Abstract
Reactive oxygen species (ROS) modulate neuronal excitability. In the present study we examined the effects of hydrogen peroxide (H2O2), a well established ROS, on neuronal activity from two neonatal mouse brain regions, i.e., the pre-Bötzinger complex (preBötC) within the ventral respiratory column (VRC) and the CA1 area of the hippocampus. In the preBötC, 2.2 mM H2O2 evoked a transient depression followed by augmentation of neuronal activity. The iron chelator deferoxamine (500 μM) did not prevent H2O2-mediated neuronal augmentation but prevented the initial depression. Combined application of Fe2+ and H2O2 only caused depression of the preBötC rhythm. In contrast, H2O2 suppressed neuronal activity in the CA1 region, and this effect was accentuated by coapplication of Fe2+ and H2O2, suggesting that hydroxyl radical generated by Fenton reaction mediates the effects of H2O2 on CA1 neuronal activity. Malondialdehyde (MDA) levels were monitored as an index of lipid peroxidation in H2O2-treated preBötC and CA1 areas. MDA levels were unaltered in H2O2-treated preBötC, whereas MDA levels were markedly elevated in the CA1 region. These findings suggest that 1) exogenous administration of H2O2 exerts differential effects on neuronal activities of preBötC versus CA1 neuronal populations and 2) H2O2 is a potent modulator of respiratory rhythmogenesis from the preBötC without affecting global oxidative status.
Keywords: neonate, oxidative stress, rhythm
a growing number of studies indicate that superoxide anion (O2·−) and hydrogen peroxide (H2O2), well-characterized reactive oxygen species (ROS), function as neuromodulators. H2O2 affects presynaptic Ca2+ entry at the neuromuscular junction (Giniatullin and Giniatullin 2003), and O2·− has been hypothesized to contribute to the long-term facilitation of phrenic and hypoglossal motor outputs (Macfarlane and Mitchell 2008). H2O2 generated from O2·− sensitizes the carotid body response to hypoxia and induces sensory plasticity manifested as sensory long-term facilitation (Peng et al. 2003, 2009). In midbrain dopaminergic neurons, H2O2 reduces neuronal excitability by affecting ATP-sensitive potassium (KATP) channels (Avshalumov et al. 2005). In adult CA1 neurons of the hippocampus, O2·− (Knapp and Klann 2002) and low concentrations of H2O2 (Kamsler and Segal 2003) induce long-term potentiation, an established form of neuronal plasticity.
Neonates experience higher levels of ROS because of transition from the low-oxygen environment in utero to air (Perrone et al. 2010), and the resulting oxidative stress has been implicated in several neuropathological conditions including reperfusion injury following resuscitation (Saugstard 2001a; Varma et al. 2003) as well as oxygen toxicity resulting from the use of supplemental oxygen (Poets 2010; Saugstard 2001b). Additionally, oxidative stress may contribute to apnea of prematurity (Pawar et al. 2009). Although the effects of ROS are well documented in the adult nervous system, relatively little is known on the effects of ROS on the neonatal nervous system. In the present study, we tested the effects of H2O2 on neuronal activity of the pre-Bötzinger complex (preBötC) in neonates, an area that is critical for breathing and hence survival (McKay et al. 2005; Ramirez and Garcia 2007). To test whether the effects of H2O2 are region selective, parallel experiments were performed on CA1, an area that is critical for learning and memory. Our results demonstrate that H2O2 causes an initial depression followed by excitation of neuronal activity in preBötC without inducing oxidative stress. In striking contrast, H2O2 depressed CA1 neuronal activity, and this effect was associated with increased oxidative stress.
METHODS
Solutions and pharmacology.
Artificial cerebrospinal fluid (aCSF; in mM: 118 NaCl, 24 NaHCO3, 1.0 NaH2PO4, 3.0 KCl, 1 MgCl2, 1.5 CaCl2, and 30 d-glucose, pH 7.4) equilibrated with carbogen (95% O2, 5% CO2) was used for control O2 conditions. Intracellular patch electrode solution contained (in mM) 140 K-gluconic acid, 1 CaCl2, 10 EGTA, 2 MgCl2, 4 Na2ATP, and 10 HEPES (pH 7.2). The NMDA receptor antagonist 3-((R)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP) and the AMPA/kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) were obtained from Tocris Cookson (Ellisville, MO). The glycine receptor antagonist strychnine (STR), the GABAA-receptor antagonist picrotoxin (PTX), FeSO4, and deferoxamine were purchased from Sigma Aldrich (St. Louis, MO). H2O2 solution (unstabilized; 6%) was from VRW International (Radnor, PA).
Slice preparation.
In accordance with National Institutes of Health guidelines, all experiments were performed either at The University of Chicago or at Seattle Children's Research Institute with protocols approved by the Animal Care and Use Committees at the respective institutions. Transverse medullary brain stem slices and coronal cortico-hippocampal brain slices were prepared from neonatal CD1 mice (P7–P12). Mice were anesthetized (via either ether or isoflurane inhalation) and rapidly decapitated, and the brain and brain stem were quickly removed. Either the cerebrum or the brain stem was mounted to an agar block with cyanoacrylate glue. The mounted tissues were submerged in ice-cold aCSF equilibrated with carbogen, and slices of the region of interest were cut with a vibratome.
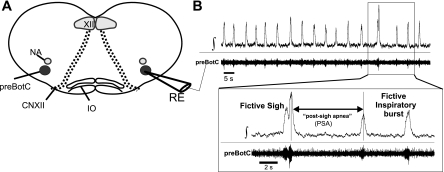
Transverse medullary brain stem slices (600 μm thick) were prepared with protocols described previously (Ramirez et al. 1996). The transverse medullary slice contained a portion of the ventral respiratory column (VRC) that included the preBötC and all anatomical landmarks previously described (Fig. 1A; Ramirez et al. 1996). In well-oxygenated conditions, two integrated waveforms are readily identified—fictive eupnea and fictive sighs. Fictive eupnea is characterized by an augmenting or bell-shaped population burst, while fictive sighs occur with a much lower frequency and are large amplitude with a biphasic burst shape (Lieske et al. 2000; Fig. 1B). Fictive sighs are also followed by a prolonged interevent interval (i.e., postsigh apnea). Only one slice per brain stem was taken and transferred immediately to the recording chamber, where it was allowed to recover for 30–60 min prior to experimentation. In a subset of experiments, either one or both VRC regions were microdissected from the brain stem slice. In these VRC islands, the intrinsic rhythmic activity from the preBötC neuron is preserved, whereas the circuitry contributing to rhythmogenesis is greatly reduced. Coronal cortico-hippocampal brain slices (400–500 μm thick) were cut from a cerebrum mounted on agar in an orientation in which the frontal lobe was parallel to the cutting blade of the vibratome. The cortico-hippocampal slices were transferred into aCSF equilibrated with carbogen at ∼21°C and bisected at the central fissure, yielding two usable slices. Experiments on the cortico-hippocampal slices were performed within 2 h after the slice preparation.
Fig. 1.
The pre-Bötzinger complex (preBötC) in the transverse medullary brain stem slice generates a population rhythm activity similar to and implicated with respiratory patterns found in vivo. A: cartoon schematic of the transverse brain stem slice containing the preBötC. This preparation also contains other neuronal regions related and unrelated to respiration. Anatomical landmarks include the nucleus ambiguus (NA), the hypoglossal nucleus (XII), fiber bundles of the hypoglossal nerve (CNXII), and the inferior olive (IO). Placing an extracellular recording electrode (RE) over the ventral respiratory column allows for the recording of the population rhythm from the preBötC. B: representative trace of the integrated (∫) and raw (preBötC) population activity originating from the preBötC. Inset: expanded timescale identifying the fictive sigh, postsigh apnea, and fictive normal inspiratory bursts (i.e., fictive eupnea).
Electrophysiological recordings.
After the transfer of hippocampal or medullary slices or VRC islands into the recording chamber (6-ml volume), carbogen-equilibrated aCSF (33 ± 2°C) was circulated and recycled (18–20 ml/min; a total volume of 100 ml) via a peristaltic pump. To initiate the fictive respiratory rhythm from the neuronal population of the VRC or spontaneous bursting from the CA1 neuronal population, the extracellular KCl concentration was elevated from 3 to 8 mM over a 20-min period. Experiments were initiated after 10-min equilibration of the slices with 8 mM KCl.
Extracellular recordings were made from either the CA1 neuronal population or the preBötC located in the VRC, using the medullary brain stem slices or VRC islands. The population rhythm from the preBötC generates distinct burst patterns similar to and implicated with in vivo patterns of breathing (Lieske et al. 2000; Pena et al. 2004). Under well-oxygenated conditions, two population burst patterns of preBötC can be discriminated: 1) fictive inspiratory bursts (i.e., fictive eupnea) and 2) fictive sighs (Fig. 1B). Using the integrated waveforms of the population bursts, we determined how H2O2 affected the burst patterns and altered the frequency of fictive inspiratory bursts as well as fictive sighs. Extracellular recordings were made with glass electrodes filled with aCSF (<2 MΩ). Population activity was amplified, filtered (low pass 1.5 kHz; high pass 250 Hz), rectified, and integrated (time constant 60 ms) in real time. In a subset of extracellular recordings, continuous unilateral microinjection of H2O2 (1.67–2.34 μl/s; 600-s duration) over one VRC region of the brain slice was performed. The injection pressure ranged between 30 and 60 Torr.
Patch-clamp recordings were made in brain stem slices from putative inspiratory neurons of the preBötC with a multiclamp amplifier (Molecular Devices, Sunnyvale, CA). For recordings, borosilicate glass electrodes (4–6 MΩ) prepared with a P-97 Flaming/Brown micropipette puller (Sutter Instrument, Novato, CA) and filled with intracellular patch electrode solution were used. A single recording from one neuron per brain stem slice was made. Inspiratory neurons were identified by their in-phase discharge pattern with the population rhythm. Experiments were performed in whole cell current-clamp configuration. Upon establishing a stable intracellular recording, the putative inspiratory neuron was isolated from the network with the AMPA receptor antagonist CNQX (10 μM), the NDMA receptor antagonist CCP (10 μM), the GABAA receptor antagonist PTX (50 μM), and the glycinergic receptor antagonist STR (1 μM). When neurons exhibited a small (≥2 mV) depolarization, a constant holding current (≥ −35 pA) was applied to return the membrane potential (Vm) back to the original steady-state Vm and the current was maintained for the entire duration of the experiment. Once a neuron was isolated, a current injection step protocol consisting of the injection of hyperpolarizing currents (−120 to 0 pA with a 20-pA step) and one depolarizing current (0 to 80 pA with a 80-pA step) was used. Measurements with this protocol were made in triplicate during 1) control conditions, 2) the early exposure to H2O2 (eH2O2; 60–180 s), and 3) the late exposure to H2O2 (lH2O2; 480–600 s). The mean value of the triplicate measurements was considered as the response of a given neuron during the aforementioned conditions. The calculated junction potential of −12 mV was subtracted post hoc from Vm. Input resistance (Rn) of each neuron was calculated using the changes in Vm that resulted from the hyperpolarizing current injections. The number of action potentials generated by injecting +80 pA was also determined.
Both extracellular and intracellular electrophysiological recordings were made with Clampex 9.0 or 10.0 (Molecular Devices). Extracellular recordings were sampled at 1.67 kHz, while intracellular recordings were sampled at 20 kHz. Typical experiments had a 600-s recording of baseline activity in control condition followed by a 600-s exposure to either experimental agent or washout. The time of switching between two different solutions was 60 ± 8 s.
Lipid peroxidation.
The degree of lipid peroxidation was determined by measuring malondialdehyde (MDA) levels in slices treated with H2O2 (2.2 mM), Fe2+-H2O2 (100 μM, 2.2 mM), or vehicle (control). The conditions and protocols used for the exposure of slices to H2O2 or Fe2+-H2O2 were identical to those used in the respective extracellular recording experiments in which the agents were bath applied. For controls, slices were incubated with aCSF containing 8 mM KCl for 1,200 s. The slices were homogenized in 20 mM phosphate buffer, pH 7.4, and the homogenates were centrifuged in an Eppendorf microcentrifuge at 500 g for 10 min at 4°C. MDA levels were determined with procedures described previously (Raghuraman et al. 2011) and expressed as nanomoles per milligram of protein.
Statistics.
Analyses of the integrated burst area, instantaneous frequency (finst), and burst count were conducted with Clampfit 9.0 (Molecular Devices). The irregularity score of finst was used as a measure of burst-to-burst variability. It was calculated with the following formula from consecutive cycle length values:
with SN = score of the Nth cycle, finstN its finst, finstN−1 the finst of the preceding burst, and ABS the absolute value (see Telgkamp et al. 2002). With this formula, a low irregularity score represents a rhythm with low burst-to-burst variability in finst.
In dose-response experiments, plots of mean finst as a function of elapsed time were made to readily identify H2O2-mediated suppression of frequency. Individual points of the mean finst represented the moving average of finst from four consecutive bursts. In some experiments, data bins were taken both prior to (t = −120 s) and during (t = 180, 300, and 600 s) experimental manipulations. In H2O2 washout and Fe2+-H2O2 experiments, additional bins were taken during experimental manipulation at t = 780, 900, and 1,200 s. Each data bin was 120 s in duration to accurately capture the transient depression caused by H2O2 in the preBötC. Integrated burst frequency (i.e., burst count) and area were normalized to control conditions (i.e., t = −120 s).
Unless stated otherwise, n values represent the number of individual slices from which the quantification was conducted. Numerical data are presented as means ± SE. Differences between two means were analyzed with Student's t-test and were considered significant at P < 0.05. Differences between three or more means were determined with one-way repeated-measures ANOVA followed by multiple comparisons testing (Dunnett's comparison). Statistics were calculated with either the KyPlot Data and Visualization software package (Kyenslab, Tokyo, Japan) or Graphpad InStat (Graphpad Software, La Jolla, CA).
RESULTS
Effects of H2O2 on inspiration-related neuronal activity in preBötC.
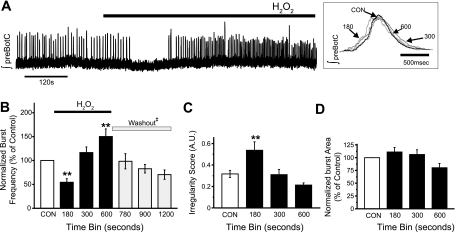
Neuronal activity in the preBötC was characterized as bursts of activity in well-oxygenated conditions, i.e., fictive inspiration. An example illustrating the effect of H2O2 (2.2 mM for 600 s) on bursting activity of preBötC is shown in Fig. 2A. Superfusion of H2O2 over the slice caused an initial depression followed by augmentation of neuronal activity, and this effect was seen in 17 of 20 preparations. On average, neuronal activity (finst) decreased from 0.21 ± 0.03 Hz (control) to 0.17 ± 0.03 Hz, resulting in a decrease in burst frequency (54 ± 7% of control). At the end of H2O2 exposure, finst increased to 0.28 ± 0.02 Hz with concomitant increase in burst frequency (150 ± 16% of control; Fig. 2B). During the early phase, H2O2 transiently increased the irregularity score of finst from 0.32 ± 0.03 to 0.54 ± 0.08 (Fig. 2C), which returned to the control level in the late phase of H2O2 exposure. On the other hand, H2O2 had no significant effect on the integrated burst area of fictive inspiration (Fig. 2D).
Fig. 2.
H2O2 biphasically affects the frequency of fictive normal inspiratory bursts. A: representative trace of the integrated population activity originating from the preBötC (∫preBötC) illustrating the biphasic effect that 2.2 mM H2O2 has on burst frequency of fictive inspiratory bursts. This biphasic effect was characterized initially by suppression followed by augmentation in burst frequency. Inset: expanded traces of single fictive normal inspiratory bursts taken during control (CON) or during H2O2 exposure (time bins 180, 300, and 600). B: effects of H2O2 on burst frequency; in a subset of experiments (n = 5) H2O2 was washed out and followed for an additional 600 s (gray bars) to demonstrate the reversibility of the effects of H2O2. C and D: irregularity score of finst [C; arbitrary units (A.U.)] and integrated burst area (D) were assessed by comparing average metric values (120-s bins) during control (CON) to those at 180, 300, and 600 s (black bars) during the 600-s exposure to H2O2 (n = 17 of 20 demonstrated a biphasic frequency response to H2O2; **P < 0.01). Washout bins were taken at 780, 900 and 1,200 s. ‡No significant differences were found between washout values and respective control values.
To assess whether the effects of H2O2 are reversible, neuronal activity was recorded for an additional 600 s after washout of H2O2 (n = 5). Inspiratory burst frequency returned toward baseline with a tendency to undershoot (74 ± 11%), which was not statistically significant (Fig. 2B). At the end of the washout period, burst area and irregularity score were 105 ± 19% and 116 ± 14% of control, respectively.
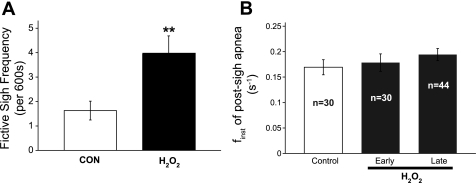
H2O2 exposure increased the number of fictive sighs from 1.63 ± 0.38 per 600 s to 3.95 ± 0.72 per 600 s (Fig. 3A; n = 20). The integrated burst area of fictive sighs was unaffected by H2O2. Unlike the biphasic effect of H2O2 on finst of fictive inspiration, the finst of the postsigh apnea during the early (0–300 s) and late (>300 s) phases of H2O2 exposures were comparable to that of untreated control animals (Fig. 3B).
Fig. 3.
H2O2 stimulates fictive sighs. A: 2.2 mM H2O2 increases fictive sigh frequency (n = 20 slices; **P < 0.01). B: finst of postsigh apnea is unaffected during the early (0–300 s) and late (>300 s) exposure to H2O2. Because of the infrequency of fictive sighs, each fictive sigh event was treated as an individual, which is reflected in the reported n values.
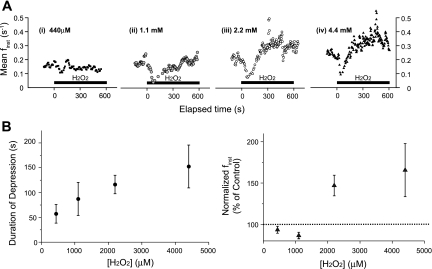
Analysis of the dose response revealed that the duration of suppressed neuronal activity depended on H2O2 concentration (Fig. 4, A and B, left). The increase in neuronal activity was evident with as low as 440 μM H2O2, and the magnitude of excitation further increased with 2.2 mM. The magnitude of excitation elicited with 4.4 mM was the same as that evoked by 2.2 mM, indicating that the response reached plateau (Fig. 4B, right).
Fig. 4.
Dose response of H2O2 on the frequency of rhythmogenesis from the preBötC. A: representative plots of the running average of finst as a function of time when exposed to 440 μM (i), 1.1 mM (ii), 2.2 mM (iii), or 4.4 mM (iv) H2O2. Using the plots of the running average of finst revealed that H2O2 caused an initial transient concentration-dependent suppression in population activity from the preBötC. B, left: plotting the duration of the suppressed finst as a function concentration revealed the H2O2-mediated transient suppression of finst at 440 μM (n = 4/6), 1.1 mM (n = 3/5), 2.2 mM (n = 17/20), and 4.4 mM (n = 6/6) H2O2. Right: plotting the mean normalized finst values for the experiments showing an transient suppression of finst illustrates the biphasic nature of the frequency response of rhythmogenesis to H2O2 and the ability of H2O2 to augment frequency at the highest concentrations tested.
Effect of H2O2 application on neuronal activity from the VRC.
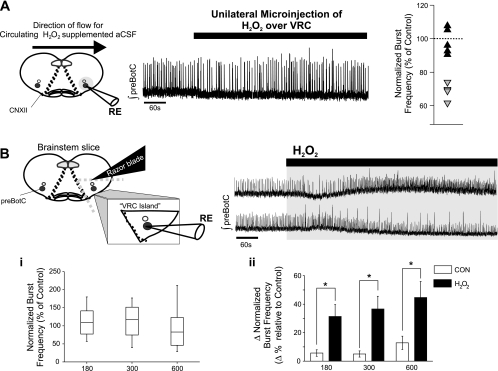
To determine whether a local administration of H2O2 rather than superfusion produces a similar biphasic change in burst frequency of VRC, effects of unilateral microinjections of H2O2 into the VRC within the brain slice were tested. H2O2 microinjections suppressed the frequency of the rhythm by ≥20% (Fig. 5A). In another series, VRC islands were microdissected and then exposed to H2O2 (2.2 mM) via superfusion. This further reduced preparation preserved stable rhythmogenesis from the preBötC when left untreated (Fig. 5B; Johnson et al. 2001). In this preparation, H2O2 did not evoke predictable changes in frequency (Fig. 5Bi). However, a comparison of the magnitude of change (independent of directionality) demonstrated that H2O2 exposure affects burst frequency of the rhythm (Fig. 5Bii).
Fig. 5.
Effects of H2O2 microinjection on rhythmogenesis in the brain stem slice and the effects of H2O2 on the frequency of rhythmogenesis from ventral respiratory column (VRC) islands. A: representative trace showing that unilateral microinjection of 2.2 mM H2O2 over the VRC can cause a biphasic frequency in the brain stem slices. An initial depression of frequency (depression ≥80% of control frequency) was observable in 4 of 9 experiments. The distribution of initial H2O2-mediated frequency effects is shown (gray triangles: experiments showing depression ≥80% of control frequency, n = 4; black triangles: experiments showing depression <80% of control frequency, n = 5). B: rhythmogenesis from microdissected VRC island is sensitive to 2.2 mM H2O2 (600 s), but as shown in the 2 representative traces the effect on frequency is not uniform. i: Box-whisker plots of normalized burst frequency from experiments with exposure to H2O2 during the 180, 300, and 600 s time bins show that H2O2 exposure produces unpredictable changes in burst frequency from rhythms recorded from VRC islands. ii: At all time bins, the magnitude of change in burst frequency, independent of directionality, is significantly different with exposure to H2O2 (n = 9; black bars) compared with VRC island rhythm time-matched controls (n = 4; open bars) left in artificial cerebrospinal fluid (aCSF). *P < 0.05.
Effects of H2O2 on individual preBötC neurons.
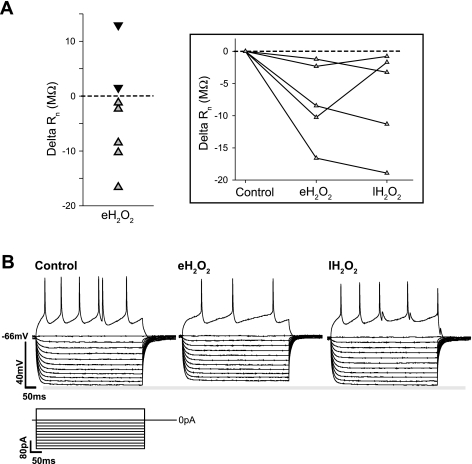
To assess the effects of H2O2 on membrane properties of preBötC neurons, whole cell current-clamp recordings were made from putative inspiratory neurons. These neurons were isolated from the network with bath application of CNQX (10 μM), CCP (10 μM), PTX (50 μM), and STR (1 μM). In isolation, all neurons (n = 7) became silent, having a steady-state Vm of −61.67 ± 2.33 mV and a Rn of 155.07 ± 20.81 MΩ. Exposure to H2O2 did not lead to significant changes in Vm (eH2O2: 61.25 ± 2.45 mV; lH2O2: 61.01 ± 2.43 mV). However, a majority of neurons (n = 5 of 7) showed decreased Rn during eH2O2 (range of ΔRn = −1.20 to −16.59 MΩ) (Fig. 6A) followed by a rebound in the late phase i.e., lH2O2 (Fig. 6A, inset). In 71% of neurons (n = 5 of 7), the number of evoked action potentials during the early phase of H2O2, i.e., eH2O2, decreased to 65 ± 18% of control (Fig. 6B) but was not statistically significant (P = 0.11). The depressed action potential frequency was transient in four of the five neurons tested (Fig. 6B).
Fig. 6.
Effect of H2O2 on individual preBötC neurons. A: although exposure to H2O2 (2.2 mM, 600 s) did not have a significantly measurable effect on the mean input resistance (Rn) of network isolated preBötC neurons (n = 7), during early exposure to H2O2 (eH2O2, t = 60–180 s of exposure) 5 of 7 neurons exhibited a decrease in Rn (gray symbols) while 2 of 7 showed an increase in Rn (black symbols). Inset: plot of the delta Rn of the 5 neurons showing a decrease in Rn during eH2O2 and their delta Rn during the last 120 s of H2O2 exposure (lH2O2). B: representative trace of an individual neuron using the step protocol to determine Rn and relative changes in excitability. In this example a decreased Rn occurs during eH2O2 that rebounds during lH2O2. This change in Rn is accompanied by an initial drop in excitability that rebounds during lH2O2 as determined by the number of evoked action potentials during each phase. The decrease in evoked excitability during eH2O2 was observed in 5 of 7 neurons; 4 of these 5 neurons exhibited a rebound in lH2O2.
Fe2+-H2O2 does not cause a biphasic change in the frequency of respiratory activity from the preBötC and deferoxamine does not prevent augmentation caused by H2O2.
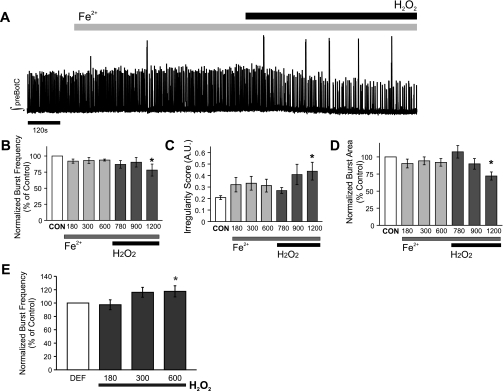
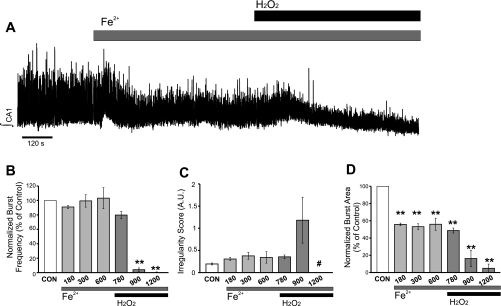
The following experiments were performed to assess the potential contribution of hydroxyl radical (•OH) generation by Fenton reaction to H2O2-induced neuronal changes in preBötC. To this end, brain slices containing preBötC were superfused with aCSF containing FeSO4 (100 μM; Fe2+). By itself, Fe2+ had no effect on the integrated burst area, pattern of regularity, or frequency of rhythmogenesis originating from the preBötC (Fig. 7A; n = 10). However, application of H2O2 in the presence of Fe2+ suppressed the frequency of fictive eupnea (78 ± 9% of control; Fig. 7B), leading to an increase in the irregularity score of finst from 0.2084 ± 0.016 to 0.4384 ± 0.077 (Fig. 7C). By the end of exposure to Fe2+-H2O2, integrated burst area was suppressed to 72 ± 6% of control (Fig. 7D).
Fig. 7.
Fe2+-H2O2 coapplication does not mimic the effects of H2O2 on rhythmogenesis from the preBötC, and deferoxamine does not prevent H2O2-mediated frequency augmentation. A: representative trace of the integrated population activity (∫) originating from the preBötC during control, supplementation of Fe2+, and addition of H2O2 (2.2 mM) in Fe2+-supplemented aCSF. Addition of H2O2 in the presence of Fe2+ yields •OH via Fenton chemistry. B–D: the effects of Fe2+ and •OH on burst frequency (B), irregularity score of finst (C), and integrated burst area (D) were determined by comparing a 120-s bin during control (CON) to bins ending at the elapsed times of 180, 300, 600 (Fe2+; light gray bars) and 780, 900, and 1,200 (Fe2+/H2O2; dark gray bars). n = 10. E: preexposure to the iron chelator deferoxamine (DEF, 500 μM) did not prevent the H2O2 (2.2 mM)-mediated frequency augmentation (n = 7 of 9). *P < 0.05.
In another series of experiments, the effects of deferoxamine (500 μM, n = 9), which chelates free Fe2+, on H2O2-induced neuronal changes in preBötC were tested. Application of deferoxamine (600 s) had no significant effect on the preBötC rhythm (P = 0.07). However, deferoxamine prevented the initial H2O2-mediated suppression of burst frequency but was unable to prevent the augmentation of burst frequency (118 ± 8%, P < 0.05; Fig. 7E).
H2O2 suppresses spontaneous bursting in the CA1 neuronal population.
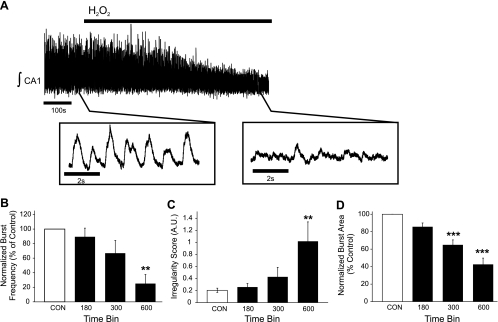
To determine whether the effects of H2O2 were regionally specific to the preBötC, the following studies were performed on CA1 neurons of the hippocampus exposed to either H2O2 or Fe2+-H2O2. H2O2 (2.2 mM bath application) suppressed spontaneous bursting activity of the CA1 neurons (Fig. 8A; n = 6). Integrated burst frequency decreased within the first bin during H2O2 exposure to 25 ± 13% (Fig. 8B), corresponding to the decrease in finst from 0.83 ± 0.08 Hz (during control) to 0.32 ± 0.14 Hz (in H2O2). By the end of the exposure, H2O2 increased irregularity score of finst from 0.20 ± 0.04 during control to 1.02 ± 0.32 in exposure (Fig. 8C) and reduced integrated burst area to 43 ± 7% (Fig. 8D). Although Fe2+ (100 μM) had no effect on burst frequency (Fig. 9B) or irregularity score of finst (Fig. 9C), it reduced the integrated burst area by 56 ± 7% of control (Fig. 9D). Addition of H2O2 to the Fe2+-supplemented aCSF suppressed burst frequency (4.03 ± 2.46% of control; Fig. 9B) and further suppressed integrated burst area (16 ± 19% of control; Fig. 9D). In a subset of experiments (n = 4), preexposure to deferoxamine (500 μM, 600 s) prevented the suppressive effects of H2O2 on the burst frequency from the CA1 neuronal population (data not shown).
Fig. 8.
H2O2 suppresses K+-induced population bursting in the CA1 neuronal population of the hippocampus. A: representative trace of the integrated activity from the CA1 neuronal population (∫ CA1) during control and in the presence of 2.2 mM H2O2. B–D: the effects of H2O2 on burst frequency (B), irregularity score of finst (C), and integrated burst area (D) were quantified by comparing 120-s bins during control (CON) to bins ending at 180, 300, and 600 s (black bars) during the 600-s exposure to H2O2. n = 6. **P < 0.01, ***P < 0.001.
Fig. 9.
Effects of Fe2+-H2O2 coapplication on K+-mediated population bursting in the CA1 neuronal population of the hippocampus. A: representative trace of the integrated population activity (∫ CA1) during control, supplementation of Fe2+, and addition of H2O2 (2.2 mM) in Fe2+-supplemented aCSF. Addition of H2O2 in the presence of Fe2+ yields •OH via Fenton chemistry. B–D: the effects of Fe2+-H2O2 on burst frequency (B), irregularity score of finst (C), and integrated burst area (D) were determined by comparing a 120-s bin during control (CON) to bins ending at the elapsed times of 180, 300, 600 (Fe2+; light gray bars) and 780, 900, and 1,200 (Fe2+-H2O2; dark gray bars). #Irregularity score of finst was not reported for time bin = 1,200 because ≤1 burst occurred during this bin window in 2 of 4 individual experiments. n = 4. **P < 0.01.
Effects of H2O2 on lipid peroxidation in CA1 and preBötC neurons.
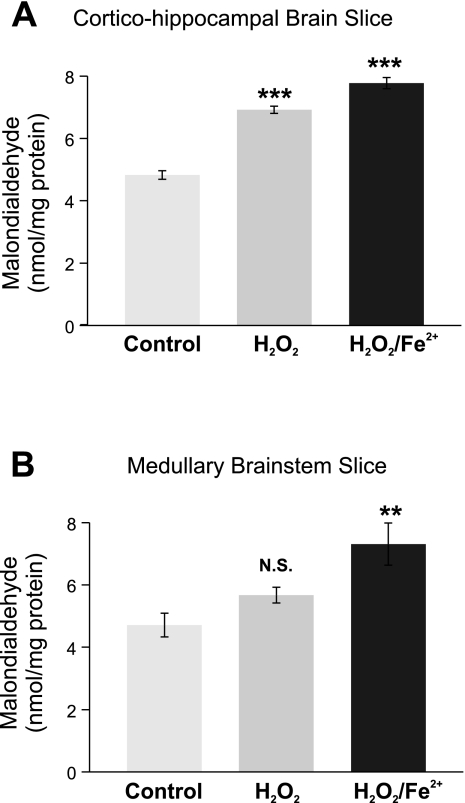
MDA levels were monitored as an index of lipid peroxidation (Peng et al. 2006; Ramanathan et al. 2005) in untreated (control), H2O2-treated (2.2 mM for 600 s), and Fe2+ (100 μM)-H2O2 (2.2 mM)-treated preBötC and cortico-hippocampal slices. Basal MDA levels were comparable between cortico-hippocampal brain slices and medullary brain slices (Fig. 10; n = 7 slices). MDA levels significantly increased in the cortico-hippocampal brain slices after exposure to either H2O2 (6.92 ± 0.12 nmol/mg protein, n = 6 slices) or Fe2+-H2O2 (7.78 ± 0.18 nmol/mg of protein, n = 7 slices; Fig. 10A). On the other hand, MDA levels in the brain stem slices treated with H2O2 (5.68 ± 0.25 nmol/mg protein, n = 6 slices; P value = 0.12) were similar to untreated control (4.71 ± 0.38 nmol/mg protein, n = 6 slices; Fig. 10B), whereas combined treatment with Fe2+-H2O2 caused a significant increase in MDA levels (7.78 ± 0.18 nmol/mg protein, n = 5 slices; P < 0.01; Fig. 10B).
Fig. 10.
While acute exposure to H2O2 or Fe2+-H2O2 increases lipid peroxidation in cortico-hippocampal brain slices, only Fe2+-H2O2 increases lipid peroxidation in brain stem slices. A: exposure to H2O2 (2.2 mM 600s; n = homogenates from 6 slices) or Fe2+-H2O2 (100 μM-2.2 mM 600s; n = homogenates from 7 slices) increased lipid peroxidation in cortico-hippocampal brain slices compared with time-matched untreated controls (n = homogenates from 7 slices). B: while exposure to Fe2+-H2O2 (100 μM-2.2 mM 600 s; n = homogenates from 5 slices) significantly increased lipid peroxidation, no significant increase (N.S.) in lipid peroxidation was observed during exposure to H2O2 (2.2 mM 600 s; n = homogenates from 6 slices) compared with time-matched untreated controls (n = homogenates from 6 slices). **P < 0.01, ***P < 0.001.
DISCUSSION
We tested the hypothesis that the neonatal central nervous system (CNS) responds to acute ROS exposure in differential and region-specific manners. We used experimental paradigms with various oxidants and juxtaposed the response to these paradigms in two different neonatal neuronal populations. The oxidants used here powerfully suppress population bursting in the CA1 region of the hippocampus. By contrast, H2O2 causes a biphasic change in the frequency of spontaneous bursting originating from the preBötC. Together, these novel findings lead to the general conclusion that the net action of ROS on neuronal function is dependent on the brain region and the type of ROS generated. The implications of this conclusion and these findings are discussed in further detail below.
Study considerations.
Simply defined, the increase in the concentration of H2O2 with either H2O2 alone or Fe2+-H2O2 could be considered a form of oxidative stress, as H2O2 in both paradigms shifts the redox state within the system to a more oxidized environment. Because of the abundance of oxidizable lipids in brain slices, lipid peroxidation, reflected by MDA levels, was the chosen metric for determining whether H2O2 or H2O2-Fe2+ significantly increased global oxidative stress (Peng et al. 2006; Ramanathan et al. 2005). Fe2+-H2O2 caused a significant shift in oxidative state and created measurable oxidative stress in both brain stem and cortico-hippocampal slices. H2O2 significantly increased lipid peroxidation in cortico-hippocampal slices. However, similar to observations reported in adult striatal slices (Chen et al. 2001), H2O2 did not significantly increase lipid peroxidation in brain stem slices. Thus H2O2 and Fe2+-H2O2 do not appear to produce the same type of oxidative state within the brain stem slice, and, furthermore, an increase in global oxidative stress does not appear to be requisite for exogenous H2O2 to profoundly impact on neuronal activity from the preBötC.
Modulation of rhythmogenesis from the neonatal preBötC.
Despite the minimal effect of H2O2 on lipid peroxidation in the brain stem slice, H2O2 caused a profound biphasic frequency effect on rhythmogenesis from the preBötC. However, H2O2 neither caused a sustained destabilization of burst to burst variability nor significantly affected integrated burst area, and upon washout the H2O2-mediated effects were reversible. Although iron chelation prevented the initial depression of frequency in the majority of experiments, the steady-state augmentation was attenuated but not blocked. Moreover, Fe2+-H2O2 could not mimic the effect of H2O2. Together these observations suggest that the effects of H2O2 on rhythmogenesis do not represent a generalized response to a shift in redox state, but rather H2O2 specifically modulates the frequency of respiratory activity from the preBötC.
We do, however, recognize that the observed effects of H2O2 on rhythmogenesis likely occur during and after nonphysiological conditions, such as that during and after apneas. In such conditions, H2O2 availability is presumably increased upon reoxygenation from hypoxia and coupled with minimal free Fe2+ availability. Thus the steady-state effect of exogenous H2O2 on the preBötC may be a functionally relevant response to an acute sudden increase in H2O2. Moreover, ROS are endogenously produced in local neuronal environments not only during upswings in oxygen but also during well-oxygenated conditions (Bao et al. 2009; D'Agostino et al. 2007), making it possible for baseline production of endogenous H2O2 to influence rhythmogenesis under more physiologically relevant conditions, but this remains to be determined.
Importance of local circuitry to effects of H2O2 on the preBötC and H2O2 sensitivity of preBötC neurons.
Rhythmicity originating from the preBötC is influenced by several presynaptic connections that are presumably still active in the brain stem slice. Bath application of H2O2 coupled with population level recordings limited the ability to discriminate between H2O2 sensitivity of presynaptic elements outside the preBötC and the direct intrinsic H2O2 sensitivity of preBötC neurons. To address this limitation, experiments using unilateral microinjection of H2O2 over the VRC respiratory islands and intracellular patch-clamp recordings were conducted. Microinjection of H2O2 over the VRC was capable of transiently suppressing burst frequency but unable to augment frequency. This suggests that the H2O2-induced augmentation may represent a more integrated response potentially involving medullary regions outside the preBötC. Indeed, experiments involving the much-reduced VRC islands demonstrated that H2O2 causes unpredictable changes in the respiratory frequency generated within the preBötC. In aggregate, these experiments show that 1) the preBötC-containing VRC region possesses a specific sensitivity to H2O2 but 2) the biphasic response is an integrated network response that potentially involves also presynaptic elements located throughout the respiratory circuit of the brain stem slice. Such areas may include input from not only the contralateral preBötC but also other regions such as the raphe, A1C1, and nucleus tractus solitarii. These other neuronal populations are potentially also sensitive to H2O2 and may indirectly influence the H2O2 response of rhythmogenesis from the preBötC.
The hypothesis that the H2O2 response emerges through an integrated network response is further corroborated by our observation that, on average, H2O2 did not significantly affect intrinsic membrane properties or excitability of the sampled preBötC neurons. However, there was a tendency for H2O2 to decrease Rn and alter excitability in the majority of these neurons. In this group, Rn and excitability initially decreased with H2O2, suggesting that H2O2 may be increasing the K+ conductance in these neurons. A potential candidate for H2O2 to increase K+ conductance in these neurons may be the KATP channel. The KATP channel has been shown to be influenced by H2O2 regulating excitability of midbrain neurons (Avshalumov et al. 2005). It is likely, however, that H2O2 has action on more than just one effector that influences neuronal excitability, since the influence of H2O2 on membrane properties of preBötC neurons and population rhythms from VRC islands is not uniform. The notion that H2O2 results in an integrated network response is reminiscent of the hypoxic response of the preBötC, which also involves a complex network reconfiguration during which the discharge pattern of respiratory neurons is differentially altered (Pena et al. 2004). When isolated from fast excitatory and inhibitory synaptic transmission, inspiratory preBötC neurons can be identified into two general groups (Pena et al. 2004)—nonpacemaking and pacemaking neurons. Pacemaking neurons can be further subdivided into cadmium-sensitive and cadmium-insensitive pacemaker neurons (Thoby-Brisson and Ramirez 2001; Pena et al. 2004). These different types of neurons are characterized not only by their heterogeneous membrane properties but also by their differential responsiveness to hypoxia. Thus it may not be surprising that H2O2 did not uniformly affect intrinsic membrane properties or excitability of individual preBötC neurons. Because our sampling of preBötC neurons was limited to nonpacemaking inspiratory neurons, it is currently unknown to what extent H2O2 affects the intrinsic membrane properties and excitability of other types of respiratory neurons including cadmium-sensitive and cadmium-insensitive pacemaker neurons. Future studies will need to characterize all types of respiratory neurons to gain insights into the network reconfiguration that governs the integrated H2O2 response.
Acute vulnerability of neonatal neuronal CA1 activity to oxidative environments.
Population activity in the adult CA1 region of the hippocampus is acutely sensitive to millimolar concentrations of H2O2 suppressing both potentiated and unpotentiated glutamatergic synaptic transmission via postsynaptic mechanisms (Avshalumov et al. 2000; Kamsler and Segal 2003; Pellmar 1986). Consistent with these reports, we showed that at millimolar concentrations H2O2 suppressed spontaneous population bursting in the CA1 region of the neonatal hippocampus. The acute effect of H2O2 appeared to be due, at least in part, to a generalized sensitivity of neonatal CA1 neurons to oxidative stress, as presynaptic bursting from the CA3 region was still observed during the H2O2-mediated depression of bursting from the CA1 region. This would be in agreement with a previous study in which the presynaptic volley from the adult CA3 neuronal population was unaffected by Fe2+-H2O2 (Pellmar 1987). However, while presynaptic action potential generation appears to be unaffected, it is possible that the amount of presynaptic neurotransmitter release decreases in oxidizing conditions (Pellmar 1986). Hence, the site of action of H2O2 in this neonatal neuronal circuit may involve presynaptic, postsynaptic, or some combination of both elements. Although our experiments did not discriminate the precise location of action, these experiments demonstrate that, as in the adult, activity originating from the neonatal CA1 neuronal population is suppressed by H2O2, which could be prevented with chelation of Fe2+ with deferoxamine. Because the effects of H2O2 could be mimicked by Fe2+-H2O2 and blocked by deferoxamine, it is likely that H2O2 and •OH (produced from Fe2+-H2O2) overlap in the mechanism(s) of action suppressing activity. Similarly, lipid peroxidation caused by either H2O2 alone or H2O2-Fe2+ was increased in neonatal cortico-hippocampal brain slices. In agreement with our findings, experiments from adult hippocampal brain slices showed that iron chelation prevents H2O2-mediated suppression of evoked activity from the adult CA1 neuronal population (Avshalumov et al. 2000). Hence, the neonatal CA1 neuronal population appears to have an acute sensitivity to a general shift in oxidative environments, responding in a manner consistent with that found in the adult population.
Rhythmogenesis from the preBötC in the presence of Fe2+-H2O2.
While under normal conditions Fe2+ is tightly regulated in the CNS (Moos 2002; Moos et al. 2007), a compromised blood-brain barrier potentially increases Fe2+ availability where the transition metal becomes readily available to catalyze H2O2 into the more deleterious species, •OH, leading to oxidative injury and damage (Halliwell 2006). For example, severe traumatic brain injury or stroke can damage the blood-brain barrier and often correlates with oxidative injury. The effect of H2O2 on the preBötC rhythm in such cases may be better reflected by the effects observed with Fe2+-H2O2 as opposed to the H2O2 paradigm. Fe2+-H2O2 suppressed frequency and integrated burst area but did not prevent the generation of rhythmogenesis from the preBötC. Compared with activity from the CA1 neuronal population, rhythmogenesis from preBötC appears to be substantially more resistant to oxidative environments. This relative resistance of the preBötC to oxidative modifications may involve differences in lipid composition, intrinsic antioxidant protection, or activity of repair enzymes between the regions studied here. While the basis of this relative resistance of rhythmogenesis from the preBötC to redox alterations is unknown, our observations emphasize the importance of continued network output from the preBötC in oxidative environments, as loss of activity would ultimately result in the cessation of breathing in vivo (McKay et al. 2005; Ramirez and Garcia 2007).
Neurophysiological implications of the action of H2O2.
Here we demonstrate that regional differences in response to acute changes in H2O2 exist between two areas in neonatal brain slices. While the activity from the neonatal CA1 neuronal population appears to share sensitivity to oxidative environments similar to its adult counterpart, rhythmogenesis from the preBötC appears to differentially respond to various oxidative environments. Whether this differential sensitivity is preserved in the adult is currently unknown. On an acute timescale, the sudden rise in ROS during reoxygenation may not always lead to immediate oxidative stress and injury. In the adult, ROS appear to be involved with long-term facilitation of the sensory and motor components of respiration (Macfarlane and Mitchell 2008; Peng et al. 2003). In these cases, ROS modulate respiratory control that may or may not lead to immediate oxidative stress. However, it is important to recognize that chronic states of intermittent hypoxia, such as that occurring in untreated sleep apnea and apneas of prematurity, often correlate to increased oxidative stress. Thus, in chronic conditions in which oxygen homeostasis oscillates even without significant changes in transition metal availability, ROS, like H2O2, can also overwhelm antioxidant defenses. In such chronic cases, oxidative stress can influence gene expression and alter physiology (Kumar et al. 2006; Peng et al. 2006; Yuan et al. 2004). These changes may ultimately redefine how a given area of the brain responds to fluctuations in ROS like H2O2. Hence, the neurophysiological response to ROS in a given brain region may be dynamically influenced by several factors including the timescale at which changes in ROS status are studied.
GRANTS
This study was supported by National Institutes of Health Grants RO1-HL/NS-68860 and P01-HL-090554-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Drs. Sebastien Zanella, Henner Koch, and Aguan Wei for their helpful suggestions and editorial comments.
REFERENCES
- Avshalumov M, Chen B, Koos T, Tepper J, Rice M. Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels. J Neurosci 25: 4222–4231, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov M, Chen B, Rice M. Mechanisms underlying H2O2-mediated inhibition of synaptic transmission in rat hippocampal slices. Brain Res 882: 86–94, 2000 [DOI] [PubMed] [Google Scholar]
- Bao L, Avshalumov M, Patel J, Lee C, Miller E, Chang C, Rice M. Mitochondria are the source of hydrogen peroxide for dynamic brain-cell signalling. J Neurosci 29: 9002–9010, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Avshalumov M, Rice M. H2O2 is a novel endogenous modulator of synaptic dopamine release. J Neurophysiol 85: 2468–2476, 2001 [DOI] [PubMed] [Google Scholar]
- D'Agostino D, Putnam R, Dean J. Superoxide (•O2) production in CA1 neurons of rat hippocampal slices exposed to graded levels of oxygen. J Neurophysiol 98: 1030–1041, 2007 [DOI] [PubMed] [Google Scholar]
- Giniatullin A, Giniatullin R. Dual action of hydrogen peroxide on synaptic transmission at the frog neuromuscular junction. J Physiol 552.1: 283–293, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem 97: 1634–1658, 2006 [DOI] [PubMed] [Google Scholar]
- Johnson S, Koshiya N, Smith J. Isolation of the kernel for respiratory rhythm generation in a novel preparation: the pre-Bötzinger complex “island.” J Neurophysiol 85: 1772–1776, 2001 [DOI] [PubMed] [Google Scholar]
- Kamsler A, Segal M. Hydrogen peroxide modulation of synaptic plasticity. J Neurosci 23: 269–276, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp L, Klann E. Role of reactive oxygen species in hippocampal long-term potentiation: contributory or inhibitory? J Neurosci Res 70: 1–7, 2002 [DOI] [PubMed] [Google Scholar]
- Kolbe K, Schonherr R, Gessner G, Sahoo N, Hoshi T, Heinemann SH. Cysteine 723 in the C-linker segment confers oxidative inhibition of hERG1 potassium channels. J Physiol 588: 2999–3009, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G, Rai V, Sharma S, Ramakrishnan D, Peng Y, Souvannakitti D, Prabhakar N. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol 575: 229–239, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieske S, Thoby-Brisson M, Ramirez J. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs, gasps. Nat Neurosci 3: 600–607, 2000 [DOI] [PubMed] [Google Scholar]
- Macfarlane P, Mitchell G. Respiratory long-term facilitation following intermittent hypoxia requires reactive oxygen species formation. Neuroscience 152: 189–197, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L, Janczewski W, Feldman J. Sleep-disordered breathing after targeted ablation of preBotzinger complex neurons. Nat Neurosci 8: 1142–1144, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos T. Brain iron homeostasis. Dan Med Bull 49: 279–301, 2002 [PubMed] [Google Scholar]
- Moos T, Nielsen T, Skjorringe T, Morgan E. Iron trafficking inside the brain. J Neurochem 103: 1730–1740, 2007 [DOI] [PubMed] [Google Scholar]
- Pawar A, Nanduri J, Guoxiang Y, Khan S, Wang N, Kumar G, Prabhakar N. Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 296: R735–R742, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellmar T. Electrophysiological correlates of peroxide damage in guinea pig hippocampus in vitro. Brain Res 364: 377–381, 1986 [DOI] [PubMed] [Google Scholar]
- Pellmar TC. Peroxide alters neuronal excitability in the CA1 region of guinea-pig hippocampus in vitro. Neuroscience 23: 447–456, 1987 [DOI] [PubMed] [Google Scholar]
- Pena F, Parkis M, Tryba A, Ramirez J. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron 43: 105–117, 2004 [DOI] [PubMed] [Google Scholar]
- Peng Y, Overholt J, Kline D, Kumar G, Prabhakar N. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA 100: 10073–10078, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Yaun G, Ramakrishnan D, Sharma S, Bosch-Marce M, Kumar G, Semenza G, Prabhakar N. Heterozygous HIF-1α deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577: 705–716, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK, Prabhakar NR. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci 29: 4903–4910, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone S, Negro S, Tataranno ML, Buonocore G. Oxidative stress and antioxidant strategies in newborns. J Matern Fetal Neonatal Med 23, Suppl 3: 63–65, 2010 [DOI] [PubMed] [Google Scholar]
- Poets C. Interventions for apnoea of prematurity: a personal view. Acta Paediatr 99: 172–177, 2010 [DOI] [PubMed] [Google Scholar]
- Raghuraman G, Kalari A, Dhingra R, Prabhakar NR, Kumar GK. Enhanced neuropeptide Y synthesis during intermittent hypoxia in the rat adrenal medulla: role of reactive oxygen species-dependent alterations in precursor peptide processing. Antioxid Redox Signal 14: 1179–1190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan L, Gozal D, Siegel J. Antioxidant responses to chronic hypoxia in the rat cerebellum. J Neurochem 93: 47–52, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J, Garcia A., III Point:Counterpoint: Medullary pacemaker neurons are essential for both eupnea and gasping in mammals vs. medullary pacemaker neurons are essential for gasping, but not eupnea, in mammals. J Appl Physiol 103: 717–718, 2007 [DOI] [PubMed] [Google Scholar]
- Ramirez J, Quellmalz U, Richter D. Postnatal changes in the mammalian respiratory network as revealed by the transverse brainstem slice of mice. J Physiol 491: 799–812, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugstard O. Resuscitation of the asphyxic newborn infant: new insight leads to new therapeutic possibilities. Biol Neonate 79: 258–260, 2001a [DOI] [PubMed] [Google Scholar]
- Saugstard O. Update on oxygen radical disease in neonatology. Curr Opin Obstet Gynecol 12: 147–153, 2001b [DOI] [PubMed] [Google Scholar]
- Schlief T, Schonherr R, Heinemann SH. Modification of C-type inactivating Shaker potassium channels by chloramine-T. Pflügers Arch 431: 483–493, 1996 [DOI] [PubMed] [Google Scholar]
- Telgkamp P, Cao Y, Basbaum A, Ramirez J. Long-term deprivation of substance P in PPT-A mutant mice alters the anoxic response of the isolated respiratory network. J Neurophysiol 88: 206–213, 2002 [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Ramirez JM. Identification of two types of inspiratory pacemaker neurons in the isolated respiratory neural network of mice. J Neurophysiol 86: 104–112, 2001 [DOI] [PubMed] [Google Scholar]
- Varma S, Janesko K, Wisniewski S, Bayir H, Adelson P, Thomas N, Kochanek P. F2-isoprostane and neuron specific enolase in cerebrospinal fluid after severe traumatic brain injury in infants and children. J Neurotrauma 8: 781–786, 2003 [DOI] [PubMed] [Google Scholar]
- Yuan G, Adhikary G, McCormick A, Holcroft J, Kumar G, Prabhakar N. Role of oxidative stress in intermittent hypoxia-induced immediate early gene activation in rat PC12 cells. J Physiol 557: 773–783, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]