Abstract
Neonatal damage to the trigeminal nerve leads to “reactive synaptogenesis” in the brain stem sensory trigeminal nuclei. In vitro models of brain injury-induced synaptogenesis have implicated an important role for astrocytes. In this study we tested the role of astrocyte function in reactive synaptogenesis in the trigeminal principal nucleus (PrV) of neonatal rats following unilateral transection of the infraorbital (IO) branch of the trigeminal nerve. We used electrophysiological multiple input index analysis (MII) to estimate the number of central trigeminal afferent fibers that converge onto single barrelette neurons. In the developing PrV, about 30% of afferent connections are eliminated within 2 postnatal weeks. After neonatal IO nerve damage, multiple trigeminal inputs (2.7 times that of the normal inputs) converge on single barrelette cells within 3–5 days; they remain stable up to the second postnatal week. Astrocyte proliferation and upregulation of astrocyte-specific proteins (GFAP and ALDH1L1) accompany reactive synaptogenesis in the IO nerve projection zone of the PrV. Pharmacological blockade of astrocyte function, purinergic receptors, and thrombospondins significantly reduced or eliminated reactive synaptogenesis without changing the MII in the intact PrV. GFAP immunohistochemistry further supported these electrophysiological results. We conclude that immature astrocytes, purinergic receptors, and thrombospondins play an important role in reactive synaptogenesis in the peripherally deafferented neonatal PrV.
Keywords: trigeminal brain stem, deafferentation, purinergic receptors, thrombospondin, gabapentin, central nervous system
in the adult brain, deafferentation results in rapid synaptic loss followed by a prolonged “reactive synaptogenesis” via sprouting of afferent axons that form new synaptic connections (Collazos-Castro and Nieto-Sampedro 2001; Cotman and Anderson 1988; Deller and Frotscher 1997; Dieringer 1995; Hamori 1990; Marrone and Petit 2002; Matthews et al. 1976). The cellular and molecular mechanisms underlying reactive synaptogenesis are poorly understood. In addition, very little is known about reactive synaptogenesis in the neonatal brain during the initial wiring of neural circuits and synaptogenesis. This is partly due to difficulties in dissociating injury-induced synaptogenesis from the normal course of developmental synaptogenesis and synaptic pruning.
In the rat, damage to the infraorbital (IO) branch of the trigeminal nerve during the early postnatal critical period of structural plasticity (Erzurumlu 2010) results in synaptic remodeling in the brain stem trigeminal principal nucleus (PrV). Whisker-specific neural patterns (barrelettes) are lost, and apoptosis in both the trigeminal ganglion and the PrV significantly increases (Henderson et al. 1993; Miller et al. 1991; Miller and Kuhn 1997; Sugimoto et al. 1998, 1999). Most IO-PrV synapses of the rat are functional at birth (Lo et al. 1999; Lo and Erzurumlu 2007). After neonatal IO transection, membrane properties of surviving PrV neurons, neurotransmitter release probability of presynaptic afferents, and postsynaptic NMDA receptor subunit composition do not change (Lo and Erzurumlu 2001, 2011; Lo and Zhao 2011). However, postsynaptic responses to stimulation of the trigeminal tract (TrV) show marked changes. Minimal stimulation of the TrV induces silent synapses without functional AMPA receptors in the deafferented PrV (Lo and Erzurumlu 2007). Maximal stimulation of the TrV elicits a sustained depolarization (plateau potential) that is mediated by high-threshold L-type Ca2+ channels and triggered by NMDA receptor-mediated excitatory postsynaptic potentials in the deafferented PrV (Lo and Erzurumlu 2002), suggesting that synaptic inputs are enhanced to depolarize the membrane to the threshold of L-type Ca2+ channels in the deafferented PrV.
In the present study, we employed a reliable electrophysiological approach, multiple input index (MII) (Arsenault and Zhang 2006; Chen and Regehr 2000; Crepel and Mariani 1976; Hooks and Chen 2006; Lo et al. 2002; Lu and Constantine-Paton 2004; Mariani and Changeux 1981; Stevens et al. 2007) to estimate the number of trigeminal inputs converging on single barrelette neurons in the developing and deafferented PrV of postnatal rats. We found that in the intact PrV, about 30% of synaptic connections are eliminated within 2 postnatal weeks. However, in the deafferented PrV, new synaptic connections are rapidly formed 3–5 days after peripheral deafferentation and remain stable up to the second postnatal week. Thus single barrelette neurons in the deafferented PrV receive 2.7 times more trigeminal inputs than those of the intact PrV.
The IO nerve injury-induced reactive synaptogenesis in the PrV is accompanied by robust astrocytosis confined to the deafferented zone of the PrV. In vitro models of brain injury-induced synaptogenesis have implicated an important role for astrocytes (Allen and Barres 2005). We tested the role of astrocytes in our in vivo reactive synaptogenesis model by pharmacological interruption of astrocyte function at various levels. Blockade of astrocyte function, purinergic receptors, and thrombospondins (TSPs) all reduced reactive synaptogenesis without interfering with developmental synaptogenesis. Thus our results reveal a major role for astrocytes in mediating peripheral nerve injury-associated reactive synaptogenesis in the developing brain.
MATERIALS AND METHODS
IO nerve transection.
The unilateral IO nerve injury and its effects on barrelette patterning in neonatal rats and mice has been amply documented for the past quarter century, and it is a well-established model for investigating the role of sensory periphery in patterning of the trigeminal central nervous system (CNS) structures, from brain stem to the neocortex. Postnatal day 0 (P0) and P4 Sprague-Dawley rat pups were anesthetized by hypothermia. The IO nerve on the right side was cut with sterile microscissors. The left side was either uninjured, or a skin incision was made between the eye and the whisker pad (sham operation) as an internal control. All animal protocols were in accordance with the National Institutes of Health guidelines and were approved by the University of Maryland at Baltimore Institutional Animal Care and Use Committee.
Brain slice preparation.
In the present study, we prepared brain slices from rat pups between P1 and P13. The animals were euthanized, and the brain was removed and immersed in cold (4°C) glycerol-based artificial cerebrospinal fluid (GACSF; modified from Ye et al. 2006) that contained (in mM) 263 glycerol, 2.5 KCl, 1.25 NaH2PO4, 4 MgSO4, 26 NaHCO3, 10 glucose, and 1 CaCl2, bubbled with 95% O2 and 5% CO2 (pH 7.4, 327 mosM). Initial incubation in GACSF prevents acute neurotoxic effects of passive chloride entry into cells and increases viability of cells in the slice (Ye et al. 2006). The brain stem was next embedded in 2% agar and cut into 400-μm-thick transverse sections with a vibratome (Leica VT 1000S) in GACSF at 4°C. Slices containing the PrV were selected under a dissecting microscope; the intact and deafferented side of each slice was marked. After 45-min incubation in warm (34°C) GACSF, slices were incubated in normal ACSF (in mM: 124 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 MgSO4, 26 NaHCO3, 10 glucose, and 2 CaCl2, pH 7.4) at room temperature. Each slice was transferred into a submerged-type recording chamber (RC-27L; Warner Instruments) and continuously perfused (>2 ml/min) with normal ACSF at room temperature. During electrophysiological recording, picrotoxin (50 μM) was added into the ACSF to block GABAergic responses.
Electrophysiology.
Whole cell patch micropipettes were backfilled with a Cs-based intracellular solution (in mM: 115 CsMeSO3, 10 NaCl, 1 KCl, 4 MgCl2, 1 CaCl2, 11 EGTA, 20 HEPES, 3 Na2-ATP, 0.5 Na2-GTP, and 0.1 spermine, pH 7.25, >290 mosM) with a tip resistance of 5–9 MΩ. Neurons in the ventral part of the PrV (barrelette region) were blindly patched with the previously described techniques (Lo et al. 1999). Barrelette neurons were identified by their prominent transient K+ (IA) conductance (Lo et al. 1999) that was gradually blocked by Cs+ dialyzed from a micropipette 3 min after whole cell recording. Fine-tip stimulating electrodes (0.5 MΩ; catalog no. IRM33A05KT; World Precision Instruments) were inserted at various points along the TrV lateral to the barrelette region. Electrical pulses (0.1–0.3 ms in duration, 0.33 Hz, 0–350 μA) were passed through the electrodes to evoke excitatory postsynaptic currents (EPSCs). Because silent synapses without AMPA receptor-mediated EPSCs were induced by minimal (threshold) stimulation in both intact and deafferented PrV (Lo and Erzurumlu 2007), barrelette neurons were voltage-clamped at +60 mV to prevent loss of EPSCs that were mediated exclusively by NMDA receptors. However, suprathreshold stimulation induced both AMPA and NMDA receptor-mediated EPSCs at +60 mV (Lo and Zhao 2011); thus EPSCs induced by suprathreshold stimuli contain both AMPA and NMDA components. All physiological data were collected with an Instrutech ITC-16 interface unit and stored on a Pentium III personal computer with Pulse (HEKA) software (Instrutech).
Determination of the MII.
Barrelette neurons were voltage-clamped at +60 mV to show EPSCs induced by stimulation of TrV at 0.33 Hz. The stimulus intensity was gradually increased from 0 to 350 μA at steps of 10 μA. The peak amplitudes of EPSCs were measured and plotted against stimulus intensity. The amplitude of EPSCs enhanced in a stepwise manner following the increase in stimulus intensity. We first measured the baseline noise of recordings and calculated the standard deviation (SD) of the noise. The variation in amplitude of EPSCs was analyzed. If the amplitude of an EPSC was larger than the prior EPSC by more than 3 times the SD, a “jumping step” was defined. The fluctuation of EPSC amplitudes induced by the same stimulus intensity was always less than 3 times that of the noise SD. For each recorded cell, we first measured the threshold intensity and then increased the intensity by 10-μA increments until the amplitude of the EPSCs reached its maximal level. The maximal EPSC was always achieved with stimulus intensity that was about 5 times the threshold intensity; the maximal stimulus intensity we used was always above 5 times the threshold. The number of jumping steps (MII) provided an estimate of the lower limit number of innervating fibers that converge onto the recorded neuron (see discussion).
Pharmacology.
Sodium fluoroacetate (SFA) was injected at 1 mg/kg ip on P2. Reactive blue-2 (RB-2; 100 mg/kg ip) or suramin (SRMN; 20 mg/kg ip) was administered on P0, P2, and P4. Gabapentin (GBPT; 100 mg/kg ip) was injected on P1–P4.
Histochemistry and immunohistochemistry.
Unilaterally P0 IO-cut control and drug-treated pups at P5–P7 were euthanized and perfused with saline followed by phosphate-buffered 4% paraformaldehyde. The brains were fixed overnight and cytoprotected in 30% sucrose. Coronal sections of 20–30 μm were cut in a cryostat and collected in serial order. Alternate sections containing the PrV were processed for cytochrome oxidase (CO) histochemistry and Wisteria floribunda agglutinin (WFA) labeling or for glial fibrillary acidic protein (GFAP) and aldehyde dehydrogenase 1 family, member L1 (ALDH1L1) immunohistochemistry. WFA is a lectin that binds to the N-acetyl-galactosamine component of the chondroitin sulfate chains and is commonly used to detect chondroitin sulfate proteoglycans (CSPGs) in brain tissue (Brückner et al. 1993; Celio et al. 1998; Härtig et al. 1994; Pantazopoulos et al. 2006). ALDH1L1 is recognized as a highly specific antigenic marker for astrocytes (Cahoy et al. 2008; Yang et al. 2011).
A series of sections from unilateral IO-cut animals were processed for synaptophysin immunoreactivity to provide morphological correlates of reactive synaptogenesis assessed by electrophysiology. For CO histochemistry, the sections were incubated with PBS containing 0.5 mg/ml cytochrome c, 0.5 mg/ml diaminobenzidine (DAB; both from Sigma), and 50 mg/ml sucrose for 6–7 h at 37°C in a shaker incubator. For GFAP immunochemistry, the sections were incubated in 0.6% H2O2 in PBS to block the endogenous peroxidase activity. After rinses in PBS, the sections were incubated at 4°C with rabbit anti-GFAP (DAKO) for 48 h, followed by incubation with biotinylated donkey anti-rabbit antibody (Jackson ImmunoResearch). The detection of GFAP immunoreactivity was performed using the ABC kit (Vector Labs) and visualized with DAB.
Quantitative analysis of GFAP expression was carried out with a Nikon Microphot microscope (Nikon Americas) under bright-field ×10 magnification. Microscope images were captured with a Nikon Digital Sight camera mounted to the microscope, and labeling intensities were obtained using NIS-Elements software (Nikon Americas). Two circular regions of interest (ROI) of 213 μm in diameter were placed on the dorsal and ventral (barrelette region) PrV separately. Mean pixel intensities were measured inside each ROI. The intensity of the dorsal ROI was taken as a control. The difference between the dorsal and ventral ROI represented GFAP labeling in the ventral PrV.
For synaptophysin and ALDH1L1 immunofluorescence and WFA labeling, the sections of P5 rats were incubated in mouse monoclonal purified IgG against synaptophysin I (SYSY), anti-ALDH1L1 (NeuroMab), and rabbit anti-GFAP (DAKO) or in a solution of biotin-conjugated lectin WFA (Sigma) in 0.01 M PBS containing 1% normal goat serum, 2% BSA, and 0.3% Triton X-100 overnight at 4°C. Next, the sections were rinsed with PBS and then incubated with Alexa Fluor 488 goat anti-mouse IgG (Invitrogen) and Alexa Fluor 594 goat anti-rabbit IgG (Invitrogen) at room temperature for 1 h. WFA was stained with a 1-h incubation in FITC-conjugated streptavidin. The slides were rinsed with PBS and covered with fluorescence mounting medium.
Synaptophysin immunostaining was evaluated using fluorescence microscopy (Nikon Eclipse 90i). A z-series of the images from the barrelette region of the PrV were acquired using a ×40 objective at 1,310,720-pixel2 density with a Nikon DS-Fil camera. Quantitative ratio measurements of synaptophysin puncta were performed by using NIS Elements software (Nikon) to detect the values of regional maxima (compares each pixel's intensity with the intensity of surrounding pixels). A series of consecutive PrV-brain stem sections (a total of 32 sections from 4 pups) from unilaterally deafferented pups was used. The values of regional maxima were obtained at the same scale of threshold, matrix, and count. The fluorescence intensity was taken as a measure of synaptic puncta density. The difference in intensity (regional maxima) was calculated as a percentage.
Western blot.
For Western blot analyses, 6 litters of P0 unilateral IO-lesioned rat pups were euthanized on P5. The brains were extracted and sliced on a McIlwain tissue chopper at 700–1,000 μm. Slices containing the PrV were selected under a stereomicroscope, and the PrV was dissected out using microscissors. The left and right (IO cut side) PrV from each animal were collected in Eppendorf tubes and rapidly frozen. In all cases, the whisker pad was dissected to ensure that the nerve cut was complete. Protein (20 μg) from each sample was separated on 10% SDS-PAGE gel (Bio-Rad) and electroblotted to nitrocellulose membrane (GE Healthcare). The membranes were incubated in blocking solution [5% milk in Tris-buffered saline with Tween 20 (TBS-T) for 1 h at room temperature] and then incubated with anti-mouse GFAP (1:10,000 Millipore) with 5% milk in TBS-T overnight at 4°C. After three washes with TBS-T, the membranes were incubated for 1 h at room temperature in goat anti-mouse IgG horseradish peroxidase (HRP; 1:5,000; Bio-Rad) in 5% milk in TBS-T. After TBS-T washes, the immunoreactivity was detected using SuperSignal West Pico chemiluminescent substrate (Thermo Scientific) for HRP. To monitor the loading and blotting of equal amounts of protein, the membranes were incubated with an antibody against actin (1:1,000; Sigma-Aldrich). The ECL-exposed films were quantified by comparing the intensity of the bands on a Western blot (ImageJ version 1; National Institutes of Health). Intensity measurements were normalized to actin levels.
Statistical analysis.
All data are means ± SE. Student's t-test and analysis of variance (ANOVA) were used.
RESULTS
MII analysis is an electrophysiological approach, commonly used to estimate the lower limit number of afferents converging onto a single neuron. We used MII analysis to estimate the number of trigeminal afferents converging on single barrelette cells during development and following neonatal IO nerve damage in rat pups.
Synaptic refinement in the developing PrV of postnatal rats.
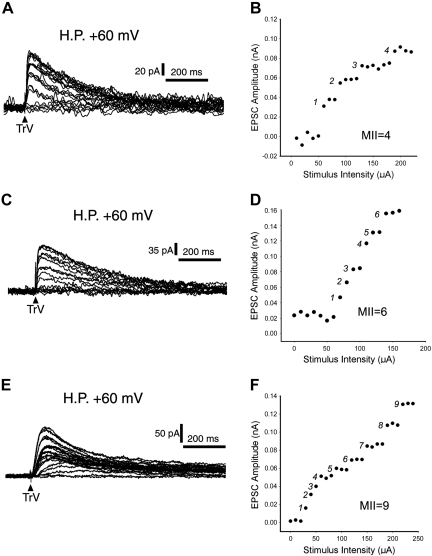
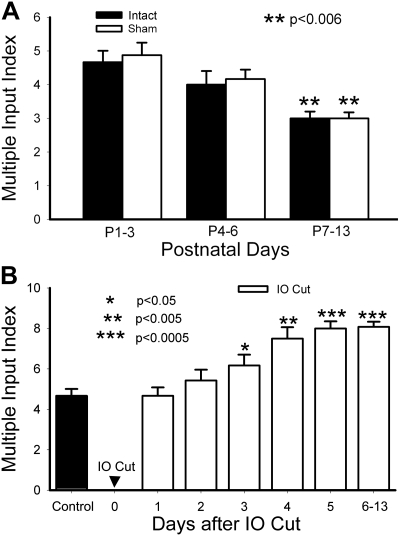
Previously, we reported that single barrelette neurons receive multiple trigeminal afferents in the developing PrV (Lo et al. 1999). However, quantitative analyses were not performed. In the present study, we voltage-clamped EPSCs at +60 mV (to accommodate the absence of functional AMPA receptors in silent synapses; see Lo and Erzurumlu 2007) and quantitatively analyzed MII in the developing PrV. In both rats and mice, any damage to the whisker follicles or to the IO nerve up to P3 (but not after P4) results in predictable and permanent alterations in whisker-specific neural patterning all the way from the trigeminal brain stem to the neocortex; thus this period is known as the critical period for structural plasticity (reviewed in Erzurumlu 2010). During the critical period, single barrelette neurons receive inputs from 3–6 trigeminal fibers with an average MII of 4.67 ± 0.34 (n = 12). Between P4 and P6, the MII slightly decreased to 4.00 ± 0.40 (n = 7), but it was not significantly different (P > 0.25) from that on P1–P3. An example of superimposed EPSCs induced by increasing stimulus intensities is shown in Fig. 1A. A plot of the EPSC amplitude against the stimulus intensity shows four jumping steps in EPSC amplitudes (Fig. 1B). In the second postnatal week (P7–P13), single barrelette neurons received significantly (P < 0.01) fewer trigeminal afferents than the first postnatal week; the MIIs ranged from 2 to 4 with a mean of 3.00 ± 0.20. This indicates synaptic pruning with elimination of about 30% synaptic connections during the second postnatal week (Fig. 2A, filled bars). Next, we used the same electrophysiological approach to assess the effects of sham operation and P0 IO nerve transection.
Fig. 1.
Examples of multiple input index (MII). A, C, and E: example records of multiple innervation of barrelette neurons. Superimposed excitatory postsynaptic currents (EPSCs) are shown at a holding potential (HP) of +60 mV along with progressively increasing stimulus intensity from 0 to 350 μA at steps of 10 μA. Note that the EPSC amplitudes fluctuate in a stepwise manner. B, D, and F: plots of EPSC amplitude against stimulus intensity. The amplitudes show abrupt jumps as the stimulus intensity increases. TrV, trigeminal tract.
Fig. 2.
MII of barrelette neurons in the trigeminal principal nucleus (PrV). A: the MII of single barrelette neurons declined during postnatal development in both intact (filled bars) and sham-operated PrV (open bars). Note that there is no difference between intact and sham-operated PrV. The MII on postnatal days 7–13 (P7–P13) is significantly (P < 0.006) smaller than that on P1–P3. B: time course of synaptogenesis after deafferentation. One day after deafferentation [infraorbital (IO) cut], the MII is the same as that in intact PrV on P1–P3 (control). The MII begins to increase from 2 days after IO cut and reaches a plateau after 5 days later. Compared with 1 day postdeafferentation, the increase in the MII is significant on 3–5 days postdeafferentation (*P < 0.05; **P < 0.005; ***P < 0.0005).
Sham operation does not alter the developmental synaptic refinement.
Synaptic refinement in the PrV of the sham-operated side was about the same as that of intact PrV (Fig. 2A, open bars). During the critical period (P1–P3), averaged MII was 4.88 ± 0.37 (n = 8), which was similar to that of intact PrV (P > 0.70). Between P4 and P6, the MII slightly decreased to 4.17 ± 0.28 (n = 6), but it was not significantly different (P > 0.20) from that on P1–P3 and from that of intact PrV (P > 0.76). In the second postnatal week (P7–P13), the averaged MII was 3.00 ± 0.18 (n = 8), which was significantly lower than that on P1–P3 (P < 0.006) and just the same as that of intact PrV. Thus sham lesions, which leave the IO nerve intact, do not affect postnatal synaptic pruning.
Neonatal deafferentation results in rapid formation of new synaptic connections in the PrV.
One day after IO nerve transection, the MII of barrelette neurons was 4.67 ± 0.41 (Fig. 2B), which is similar to that of intact P1–P3 PrV (4.67 ± 0.34; Fig. 2B, filled bar). Two days after deafferentation, the MII increased to 5.43 ± 0.53, but this was not significant (P > 0.1). A significant increase in MII (6.17 ± 0.54, P < 0.05) occurred 3 days after deafferentation (Fig. 2B). An example of six trigeminal inputs is presented in Fig. 1, C and D. On the 4th and 5th days following IO lesion, the MII continued to increase (7.50 ± 0.50 and 8.00 ± 0.35, respectively; P < 0.005) and reached a plateau after the 6th day. The MIIs remained at the same level (8.08 ± 0.25) between 6 and 13 days after deafferentation. There was no significant difference (P > 0.85) from that on the 5th postdeafferentation day (Fig. 2B).
The MII on postdeafferentation days 6–13 was about 2.7 times (P < 0.0001) that of the MII (3.00 ± 0.20) in intact PrV at the same age. An example of innervation by nine trigeminal fibers is presented in Fig. 1, E and F. Thus reactive synaptogenesis occurs mainly 3–5 days after IO nerve transection. These newly formed synapses are developmentally stabilized in the deafferented PrV. Our results are in line with previous findings that PrV cells display larger, complex receptive fields in neonatally lesioned animals (Waite 1984).
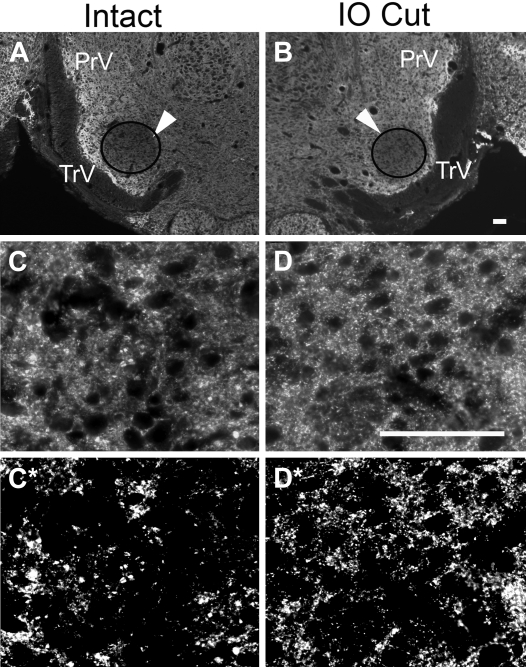
The synaptogenesis revealed by electrophysiology was also supported by immunohistochemistry for synaptophysin, a marker for presynaptic terminals (synaptic puncta). The density of synaptic puncta in the deafferented PrV was significantly higher than that in the intact PrV (Fig. 3). Synaptic puncta visualized by immunohistochemistry represent all synaptic inputs to the PrV (from the trigeminal nerve, inter- and intranuclear synapses, corticotrigeminal synapses, etc.) Hence, for quantifications, we normalized the fluorescence intensity with respect to the uninjured control PrV. We first designated a standardized, circular ROI placed on the barrelette region of the PrV (faded circular regions in low-power micrographs in Fig. 3) and performed quantitative measurements. Normalized synaptophysin labeling in the control PrV ROI was 100.0 ± 2.6%, whereas the labeling on the IO cut side was 121.4 ± 2.1%, which is significantly higher (P < 0.005).
Fig. 3.
Synaptophysin immunohistochemistry. A and B: low-power view of synaptophysin immunohistochemistry in the intact (A) and deafferented PrV (B). Arrowheads point to the regions of interest (ROI) over the barrelette region of the PrV from where the micrographs were taken at higher magnification. C and D: higher magnification views from intact (C) and deafferented sides (D) show the density of synaptic puncta in deafferented PrV under fluorescence. C* and D* show the same fields after thresholding (see materials and methods) for intensity measurements with the NIS Elements program. The density of the fluorescence is higher than that in the intact PrV. Scale bar, 100 μm.
Reactive synaptogenesis in the deafferented PrV occurs beyond critical period for barrelette plasticity.
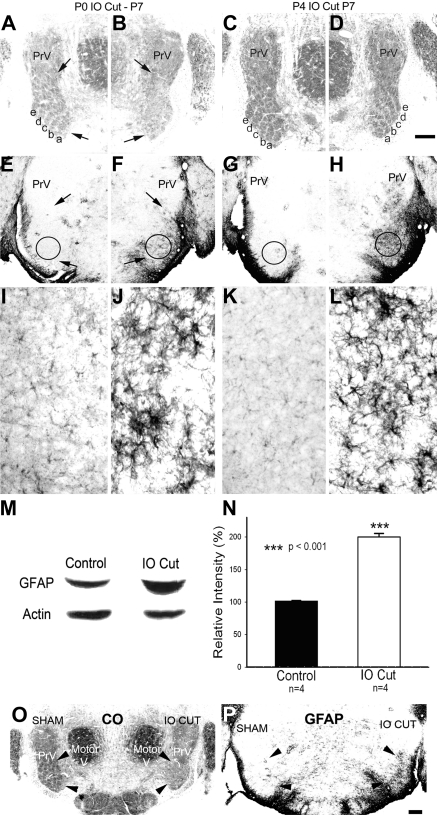
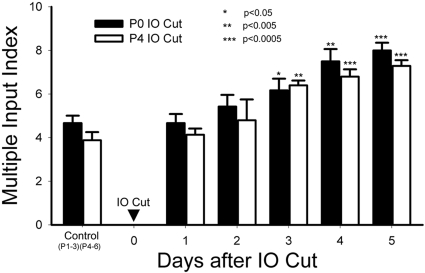
IO nerve damage during the neonatal critical period leads to loss of trigeminal afferent terminal patterns (Fig. 5, B vs. A) and nonpolarized dendritic orientation of barrelette neurons (Lo and Erzurumlu 2001). In the next series of experiments, we tested whether IO nerve transection-induced reactive synaptogenesis is restricted to the critical period or extends beyond it. When we compared the time course of reactive synaptogenesis after P0 and P4 lesions, both were similar (Fig. 4). There was no significant difference (P > 0.35) between the two groups at each postinjury day. Because IO nerve lesion on P4 did not alter barrelette patterns (Fig. 5, D vs. C), we conclude that the mechanisms underlying synapse formation induced by peripheral nerve injury are different from the mechanisms underlying barrelette pattern formation and consolidation in the PrV.
Fig. 5.
Effects of P0 and P4 IO nerve cut on the PrV and astrocytosis. A: cytochrome oxidase (CO) histochemistry revealed the barrelette patterns in the intact control side at P7. Arrows point to the IO nerve-recipient zone of the PrV in this and other micrographs. Whisker-specific barrelette rows (a–e) are indicated. B: the deafferented side of the same section shown in A. Note that the barrelettes have dissolved or did not form. C and D: CO staining in the intact (C) and deafferented sides (D) of the same P7 brain stem from a pup that underwent IO cut on P4 (after the critical period). Note that 5 rows of barrelettes are present in the deafferented PrV. E: glial fibrillary acidic protein (GFAP) labeling in the control PrV at P5. F: marked astrocytosis and high levels of GFAP expression in the IO nerve-recipient zone of the PrV in the deafferented side 4 days after P0 IO cut. Intense astrocytosis and GFAP staining is also visible after the critical period. G and H: control (G) and deafferented sides (H) of the same P7 brain stem after P4 IO cut. Note the high level of GFAP immunostaining in the IO nerve-recipient zone of the deafferented PrV (H). Higher magnification views of GFAP immunostaining are shown in I–L; these micrographs are from ROIs (marked by circles) of the PrV of the same sections shown in E–H. Scale bar in D is 200 μm for A–H and 20 μm for I–L. M: Western blots of GFAP expression on the ventral PrV (barrelette region) of P5 rat pups. N: the relative intensity of GFAP in the deafferented PrV was significantly higher (***P < 0.001) than that in the intact PrV. O: sham operation did not change barrelette patterns. P: sham operation did not upregulate GFAP expression. Scale bar, 100 μm.
Fig. 4.
Time course of reactive synaptogenesis after IO cut on P0 and P4 (during and after the critical period for structural plasticity). The MII of the first day after IO cut on P0 (filled bar) and on P4 (open bar) is similar to the control value. Compared with 1 day postdeafferentation, the increase in the MII is significant on 3–5 days postdeafferentation in both cases (*P < 0.05; **P < 0.005; ***P < 0.0005). These results indicate that IO nerve injury-induced reactive synaptogenesis is not confined to the critical period.
Neonatal deafferentation leads to upregulation of astrocyte-specific protein expression.
Immunohistochemistry experiments revealed a marked astrocytosis and GFAP expression in the IO nerve-recipient zone of the PrV (Fig. 5, E–L). Interestingly, this intense GFAP immunostaining was seen after both P0 and P4 IO nerve lesions (Fig. 5, E–L), suggesting that reactive gliosis is not restricted to the critical period for structural plasticity. Western blot analyses also revealed a marked increase in GFAP expression in the barrelette region of the deafferented PrV (Fig. 5M). The deafferented PrV (IO cut) showed much higher GFAP expression than the intact PrV (control). The intensity of the deafferented PrV was 200.20 ± 5.42%, which is significantly higher (P < 0.001) than that of the intact PrV (101.45 ± 0.97%; Fig. 5N). On the sham-operated side, the barrelette patterns and GFAP expression in the PrV were similar to the intact PrV (Fig. 5, O and P).
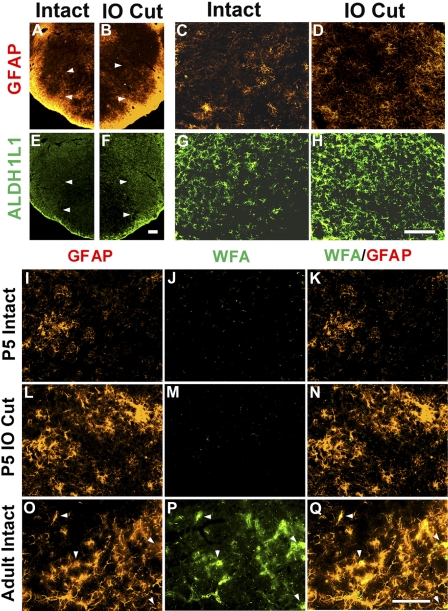
Because astrocytes are highly heterogeneous in their gene expression profiles (for reviews, see Kimelberg 2009; Zhang and Barres 2010), GFAP, once thought to be the “pan-astrocyte marker,” is not expressed in all classes of astrocytes (Kimelberg 2009). Thus we examined the expression in the PrV of ALDH1L1, which was proposed to be a “highly, broadly and specifically antigenic marker for astrocytes” (Cahoy et al. 2008; Yang et al. 2011). Both GFAP and ALDH1L1 labeled more astrocytes on the deafferented PrV than on the intact PrV (Fig. 6, A and C vs. B and D, and E and G vs. F and H). On each side, ALDH1L1 labeled more astrocytes than GFAP did (Fig. 6, A–D vs. E–H).
Fig. 6.
Expression of glial markers and chondroitin sulfate proteoglycans (CSPGs). Top: both GFAP and ALDH1L1 expression are upregulated in the deafferented PrV. Low- and high-power photomicrographs of the intact PrV (A and C) show low GFAP expression, whereas in the deafferented PrV (B and D) GFAP expression is upregulated. Similarly, ALDH1L1 expression is upregulated in the denervated PrV (F and H) compared with the intact side (E and G). Note that ALDH1L1 labeled more astrocytes than GFAP, indicating that it is a better pan-astrocyte marker. Bottom micrographs illustrate the absence of Wisteria floribunda agglutinin (WFA) staining for CSPGs in the neonatal PrV (J and M), whereas the same procedure shows high levels of CSPG staining in the adult PrV (P). The same sections were immunostained with GFAP (I, L, and O), and double labeling is shown in K, N, and Q. Arrowheads point to astrocytes double-labeled with both WFA and GFAP. Scale bar, 100 μm.
Astrocytes of postnatal rats are immature during reactive synaptogenesis following neonatal deafferentation.
Astrocytes are generated from radial glia after neurogenesis is completed. In postnatal animals, astrocytes are immature with short processes and complex, nonlinear current-voltage (I–V) profiles. As they mature, they acquire stellate morphology and linear I–V relationships (Béchade et al. 2011; Zhou et al. 2006). Interestingly, immature and mature astrocytes have different functions in synaptogenesis and synaptic plasticity (for reviews, see Bradbury and Carter 2011; Ullian et al. 2004). For example, immature astrocytes promote CNS axonal regeneration, whereas mature ones inhibit axon regeneration by secreting CSPGs. Injection of immature astrocytes or CSPG degradation in perineuronal nets reactivates ocular dominance plasticity in the adult visual cortex (Muller and Best 1989; Pizzorusso et al. 2002). CSPG expression is an important index to differentiate immature and mature astrocytes. We used WFA labeling to determine whether CSPGs related to mature astrocytes play a role in reactive synaptogenesis in the PrV. Curiously, WFA did not label the extracellular matrix (ECM) or astrocytes in the PrV of rat pups during the first postnatal week (Fig. 6, J, K, M, N) but labeled astrocytes in the PrV of adult rats (Fig. 6, P and Q). This result suggests that astrocytes are immature during reactive synaptogenesis induced by neonatal IO nerve transection.
Several in vitro studies have indicated a role for astrocytes in synaptogenesis (reviewed in Allen and Barres 2005; Freeman 2006; Pfrieger 2010; Stevens 2008). These reports and increased GFAP and ALDH1L1 expression in the deafferented PrV prompted us to utilize various pharmacological blocking agents to investigate the role of astrocytes in IO nerve injury-induced reactive synaptogenesis.
Synaptogenesis induced by neonatal deafferentation requires activation of purinergic receptors.
In the CNS, reactive astrocytic response is initiated by the activation of P2 class of purinergic receptors by extracellular ATP released on tissue damage (Washburn and Neary 2006). RB-2 is more effective at blocking rat P2Y4 receptors (Abbracchio et al. 2006; Burnstock 2006; Melani et al. 2006; Von Kugelgen 2006; Wildman et al. 2003). We administered RB-2 (100 mg/kg ip injections on P0, P2, and P4) to test the effect of P2Y4 receptor blockade on normal developmental and reactive synaptogenesis in the PrV. In all pharmacological blockade experiments, IO nerve lesions were performed unilaterally. Thus the uninjured side of the same brain served as an internal control for the effects of drug manipulations on normal synaptogenesis.
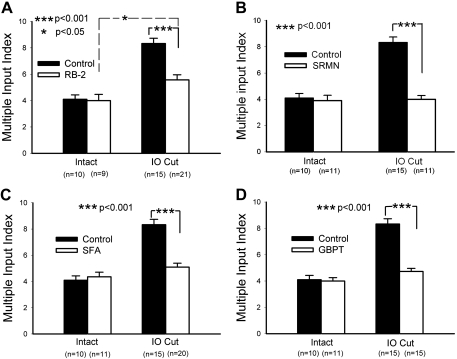
In the intact PrV, the averaged MII for barrelette cells was 4.10 ± 0.33 (Fig. 7A, intact, filled bar), and after RB-2 injections, the MII in the intact PrV was 4.00 ± 0.47 (Fig. 7A, intact, open bar); there was no significant difference between them (P > 0.85). Thus RB-2 injections do not affect normal developmental synaptogenesis. In the deafferented PrV without RB-2 injection, the MII was 8.33 ± 0.41 (Fig. 7A, IO cut, filled bar). In the deafferented PrV of RB-2-injected pups, the MII was 5.57 ± 0.39 (Fig. 7A, IO cut, open bar), which was larger (P < 0.05) than that in the intact side (Fig. 7A, intact, open bar) but smaller (P < 0.001) than that without RB-2 injection (Fig. 7A, IO cut, filled bar). These results indicate that RB-2 partially blocks reactive synaptogenesis. This is in line with in vitro studies showing that ATP-induced TSP-1 expression in cultured astrocytes is not completely blocked by RB-2 (Tran and Neary 2006).
Fig. 7.
MII analysis after various drug treatments in the intact (control) and deafferented (IO cut) PrV. A: application of reactive blue-2 (RB-2) partially blocked reactive synaptogenesis (IO cut, filled vs. open bars) without affecting developmental synaptogenesis in the control side (intact, filled vs. open bars). B: application of suramin (SRMN), a broad-spectrum antagonist for P2 class receptors, completely blocked the reactive synaptogenesis (IO cut, filled vs. open bars) with no effects on the control side (intact, filled vs. open bars). C: application of sodium fluoroacetate (SFA), an inhibitor of astrocyte metabolism, completely blocked the reactive synaptogenesis in the IO cut side (filled vs. open bars) but did not affect the control side (filled vs. open bars). D: application of gabapentin (GBPT), an antagonist for thrombospondin (TSP) receptor, also completely blocked the reactive synaptogenesis (IO cut, filled vs. open bars), yet it did not affect the MII in the control side (intact, filled vs. open bars). *P < 0.05; ***P < 0.001.
To further investigate the involvement of purinergic receptors in reactive synaptogenesis, we administered SRMN, a broad spectrum antagonist of P2X and P2Y receptors (Abbracchio et al. 2006; Burnstock 2006; Friedle et al. 2010; Lambrecht et al. 2002). After SRMN injections (20 mg/kg ip on P0, P2, and P4), the MII of barrelette cells on the intact side was 3.91 ± 0.40 (Fig. 7B, intact, open bar), similar to that of normal control value (P > 0.73, Fig. 7B, intact, filled bar). Thus SRMN injections do not affect developmental synaptogenesis in the PrV. After SRMN injections, the MII of deafferented PrV barrelette cells was 4.00 ± 0.29, which was about the same as that of the intact PrV (P > 0.86, Fig. 7B, IO cut, open bar), suggesting that the reactive synaptogenesis is completely blocked by SRMN (P < 0.001, Fig. 7B, IO cut, filled vs. open bar).
Synaptogenesis induced by deafferentation requires functional astrocytes.
The upregulation of GFAP and ALDH1L1 in the deafferented PrV (Fig. 5, E–N, and Fig. 6, B, D, F, H) suggests that astrocytes play an important role in reactive synaptogenesis induced by neonatal IO transection. Because SFA is selectively taken up by astrocytes and leads to inhibition of their aconitase in the tricarboxylic acid cycle, it is extensively used as an astrocyte-specific function inhibitor (Andersson et al. 2007; Dopico et al. 2006; Fonnum et al. 1997; Hassel et al. 1997; Henneberger et al. 2010; Hulsmann et al. 2003; Lian and Stringer 2004; Okada-Ogawa et al. 2009; Shigetomi et al. 2008; Zhang et al. 2003; Zielinska et al. 2007). We administered SFA (1 mg/kg ip injection on P2) to perturb astrocyte metabolism to determine any changes in synaptogenesis in both intact and deafferented PrV. After SFA injection, the MII was 4.36 ± 0.35 on the intact, control side (Fig. 7C, intact, open bar). It was not significantly different from that on the intact PrV without SFA injection (P > 0.60, Fig. 7C, intact, filled bar), indicating that SFA injection does not alter normal development of synaptic connections. After IO transection with SFA injection, the MII for barrelette cells was 5.10 ± 0.30 (Fig. 7C, IO cut, open bar), which was significantly (P < 0.001) less than that for IO cut alone (Fig. 7C, IO cut, filled bar), but it was not significantly different (P > 0.05) from that for the intact side with or without SFA injection (Fig. 7C, intact, filled and open bars). Thus SFA injection completely blocks reactive synaptogenesis in the deafferented PrV.
Synaptogenesis induced by deafferentation involves TSPs.
In vitro studies have shown that TSPs promote synapse formation (Christopherson et al. 2005; Eroglu et al. 2009; Xu et al. 2009). Interestingly, TSP-induced synapses are presynaptically active but are postsynaptically silent (Christopherson et al. 2005; Xu et al. 2009). We have shown that in the deafferented PrV, most barrelette neurons show silent synapses (Lo and Erzurumlu 2007), suggesting that IO nerve injury-induced reactive synaptogenesis in the PrV is mediated by TSPs. A recent study showed that GBPT blocks TSP-induced synaptogenesis in vitro (Eroglu et al. 2009). We tested the effect of GBPT (100 mg/kg ip injections on P1–P4) in our in vivo model of reactive synaptogenesis.
After GBPT injections, the averaged MII in the intact PrV was similar to that for the control value (4.00 ± 0.26, P > 0.82; Fig. 7D, intact, filled vs. open bar). In the deafferented PrV, the MII was 4.73 ± 0.24 (Fig. 7D, IO cut, open bar), which was significantly lower (P < 0.001) than that without GBPT injections (Fig. 7D, IO cut, filled bar) but not different from that for the intact PrV (P > 0.059; Fig. 7D, intact, open bar). Therefore, GBPT completely blocks reactive synaptogenesis but not the normal course of developmental synaptogenesis. After completing these Student's t-tests, we performed ANOVA tests for all data sets in Fig. 7. Only values for IO cut controls (P < 0.001) and IO cut RB-2-injected intact PrV (P < 0.05) are different from other values. This supported our Student's t-test results.
Quantitative analysis of GFAP immunohistochemistry.
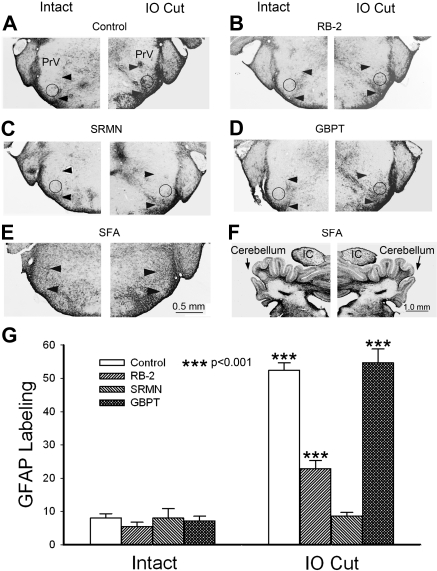
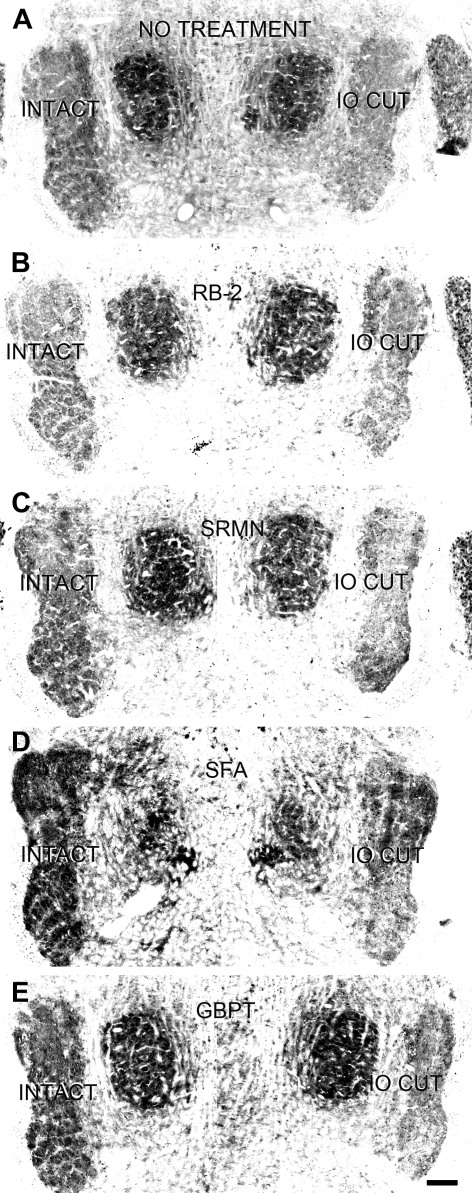
We took GFAP expression in the ventral part (barrelette region, marked by 2 arrowheads in Fig. 8, A–E) of the PrV to reveal the effect of different antagonists. In the control animals, the GFAP labeling in the ROI (circle in Fig. 8, A–E) of the deafferented PrV was 52.4 ± 2.3 (Fig. 8G, IO cut, control), which was much higher (P < 0.001) than that of the intact PrV (8.1 ± 1.3, n = 13, Fig. 8G, intact, control). Example micrographs for control animals are presented in Fig. 8A and Fig. 5, E–L. After application of RB-2, the GFAP labeling in the deafferented PrV was reduced (Fig. 8B). GFAP labeling in the barrelette region of the intact PrV was 5.5 ± 1.3, which was not different (P > 0.16) from that of control animals, whereas the labeling in the deafferented PrV was 22.9 ± 2.4 (n = 13), which was significantly higher (P < 0.001) than that of intact PrV (Fig. 8G, intact, RB-2) but significantly lower than that of control deafferented PrV (P < 0.001; Fig. 8G, IO cut, RB-2 vs. control). Thus RB-2 injections partially blocked GFAP expression in the deafferented PrV, similar to our electrophysiological results. Application of SRMN abolished the increased GFAP expression in the deafferented PrV (Fig. 8C). The GFAP labeling on both sides were about the same (8.0 ± 2.8 vs. 8.6 ± 1.1, n = 8, P > 0.62; Fig. 8G, SRMN); this is in line with our finding that SRMN completely blocked synaptogenesis. Application of GBPT did not affect GFAP expression on both intact and deafferented PrV (Fig. 8D). The GFAP labeling in the intact side was 7.1 ± 1.5 (n = 10), which did not differ from that of control animals (P > 0.62; Fig. 8G, intact, GBPT vs. control), whereas the labeling in the deafferented PrV was 54.7 ± 4.2, similar to that of control animals (P > 0.61; Fig. 8G, IO cut, GBPT vs. control). This result did not contradict our electrophysiological results showing that GBPT completely blocked reactive synaptogenesis, because GBPT blocks the effect of TSPs on synaptogenesis, downstream of astrocyte reaction. ANOVA analysis supported t-test results showing control, RB-2, and GBPT values on the IO cut side were different (P < 0.001) from other values (Fig. 8G). Notably, application of SFA resulted in a great increase in GFAP expression nonselectively. Swollen astrocytes were distributed all across brain stem sections. There was no difference between the intact and deafferented PrV (Fig. 8E). The inferior colliculi and cerebellum on both sides were also heavily GFAP positive (Fig. 8F). Despite widespread GFAP expression in the brain stem, the physiological function of astrocytes in reactive synaptogenesis is completely blocked by SFA in the deafferented PrV without affecting normal developmental synaptogenesis in the control PrV.
Fig. 8.
Analysis of GFAP expression after various drug treatments in the intact (control) and deafferented (IO cut) PrV. A: example micrographs of a control animal showing higher GFAP labeling in the ROI (circles) of the barrelette region (arrowheads) in the deafferented (IO cut) PrV than in the intact PrV. B: after RB-2 injections, the GFAP labeling in the deafferented side was less than that in control animals, but the labeling in the intact side was not changed. C: after SRMN injections, the GFAP labeling in the deafferented side was similar to that of the intact side, which was also similar to that of control PrV. D: after GBPT injections, the GFAP labeling in both sides was similar to that of controls, suggesting GBPT does not alter GFAP expression. E: application of SFA led to a conspicuous increase in GFAP labeling all over the brain stem. F: upregulation of GFAP expression was also seen in the cerebellum and inferior colliculus (IC). G: averaged measurements of GFAP labeling show that drug treatments did not affect GFAP expression in the intact PrV. In the deafferented PrV, RB-2 decreased GFAP labeling, SRMN completely blocked the increased GFAP labeling, and GBPT had no effect on GFAP labeling. ANOVA showed that only control, RB-2, and GBPT values are higher (***P < 0.001) than other values.
The disruption of whisker-specific patterns is independent of astrocyte function.
IO nerve transection at birth leads to failure in whisker-specific neural patterning, barrelette formation, in the rodent PrV. There is a critical period for this effect that ends abruptly by P3 in both mice and rats (Erzurumlu 2010). Initially, we thought that P0 IO nerve transection-induced reactive synaptogenesis and astrocytosis might also be related to critical period plasticity for barrelette formation. However, our experiments involving IO nerve lesions after the end of the critical period still showed reactive synaptogenesis and upregulated GFAP expression. In the visual system, the presence of immature astrocytes is a prerequisite for visual cortical plasticity (Muller and Best 1989). Our pharmacological intervention experiments aimed at blocking various aspects of astrocyte function did not alter barrelette patterns in the developing PrV. After application of RB-2, SRMN, SFA, and GBPT, the intact side of the PrV showed normal barrelettes (Fig. 9, A, C, E, and G) and the deafferented side revealed their absence (Fig. 9, B, D, F, and H). These observations indicate that neonatal IO nerve damage-induced barrelette pattern failure may not involve astrocytes but that the ensuing reactive synaptogenesis does.
Fig. 9.
Pharmacological interventions aimed at perturbing astrocyte function do not interfere with formation and maintenance of barrelette patterns in the intact PrV. A comparison of intact and IO cut sides in the same brains from P5 rat pups that underwent P0 IO damage and includes images of controls (no drug treatment; A) and pups injected with RB-2 (B), SRMN (C), SFA (D), and GBPT (E). Note that in all cases the intact side has barrelette patterns, whereas the IO cut side has no patterns. Scale bar, 100 μm.
DISCUSSION
The electrophysiological method to estimate the number of innervating fibers to each target neuron was first used at the neuromuscular junctions of newborn rats (Redfern 1970). This approach was later used in the studies on postnatal synaptic refinement in the olivocerebellar (Crepel and Mariani 1976; Mariani and Changeux 1981), retinogeniculate (Chen and Regehr 2000; Hooks and Chen 2006; Lo et al. 2002; Stevens et al. 2007), retinocollicular (Lu and Constantine-Paton 2004), and medial lemniscal pathways (Arsenault and Zhang 2006). The criterion for multiple innervation is the “stepwise graded character” or “abrupt jump in the amplitude” of postsynaptic responses on progressively increasing stimulus intensity. The number of jumping steps provides an estimation of the lower limit number of innervating fibers, because a jumping step represents additional activation of a group of afferent fibers that have similar stimulus threshold. We know that unitary EPSCs induced by a single fiber fluctuate in amplitude (Laurent et al. 2002). The amplitudes of EPSCs induced by a group of fibers fluctuate to a much less extent, because the fluctuations in EPSC amplitude for different fibers counteract each other. That is why the amplitude variation of EPSCs induced by the same stimulus intensity is always less than three times the noise SD in our study. It is important to note that multiple input index analysis cannot reveal the exact number of fibers innervating a given neuron. However, despite its limitations, this technique is an effective physiological means to illustrate the changes in the convergence of afferent fibers onto single neurons. Using this approach, we have demonstrated that about 30% of synaptic connections are eliminated during the postnatal development in the intact PrV. In addition, we have shown emergence of rapid synaptogenesis in the neonatally deafferented PrV and effects of different blockers on it.
Our immunohistochemical studies also supported the electrophysiology findings and presence of intense GFAP expression as a result of neonatal peripheral nerve damage. The intensity of immunofluorescence for synaptophysin (a marker for presynaptic puncta) was significantly higher than that in the intact PrV. It is worth noting that the quantitative analyses of immunostaining results and their statistical evaluation do not compare the actual physical number of synapses or GFAP-labeled astrocytes in the control and the denervated PrV. Our results merely compare fluorescence intensity of immunolabeling in the control and denervated PrV and in the denervated and enervated portions of the PrV. Use of array tomography or superresolution fluorescence imaging with multicolor, three-dimensional stochastic optical reconstruction microscopy would give the actual number of synapses within nanometer precision (Dani et al. 2000; Micheva and Smith 2007). Another caveat to our immunohistochemistry results is that we were able to use only a presynaptic marker, synaptophysin. We tested several commercially available postsynaptic density protein markers, but although they worked fine in the cortex, superior colliculus, cerebellum, and hippocampus, they did not work in the brain stem regions where the PrV is localized. Despite these shortcomings, the immunofluorescence comparisons between the control and denervated portions of the PrV revealed clear differences supporting our electrophysiological findings.
Brain injury leads to an increase in extracellular adenosine 5′-triphosphate (ATP) (Braun et al. 1998; Fields and Stevens 2000). ATP is released from various sources (Franke et al. 2006; Vizi et al. 2001), including the damaged cells (Fields and Stevens 2000). ATP activates purinergic receptors of astrocytes so that astrocytes switch from quiescent (resting) to reactive status (Collazos-Castro and Nieto-Sampedro 2001; Tran and Neary 2006). Reactive astrocytes release soluble factors such as TSPs (Christopherson et al. 2005; Tran and Neary 2006), cholesterol (Slezak and Pfrieger 2003), and other factors (see for review Pfrieger 2010; Stevens 2008). Among these factors, TSPs mediate cell-cell and cell-extracellular matrix interactions (Adams 2001) and have been reported to induce the formation of ultrastructurally normal synapses but postsynaptically silent synapses (Christopherson et al. 2005). A role of astrocytes in synaptogenesis is further strengthened by observations from TSP1/2 knockout mice, which develop significantly fewer numbers of excitatory synapses (Christopherson et al. 2005).
In the present study, IO nerve damage only affected the presynaptic central trigeminal axons and terminals. We do not know whether this also results in ATP increase in the PrV. Previous studies have shown that IO nerve injury at birth induces increased apoptosis in the trigeminal ganglion and the PrV starting 2–24 h after peripheral injury (Miller and Kuhn 1997; Sugimoto et al. 1999). It is reasonable to assume that the dying PrV cells release ATP that activates purinergic receptors and induces robust astrocytic responses in the deafferented zone of the PrV. Systematic application of RB-2 partially blocks synaptogenesis, similar to the results of in vitro studies (Tran and Neary 2006; Wildman et al. 2003). Reactive synaptogenesis is completely blocked by application of SRMN, a broad-spectrum antagonist for P2 receptors. These results are further supported by GFAP immunohistochemistry. Notably, peripheral nerve injury leads to neuropathic pain by activating purinergic receptors (P2X4) of astrocytes and microglia in other nuclei (Beggs and Salter 2010; Okada-Ogawa et al. 2009; Piao et el. 2006). Because microglial response triggered by nerve injury is not mature before P16 (Moss et al. 2007) and synaptogenesis in the PrV occurs mainly in the first postnatal week, we believe the effects of RB-2 and SRMN on synaptogenesis are caused by blocking the activation of astrocytes.
Astrocyte activity can be selectively impaired with SFA, a specific inhibitor of the Krebs cycle in astrocytes (Fonnum et al. 1997; Hassel et al. 1997). SFA has been used to inhibit astrocyte function (Andersson et al. 2007; Dopico et al. 2006; Henneberger et al. 2010; Hulsmann et al. 2003; Lian and Stringer 2004; Okada-Ogawa et al. 2009; Shigetomi et al. 2008; Zhang et al. 2003; Zielinska et al. 2007). However, its effect on synaptogenesis is still unknown. In our in vivo model, application of SFA did not alter synaptic connections in the intact PrV but completely blocked reactive synaptogenesis in the deafferented PrV, indicating that functional astrocytes play a critical role in reactive synaptogenesis. Interestingly, systemic application of SFA induces a robust, ubiquitous GFAP expression in the CNS. Our electrophysiological results show that this upregulated GFAP expression does not affect normal developmental synaptogenesis in the control PrV but abolishes reactive synaptogenesis in the deafferented PrV. These results suggest that SFA switches astrocytes into reactive status but also abolishes their function in reactive synaptogenesis.
Brain injury also upregulates TSPs in astrocytes (Tran and Neary 2006). TSP family members TSP1 and TSP2 are trimers and interact with cell-surface receptors, cytokines, growth factors, and proteases and have been reported to induce synapse formation (Christopherson et al. 2005; Xu et al. 2009). Genetic deletion of these proteins in mice results in 30% fewer synapses in their brains (Allen and Barres 2005; Christopherson et al. 2005). TSP-induced synapses are apparently “silent synapses” that contain NMDA receptors but lack AMPA receptors (Christopherson et al. 2005), much like what we found in the neonatally deafferented PrV (Lo and Erzurumlu 2007). Recently, GBPT (and pregabalin) was identified as a ligand for calcium channel subunit α2δ-1 and was reported to block TSP-induced synaptogenesis (Eroglu et al. 2009). Our in vivo results support this finding. GBPT treatment of rat pups completely blocks reactive synaptogenesis in the deafferented PrV but does not affect the normal course of developmental synaptogenesis in the same brain.
Taken together, our results indicate that peripheral sensory nerve damage in neonates leads to robust synaptic plasticity in the brain stem and that astrocytes play a major role in this process through purinergic receptors and TSP secretion. We do not know the morphological substrates of this synaptic plasticity. The most likely scenario would be sprouting of surviving trigeminal afferent terminals in the PrV and their convergence on a reduced number of PrV neurons (due to transsynaptic cell death). In this context, astrocytes could be inducing both sprouting and synapse formation. Finally, the consequences of this neonatal reactive synaptogenesis in the adult state are not known but may lead to hypersensitivity of the pathway or neuropathic pain conditions (Moss et al. 2007; Okada-Ogawa et al. 2009). Pharmacological intervention of astrocyte signaling at various levels offers venues of ameliorating the peripheral nerve injury-induced reactive synaptogenesis in the neonatal brain. The present study also allows differentiation of developmental synaptogenesis, which is clearly not affected by pharmacological blockade of astrocyte function, from injury-induced reactive synaptogenesis.
GRANTS
This work was supported by the National Institute of Neurological Disorders and Stroke Grant R01 NS037070 (to R. S. Erzurumlu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
F.-S. L. conception and design of research; F.-S. L. and S. Z. performed experiments; F.-S. L. and S. Z. analyzed data; F.-S. L. interpreted results of experiments; F.-S. L. prepared figures; F.-S. L. drafted manuscript; R. S. E. edited and revised manuscript; R. S. E. approved final version of manuscript.
REFERENCES
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58: 281–341, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JC. Thrombospondins: multifunctional regulators of cell interactions. Annu Rev Cell Dev Biol 17: 25–51, 2001 [DOI] [PubMed] [Google Scholar]
- Allen NJ, Barres BA. Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol 15: 542–548, 2005 [DOI] [PubMed] [Google Scholar]
- Andersson M, Blomstrand F, Hanse E. Astrocytes play a critical role in transient heterosynaptic depression in the rat hippocampal CA1 region. J Physiol 585: 843–852, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault D, Zhang ZW. Developmental remodeling of the lemniscal synapses in the ventral basal thalamus of the mouse. J Physiol 573: 121–132, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béchade C, Pascual O, Triller A, Bessis A. Nitric oxide regulates astrocyte maturation in the hippocampus: involvement of NOS2. Mol Cell Neurosci 46: 762–769, 2011 [DOI] [PubMed] [Google Scholar]
- Beggs S, Salter MW. Microglia-neuronal signaling in neuropathic pain hypersensitivity 2.0. Curr Opin Neurobiol 20: 474–480, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Carter LM. Manipulating the glia scar: chondroitinase ABC as a therapy for spinal cord injury. Brain Res Bull 84: 306–316, 2011 [DOI] [PubMed] [Google Scholar]
- Braun N, Zhu Y, Krieglstein J, Culmsee C, Zimmermann H. Upregulation of the enzyme chain hydrolyzing extracellular ATP after transient forebrain ischemia in the rat. J Neurosci 18: 4891–4900, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brückner G, Brauer K, Härtig W, Wolff JR, Rickmann MJ, Derouiche A, Delpech B, Girard N, Oertel WH, Reichenbach A. Perineuronal nets provide a olyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia 8: 183–200, 1993 [DOI] [PubMed] [Google Scholar]
- Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci 27: 166–176, 2006 [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamian JL, Christophersom KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28: 264–278, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L. Perineuronal nets: past and present. Trends Neurosci 21: 510–515, 1998 [DOI] [PubMed] [Google Scholar]
- Chen C, Rehehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron 28: 955–966, 2000 [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokees CCA, Mullowney E, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120: 421–433, 2005 [DOI] [PubMed] [Google Scholar]
- Collazos-Castro JE, Nieto-Sampedro M. Developmental and reactive growth of dentate gyrus afferents: cellular and molecular interactions. Restor Neurol Neurosci 19: 169–187, 2001 [PubMed] [Google Scholar]
- Cotman CW, Anderson KJ. Synaptic plasticity and functional stabilization in the hippocampal formation: possible role in Alzheimer's disease. Adv Neurol 47: 313–335, 1988 [PubMed] [Google Scholar]
- Crepel F, Mariani J. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol 7: 567–578, 1976 [DOI] [PubMed] [Google Scholar]
- Dani A, Huang B, Bergan J, Dulac C, Zhuang X. Superresolution imaging of chemical synapses in the brain. Neuron 68: 843–856, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deller T, Frotscher M. Lesion-induced plasticity of central neurons: sprouting of single fibres in the rat hippocampus after unilateral entorhinal cortex lesion. Prog Neurobiol 53: 687–727, 1997 [DOI] [PubMed] [Google Scholar]
- Dieringer N. ‘Vestibular compensation’: neural plasticity and its relations to functional recovery after labyrinthine lesions in frog and other vertebrates. Prog Neurobiol 46:97–125, 1995 [PubMed] [Google Scholar]
- Dopico JG, González-Hernández T, Pérez IM, García IG, Abril AM, Inchausti JO, Díaz MR. Glycine release in the substantia nigra: Interaction with glutamate and GABA. Neuropharmacology 50: 548–557, 2006 [DOI] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O'Rourke NA, Park CY, Özkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, Green EM, Lawler J, Doimetsch R, Garcia KC, Smith SJ, Luo ZD, Rosenthal A, Mosher DF, Barres BA. Gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 139: 1–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS. Critical period for the whisker-barrel system. Exp Neurol 222: 10–12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields R, Stevens B. ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci 23: 625–633, 2000 [DOI] [PubMed] [Google Scholar]
- Fonnum F, Johnsen A, Hassel B. Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia 21: 106–113, 1997 [PubMed] [Google Scholar]
- Franke H, Grummich B, Härtig W, Grosche J, Regenthal R, Edwards RH, Illes P, Krügel U. Changes in purinergic signaling after cerebral injury -involvement of glutamatergic mechanisms? Int J Dev Neurosci 24: 123–132, 2006 [DOI] [PubMed] [Google Scholar]
- Freeman MR. Sculpting the nervous system: glia control of neuronal development. Curr Opin Neurobiol 16: 119–125, 2006 [DOI] [PubMed] [Google Scholar]
- Friedle SA, Curet MA, Watters JJ. Recent patents on novel P2X7 receptor antagonists and their potential for reducing central nervous system inflammation. Recent Pat CNS Drug Discov 5: 35–45, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamori J. Morphological plasticity of postsynaptic neurones in reactive synaptogenesis. J Exp Biol 153: 251–260, 1990 [DOI] [PubMed] [Google Scholar]
- Härtig W, Brauer K, Bigl V, Brückner G. Chondroitin sulfate proteoglycan-immunoreactivity of lectin-labeled perineuronal nets around parvalbumin-containing neurons. Brain Res 635: 301–311, 1994 [DOI] [PubMed] [Google Scholar]
- Hassel B, Bachelard H, Jones P, Fonnum F, Sonnewald U. Trafficking of amino acid between neurons and glia in vivo. Effects of inhibition of glial metabolism by fluoroacetate. J Cereb Blood Flow Metab 17: 1230–1238, 1997 [DOI] [PubMed] [Google Scholar]
- Henderson TA, Rhoades RW, Bennett-Clarke CA, Osborne PA, Johnson EM, Jacquin MF. NGF augmentation rescues trigeminal ganglion and principalis neurons, but not brainstem or cortical whisker patterns, after infraorbital nerve injury at birth. J Comp Neurol 336: 243–260, 1993 [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusalov DA. Long-term potentiation depends on release of d-serine from astrocytes. Nature 463: 232–236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron 52: 281–291, 2006 [DOI] [PubMed] [Google Scholar]
- Hulsmann S, Straub H, Richter DW, Speckmann EJ. Blockade of astrocyte metabolism causes delayed excitation as revealed by voltage-sensitive dyes in mouse brainstem slices. Exp Brain Res 150: 117–121, 2003 [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Astrocyte heterogeneity or homogeneity? In: Astrocytes in (Patho)Physiology of the Nervous System, edited by Parpura V, Heydon PG. New York: Springer, 2009 [Google Scholar]
- Lambrecht G, Braun K, Damer S, Ganso M, Hildebrandt C, Ullmann H, Kassack MU, Nickel P. Structure-activity relationships of SRMN and pyridoxal-5′-phosphate derivatives as P2 receptor antagonists. Curr Pharm Des 8: 2371–2399, 2002 [DOI] [PubMed] [Google Scholar]
- Laurent A, Goaillard JM, Cases O, Lebrand C, Gaspar P, Ropert N. Activity-dependent presynaptic effect of serotonin 1B receptors on the somatosensory thalamocortical transmission in neonatal mice. J Neurosci 22: 886–900, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian X-Y, Stringer JL. Astrocytes contribute to regulation of extracellular calcium and potassium in the rat cerebral cortex during spreading depression. Brain Res 1012: 177–184, 2004 [DOI] [PubMed] [Google Scholar]
- Lo FS, Erzurumlu RS. L-Type calcium channel-mediated plateau potentials in barrelette cells of the principal trigeminal nucleus during structural plasticity. J Neurophysiol 88: 794–801, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Erzurumlu RS. Neonatal deafferentation does not alter membrane properties of developing trigeminal principal sensory nucleus neurons. J Neurophysiol 85: 1088–1096, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Erzurumlu RS. Peripheral nerve damage does not alter release properties of developing central trigeminal afferents. J Neurophysiol 105: 1681–1688, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Erzurumlu RS. Conversion of functional synapses into silent synapses in the trigeminal brainstem following neonatal peripheral nerve transection. J Neurosci 27: 4929–4934, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Guido W, Erzurumlu RS. Electrophysiological properties and synaptic responses of cells in the whisker representation area of the postnatal rat trigeminal principal sensory nucleus. J Neurophysiol 82: 2765–2775, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Zhao S. N-methyl-d-aspartate receptor subunit composition in the rat trigeminal principal nucleus remains constant during postnatal development and following neonatal denervation. Neuroscience 178: 240–249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Ziburkus J, Guido W. Synaptic mechanisms regulating the activation of a Ca2+-mediated plateau potential in developing relay cells of the LGN. J Neurophysiol 87: 1175–1185, 2002 [DOI] [PubMed] [Google Scholar]
- Lu W, Constantine-Paton M. Eye opening rapidly induces synaptic potentiation and refinement. Neuron 43: 237–249, 2004 [DOI] [PubMed] [Google Scholar]
- Mariani J, Changeux JP. Ontogenesis of olivocerebellar relationships. I. Studies by intracellular recordings of the multiple innervation of Purkinje cells by climbing fibers in the developing rat cerebellum. J Neurosci 1: 696–702, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone DF, Petit TL. The role of synaptic morphology in neural plasticity: structural interactions underlying synaptic power. Brain Res Rev 38: 291–308, 2002 [DOI] [PubMed] [Google Scholar]
- Matthews DA, Cotman C, Lynch G. An electron microscopic study of lesion induced synaptogenesis in the dentate gyms of the adult rat. II. Reappearance of morphologically normal synaptic contacts. Brain Res 115: 23–41, 1976 [DOI] [PubMed] [Google Scholar]
- Melani A, Amadio S, Gianfriddo M, Vannucchi MG, Volonte G, Bernardi G, Pedata F, Sancesario G. P2X7 receptor modulation on microglial cells and reduction of brain infarct caused by middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab 26: 974–982, 2006 [DOI] [PubMed] [Google Scholar]
- Micheva KD, Smith SJ. Array tomography: a new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron 55: 25–36, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, al-Ghoul WM, Murtaugh M. Expression of ALZ-50 immunoreactivity in the developing principal sensory nucleus of the trigeminal nerve: effect of transecting the infraorbital nerve. Brain Res 560: 132–138, 1991 [DOI] [PubMed] [Google Scholar]
- Miller MW, Kuhn P. Neonatal transection of the infraorbital nerve increases the expression of protein related to neuronal death in the principal sensory nucleus of the trigeminal nerve. Brain Res 796: 233–244, 1997 [DOI] [PubMed] [Google Scholar]
- Moss A, Beggs S, Vega-Avelaira D, Costigan M, Hathway GJ, Salter MW, Fitzgerald M. Spinal microglia and neuropathic pain in young rats. Pain 128: 215–224, 2007 [DOI] [PubMed] [Google Scholar]
- Muller CM, Best J. Ocular dominance plasticity in adult cat visual cortex after transplantation of cultured astrocytes. Nature 342: 427–430, 1989 [DOI] [PubMed] [Google Scholar]
- Okada-Ogawa A, Suzuki I, Sessle BJ, Chiang CY, Salter MW, Dostrovsky JO, Tsuoi Y, Kondo M, Kitagawa J, Kobayashi A, Noma N, Imamura Y, Iwata K. Astroglia in medullary dorsal horn (trigeminal spinal subnucleus caudalis) are involved in trigeminal neuropathic pain mechanisms. J Neurosci 29: 11161–11171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazopoulos H, Lange N, Hassinger L, Berretta S. Subpopulations of neurons expressing parvalbumin in the human amygdala. J Comp Neurol 496: 706–722, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrieger FW. Role of glial cells in the formation and maintenance of synapses. Brain Res Rev 63: 39–46, 2010 [DOI] [PubMed] [Google Scholar]
- Piao ZG, Cho IH, Park CK, Hong JP, Choi SY, Lee SJ, Lee S, Park K, Kim JS, Oh SB. Activation of glia and microglial p38 MAPK in medullary dorsal horn contributes to tactile hypersensitivity following trigeminal sensory nerve injury. Pain 121: 219–231, 2006 [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medina P, Beardy N, Cheri S, Fawcett JW, Mafia L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298: 1248–1251, 2002 [DOI] [PubMed] [Google Scholar]
- Redfern PA. Neuromuscular transmission in new-born rats. J Physiol 209: 701–709, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Bowser DN, Sofroniew MV, Khakh B. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J Neurosci 28: 6659–6663, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slezak M, Pfrieger FW. New roles for astrocytes: regulation of CNS synaptogenesis. Trends Neurosci 26: 531–535, 2003 [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SWM, Barres BA. The classical complement Cascade mediates CNS synapse elimination. Cell 131: 1164–1178, 2007 [DOI] [PubMed] [Google Scholar]
- Stevens B. Neuron-astrocyte signaling in the development and plasticity of neural circuits. Neurosignals 16: 278–288, 2008 [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Xiao C, Takeyama A, He YF, Takano-Yamamoto T, Ichikawa H. Apoptotic cascade of neurons in the subcortical sensory relay nuclei following the neonatal infraorbital nerve transection. Brain Res 824: 284–290, 1999 [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Xiao C, Ichikawa H. Neonatal primary neuronal death induced by capsaicin and axotomy involves an apoptotic mechanism. Brain Res 807: 147–154, 1998 [DOI] [PubMed] [Google Scholar]
- Tran MD, Neary JT. Purinergic signaling induces thrombospondin-1 expression in astrocytes. Proc Natl Acad Sci USA 103: 9321–9326, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Christopherson KS, Barres BA. Role for glia in synaptogenesis. Glia 47: 209–216, 2004 [DOI] [PubMed] [Google Scholar]
- Vizi ES, Hasko G, Lendvai B, Sperlagh B. Role of endogenous ATP in the regulation of pro- and anti-inflammatory mediator production. Drug Dev Res 53: 117–125, 2001 [Google Scholar]
- Von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptors subtypes. Pharmacol Ther 110: 415–432, 2006 [DOI] [PubMed] [Google Scholar]
- Waite PM. Rearrangement of neuronal responses in the trigeminal system of the rat following peripheral nerve section. J Physiol 352: 425–445, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn KB, Neary JT. P2 purinergic receptors signal to STAT3 in astrocytes: difference in STAT3 responses to P2Y and P2X receptor activation. Neuroscience 142: 411–423, 2006 [DOI] [PubMed] [Google Scholar]
- Wildman SS, Unwin RJ, King BF. Extended pharmacological profiles of rat P2Y2 and rat P2Y4 receptors and their sensitivity to extracellular H+ and Zn2+ ions. Br J Pharmacol 140: 1177–1186, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xiao N, Xia J. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat Neurosci 13: 221–224, 2009 [DOI] [PubMed] [Google Scholar]
- Yang Y, Vidensky S, Jin L, Jie C, Lorenzini I, Franki M, Rothstein JD. Molecular comparison of GLT1+ and ALDH1L1+ astrocytes in vivo in astroglial reporter mice. Glia 59: 200–207, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JH, Zhang J, Xiao C, Kong JQ. Patch-clamp studies in the CNS illustrate a simple new method for obtaining viable neurons in rat brain slices: glycerol replacement of NaCl protects CNS neurons. J Neurosci Methods 158: 251–259, 2006 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol 20: 588–594, 2010 [DOI] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40: 971–982, 2003 [DOI] [PubMed] [Google Scholar]
- Zhou M, Schools GP, Kimelberg HK. Development of GLAST+ astrocytes and NG2+ glia in rat hippocampus CA1: mature astrocytes are electrophysiologically passive. J Neurophysiol 95: 134–143, 2006 [DOI] [PubMed] [Google Scholar]
- Zielinska M, Fresko I, Konopacka A, Felipo V, Albrecht J. Hyperammonemia inhibits the natriuretic peptide receptor 2 (NPR-2)-mediated cyclic GMP synthesis in the astrocytic compartment of rat cerebral cortex slices. Neurotoxicology 28: 1260–1263, 2007 [DOI] [PubMed] [Google Scholar]