRAP-positive cells were counted in 3–5 fields (20× objective), and the percentage of TRAP-positive cells was calculated.
Quantitative real-time polymerase chain reaction
Cells were centrifuged after wash with PBS for twice. Cell pellet was resuspended in 1 ml Trizol reagent and the total RNA was extracted with chloroform and isopropanol, purified on Qiagen Mini-Prep column, and treated with DNase. High Capacity cDNA Reverse Transcription kit (Applied Biosystems) was used for cDNA preparation from 1 μg total RNA. qRT-PCR was done in triplicate in an ABI PRISM 7900HT Sequence Detection System with 5% cDNA product, primers (Supplementary Table S1) at 125 nM, and Fast SYBR Green Master Mix (Applied Biosystems). Relative quantitation of PCR products was done by the 2−▵▵CT method, CT = cycles to threshold, and ▵▵CT = (target gene CT) − (β-actin reference gene CT). Final data were described as fold changes against control cells.
ROS production
Oxidant production in OC precursor cells was assessed by measuring the oxidation of intracellular DHR123 (Molecular Probes) as described previously (Younce et al., 2010). ROS production was expressed as folds compared with control cells expressing GFP alone.
Membrane isolation
OC precursor cells were harvested, sonicated on ice in a buffer containing 100 mM KCl, 3 mM NaCl, 3.5 mM MgCl2, 10 mM HEPES, 1 mM EGTA, 10 µg/ml pepstatin, 10 µg/ml leupeptin, and 0.5 mM PMSF; lysates were centrifuged at 600 g for 10 min at 4°C to remove nuclei and unbroken cells. The supernatant was then ultracentrifuged at 100000 g for 1 h at 4°C. Membranes were washed in the same buffer, quantified (Lemarie), and resuspended in Laemmli sample buffer, before western blot analysis.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and western blotting
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and western blotting were performed as described elsewhere. Samples (30 μg of protein) were subjected to 12% SDS–PAGE for most of target protein and 18% SDS–PAGE for LC3-II and LC3, transferred onto PVDF membranes (Millipore), and assayed for MCPIP, p47PHOX, TRAP, CTSK, GRP78, IRE-1, Beclin-1, LC3-II, and β-actin or Fas (loading control) protein expression by chemiluminescence detection (Pierce ECL kit) according to the manufacturer's instructions. The specific protein bands were quantified by densitometric analysis with GS-690 Image Densitometer (Bio-Rad).
Statistical analysis
Data are represented as mean ± SD of experiments performed on at least three separate occasions. Student's t-test was used to compare the means of normally distributed continuous variables. P< 0.05 indicated statistical significance.
Supplementary material
Supplementary material is available at Journal of Molecular Cell Biology online.
Funding
This work was supported in part by National Institutes of Health Grant HL-69458.
Conflict of interest: none declared.
Supplementary Material
References
- Azfer A., Niu J., Rogers L.M., et al. Activation of endoplasmic reticulum stress response during the development of ischemic heart disease. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1411–H1420. doi: 10.1152/ajpheart.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerga R., Zhang Y., Chen P.H., et al. Targeted deletion of autophagy-related 5 (atg5) impairs adipogenesis in a cellular model and in mice. Autophagy. 2009;5:1118–1130. doi: 10.4161/auto.5.8.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Blommaart E.F., Krause U., Schellens J.P., et al. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur. J. Biochem. 1997;243:240–246. doi: 10.1111/j.1432-1033.1997.0240a.x. [DOI] [PubMed] [Google Scholar]
- Boyce B.F., Yao Z., Zhang Q., et al. New roles for osteoclasts in bone. Ann. NY Acad. Sci. 2007;1116:245–254. doi: 10.1196/annals.1402.084. [DOI] [PubMed] [Google Scholar]
- Colosetti P., Puissant A., Robert G., et al. Autophagy is an important event for megakaryocytic differentiation of the chronic myelogenous leukemia K562 cell line. Autophagy. 2009;5:1092–1098. doi: 10.4161/auto.5.8.9889. [DOI] [PubMed] [Google Scholar]
- Decoursey T.E., Ligeti E. Regulation and termination of NADPH oxidase activity. Cell. Mol. Life Sci. 2005;62:2173–2193. doi: 10.1007/s00018-005-5177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerszten R.E., Friedrich E.B., Matsui T., et al. A role of phosphoinositide 3-kinase in monocyte recruitment under flow conditions. J. Biol. Chem. 2001;276:26846–26851. doi: 10.1074/jbc.M011235200. [DOI] [PubMed] [Google Scholar]
- Glick D., Barth S., Macleod K.F. Autophagy: cellular and molecular mechanisms. J. Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S., Zhang Y., Jin S. Autophagy and adipogenesis: implications in obesity and type II diabetes. Autophagy. 2010;6:179–181. doi: 10.4161/auto.6.1.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha J., Choi H.S., Lee Y., et al. CXC chemokine ligand 2 induced by receptor activator of NF-kappa B ligand enhances osteoclastogenesis. J. Immunol. 2010;184:4717–4724. doi: 10.4049/jimmunol.0902444. [DOI] [PubMed] [Google Scholar]
- Harris E.D., Jr Rheumatoid arthritis: pathophysiology and implications for therapy. N. Engl. J. Med. 1990;322:1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Huang H., Ryu J., Ha J., et al. Osteoclast differentiation requires TAK1 and MKK6 for NFATc1 induction and NF-kappaB transactivation by RANKL. Cell Death Differ. 2006;13:1879–1891. doi: 10.1038/sj.cdd.4401882. [DOI] [PubMed] [Google Scholar]
- Kim M.S., Day C.J., Morrison N.A. MCP-1 is induced by receptor activator of nuclear factor-κB ligand, promotes human osteoclast fusion, and rescues granulocyte macrophage colony-stimulating factor suppression of osteoclast formation. J. Biol. Chem. 2005;280:16163–16169. doi: 10.1074/jbc.M412713200. [DOI] [PubMed] [Google Scholar]
- Kim M.S., Day C.J., Selinger C.I., et al. MCP-1-induced human osteoclast-like cells are tartrate-resistant acid phosphatase, NFATc1, and calcitonin receptor-positive but require receptor activator of NFkappaB ligand for bone resorption. J. Biol. Chem. 2006a;281:1274–1285. doi: 10.1074/jbc.M510156200. [DOI] [PubMed] [Google Scholar]
- Kim M.S., Magno C.L., Day C.J., et al. Induction of chemokines and chemokine receptors CCR2b and CCR4 in authentic human osteoclasts differentiated with RANKL and osteoclast like cells differentiated by MCP-1 and RANTES. J. Cell. Biochem. 2006b;97:512–518. doi: 10.1002/jcb.20649. [DOI] [PubMed] [Google Scholar]
- Kim D.S., Kim J.H., Lee G.H., et al. p38 mitogen-activated protein kinase is involved in endoplasmic reticulum stress-induced cell death and autophagy in human gingival fibroblasts. Biol. Pharm. Bull. 2008;33:545–549. doi: 10.1248/bpb.33.545. [DOI] [PubMed] [Google Scholar]
- Kim K.W., Moretti L., Mitchell L.R., et al. Endoplasmic reticulum stress mediates radiation-induced autophagy by perk-eIF2alpha in caspase-3/7-deficient cells. Oncogene. 2010;29:3241–3251. doi: 10.1038/onc.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviranta R., Morko J., Uusitalo H., et al. Accelerated turnover of metaphyseal trabecular bone in mice overexpressing cathepsin K. J. Bone Miner. Res. 2001;16:1444–1452. doi: 10.1359/jbmr.2001.16.8.1444. [DOI] [PubMed] [Google Scholar]
- Leto T.L., Morand S., Hurt D., et al. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid. Redox Signal. 2009;11:2607–2619. doi: 10.1089/ars.2009.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Wang J., Azfer A., et al. A novel CCCH-zinc finger protein family regulates proinflammatory activation of macrophages. J. Biol. Chem. 2008;283:6337–6346. doi: 10.1074/jbc.M707861200. [DOI] [PubMed] [Google Scholar]
- Lu Y., Cai Z., Xiao G., et al. Monocyte chemotactic protein-1 mediates prostate cancer-induced bone resorption. Cancer Res. 2007;67:3646–3653. doi: 10.1158/0008-5472.CAN-06-1210. [DOI] [PubMed] [Google Scholar]
- Malhotra J.D., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- Malhotra J.D., Miao H., Zhang K., et al. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc. Natl Acad. Sci. USA. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K., Takeuchi O., Standley D.M., et al. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature. 2009;458:1185–1190. doi: 10.1038/nature07924. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Ninomiya K., Sonoda K.H., et al. MCP-1 expressed by osteoclasts stimulates osteoclastogenesis in an autocrine/paracrine manner. Biochem. Biophys. Res. Commun. 2009;383:373–377. doi: 10.1016/j.bbrc.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Mundy G.R. Osteoporosis and inflammation. Nutr. Rev. 2007;65:S147–S151. doi: 10.1111/j.1753-4887.2007.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Niu J., Azfer A., Rogers L.M., et al. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc. Res. 2007;73:549–559. doi: 10.1016/j.cardiores.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J., Azfer A., Zhelyabovska O., et al. Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP) J. Biol. Chem. 2008;283:14542–14551. doi: 10.1074/jbc.M802139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petiot A., Ogier-Denis E., Blommaart E.F., et al. Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J. Biol. Chem. 2000;275:992–998. doi: 10.1074/jbc.275.2.992. [DOI] [PubMed] [Google Scholar]
- Sakaki K., Kaufman R.J. Regulation of ER stress-induced macroautophagy by protein kinase C. Autophagy. 2008;4:841–843. doi: 10.4161/auto.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakiyama H., Masuda R., Inoue N., et al. Establishment and characterization of macrophage-like cell lines expressing osteoclast-specific markers. J. Bone Miner. Metab. 2001;19:220–227. doi: 10.1007/s007740170024. [DOI] [PubMed] [Google Scholar]
- Sato K., Takayanagi H. Osteoclasts, rheumatoid arthritis, and osteoimmunology. Curr. Opin. Rheumatol. 2006;18:419–426. doi: 10.1097/01.bor.0000231912.24740.a5. [DOI] [PubMed] [Google Scholar]
- Singh R., Xiang Y., Wang Y., et al. Autophagy regulates adipose mass and differentiation in mice. J. Clin. Invest. 2009;119:3329–3339. doi: 10.1172/JCI39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalniak L., Mizgalska D., Zarebski A., et al. Regulatory feedback loop between NF-kappaB and MCP-1-induced protein 1 RNase. FEBS J. 2009;276:5892–5905. doi: 10.1111/j.1742-4658.2009.07273.x. [DOI] [PubMed] [Google Scholar]
- Stappenbeck T.S. The role of autophagy in Paneth cell differentiation and secretion. Mucosal. Immunol. 2010;3:8–10. doi: 10.1038/mi.2009.121. [DOI] [PubMed] [Google Scholar]
- Steinbeck M.J., Kim J.K., Trudeau M.J. Involvement of hydrogen peroxide in the differentiation of clonal HD-11EM cells into osteoclast-like cells. J. Cell. Physiol. 1998;176:574–587. doi: 10.1002/(SICI)1097-4652(199809)176:3<574::AID-JCP14>3.0.CO;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura R., Li L. Shifting in balance between osteogenesis and adipogenesis substantially influences hematopoiesis. J. Mol. Cell Biol. 2010;2:61–62. doi: 10.1093/jmcb/mjp030. [DOI] [PubMed] [Google Scholar]
- Takami M., Woo J.T., Takahashi N., et al. Ca2+-ATPase inhibitors and Ca2+-ionophore induce osteoclast-like cell formation in the cocultures of mouse bone marrow cells and calvarial cells. Biochem. Biophys. Res. Commun. 1997;237:111–115. doi: 10.1006/bbrc.1997.7090. [DOI] [PubMed] [Google Scholar]
- Tsai Y.Y., Oca-Cossio J., Agering K., et al. Novel synthesis of cerium oxide nanoparticles for free radical scavenging. Nanomedicine (Lond) 2007;2:325–332. doi: 10.2217/17435889.2.3.325. [DOI] [PubMed] [Google Scholar]
- Vrotsos E.G., Kolattukudy P.E., Sugaya K. MCP-1 involvement in glial differentiation of neuroprogenitor cells through APP signaling. Brain Res. Bull. 2009;79:97–103. doi: 10.1016/j.brainresbull.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Beclin 1 bridges autophagy, apoptosis and differentiation. Autophagy. 2008;4:947–948. doi: 10.4161/auto.6787. [DOI] [PubMed] [Google Scholar]
- Wise G.E., Frazier-Bowers S., D'Souza R.N. Cellular, molecular, and genetic determinants of tooth eruption. Crit. Rev. Oral Biol. Med. 2002;13:323–334. doi: 10.1177/154411130201300403. [DOI] [PubMed] [Google Scholar]
- Wolf G. Role of reactive oxygen species in angiotensin II-mediated renal growth, differentiation, and apoptosis. Antioxid. Redox Signal. 2005;7:1337–1345. doi: 10.1089/ars.2005.7.1337. [DOI] [PubMed] [Google Scholar]
- Xia C., Meng Q., Liu L.Z., et al. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- Xue X., Piao J.H., Nakajima A., et al. Tumor necrosis factor alpha (TNFα) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFα. J. Biol. Chem. 2005;280:33917–33925. doi: 10.1074/jbc.M505818200. [DOI] [PubMed] [Google Scholar]
- Yamasaki N., Tsuboi H., Hirao M., et al. High oxygen tension prolongs the survival of osteoclast precursors via macrophage colony-stimulating factor. Bone. 2009;44:71–79. doi: 10.1016/j.bone.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Yip K.H., Zheng M.H., Steer J.H., et al. Thapsigargin modulates osteoclastogenesis through the regulation of RANKL-induced signaling pathways and reactive oxygen species production. J. Bone Miner. Res. 2005;20:1462–1471. doi: 10.1359/JBMR.050324. [DOI] [PubMed] [Google Scholar]
- Younce C.W., Kolattukudy P.E. MCP-1 causes cardiomyoblast death via autophagy resulting from ER stress caused by oxidative stress generated by inducing a novel zinc-finger protein, MCPIP. Biochem. J. 2010;426:43–53. doi: 10.1042/BJ20090976. [DOI] [PubMed] [Google Scholar]
- Younce C.W., Azfer A., Kolattukudy P.E. MCP-1 (monocyte chemotactic protein-1)-induced protein, a recently identified zinc finger protein, induces adipogenesis in 3T3-L1 pre-adipocytes without peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2009;284:27620–27628. doi: 10.1074/jbc.M109.025320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younce C.W., Wang K., Kolattukudy P.E. Hyperglycemia-induced cardiomyocyte death is mediated via MCP-1 production and induction of a novel zinc-finger protein MCPIP. Cardiovasc. Res. 2010;87:665–674. doi: 10.1093/cvr/cvq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng M., Zhou J.N. Roles of autophagy and mTOR signaling in neuronal differentiation of mouse neuroblastoma cells. Cell. Signal. 2008;20:659–665. doi: 10.1016/j.cellsig.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Zhang K.H., Tian H.Y., Gao X., et al. Ferritin heavy chain-mediated iron homeostasis and subsequent increased reactive oxygen species production are essential for epithelial–mesenchymal transition. Cancer Res. 2009;69:5340–5348. doi: 10.1158/0008-5472.CAN-09-0112. [DOI] [PubMed] [Google Scholar]
- Zhou L., Azfer A., Niu J., et al. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ. Res. 2006;98:1177–1185. doi: 10.1161/01.RES.0000220106.64661.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.