Abstract
Since the environment is in constant flux, decision-making capabilities of the brain must be rapid and flexible. Yet in sensory motion processing pathways of the primate brain where decision making has been extensively studied, the flexibility of neurons is limited by inherent selectivity to motion direction and speed. The supplementary eye field (SEF), an area involved in decision making on moving stimuli, is not strictly a sensory or motor structure, and hence may not suffer such limitations. Here we test whether neurons in the SEF can flexibly interpret the rule of a go/nogo task when the decision boundary in the task changes with each trial. The task rule specified that the animal pursue a moving target with its eyes if and when the target entered a visible zone. The size of the zone was changed from trial to trial in order to shift the decision boundary, and thereby assign different go/nogo significance to the same motion trajectories. Individual SEF neurons interpreted the rule appropriately, signaling go or nogo in compliance with the rule and not the direction of motion. The results provide the first evidence that individual neurons in frontal cortex can flexibly interpret a rule that governs the decision to act.
Keywords: smooth pursuit, eye movement, rule learning, decision making, motion
successful decision making is a hallmark of higher intelligence. Decisions are often based upon learned rules that link arbitrary stimuli to behaviors that lead to positive outcomes. Often decisions must be made based on an indeterminate range of stimuli that conform to the same rule, and require neural architecture that is flexible in this regard. Motion perception has been commonly adopted as a vehicle to understand sensorimotor decision making because of its well-understood neurophysiology. Recent work has implicated many motion-related structures in visuoocular decision making, including areas MT (medial-temporal cortex) (Britten et al. 1996; Newsome et al. 1989), lateral-inferior parietal (LIP) (Shadlen and Newsome 2001), the frontal eye field (FEF) (Gold and Shadlen 2000, 2003), and the superior colliculus (SC) (Horwitz et al. 2004). However, a neural architecture supporting flexible rule-guided decisions has remained elusive.
In studies that have probed sensory decision making in MT, monkeys decided whether a stimulus moved in one direction or another relative to a decision boundary (e.g., Newsome et al. 1989). In this work, the activity of MT neurons was found to signal the decision (making a leftward or rightward saccade), based on the direction of stimulus motion in relation to the decision boundary. However, since MT is a sensory structure, the response of MT neurons to stimulus motion does not systematically change to reflect a change in the decision boundary (Britten et al. 1996). In LIP, neurons can also signal to which side of a decision boundary stimulus motion is directed (Freedman and Assad 2006). In contrast to MT neurons, the tuning of LIP neurons can change to signal different motion directions after a decision boundary is changed. Specifically, these neurons initially categorized motions with respect to a single decision boundary and, after retraining with a second boundary, categorized the motions with respect to the second boundary but not the first. However, no evidence was provided in this study that LIP neurons have the ability to flexibly categorize motions based on a rapidly changing decision boundary.
We asked whether the supplementary eye field (SEF), a structure further downstream from MT and LIP in sensorimotor processing, might flexibly interpret a go/nogo rule for multiple decision boundaries in a task that we used previously to demonstrate the ability of neurons here to interpret a single boundary (Kim et al. 2005; Yang et al. 2010). The SEF is not directly linked to sensory encoding or motor execution, and is therefore a potential candidate for flexibly encoding decision boundaries. Directional tuning, a prerequisite for sensory motion neurons such as those in MT or LIP and for neurons involved in movement generation such as those in the FEF and SC, is absent or less prominent in the SEF (Schall 1991a; Yang et al. 2010). Evidence that the SEF is more involved in eye movement preparation than execution is the finding that preparatory activity occurs here even if the eye movement is eventually withheld (Schall 1991a). Furthermore, lesions of the SEF have minimal effects on simple pursuit (Fukushima et al. 2003; Tehovnik et al. 2000) or saccade (Schiller and Chou 1998) execution.
In fact, the SEF seems to be a predominantly cognitive structure and therefore a prime candidate for flexible decision making. For example, neurons here are active in a go/nogo ocular decision in which a cue specifies whether or not a saccade is to be suppressed (Stuphorn and Schall 2006). Our previous work has shown that the activity of some SEF neurons conforms to the rule of a go/nogo ocular decision (Kim et al. 2005) and continues to signal the rule even when the monkey makes decision errors (Yang et al. 2010). Furthermore, in tasks where the animal must decide whether to make a saccade to the left or right end of a visual bar, SEF neurons code the saccade to one end of the bar, but not the other, independent of the absolute location of the bar in space or the saccade trajectory (Olson and Gettner 1995, 1999). These results support the ability of the SEF to interpret a decision rule that applies to a specific decision boundary independent of the low-level physical properties of the task stimulus or the ocular response. However, the possibility that neurons here can flexibly interpret a decision rule when the decision boundary changes from trial to trial has not been directly tested.
To this end, in the present study monkeys first performed the standard version of ocular baseball, our go/nogo decision task (Heinen et al. 2006; Kim et al. 2005; Yang et al. 2010). In the task, the monkey decided whether to pursue a moving target given the following rule: If the target intersects a visible square on the screen (the plate), smooth pursuit is required; if not, fixation must be maintained. The corner of the plate determined the decision boundary, i.e., the angle of a virtual trajectory that separates go (strike) trajectories from nogo (ball) trajectories. Neurons that displayed higher activity for strike trials than for ball trials (or vice versa) were classified as task related and were tested further with a modified version of the ocular baseball task. In this version, we changed the decision boundary randomly from trial to trial by manipulating plate size so that the same trajectory specified strike or ball on different trials. Activity of task-related SEF neurons was then analyzed to determine whether it reflected the changing decision boundary.
METHODS
Surgery
Surgery was performed on two adolescent Macaca mulatta monkeys under aseptic conditions in order to implant a recording chamber over the SEF, a head holder, and an eye coil. With the monkey under isoflurane gas anesthesia, a 2-cm craniotomy was trephined in the skull centered at 24 mm anterior in Horsley-Clark stereotaxic coordinates (Richmond and Optican 1987). A stainless steel recording chamber with an inner diameter of 1.4 cm was positioned over the craniotomy and secured along with the other implants with dental acrylic. The eye coil was implanted under the conjunctiva of one eye. All procedures were approved by the Institutional Animal Care and Use Committee and were in compliance with the guidelines set forth in the United States Public Health Service Guide for the Care and Use of Laboratory Animals.
Tasks
Standard ocular baseball task.
Each trial began with the appearance of a 0.5° diameter white spot located at the center of the screen and surrounded by a visible square zone that we refer to as the “plate.” In the standard task, the plate was 12° by 12° and constant across trials (see Fig. 1A, top). To trigger the onset of the moving target (also a white spot of 0.5° diameter), the monkey had to maintain its gaze within a 4° invisible square window centered at the fixation point for 500 ms. At the end of the fixation period, a target appeared 20° to the left or right of the fixation point and moved at a constant velocity from the periphery with a trajectory that either intersected or bypassed the plate. The total motion duration in each trial was 1,200 ms. The fixation point and plate remained visible throughout the trial.
Fig. 1.
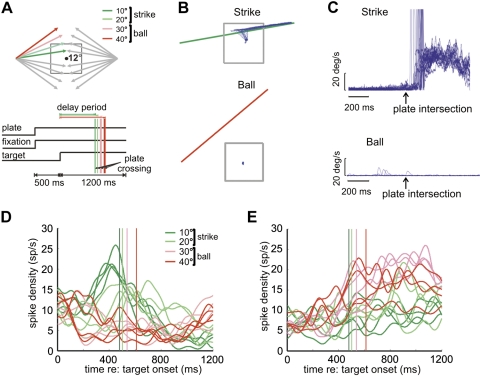
Standard ocular baseball. A: task schematics. Top: spatial layout, showing 16 possible target trajectories in relation to the visible 12° plate on the screen. The dot at the center is the fixation spot. Trajectory angles are 10°, 20°, 30°, and 40° with respect to the horizontal meridian. Four trajectories are color coded to illustrate strike (green) and ball (red) trajectories. In each trial, a target appeared at 20° left or right in the periphery and moved inward at 30°/s. Bottom: the temporal events for each trial. The vertical lines show plate intersection times for trials with different angles of horizontal deviation. B: Cartesian eye position from a single block of strike and ball trials. C: radial eye velocity for the same block of trials. D: mean spike rate for a strike neuron in strike (green) and ball (red) trials. The vertical lines indicate corresponding plate intersection times. E: mean spike rate for a ball neuron.
In Fig. 1A, top, are shown the possible target trajectories in the standard paradigm. There are four possible angles of target motion relative to horizontal, and for each angle the target can move either left or right, up or down, resulting in 16 total trajectories. Only one target appears in each trial, and the trajectory that it takes is randomly selected from trial to trial. The animal is required to pursue targets that intersect the plate (go or “strike” trials) or maintain fixation for targets that do not (nogo or “ball” trials); these two types of trials were used to classify strike and ball neurons. In strike trials, the plate-crossing time is simply the time when the target intersects the plate. In ball trials, plate-crossing time is defined as the intersection of the extended vertical edge of the plate. Note that plate-crossing time differs between trajectory angles. In strike trials, the target had to be acquired (within 3° of the target) within 300 ms after plate crossing, and eye position had to remain within 3° of the moving target until it disappeared. Liquid reward was given to the monkey at the end of a successful trial. Intertrial interval was variable (300–700 ms). A critical aspect of ocular baseball is the delay period, which extends from the time when the target starts to move to the plate-crossing time (see Fig. 1A, bottom). During the delay period, the monkey is required to maintain fixation so that the character of neural activity can be assessed when no movement is ongoing.
Modified ocular baseball.
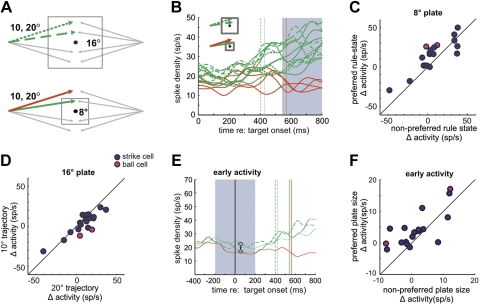
In the modified task, plate size was alternated randomly between 8° and 16° from trial to trial. Two separate conditions were used in separate blocks of trials, the two-plate, one-target condition (2P1T) and the two-plate, two-target condition (2P2T). In the 2P1T condition there were four potential trajectories, each with an angle that was 20° relative to horizontal (see Fig. 3A). Here, an identical trajectory specified strike with a 16° plate and ball with an 8° plate. Therefore, in this experiment the motion trajectory did not uniquely specify the rule state (strike or ball). In the 2P2T condition, both the 20° angle and an additional 10° angle were used (8 total trajectories). Here, the 10° angle trajectories specified strikes regardless of plate size, and plate size did not uniquely specify the rule state. In both conditions, all other parameters were the same as in the standard ocular baseball task.
Fig. 3.
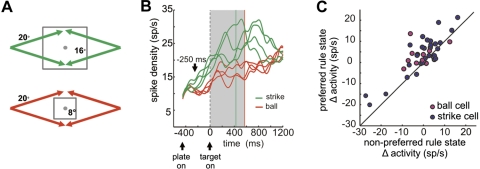
Results of the 2-plate task with a constant trajectory angle (2P1T). A: spatial schematic. Plate size was randomly chosen as either 16° (top) or 8° (bottom), and the target trajectory was always 20°, which resulted in either strike (green) or ball (red) trials, respectively. B: typical neuronal activity recorded in 2P1T trials. Activity is shown as average spike density for all 8 combinations of plate size and trajectory direction in 1 block. Green and red traces are activity for the large (strike) and small (ball) plates, respectively. Green and red vertical lines indicate plate intersection times for strike and ball trials, respectively. Note greater activity for the large plate when all targets specified strike (green curves). The gray shaded region indicates the analysis period (0–570 ms after target motion onset). C: population summary for strike and ball neurons. Each point represents neuronal activity for preferred vs. nonpreferred rule states, where the preferred rule state is strike (large plate) for strike neurons and ball (small plate) for ball neurons.
Data Acquisition and Analysis
Experimental control and data acquisition were performed with a Pentium IV 3.4 GHz PC (Windows XP) running National Instruments (NI) LabVIEW Express 7.0 with the real-time module. Visual stimuli were displayed on a 24-in. computer monitor driven by a Macintosh G4 (MacOS 9) system, using Matlab (The MathWorks) software and the Psychophysics Toolbox real-time visual display tools (Brainard 1997). Eye position signals were recorded with a magnetic-field system (CNC Systems). Horizontal and vertical eye velocity were calculated off-line by differentiating and filtering the recorded eye position signals, using a two-pole Butterworth noncausal filter and a cutoff of 50 Hz. The computer acquired the eye position signals at a sampling rate of 1.0 kHz. Single neurons were recorded from the SEF with tungsten microelectrodes (FHC) with an impedance of 1.0–2.0 MΩ, tested at 1,000 Hz. Neural spikes were then amplified, filtered, and detected with the amplifier (TDT, Pentusa).
All data analysis was conducted off-line with Matlab. The raw spike counts were converted into a continuous smoothed spike-density function by convolving a Gaussian having a fixed temporal width of 30 ms (the time from signal peak amplitude to ⅓ peak) with the raw spike count.
To determine the time that neuronal activity first became different for strike and ball trials (separation time), spike density values were first smoothed with a moving average window (width = 20 ms, step size = 10 ms). A Wilcoxon rank sum test was then applied to all the trials for each time bin. Separation time is defined as the time when the P value of the strike/ball activity difference first reaches significance (P < 0.05) and remains significant for longer than 100 consecutive milliseconds. A neuron with higher activity during strike trials at the initial separation time is defined as a strike neuron, and a neuron with higher activity during ball trials is defined as a ball neuron. Separation times were also computed with an alpha function (Gabbiani and Cox 2010).
Psycho- and neurometric functions of the strike/ball decision performance for the monkey and for individual neurons were constructed based on signal detection theory (Green and Swets 1966; Swets 1964) from behavioral and neural data collected in the same block of trials. Briefly, signal detection theory posits that when an observer must determine which of two noisy stimuli contains the signal, the internal responses to the “noise” and “signal + noise” can be conceptualized as Gaussian-shaped likelihood distributions. The ability of an observer to detect the signal depends upon the standard deviations of these two distributions and the distance between their means. A z score transformation is used to standardize detectability measures by expressing them as a function of the standard deviation of the hypothetical likelihood distributions with the equation
| (1) |
where MN is the mean of the “noise” distribution, MS+N is the mean of the “signal + noise” distribution, and SDS+N is the standard deviation of the “signal + noise” distribution.
In the present study, the data for the psychometric functions were obtained directly from the probability that the monkey would pursue a target with a strike or ball motion angle. Neurometric functions are analogous to these in that they yield the probability that a neuron's activity correctly signals a target with a strike or ball trajectory angle. In perceptual discrimination tasks used in previous studies (e.g., Newsome et al. 1989), the observer must detect the difference between a standard and a test, the standard being considered “noise” and the test as “signal + noise.” In our study, the activity from each trial was used to construct a frequency distribution of firing rate for each target angle. The z score was calculated for each test distribution with Eq. 1, where MN is the mean firing rate of the strike target distribution for ball neurons and ball target distribution for strike neurons and MS+N and SDS+N are the mean and standard deviation of the strike target distribution for strike neurons and ball target distribution for ball neurons. A table of the standard normal distribution was then used to determine the proportion of the distribution that was located below each z score. These proportions are analogous to the proportion of strike and ball judgments that are used to plot the monkey's psychometric functions and, as in the construction of the psychometric functions, can be plotted as a function of target trajectory, creating a neurometric function. The data points were fit by a Weibull function of the form
| (2) |
where x ranges from the minimum to the maximum angle of the target trajectory, α is the subjective decision boundary, γ is the maximum value of the function, δ is the minimum value, and β is the slope.
We first fit the data in the single-plate condition of standard baseball [mean R2 of fit (±SD): neurometric 0.91 ± 0.21, psychometric 0.99 ± 0.01]. Since we wished to know whether the monkey and neuron could adjust to a decision boundary change in a similar fashion, we then compared the shift of the psychometric and neurometric functions after the decision boundary was changed in the two-plate experiment. The large plate in the two-plate experiment could not be used to compute the decision boundary because both trajectories were strike trajectories; we only used the small plate data, which produced strike and ball data points from the 10° and 20° trajectories, respectively. To quantify the change in the probability of ball response due to change in the decision boundary, we substituted the data points corresponding to 10° and 20° in the single-plate condition with that in the two-plate condition and fit them with a Weibull function [mean R2 of fit (±SD): neurometric 0.74 ± 0.28, psychometric 0.99 ± 0.004]. We quantified the amount of angular shift of the decision boundary in the psychometric and neurometric functions by subtracting α of the best-fit Weibull function in the one-plate condition from that in the two-plate condition for both behavioral and neural data.
RESULTS
SEF neurons were screened to identify those related to the strike/ball ocular decision. Figure 1B shows the Cartesian representation of eye position in relation to the plate and target trajectory for a typical block of trials with 10° strike angles and 40° ball angles, respectively, illustrating that the monkey successfully observed the rule of ocular baseball. Figure 1C plots eye velocity traces aligned on plate intersection, showing that the onset of pursuit eye movements, which were sometimes preceded by saccades, often predicted the time of plate intersection (median latency = 112 ms after plate intersection).
Of 153 neurons recorded from the SEF of two monkeys, 48 were differentially active in strike and ball trials in the delay period during standard ocular baseball. Consistent with previous literature (Kim et al. 2005), the activity of one population of SEF neurons was higher during strike than during ball trials in the delay period (n = 37) (see example in Fig. 1D). A second, smaller, complementary population had higher activity during ball trials (n = 11) (see example in Fig. 1E). Note that strike- or ball-related activity of a given neuron could occur if the cell were merely tuned to a single motion trajectory and/or movement direction because its activity could weight the mean toward either option. However, for the example strike and ball neurons depicted in Fig. 1, D and E, it can be seen that this is not the case. For the strike neuron here, activity for most of the strike trajectories was higher than that observed for the ball trajectories, and vice versa for the ball neuron.
To determine directional tuning for the population of task-related neurons that we recorded, we computed a directional index (DI) for each cell, using the following formula:
| (3) |
where μpref is the mean firing rate for the preferred direction in the delay period, derived from the two trajectories that specify the same rule and have the highest pooled mean spike rate compared with the other right pairs of trajectories. μopp is the mean firing rate for the opposite direction in the delay period, derived from the two opposite trajectories.
We also computed a “rule index” (RI) for each neuron, using logic analogous to that used to compute DI. Conceptually, we tested whether the activity for the preferred direction of the neuron in the delay period was different from the activity obtained for the nearest angles that conformed to the opposite rule. The formula for computing RI is
| (4) |
where μrule.diff is the mean firing rate for the two trajectories adjacent to the preferred trajectories, which specify a different ocular decision.
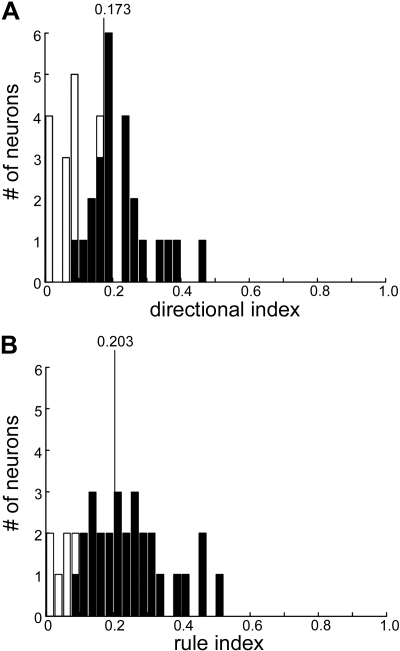
Figure 2 shows the population DIs and RIs for the population of task-related neurons. The mean DI was 0.173, and 39% of the cells we recorded did not have significant directional tuning. The mean RI was 0.203, and only 18% of the neurons did not have significant rule tuning. Therefore, activity of these cells before plate intersection is consistent with the neurons interpreting the rule and signaling appropriate behavior. For simplicity, we will discuss in the text the results for strike neurons, although population data will be shown for both types of cells.
Fig. 2.
Directional and rule tuning during the delay period of standard ocular baseball. A: directional index (DI) values for recorded supplementary eye field (SEF) neurons, with filled bars indicating significant directional tuning. The vertical line indicates the mean of DI distribution. B: rule index (RI) values for the same group of SEF neurons.
We sought to determine whether SEF neurons that specify the rule of ocular baseball are interpreting the rule online, as opposed to learning to classify a set of specific motion trajectories as occurs with LIP neurons (Freedman and Assad 2006). If the neurons are classifying motion trajectories, they should respond the same for a given trajectory regardless of whether it signals strike or ball. Conversely, if they are interpreting the rule, they should respond differently for the same trajectory when the decision boundary is changed and that trajectory specifies a different behavior. To distinguish between these alternatives, results from the 2P1T tasks for the same population of SEF neurons were compared with those in the standard baseball task. Figure 3A shows the spatial diagram of the 2P1T task. In it, plate size was alternated randomly between 8° and 16° on each trial, and the trajectory angle remained fixed at 20° relative to the horizontal meridian. Here, the identical trajectory specifies strike with the large plate and ball with the small plate.
Figure 3B shows the activity of an example baseball neuron for the four trajectories for the large plate and the small plate. The activity for the strike trajectories (with the large plate) was always higher than that accompanying the ball trajectories (with the small plate), suggesting that the neuron was interpreting the baseball rule rather than encoding a specific motion angle or trajectory. Figure 3C shows this was the case across the population of neurons. Here it can be seen that most neurons that reflected the rule in standard baseball continued to signal strike and ball despite the unchanging trajectory, as indicated by most points being above the diagonal line in Fig. 3C. A MANOVA test showed that the population of neurons as a whole was more likely to signal its preferred rule state (d = 1, λ = 0.192, P < 0.01, df = 83).
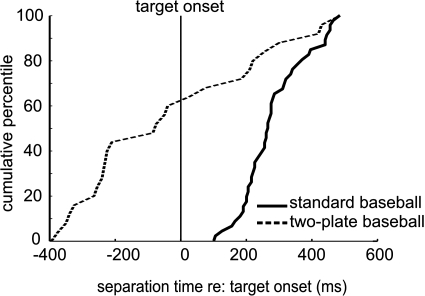
Interestingly, the neurons in the two-plate experiment appeared to discriminate strike and ball trials after the plate appeared and before the target began to move (see Fig. 3B). Note that in standard baseball all separation times occurred after the target moved (spike density: median = 250 ms; alpha function: median = 260 ms). In 2P1T, separation time often occurred before the target moved (spike density: median = −80 ms; alpha function: median = −45 ms), when only the plate was present, suggesting that the neurons were interpreting the ocular baseball rule based on plate size. Since plate size uniquely specified strike or ball in that experiment, it would be possible for a neuron to discriminate strike and ball trials based on plate size alone without interpreting the ocular baseball rule that concerns the plate-trajectory relationship. In fact, as shown in Fig. 4, most neurons tested in this experiment (30/41) showed higher activity for the large plate than the small plate before the target moved. This result implies that the cells, while not classifying motion trajectories, might still be classifying another simple sensory property, e.g., plate size, and are still unable to interpret flexibly the relationship between the target and the plate specified by the ocular baseball rule. Note that the lower number of cells in this experiment (41 instead of 48) resulted from too few trials being recorded during this task from some SEF neurons.
Fig. 4.
Cumulative percentile of separation times in standard and 2-plate (2P1T) baseball. Separation time is defined as the time at which the activity of a neuron first became statistically different between strike and ball trials (see methods). The solid vertical line indicates when the target began to move.
To test whether the plate size was being classified by these cells, some SEF neurons were subjected to a second version of the two-plate task, the 2P2T, in which plate size and trajectory angle were randomly varied from trial to trial so that neither by itself uniquely specified the rule. As shown in Fig. 5, the 2P2T task requires that the monkey (and neurons) account for the relationship between the trajectory and plate to generate a proper strike or ball signal. Here, with the smaller 8° plate, the 10° trajectory specified strike and the 20° trajectory specified ball, as indicated respectively by the green and red trajectories in Fig. 5A, bottom. For the larger 16° plate, both the 10° and 20° trajectories specified strike. In this paradigm, if neuronal activity merely reflected plate size, activity for the 10° and 20° trajectories should be the same for the same size plate, and activity for the 10° trajectories should be different for the small and large plates.
Fig. 5.
Results of 2-plate, 2-trajectory (2P2T) task. A: spatial schematic. Plate size was either 16° (top) or 8° (bottom), and target trajectories were 10° or 20°, all randomized within a block. B: activity of a typical neuron in the 2P2T experiment. Average activity for each trajectory is shown for the large plate (dashed green traces, 10° trajectory; dotted, 20°) and for the small plate (solid green traces, 10°; solid red, 20°). Gray shaded region indicates the analysis period (550–800 ms). The cells were initially classified as baseball related based on delay period activity in standard baseball. A late analysis period is used here because many cells signaled plate size before the target appeared, possibly contaminating the earlier part of delay period. C: summary data for the small plate. Each point represents activity for a single neuron in 2P2T for the trajectories that conformed to its preferred and nonpreferred rule states as determined by their activity in standard ocular baseball. Note higher activity for most cells when the trajectory conformed to the preferred rule state, indicating that neurons were discriminating the different rule states. D: summary data for the large plate. Here the x-axis shows normalized activity for the 10° trajectory and the y-axis for the 20° trajectory. Note that overall there was no significant difference in activity for the 2 trajectories, indicating that neurons classified them as conforming to the same rule state. E: activity of a strike cell averaged over combinations of same angle and plate size from a block of trials showing putative probabilistic encoding of the rule. Green traces are strike trials with the small plate (solid) and large plate, 10° (dashed) and 20° (dotted). The red trace represents the small-plate ball trials. Vertical lines represent plate intersection and are color coded correspondingly. Note that this neuron first responds more for the large plate (top circled traces) than the small plate (bottom circled traces) but later in the trial signals strike and ball. Shaded region shows the analysis period (±200 ms relative to target onset). F: summary data of early activity for all recorded SEF neurons. Activity for the preferred plate size is plotted against that observed for the nonpreferred plate size for both strike and ball neurons. Note that most cells signaled plate size. The difference in activity, Δ activity, for the preferred plate size refers to the spike rate for large-plate trials minus that for small-plate trials for strike neurons and the small-plate spike rate minus the large-plate spike rate for ball neurons.
Figure 5B shows an example neuron's response during the 2P2T task. The neuron appears not merely to classify plate size, because its activity is similar for the 10° strike trajectories for the two plate sizes and different for the ball and strike trajectories in the 8° plate condition. Therefore, the neuron apparently used both plate size and trajectory information to signal strike or ball. This was true for the population: Fig. 5C shows that with the small plate the activity of most strike neurons in the delay period was higher for the 10° than 20° trajectories, and vice versa for ball neurons. A MANOVA test showed that the population of neurons as a whole was significantly more likely to signal its preferred rule state (d = 1, λ = 0.170, P < 0.01, df = 31). For the large plate, neural activity was not significantly different between the 10° and 20° trajectories (Fig. 5D), as shown by a MANOVA test (d = 0, λ = 0.260, P > 0.01, df = 35).
Interestingly, these neurons also appear to utilize plate size to signal the likelihood of strike and ball trials at an early time, similar to how LIP neurons encode the probability of appearance of different stimuli (Yang and Shadlen 2007). Figure 5E shows activity of the same strike neuron as in Fig. 5b, but now collapsed so that only one trace per angle is shown. Note that the early activity of the neuron is higher for the large plate than for the small plate, presumably because of the higher probability of a strike trial with the large plate. Note that the activity of the neuron then changes to reflect the rule after the target begins to move. Figure 5F summarizes the population data for the early activity (before target onset). Note that the activity of most neurons is correlated with the probability of a strike or ball trial associated with a given plate size. A MANOVA test showed the population of neurons to be significantly more active when the plate size correlated with the cell's preferred rule state (d = 1, λ = 0.409, P < 0.01, df = 67).
A goal of decision-making research is to relate the activity of single neurons to the behavior of the animal. In the Newsome et al. (1989) task, the monkey indicates with a saccade whether a stimulus moves with an angle clockwise or counterclockwise to a decision boundary. An MT neuron with appropriate directional selectivity can reflect the animal's decision in the task if the decision boundary remains fixed. However, rotating the decision boundary affects how well the activity of the neuron echoes the behavioral decision, and can even render it unrelated to that decision (Britten et al. 1996).
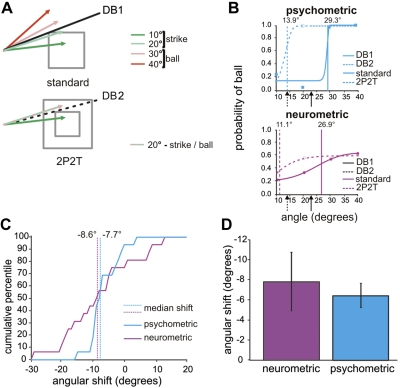
We wished to know how well SEF neurons interpreted the decision rule when the boundary was shifted in our experiment, and to compare neuronal activity with the monkeys' behavior when the shift occurred. To quantify and compare the decisions of the neurons and the monkeys, we computed neurometric functions and psychometric functions from the activity of neurons and behavior of the monkeys in 16 recording sessions (see methods). Figure 6A shows the potential trajectory angles and the objective decision boundary (the angle that differentiates strike and ball trajectories) based on the plate-trajectory relationship. The decision boundary was 23.2° for the 12° plate in the standard ocular baseball experiment, different from the 16° and 8° plate sizes in the 2P2T experiment, which had decision boundaries of 33.7° and 14.0°, respectively (only the decision boundaries for the small plate are shown in Fig. 6A). The large plate in the 2P2T experiment was not used because both trajectories were strikes.
Fig. 6.
Comparing choices of neuron and monkey in ocular baseball relative to a decision boundary change. A: decision boundaries in standard ocular baseball (top) and the 2P2T experiment (bottom). Green and red arrows indicate strike and ball trajectories (alternating red/green trajectory is either a strike or ball trajectory depending on plate size), and black solid and dashed lines show objective decision boundaries for the 12° plate in standard ocular baseball (DB1) and for the 8° plate in 2P2T baseball with the small plate size (DB2), respectively. B: typical psychometric and neurometric functions for standard ocular baseball (solid curves) and small-plate trials in the 2P2T experiment (dashed curves), obtained from an example SEF strike neuron. The solid and dashed arrows below the x-axis mark objective decision boundaries for standard baseball (23.2°) and small-plate 2P2T (14.0°) baseball, respectively. Solid and dashed vertical lines indicate the corresponding subjective decision boundaries derived from the computed coefficients of fits (see methods). C: cumulative percentiles for the shifts in psychometric and SEF cell neurometric functions following the decision boundary change from DB1 to DB2. Vertical lines indicate the median angular shift in the subjective decision boundary. Note that both functions are shifted to the left, consistent with a shift in the objective decision boundary from the 12° plate to the smaller 8° plate. Note also that both functions are shifted by a similar amount, indicating that the animal and neuron interpreted the decision boundary shift similarly. D: summary of the shift. Error bars indicate the SE of the mean degree of angle in shift.
Figure 6B shows typical neurometric and psychometric functions for a representative neuron and the accompanying behavior of the monkey in the standard baseball task and after the decision boundary was shifted in the 2P2T task. Both the neuron and the monkey appear to interpret the rule correctly despite the shift in decision boundary. To quantify the extent to which the population of neurons agreed with the behavior in observing the decision boundary, we first took the angle of α produced by the Weibull fit (see methods) as an indicator of the subjective decision boundary that the monkey or a given neuron chose. Next, we constructed a cumulative distribution of the difference in α for each pair of psychometric and neurometric functions for all 16 recording sessions (Fig. 6C). The medians of angular shift in the subjective decision boundaries are comparable for the psychometric and neurometric functions, as indicated by the vertical lines in Fig. 6C. A paired t-test showed that the mean (±SD) angular shifts in the subjective decision boundaries for the psychometric (−7.22 ± 5.27°) and neurometric (−5.72 ± 16.28°) functions over all recording sessions were not significantly different (P = 0.73), as shown in Fig. 6D. Therefore, the activity of SEF neurons appears to interpret the decision rule of ocular baseball online on a trial-by-trial basis consistent with both the shifting decision boundary and the decision boundary for the animal's behavioral choice.
DISCUSSION
In the present study we used our ocular baseball decision paradigm to determine whether the SEF, a structure involved in the preparation but not execution of eye movements, could flexibly interpret a strike-ball task rule governing motion trajectories when the same trajectories specified either strike or ball in different contexts. Specifically, we changed the decision boundary from trial to trial in the task by altering the size of the plate that dictated whether a given trajectory specified strike (pursue the target) or ball (fixate). As a result, the same motion trajectory could specify a different rule state (strike or ball) based on the decision boundary that was specified in a given trial. We found that SEF neurons continued to interpret the task rule reliably when the decision boundary was changed, and did so by shifting their subjective decision boundary on a trial-by-trial basis. Our work thus provides evidence of a neural structure that flexibly interprets motion stimuli within the context of a task rule in order to rapidly signal different motor decisions to the same motion stimulus and the same motor decision to different motion stimuli.
Sensorimotor decision making involves a cascade of stages, from deciding the perceptual category of stimuli to selecting a response from multiple alternatives. Some neural structures, such as MT, are at the perceptual stage. In experiments conducted in MT, monkeys indicate whether a stimulus moves in a direction with an angle clockwise or counterclockwise to a decision boundary [e.g., decide whether the motion has a rightward or leftward component relative to up (Newsome et al. 1989)]. Sensory motion neurons such as those in MT can signal perceptual decisions by their activity level, since activity is higher for a single “preferred” direction than others. Therefore, if a given neuron is sensitive to a certain range of motion directions relative to a decision boundary, it can signal the appropriate decision with a high degree of precision (Jazayeri and Movshon 2007). However, if the decision boundary is changed (e.g., decide if the motion is up or down relative to horizontal), the same neuron continues to respond best to the motion direction it preferred before the change (Britten et al. 1996), and therefore does not necessarily continue to reflect the decision.
For the results of perceptual categorization to affect movement decisions, additional transformations must occur beyond MT. LIP, unlike MT, has neurons that can classify the same motion directions differently when a decision boundary is changed (Freedman and Assad 2006). However, in this work the animals were retrained before the activity of neurons there was found to calibrate to the new boundary, and the task only required the animals to encode a single decision boundary at the time they were tested. Therefore, although LIP neurons can adjust to encode different decision boundaries, there is no evidence that they can simultaneously encode multiple boundaries and continue to interpret them when they change rapidly. Given that the SEF appears to be at a stage in the decision hierarchy with capabilities that surpass those observed in MT and LIP, SEF neurons in our study were relatively insensitive to specific angles or trajectories of target motion and continued to interpret the rule of our task when the decision boundary was changed. In addition, the neurons adjusted rapidly to the change from trial to trial. Therefore, the SEF likely lies beyond the relatively inflexible motion perception decision circuitry in MT and LIP.
Furthermore, the SEF does not appear to be strictly a component of motor decision circuitry for several reasons. For one, it appears that neurons here are not involved in specifying movement parameters, since SEF neurons related to ocular baseball show weak or no directional tuning (see Fig. 2), whereas movement cells should be tuned to specific movement directions. Additionally, the neurons we report here were differentially active during the delay period in standard baseball on average 324 ms before the movement, and earlier still in the two-plate experiment (170 ms before the target moved), which is too early to generate a movement command given that the latency of smooth pursuit in monkeys is ∼80 ms (Lisberger and Westbrook 1985). Moreover, in the 2P2T experiment, many neurons reversed their decision in the course of the trial (see Fig. 5E). These neurons signaled early a decision that appeared to reflect the different probabilities of balls and strikes for a given plate size, analogous to probabilistic reasoning by LIP neurons (Shadlen and Newsome 2001). Shortly after target motion began, but still long before the pursuit movement was executed, their activity changed to reflect the rule governing the target/plate relationship. Finally, and most critically, while SEF neurons signal the rule of the task, they do not reflect the behavior when errors are made (Yang et al. 2010).
If not involved in perceptual or motor aspects of decision making, where does the SEF lie in the neural decision cascade given our results? SEF neurons appear unique in that they do not encode sensory motion or issue motor commands; rather, they seem to interpret an abstract rule for a movement decision, and do so in a flexible fashion. We believe that the SEF is at a “perceptual-motor interface” in the decision-making process, lying between perceptual decision-making structures MT and likely LIP and motor output structures such as the FEF, which encodes the eventual decision response (Gold and Shadlen 2000). Because of this, and because the SEF is involved in movement preparation even when the movement is withheld (Schall 1991a), this structure appears to be distinctively suited to interpret a strike/ball decision rule flexibly. Using trajectory information conveyed from sensory motion regions, and/or through an interaction with the FEF where neurons have been shown to extrapolate target trajectory (Ferrera et al. 2010), the SEF might interpret the ocular baseball rule by specifying the desired decision in the context of the if/then contingencies that the rule imposes. Specifically, if the trajectory is predicted to cross the plate, the neurons interpret that movement is appropriate and generate preparatory activity. If the trajectory is predicted not to cross the plate, the neurons interpret that movement is inappropriate and signal that it be withheld. Thus the SEF bridges the margin between the strike/ball perception and the go/nogo motor response because neurons here interpret strike/ball to mean go/nogo.
There is evidence that the FEF, like LIP, can categorize motion and does so in a flexible fashion (Ferrera et al. 2009). In that study, monkeys classified a given speed motion with respect to either a low or high speed category boundary and signaled their choice by making a saccade to one of two spatially separated targets. While the animal viewed the moving stimuli, the activity of a population of FEF neurons was modulated by the category boundary from trial to trial. This result suggests that FEF neurons flexibly categorize speeds with respect to different decision boundaries, similar to how SEF neurons in the present study flexibly interpret the rule of our task when a decision boundary rapidly changes.
However, we do not think that the FEF and the SEF play similar roles in decision making, since the FEF is more related to generating eye movements than the premotor SEF (Schall 1991b). In accordance with the motor character of the FEF, in Ferrera et al. (2009) when a saccade was used to signal the decision, a significant amount of the activity of FEF neurons had a motor component. Saccades toward one chosen target location evoked a response that averaged 19% stronger than the response toward the other location (Ferrera et al. 2009, supplementary material). Even though target position was randomized, target position and target direction still significantly contributed to the response in 33% and 45% of the neurons, respectively. Moreover, the FEF provides a readout of the results of the decision process and is therefore likely located beyond the SEF, toward the end of the neural decision cascade (Gold and Shadlen 2000). Consistent with this, FEF neurons discriminate the rule state in our ocular baseball task significantly later than SEF neurons (Yang et al. 2009). A final difference between the FEF and SEF in decision making is that the FEF activity in Ferrera et al. (2009) reflects motion classification rather than rule interpretation, which our results suggest is an SEF function.
Given that the SEF does not ultimately enforce ocular decisions, how might its activity be used in the decision process? Our data suggest that this region interprets stimulus contingencies when rapid ocular decisions are required. If the contingencies are favorable for an ocular response, the SEF relays a “tentative” strike signal in the form of preparatory activity to a structure that is anatomically and functionally closer to where movement is generated. If the contingencies are unfavorable, a tentative ball signal would instead be relayed. In this scheme, the rule-related information is summed in the movement structure with other neural information relevant to the decision.
A potential summing junction for ocular decision making is the FEF, which has strong reciprocal connections with the SEF (Barbas and Pandya 1991). The FEF is likely closer to the motor output than the SEF, because neurons here have sharper tuning for visual stimuli and saccades than those in the SEF (Schall 1991b), and smaller currents are required to evoke saccades in the FEF during fixation (Tehovnik and Sommer 1997). Furthermore, the FEF pursuit region (FEFsem) appears downstream from decision making processes and is involved in generating pursuit dynamics (Mahaffy and Krauzlis 2011a, 2011b). Pursuit can also be electrically evoked in the FEFsem (e.g., Tehovnik and Sommer 1997), whereas it can only be modulated by SEF stimulation in alert primates (Missal and Heinen 2001). The FEF also receives input from the substantia nigra of basal ganglia (Lynch et al. 1994), a region that encodes expected reward, which is another component of decision making (Sato and Hikosaka 2002). Neural signals generated in the SEF, the basal ganglia, and other neural substrates are likely summed in the FEF in the process of rendering the eventual ocular decision.
The SEF is also involved in anticipatory eye movement generation (Heinen and Liu 1997; Missal and Heinen 2004) and eye movement control guided by expectation (de Hemptinne et al. 2008). Appropriate anticipation and expectation might also benefit from knowing contingencies that are imposed by rules, and given that the SEF interprets the ocular baseball rule, the efficacy of anticipation and expectation processes could be enhanced with this information. That anticipation and expectation interact with rule interpretation is supported by the putative probabilistic coding of plate size and target trajectories shown in Fig. 5E.
In conclusion, the ball and strike activity generated by the SEF might provide primates with an option to override reflexive behavior by facilitating decision making in situations where behaving according to learned rules maximizes reward. Furthermore, the flexible nature of rule interpretation exhibited by SEF neurons allows rapid behavior that could minimize penalties imposed by reaction time delays.
GRANTS
This work was supported by National Eye Institute Grant EY-117720 and the R. C. Atkinson Fellowship at the Smith-Kettlewell Eye Research Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.J.H., H.H., and S.N.Y. conception and design of research; S.J.H., H.H., and S.N.Y. performed experiments; S.J.H., H.H., and S.N.Y. analyzed data; S.J.H., H.H., and S.N.Y. interpreted results of experiments; S.J.H., H.H., and S.N.Y. prepared figures; S.J.H., H.H., and S.N.Y. drafted the manuscript; S.J.H., H.H., and S.N.Y. edited and revised the manuscript; S.J.H., H.H., and S.N.Y. approved the final version of the manuscript.
REFERENCES
- Barbas H, Pandya D. Patterns of connections of the prefrontal cortex in the rhesus monkey associated with cortical architecture. In: Frontal Lobe Function and Dysfunction, edited by Levin HS, Eisenberg HM, Benton AL. New York: Oxford Univ. Press, 1991, p. 35–58 [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis 10: 433–436, 1997 [PubMed] [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci 13: 87–100, 1996 [DOI] [PubMed] [Google Scholar]
- de Hemptinne C, Lefèvre P, Missal M. Neuronal bases of directional expectation and anticipatory pursuit. J Neurosci 28: 4298–4310, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrera VP, Yanike M, Cassanello C. Frontal eye field neurons signal changes in decision criteria. Nat Neurosci 12: 1458–1462, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrera VP, Barborica A. Internally generated error signals in monkey frontal eye field during an inferred motion task. J Neurosci 30: 11612–11623, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DJ, Assad JA. Experience-dependent representation of visual categories in parietal cortex. Nature 443: 85–88, 2006 [DOI] [PubMed] [Google Scholar]
- Fukushima J, Akao T, Takeichi N, Kaneko CRS, Fukushima K. Involvement of the frontal oculomotor areas in developmental compensation for the directional asymmetry in smooth pursuit eye movements in young primates. Ann NY Acad Sci 1004: 451–456, 2003 [Google Scholar]
- Gabbiani F, Cox SJ. Mathematics for Neuroscientists. New York: Academic, 2010 [Google Scholar]
- Gold JI, Shadlen MN. Representation of a perceptual decision in developing oculomotor commands. Nature 404: 390–394, 2000 [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The influence of behavioral context on the representation of a perceptual decision in developing oculomotor commands. J Neurosci 23: 632–651, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci 30: 374–535, 2007 [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley, 1966 [Google Scholar]
- Heinen SJ, Liu M. Single-neuron activity in the dorsomedial frontal cortex during smooth-pursuit eye movements to predictable target motion. Vis Neurosci 14: 853–865, 1997 [DOI] [PubMed] [Google Scholar]
- Heinen SJ, Rowland J, Lee BT, Wade AR. An oculomotor decision process revealed by functional magnetic resonance imaging. J Neurosci 26: 13515–13522, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz GD, Batista AP, Newsome WT. Representation of an abstract perceptual decision in macaque superior colliculus. J Neurophysiol 91: 2281–2296, 2004 [DOI] [PubMed] [Google Scholar]
- Jazayeri M, Movshon JA. A new perceptual illusion reveals mechanisms of sensory decoding. Nature 446: 912–915, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller EL, Heinen SJ. Generation of smooth-pursuit eye movements: neuronal mechanisms and pathways. Neurosci Res 11: 79–107, 1991 [DOI] [PubMed] [Google Scholar]
- Kim YG, Badler JB, Heinen SJ. Trajectory interpretation by supplementary eye field neurons during ocular baseball. J Neurophysiol 94: 1385–1391, 2005 [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Lisberger SG. A model of visually-guided smooth pursuit eye movements based on behavioral observations. J Comput Neurosci 1: 265–283, 1994 [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ. Recasting the smooth pursuit eye movement system. J Neurophysiol 91: 591–603, 2004 [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Morris EJ, Tychsen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu Rev Neurosci 10: 97–129, 1987 [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Westbrook LE. Properties of visual inputs that initiate horizontal smooth pursuit eye movements in monkeys. J Neurosci 5: 1662–1673, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JC, Hoover JE, Strick PL. Input to the primate frontal eye field from the substantia nigra, superior colliculus, and dentate nucleus demonstrated by transneuronal transport. Exp Brain Res 100: 181–186, 1994 [DOI] [PubMed] [Google Scholar]
- Mahaffy S, Krauzlis RJ. Neural activity in the frontal pursuit area does not underlie pursuit target selection. Vision Res 51: 853–866, 2011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffy S, Krauzlis RJ. Inactivation and stimulation of the frontal pursuit area change pursuit metrics without affecting pursuit target selection. J Neurophysiol 106: 347–360, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell JH, van Essen DC. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J Neurophysiol 49: 1127–1147, 1983 [DOI] [PubMed] [Google Scholar]
- Missal M, Heinen SJ. Facilitation of smooth pursuit initiation by electrical stimulation in the region of the supplementary eye fields. J Neurophysiol 86: 2413–2425, 2001 [DOI] [PubMed] [Google Scholar]
- Missal M, Heinen SJ. Supplementary eye fields stimulation facilitates anticipatory pursuit. J Neurophysiol 92: 1257–1262, 2004 [DOI] [PubMed] [Google Scholar]
- Newsome WT, Britten KH, Movshon JA. Neuronal correlates of a perceptual decision. Nature 341: 52–54, 1989 [DOI] [PubMed] [Google Scholar]
- Olson CR, Gettner SN. Macaque SEF neurons encode object-centered directions of eye movements regardless of the visual attributes of instructional cues. J Neurophysiol 81: 2340–2346, 1999 [DOI] [PubMed] [Google Scholar]
- Olson CR, Gettner SN. Object-centered direction selectivity in the macaque supplementary eye field. Science 269: 985–988, 1995 [DOI] [PubMed] [Google Scholar]
- Richmond BJ, Optican LM. Temporal encoding of two-dimensional patterns by single units in primate inferior temporal cortex. II. Quantification of response waveform. J Neurophysiol 57: 147–161, 1987 [DOI] [PubMed] [Google Scholar]
- Robinson DA, Gordon JL, Gordon SE. A model of smooth pursuit eye movement system. Biol Cybern 55: 43–58, 1986 [DOI] [PubMed] [Google Scholar]
- Rorie AE, Newsome WT. A general mechanism for decision-making in the human brain? Trends Cogn Sci 9: 41–43, 2005 [DOI] [PubMed] [Google Scholar]
- Sato M, Hikosaka O. Role of primate substantia nigra pars reticulata in reward-oriented saccadic eye movement. J Neurosci 22: 2363–2373, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD. Neuronal activity related to visually guided saccadic eye movements in the supplementary motor area of rhesus monkeys. J Neurophysiol 66: 530–558, 1991a [DOI] [PubMed] [Google Scholar]
- Schall JD. Neuronal activity related to visually guided saccades in the frontal eye fields of rhesus monkeys: comparison with supplementary eye fields. J Neurophysiol 66: 559–579, 1991b [DOI] [PubMed] [Google Scholar]
- Schiller PH, Chou I. The effects of frontal eye field and dorsomedial frontal cortex lesions on visually guided eye movements. Nat Neurosci 1: 248–253, 1998 [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol 86: 1916–1936, 2001 [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci 9: 925–931, 2006 [DOI] [PubMed] [Google Scholar]
- Swets JA. Signal Detection and Recognition by Human Observers. New York: Wiley, 1964 [Google Scholar]
- Tehovnik EJ, Sommer MA. Electrically evoked saccades from the dorsomedial frontal cortex and frontal eye fields: a parametric evaluation reveals differences between areas. Exp Brain Res 117: 369–378, 1997 [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, Chou IH, Slocum WM, Schiller PH. Eye fields in the frontal lobes of primates. Brain Res Rev 32: 413–448, 2000 [DOI] [PubMed] [Google Scholar]
- Watamaniuk SNJ, Heinen SJ. Human smooth pursuit direction discrimination. Vision Res 39: 59–70, 1999 [DOI] [PubMed] [Google Scholar]
- Yang SN, Ford JS, Heinen SJ. Contrasting supplementary and frontal eye field involvement in ocular decision making. J Vis 9: 80, 2009 [Google Scholar]
- Yang S, Hwang H, Ford JS, Heinen SJ. Supplementary eye field activity reflects a decision rule governing smooth pursuit but not the decision. J Neurophysiol 103: 2458–2469, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Shadlen MN. Probabilistic reasoning by neurons. Nature 447: 1075–1080, 2007 [DOI] [PubMed] [Google Scholar]