Abstract
Gonadotropin-releasing hormone (GnRH) neurons form the final common pathway for central control of fertility. Regulation of GnRH neurons by long-loop gonadal steroid feedback through steroid receptor-expressing afferents such as GABAergic neurons is well studied. Recently, local central feedback circuits regulating GnRH neurons were identified. GnRH neuronal depolarization induces short-term inhibition of their GABAergic afferents via a mechanism dependent on metabotropic glutamate receptor (mGluR) activation. GnRH neurons are enveloped in astrocytes, which express mGluRs. GnRH neurons also produce endocannabinoids, which can be induced by mGluR activation. We hypothesized the local GnRH-GABA circuit utilizes glia-derived and/or cannabinoid mechanisms and is altered by steroid milieu. Whole cell voltage-clamp was used to record GABAergic postsynaptic currents (PSCs) from GnRH neurons before and after action potential-like depolarizations were mimicked. In GnRH neurons from ovariectomized (OVX) mice, this depolarization reduced PSC frequency. This suppression was blocked by inhibition of prostaglandin synthesis with indomethacin, by a prostaglandin receptor antagonist, or by a specific glial metabolic poison, together suggesting the postulate that prostaglandins, potentially glia-derived, play a role in this circuit. This circuit was also inhibited by a CB1 receptor antagonist or by blockade of endocannabinoid synthesis in GnRH neurons, suggesting an endocannabinoid element, as well. In females, local circuit inhibition persisted in androgen-treated mice but not in estradiol-treated mice or young ovary-intact mice. In contrast, local circuit inhibition was present in gonad-intact males. These data suggest GnRH neurons interact with their afferent neurons using multiple mechanisms and that these local circuits can be modified by both sex and steroid feedback.
Keywords: gonadotropin-releasing hormone, androgen, estradiol, γ-aminobutyric acid, local circuit, glia
gonadotropin-releasing hormone (GnRH) neurons play a major role in the control of reproduction (Cattanach et al. 1977). GnRH causes the anterior pituitary to synthesize and secrete the gonadotropic hormones, which activate steroidogenesis and gametogenesis. Gonadal steroid milieu alters the release of GnRH in vivo (Levine and Ramirez 1982; Levine et al. 1985a, 1985b; Moenter et al. 1990, 1991) and the activity of GnRH neurons in brain slices (Christian et al. 2005; Pielecka and Moenter 2006; Pielecka et al. 2006). This long-loop feedback modulation of GnRH neurons largely appears to be via steroid-sensitive afferent networks (Wintermantel et al. 2006), because GnRH neurons have only rarely been found to express steroid hormone receptors other than the β-form of the estradiol receptor (Hrabovszky et al. 2000, 2001), which does not appear to be critical for fertility (Krege et al. 1998).
GABAergic transmission to GnRH neurons is particularly important in conveying steroid feedback regulation (Chen and Moenter 2009; Christian and Moenter 2007; Penatti et al. 2010; Sullivan and Moenter 2003, 2005). Of note, GABA largely provides a depolarizing/excitatory action in GnRH neurons due to elevated chloride levels maintained in these cells in adulthood (DeFazio et al. 2002; Herbison and Moenter 2011; Moenter and DeFazio 2005; Nakane and Oka 2010; Watanabe et al. 2009; Yin et al. 2008). GnRH neurons have been shown to interact with their GABAergic afferents rapidly via metabotropic glutamate receptor (mGluR)-mediated local circuit interactions (Chu and Moenter 2005), via retrograde endocannabinoid signaling (Farkas et al. 2010), and more slowly via the GnRH decapeptide (Chen and Moenter 2009), with all of these interactions tending to quiet the GABAergic afferents for different durations.
In previous work examining these local circuit interactions, repeated action potential-like depolarization of GnRH neurons caused a short-term reduction of GABAergic transmission to that same GnRH neuron. This reduction was blocked by antagonists of mGluRs, suggesting their involvement (Chu and Moenter 2005). GnRH neurons have been reported to express vesicular glutamate transporters (Hrabovszky et al. 2004), and activation of mGluRs depresses GABAergic transmission to GnRH neurons (Chu and Moenter 2005), suggesting a direct interaction is possible via this mechanism; however, further studies are needed to determine whether this circuit contains other elements.
Two mechanisms of particular relevance to GnRH neurons that can be engaged by mGluRs are glia and endocannabinoids. GnRH neurons are surrounded by astrocytes (Baroncini et al. 2007; Cashion et al. 2003; Witkin et al. 1991), and astrocytes, including those in the hypothalamus, express mGluRs (Condorelli et al. 1997; Dziedzic et al. 2003; Nakanishi and Masu 1994). Astrocytes are increasingly recognized to play a role in synaptic transmission and also can regulate extracellular glutamate uptake and release (Bergles and Jahr 1998; Rothstein et al. 1996; Tanaka et al. 1997; see for review Haydon 2001; Perea and Araque 2007). Astrocytes communicate with neurons via gliotransmitters, a major class of which is prostaglandins, and prostaglandins can depress synaptic transmission (Laaris and Weinreich 2007). Endocannabinoids reduce basal GABAergic transmission to GnRH neurons (Farkas et al. 2010), but their role in depolarization-induced suppression has not been studied.
In this study we examined the role of prostaglandins and endocannabinoids in depolarization-induced suppression of GABAergic transmission to GnRH neurons, and determined the steroid feedback sensitivity of this circuit.
MATERIALS AND METHODS
Animals.
GnRH neurons were recorded from transgenic mice expressing enhanced green fluorescent protein (eGFP) under the control of GnRH promoter (Suter et al. 2000). Mice were housed under a 14:10-h light-dark photoperiod with Harlan 2916 chow (Harlan, Indianapolis, IN) and water available ad libitum. Adult female or male mice (42–60 days) or ovary-intact prepubertal females from 7–22 days of age were used. To control the effects of ovarian steroids in adult females, ovariectomy (OVX) was performed under isoflurane anesthesia (Burns Veterinary Supply, Westbury, NY). Bupivicaine (0.25%, 7 μl per surgical site; Abbott Labs, North Chicago, IL), a long-lasting local anesthetic, was applied to surgery sites to reduce postoperative pain and distress. At the time of OVX, some animals received subcutaneous Silastic implants (Dow-Corning, Midland, MI) containing 0.625 μg of 17β-estradiol (E) and/or 400 μg of dihydrotestosterone (DHT) in sesame oil. Surgery was performed 5–10 days before experimentation. Doses of steroids and time between surgery and electrophysiological experiments were chosen according to the models previously described (DeFazio and Moenter 2002; Sullivan and Moenter 2005). The estradiol level is a low physiological level, and the DHT implant produces a mild elevation in androgen that is insufficient to restore mass of seminal vesicles in male mice, thus mimicking the mild hyperandrogenemia of the common fertility disorder polycystic ovary syndrome, in which GnRH neuron activity is elevated. All procedures were approved by the University of Virginia Animal Care and Use Committee.
Brain slice preparation.
All chemicals were purchased from Sigma Chemical (St. Louis, MO) unless otherwise noted. Brain slices were prepared as previously described (Chu and Moenter 2005; Nunemaker et al. 2002). All buffers were continuously bubbled with a mixture of 95% O2 and 5% CO2, beginning at least 15 min before use, to maintain proper saturation; pH was also monitored. Sagittal 300-μm brain slices were cut using a Vibratome 3000 (Ted Pella, Redding, CA) in ice-cold sucrose saline containing (in mM) 250 sucrose, 3.5 KCl, 26 NaHCO3, 10 glucose, 1.25 NaH2PO4, 1.2 MgSO4, and 3.8 MgCl2. Slices were incubated in a 1:1 mixture of sucrose saline and artificial cerebrospinal fluid (ACSF) containing (in mM) 125 NaCl, 3.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2.5 CaCl2, 1.2 MgSO4, and 10 d-glucose, pH 7.4, for 30 min at 31°C and then transferred to 100% ACSF and incubated for at least an additional 30 min at room temperature (22–24°C) until study.
Electrophysiological recordings.
Individual brain slices were transferred to a recording chamber mounted on the stage of an upright microscope (Olympus BX50WI; Opelco, Dulles, VA). The chamber was perfused continuously with ACSF containing 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione and 20 μM d(−)2-amino-5-phosphonovaleric acid to block ionotropic glutamate currents at a rate of 5–6 ml/min at 31–32°C. Slices were stabilized in the chamber for at least 5 min before recording. All recordings were performed from GnRH neurons located in the preoptic area and ventral anterior hypothalamic regions; no differences were noted based on location of cells. Recording micropipettes were pulled from capillary glass (type 7052, outer diameter/inner diameter 1.65/1.1 mm; World Precision Instruments, Sarasota, FL) using a Flaming/Brown P-97 pipette puller (Sutter Instruments, Novato, CA) to obtain pipettes with a resistance of 2.5–4.0 MΩ. Whole cell recordings were made in voltage-clamp mode of an EPC-10 or EPC-10 USB amplifier with Patchmaster software (HEKA Elektronik, Lambrecht/Pfalz, Germany) on a Macintosh MacPro computer (Apple Computers, Cupertino, CA). GnRH-GFP neurons were identified by brief illumination at 470 nm.
To facilitate detection of GABAergic currents at a holding potential of −60 mV, pipettes were filled with high-chloride solution containing (in mM) 140 KCl, 10 HEPES, 5 EGTA, 4.0 MgATP, 0.4 NaGTP, and 1.0 CaCl2, pH 7.3, 290 mOsm. Cells were stabilized for 3–5 min after the whole cell configuration was achieved before data were recorded. Electrical passive properties [input resistance (Rin), series resistance (Rs), and membrane capacitance (Cm)] were monitored every 2–3 min. Only recordings with Rin > 500 MΩ, Rs < 20 MΩ, and stable Cm were accepted for further treatment and analysis. Furthermore, a minimum of 0.5-Hz GABAergic postsynaptic currents (PSCs) was required to allow detection of any suppression of the frequency of these events by treatments.
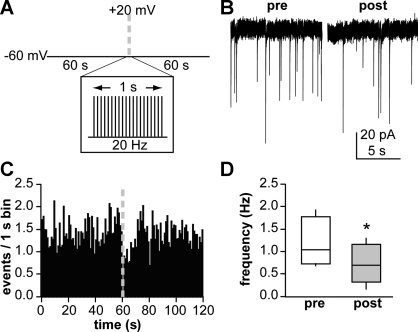
Figure 1A illustrates the voltage protocol utilized to determine how GnRH neuronal depolarization (GND) regulates GABAergic transmission to the depolarized GnRH neuron. Cells were clamped at −60 mV for 60 s to monitor PSC frequency. The cell was then depolarized to +20 mV for 2 ms every 50 ms for 1 s. This was followed by an additional 60 s of recording of PSC frequency at −60 mV. This protocol was repeated in control solution three to four times. Cells were then treated, and the protocol was repeated three to four times after drug wash in. Treatments and duration required for drug wash in were as follows: 5 μM indomethacin (5 min), 10 μM AH6809 (5 min; antagonist of prostaglandin receptor EP2, weak activity at EP1 and DP1), 5 μM fluorocitrate (30 min), or 1 μM SR141716 [5 min; cannabinoid receptor type 1 (CB1R) antagonist (generous gift from the National Institute of Drug Abuse)]. To block endocannabinoid synthesis in GnRH neurons, we added 10 μM orlistat (a diacylglycerol lipase inhibitor) to the recording pipette solution. Only one cell was recorded in each slice (to avoid any altered results due to previous pharmacological treatments), and no more than two cells per animal.
Fig. 1.
Repeated gonadotropin-releasing hormone (GnRH) neuronal depolarization (GND) results in a short-term suppression of GABAergic transmission to that GnRH neuron. A: voltage protocol used to record GABAergic postsynaptic currents (PSCs), consisting of a 60-s pre-GND segment, 1 s of 20-Hz depolarization to +20 mV to simulate action potential firing (GND), and 60 s of post-GND recording. B: representative recording of GABAergic PSCs from a GnRH neuron from an ovariectomized (OVX) mouse during the 10 s before (pre) and after (post) GND. C: mean PSC frequency data binned in 1-s intervals (n = 9 cells that responded to GND with suppression out of 12 tested). D: box and whisker plot showing full range of values (whiskers), 25th–75th percentile (box), and median PSC frequency (horizontal line within box) during the 10 s before vs. 10 s after GND. *P < 0.05.
Analysis.
Data collected during experiments were analyzed off-line using software developed in Igor Pro (Sullivan et al. 2003) to identify PSCs. Spontaneous PSCs were detected automatically and confirmed by eye. Both false positive and false negative detection errors were corrected manually. Data generated were transferred to a spreadsheet for additional data and statistical analysis (OpenOffice.org 3.0.0 Beta, Sun Microsystems; Prism 4, GraphPad Software). The number of GABAergic postsynaptic events per second was counted for each recording trace and then averaged for each cell to obtain cell mean values, which were then averaged to obtain group mean values. Averaged PSC frequencies during 10 s before and 10 s after GND were compared using two-tailed paired Student's t-test or two-way ANOVA followed by Bonferroni post hoc tests; P < 0.05 was considered significant. Data in text are means ± SE; summary data are shown as a full range of values, medians, and 25th–75th percentiles where indicated.
RESULTS
Local circuit feedback regulation of GnRH neuron activity is modulated by endocannabinoids.
To examine GnRH-GABA neuron local feedback, we recorded GABAergic PSCs in GnRH neurons from OVX female mice for 60 s before and after repeated depolarization of the GnRH neuron (GND; −60 to +20 mV for 2 ms, 20 Hz, 1 s). A subpopulation of GnRH neurons (9 of 12) exhibited a short-term reduction (P < 0.05) in GABAergic PSC frequency directly after GND in control solution (ACSF) as previously described (Chu and Moenter 2005). A representative example is shown in Fig. 1B, PSC frequency vs. time in Fig. 1C, and PSC frequency in 10 s immediately before and after GND in Fig. 1D (1.2 ± 0.2 vs. 0.8 ± 0.2 Hz, n = 9). The suppression in PSC frequency lasted ∼9–11 s. Weaker depolarizations (e.g., to 0 mV) at the same frequency did not induce a suppression, indicating an action potential-like depolarization, i.e., crossing 0 mV, was required (not shown).
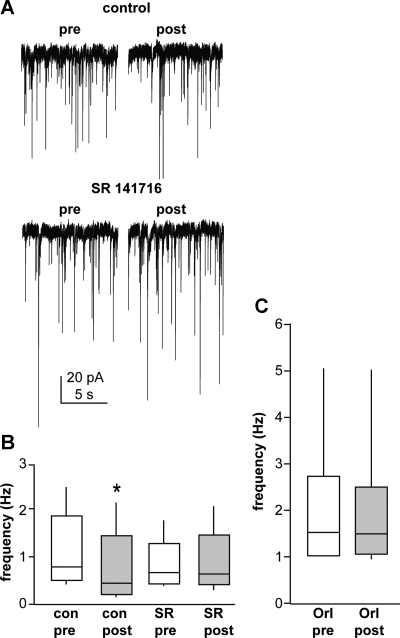
Because our goal was to test the mechanisms of this suppression, only cells responding to GND with suppression of GABAergic transmission were used in further studies. Depolarization-stimulated inhibition (DSI), which resembles this response, often involves endocannabinoid signaling (Diana and Marty 2004). Recent studies by Farkas et al. (2010) demonstrated that GABAergic afferents to GnRH neurons express CB1Rs and that basal GABAergic transmission to GnRH neurons is regulated by endocannabinoids. We tested whether cannabinoid signaling was also involved in modulation of the GnRH neuron-GABA afferent local circuitry. SR141716, a specific blocker of CB1Rs (Fig. 2, A and B) blocked the inhibitory effect of GND (control pre-GND 1.6 ± 0.5 Hz, post-GND 1.1 ± 0.4 Hz, P < 0.05; drug pre-GND 1.3 ± 0.5 Hz, post-GND 1.3 ± 0.5 Hz, n = 7 cells), suggesting endocannabinoid receptor signaling is another component of the local feedback circuit. To examine the source of endocannabinoids, we blocked their synthesis in the recorded cell by including orlistat (10 μM) within the recording pipette solution. As expected from previous work (Farkas et al. 2010), this increased basal GABAergic transmission to GnRH neurons. This treatment also blocked GND-induced suppression of GABAergic transmission (Fig. 2C; n = 9 cells, pre-GND 2.2 ± 0.5 Hz, post-GND 2.1 ± 0.5 Hz). These data suggest endocannabinoids produced by GnRH neurons play an important role in the GnRH-GABA circuit.
Fig. 2.
Endocannabinoid signaling is involved in GND-induced suppression of GABAergic transmission. A: representative recording of GABAergic PSCs from a GnRH neuron treated with SR141716 (SR) during the 10 s before and after GND. B: comparison of pre- and post-GND GABAergic PSC frequency in the presence and absence of cannabinoid receptor type 1 (CB1R) antagonist SR (n = 7 cells). C: comparison via t-test of pre- and post-GND GABAergic PSC frequency in the presence of 10 μM orlistat (Orl; an inhibitor of endocannabinoid synthesis) in the recording pipette (n = 9 cells). *P < 0.05 vs. pre-GND values for that same condition. See Fig. 1 for details on box and whisker plots. con, Control.
Local circuit feedback regulation of GABAergic transmission is modulated by prostaglandins.
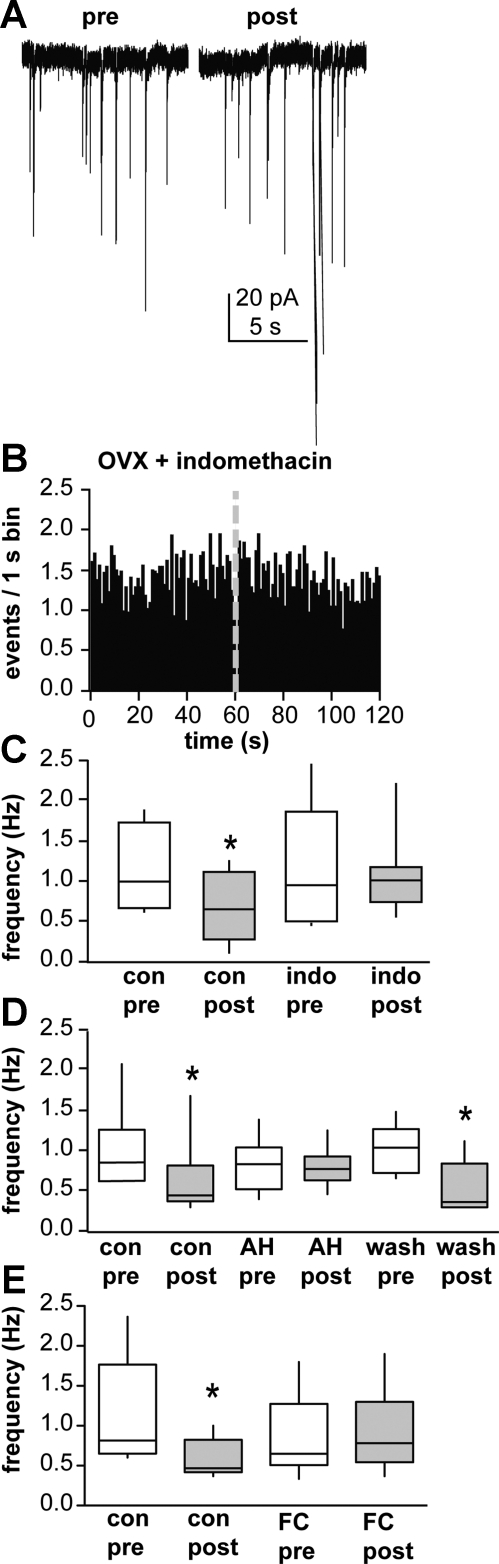
GnRH neurons are encased in glia (Witkin et al. 1991), which can express mGluRs (Condorelli et al. 1997; Dziedzic et al. 2003; Nakanishi and Masu 1994) and modulate synaptic transmission. Thus we examined glia as an additional intermediate in this local circuit. As a first step, we tested the hypothesis that prostaglandins, a common gliotransmitter, alter this circuit. Slices were treated with 5 μM indomethacin to block cyclooxygenase, a critical step in prostaglandin biosynthesis. Indomethacin blocked the GND-induced reduction in GABAergic PSCs (Fig. 3, A–C; n = 9 cells, control pre-GND 1.2 ± 0.2 Hz, post-GND 0.8 ± 0.2 Hz, P < 0.05; drug pre-GND 1.2 ± 0.3 Hz, post-GND 1.1 ± 0.2 Hz) but did not alter spontaneous PSC frequency (Fig. 3C). This suggests that blockade of prostaglandin synthesis with indomethacin disrupted the mechanism underlying communication between GnRH neuron depolarization and inhibition of GABAergic transmission.
Fig. 3.
Prostaglandins mediate the local depolarization-induced feedback circuit. A: representative recording of GABAergic PSCs from a GnRH neuron treated with indomethacin (indo; 5 μM) during the 10 s before and after GND. B: mean PSC frequency data binned in 1-s intervals (n = 9 cells). C: comparison via 2-way ANOVA of pre- and post-GND GABAergic PSC frequency in the presence and absence of indo (n = 9 cells). D: comparison via 2-way ANOVA of pre- and post-GND GABAergic PSC frequency in the presence and absence of 10 μM AH6809 (AH; n = 6 cells). E: comparison via 2-way ANOVA of pre-and post-GND GABAergic PSC frequency in the presence and absence of 5 μM fluorocitrate (FC; n = 6 cells). *P < 0.05 vs. pre-GND values for that same condition. See Fig. 1 for details on box and whisker plots.
In addition to being involved in prostaglandin synthesis, cyclooxygenase can degrade endocannabinoids (Kozak et al. 2004), which can modulate GABAergic transmission to GnRH neurons (Farkas et al. 2010). One possible interpretation of the above data is that blockade of cyclooxygenase with indomethacin causes accumulation of endocannabinoids. Although endocannabinoids decrease GABAergic transmission, it is possible that a sustained exposure to endocannabinoids could cause desensitization of the CB1R and a reduced response. To test more specifically whether prostaglandin signaling is involved, we examined the effect of a prostaglandin receptor antagonist (10 μM AH6809) on GND-induced suppression. AH6809 blocked suppression of GABAergic transmission to GnRH neurons following GND in a manner similar to indomethacin (Fig. 3D; n = 6 cells, control pre-GND 1.0 ± 0.2 Hz, post-GND 0.6 ± 0.2 Hz, P < 0.05; drug pre-GND 0.8 ± 0.1 Hz, post-GND 0.8 ± 0.1 Hz; wash pre-GND 1.0 ± 0.1 Hz, post-GND 0.5 ± 0.2 Hz, P < 0.05).
Prostaglandins are common gliotransmitters but also can be synthesized by neurons (Yamagata et al. 1993). To attempt to more directly implicate glia in this circuit, we used fluorocitrate to disrupt glial function. Fluorocitrate blocks the Krebs cycle exclusively in glia cells (Fonnum et al. 1997) and thus is a specific metabolic inhibitor of glia. Fluorocitrate at 5 μM, a dose that does not appear to alter neuronal function (Hassel et al. 1992), blocked GND-induced reduction in GABAergic transmission (Fig. 3E; control pre-GND 1.1 ± 0.3 Hz, post-GND 0.7 ± 0.2 Hz, P < 0.05; drug pre-GND 0.9 ± 0.2 Hz, post-GND 0.9 ± 0.2 Hz, n = 6 cells). At this dose, fluorocitrate had no effect on spontaneous PSC frequency (paired t-test between control pre-GND and drug pre-GND). Together with the above data, these results suggest prostaglandin signaling alters this local circuit and that glia are a potential source of these prostaglandins.
Estradiol alters local circuit modulation of GABAergic transmission to GnRH neurons.
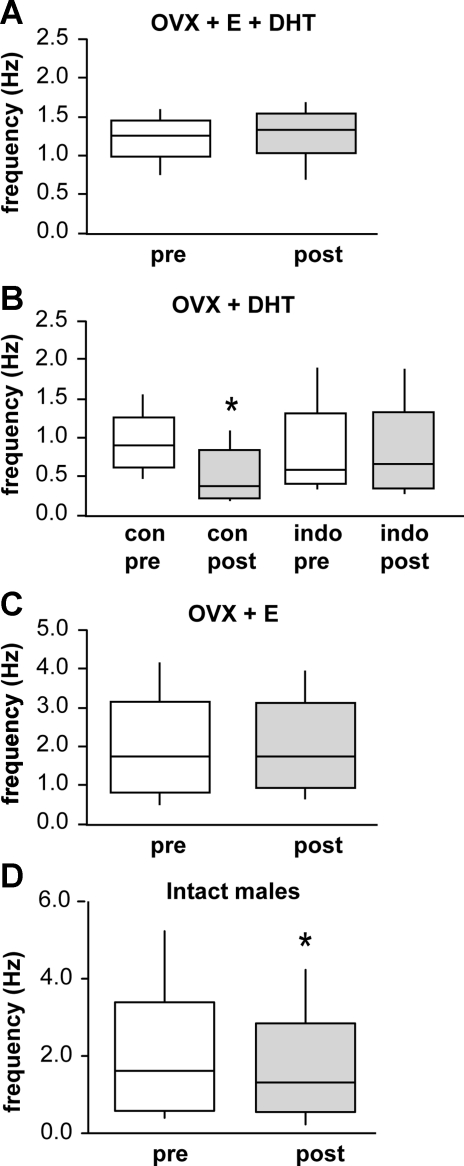
Because of the importance of GABAergic afferents in conveying gonadal steroid feedback, we investigated the role of steroids in this local circuit. Previous work had established that treating OVX female mice with physiological levels of estradiol and mild elevations of androgen provided by DHT (OVX+E+DHT) increased both GnRH neuron activity and GABAergic transmission to GnRH neurons (Pielecka et al. 2006; Sullivan and Moenter 2005). The most common endocrine presentation for infertility, polycystic ovary syndrome, is characterized by hyperandrogenemia, increased frequency of GnRH/luteinizing hormone release, and elevated CSF levels of GABA (Blank et al. 2006; Loucks et al. 2002). Interestingly, in OVX+E+DHT females, there was no GND-induced reduction in GABAergic transmission (Fig. 4A; pre-GND 1.2 ± 0.1 Hz, post-GND 1.3 ± 0.1 Hz, n = 6 cells), suggesting that steroids induce central changes that impact this circuit. We next tested the hypothesis that DHT blocks the ability of GND to inhibit GABAergic afferents, which provide a stimulatory input to GnRH neurons (DeFazio et al. 2002), hence leading to failure of an important homeostatic mechanism in hyperandrogenemia. In brain slices from animals treated with DHT alone, however, the GND-induced reduction of GABAergic transmission to GnRH neurons persisted and was of a similar magnitude to that observed in untreated OVX animals; depolarization of GnRH neurons from OVX+DHT mice significantly lowered GABA PSC frequency during the first 10 s following stimulation (Fig. 4B; pre-GND 0.9 ± 0.1 Hz, post-GND 0.4 ± 0.1 Hz, P < 0.05, n = 8 cells). In addition, indomethacin (5 μM) treatment blocked this reduction without influencing spontaneous PSC frequency (Fig. 4B; drug pre-GND 0.7 ± 0.2 Hz, post-GND 0.7 ± 0.2 Hz, n = 8 cells). These data indicate that androgen interruption of this circuit does not play a role in the excitatory effects of hyperandrogenemia on the GnRH neuronal network.
Fig. 4.
Effect of steroid feedback and sex on the local circuit. A: comparison of PSC frequency during the 10 s before and after GND in cells from OVX mice treated in vivo with both estradiol and dihydrotestosterone (OVX+E+DHT; n = 6). B: comparison of pre- and post-GND GABAergic PSC frequency in DHT-treated mice in the presence and absence of indo (OVX+DHT; n = 8 cells that responded to GND with suppression out of 13 studied). C: comparison of PSC frequency during the 10 s before and after GND in cells from OVX mice treated in vivo with estradiol (OVX+E; n = 6 cells). D: comparison of PSC frequency during the 10 s before and after GND in cells from adult male mice (n = 10 cells). *P < 0.05. See Fig. 1 for details on box and whisker plots.
In contrast to DHT alone, treatment with estradiol alone abolished the inhibitory effect of GND on GABAergic transmission (Fig. 4C; pre-GND 1.9 ± 0.5 Hz, post-GND 1.9 ± 0.4 Hz, n = 6 cells). This suggests that estradiol is responsible for disruption of the local feedback circuit from GnRH neurons to their GABAergic afferents. On the basis of suppression of this local feedback circuit by estradiol, we hypothesized that this circuit might be developmentally regulated, being absent in immature animals. To test this we examined the ability of GND to suppress GABAergic transmission in prepubertal females. Before the age of ∼19 days, GABAergic transmission was very low frequency, precluding monitoring a suppression. In ovary-intact females ages 19–22 days, which is about 1 wk after the initial rise in circulating levels of GnRH-dependent pituitary gonadotropins (Selmanoff et al. 1977) and just before estradiol-dependent vaginal opening (Brill and Moenter 2009), which is the first outward sign of puberty, there was no GND-induced inhibition of GABAergic PSC frequency (pre-GND 0.6 ± 0.1 Hz, post-GND 0.7 ± 0.1 Hz, n = 9 cells). Another possible explanation for the blockade of this circuit by estradiol is that inhibition of this circuit is part of the mechanism by which estradiol induces the surge mode of GnRH secretion that initiates the ovulatory cascade. This feedback effect is diurnal in mice, and the above studies were done in the afternoon, which is the time of estradiol positive feedback to generate the surge mode (Christian et al. 2005). Unfortunately, during estradiol negative feedback, PSC frequency is too low to test for the existence of this circuit. Likewise, PSC frequency was low in diestrus females; thus the apparent lack of GND-induced inhibition in ovary-intact females (pre-GND 0.26 ± 0.09 Hz, post-GND 0.28 ± 0.07 Hz, P = 0.75, n = 6 cells) must be interpreted with caution as a possible basement effect. As an alternative, we examined adult males, which lack a surge mode and have higher basal GABAergic transmission than females (Chen and Moenter 2009). In gonad-intact adult males, this circuit is functional, suggesting a sex difference (pre-GND 2.0 ± 0.5 Hz, post-GND 1.7 ± 0.4 Hz, n = 10 cells).
DISCUSSION
The classic view of the elements of the hypothalamo-pituitary-gonadal axis has been a one-way coupling through mainly homeostatic feedback from peripheral hormones. More recently, central interactions among GnRH neurons and components of their afferent network have been described, including mGluR-, GnRH-, and CB1R-dependent mechanisms (Chen and Moenter 2009; Chu and Moenter 2005; Farkas et al. 2010). These findings helped establish that GnRH neurons are not merely central output neurons but also play a key role in local circuit function. In this study we have demonstrated that GnRH neuron depolarization induces short-term inhibition of their GABAergic afferents via multiple mechanisms and that this interaction is steroid and likely sex dependent.
Action potential-like depolarization of GnRH neurons was previously shown to regulate GABAergic transmission to the recorded GnRH neuron in a manner dependent on activation of mGluRs (Chu and Moenter 2005). This mode of short-term plasticity bears resemblance to DSI (Diana and Marty 2004). It is important to bear in mind that the depolarizing/excitatory effect of GABAA receptor activation on GnRH neurons renders this circuit as a negative feedback loop, in which depolarization of GnRH neurons reduces an excitatory drive (DeFazio et al. 2002; Herbison and Moenter 2011). Classic DSI utilizes endocannabinoid signaling and is modulated by mGluR activation (Diana and Marty 2004). Endocannabinoids serve as retrograde transmitters to both neurons (Diana and Marty 2004) and glia (Navarrete and Araque 2008). The latter are of particular interest, because GnRH neurons receive relatively few inputs compared with neighboring neurons, are ensheathed in astrocytes, and are regulated by mGluR-dependent glial signals (Baroncini et al. 2007; Cashion et al. 2003; Dziedzic et al. 2003; Witkin et al. 1991).
In the current study, we extended previous observations by providing evidence for at least two signaling mechanisms involved in this local circuit. Blocking CB1Rs or blocking endocannabinoid synthesis in GnRH neurons inhibited depolarization-induced suppression of GABAergic transmission. Direct retrograde signaling via endocannabinoids from GnRH neurons to GABAergic afferents was recently reported (Farkas et al. 2010), but that study focused on basal levels of neurotransmission rather than mechanisms of short-term plasticity. The present data support and extend those findings to another mode of synaptic regulation.
Because of the extensive interactions between GnRH neurons and glia, and the ability of endocannabinoids to increase intracellular calcium in glia (Navarrete and Araque 2008), we also examined a potential role for glia in modulating this circuit. Blockade of prostaglandin synthesis with indomethacin or antagonism of prostaglandin receptors eliminated the reduction in GABAergic PSC frequency that occurs after GND. Furthermore, disruption of astrocyte metabolic function with fluorocitrate also blocked the reduction. This latter finding suggests a potential modulatory effect of glia via prostaglandins on GND-induced inhibition.
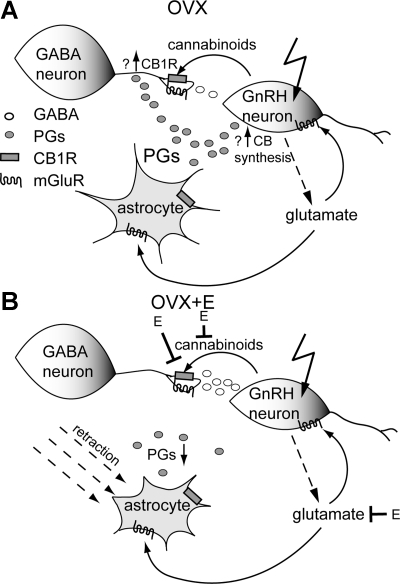
The possible involvement of both endocannabinoids and glia in this circuit is substantiated by other studies of interactions among these systems. Astrocytes are important regulators of synaptic transmission (Newman 2003; Oliet et al. 2001; Perea and Araque 2007; Rothstein et al. 1996). Endocannabinoids can alter astrocyte transmitter uptake (Shivachar 2007), and hypothalamic astrocytes express CB1Rs (Moldrich and Wenger 2000). On the basis of current knowledge, we propose a simplified model of GnRH local circuit regulation of GABAergic inputs (Fig. 5). GnRH neuron depolarization causes synthesis of endocannabinoids, which suppress GABAergic transmission via both direct retrograde transmission and alterations in glial function. Normal glial function, including production of prostaglandins, is required for this inhibitory circuit. Two possible prostaglandin actions are to facilitate production of endocannabinoids by GnRH neurons and/or to promote trafficking of CB1Rs to the presynaptic GABAergic terminal membrane, increasing responsiveness. Cessation of prostaglandin signaling by blockade of either their synthesis or receptor function would thus disrupt endocannabinoid-dependent GND-induced suppression of GABAergic transmission.
Fig. 5.
Model for proposed local circuit and possible mechanisms for estradiol blockade in female mice. A: GND causes synthesis of endocannabinoids, which bind to presynaptic terminals of afferent GABAergic neurons and inhibit GABA release. Glutamate release, possibly by GnRH neurons, may stimulate astrocytes to produce prostaglandins (PGs), promote cannabinoid synthesis by GnRH neurons or glia, and/or modulate GABA release. The possible actions of PGs include promotion of cannabinoid synthesis and/or CB1R trafficking to the presynaptic membrane of GABAergic terminals. B: possible actions of estradiol in blocking the circuit include reduced metabotropic glutamate receptor (mGluR) activation of astrocytes (decreasing PG levels) and/or GABAergic neurons, retraction of astrocytic coverage of GnRH neurons, and decreasing GnRH neuron cannabinoid production.
An interesting feature of this circuit is that it is blocked by treatment of female mice with estradiol. Estradiol is a major feedback regulator of GnRH neurons, having both negative and positive effects in females dependent on interactions with the circadian clock (Christian and Moenter 2010). This positive feedback action occurs near lights off and causes a surge of GnRH release that is mandatory in most species for inducing a pituitary surge of gonadotropins and subsequent ovulation.
One of the neurobiological mechanisms employed by estradiol to induce these changes is a diurnal change in GABA transmission (Christian and Moenter 2007). Because of the elevated chloride levels maintained in adult GnRH neurons, GABAA receptor activation is depolarizing and can initiate action potential firing (DeFazio et al. 2002). Consistent with this, estradiol treatment suppresses GABAergic transmission during negative feedback and increases it during positive feedback. This can be observed in the trend for higher basal PSC frequency in the OVX+E group compared with OVX animals in the present study. Although this diurnal action of estradiol is strongest on days 2–5 postimplantation with steroid, diurnal effects remain for the duration of treatment used in the present study (Christian et al. 2005). It is possible that an additional positive feedback action of estradiol is to block this local inhibitory feedback circuit. The higher activity of GnRH neurons during positive feedback would tend to increase activity of this feedback circuit, which would be counterproductive to GnRH surge generation. Therefore, this mechanism may be invoked in a diurnal manner. Unfortunately, the very low frequency of GABAergic PSCs in estradiol-treated animals in the morning during negative feedback precludes testing directly this hypothesis (Christian and Moenter 2007).
To begin to ask this question from another angle, we examined this circuit in adult males, which lack a surge mechanism (Gorski 1973) and in which estradiol provides the major inhibitory feedback to GnRH neuron activity (Pielecka and Moenter 2006). Unlike in estradiol-treated females, GND in gonad-intact adult males inhibited GABAergic transmission, suggesting physiological levels of estradiol do not inhibit this mechanism in males and pointing to an interesting potential sex difference in terms of the mechanisms altered by organizational effects of steroid hormones and their later activational effects. Preliminary data in ovary-intact, diestrus females suggest no GND-induced suppression exists, supporting this postulate; however, low basal rates of GABAergic transmission preclude a firm conclusion based on these data alone.
Another possibility is that the development of reproductive capacity and steroidogenesis is associated with the removal of this inhibitory circuit during the pubertal process. We attempted to test this hypothesis by recording PSCs from young animals before puberty. As with some other neuronal types (Baccei and Fitzgerald 2004), frequency of synaptic inputs was too low before about postnatal day 17 to test for the inhibitory circuit. When examined in animals from 18 to 22 days of age, before estrogen-dependent vaginal opening, the first outward sign of puberty, but after activation of the reproductive neuroendocrine system (Selmanoff et al. 1977), the estradiol-induced blockade was already in place.
Estradiol can influence many of the proposed elements of this local circuit (Fig. 5B). For example, estradiol can block cannabinoid-induced presynaptic inhibition of fast synaptic transmission in pro-opiomelanocortin neurons (Nguyen and Wagner 2006). Estradiol reduced the ability of neuronal activity to increase intracellular calcium levels in astrocytes in hippocampal cultures (Rao and Sikdar, 2006). This process is dependent upon activation of astrocyte mGluRs; consistent with this, the ability of 1-aminocyclopentane-1,3-dicarboxylic acid (ACPD), a broad-spectrum mGluR agonist, to increase astrocytic calcium levels was also decreased in estradiol-treated cultures. Furthermore, estradiol acts on endothelial cells to induce retraction of tanycyte processes from GnRH neuron terminals (de Seranno et al. 2010); similar mechanisms may exist to regulate astrocyte apposition near the somata of these neurons. In this regard, several studies have shown that changes in steroid milieu, and in particular estradiol, alter glial apposition to GnRH neurons in a manner correlated with number of synapses (Baroncini et al. 2007; King and Letourneau 1994; Kozlowski and Coates 1985; Meister et al. 1988; Prevot et al. 1998, 1999; Witkin et al. 1991, 1995, 1997; Xiong et al. 1997; Yin et al. 2009a, 2009b). This apposition can change in a diurnal manner consistent with the estradiol-induced changes in GnRH neuron activity (Cashion et al. 2003).
The existence of local feedback circuits among GnRH neurons, glia, and GABAergic neurons adds to our understanding of how these central elements interact. The feedback modulation of these interactions by estradiol, and the potential sex difference in this circuit, also provide new directions for future research on central and peripheral control of GnRH neurobiology.
GRANTS
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grants R01 HD34860 and U54 HD28934.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K. M. G. performed experiments; K. M. G. analyzed data; K. M. G. and S. M. M. interpreted results of experiments; K. M. G. prepared figures; K. M. G. drafted manuscript; K. M. G. and S. M. M. approved final version of manuscript; S. M. M. conception and design of research; S. M. M. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Debra Fisher and Laura Burger for expert technical assistance and Catherine Christian for valuable editorial comments.
REFERENCES
- Baccei ML, Fitzgerald M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. J Neurosci 24: 4749–4757, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroncini M, Allet C, Leroy D, Beauvillain JC, Francke JP, Prevot V. Morphological evidence for direct interaction between gonadotrophin-releasing hormone neurones and astroglial cells in the human hypothalamus. J Neuroendocrinol 19: 691–702, 2007 [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Glial contribution to glutamate uptake at Schaffer collateral-commissural synapses in the hippocampus. J Neurosci 18: 7709–7716, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update 12: 351–361, 2006 [DOI] [PubMed] [Google Scholar]
- Brill DS, Moenter SM. Androgen receptor antagonism and an insulin sensitizer block the advancement of vaginal opening by high-fat diet in mice. Biol Reprod 81: 1093–1098, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashion AB, Smith MJ, Wise PM. The morphometry of astrocytes in the rostral preoptic area exhibits a diurnal rhythm on proestrus: relationship to the luteinizing hormone surge and effects of age. Endocrinology 144: 274–280, 2003 [DOI] [PubMed] [Google Scholar]
- Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G. Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature 269: 338–340, 1977 [DOI] [PubMed] [Google Scholar]
- Chen P, Moenter SM. GABAergic transmission to gonadotropin-releasing hormone (GnRH) neurons is regulated by GnRH in a concentration-dependent manner engaging multiple signaling pathways. J Neurosci 29: 9809–9818, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA 102: 15682–15687, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci 27: 1913–1921, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev 31: 544–577, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Moenter SM. Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: a possible local feedback circuit. J Neurosci 25: 5740–5749, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli DF, Dell'Albani P, Corsaro M, Giuffrida R, Caruso A, Trovato Salinaro A, Spinella F, Nicoletti F, Albanese V, Giuffrida Stella AM. Metabotropic glutamate receptor expression in cultured rat astrocytes and human gliomas. Neurochem Res 22: 1127–1133, 1997 [DOI] [PubMed] [Google Scholar]
- de Seranno S, d' Anglemont de Tassigny X, Estrella C, Loyens A, Kasparov S, Leroy D, Ojeda SR, Beauvillain JC, Prevot V. Role of estradiol in the dynamic control of tanycyte plasticity mediated by vascular endothelial cells in the median eminence. Endocrinology 151: 1760–1772, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-type gamma-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol 16: 2872–2891, 2002 [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Moenter SM. Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol Endocrinol 16: 2255–2265, 2002 [DOI] [PubMed] [Google Scholar]
- Diana MA, Marty A. Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE). Br J Pharmacol 142: 9–19, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziedzic B, Prevot V, Lomniczi A, Jung H, Cornea A, Ojeda SR. Neuron-to-glia signaling mediated by excitatory amino acid receptors regulates ErbB receptor function in astroglial cells of the neuroendocrine brain. J Neurosci 23: 915–926, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas I, Kallo I, Deli L, Vida B, Hrabovszky E, Fekete C, Moenter SM, Watanabe M, Liposits Z. Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology 151: 5818–5829, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F, Johnsen A, Hassel B. Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia 21: 106–113, 1997 [PubMed] [Google Scholar]
- Gorski RA. Perinatal effects of sex steriods on brain development and function. Prog Brain Res 39: 149–163, 1973 [DOI] [PubMed] [Google Scholar]
- Hassel B, Paulsen RE, Johnsen A, Fonnum F. Selective inhibition of glial cell metabolism in vivo by fluorocitrate. Brain Res 576: 120–124, 1992 [DOI] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci 2: 185–193, 2001 [DOI] [PubMed] [Google Scholar]
- Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABAA receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol 23: 557–569, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabás K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 142: 3261–3264, 2001 [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-β messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat Brain. Endocrinology 141: 3506–3509, 2000 [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Turi GF, Kallo I, Liposits Z. Expression of vesicular glutamate transporter-2 in gonadotropin-releasing hormone neurons of the adult male rat. Endocrinology 145: 4018–4021, 2004 [DOI] [PubMed] [Google Scholar]
- King JC, Letourneau RJ. Luteinizing hormone-releasing hormone terminals in the median eminence of rats undergo dramatic changes after gonadectomy, as revealed by electron microscopic image analysis. Endocrinology 134: 1340–1351, 1994 [DOI] [PubMed] [Google Scholar]
- Kozak KR, Prusakiewicz JJ, Marnett LJ. Oxidative metabolism of endocannabinoids by COX-2. Curr Pharm Des 10: 659–667, 2004 [DOI] [PubMed] [Google Scholar]
- Kozlowski GP, Coates PW. Ependymoneuronal specializations between LHRH fibers and cells of the cerebroventricular system. Cell Tissue Res 242: 301–311, 1985 [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JÅ, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95: 15677–15682, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaris N, Weinreich D. Prostaglandin E2 depresses solitary tract-mediated synaptic transmission in the nucleus tractus solitarius. Neuroscience 146: 792–801, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JE, Bethea CL, Spies HG. In vitro gonadotropin-releasing hormone release from hypothalamic tissues of ovariectomized estrogen-treated cynomolgus macaques. Endocrinology 116: 431–438, 1985a [DOI] [PubMed] [Google Scholar]
- Levine JE, Norman RL, Gliessman PM, Oyama TT, Bangsberg DR, Spies HG. In vivo gonadotropin-releasing hormone release and serum luteinizing hormone measurements in ovariectomized, estrogen-treated rhesus macaques. Endocrinology 117: 711–721, 1985b [DOI] [PubMed] [Google Scholar]
- Levine JE, Ramirez VD. Luteinizing hormone-releasing hormone release during the rat estrous cycle and after ovariectomy, as estimated with push-pull cannulae. Endocrinology 111: 1439–1448, 1982 [DOI] [PubMed] [Google Scholar]
- Loucks T, Rohan L, Kalro B, Berga S. Increased gamma-amino-butyric acid (GABA) levels in lean women with polycystic ovary syndrome (PCOS) (Abstract). In: Endocrine Society 84th Annual Meeting Programs & Abstracts Bethesda, MD: Endocrine Society, 2002, p. 108–109 [Google Scholar]
- Meister B, Hökfelt T, Tsuruo Y, Hemmings H, Ouimet C, Greengard P, Goldstein M. DARPP-32, a dopamine- and cyclic AMP-regulated phosphoprotein in tanycytes of the mediobasal hypothalamus: distribution and relation to dopamine and luteinizing hormone-releasing hormone neurons and other glial elements. Neuroscience 27: 607–622, 1988 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Karsch FJ. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology 127: 1375–1384, 1990 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Locatelli A, Karsch FJ. Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology 129: 1175–1182, 1991 [DOI] [PubMed] [Google Scholar]
- Moenter SM, DeFazio RA. Endogenous gamma-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology 146: 5374–5379, 2005 [DOI] [PubMed] [Google Scholar]
- Moldrich G, Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides 21: 1735–1742, 2000 [DOI] [PubMed] [Google Scholar]
- Nakane R, Oka Y. Excitatory action of GABA in the terminal nerve gonadotropin-releasing hormone neurons. J Neurophysiol 103: 1375–1384, 2010 [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Masu M. Molecular diversity and functions of glutamate receptors. Annu Rev Biophys Biomol Struct 23: 319–348, 1994 [DOI] [PubMed] [Google Scholar]
- Navarrete M, Araque A. Endocannabinoids mediate neuron-astrocyte communication. Neuron 57: 883–893, 2008 [DOI] [PubMed] [Google Scholar]
- Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci 23: 1659–1666, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QH, Wagner EJ. Estrogen differentially modulates the cannabinoid- induced presynaptic inhibition of amino acid neurotransmission in proopiomelanocortin neurons of the arcuate nucleus. Neuroendocrinology 84: 123–137, 2006 [DOI] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Moenter SM. Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology 143: 2284–2292, 2002 [DOI] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science 292: 923–926, 2001 [DOI] [PubMed] [Google Scholar]
- Penatti CAA, Davis MC, Porter DM, Henderson LP. Altered GABAA receptor-mediated synaptic transmission disrupts the firing of gonadotropin-releasing hormone neurons in male mice under conditions that mimic steroid abuse. J Neurosci 30: 6497–6506, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317: 1083–1086, 2007 [DOI] [PubMed] [Google Scholar]
- Pielecka J, Moenter SM. Effect of steroid milieu on gonadotropin-releasing hormone-1 neuron firing pattern and luteinizing hormone levels in male mice. Biol Reprod 74: 931–937, 2006 [DOI] [PubMed] [Google Scholar]
- Pielecka J, Quaynor SD, Moenter SM. Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology 147: 1474–1479, 2006 [DOI] [PubMed] [Google Scholar]
- Prevot V, Croix D, Bouret S, Dutoit S, Tramu G, Stefano GB, Beauvillain JC. Definitive evidence for the existence of morphological plasticity in the external zone of the median eminence during the rat estrous cycle: implication of neuro-glio-endothelial interactions in gonadotropin-releasing hormone release. Neuroscience 94: 809–819, 1999 [DOI] [PubMed] [Google Scholar]
- Prevot V, Dutoit S, Croix D, Tramu G, Beauvillain JC. Semi-quantitative ultrastructural analysis of the localization and neuropeptide content of gonadotropin releasing hormone nerve terminals in the median eminence throughout the estrous cycle of the rat. Neuroscience 84: 177–191, 1998 [DOI] [PubMed] [Google Scholar]
- Rao SP, Sikdar SK. Astrocytes in 17beta-estradiol treated mixed hippocampal cultures show attenuated calcium response to neuronal activity. Glia 53: 817–826, 2006 [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16: 675–686, 1996 [DOI] [PubMed] [Google Scholar]
- Selmanoff MK, Goldman BD, Ginsburg BE. Developmental changes in serum luteinizing hormone, follicle stimulating hormone and androgen levels in males of two inbred mouse strains. Endocrinology 100: 122–127, 1977 [DOI] [PubMed] [Google Scholar]
- Shivachar AC. Cannabinoids inhibit sodium-dependent, high-affinity excitatory amino acid transport in cultured rat cortical astrocytes. Biochem Pharmacol 73: 2004–2011, 2007 [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM. Neurosteroids alter gamma-aminobutyric acid postsynaptic currents in gonadotropin-releasing hormone neurons: a possible mechanism for direct steroidal control. Endocrinology 144: 4366–4375, 2003 [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Moenter SM. GABAergic integration of progesterone and androgen feedback to gonadotropin-releasing hormone neurons. Biol Reprod 72: 33–41, 2005 [DOI] [PubMed] [Google Scholar]
- Sullivan SD, DeFazio RA, Moenter SM. Metabolic regulation of fertility through presynaptic and postsynaptic signaling to gonadotropin-releasing hormone neurons. J Neurosci 23: 8578–8585, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141: 412–419, 2000 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276: 1699–1702, 1997 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Sakuma Y, Kato M. GABAA receptors mediate excitation in adult rat GnRH neurons. Biol Reprod 81: 327–332, 2009 [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52: 271–280, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JW, Ferin M, Popilskis SJ, Silverman AJ. Effects of gonadal steroids on the ultrastructure of GnRH neurons in the rhesus monkey: synaptic input and glial apposition. Endocrinology 129: 1083–1092, 1991 [DOI] [PubMed] [Google Scholar]
- Witkin JW, O'Sullivan H, Ferin M. Glial ensheathment of GnRH neurons in pubertal female rhesus macaques. J Neuroendocrinol 7: 665–671, 1995 [DOI] [PubMed] [Google Scholar]
- Witkin JW, O'Sullivan H, Miller R, Ferin M. GnRH perikarya in medial basal hypothalamus of pubertal female rhesus macaque are ensheathed with glia. J Neuroendocrinol 9: 881–885, 1997 [DOI] [PubMed] [Google Scholar]
- Xiong JJ, Karsch FJ, Lehman MN. Evidence for seasonal plasticity in the gonadotropin-releasing hormone (GnRH) system of the ewe: changes in synaptic inputs onto GnRH neurons. Endocrinology 138: 1240–1250, 1997 [DOI] [PubMed] [Google Scholar]
- Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron 11: 371–386, 1993 [DOI] [PubMed] [Google Scholar]
- Yin C, Ishii H, Tanaka N, Sakuma Y, Kato M. Activation of A-type gamma-amino butyric acid receptors excites gonadotrophin-releasing hormone neurones isolated from adult rats. J Neuroendocrinol 20: 566–575, 2008 [DOI] [PubMed] [Google Scholar]
- Yin W, Mendenhall JM, Monita M, Gore AC. Three-dimensional properties of GnRH neuroterminals in the median eminence of young and old rats. J Comp Neurol 517: 284–295, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Wu D, Noel ML, Gore AC. Gonadotropin-releasing hormone neuroterminals and their microenvironment in the median eminence: effects of aging and estradiol treatment. Endocrinology 150: 5498–5508, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]