Abstract
The mechanism by which distinct subprocesses in the brain are coordinated is a central conundrum of systems neuroscience. The parietal lobe is thought to play a key role in visual feature integration, and oscillatory activity in the gamma frequency range has been associated with perception of coherent objects and other tasks requiring neural coordination. Here, we examined the neural correlates of integrating mental representations in working memory and hypothesized that parietal gamma activity would be related to the success of cognitive coordination. Working memory is a classic example of a cognitive operation that requires the coordinated processing of different types of information and the contribution of multiple cognitive domains. Using magnetoencephalography (MEG), we report parietal activity in the high gamma (80–100 Hz) range during manipulation of visual and spatial information (colors and angles) in working memory. This parietal gamma activity was significantly higher during manipulation of visual-spatial conjunctions compared with single features. Furthermore, gamma activity correlated with successful performance during the conjunction task but not during the component tasks. Cortical gamma activity in parietal cortex may therefore play a role in cognitive coordination.
Keywords: magnetoencephalography, binding, oscillations, color, orientation
in perception, visual information is transmitted to the primary visual cortex via spatially segregated processing streams, with spatial information in dorsal areas and visual information in ventral areas (Ungerleider and Mishkin 1982). This ventral/dorsal “what/where” segregation of perceptual processing is thought to continue into frontal areas associated with higher cognitive functions (Goldman-Rakic 1987; Ungerleider et al. 1998). However, it is still poorly understood how the activity of specialized areas is integrated to perform complex cognitive tasks.
It is possible that feature binding is mediated by convergence of different processing streams in higher cortical areas. For example, Damasio (1989a, 1989b) proposed the existence of multiple “convergence zones,” which integrate different attributes of objects. These convergence zones are organized hierarchically, in a caudo-rostral fashion according to the level of task complexity. In particular, the parietal cortex is thought to play an important role in feature binding (Shadlen and Movshon 1999). This idea is consistent with neuropsychological findings showing that patients with parietal lesions have selective impairments in this domain (Humphreys et al. 2009; Robertson 2003; Treisman 1998).
Other work suggests that neural oscillations play a role in the integration of featural information into a coherent percept. Single-unit and multiunit recordings in visual cortex of anesthetized cats have shown synchronous gamma band oscillatory firing of neurons sensitive to different features particularly when these features combined into a single object (Engel et al. 1991; Gray et al. 1989). In humans, local increases in oscillatory activity in the gamma frequency range were associated with feature binding during visual perception in studies using electroencephalography (EEG; Tallon-Baudry et al. 1996) and magnetoencephalography (MEG; Vidal et al. 2006). Gamma activity has also been associated with selective attention, memory recall, and memory storage (Herrmann et al. 2004; Jensen et al. 2007).
However, previous work has only examined oscillatory activity during attention to single domains, e.g., color (Müller and Keil 2004), or short-term memory of visual or spatial information (Jokisch and Jensen 2007). An important function of normal human cognition is the ability to integrate different types of information. Working memory (WM) is a classic example of a cognitive operation that requires the coordinated processing of different types of information and the contribution of multiple cognitive domains, but the way in which information is integrated in WM is not well understood and is the focus of the present study. We recorded MEG during a WM task in which participants had to mentally manipulate the colors and/or rotation angles of two briefly presented sample stimuli to find the average and then indicate whether a subsequent test stimulus matched or mismatched the average of the two samples (see Fig. 1). On the basis of the research discussed above, we hypothesized that integration of color and spatial information in WM would be associated with oscillatory activity in the gamma frequency range, and that this activity would likely be localized in parietal cortex.
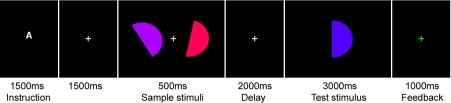
Fig. 1.
An example of the sequence of events in a typical trial. An instruction letter indicated which task to perform. Participants had to manipulate the colors, rotation angles, or both colors and angles (dual task) of the sample stimuli to determine whether the test stimulus matched or mismatched the average color and/or angle.
MATERIALS AND METHODS
Participants.
Sixteen neurologically healthy volunteers (5 men, 11 women) aged between 23 and 36 yr (mean age 28 yr) took part in the study in return for payment. Participants all had normal color vision and normal or corrected-to-normal visual acuity. The study was approved by the ethics committee of Cardiff University, and all participants gave informed consent.
Stimuli and procedure.
The stimuli were colored semicircles (visual angle = 1.0° × 1.7°) on a black background. Each trial began with a central instruction letter, indicating which task to perform. “A” indicated the angle task, “C” indicated the color task, and “D” indicated the dual task (i.e., the combination of both angle and color tasks). A white fixation cross appeared for 1,500 ms, and then two sample stimuli with different colors and rotation angles appeared for 500 ms on the left and right of the fixation cross (distance from fixation = 1.4°). There was a 2,000-ms delay in which only the fixation cross was present. Then a test stimulus appeared in the center of the screen for 3,000 ms, during which participants had to indicate with a left- or right-hand button press whether the test stimulus matched or mismatched the average of the two sample stimuli in terms of color, rotation angle, or both color and angle. This was followed by a feedback display for 1,000 ms, in which the fixation cross turned green for a correct response, red for an incorrect response, and gray if no response was made during the 3,000-ms presentation of the test stimulus. Participants were instructed to respond as accurately as possible. They were instructed that speed of response was not important, provided that they responded within 3 s from the onset of the test stimulus. The assignment of match and mismatch to the left and right response buttons was counterbalanced across participants. The intertrial interval, which contained only the fixation cross, was 2 s. The experiment was divided into three blocks of 90 trials, with 30 trials per task in each block. In total, there were 90 trials per task. For each task, the test stimulus matched the average of the two samples on one-third of trials and mismatched on two-thirds of trials. The uneven distribution of match and mismatch trials was chosen in order to prevent participants from focusing on one dimension exclusively during the conjunction task, a method employed by Mohr and Linden (2005). Note that correct responses for both match and mismatch trials were included in the MEG analysis. The order of conditions within each block was randomized.
The rotation angles of the two sample stimuli differed by a rotation of 60°. In the “match” condition of the angle task, the rotation angle of the test stimulus differed from each sample stimulus by a rotation of 30°. In the “mismatch” condition, the rotation angle of the test stimulus differed from the matching rotation angle by 20° (50%) or 30° (50%). Colors were defined in hue saturation value (HSV) color space, in which the hue is represented by 0–360°. Previous work has shown that average colors calculated in HSV color space correspond well to perceived average colors (Mohr and Linden 2005), suggesting that HSV is a suitable color space for defining stimuli. The colors of the sample and test stimuli on each trial were matched in luminance. We ensured that the colors of the stimuli in each trial were equiluminant by using a hand-held photometer (Konica Minolta) to measure the luminance in candelas per square meter for each combination of sample and test stimuli as they appeared on the computer monitor. The value on the “luminance” axis in HSV color space was adjusted until the stimuli were equiluminant, as measured by the photometer. The two sample stimuli differed in hue by 60°, and the hue of the test stimulus in the “match” condition differed from each sample by 30°. In the “mismatch” condition, the hue of the test stimulus differed from the matching hue by either 50° (50%) or 30° (50%). Stimuli were presented on a 22-in. Mitsubishi Diamond Pro 2070 monitor with a refresh rate of 60 Hz. The monitor was positioned 208 cm from the eyes and viewed through a cut-away in the magnetically shielded room. Participants' responses were registered by using two button boxes placed on the left and right. A short practice, consisting of 27 trials (9 trials for each task), was given before each recording session. The practice session lasted for 4.5 min, and the three experimental blocks lasted for 45 min in total (15 min each).
Behavioral data analysis.
Accuracy was calculated by using the A′ score as a measure of signal detection sensitivity (Grier 1971).1 A′ was used because it is more robust than d′ against violations of the assumption that the hypothetical noise and signal plus noise distributions have equal variances (Donaldson 1993; Grier 1971).2 A′ scores and response times (RTs) were analyzed by one-way repeated-measures ANOVA. Significant effects were followed up with Bonferroni-corrected post hoc tests.
MEG acquisition.
Whole head MEG recordings were made with a CTF 275-channel axial gradiometer system sampled at 1,200 Hz (0–300 Hz band pass). Twenty-nine reference channels were recorded for noise cancellation purposes, and the primary sensors were analyzed as synthetic third-order gradiometers. Three of the 275 channels were turned off because of excessive noise. Head position relative to the sensor array was monitored by placing three coils at the cardinal landmarks of the head (nasion, left and right preauricular). At the end of each block, if head movement exceeded 5 mm from the starting position, participants were instructed to move their head to within 5 mm of the initial position before beginning the next block. Only trials with correct responses were used in the MEG analysis.
Source analysis.
For each participant, MEG data were coregistered with the anatomical MRI recorded with a 3-T General Electric HDx Scanner with a T1-weighted sequence and a resolution of 1 mm3. MRI/MEG coregistration was achieved by matching the locations of the fiduciary coils (nasion, left and right preauricular) to corresponding points on the MRI. For source modeling a multiple local-spheres forward model was used (Huang et al. 1999) derived by fitting spheres to the brain surface extracted by FSL's Brain Extraction Tool (Smith 2002). Three-dimensional images of MEG source power were obtained by synthetic aperture magnetometry (SAM; Robinson and Vrba 1999; Vrba and Robinson 2001). For each subject and condition data were band-pass filtered with fourth-order bidirectional IIR Butterworth filters into seven frequency bands (5–15 Hz, 15–25 Hz, 25–40 Hz, 40–60 Hz, 60–80 Hz, 80–100 Hz, and 100–120 Hz). Note that relatively broad bandwidths in the lower-frequency ranges were chosen to increase the accuracy of the beamformer analysis (Brookes et al. 2008). Estimates of source power were derived for the whole brain at 4-mm isotropic voxel resolution. For each voxel in the brain (r), SAM generates a set of weighting coefficients w(r), one for each sensor (m), by minimizing the total source power S2 while retaining unit gain for the forward solution B(r,u). That is,
| (1) |
Here C is the covariance matrix of the band-pass filtered data, superscript T is transposition, and u is the dipole orientation that produces maximal power at that location (Cheyne et al. 2006; Robinson and Vrba 1999). The weighting coefficients can be determined by
| (2) |
We used no regularization for our covariance matrix inversion. A time series of electromagnetic source activity at r can then be reconstructed by
| (3) |
Images of source power (Student's t-statistics) were reconstructed for two 1-s active intervals during WM retention (500 ms to 1,500 ms and 1,500 ms to 2,500 ms from the onset of the sample stimuli) compared with a control period (−1,000 to 0 ms) by calculating the power of band-pass filtered virtual sensor data.
We chose a single dipole beamformer, SAM, in order to examine local task-related changes in neural oscillations within specific regions of the cortex. For this application, SAM is a well-proven technique that has demonstrated its efficacy in several studies (Hillebrand et al. 2005). A possible limitation of beamforming approaches such as SAM is that they assume that the time series of the underlying sources are uncorrelated. This means that synchronous activity in two separated brain regions would be self-canceling, if the sources were perfectly correlated, and would therefore not appear in the final source reconstruction. However, studies using simulated and real data have shown that beamforming methods are robust to moderate and/or transient levels of correlation between sources (Gross et al. 2001; Hadjipapas et al. 2005; van Veen et al. 1997). Although it is still theoretically possible that very highly correlated sources may exist and hence self-cancel in a beamformer reconstruction, there is little neurophysiological evidence for very high and sustained interregional correlation in normal oscillatory activity. Highly stable interregional synchrony is in fact most likely to be a reflection of pathological conditions such as Parkinson's disease (Schnitzler and Gross 2005). Although the beamforming method can be extended to allow the existence of two or more distant correlated sources (Schoffelen et al. 2008; Siegel et al. 2008), these methods are dependent on an a priori model for the number of simultaneously active sources (e.g., two), and we have no such hypothesis in this experiment. Unlike traditional multidipole modeling approaches SAM requires no a priori specification of the number and location of sources (Huang et al. 2004).
For group analysis, MRI and SAM images for each condition and frequency band were spatially normalized to the MNI template brain with FMRIB's Linear Image Registration Tool (FLIRT) (Jenkinson et al. 2002). Statistical analysis was performed by nonparametric permutation testing (Nichols and Holmes 2002; Singh et al. 2003), and a 5-mm3 Gaussian smoothing kernel was applied to the variance maps to generate pseudo-t images. These images were created for each condition and frequency band and thresholded using the omnibus test statistic value at P < 0.05 (2-tailed) to correct for multiple comparisons. To statistically compare the active versus baseline power obtained from the SAM analysis across the three conditions, pseudo-t images of contrasts between conditions were created with cluster-based thresholding with an initial cluster mass threshold of t = 2.5. Only clusters significant at P < 0.05 (2-tailed) were accepted. Subsequently, the contrast images were masked using the statistical maps of each condition included in the contrast, so that significant effects in the contrast image could only be found for voxels that were significantly activated in one of the conditions.3 Cortical surface meshes were extracted from an average template MRI with FreeSurfer (http://surfer.nmr.mgh.harvard.edu/). SAM source-reconstruction images were then superimposed on these meshes and visualized with mri3dX (https://www.jiscmail.ac.uk/lists/MRI3DX.html).
Virtual sensor analysis.
Virtual sensors were used to show the time-frequency representation of activity in regions found to be significantly activated in the source analysis. Virtual sensors were generated with SAM coefficients obtained from the individual condition covariance matrices. Time-frequency analysis was performed with the Fieldtrip toolbox (Oostenveld et al. 2011). Time-frequency representations of power were obtained by using a multitaper method (Mitra and Pesaran 1999) applied to time windows sliding in 10-ms steps. For the lower-frequency band (0–40 Hz), one Slepian taper was applied and a fixed time window of 500 ms was used. For the higher-frequency band (40–120 Hz), a fixed time window of 250 ms was used. With three Slepian tapers, this produced frequency smoothing of 8 Hz. In each trial, power values obtained from the Fourier transforms of the individual tapers were averaged, and power values were subsequently averaged across trials for each condition. Power values were baseline corrected by computing the relative change from baseline power for each frequency.
RESULTS
Accuracy (A′) and RT data are shown in Table 1. There was a main effect of task on accuracy [F (2, 30) = 11.6, P < 0.001], with higher accuracy in the angle task compared with the color task (P = 0.01) and the dual task (P < 0.001). There was also a main effect of task on RT [F (2, 30) = 14.6, P < 0.001], with faster responses in the angle task than in the color task (P = 0.008) and the dual task (P = 0.001). Note that performance of the dual task was not significantly worse (P = 0.9) or slower (P = 0.3) than that of the more difficult single task (color), fulfilling the behavioral criterion for parallel processing in working memory (Mohr and Linden 2005). We also calculated a normalized response criterion (c′), using the methods described by MacMillan and Creelman (1990).4 One-sample t-tests revealed that the response criterion did not significantly differ from 0 (ideal observer) in the angle task [t(15) = −0.6, not significant (ns)], the color task [t(15) = 1.4, ns], or the dual task [t(15) = −1.9, ns].
Table 1.
Mean response time on correct-response trials, hit rate, false alarm rate, and A′ for each condition
| RT | Hits | False Alarms | A′ | |
|---|---|---|---|---|
| Angle | 1,075 (37) | 0.82 (0.03) | 0.22 (0.02) | 0.87 (0.01) |
| Color | 1,178 (36) | 0.72 (0.03) | 0.24 (0.02) | 0.82 (0.01) |
| Dual | 1,208 (37) | 0.78 (0.03) | 0.34 (0.02) | 0.80 (0.02) |
Values are mean (SE) response time (RT; in ms) on correct-response trials, hit rate, false alarm rate, and A′ for each condition. Participants performed significantly faster and more accurately in the angle task. Importantly, performance of the dual task was not significantly worse or slower than that of the more difficult single task (color).
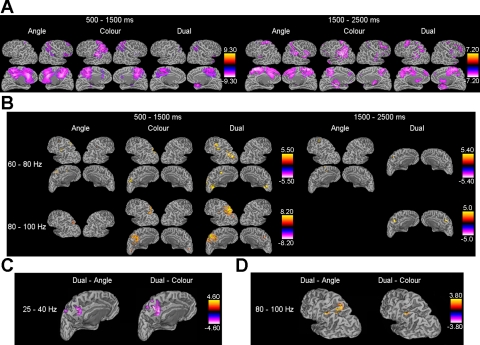
SAM was used to obtain three-dimensional images of cortical power changes within alpha, beta, and gamma frequency bands during the delay interval. Note that MEG is not contaminated by microsaccade artifacts that may affect high-frequency oscillations recorded with EEG (Yuval-Greenberg et al. 2008). This is because EEG is susceptible to eye movement artifacts due to volume conduction, particularly when using a nose reference (Melloni et al. 2009). By contrast, MEG has relatively little field spread and is reference free. Therefore, electric fields from eye muscle contractions are unlikely to influence activity at other recording sites. Lower-frequency bands (5–40 Hz) showed event-related desynchronization (ERD), and the 40–60 Hz and 100–120 Hz frequency bands did not show significant source power changes. Alpha (5–15 Hz) and low beta (15–25 Hz) activity were located mainly in parietooccipital regions, whereas high beta (25–40 Hz) activity extended into more anterior regions (see Fig. 2A). Significant event-related synchronization (ERS) was found in the high gamma band (60–100 Hz) throughout the delay interval, mainly in the left hemisphere (see Fig. 2B). Because the purpose of this study is to examine oscillatory activity associated with WM integration, we focus on the high beta and gamma frequency bands, in which dual-task activity differed from both single tasks. Locations of activation foci in each condition are shown in Tables 2 and 3. Images of contrasts between conditions were created by using cluster-based thresholding, and the locations of significant activation foci (P < 0.05, 2-tailed) for the contrast images can be found in Tables 4 and 5. We now discuss the results according to frequency band.
Fig. 2.
A: group-level images showing areas of significant (P < 0.05, 2-tailed) event-related desynchronization (ERD) in the 25–40 Hz frequency band for each task. Changes in source power (pseudo-t values) during the 1st and 2nd parts of the delay interval compared with baseline are superimposed on 50% inflated cortical surfaces of the template brain (Dale et al. 1999). B: group-level images showing areas of significant (P < 0.05, 2-tailed) event-related synchronization (ERS) for each task. C: group-level pseudo-t images of contrasts between conditions in the 25–40 Hz frequency band during the 2nd part of the delay. Areas of significantly greater (P < 0.05, 2-tailed) ERD in the dual task compared with the color and angle tasks are superimposed on the left hemisphere of the template brain. D: group-level pseudo-t images of contrasts between conditions in the 80–100 Hz frequency band during the 1st part of the delay. Areas of significantly greater (P < 0.05, 2-tailed) ERS in the dual task compared with the color and angle tasks are superimposed on the left hemisphere of the template brain.
Table 2.
Talairach coordinates, pseudo-t values, region, and hemisphere of significant activation foci in each condition during first 1,000 ms of delay
| Region | H | T | x | y | z |
|---|---|---|---|---|---|
| 25–40 Hz | |||||
| Angle | |||||
| AC | R | −8.2 | 3 | 29 | 15 |
| Cingulate | R | −8.2 | 7 | −29 | 35 |
| SFG | R | −7.6 | 17 | 49 | 47 |
| Color | |||||
| SG | L | −9.3 | −39 | −45 | 31 |
| PL | R | −7.6 | 1 | −31 | 49 |
| Dual | |||||
| IFG | R | −7.3 | 27 | 9 | −17 |
| MedFG | R | −6.6 | 1 | −19 | 53 |
| SPL | R | −5.9 | 29 | −53 | 51 |
| SG | L | −4.9 | −39 | −49 | 29 |
| 60–80 Hz | |||||
| Angle | |||||
| IPL | L | 5.5 | −55 | −37 | 49 |
| SFG | L | 5.2 | −17 | −5 | 55 |
| PG | L | 5.2 | −13 | −57 | 67 |
| PG | R | 5.1 | 29 | −31 | 47 |
| Color | |||||
| Precuneus | L | 5.6 | −11 | −81 | 47 |
| Dual | |||||
| PG | L | 5.5 | −41 | −23 | 47 |
| Cuneus | L | 5.4 | −27 | −93 | 23 |
| Cuneus | L | 5.4 | −1 | −79 | 11 |
| MedFG | L | 5.3 | −9 | −17 | 51 |
| 80–100 Hz | |||||
| Angle | |||||
| Precuneus | L | 5.2 | −21 | −75 | 55 |
| Color | |||||
| Precuneus | L | 6.7 | −13 | −55 | 43 |
| Cuneus | R | 5.3 | 3 | −91 | 9 |
| Dual | |||||
| SPL | L | 8.3 | −19 | −69 | 63 |
| IPL | L | 5.4 | −57 | −37 | 51 |
Values are Talairach coordinates (x, y, z), pseudo-t values (T), region, and hemisphere (H) of significant activation foci (P < 0.05, 2-tailed) in each condition during the first 1,000 ms of the delay. AC, anterior cingulate; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; MedFG, medial frontal gyrus; PG, postcentral gyrus; PL, paracentral lobule; SFG, superior frontal gyrus; SG, supramarginal gyrus; SPL, superior parietal lobule.
Table 3.
Talairach coordinates, pseudo-t values, region, and hemisphere of significant activation foci in each condition during second 1,000 ms of delay
| Region | H | T | X | y | z |
|---|---|---|---|---|---|
| 25–40 Hz | |||||
| Angle | |||||
| Insula | R | −6.7 | 39 | 5 | 7 |
| PL | L | −6.6 | −3 | −33 | 69 |
| IPL | R | −6.0 | 61 | −39 | 39 |
| Cingulate | L | −6.9 | −1 | 27 | 29 |
| Color | |||||
| IPL | L | −7.2 | −41 | −47 | 35 |
| IFG | R | −6.2 | 61 | 19 | 11 |
| MidFG | R | −6.1 | 47 | 27 | 43 |
| STG | R | −6.1 | 69 | −17 | 11 |
| Dual | |||||
| SG | L | −6.8 | −41 | −47 | 25 |
| MedFG | R | −6.0 | 7 | −15 | 59 |
| MidFG | L | −5.8 | −35 | 9 | 57 |
| Precuneus | L | −5.7 | −15 | −51 | 45 |
| IFG | R | −5.5 | 37 | 29 | 13 |
| MidFG | R | −5.4 | 39 | 19 | 57 |
| MedFG | L | −5.3 | −5 | 57 | 15 |
| PrG | R | −5.0 | 63 | −13 | 37 |
| 60–80 Hz | |||||
| Angle | |||||
| SFG | L | 5.4 | −17 | −3 | 59 |
| Dual | |||||
| Precuneus | L | 5.2 | −5 | −65 | 65 |
| 80–100 Hz | |||||
| Dual | |||||
| Precuneus | R | 5.1 | 1 | −61 | 53 |
Values are Talairach coordinates (x, y, z), pseudo-t values (T), region, and hemisphere (H) of significant activation foci (P < 0.05, 2-tailed) in each condition during the second 1,000 ms of the delay. MidFG, middle frontal gyrus; PrG, precentral gyrus; STG, superior temporal gyrus.
Table 4.
Talairach coordinates, pseudo-t values, region, and hemisphere of significant activation foci for contrasts between conditions during first 1,000 ms of delay
| Region | H | T | x | y | z |
|---|---|---|---|---|---|
| 25–40 Hz | |||||
| Color-angle | |||||
| Precuneus | L | −3.4 | −37 | −77 | 45 |
| 60–80 Hz | |||||
| Angle-color | |||||
| PG | R | 3.4 | 33 | −31 | 47 |
| SPL | L | 3.2 | −17 | −59 | 65 |
| Dual-color | |||||
| MedFG | L | 4.6 | −11 | −19 | 57 |
| MOG | L | 4.1 | −29 | −91 | 17 |
| 80–100 Hz | |||||
| Dual-angle | |||||
| SPL | L | 3.8 | −33 | −57 | 61 |
| IPL | L | 3.3 | −55 | −37 | 51 |
| Dual-color | |||||
| IPL | L | 3.5 | −57 | −35 | 53 |
Values are Talairach coordinates (x, y, z), pseudo-t values (T), region, and hemisphere (H) of significant activation foci (P < 0.05, 2-tailed) for the contrasts between conditions during the first 1,000 ms of the delay. MOG, middle occipital gyrus.
Table 5.
Talairach coordinates, pseudo-t values, region, and hemisphere of significant activation foci for contrasts between conditions during second 1,000 ms of delay
| Region | H | T | x | y | z |
|---|---|---|---|---|---|
| 25–40 Hz | |||||
| Angle-color | |||||
| Precuneus | R | −4.1 | 17 | −49 | 53 |
| PL | L | −3.1 | −5 | −45 | 61 |
| Dual-angle | |||||
| Precuneus | L | −4.4 | −13 | −53 | 41 |
| Dual-color | |||||
| Precuneus | L | −4.6 | −13 | −51 | 57 |
| 60–80 Hz | |||||
| Angle-color | |||||
| SFG | L | 2.9 | −13 | −7 | 65 |
| Dual-color | |||||
| SPL | L | 3.4 | −1 | −69 | 61 |
Values are Talairach coordinates (x, y, z), pseudo-t values (T), region, and hemisphere (H) of significant activation foci (P < 0.05, 2-tailed) for the contrasts between conditions during the second 1,000 ms of the delay.
In the 25–40 Hz frequency band significant ERD was found throughout the delay interval in all tasks, mainly in medial superior frontal and parietal regions (see Fig. 2 and Tables 2 and 3). The contrast analysis revealed that during the early delay period (500–1,500 ms) this ERD was stronger for the color task compared with the angle task in left precuneus (see Table 4), whereas during the second part of the delay (1,500–2,500 ms) ERD was stronger for the angle task compared with the color task in right precuneus and left paracentral lobule (see Table 5). ERD in the dual task was significantly stronger than in the angle and color tasks in left precuneus during the late delay period (see Fig. 2C and Table 5). To examine this effect further we performed a correlation analysis on individual peak 25–40 Hz power delay (Fig. 2A) and accuracy (A′) during the second part of the delay. Correlations between parietal ERD and accuracy were not significant in the dual task (R = −0.23, ns) or the color task (R = −0.40, ns) but almost reached significance in the angle task (R = −0.44, P = 0.09).
In the 60–80 Hz frequency band ERS was observed in the angle task in left inferior parietal lobule (IPL), bilateral postcentral gyrus (PG), and left superior frontal gyrus (SFG) in the early delay period (see Table 2). The SFG activity persisted into the late delay period (see Table 3). In the color task, there was 60–80 Hz activity in the left precuneus extending into superior parietal lobule (SPL), but this did not continue into the second part of the delay (see Table 2). In the dual task, 60–80 Hz activity was concentrated in the left hemisphere in postcentral gyrus (PG), cuneus extending into middle occipital gyrus (MOG), and medial frontal gyrus (medFG) during the early delay period (see Table 2) and in left precuneus extending into SPL in the second part of the delay (see Table 3). These areas can be seen in Fig. 2B. The contrast analysis showed that source power increases were significantly stronger for the angle task compared with the color task in left SPL and right PG during the first part of the delay (see Table 4) and in left SFG during the second part of the delay (see Table 5). Significantly stronger source power increases for the dual task relative to the color task were found in left medFG and left MOG during the first part of the delay (see Table 4) and in left SPL during the second part of the delay (see Table 5).
In the 80–100 Hz frequency band, significant ERS was found in regions of parietal cortex, mainly on the left, for all conditions during the first part of the delay interval. ERS was observed in left precuneus in both the angle and color tasks and in right cuneus in the color task. In the dual task a large left parietal region showed significant ERS; this region had a peak voxel in SPL and extended into precuneus. Significant ERS in left IPL was also observed in the dual task. These areas are shown in Fig. 2B and Table 2. The contrast analysis showed that this left parietal gamma synchronization was significantly greater in the dual task compared with the angle and color tasks. That is, the increase in source power was significantly greater (P < 0.05, 2-tailed) for the dual task compared with the angle task in left IPL and SPL and compared with the color task in left IPL (see Fig. 2D and Table 4). During the second part of the delay significant ERS in the precuneus was observed for the dual task but not for the angle and color tasks (see Fig. 2B and Table 3).
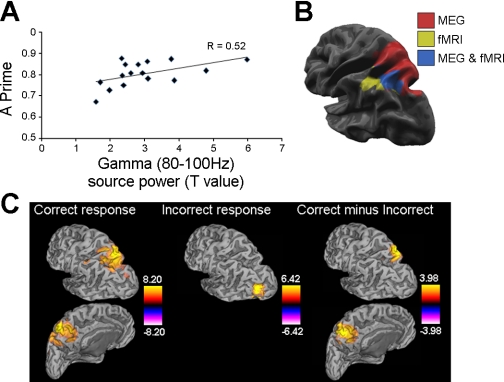
To further examine the relationship between gamma ERS and WM for conjunctions we performed a Pearson's r correlation analysis on peak gamma power during the first part of the delay, in which gamma ERS was significantly greater in the dual compared with the single feature conditions (Fig. 2B). We also calculated the 95% confidence intervals (CIs) for the Fisher's z-transformed Pearson's r values, using a bootstrap procedure with 5,000 replications (Efron and Tibshirani 1993). This revealed a significant correlation between individual peak gamma source power and A′ in the dual task (R = 0.52, P < 0.04, CI = [0.26, 0.96]) (see Fig. 4A). No significant correlations were found in the angle and color tasks (both R < 0.4, P > 0.1). There were also no significant correlations between c′ and peak gamma power in the angle task (R = 0.06), the color task (R = 0.07), or the dual task (R = −0.19), showing that gamma activity was not modulated by a change in the response criterion.
Fig. 4.
A: correlation between left parietal gamma (80–100 Hz) source power and accuracy (A′) in the dual task. B: locations of left parietal gamma (80–100 Hz) activity (red), fMRI activity (yellow), and the area of overlap (blue) in the dual task. The fMRI image was obtained from a dual-task minus single-task conjunction analysis within a whole brain, random-effects GLM. Further details of the fMRI methods and results can be found in Jackson et al. (2011). C: group-level images showing areas of significant (P < 0.05, 2-tailed) ERS for correct (see also Fig. 2B) and incorrect responses and a significant (P < 0.05, 2-tailed) contrast between correct and incorrect responses in the 80–100 Hz frequency band during the 1st part of the delay.
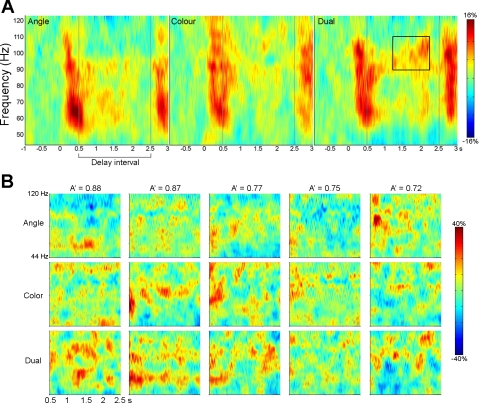
To visualize the time course of induced gamma activity, we performed a time-frequency analysis. Figure 3A shows time-frequency spectrograms of virtual sensors placed at the peak location of the left parietal source power increase in the high gamma band (80–100 Hz) during the first part of the delay for each condition. The spectrograms show an increase in high gamma power throughout the delay interval in the dual task, but not in the angle and color tasks, and this increase is highlighted by the box on the dual-task spectrogram. Time-frequency spectrograms during the delay interval for five participants ranging from high dual-task accuracy (on left) to low dual-task accuracy (on right) can be seen in Fig. 3B.
Fig. 3.
A: time-frequency spectrograms for virtual sensors placed in the peak location of increased 80–100 Hz left parietal gamma source power in each task. Virtual sensors were obtained by identifying the location of peak activity in each individual subject/condition that was closest to the location of peak activity in the group analysis. Vertical lines indicate the beginning and end of the delay interval. B: time-frequency spectrograms showing the delay interval activity in each task for a sample of 5 individual participants with dual-task A′ scores ranging from high (on left) to low (on right).
The analyses reported above were conducted on correct-response trials only. To examine whether gamma activity was associated with correct performance in the conjunction task, we used SAM to obtain images of source power for the 80–100 Hz frequency band on trials in which participants made an incorrect response. This analysis found a significant (P < 0.05) increase in gamma source power in left MOG in the conjunction condition but no significant power changes in the color and angle conditions. Contrasts between correct and incorrect trials for each condition found that 80–100 Hz gamma ERS was significantly higher (P < 0.05) in left SPL in the dual task for correct compared with incorrect responses (see Fig. 4C). There were no significant differences between correct and incorrect responses in the angle and color tasks. To further examine the left parietal difference in 80–100 Hz gamma between correct and incorrect trials in the dual task, we also conducted single-subject analyses by computing the difference between the SAM images for correct compared with incorrect trials within each individual. However, the correlation between individual correct minus incorrect gamma source power and A′ in the dual task did not reach significance (R = 0.28, P = 0.3).
DISCUSSION
Working memory (WM), the ability to temporarily maintain and manipulate information, requires coordination of activity from different neural subsystems. The results of this study provide the first evidence for increased gamma band activity during coordination of visual (color) and spatial (orientation) WM. Gamma activity was significantly higher during manipulation of color-angle conjunctions compared with single features and correlated with performance on the conjunction task. This gamma activity was also higher during trials in which participants made correct compared with incorrect responses. Gamma band activity associated with WM for conjunctions was localized in left parietal cortex, consistent with the idea that the parietal lobe is a key region for feature binding (Shadlen and Movshon 1999).
The behavioral results reported here are consistent with previous work showing that WM maintenance processes for visual and spatial information do not interfere (e.g., Della Sala et al. 1999; Finke et al. 2005; Klauer and Zhao 2004). Using a paradigm similar to the present study, Mohr and Linden (2005) found no dual-task relative to single-task performance cost when colors and angles were manipulated in WM. Importantly, they also found no dual-task costs in RT or accuracy when the sample stimuli were presented at the same time as the test stimulus, suggesting that participants were not performing the dual task in a serial fashion. Conversely, when both tasks in Mohr and Linden (2005) required spatial WM (spatial rotation and distance judgments), dual-task performance was impaired compared with single-task performance. In the present study, performance of the dual task was not significantly worse than that of the more difficult single task (color); therefore it seems reasonable to assume that WM representations of colors and angles were manipulated in parallel, requiring cognitive coordination. As with standard maintenance paradigms that require conjunctions, for example, of face identity and color (Piekema et al. 2010), participants might have formed intrinsic intra-item bindings between color and orientation. The present paradigm does not allow us to specify the exact stage at which participants moved from the parallel maintenance of color and spatial information to its manipulation and, presumably, to the integration into a mental image for comparison with the test stimulus. However, the absence of RT costs for the dual condition at retrieval suggests that color and spatial information were combined into an integrated representation already during the delay period.
Gamma activity is correlated with numerous cognitive processes (Herrmann et al. 2010). However, it is unlikely that the increased gamma in the dual task relative to the single tasks reflects nonspecific attentional demands. Increased demands on attentional or memory resources are typically associated with a significant increase in reaction time (see, e.g., Pashler 1994) and decrease in accuracy (see, e.g., Luck and Vogel 1997), which we did not observe in the present study. WM capacity for simple object features, such as colors, is around four items (Alvarez and Cavanagh 2004; Eng et al. 2005; Jackson and Raymond 2008; Luck and Vogel 1997; Wheeler and Treisman 2002), which suggests that the requirements of the dual task were within normal capacity limits. Increased subjective difficulty or low performance cannot explain the higher gamma activity in the dual task, because the correlation analysis showed that higher gamma was associated with higher accuracy. Rather, it is likely that cognitive coordination in the dual task involved specific attentional demands for integrating information (see, e.g., Treisman and Gelade 1980). Although we cannot disentangle the neural correlates of the different cognitive control processes that can be implicated in the specific demands of the dual task, we can identify the neural activation that differentiates the dual from the single tasks. This differential activation was mainly reflected in parietal gamma power, allowing us to conclude that at least some of the processes of cognitive coordination are related to oscillatory gamma activity.
fMRI studies have shown that dorsolateral prefrontal or premotor cortex and parietal cortex are preferentially involved in WM for spatial information, whereas ventrolateral prefrontal cortex (PFC) is preferentially involved in WM for nonspatial information (Courtney et al. 1998; Munk et al. 2002; Sala et al. 2003). Of most interest, visual-preferred and spatial-preferred brain regions are involved in WM for visual-spatial conjunctions, but conjunction-related activity in each domain-specific region is intermediate to the activity produced by the preferred and nonpreferred information (Munk et al. 2002; Sala and Courtney 2007). Therefore, neural activity during WM for visual-spatial conjunctions does not seem to depend on simple addition of the visual and spatial responses.
Recent fMRI work using the same paradigm as the present study has shown increased BOLD response during the dual task compared with the single tasks in left parietal cortex (Jackson et al. 2011; see Fig. 4). To quantitatively compare the peaks of left parietal activity found with fMRI and MEG, we used the following formula to test for Euclidean distance in three-dimensional space: square root [(p1 − q1)2 + (p2 − q2)2 + (p3 − q3)2], where p = the present MEG study, q = Jackson et al.'s (2011) fMRI study, p1/q1 = x-coordinates, p2/q2 = y-coordinates, and p3/q3 = z-coordinates. This showed that the focal points of the areas of BOLD response and gamma activity in left parietal cortex were 23 mm apart. As shown in Fig. 4, the region found by fMRI overlaps with the region found by MEG; 42% of the active fMRI voxels fall within the active MEG region. Given that the two studies used separate participant groups, this indicates relatively good correspondence between the MEG and fMRI results. The finding that oscillatory gamma activity corresponds spatially to the BOLD response is consistent with evidence that the BOLD signal reflects neural activity. Intracranial recordings in anesthetized (Logothetis et al. 2001) and awake (Goense and Logothetis 2008) animals have shown that blood oxygenation correlates with local field potentials, particularly in the gamma range (Niessing et al., 2005). Furthermore, MEG studies in humans have shown spatial correspondence between gamma synchronization and the BOLD response in primary visual cortex (Brookes et al. 2005; Muthukumaraswamy and Singh 2008). The present study extends these findings by showing similarities between the BOLD signal and gamma activity in parietal regions during a cognitive task.
The fMRI work using this paradigm (Jackson et al. 2011) also found increased BOLD signal for dual compared with single tasks in right ventro-lateral and dorso-lateral PFC, which was interpreted to reflect a greater allocation of attentional resources for processing conjunctions. However, the present study did not observe an increase in oscillatory activity in these areas for the dual task relative to the single tasks in any frequency band. It is possible that frontal areas were involved mainly during the encoding of the sample stimuli and during the evaluation of the test stimulus and selection of response, whereas the MEG analysis was conducted on activity during the WM delay interval. Indeed, Jackson et al. (2011) showed that the BOLD signal peaked later in parietal cortex compared with frontal regions. Alternatively, it is possible that MEG was not sufficiently sensitive to detect frontal sources of oscillatory activity. The fMRI prefrontal regions of activity were deeper than the parietal region, and did not extend as much onto the cortical surface, which would make them harder to detect with MEG. There may have also been greater interindividual variability in frontal areas.
The parietal lobe has been shown to play an important role in WM storage of both visual and spatial information (Linden et al. 2003; Todd and Marois 2004), which makes it a logical site for coordinating the processing of mental object representations. Information in posterior parietal cortex is thought to be organized in terms of its relevance for behavior, with objects represented in multiple spatial reference frames depending on the type of action to be performed (Colby and Goldberg 1999; Rizzolatti et al. 1997). This requires spatial representations, such as orientation and location, to be integrated with object identity. In support of this idea, single-unit recordings in monkeys have found both shape- and location-selective neurons in the lateral intraparietal area (Janssen et al. 2008; Sereno and Amador 2006), and fMRI in humans has confirmed that visual object information is represented in parietal areas (Konen and Kastner 2008).
There is also substantial neuropsychological evidence to support the idea that parietal lobe is critical for forming integrated representations. Patients with Balint's syndrome, with bilateral parietal damage, show binding problems on tasks requiring conjunctions of simple visual features (see, e.g., Friedman-Hill et al. 1995; Robertson et al. 1997). Other work has found that patients with unilateral parietal lesions are selectively impaired on conjunction search in their contralesional visual field (Humphreys et al. 2009). Such binding deficits are thought to reflect impairments in the parietal spatial attention network, consistent with the view that feature integration depends on spatial attention (Treisman 1998; Treisman and Gelade 1980). Further support for this idea comes from brain imaging work showing that conjunction tasks activate regions of parietal cortex associated with spatial attention (Shafritz et al. 2002).
High gamma activity during WM manipulation of conjunctions extended into left precuneus, and we also found a stronger decrease in source power in the high beta band (25–40 Hz) during WM manipulation of conjunctions relative to single features, which was localized to left precuneus. The precuneus is thought to be involved in a wide range of cognitive functions, including visuospatial imagery and episodic memory retrieval (see Cavanna and Trimble 2006). Activity in precuneus has also been reported in tasks involving complex WM operations, such as mental arithmetic (Fehr et al. 2007), which suggests that activity in this region is related to cognitive coordination processes. However, unlike high gamma activity, the high beta activity observed in the present study did not correlate with performance, showing that beta activity in precuneus was not specifically associated with successful WM integration.
Cortical gamma oscillations may be generated by synaptic interactions of excitatory and inhibitory neuronal populations that cause rhythmic changes in neural firing or by activity in a subpopulation of inherently oscillatory neurons (Singer and Gray 1995). Recently, Fries and colleagues (Fries et al. 2007) have suggested that cortical information processing relies on rhythmic gamma activity generated by cortical microcircuits of pyramidal cells and interneurons. That is, cognitive processes such as information integration may be achieved by adjusting phase relationships and oscillatory frequencies among cell populations. During the conjunction task in the present study, a coordinated representation may have been formed by synchronous oscillations of cell populations coding color and orientation. Alternatively, the increased parietal gamma power observed here could simply be due to more neurons contributing to the local field potential. Techniques for measuring neural oscillations in humans, such as MEG, do not have the spatial resolution to specifically examine the synchrony within regions that was shown in animal studies (Engel et al. 1991; Gray et al. 1989).
The increased parietal gamma activity associated with WM for visual-spatial conjunctions in this study was observed in a high-frequency band. Other work has shown high-frequency (>70 Hz) gamma activity during cognitive tasks such as visual grouping (Vidal et al. 2006) and WM maintenance (Jokisch and Jensen 2007). By contrast, low-frequency (<70 Hz) gamma has been reported in simple visual tasks (Brookes et al. 2005; Muthukumaraswamy and Singh 2008). It is possible that the frequency of gamma oscillations depends on the nature of the task and/or the properties of the cortical areas involved in the task. Alternatively, recent work has shown that gamma frequency in primary visual cortex varies across individuals and depends on resting GABA concentration (Muthukumaraswamy et al. 2009).
To summarize, we have shown that parietal gamma activity was increased during WM for visual-spatial conjunctions compared with single features, and this gamma activity correlated with successful performance on the conjunction task. High beta activity in precuneus was also increased in the conjunction compared with the single tasks. These findings are compatible with the idea that parietal cortex supports integration of visual features (Shadlen and Movshon 1999). Theories of binding by neural synchrony may also explain our findings, although, as discussed above, we cannot be certain that the increased gamma power in this study reflects a binding-related increase in local synchronization. Note that these accounts are not mutually exclusive and it is possible that cognitive integration is mediated by a combination of spatial selection in parietal cortex and intra- or interareal neural synchrony (see, e.g., Hummel and Biederman 1992; Hummel and Stankiewicz 1998). In conclusion, our results show that parietal cortex plays a role in the integration of mental representations of color and orientation, and this integration is associated with oscillatory neural activity in the gamma frequency band.
GRANTS
This research was supported by the Wales Institute of Cognitive Neuroscience (grant no. WBC021), the Wellcome Trust (grant no. 077185/Z/05/Z), and the Future and Emerging Technologies (FET) program within the Seventh Framework Programme for Research of the European Commission (FET-Open grant no. 222079).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.M.M., K.L.S., and D.E.J.L. conception and design of research; H.M.M., S.D.M., and K.D.S. analyzed data; H.M.M. interpreted results of experiments; H.M.M. prepared figures; H.M.M. and D.E.J.L. drafted manuscript; H.M.M., S.D.M., K.L.S., R.M.B., K.D.S., and D.E.J.L. edited and revised manuscript; H.M.M., S.D.M., C.S.H., K.L.S., R.M.B., K.D.S., and D.E.J.L. approved final version of manuscript; C.S.H. performed experiments.
ACKNOWLEDGMENTS
We thank Dr. Stephen Robinson for providing the SAMspm beamformer software.
Footnotes
A′ was calculated with the following formula: A′ = 0.5 + [(H − FA) × (1 + H − FA)]/[4 × H × (1 − FA)], where H is hit rate (proportion of correct responses on match trials) and FA is false alarm rate (proportion of mismatching trials incorrectly identified as matching). If FA > H the following formula is used: A′ = 0.5 − [(FA − H) × (1 + FA − H)]/[4 × FA × (1 − H)]. A′ of 0.5 indicates chance performance, and A′ of 1 indicates perfect performance.
Note that the analysis produced the same pattern of results when d′ was used as a measure of accuracy.
To ensure that significant effects in the contrast images were not due to baseline differences, SAM images of contrasts between conditions were calculated for each frequency band during the baseline interval. This analysis found no significant baseline differences in any of the regions that showed significant differences between conditions during the delay interval.
c′ = −0.5[z(H) + z(F)]/[z(H) − z(F)], where c′ = normalized response criterion, H = hit rate, and F = false alarm rate. A negative value of c′ indicates a liberal response criterion (e.g., more likely to respond “match”), whereas a positive value of c′ indicates a conservative response criterion (e.g., more likely to respond “mismatch”).
REFERENCES
- Alvarez GA, Cavanagh P. The capacity of visual short term memory is set both by visual information load and by number of objects. Psychol Sci 15: 106–111, 2004 [DOI] [PubMed] [Google Scholar]
- Brookes M, Gibson A, Hall S, Furlong P, Barnes G, Hillebrand A, Singh K, Holliday I, Francis S, Morris P. GLM-beamformer method demonstrates stationary field, alpha ERD and gamma ERS co-localisation with fMRI BOLD response in visual cortex. Neuroimage 26: 302–308, 2005 [DOI] [PubMed] [Google Scholar]
- Brookes MJ, Vrba J, Robinson SE, Stevenson CM, Peters AM, Barnes GR, Hillebrand A, Morris PG. Optimising experimental design for MEG beamformer imaging. Neuroimage 39: 1788–1802, 2008 [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129: 564–583, 2006 [DOI] [PubMed] [Google Scholar]
- Cheyne D, Bakhtazad L, Gaetz W. Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event-related beamforming approach. Hum Brain Mapp 27: 213–229, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci 22: 319–349, 1999 [DOI] [PubMed] [Google Scholar]
- Courtney S, Petit L, Haxby J, Ungerleider L. The role of prefrontal cortex in working memory: examining the contents of consciousness. Philos Trans R Soc Lond B Biol Sci 353: 1819–1828, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A, Fischl B, Sereno M. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9: 179–194, 1999 [DOI] [PubMed] [Google Scholar]
- Damasio A. Time-locked multiregional retroactivation: a systems-level proposal for the neural substrates of recall and recognition. Cognition 33: 25–62, 1989a [DOI] [PubMed] [Google Scholar]
- Damasio AR. The brain binds entities and events by multiregional activation from convergence zones. Neural Comput 1: 123–132, 1989b [Google Scholar]
- Della Sala S, Gray C, Baddeley A, Allamano N, Wilson L. Pattern span: a tool for unwelding visuo-spatial memory. Neuropsychologia 37: 1189–1199, 1999 [DOI] [PubMed] [Google Scholar]
- Donaldson W. Accuracy of d′ and A′ as estimates of sensitivity. Bull Psychon Soc 31: 271–274, 1993 [Google Scholar]
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman and Hall, 1993 [Google Scholar]
- Eng HY, Chen D, Jiang Y. Visual working memory for simple and complex visual stimuli. Psychon Bull Rev 12: 1127–1133, 2005 [DOI] [PubMed] [Google Scholar]
- Engel A, Kreiter A, König P, Singer W. Synchronization of oscillatory neuronal responses between striate and extrastriate visual cortical areas of the cat. Proc Natl Acad Sci USA 88: 6048–6052, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr T, Code C, Herrmann M. Common brain regions underlying different arithmetic operations as revealed by conjunct fMRI-BOLD activation. Brain Res 1172: 93–102, 2007 [DOI] [PubMed] [Google Scholar]
- Finke K, Bublak P, Neugebauer U, Zihl J. Combined processing of what and where information within the visuospatial scratchpad. Eur J Cogn Psychol 17: 1–22, 2005 [Google Scholar]
- Friedman-Hill SR, Robertson LC, Treisman A. Parietal contributions to visual feature binding: evidence from a patient with bilateral lesions. Science 269: 853–855, 1995 [DOI] [PubMed] [Google Scholar]
- Fries P, Nikolić D, Singer W. The gamma cycle. Trends Neurosci 30: 309–316, 2007 [DOI] [PubMed] [Google Scholar]
- Goense J, Logothetis N. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol 18: 631–640, 2008 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of primate prefrontal cortex, and regulation of behavior by representational memory. In: Handbook of Physiology. The Nervous System. Higher Functions of the Brain. Bethesda, MD: Am Physiol Soc, 1987, sect. 1 vol. V, p. 373–417 [Google Scholar]
- Gray C, König P, Engel A, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338: 334–337, 1989 [DOI] [PubMed] [Google Scholar]
- Grier JB. Nonparametric indexes for sensitivity and bias: computing bias. Psychol Bull 75: 424–429, 1971 [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci USA 98: 694–699, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjipapas A, Hillebrand A, Holliday IE, Singh KD, Barnes GR. Assessing interactions of linear and nonlinear neuronal sources using MEG beamformers: a proof of concept. Clin Neurophysiol 116: 1300–1313, 2005 [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Fründ I, Lenz D. Human gamma-band activity: a review on cognitive and behavioral correlates and network models. Neurosci Biobehav Rev 34: 981–992, 2010 [DOI] [PubMed] [Google Scholar]
- Herrmann C, Munk M, Engel A. Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn Sci 8: 347–355, 2004 [DOI] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp 25: 199–211, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Mosher J, Leahy R. A sensor-weighted overlapping-sphere head model and exhaustive head model comparison for MEG. Phys Med Biol 44: 423–440, 1999 [DOI] [PubMed] [Google Scholar]
- Huang MX, Shih JJ, Lee RR, Harrington DL, Thoma RJ, Weisend MP, Hanlon F, Paulson KM, Li T, Martin K, Millers GA, Canive JM. Commonalities and differences among vectorized beamformers in electromagnetic source imaging. Brain Topogr 16: 139–158, 2004 [DOI] [PubMed] [Google Scholar]
- Hummel JE, Biederman I. Dynamic binding in a neural network for shape recognition. Psychol Rev 99: 480–517, 1992 [DOI] [PubMed] [Google Scholar]
- Hummel JE, Stankiewicz BJ. Two roles for attention in shape perception: a structural description model of visual scrutiny. Vis Cogn 5: 49–79, 1998 [Google Scholar]
- Humphreys G, Hodsoll J, Riddoch M. Fractionating the binding process: neuropsychological evidence from reversed search efficiencies. J Exp Psychol Hum Percept Perform 35: 627–647, 2009 [DOI] [PubMed] [Google Scholar]
- Jackson MC, Morgan HM, Mohr H, Shapiro KL, Linden DEJ. Strategic resource allocation in the human brain supports cognitive coordination of visual and spatial working memory. Hum Brain Mapp 32: 1330–1348, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MC, Raymond JE. Familiarity enhances visual working memory for faces. J Exp Psychol Hum 34: 556–568, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen P, Srivastava S, Ombelet S, Orban G. Coding of shape and position in macaque lateral intraparietal area. J Neurosci 28: 6679–6690, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841, 2002 [DOI] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman J. Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex 12: 877–882, 2002 [DOI] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux J. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci 30: 317–324, 2007 [DOI] [PubMed] [Google Scholar]
- Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci 27: 3244–3251, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauer KC, Zhao Z. Double dissociations in visual and spatial short-term memory. J Exp Psychol Gen 133: 355–381, 2004 [DOI] [PubMed] [Google Scholar]
- Konen C, Kastner S. Two hierarchically organized neural systems for object information in human visual cortex. Nat Neurosci 11: 224–231, 2008 [DOI] [PubMed] [Google Scholar]
- Kosslyn S, DiGirolamo G, Thompson W, Alpert N. Mental rotation of objects versus hands: neural mechanisms revealed by positron emission tomography. Psychophysiology 35: 151–161, 1998 [PubMed] [Google Scholar]
- Linden D, Bittner R, Muckli L, Waltz J, Kriegeskorte N, Goebel R, Singer W, Munk M. Cortical capacity constraints for visual working memory: dissociation of fMRI load effects in a fronto-parietal network. Neuroimage 20: 1518–1530, 2003 [DOI] [PubMed] [Google Scholar]
- Logothetis N, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157, 2001 [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature 390: 279–281, 1997 [DOI] [PubMed] [Google Scholar]
- MacMillan NA, Creelman CD. Response bias: characteristics of detection theory, threshold theory, and “nonparametric” indexes. Psychol Bull 107: 401–413, 1990 [Google Scholar]
- Melloni L, Schwiedrzik CM, Wibral M, Rodriguez E, Singer W. Response to: Yuval-Greenberg et al., “Transient induced gamma-band response in EEG as a manifestation of miniature saccades.” Neuron 62: 8–10, 2009 [DOI] [PubMed] [Google Scholar]
- Mitra P, Pesaran B. Analysis of dynamic brain imaging data. Biophys J 76: 691–708, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr H, Linden D. Separation of the systems for color and spatial manipulation in working memory revealed by a dual-task procedure. J Cogn Neurosci 17: 355–366, 2005 [DOI] [PubMed] [Google Scholar]
- Müller M, Keil A. Neuronal synchronization and selective color processing in the human brain. J Cogn Neurosci 16: 503–522, 2004 [DOI] [PubMed] [Google Scholar]
- Munk M, Linden D, Muckli L, Lanfermann H, Zanella F, Singer W, Goebel R. Distributed cortical systems in visual short-term memory revealed by event-related functional magnetic resonance imaging. Cereb Cortex 12: 866–876, 2002 [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S, Singh K. Spatiotemporal frequency tuning of BOLD and gamma band MEG responses compared in primary visual cortex. Neuroimage 40: 1552–1560, 2008 [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S, Edden R, Jones D, Swettenham J, Singh K. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci USA 106: 8356–8361, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Holmes A. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15: 1–25, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt K, Niessing M, Singer W, Galuske R. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science 309: 948–951, 2005 [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011: 156869, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H. Dual-task interference in simple tasks: data and theory. Psychol Bull 116: 220–244, 1994 [DOI] [PubMed] [Google Scholar]
- Piekema C, Rijpkema M, Fernandez G, Kessels RPC. Dissociating the neural correlates of intra-item and inter-item working-memory binding. PLoS One 5: e10214, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Parietal cortex: from sight to action. Curr Opin Neurobiol 7: 562–567, 1997 [DOI] [PubMed] [Google Scholar]
- Robertson L. Binding, spatial attention and perceptual awareness. Nat Rev Neurosci 4: 93–102, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson LC, Treisman A, Friedman-Hill S, Grabowecky M. The interaction of spatial and object pathways: evidence from Balint's syndrome. J Cogn Neurosci 9: 295–317, 1997 [DOI] [PubMed] [Google Scholar]
- Robinson SE, Vrba J. Functional neuroimaging by synthetic aperture magnetometry (SAM). In: Recent Advances in Biomagnetism, edited by Yoshimoto T, Kotani M, Kuriki S, Karibe H, Nakasato N. Sendai, Japan: Tohoku Univ. Press, 1999, p. 302–305 [Google Scholar]
- Sala J, Courtney S. Binding of what and where during working memory maintenance. Cortex 43: 5–21, 2007 [DOI] [PubMed] [Google Scholar]
- Sala J, Rämä P, Courtney S. Functional topography of a distributed neural system for spatial and nonspatial information maintenance in working memory. Neuropsychologia 41: 341–356, 2003 [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci 6: 285–296, 2005 [DOI] [PubMed] [Google Scholar]
- Schoffelen JM, Oostenveld R, Fries P. Imaging the human motor system's beta-band synchronization during isometric contraction. Neuroimage 41: 437–447, 2008 [DOI] [PubMed] [Google Scholar]
- Sereno A, Amador S. Attention and memory-related responses of neurons in the lateral intraparietal area during spatial and shape-delayed match-to-sample tasks. J Neurophysiol 95: 1078–1098, 2006 [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Movshon JA. Synchrony unbound: a critical evaluation of the temporal binding hypothesis. Neuron 24: 67–77, 1999 [DOI] [PubMed] [Google Scholar]
- Shafritz K, Gore J, Marois R. The role of the parietal cortex in visual feature binding. Proc Natl Acad Sci USA 99: 10917–10922, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Oostenveld R, Fries P, Engel AK. Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron 60: 709–719, 2008 [DOI] [PubMed] [Google Scholar]
- Singer W, Gray C. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci 18: 555–586, 1995 [DOI] [PubMed] [Google Scholar]
- Singh K, Barnes G, Hillebrand A. Group imaging of task-related changes in cortical synchronisation using nonparametric permutation testing. Neuroimage 19: 1589–1601, 2003 [DOI] [PubMed] [Google Scholar]
- Smith S. Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J Neurosci 16: 4240–4249, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature 428: 751–754, 2004 [DOI] [PubMed] [Google Scholar]
- Treisman A. Feature binding, attention and object perception. Philos Trans R Soc Lond B Biol Sci 353: 1295–1306, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman A, Gelade G. A feature integration theory of attention. Cogn Psychol 12: 97-–l36, 1980 [DOI] [PubMed] [Google Scholar]
- Tuladhar A, ter Huurne N, Schoffelen J, Maris E, Oostenveld R, Jensen O. Parieto-occipital sources account for the increase in alpha activity with working memory load. Hum Brain Mapp 28: 785–792, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Courtney SM, Haxby JV. A neural system for human visual working memory. Proc Natl Acad Sci USA 95: 883–890, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider MG, Mishkin M. Two cortical visual systems. In: Analysis of Visual Behavior, edited by Ingle DJ, Goodale MA, Mansfield RJW. Cambridge, MA: MIT Press, 1982, p. 549–586 [Google Scholar]
- Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 44: 867–880, 1997 [DOI] [PubMed] [Google Scholar]
- Vidal J, Chaumon M, O'Regan J, Tallon-Baudry C. Visual grouping and the focusing of attention induce gamma-band oscillations at different frequencies in human magnetoencephalogram signals. J Cogn Neurosci 18: 1850–1862, 2006 [DOI] [PubMed] [Google Scholar]
- Vrba J, Robinson S. Signal processing in magnetoencephalography. Methods 25: 249–271, 2001 [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Treisman AM. Binding in short-term visual memory. J Exp Psychol Gen 131: 48–64, 2002 [DOI] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren A, Nelken I, Deouell L. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron 58: 429–441, 2008 [DOI] [PubMed] [Google Scholar]