Abstract
Spatial and temporal properties of head movement are encoded by vestibular hair cells in the inner ear. One of the most striking features of these receptors is the orderly structural variation in their mechanoreceptive hair bundles, but the functional significance of this diversity is poorly understood. We tested the hypothesis that hair bundle structure is a significant contributor to hair bundle mechanics by comparing structure and steady-state stiffness of 73 hair bundles at varying locations on the utricular macula. Our first major finding is that stiffness of utricular hair bundles varies systematically with macular locus. Stiffness values are highest in the striola, near the line of hair bundle polarity reversal, and decline exponentially toward the medial extrastriola. Striolar bundles are significantly more stiff than those in medial (median: 8.9 μN/m) and lateral (2.0 μN/m) extrastriolae. Within the striola, bundle stiffness is greatest in zone 2 (106.4 μN/m), a band of type II hair cells, and significantly less in zone 3 (30.6 μN/m), which contains the only type I hair cells in the macula. Bathing bundles in media that break interciliary links produced changes in bundle stiffness with predictable time course and magnitude, suggesting that links were intact in our standard media and contributed normally to bundle stiffness during measurements. Our second major finding is that bundle structure is a significant predictor of steady-state stiffness: the heights of kinocilia and the tallest stereocilia are the most important determinants of bundle stiffness. Our results suggest 1) a functional interpretation of bundle height variability in vertebrate vestibular organs, 2) a role for the striola in detecting onset of head movement, and 3) the hypothesis that differences in bundle stiffness contribute to diversity in afferent response dynamics.
Keywords: mechanics, utricle, turtle
the utricle is a mechanoreceptive organ in the vestibular labyrinth of vertebrates that detects linear head accelerations in the horizontal plane and head tilt with respect to gravity. Its sensory epithelium, the macula, is a sheet of receptors separated by supporting cells. These receptors are called hair cells because of the hairlike processes (the hair bundle) that extend upward from the hair cells' apical surfaces. The hair bundles are surrounded by an otoconial membrane (OM), which comprises three layers (Kachar et al. 1990): a layer of otoconia (the otoconial layer, OL), a compact gel layer (GL), and a column filament layer (CFL) that extends from the underside of the GL to the apical surface of the macula. Hair bundles occupy cylindrical interruptions in the CFL. When the head moves, the OM shears relative to the macular surface, causing the hair bundles to deflect. Bundle deflection alters the open probability of mechanically gated transduction channels, which modulates the hair cell membrane potential and, thus, the firing rate of postsynaptic afferents. This modulation of afferent spiking is the signal that vestibular organs send to the central nervous system.
The OM, hair cells, and afferents are spatially heterogeneous (see for reviews Eatock and Lysakowski 2006; Eatock and Songer 2011; Fermin et al. 1998; Furness and Hackney 2006; Lysakowski and Goldberg 2004; Popper and Platt 1993). Their properties often covary, suggesting that the utricle may be subdivided into regions that play different roles in encoding and signaling head movement. One of the most striking forms of regional variation is seen in the structure of hair bundles. Each hair bundle is composed of a single microtubule-based kinocilium and multiple actin-based stereocilia, all interconnected by filamentous links (Furness and Hackney 2006; Nayak et al. 2007). Hair bundle structure (e.g., bundle shapes, heights, stereocilia numbers, stereocilia spacing) varies greatly between species, between vestibular endorgans (e.g., utricle vs. semicircular canals), and within the same endorgan at different epithelial loci. This structural variation has been documented in a wide range of aquatic and terrestrial vertebrates (Lewis et al. 1985; Platt 1983). Its functional role is unclear, but theoretical arguments suggest it may lead to differences in hair bundle stiffness (Howard et al. 1988).
Bundle stiffness (force/unit deflection), a measure of a bundle's resistance to deflection, is a fundamental mechanical property that shapes a hair cell's response to mechanical stimuli caused by head movement. For purposes of analysis, it is useful to distinguish between dynamic and steady-state contributions to bundle stiffness. A variety of dynamic processes modulate bundle stiffness during mechanotransduction, and considerable attention has been devoted to understanding their underlying mechanisms, particularly in auditory organs or quasi-auditory organs such as frog saccule (see for reviews Fettiplace and Ricci 2006; Hudspeth 2008; Vollrath et al. 2007). Much less is known about determinants of steady-state stiffness. This is unfortunate because steady-state stiffness is important for vestibular organs, such as the utricle, that must transduce the lower-frequency signals associated with head movements (usually below 100 Hz) as well as static head tilts.
Steady-state stiffness is thought to arise from the structure and material properties of hair bundles (see for review Howard et al. 1988), but there have been few systematic attempts to investigate this hypothesis. There are many reports of steady-state hair bundle stiffness of both cochlear and vestibular bundles from a variety of species (Ashmore 1984; Bashtanov et al. 2004; Crawford and Fettiplace 1985; Denk et al. 1989; Flock and Strelioff 1984; Géléoc et al. 1997; Howard and Ashmore 1986; Howard and Hudspeth 1987; Jaramillo and Hudspeth 1993; Kössl et al. 1990; Marquis and Hudspeth 1997; Strelioff and Flock 1984; Szymko et al. 1992). With limited exceptions (Flock and Strelioff 1984; Géléoc et al. 1997; Howard and Ashmore 1986; Ricci et al. 2000; see discussion) these studies measured the stiffness of bundles with similar structures, so the role of bundle structure in shaping steady-state stiffness is still unclear.
In this study, we took advantage of the orderly variation in hair bundle structure across the turtle macula (Fontilla and Peterson 2000; Jorgensen 1974, 1988; Moravec and Peterson 2004; Rowe and Peterson 2006; Severinsen et al. 2003; Xue and Peterson 2006) to examine covariation of bundle structure and stiffness. We had two goals. First, we sought to learn how bundle stiffness varies with epithelial locus, because this may shed light on the role of different macular regions in detecting and encoding head movements. Our second goal was to learn which features of hair bundle structure best predict steady-state stiffness. This is important for understanding determinants of bundle stiffness, and, in addition, such correlated information about bundle structure and stiffness can provide a valuable test of stiffness predictions from computational models of hair bundles having different structures. We reported some of these data previously in abstract form (Moravec et al. 2005; Spoon et al. 2005).
MATERIALS AND METHODS
Tissue Preparation
We tested bundles from isolated utricular maculae of turtles (Trachemys scripta elegans) of both sexes (carapace lengths: 3.75–5.75 in.). We euthanized turtles via 0.5-ml injections of Euthosal (390 mg pentobarbital sodium and 50 mg phenytoin sodium per ml) in accordance with protocols reviewed and approved by the Virginia Tech Institutional Animal Care and Use Committee, extracted the utricles, and maintained them at room temperature in oxygenated Hanks' balanced salt solution (without phenol red; in mM: 1.26 CaCl2, 0.493 MgCl2, 0.407 MgSO4, 5.33 KCl, 0.441 KH2PO4, 4.17 NaHCO3, 137.93 NaCl, 0.338 Na2HPO4, 5.56 d-glucose; pH of 7.2, osmolarity of 300 mosM) buffered with 10 mM HEPES, hereafter referred to as buffered HBSS. With the OM intact, we folded utricles along a central medio-lateral transect (Fig. 1A) and pinned them in an experimental chamber. Then we used an eyelash to gently peel the OM away without subtilisin proteinase digestion because it is known to damage interciliary links that affect bundle stiffness (Bashtanov et al. 2004; Goodyear and Richardson 1999; Jacobs and Hudspeth 1990; Karavitaki and Corey 2010). This preparation provided a side view of the hair bundles (Fig. 1B) from which we could measure bundle stiffness, dimensions, and distances from the line of hair cell polarity reversal (LPR; Fig. 1B). We viewed bundles with differential interference contrast (DIC) optics, using a Zeiss Axioskop with ×10 eyepieces, a ×100 immersible objective (1.0 NA), and an oil immersion condenser (1.4 NA). The microscope was located on a vibration isolation table. We restricted measurements of bundle stiffness and dimensions to hair cells that appeared healthy, with no signs of swelling, blebbing, or damage to the hair bundle. Finally, we varied the starting position of stiffness measurements (relative to the LPR) across experimental sessions to ensure that differences in measured stiffness values were not confounded by the passage of time. In this way we measured the stiffness of 48 hair bundles in the striola, 23 bundles in the medial extrastriola (MES), and 2 bundles in the lateral extrastriola [LES; the LES is covered by a “shelf,” i.e., a connective tissue falx (see Fig. 2A in Xue and Peterson 2006), which makes these bundles difficult to access]. Here and elsewhere, the word “bundle” refers to both kinocilium and stereocilia and “bundle stiffness” includes contributions of kinocilium and stereocilia.
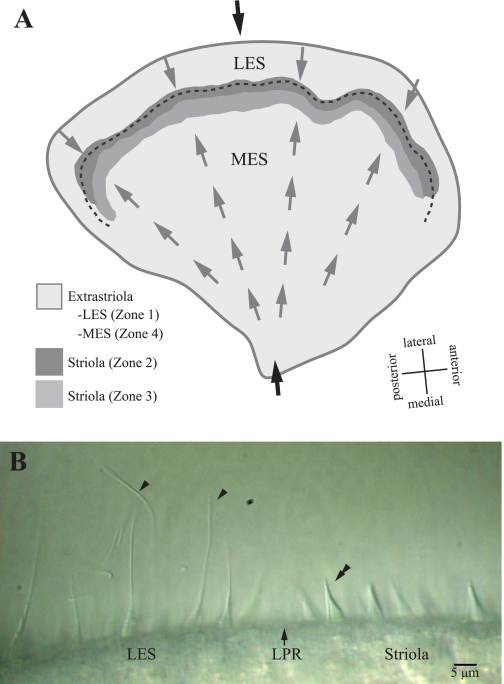
Fig. 1.
Turtle utricle. A: the macula is divided into 4 zones. Zone 1 corresponds to the lateral extrastriola (LES). Zones 2 and 3 form the striola: zone 2 (dark gray shading) is a 30- to 40-μm-wide band of type II hair cells with distinctive bundles; zone 3 (medium gray shading) is a 50- to 75-μm-wide band of type I hair cells. Zone 4 corresponds to the medial extrastriola (MES). The juxtastriola (not labeled) is an ∼100-μm-wide band just medial to zone 3. Hair bundle dimensions change gradually across zone 3 and the juxtastriola, from zone 2-like to zone 4 (MES)-like. The shapes and relative heights of bundles in different zones are summarized in Fig. 9 of Xue and Peterson (2006). Gray arrows indicate hair bundle polarity (axis of maximal sensitivity), and the dashed line indicates the line of polarity reversal (LPR). This LPR runs through zone 2, close to its lateral margin. Black arrows indicate the medial-to-lateral transect along which we folded the utricle to make stiffness measurements; it includes all macular subdivisions. B: hair bundles at the LPR. This differential interference contrast image shows the morphology of hair bundles immediately lateral (toward left in image) and medial (toward right) to the LPR (arrow). The LPR lies near the lateral edge of the striola and defines the line about which the excitatory directions of the hair bundles reverse orientation. Arrowheads mark 2 of the long kinocilia coupled to short stereocilia that are characteristic of LES hair bundles. The double arrowhead indicates a typical zone 2 hair bundle with a short kinocilium approximately as tall as the tallest stereocilia (KS ratio ∼1). Image contrast adjusted with ImageJ software.
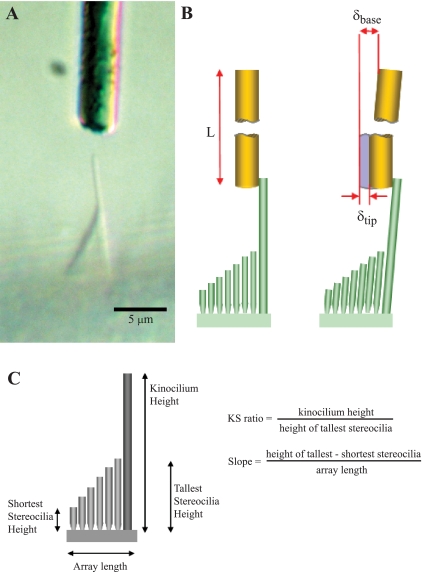
Fig. 2.
Measuring bundle stiffness and bundle dimensions. A: a bundle along the folded edge of the macula is viewed from the side, and a horizontally oriented flexible glass fiber (gold rod) is advanced toward the kinocilium. This bundle was 73 μm from the LPR (zone 3). Image contrast adjusted with ImageJ software. B, left. The glass fiber is brought into contact with the tip of the kinocilium. The fiber's length (L) is much greater than the height of the kinocilium. Right: displacement of the fiber base (δbase) results in bending of the bundle and, thus, displacement of the fiber tip (δtip). We measured displacement of the fiber base with an interferometer and displacement of the fiber/kinocilium tip with photodiodes. C: bundle dimensions. Schematic hair bundle in side view showing the 4 bundle dimensions measured in this study and 2 variables calculated from these measurements (KS ratio and Slope). We measured stiffness of the same bundles to investigate the contributions of bundle structure to bundle stiffness.
Stiffness Measurements
We used a well-established force-deflection technique to measure steady-state stiffness (Fig. 2, A and B; Ashmore 1984; Crawford and Fettiplace 1985; Howard and Ashmore 1986; Howard and Hudspeth 1987, 1988; Kössl et al. 1990; Jaramillo and Hudspeth 1993; Strelioff and Flock 1984). We deflected bundles by placing the tip of a flexible glass fiber in contact with the distal end of the kinocilium and displacing the base of the fiber. Fabrication, calibration, and selection of glass fibers are described in Glass Fibers, below. From the fiber's base displacement (Fig. 2B; δbase) and the resulting bundle deflection (Fig. 2B; δtip), we determined the deflection of the fiber (δF) (Eq. 1).
| (1) |
Stiffness (k) is defined as force per unit deflection, k = F/δ, where the force (F) is applied at the tip of the bundle and deflection under the action of this force is measured at the tip. Equating the contact force between the bundle and fiber, kBδtip = kFδF, where kB and kF are the stiffness of the bundle and fiber, respectively, and combining with Eq. 1 gives the expression used to calculate bundle stiffness:
| (2) |
We measured displacements of the fiber tip (Eq. 2: δtip), using a paired set of light-sensitive photodiodes (dual-element silicon photodetectors) housed on the camera port of the microscope, and we aligned the gap separating these rectangular photodiodes (0.127 mm) with the magnified (×100) image of the fiber. As the image of the glass fiber moves from one photodiode to the other during bundle deflection, their differential output voltage is linearly proportional to the displacement of the fiber. We determined the linear range of displacement voltages in each experiment and used only this range.
We oscillated the fiber base sinusoidally, using a piezoelectric linear actuator (Burleigh, PZS-050) with a peak-to-peak amplitude of 1 μm and a frequency of 0.5 Hz. At this frequency, fluid drag did not inhibit fiber movement (δbase = δtip) with the fiber immersed only in fluid and not in contact with a bundle. We measured displacements of the fiber's base (Eq. 2: δbase) with a resolution of 1 nm, using a fiber-optic displacement sensor [extrinsic Fabry-Perot interferometer (EFPI), Luna Innovations, Blacksburg, VA] that was secured to the linear actuator. We brought the fiber into contact with bundles projecting from the folded edge of the macula such that the long axis of the fiber was parallel with that of the kinocilia (Fig. 2B). This orientation was necessary to prevent the fiber from contacting more than one bundle. We positioned the tip of the fiber as close to the top of the bundle as possible while still maintaining contact, typically 1–2 μm from the top. This was necessary to maintain contact with the bundle and generate reproducible stiffness measurements. In early experiments we attempted other methods of applying force to the bundle, but these were not successful. We were unable to deflect bundles reliably by pulling on the kinocilium, a method that has been used successfully on the bulbed kinocilia of frog saccular hair cells (see, e.g., Karavitaki and Corey 2010). In some experiments, we also attempted to move the probe down the kinocilium to contact the tallest stereocilia (for bundles with kinocilia much taller than the stereocilia), but under these conditions it proved impossible to identify the effective point of force application with certainty. For all these reasons, we limited force application to pushing the bundle tip. In most cases, the probe contacted the bundle at the tip of the kinocilium; where the tallest stereocilia were as tall as the kinocilium, the probe also contacted the tips of a few tall stereocilia. We pushed bundles in their excitatory direction from their resting position with each oscillation (Fig. 3).
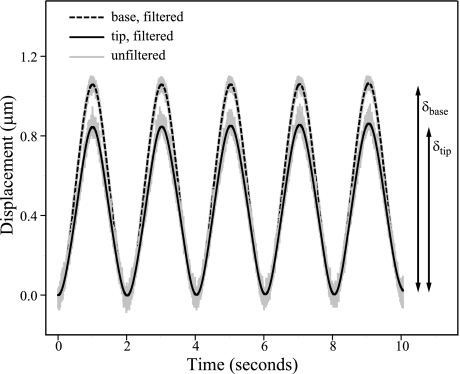
Fig. 3.
Sinusoidal displacement of bundle tip by probe. We oscillated the base of the glass fiber at 0.5 Hz, and we measured its displacement (dashed black line) with a fiber-optic sensor. We measured the displacement of the fiber tip (solid black line), which was in contact with the bundle tip, using a pair of photodiodes. The minimum of the sine wave corresponds with the resting position of the bundle and the maximum to the peak displacement in the bundle's excitatory direction. The peak-to-peak amplitude of the fiber's base (δbase) and tip (δtip) were used to calculate stiffness (Eq. 2). Unfiltered recordings of both base and tip displacements are shown in gray. The measurement shown is from a striolar hair cell located just medial to the line of polarity reversal, i.e., in zone 2 (K = 102 ± 14 μN/m).
The oscillating fiber was in contact with the bundle during each 30-s trial. Immediately after each trial, we retracted the fiber to a position just above the bundle in the same horizontal plane. Then we oscillated the uninhibited fiber at the same amplitude and frequency for 30 s to calibrate the photodiodes (i.e., to determine the change in photodiode voltage per change in fiber position). We recorded signals from both photodiodes and EFPI in LabVIEW 7.0 (National Instruments) and sampled them at 1 kHz. The data were filtered in MATLAB 7.0.4 (The MathWorks) with a 4th-order low-pass digital Butterworth filter. We also used MATLAB to extract peak-to-peak displacements of the fiber's base and tip from the recorded data. Finally, we calculated bundle stiffness from the deflection during each oscillation and averaged across oscillations to determine bundle stiffness from that trial. We performed at least three trials (30 s each) on every tested hair bundle.
This procedure yields a steady-state measurement of bundle stiffness (kB). Because kinocilium and stereocilia move as a unit when a hair bundle is deflected by a force applied to the kinocilium (Karavitaki and Corey 2010; Kozlov et al. 2007), kB reflects the contribution of both kinocilium and stereocilia. The time course of bundle deflections was 1 s, which is 20–100 times slower than that required for active responses of the bundle, i.e., slow and fast adaptation (Eatock et al. 1987; Holt et al. 2002; Ricci et al. 1998). Such dynamic changes in stiffness are measured within 0.75–1.25 ms after deflection (Howard and Hudspeth 1987, 1988; Jaramillo and Hudspeth 1993; Marquis and Hudspeth 1997; Ricci et al. 2000). Thus tip links were tensioned, but bundles were fully adapted at the peak of displacement, where we made our measurements. Because no dynamic changes were occurring, our measurements reflected steady-state bundle stiffness.
To estimate stiffness in vivo, i.e., the stiffness of hair cell bundles at the height of their attachment to the OM, we calculated what we call physiological stiffness (kP), using Eq. 3,
| (3) |
where ha is the height of force application and he is the height of OM attachment above the bundle's insertion. Values of he in turtle utricle were measured in a previous confocal microscopy study that measured the height at which individual kinocilia attach to the gel layer of the OM along the same medial-to-lateral transect that we used in our stiffness experiments (Xue and Peterson 2003). Preliminary estimates of he are reported in Davis et al. (2007). Equation 3 is based on a model in which the bundle acts as a rigid rod pivoting about a rotational spring with a rotational stiffness (kR) equal to kBha2 (Crawford and Fettiplace 1985; Howard and Ashmore 1986). This relationship assumes that the bundle's angular rotation is small, that kR is constant for a given bundle, and that the stereocilia and kinocilia do not bend. In the zone 4 and zone 1 bundles, the kinocilium is much taller than the stereocilia and likely bends during OM deflection, violating the rigid bar assumption (Spoon and Grant 2011). This introduces error in determining kP, which is small for zone 4 bundles where ha is close to he and larger in zone 1 where ha is much greater than he. This error has the effect of underestimating kP in zone 1 by at least a factor of four.
Glass Fibers
We made the flexible glass fibers used for stiffness measurements from borosilicate glass rods pulled to tip diameters of 2.3–5.9 μm and trimmed to lengths of 1.34–4.12 mm. We designed glass fibers to have stiffness values (19–433 μN/m) comparable to the stiffness of measured hair bundles. We also sputter coated the fibers with 100–200 Å of gold to make the fiber opaque and thus improve its optical contrast; this produced a threefold increase in the photodiode linear operating region.
Calibration.
We measured the stiffness of the glass fibers (Eq. 4: kF) by statically attaching polymethyl methacrylate microbeads (30- to 100-μm diameter; Bangs Laboratory, no. BB05N) to the horizontally positioned fiber. Then, while viewing the fiber with a microforge at ×35, we measured its vertical deflection (δ) and length (l), the bead diameters, and the distance of the beads from the fiber tip (ai). We determined the fiber's stiffness (kF) from Eq. 4
| (4) |
for a cantilevered beam with multiple point loads, and we calculated the weight of each bead (Wi) from the bead's diameter and known density (1,190 kg/m3). The number of beads attached to the fiber (n) typically ranged from 1 to 4.
To validate this method of determining fiber stiffness, we used an equation representing a cantilevered beam (Eq. 5)
| (5) |
based on the Young's modulus of glass (E), fiber length (L), and diameter (d). Equation 5 assumes that the diameter is constant along the length. We fabricated three fibers from fiberglass insulation because these fibers have nearly constant diameters (≈10 μm, determined by measuring diameters at several locations along their length). These fibers had a Young's modulus of 77 GPa (Wallenberger et al. 2001). We compared their calculated stiffnesses (from Eq. 5) with their bead-calibrated stiffness (Eq. 4). Results from the two techniques differed by 8–18%, with the highest difference occurring for the fiber with greatest longitudinal variation in diameter (SD ± 0.34 μm). This estimates the error associated with bead calibrations.
Selection.
To measure hair bundle stiffness accurately, the relative stiffnesses of forcing fiber and hair bundle must fall within a restricted range: the fiber must be compliant enough to flex slightly when deflecting the bundle, but stiff enough to bend the bundle. To determine the optimal ratio of bundle to forcing fiber stiffness, we paired seven bead-calibrated fibers (k = 7.6–1,107 μN/m) with each other 17 ways (with one fiber as the forcing fiber and one fiber as the stationary fiber) to produce ratios of forcing fiber to stationary fiber stiffness (kF/kS) ranging from 0.01 to 22.11. Then we used the force-deflection technique to measure the stiffness of each stationary glass fiber relative to its forcing fiber. We found that the percent difference between the force-deflection and bead-calibrated stiffness measurements of the stationary fiber depended on the kF-to-kS ratio. For kF/kS > 0.17 the percent difference was 10% or less. Below 0.17, the percent difference steadily increased from 30% to 90% as kF/kS decreased. These results are similar to reports (Howard and Ashmore 1986) that force deflection measurements were most accurate when the ratio of fiber to bundle stiffness was between 0.2 and 2. As a precaution, we made all bundle stiffness measurements reported here with a kF/kS ≥ 1.
Controls for Bundle Integrity
Untreated bundles.
To check the stability of stiffness values over time, we measured the stiffness of two striolar bundles over 5 h (33 and 40 measurements, respectively); this time frame is longer than our testing sessions on individual utricles. We used a linear regression analysis to assess any stiffness change over this time frame. We also used these data to assess stiffness variability due to measurement error.
BAPTA and subtilisin treatment.
The stereocilia in a hair bundle are connected to each other and to the kinocilium through several types of links, which have been shown to contribute to bundle stiffness in bullfrog saccule (Marquis and Hudspeth 1997) and chick utricle (Bashtanov et al. 2004). These links can be selectively removed by treating bundles with the Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) to remove tip, kinocilium, and ankle links (Assad et al. 1991; Bashtanov et al. 2004; Marquis and Hudspeth 1997) and the proteolytic enzyme subtilisin to remove shaft and ankle links (Bashtanov et al. 2004; Jacobs and Hudspeth 1990). To examine whether these links remained intact during our stiffness experiments, we measured the reduction in stiffness of striolar bundles following independent treatments with BAPTA and subtilisin. A stiffness reduction comparable to previous works suggests that links are undamaged in our untreated utricle preparations and that, consequently, our stiffness measurements were not compromised by broken bundle links.
We prepared a 50 mg/l solution of subtilisin (protease type XXIV, Sigma-Aldrich) in buffered HBSS, and we prepared a 5 mM solution of BAPTA (tetrasodium salt, Sigma-Aldrich) in a Ca2+- and Mg2+-free HBSS (in mM: 5.33 KCl, 0.441 KH2PO4, 4.17 NaHCO3, 137.93 NaCl, 0.338 Na2HPO4, 5.56 d-glucose, and no phenol red) with 0.9 mM MgCl2 and 10 mM HEPES added (pH 7.2, osmolarity of 300 mosM). Both solutions are taken from Bashtanov et al. (2004) to facilitate comparisons between their results and ours. We made initial bundle stiffness measurements while the utricles were bathed in buffered HBSS. Then, using a peristaltic pump, we exchanged the fluid volume in the chamber with the treatment solution three times to ensure a complete exchange. The exposure time record began with the start of the exchange, and the total of three exchanges was completed in ∼9 min. We then repeated bundle stiffness measurements over time for the treated bundles (14–25 measurements per bundle over the course of ∼120 min).
Bundle Morphometry
We took high-resolution digital pictures of each bundle tested, and we measured (using ImageJ software; National Institutes of Health, public domain) its location relative to the LPR and its dimensions (Fig. 2C): height of the kinocilium (Kino), tallest (TallSt) and shortest (ShortSt) stereocilia, and array length (Array). We also calculated a KS ratio (height of the kinocilium divided by the height of the tallest stereocilia) and a Slope (difference in the height of the tallest and shortest stereocilia divided by array length) for each bundle (Xue and Peterson 2006). We defined distance from the LPR as the distance from the kinocilium of the tested bundle to the kinocilium of a bundle immediately medial to the LPR in the same focal plane.
Statistical Analysis
We implemented exploratory and inferential statistics in Spotfire S+ (TIBCO; version 2.8) or Statistica (Statsoft; version 9). For most analyses, we used nonparametric or robust statistics (Wilcox 2005) because distributions for many independent (structural) and dependent (stiffness) variables were not normal. To compare hair bundle stiffness across zones we used a robust analog of a one-way ANOVA (t1way) with multiple comparisons (lincon; Wilcox 2005).
To examine the ability of four structural variables (Kino, TallSt, ShortSt, Array) to predict measures of hair bundle stiffness (kB, kP) we used two types of multiple regression analysis and a radial basis function (RBF) network. Each approach offers unique advantages. Unless otherwise specified, 1) all stiffness values in our analyses were log10 transformed to help linearize the relation between independent (structural) and dependent (stiffness) variables and 2) we restricted our statistical tests to 71 bundles in the striola and MES because inclusion of the two LES bundles with their very tall kinocilia distorted the relation between kinocilium height and bundle stiffness for the great bulk of the data.
Regression models.
A general linear regression (GLR) model offers well-understood methods for assessing the contribution of multiple predictors to a dependent variable. We used one of these, standardized beta coefficients, to assess the relative contribution of structural variables to bundle stiffness. In addition, we used a linear robust MM regression model (Yohai 1987), which makes fewer assumptions about the variables in the analysis. This approach also minimizes the effects of outliers on regression results (Wilcox 2005), which enabled us to perform the analysis on the full data set (73 bundles). For the robust regression model, we calculated an R2 value for a model using all significant structural contributors to stiffness (the full model) and for a series of probe models that omitted each significant contributor in turn. The probe model that showed the greatest decrease in R2 relative to the full model was judged to identify the most important contributor to stiffness. For example, if omitting Kino caused a greater decrease in the model R2 value than omitting TallSt, then we judged that kinocilium height contributed more to stiffness than the height of the tallest stereocilia.
Radial basis function network.
RBF networks are widely used for estimating multivariate probability density functions based on sampled data (Bishop 1995). They are nonparametric and nonlinear, and they allow one to predict stiffness from structure in previously unseen data (i.e., data different from that used to generate the network). RBF networks involve multivariate Gaussian kernels centered on the data points, and they generally consist of two layers: one containing the set of Gaussian kernels that sum the appropriately weighted input variables and an output layer that linearly sums the weighted outputs of the RBFs. Each input variable is first rescaled to have a mean of 0 and standard deviation of 1, so that a principal component analysis (PCA) can be performed, which has the effect of maximizing the information in each input variable.
Data processing proceeded in two steps, both implemented in MATLAB. First we determined the optimal size of the Gaussian kernels using all four structural variables. This involved a full n − 1 cross-validation procedure in which we iteratively removed data for individual hair cells to form a test set and used data from the remaining hair cells (n = 70) as a training set. For each iteration, we used the training set to generate optimal weights for the RBF network, and the RBF network then used these weights to predict stiffness of the test cell from the structural measurements for that cell. Then we compared the set of predicted stiffness values for all cells with the measured stiffness values, using a linear regression. We repeated this for a range of kernel sizes, and we used the size that yielded the highest regression coefficient (R) for all subsequent analyses.
In the second step, we assessed the contribution of each structural variable to the stiffness prediction by removing each one from the input data set to the network and repeating the n − 1 cross-validation procedure described above (using the optimal kernel size). We interpreted the structural variables whose removal resulted in the greatest reduction in the linear regression coefficient as the most important predictors of stiffness.
For a statistical evaluation of R values for the full model (one using the optimal kernel size and all four structural predictor variables), we used a Monte Carlo method in which, for 10,000 iterations, we calculated R values after the predictor and stiffness values for the training sets were randomly shuffled. The resulting distribution of simulated R values is Gaussian, describable by a mean (at or near 0) and a standard deviation (σ). R values obtained with the full model are significant (P < 0.001) if they are greater than the mean ± 3.29σ of the Gaussian distribution generated by the Monte Carlo simulation.
RESULTS
Steady-State Stiffness Varies with Macular Locus
The utricle of T. scripta can be divided into multiple zones based on differences in hair cell and afferent type and in hair bundle and OM structure (Fig. 1A). Zone 1 corresponds to the LES. Zones 2 and 3 correspond to the striola. Zone 2 is a band of type II hair cells; the LPR runs along zone 2, ∼20 μm medial to its lateral margin. Zone 3 is a band of type I hair cells plus a small number of intercalated type II hair cells. The type I hair cells are identifiable by their postsynaptic calyces; thus “calyx band” always refers to zone 3. Zone 4 corresponds to the MES. At the lateral edge of zone 4, near its border with the calyx band (zone 3), hair bundle structure changes gradually from a striola-like to an MES-like configuration (Rowe and Peterson 2006; Xue and Peterson 2006); we refer to this band as the juxtastriola. A medial-to-lateral transect (Fig. 1A) includes all these macular zones.
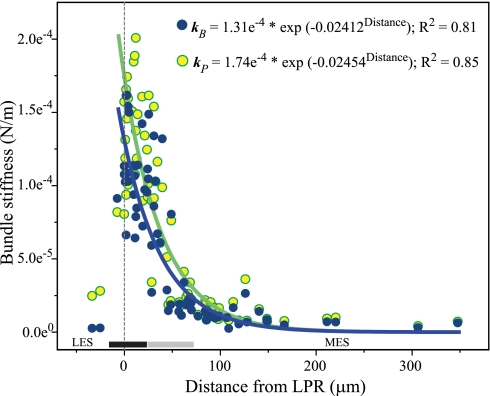
We measured the stiffness of 73 bundles (21 utricles) along this medial-to-lateral transect, concentrating on the striola and adjacent extrastriolae because hair bundle structure is markedly heterogeneous in this region (it is nearly constant in the remainder of the LES and MES; Rowe and Peterson 2006; Xue and Peterson 2006). Thus stiffness measurements within this region are most likely to reveal any dependence of bundle stiffness on bundle structure. Figure 4 shows the results in two ways: bundle stiffness (kB) and physiological stiffness (kP, i.e., bundle stiffness corrected for the height of probable force application in vivo). Solid lines are fits of kB and kP data points. Equations and R2 values for the fits are shown at top right. Both fits are significant (kB: F = 176, kP: F = 214; P < 0.001 for both).
Fig. 4.
Bundle stiffness varies with macular locus. Bundle stiffness is plotted against distance from the LPR (0 on x-axis) for 2 ways of calculating stiffness: measured stiffness (kB; dark blue circles) and physiological stiffness (kP; yellow circles). Shaded bars at bottom show approximate boundaries of striolar zones 2 (black bar) and 3 (gray bar). In both plots, bundle stiffness declines exponentially from far lateral striola through the juxtastriola (i.e., to a point ∼150 μm medial to the LPR). Lines are fits for kB (blue) and kP (green) data points (n = 71; 2 LES bundles excluded); equations for fits are at top right, together with their R2 values. Both fits are significant (P < 0.001; see text). These zonal differences in bundle stiffness are summarized in Fig. 5.
In each plot, steady-state stiffness drops exponentially from the lateral margin of the striola (∼20 μm lateral to the LPR) through the juxtastriola (125–150 μm medial to the LPR). In contrast, stiffness values show no systematic variation within the MES. Finally, two bundles with very tall kinocilia 24 μm and 32.5 μm lateral to the LPR had measured stiffness values similar to those in the MES but were slightly more stiff when an estimate of physiological stiffness was used. The reason for this is that we applied the glass probe near the tips of their tall kinocilia when measuring stiffness, whereas in vivo the kinocilia attach to the OM further down their shafts. Thus the height of force application is lower in vivo than when measuring stiffness experimentally and, accordingly, stiffness is greater. There appears to be a break in stiffness values at ∼50 μN/m (especially clear for kB). This break corresponds to a distance from the LPR of 40–50 μm. Hereafter we use the terms “stiff” and “compliant” to refer to these two stiffness groups. The stiff and compliant groups differ significantly from each other in stiffness (Mann-Whitney U: P < 0.0001 for kB and kP).
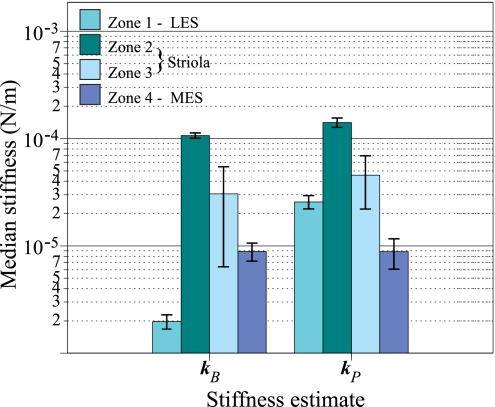
Figure 4 suggests that there are differences in the steady-state stiffness of hair bundles in the four major subdivisions of the macula: zone 1 (LES), striolar zones 2 and 3, and zone 4 (MES). These zonal differences are quantified in Fig. 5. In this grouped bar graph, each group corresponds to one calculation of bundle stiffness, kB or kP. Within each group, we represent the four macular zones by different colors. Bar heights are medians, and error bars are confidence intervals of the median. This figure makes two points. First, there are significant differences in stiffness across zones. This is indicated by nonoverlapping confidence intervals of the median, and we confirmed these differences statistically (all comparisons significant, P < 0.01, except zone 1 vs. zone 3 for kP). Bundles in zone 2 are significantly more stiff than bundles in all other zones; zone 3 hair bundles typically exhibit the greatest range in steady-state stiffness, and extrastriolar bundles are generally more compliant than striolar bundles. The second point is that the relative stiffness values for bundles in the four zones are similar for both methods of assessing steady-state stiffness. Thus our measured stiffness values (kB) are very close to the presumed condition in vivo (kP). The only exception is that stiffness values for the two LES bundles are lower than MES values when assessed with kB, whereas the relative stiffness of LES and MES bundles are reversed when the height of force application is corrected for the probable height of force application in vivo (kP). As noted above, the reason for this is that the two LES bundles had very tall kinocilia, which greatly reduced their stiffness values when kB was used to calculate bundle stiffness. Table 1 summarizes differences among zones for the two measures of hair bundle stiffness, kB and kP.
Fig. 5.
Differences in stiffness of hair bundles from 4 macular zones. This grouped bar plot shows zonal differences in hair bundle stiffness. Each group of 4 bars shows bundle stiffness determined by a different method: measured stiffness (kB) and physiological stiffness (kP). Within each group, zones are represented by different colors (see key). Bar heights are medians; error bars are confidence intervals of the median. Medians are plotted on a log axis to expand values at the low end of the stiffness scale. Both methods of determining bundle stiffness give comparable results except that the MES bundles are stiffer than those in the LES when measured values (kB) are used, but their relative stiffnesses are reversed when physiological measures (kP) are used because this method reduces the effects of the very long kinocilia in the LES (Fig. 1B). These bar graphs summarize the individual data points shown in Fig. 4.
Table 1.
Zonal differences in hair bundle stiffness
| Zone 1 | Zone 2 | Zone 3 | Zone 4 | |
|---|---|---|---|---|
| kB, μN/m | ||||
| Median | 1.97 | 106.41 | 30.57 | 8.88 |
| CIs | 1.66–2.29 | 99.56–113.26 | 6.30–54.84 | 7.11–10.65 |
| Range | 1.83–2.11 | 63.50–161.02 | 10.66–147.85 | 1.77–25.69 |
| kP, μN/m | ||||
| Median | 25.67 | 140.85 | 45.49 | 8.85 |
| CIs | 21.85–29.49 | 126.76–156.94 | 21.78–69.20 | 6.00–11.69 |
| Range | 23.95–27.38 | 79.82–200.08 | 17.84–160.74 | 3.43–35.36 |
Values are median, 95% confidence intervals of the median (CIs), and range for 2 measures of hair bundle stiffness [measured bundle stiffness (kB), physiological stiffness (kP)] in zones 1–4. Nonoverlapping CIs suggest significant differences between groups.
Controls for Hair Bundle Integrity
Kinocilia and stereocilia in hair bundles are connected by several types of links that, when intact, increase bundle stiffness. To assess the integrity of these links in our experimental preparation, we prepared maculae for stiffness measurements as described above, bathed them in solutions known to break bundle links, and made repeated stiffness measurements of the same bundles over a time frame comparable to, or longer than, that of our primary stiffness measurements (2–5 h; Fig. 6).
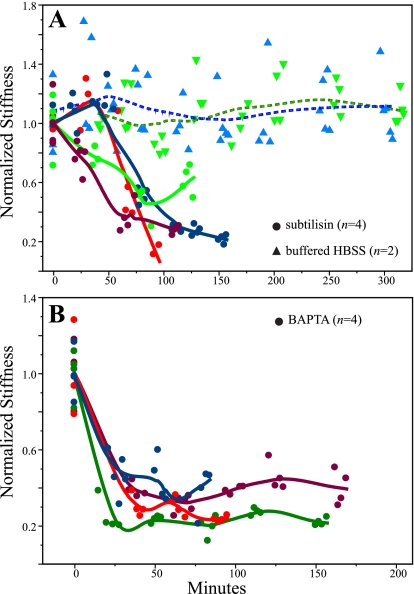
Fig. 6.
Controls for bundle integrity. Normalized bundle stiffness (kB/kmean, where kmean is the mean of 4 trials and each trial is plotted in the figure) as a function of time for 2 control conditions. A: bundle stiffness declines over time in the presence of subtilisin (●; n = 4). In contrast, bundle stiffness in a control solution (the same solution minus subtilisin; ▴; n = 2) is stable for up to 5 h. B: bundle stiffness declines precipitously in BAPTA (●; n = 4). Lines in A and B are Loess fits, which summarize trends in the data.
Bundles treated with subtilisin in buffered HBSS showed stiffness values near control levels during early measurements (20–30 min after the start of exposure), but stiffnesses then declined markedly by an average of 63 ± 11% (SD) reduction after 60 min (Fig. 6A). This is comparable to the results of Bashtanov et al. (2004), who reported a delayed decrease in stiffness of ∼48% after 40 min, with a further stiffness reduction of 25% during the following 50 min. In contrast to these results with subtilisin treatment, measurements on two bundles maintained in our standard solution (buffered HBSS) showed no decline in stiffness over 5 h of measurements (Fig. 6A; for both bundles, linear regression not significant). With two exceptions (2/17 measurements; 1 per bundle), average values at each time point (3 or 4 trials per time point) were ≤10% of the average value across time points; this indicates a measurement error of ≤10%. Together these results suggest that, under the conditions we used to measure stiffness, 1) shaft and probably ankle links are intact during our experiments (breaking ankle links does not affect bundle stiffness, so breaking them would not be detected in our test; Bashtanov et al. 2004) and 2) there is no change in bundle stiffness over the time required for stiffness measurements due to deterioration of our experimental preparation.
For four striolar bundles treated with BAPTA (Fig. 6B), stiffnesses dropped quickly (before the first posttreatment measurements ∼20 min after the bath exchange), reaching an average reduction of 65 ± 10%. Because the tallest stereocilia in these striolar bundles are as tall as the kinocilium, the probe necessarily contacted a few tall stereocilia as well as the kinocilium. Thus the stiffness reduction we saw was not simply due to severing of the links between kinocilium and stereocilia. Bashtanov et al. (2004) reported a drop of ∼43% in stiffness within 10 min of BAPTA exposure; Marquis and Hudspeth (1997) reported a stiffness decrease of 40–70%. Thus results of our BAPTA experiments are within the range of previous reports, and they suggest that tip, ankle, and kinocilial links were intact in our experimental preparation.
Bundle Structure Predicts Steady-State Stiffness
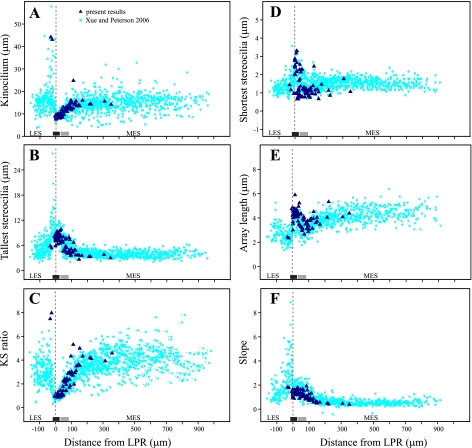
Hair bundles exhibited the same systematic regional differences in structure reported previously for turtle utricle. Figure 7 shows four measured (Fig. 7, A, B, D, and E) and two calculated (Fig. 7, C and F) features of bundle structure for the 73 bundles we used to measure steady-state stiffness. We superimpose these data on the corresponding measurements from an earlier study, which quantified bundle structure along the entire medial-to-lateral transect in fixed but not dehydrated maculae (Xue and Peterson 2006). The close correspondence between the two data sets provides additional evidence that bundle structure was not compromised in the present study. Note that spatial variation in hair bundle structure is greatest within the striola and adjacent extrastriolae. In the far LES and MES, bundle structure does not vary systematically with macular locus.
Fig. 7.
Structure of tested hair bundles: variation in 6 structural features (dark blue triangles) as a function of distance from the LPR for the 73 hair bundles evaluated in this study. Corresponding measurements from a study of fixed but not dehydrated utricular bundles (Xue and Peterson 2006) are shown with light blue circles for comparison. Dimensions from the 2 data sets are very similar. A partial exception is that array length is longer in the present study (and therefore Slopes are shallower) because we included the kinocilium in our measure of array length, whereas Xue and Peterson (2006) did not. A: kinocilium height. B: height of tallest stereocilia. C: KS ratio. D: height of shortest stereocilia. E: array length. F: Slope. Bars above the x-axis show the approximate location of zone 2 (black bar) and zone 3 (gray bar).
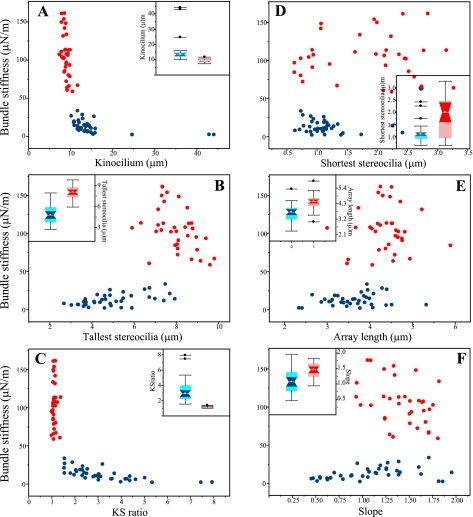
Comparison of spatial variation in bundle stiffness (Fig. 4) and bundle structure (Fig. 7) suggests that the two measures covary. For example, bundle stiffness changes systematically from the LPR to the medial margin of the juxtastriola, as do the structural variables. This covariation is addressed more directly in Fig. 8, where bundle stiffness is plotted against the six structural variables assessed in this study; bundles with kB > 50 μN/m (“stiff” group) and those with kB < 50 μN/m (“compliant” group) are indicated by different colors. The stiffest hair bundles have short kinocilia (Fig. 8A), their tallest stereocilia are relatively tall (Fig. 8B), and therefore their KS ratios are near 1 (Fig. 8C). The shortest stereocilia of the stiffest bundles are relatively tall (Fig. 8D), their arrays are long (Fig. 8E), and the falloff from the tallest to the shortest stereocilia is relatively steep (Fig. 8F). Insets for each plot compare data for the two groups with box plots. In each case, differences between the two groups are significant (confidence intervals nonoverlapping). The most striking difference between the stiff and compliant groups is in the ratio of kinocilium height to the height of the tallest stereocilia (KS ratio; Fig. 8C). Without exception, stiff bundles have KS ratios ≤1.4, and all bundles with KS ratios >1.4 are significantly more compliant. Table 2 compares summary statistics for stiff and compliant groups on each measured or calculated structural variable.
Fig. 8.
Stiff hair bundles have distinctive morphologies. Measured hair bundle stiffness as a function of the 6 morphological features evaluated in this study. The stiffest bundles (kB ≥ 50 μN/m) and the more compliant bundles are indicated by red circles and blue circles, respectively. In the stiffest bundles, the kinocilia are relatively short (A), the tallest stereocilia are tall (B), kinocilia and the tallest stereocilia are approximately the same height (KS ratio approximately equal to 1; C), the shortest stereocilia are often taller than in more compliant bundles (D), array lengths are longer (E), and Slopes are steeper (F). Insets for each plot are box plots that compare summary statistics for stiff (red) and compliant (blue) hair bundle groups. Gray circles, medians; light shading, interquartile range; dark shading, 95% confidence intervals of median; whiskers, 1.5 × interquartile range; isolated points, outliers. Nonoverlapping confidence intervals indicate that differences between the 2 stiffness groups are significant for all 6 structural features. Note that some symbols in D and E overlap the insets.
Table 2.
Structure of stiff and compliant hair bundle groups
| Stiff (kB > 50 μN/m) | Compliant (kB < 50 μN/m) | |
|---|---|---|
| Kino, μm | ||
| Median | 8.9 | 13.4 |
| CIs | 8.7–9.2 | 12.7–14.1 |
| Range | 7.4–11.7 | 10.2–44.1 |
| TallSt, μm | ||
| Median | 8.1 | 4.8 |
| CIs | 7.7–8.4 | 4.2–5.3 |
| Range | 5.9–9.8 | 2.7–7.9 |
| KS ratio | ||
| Median | 1.1 | 2.9 |
| CIs | 1.08–1.13 | 2.5–3.4 |
| Range | 1.0–1.4 | 1.5–7.9 |
| ShortSt, μm | ||
| Median | 2.0 | 1.1 |
| CIs | 1.6–2.4 | 1.0–1.2 |
| Range | 0.6–3.3 | 0.6–2.4 |
| Array, μm | ||
| Median | 4.5 | 3.7 |
| CIs | 4.3–4.6 | 3.5–3.9 |
| Range | 3.0–5.9 | 2.4–5.3 |
| Slope | ||
| Median | 1.5 | 1.1 |
| CIs | 1.3–1.6 | 0.9–1.2 |
| Range | 0.9–1.8 | 0.5–2.0 |
Values are median, 95% CIs, and range for 6 measures of hair bundle structure in stiff and compliant bundle groups [height of kinocilium (Kino), height of tallest (TallSt) and shortest (ShortSt) stereocilia, height of kinocilium divided by height of tallest stereocilia (KS ratio), array length (Array), difference in height of tallest and shortest stereocilia divided by array length (Slope)]. Nonoverlapping CIs suggest significant differences between groups.
Finally, we asked which structural variables are the most important determinants of bundle stiffness. Results for the GLR model and the RBF network were the same (Tables 3 and 4). R2 values for the full model (the proportion of variance in stiffness accounted for by the structural variables) ranged from 0.88 to 0.84 for the GLR model and from 0.85 to 0.84 for the RBF network. In the GLR model, standardized beta coefficients were significant for Kino and TallSt; in the RBF network, omission of these two structural variables caused the greatest decrease in R2 values, indicating that they contribute most to bundle stiffness. Finally, Kino was more important than TallSt in predicting kB, while TallSt was the more important variable in predicting kP (see absolute value of standardized beta coefficients in Table 3 and Rank in Table 4). Results of the Monte Carlo simulations for each stiffness measure indicated that R values obtained from the RBF network analysis were significantly greater than chance (Table 4). Finally, results of a robust MM multiple regression analysis on the full data set (i.e., with the two LES cells included) were the same as in the GLR analysis: only Kino and TallSt contributed significantly to stiffness, and the relative importance of the two structural features to kB and kP was also the same as in the GLR analysis. These results suggest that the calculated variable KS ratio best separates stiff and compliant bundle groups (Fig. 8C) because it captures the effects of both significant contributors to bundle stiffness: the heights of a bundle's kinocilium and tallest stereocilia. In contrast, the calculated variable Slope is less effective in separating stiff and compliant bundle groups (Fig. 8F), probably because only one of the three independent variables used to calculate Slope (TallSt; Fig. 2C) contributes significantly to bundle stiffness.
Table 3.
Structural predictors of hair bundle stiffness: GLR results
| Log10kB | Log10kP | |
|---|---|---|
| R2 | 0.875* | 0.844* |
| Kino | −0.590* | −0.378* |
| TallSt | 0.413* | 0.580* |
| ShortSt | 0.013 | 0.046 |
| Array | 0.156 | 0.068 |
R2 values are values for overall fit of multiple regression model that predicts log-transformed stiffness measures (kB, kP) from 4 structural variables (Kino, TallSt, ShortSt, Array). Other values are standardized beta weights, which indicate the relative contribution of the structural variables to each measure of stiffness. GLR, general linear regression.
P < 0.0001.
Table 4.
Structural predictors of hair bundle stiffness: RBF network results
| IV Omitted | Rank | R | R2 | % Decrease |
|---|---|---|---|---|
| kB | ||||
| None | 0.9187* | 0.8440 | 0.0000 | |
| Kino | 1 | 0.8391 | 0.7041 | 0.1657 |
| TallSt | 2 | 0.8781 | 0.7711 | 0.0864 |
| ShortSt | 4 | 0.9283 | 0.8617 | −0.0210 |
| Array | 3 | 0.9224 | 0.8508 | −0.0080 |
| kP | ||||
| None | 0.9241* | 0.8540 | 0.0000 | |
| Kino | 2 | 0.8803 | 0.7750 | 0.0925 |
| TallSt | 1 | 0.8578 | 0.7358 | 0.1384 |
| ShortSt | 4 | 0.9298 | 0.8644 | −0.0122 |
| Array | 3 | 0.9205 | 0.8473 | 0.0079 |
Values are results of radial basis function (RBF) network analysis for 2 measures of stiffness (kB, kP). For each stiffness measure, the first row gives R and R2 values for the full model, i.e., all 4 independent variables (IVs) included, and the next 4 rows give R and R2 values when each of the IVs is omitted in turn. The last column gives the % decrease in R value for each model compared with the value for the full model, which indicates the relative importance of each IV (Kino, TallSt, ShortSt, Array) in setting bundle stiffness. Relative importance is summarized in the Rank column. Asterisks for full models (no IVs omitted) give the probability of achieving this result by chance, based on our Monte Carlo simulations.
P < 0.001.
DISCUSSION
We have measured the steady-state stiffness of hair bundles at different locations on the utricular macula of a turtle, T. scripta. We also asked whether a hair bundle's structure predicts its steady-state stiffness. Our major findings are that 1) utricular bundle stiffness varies by nearly two orders of magnitude and changes systematically with macular locus and 2) this stiffness variation is well predicted by hair bundle structure, particularly kinocilium and stereocilia heights.
Methodological Issues
We used a well-understood force-deflection method to measure bundle stiffness, and we took several steps to maximize the accuracy of these measurements. We used an analytical technique to validate our method of calculating the stiffness of our glass fiber probes, and we determined the optimal ratio of glass fiber stiffness to bundle stiffness experimentally. These steps also provided an estimate of measurement error attributable to our apparatus and procedure (≤10%).
Second, we implemented three controls to ensure tissue integrity during our experiments. 1) We randomized the start location for stiffness measurements to ensure that differences in stiffness with macular locus did not simply reflect tissue changes over time. 2) We tested the stiffness of two bundles repeatedly over 5 h, i.e., a period longer than our experimental sessions. The stiffness of both bundles remained the same over the 5-h period (Fig. 6A), indicating that stiffness values in our experimental sessions are not likely to be compromised by the passage of time. 3) We showed that interciliary links are most likely intact during our experiments, because treatments designed to break these links produced stiffness changes whose time course and magnitude were predictable, i.e., very close to those reported for chick utricular bundles (Bashtanov et al. 2004) and frog saccular bundles (Marquis and Hudspeth 1997) undergoing the same manipulations. Our microscope was not fitted for fluorescence, which precluded fluorescence indicators of viability (e.g., FM1-43, Sytox Green) during our experiments. But the results of our control experiments strongly suggest that hair bundles remained healthy, with intact links, during our experiments. Finally, we note that the orderly covariation of bundle stiffness with macular location (Fig. 4) and its strong dependence on bundle structure (Fig. 8; Tables 3 and 4) both argue against random fluctuations in stiffness values due to tissue condition. Rather, they suggest that our methods yielded biologically meaningful measures of bundle stiffness variation across the turtle utricle.
Steady-State Stiffness Varies with Macular Locus
The stiffest bundles (steady-state stiffness > 50 μN/m) occur within a band that begins just lateral to the LPR and extends to a point 40–50 μm medial to the LPR (Fig. 4). This region encompasses all of zone 2 and the lateral margin of zone 3. Thus bundle stiffness does not appear to follow zonal boundaries precisely. There are two possible interpretations. First, our assignment of hair bundles to zones was approximate because we could not reliably visualize calyces in our DIC images to distinguish between type I and type II hair cells. Instead we identified zone boundaries by distance from the LPR, based on previous work (Moravec and Peterson 2004; Xue and Peterson 2006). This, together with the fact that such boundaries are naturally irregular, means that we may have made errors in assigning hair cells at the boundary between zones 2 and 3. A second interpretation is that bundles in zone 2 and the lateral margin of zone 3 share structural features that result in high stiffness values in both locations (see Bundle Structure Predicts Steady-State Stiffness, below).
Medial to this band of stiff bundles, steady-state stiffness values decrease systematically through the remainder of zone 3 and the juxtastriola, to reach asymptotic values in the MES (Fig. 4). These MES stiffnesses are almost two orders of magnitude lower than average values in zone 2 (Table 1). In the LES, we were able to test only two bundles because the connective tissue falx overlying the LES makes its bundles difficult to access, but, interestingly, measured stiffness values for LES bundles were similar to values for MES bundles (Fig. 4). Thus zone 2 bundles have the highest stiffness values in the macula, zone 3 and juxtastriolar bundles exhibit a lateral-to-medial stiffness gradient (reflected in large confidence intervals for zone 3 in Fig. 5), and extrastriolar bundles (MES and LES) are similarly compliant (Fig. 4).
Bundle Structure Predicts Steady-State Stiffness
In the present study, we implemented parametric and robust regression analyses and a RBF network to determine how well four structural features of bundles (Kino, TallSt, ShortSt, Array) predict bundle stiffness and which structural variables are the most important contributors to stiffness. Results of these analyses were the same. They indicate that bundle structure predicts bundle stiffness (R2 values 0.84–0.88) and that the most important predictors of stiffness are the heights of the kinocilium and the tallest stereocilia. These results are consistent with Géléoc et al. (1997), who reported that the stiffness of mouse cochlear bundles is greater than that of mouse vestibular bundles, a difference they attributed primarily to the shorter heights of cochlear bundles.
This result suggests two reasons why bundles just medial to the LPR are especially stiff. First, these bundles have the shortest kinocilia, and measured stiffness is inversely proportional to kinocilium height (i.e., to the height of applied force). Second, bundles just medial to the LPR have the smallest KS ratios in the macula. KS ratio is important for two reasons. It quantifies the moment arm by which tall stereocilia resist kinocilial deflection: the smaller the KS ratio, the more the tallest stereocilia will resist kinocilial deflection and the greater will be the bundle's stiffness. KS ratio is also important because kinocilia are not rigid (Spoon and Grant 2011). Small KS ratios mean that the kinocilium is buttressed along its length; this prevents kinocilium bending, which would decrease measured stiffness. Interestingly, KS ratio is near 1 throughout zone 2 and into the most lateral part of zone 3 (Fig. 7; see also Fig. 6C in Xue and Peterson 2006), the same region over which utricular bundles are especially stiff.
It is important to note that other features of utricular bundles may contribute to their stiffness, in addition to kinocilium and stereocilia heights. Interciliary links, when intact, clearly increase bundle stiffness (Bashtanov et al. 2004; Marquis and Hudspeth 1997; present results). Stereocilia number is thought to be proportional to bundle stiffness based on theoretical calculations (Howard et al. 1988) and inferences about stereocilia number from bundle base dimensions (Howard and Ashmore 1986). There are several additional possibilities, e.g., stereocilia dimensions or rootlet spacing (Pickles 1993), the distribution of stereocilia over the hair cell's apical surface (Bagger-Sjöbäck and Takumida 1988), the shape of the height falloff between adjacent rows of stereocilia (Bagger-Sjöbäck and Takumida 1988; Ricci et al. 1997), the degree of stereocilia “tenting” (well developed in bullfrog saccule; Jacobs and Hudspeth 1990), and dimensions or material properties of the stereocilia rootlets that insert into the cuticular plate (Furness et al. 2008; Kitajiri et al. 2010). To our knowledge, there is no direct experimental evidence for the contribution of these factors to steady-state stiffness.
In contrast, the present results provide experimental evidence that the heights of kinocilia and the tallest stereocilia are significant determinants of steady-state bundle mechanics: specifically, short kinocilia coupled to tall stereocilia (i.e., low KS ratios) predict stiff hair bundles. This finding suggests functional sequelae for well-known hair cell classifications that are based on bundle heights (e.g., Lewis and Li 1975; Lim 1976; Platt 1983; Popper and Platt 1993). A common finding in these studies is that KS ratios tend to be lower in the striola of otoconial organs (see discussion in Xue and Peterson 2006). This suggests the testable hypothesis that hair bundles in the striola of many vertebrates will have higher steady-state stiffnesses than bundles in the extrastriola.
Comparison with Steady-State Stiffness in Other Hair Bundles
Steady-state stiffness has been measured in auditory and vestibular organs from a variety of species (Table 5). Comparisons are problematic because of known and unknown differences in bundle structure as well as differences in experimental techniques. For example, only three studies have measured the stiffness of utricular bundles: one used detection of Brownian motion (Bashtanov et al. 2004); one used a fluid jet stimulus applied to the middle of the bundle (Géléoc et al. 1997); and one used a force-deflection technique (present results). But despite any structural or methodological differences, measured stiffness values appear to fall into two broad groups. Stiffness values for otoconial organs and nonmammalian cochleae are of the same order of magnitude. The stiffest bundles in turtle utricle (zone 2; median: kB = 106 μN/m; range: 64–161 μN/m) have stiffnesses near the bottom of this range. The stiffness of turtle extrastriolar bundles are approximately two orders of magnitude lower, but bundles of similar structure (tall kinocilium and short stereocilia) have not been tested in other preparations, so comparisons are not possible. The second broad group of stiffness values is for mammalian (rodent) cochleae; stiffnesses of mammalian auditory bundles are generally an order of magnitude higher than those of other inner ear organs, perhaps because of their unique structure. To our knowledge, the only studies that measured stiffness covariation with epithelial location (Flock and Strelioff 1984; Strelioff and Flock 1984) reported that bundle stiffness decreased in guinea pig cochlea with distance from the stapes, a decrease that they attributed to greater compliance of stereociliary rootlets and to increasing bundle heights.
Table 5.
Steady-state stiffness of vestibular and auditory hair bundles
| Source | Species | Organ | Stimulus | Stiffness (original) | Stiffness, μN/m |
|---|---|---|---|---|---|
| Ashmore 1984 | Frog | Saccule | Flexible probe | 132 pN/μm | 132 |
| Denk et al. 1989 | Frog | Saccule | Brownian motion | 341 μN/m | 341 |
| Howard and Ashmore 1986 | Frog | Saccule | Flexible probe | 256 pN/μm | 256 |
| Howard and Hudspeth 1987 | Frog | Saccule | Flexible probe | 0.63 mN/m | 630 |
| Jaramillo and Hudspeth 1993 | Frog | Saccule | Flexible probe | 750 μN/m | 750 |
| Marquis and Hudspeth 1997 | Frog | Saccule | Flexible probe | 900 μN/m | 900 |
| Bashtanov et al. 2004 | Chick | Utricle | Brownian motion | 0.9 mN/m | 900 |
| Géléoc et al. 1997 | Mouse | Utricle | Fluid jet | 0.67 mN/m | 670 |
| Spoon et al. (present results) | Turtle | Utricle | Flexible probe | 2–161 | |
| Crawford and Fettiplace 1985 | Turtle | Cochlea | Flexible probe | 0.0006 N/m | 600 |
| Ricci et al. 2000 | Turtle | Cochlea | Flexible probe | 2.2 mN/m | 2,200 |
| Szymko et al. 1992 | Chick | Cochlea | Fluid jet | 0.0005 N/m | 504 |
| Beurg et al. 2008 | Rat | Cochlea | Flexible probe | 3 mN/m | 3,000 |
| Flock and Strelioff 1984 | Guinea pig | Cochlea | Flexible probe | 0.78-3.47 dyn/cm | 780–3,470 |
| Strelioff and Flock 1984 | Guinea pig | Cochlea | Flexible probe | 0.69-1.66 dyn/cm | 690–1,660 |
| Géléoc et al. 1997 | Mouse | Cochlea | Fluid jet | 4.5 mN/m | 4,500 |
| Kössl et al. 1990 | Mouse | Cochlea | Flexible probe | 1.69 nN/μm | 1,690 |
| Russell et al. 1989 | Mouse | Cochlea | Flexible probe | 1.6-3.5 mN/m | 1,600–3,500 |
| Russell et al. 1992 | Mouse | Cochlea | Flexible probe | 1.42 pN/nm | 1,420 |
Functional Significance of Hair Bundle Stiffness
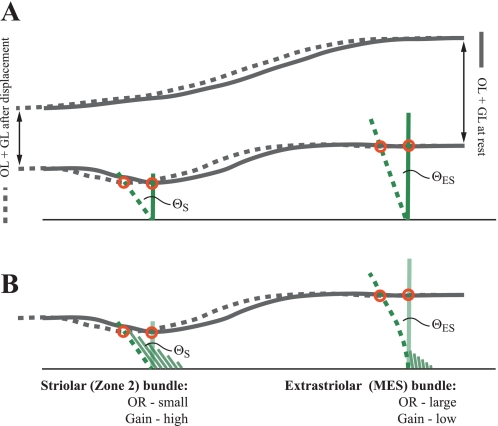
In otoconial organs in vivo, a force generated by head acceleration is converted to a proportional displacement of the OM (Fig. 9). If this displacement is applied to a short kinocilium, it will produce a greater angular deflection of the bundle, and therefore greater tension on transduction channels, than the same displacement applied to a taller kinocilium (Fig. 9A: ΘS > ΘES). Accordingly, a bundle with a short kinocilium will have a steeper current (I)-bundle displacement (X) curve (higher I/X gain) and a shorter operating range (OR; displacement necessary to produce 90% of maximum current) than a bundle with a tall kinocilium. In support of this suggestion, preliminary physiological data on turtle utricle indicate that striolar hair bundles have shorter (steeper) operating ranges than extrastriolar bundles (Meyer and Eatock 2011). Similarly, Baird (1994) reported that type E and F hair bundles, which have short kinocilia and KS ratios close to 1, show greater gains to step displacement than bundles with taller kinocilia and higher KS ratios (types B and C).
Fig. 9.
Effects of kinocilium height (A) and KS ratio (B) in vivo on a striolar bundle (S, left) and a bundle from the MES (ES, right). Dark gray lines represent the otoconial membrane (OM), from the top of the otoconial layer to the bottom of the gel layer. Relative dimensions of the OM in the striola and MES are approximated from Xue and Peterson (2003). Kinocilia attach at the bottom of the gel layer (red circles). Accelerations generated by head movement are converted to a proportional displacement of the OM and kinocilia from their start position (solid lines) to a new position (dashed lines). This produces an angular rotation of hair bundles (Θ), the size of which depends on the height of the applied force (A, i.e., kinocilium height) and KS ratio (B). In this model, we assume that bundle resistance to deflection produces no deformation of the OM. A: a unit displacement of the OM (distance between red circles for each bundle) generates a greater angular rotation of the striolar bundle than the extrastriolar bundle because the kinocilium is shorter: ΘS > ΘES. This yields a greater transduction current in the striolar bundle per unit displacement, i.e., its current/bundle displacement (I/X) gain (sensitivity) is greater than that of the extrastriolar bundle and consequently its operating range (OR) is relatively small. Stereocilia are omitted for clarity. B: the hair bundles in A are depicted with rigid kinocilia, and so the angular rotation produced by a unit tip displacement of the kinocilium depends on bundle geometry alone (i.e., kinocilium height). But real kinocilia can bend and shear when deflected (Spoon and Grant 2011), and kinocilia on bundles with high KS ratios will bend because they are not buttressed by their short stereocilia (right). This yields a smaller angular rotation of the bundle (ΘES) in response to a unit displacement (compared with the rigid kinocilium condition depicted in A, i.e., ΘES in A is greater than ΘES in B) because deflection of the kinocilium tip is not efficiently relayed to the ciliary insertions at the bundle base. Thus high KS ratios combined with compliant kinocilia further decrease the gain and increase the OR of extrastriola bundles. For clarity, only the bottom layer of the OM is depicted in B, and bundles at rest are represented by the kinocilium only. Note that the features that contribute to high bundle stiffness under experimental conditions (short kinocilia and low KS ratios) also help shape the sensitivity (I/X gain) and OR of hair cells in vivo. In this example, we depict the 2 extremes of bundle stiffness (striolar zone 2 and the MES). In real utricles, a gradient of kinocilium heights and KS ratios provides the broad spectrum of hair cell gains and ORs necessary to transduce the full complement of accelerations produced during natural head movements. OL, otoconial layer; GL, gel layer.
Since bundles with short kinocilia also tend to be stiff, as explained above, stiff bundles will have higher I/X gains and shorter operating ranges than more compliant bundles. Thus stiff bundles, such as those in the striola, will signal small OM displacements more effectively than the more compliant extrastriolar bundles, i.e., they will have greater sensitivity (smaller minimum detectable displacement/lower threshold). Such small OM displacements occur with low head accelerations or at the start of larger accelerations, suggesting a role for the striola in detecting weak stimuli and the onset of head movements. Extrastriolar bundles, in contrast, have a broader operating range because of their tall kinocilia and high KS ratios (which allow kinocilial bending; Fig. 9B). They can report larger values of acceleration (greater OM displacements), and thereby they extend the dynamic range of the utricle. Thus the spectrum of bundle stiffnesses provides the sensitivity and large dynamic range necessary for animals to transduce the full complement of accelerations that occur during natural head movements. Note that the above analysis assumes that the OM moves as a rigid sheet, as is often supposed (e.g., Kachar et al. 1990). In turtle utricle, the gel layer of the OM is thicker over the striola and LES than over the MES (Xue et al. 2007), which could introduce regional differences in the compliance of the OM. Further experimental and computational analyses of the OM and OM-bundle coupling will be necessary to assess any effects of OM compliance on hair bundle responses to head movement.
Finally, we suggest that regional differences in bundle stiffness may help explain observed differences in the response dynamics of utricular afferents. In mammals, striolar afferents are reported to have higher gains (spikes/s/unit acceleration) than extrastriolar afferents (Lysakowski and Goldberg 2004). If regional differences in the stiffness of turtle utricular hair cells are also present in mammals, as their bundle structures suggest (Li et al. 2008), then striolar bundles in mammals may have higher gains (steeper current-displacement curves) than extrastriolar bundles and so may contribute to the higher gains of their postsynaptic afferents. An additional possibility is that stiffer hair bundles in the striola may contribute to the phasic response properties and high-pass filtering of striolar afferents (Eatock and Songer 2011).
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grant DC-05063.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.S., W.J.M., J.W.G., and E.H.P. conception and design of research; C.S. performed experiments; C.S., W.J.M., M.H.R., J.W.G., and E.H.P. analyzed data; C.S., W.J.M., M.H.R., J.W.G., and E.H.P. interpreted results of experiments; C.S. and E.H.P. prepared figures; C.S., J.W.G., and E.H.P. drafted the manuscript; C.S., M.H.R., J.W.G., and E.H.P. edited and revised the manuscript; C.S., J.W.G., and E.H.P. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Drs. Jong-Hoon Nam and Ruth Anne Eatock for many valuable discussions. Dr. Nam also contributed thoughtful comments on this manuscript.
REFERENCES
- Ashmore JF. The stiffness of the sensory hair bundle of frog saccular hair cells. J Physiol 350: 20P, 1984 [Google Scholar]
- Assad JA, Shepherd GMG, Corey DP. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron 7: 985–994, 1991 [DOI] [PubMed] [Google Scholar]
- Bagger-Sjöbäck D, Takumida M. Geometrical array of vestibular sensory hair bundle. Acta Otolaryngol 106: 393–403, 1988 [DOI] [PubMed] [Google Scholar]
- Baird RA. Comparative transduction mechanisms of hair cells in the bullfrog utriculus. II. Sensitivity and response dynamics to hair bundle displacement. J Neurophysiol 71: 685–705, 1994 [DOI] [PubMed] [Google Scholar]
- Bashtanov ME, Goodyear RJ, Richardson GP, Russell IJ. The mechanical properties of chick (Gallus domesticus) sensory hair bundles: relative contributions of structures sensitive to calcium chelation and subtilisin treatment. J Physiol 559: 287–299, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Nam JH, Crawford A, Fettiplace R. The actions of calcium on hair bundle mechanics in mammalian cochlear hair cells. Biophys J 94: 2639–2653, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop CM. Neural Networks for Pattern Recognition. New York: Oxford Univ. Press, 1995 [Google Scholar]
- Crawford AC, Fettiplace R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol 364: 359–379, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JL, Xue J, Peterson EH, Grant JW. Layer thickness and curvature effects on utricle deformation in the red ear slider turtle: static and modal analysis. J Vestib Res 17: 145–162, 2007 [PMC free article] [PubMed] [Google Scholar]
- Denk W, Webb WW, Hudspeth AJ. Mechanical properties of sensory hair bundles are reflected in their Brownian motion measured with a laser differential interferometer. Proc Natl Acad Sci USA 86: 5371–5375, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock RA, Lysakowski A. Mammalian vestibular hair cells. In: Vertebrate Hair Cells, edited by Eatock RA, Fay PR, Popper AN. New York: Springer, 2006, p. 348–442 [Google Scholar]
- Eatock RA, Songer JE. Vestibular hair cells and afferents: two channels for head motion signals. Annu Rev Neurosci 34: 501–534, 2011 [DOI] [PubMed] [Google Scholar]
- Eatock RA, Corey DP, Hudspeth AJ. Adaptation of mechanoelectrical transduction in hair cells of the bullfrog's sacculus. J Neurosci 7: 2821–2836, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermin CD, Lychakov DV, Campos A, Hara H, Sondag E, Jones T, Jones S, Taylor M, Meza-Ruiz G, Martin DS. Otoconia biogenesis, phylogeny, composition and functional attributes. Histol Histopathol 13: 1103–1154, 1998 [DOI] [PubMed] [Google Scholar]
- Fettiplace R, Ricci AJ. Mechanoelectrical transduction in auditory hair cells. In: Vertebrate Hair Cells, edited by Eatock RA, Fay PR, Popper AN. New York: Springer, 2006, p. 154–203 [Google Scholar]
- Flock A, Strelioff D. Graded and nonlinear mechanical properties of sensory hairs in the mammalian hearing organ. Nature 310: 597–599, 1984 [DOI] [PubMed] [Google Scholar]
- Fontilla MF, Peterson EH. Kinocilia heights on utricular hair cells. Hear Res 145: 8–16, 2000 [DOI] [PubMed] [Google Scholar]
- Furness DN, Hackney CM. The structure and composition of the stereociliary bundle in vertebrate hair cells. In: Vertebrate Hair Cells, edited by Eatock RA, Fay RR, Popper AN. New York: Springer, 2006, p. 95–153 [Google Scholar]
- Furness DN, Mahendrasingam S, Ohashi M, Fettiplace R, Hackney CM. The dimensions and composition of stereociliary rootlets in mammalian cochlear hair cells: comparison between high- and low-frequency cells and evidence for a connection to the lateral membrane. J Neurosci 28: 6342–6353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Géléoc GSG, Lennan GWT, Richardson GP, Kros CJ. A quantitative comparison of mechanoelectrical transduction in vestibular and auditory hair cells of neonatal mice. Proc R Soc Lond B 264: 611–621, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear R, Richardson G. The ankle-link antigen: an epitope sensitive to calcium chelation associated with the hair cell surface and the calycal processes of photoreceptors. J Neurosci 19: 3761–3772, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JR, Gillespie SKH, Provance DW, Shah K, Shokat KM, Corey DP, Mercer JA, Gillespie PG. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell 108: 371–381, 2002 [DOI] [PubMed] [Google Scholar]
- Howard J, Ashmore JF. Stiffness of sensory hair bundles in the sacculus of the frog. Hear Res 23: 93–104, 1986 [DOI] [PubMed] [Google Scholar]
- Howard J, Hudspeth AJ. Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by the bullfrog's saccular hair cell. Proc Natl Acad Sci USA 84: 3064–3068, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J, Hudspeth AJ. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog's saccular hair cell. Neuron 1: 189–199, 1988 [DOI] [PubMed] [Google Scholar]
- Howard J, Roberts WM, Hudspeth AJ. Mechanoelectrical transduction by hair cells. Annu Rev Biophys Biophys Chem 17: 99–124, 1988 [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ. Making an effort to listen: mechanical amplification in the ear. Neuron 59: 530–545, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RA, Hudspeth AJ. Ultrastructural correlates of mechanoelectrical transduction in hair cells of the bullfrog's internal ear. Cold Spring Harb Symp Quant Biol 55: 547–562, 1990 [DOI] [PubMed] [Google Scholar]
- Jaramillo F, Hudspeth AJ. Displacement-clamp measurement of the forces exerted by gating springs in the hair bundle. Proc Natl Acad Sci USA 90: 1330–1334, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JM. The sensory epithelia of the inner ear of two turtles, Testudo graeca L. and Pseudemys scripta. Acta Zool 55: 289–298, 1974 [Google Scholar]
- Jorgensen JM. The number and distribution of calyceal hair cells in the inner ear utricular macula of some reptiles. Acta Zool 69: 169–175, 1988 [Google Scholar]
- Kachar B, Parakkal M, Fex J. Structural basis for mechanical transduction in the frog vestibular sensory apparatus. I. The otolithic membrane. Hear Res 45: 179–190, 1990 [DOI] [PubMed] [Google Scholar]
- Karavitaki KD, Corey DP. Sliding adhesion confers coherent motion to hair cell stereocilia and parallel gating to transduction channels. J Neurosci 30: 9051–9063, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajiri S, Sakamoto T, Belyantseva IA, Goodyear RJ, Stepanyan R, Fujiwara I, Bird JE, Riazuddin S, Riazuddin S, Ahmed ZM, Hinshaw JE, Sellers J, Bartles JR, Hammer JA, Richardson GP, Griffith AJ, Frolenkov GI, Friedman TB. Actin-bundling protein TRIOBP forms resilient rootlets of hair cell stereocilia essential for hearing. Cell 141: 786–798, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kössl M, Richardson GP, Russell IJ. Stereocilia bundle stiffness: effects of neomycin sulphate, A23187 and concanavalin A. Hear Res 44: 217–230, 1990 [DOI] [PubMed] [Google Scholar]
- Kozlov AS, Risler T, Hudspeth AJ. Coherent motion of stereocilia assures the concerted gating of hair-cell transduction channels. Nat Neurosci 10: 87–92, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis ER, Li CW. Hair cell types and distributions in the otolithic and auditory organs of the bullfrog. Brain Res 83: 35–50, 1975 [Google Scholar]
- Lewis ER, Leverenz EL, Bialek WS. The Vertebrate Inner Ear. Boca Raton, FL: CRC, 1985 [Google Scholar]
- Li A, Xue J, Peterson EH. Architecture of the mouse utricle: macular organization and hair bundle heights. J Neurophysiol 99: 718–733, 2008 [DOI] [PubMed] [Google Scholar]
- Lim DJ. Morphological and physiological correlates in cochlear and vestibular sensory epithelia. Scan Elec Micro V: 269–276, 1976 [Google Scholar]
- Lysakowski A, Goldberg JM. Morphophysiology of the vestibular periphery. In: The Vestibular System, edited by Highstein SM, Fay RR, Popper AN. New York: Springer, 2004, p. 57–152 [Google Scholar]
- Marquis RE, Hudspeth AJ. Effects of extracellular Ca2+ concentration on hair-bundle stiffness and gating-spring integrity in hair cells. Proc Natl Acad Sci USA 94: 11923–11928, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Eatock RA. Transduction in the turtle utricle (Abstract). Assoc Res Otolaryngol Abstr 34: 86, 2011 [Google Scholar]
- Moravec WJ, Peterson EH. Differences between stereocilia numbers on type I and type II vestibular hair cells. J Neurophysiol 92: 3153–3160, 2004 [DOI] [PubMed] [Google Scholar]
- Moravec WJ, Spoon C, Grant JW, Peterson EH. Bundle mechanics depend on bundle structure. II. Using artificial neural networks to perform non-linear statistical analyses (Abstract). Soc Neurosci Abstr 2005: 47.1, 2005 [Google Scholar]
- Nayak GD, Ratnayaka HS, Goodyear RJ, Richardson GP. Development of the hair bundle and mechanotransduction. Int J Dev Biol 51: 597–608, 2007 [DOI] [PubMed] [Google Scholar]
- Pickles JO. A model for the mechanics of the stereociliar bundle on acousticolateral hair cells. Hear Res 68: 159–172, 1993 [DOI] [PubMed] [Google Scholar]
- Platt C. The peripheral vestibular system of fishes. In: Fish Neurobiology, edited by Northcutt RG, Davis RE. Ann Arbor, MI: Univ. of Michigan Press, 1983, p. 89–123 [Google Scholar]
- Popper AN, Platt C. Inner ear and lateral line. In: The Physiology of Fishes, edited by Evans DH. Boca Raton, FL: CRC, 1993, p. 99–136 [Google Scholar]
- Ricci AJ, Cochran SL, Rennie KJ, Correia MJ. Vestibular type I and type II hair cells. 2. Morphometric comparisons of dissociated pigeon hair cells. J Vestib Res 7: 407–420, 1997 [PubMed] [Google Scholar]
- Ricci AJ, Wu YC, Fettiplace R. The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. J Neurosci 18: 8261–8277, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Crawford AC, Fettiplace R. Active hair bundle motion linked to fast transducer adaptation in auditory hair cells. J Neurosci 20: 7131–7142, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe MH, Peterson EH. Autocorrelation analysis of hair bundle structure in the utricle. J Neurophysiol 96: 2653–2669, 2006 [DOI] [PubMed] [Google Scholar]
- Russell IJ, Richardson GP, Kössl M. The responses of cochlear hair cells to tonic displacements of the sensory hair bundle. Hear Res 43: 55–70, 1989 [DOI] [PubMed] [Google Scholar]
- Russell IJ, Kössl M, Richardson GP. Nonlinear mechanical responses of mouse cochlear hair bundles. Proc R Soc Lond B 250: 217–227, 1992 [DOI] [PubMed] [Google Scholar]
- Severinsen SA, Jorgensen JM, Nyengaard JR. Structure and growth of the utricular macula in the inner ear of the slider turtle Trachemys scripta. J Assoc Res Otolaryngol 4: 505–520, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoon C, Grant W. Biomechanics of hair cell kinocilia: experimental measurement of kinocilium shaft stiffness and base rotational stiffness with Euler-Bernoulli and Timoshenko beam analysis. J Exp Biol 214: 862–870, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoon C, Peterson EH, Grant JW. Bundle mechanics depend on bundle structure in turtle utricle (Abstract). Assoc Res Otolaryngol Abstr 28: 300, 2005 [Google Scholar]
- Strelioff D, Flock A. Stiffness of sensory-cell hair bundles in the isolated guinea pig cochlea. Hear Res 15: 19–28, 1984 [DOI] [PubMed] [Google Scholar]
- Szymko YM, Dimitri PS, Saunders JC. Stiffness of hair bundles in chick cochlea. Hear Res 59: 241–249, 1992 [DOI] [PubMed] [Google Scholar]
- Vollrath MA, Kwan KY, Corey DP. The micromachinery of mechanotransduction in hair cells. Annu Rev Neurosci 30: 339–365, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenberger FT, Watson JC, Hong L. Glass fibers. In ASM Handbook. Vol. 21: Composites, edited by Miracles DB, Donaldson SL. Material Park, OH: ASM International, 2001, p. 27–34 [Google Scholar]
- Wilcox RR. Introduction to Robust Estimation and Hypothesis Testing. Amsterdam: Elsevier, 2005 [Google Scholar]
- Xue J, Peterson EH. Spatial patterns in the structure of the otolithic membranes (Abstract). Assoc Res Otolaryngol Abstr 26: 32, 2003 [Google Scholar]
- Xue J, Peterson EH. Hair bundle heights in the utricle: differences between macular locations and hair cell types. J Neurophysiol 95: 171–186, 2006 [DOI] [PubMed] [Google Scholar]
- Xue J, Miller I, Peterson EH. Coupling between hair bundles and otoconial membrane in turtle utricle (Abstract). Assoc Res Otolaryngol Abstr 30: 326, 2007 [Google Scholar]
- Yohai VJ. High breakdown-point and high efficiency estimates for regression. Ann Stat 15: 642–665, 1987 [Google Scholar]