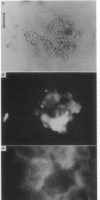
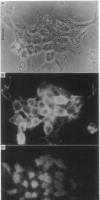
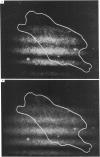
Abstract
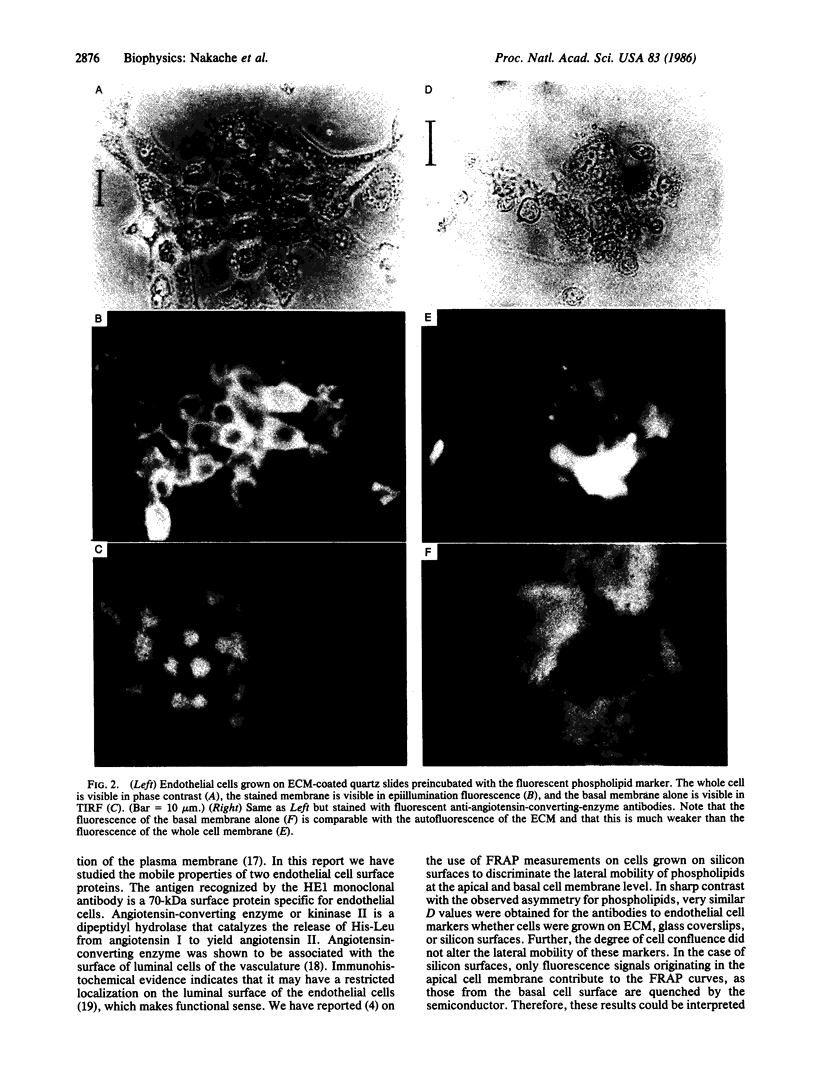
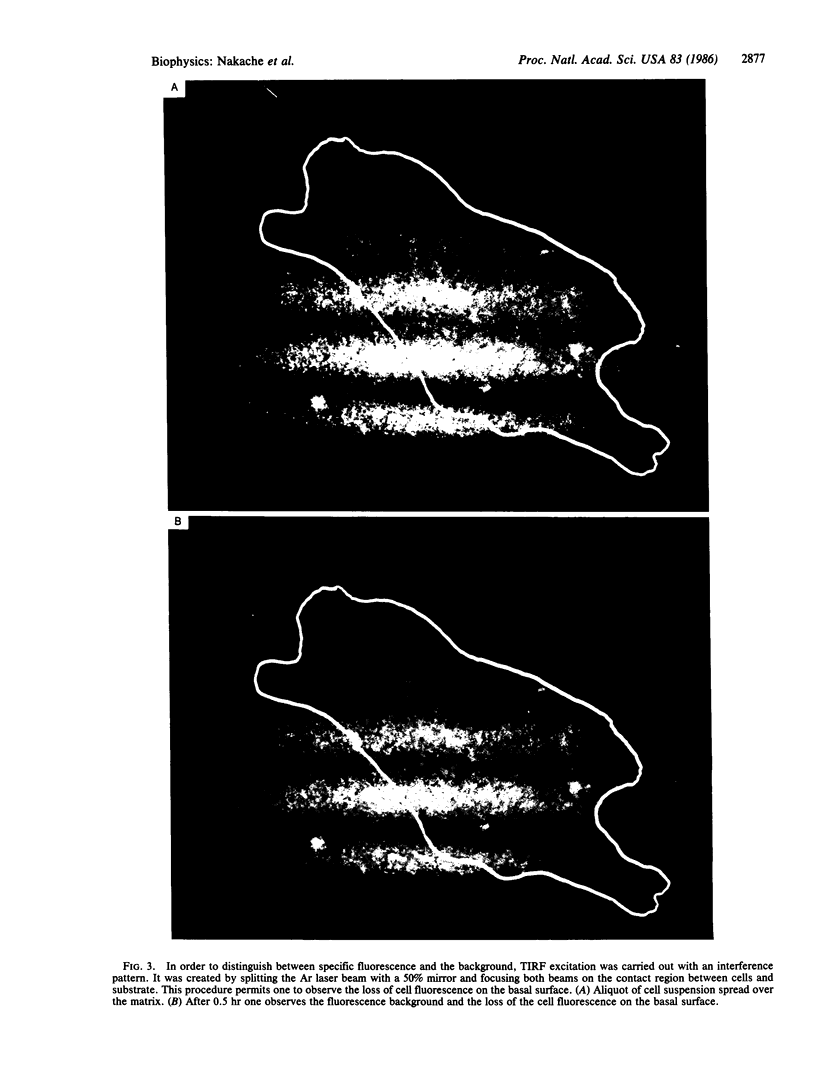
The mobility and distribution of angiotensin-converting enzyme (peptidyl-dipeptide hydrolase, EC 3.4.15.1) and a specific endothelial cell surface protein was assessed by fluorescein-conjugated monoclonal antibodies on bovine and murine endothelial cells grown on their extracellular matrix. The combination of data obtained from fluorescence recovery after photobleaching measurements and observations under epifluorescence and total internal reflection fluorescence reveals a restriction of these protein markers to the apical membrane of endothelial cell. This asymmetry is evident both when cells are grown at a sparse density or at confluence. When cells are brought into suspension, the fluorescein-conjugated antibody is found over the entire cell surface. The fluorescence disappears from the basal part of the cell when the cells are again spread on coverslips coated with a layer of extracellular matrix. Conversely, cells spread on glass coverslips without extracellular matrix do not show this restriction phenomenon. It is suggested that the extracellular matrix provides the signal to induce the restricted topology of membrane protein markers on endothelial cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Stirling C. Distribution of transport proteins over animal cell membranes. J Membr Biol. 1984;77(3):169–186. doi: 10.1007/BF01870567. [DOI] [PubMed] [Google Scholar]
- Auerbach R., Alby L., Grieves J., Joseph J., Lindgren C., Morrissey L. W., Sidky Y. A., Tu M., Watt S. L. Monoclonal antibody against angiotensin-converting enzyme: its use as a marker for murine, bovine, and human endothelial cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7891–7895. doi: 10.1073/pnas.79.24.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avnur Z., Geiger B. Substrate-attached membranes of cultured cells isolation and characterization of ventral cell membranes and the associated cytoskeleton. J Mol Biol. 1981 Dec 5;153(2):361–379. doi: 10.1016/0022-2836(81)90283-7. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Burghardt T. P., Thompson N. L. Total internal reflection fluorescence. Annu Rev Biophys Bioeng. 1984;13:247–268. doi: 10.1146/annurev.bb.13.060184.001335. [DOI] [PubMed] [Google Scholar]
- Axelrod D. Lateral motion of membrane proteins and biological function. J Membr Biol. 1983;75(1):1–10. doi: 10.1007/BF01870794. [DOI] [PubMed] [Google Scholar]
- Caldwell P. R., Seegal B. C., Hsu K. C., Das M., Soffer R. L. Angiotensin-converting enzyme: vascular endothelial localization. Science. 1976 Mar 12;191(4231):1050–1051. doi: 10.1126/science.175444. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion and the molecular processes of morphogenesis. Annu Rev Biochem. 1985;54:135–169. doi: 10.1146/annurev.bi.54.070185.001031. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Fraser S. E., Poo M. Development, maintenance, and modulation of patterned membrane topography: models based on the acetylcholine receptor. Curr Top Dev Biol. 1982;17(Pt 3):77–100. doi: 10.1016/s0070-2153(08)60519-0. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Monoclonal antibodies to sucrase/isomaltase: probes for the study of postnatal development and biogenesis of the intestinal microvillus membrane. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6629–6633. doi: 10.1073/pnas.77.11.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P., Schindler M. Matrix control of protein diffusion in biological membranes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3576–3580. doi: 10.1073/pnas.78.6.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakache M., Schreiber A. B., Gaub H., McConnell H. M. Heterogeneity of membrane phospholipid mobility in endothelial cells depends on cell substrate. Nature. 1985 Sep 5;317(6032):75–77. doi: 10.1038/317075a0. [DOI] [PubMed] [Google Scholar]
- Pisam M., Ripoche P. Redistribution of surface macromolecules in dissociated epithelial cells. J Cell Biol. 1976 Dec;71(3):907–920. doi: 10.1083/jcb.71.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J. Intramembranous particle distribution at the node of Ranvier and adjacent axolemma in myelinated axons of the frog brain. J Neurocytol. 1976 Dec;5(6):731–745. doi: 10.1007/BF01181584. [DOI] [PubMed] [Google Scholar]
- Ryan J. W., Ryan U. S., Schultz D. R., Whitaker C., Chung A. Subcellular localization of pulmonary antiotensin-converting enzyme (kininase II). Biochem J. 1975 Feb;146(2):497–499. doi: 10.1042/bj1460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. A., McConnell H. M. Determination of molecular motion in membranes using periodic pattern photobleaching. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2759–2763. doi: 10.1073/pnas.75.6.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]