Abstract
In randomly bred rats, orally applied ethanol stimulates neural substrates for appetitive sweet taste. To study associations between ethanol's oral sensory characteristics and genetically mediated ethanol preference, we made electrophysiological recordings of oral responses (spike density) by taste-sensitive nucleus tractus solitarii neurons in anesthetized selectively bred ethanol-preferring (P) rats and their genetically heterogeneous Wistar (W) control strain. Stimuli (25 total) included ethanol [3%, 5%, 10%, 15%, 25%, and 40% (vol/vol)], a sucrose series (0.01, 0.03, 0.1, 0.3, 0.5, and 1 M), and other sweet, salt, acidic, and bitter stimuli; 50 P and 39 W neurons were sampled. k-means clustering applied to the sucrose response series identified cells showing high (S1) or relatively low (S0) sensitivity to sucrose. A three-way factorial analysis revealed that activity to ethanol was influenced by a neuron's sensitivity to sucrose, ethanol concentration, and rat line (P = 0.01). Ethanol produced concentration-dependent responses in S1 neurons that were larger than those in S0 cells. Although responses to ethanol by S1 cells did not differ between lines, neuronal firing rates to ethanol in S0 cells increased across concentration only in P rats. Correlation and multivariate analyses revealed that ethanol evoked responses in W neurons that were strongly and selectively associated with activity to sweet stimuli, whereas responses to ethanol by P neurons were not easily associated with activity to representative sweet, sodium salt, acidic, or bitter stimuli. These findings show differential central neural representation of oral ethanol between genetically heterogeneous rats and P rats genetically selected to prefer alcohol.
Keywords: genetics, neural coding, solitary tract, taste, alcohol
the consumption of alcohol engages and is regulated by neural systems mediating responses to naturally reinforcing ingestive stimuli (Anstrom et al. 2003; Freedland et al. 2001; Reid 1996; Thiele et al. 1998; Zhang and Kelley 2002). Genetic variation in such systems may contribute to differential susceptibility to excessive alcohol intake (Murphy et al. 2002). Within this context, one of the most salient predictors of alcohol intake observed across species is preference for and consumption of naturally rewarding sweet substances. Human alcoholics exhibit preference for more highly concentrated sucrose solutions than nonalcoholic control subjects (Kampov-Polevoy et al. 1997, 1998), and this relationship is most prominent in subtypes of alcoholism with a familial or genetic component (Kampov-Polevoy et al. 1998, 2001). Positive relationships between ethanol and sweet preference also exist in multiple lines of rodents selectively bred for divergent ethanol intake (Dyr and Kostowski 2000; Sinclair et al. 1992; Stewart et al. 1994; Woods et al. 2003) and in the F2 progeny derived from crossbreeding ethanol-preferring and -nonpreferring lines (Foroud et al. 2002; Overstreet et al. 1993). These data suggest that inherited variation in a common neurobiological mechanism(s) mediates associations between ethanol and sweet intake.
Several lines of evidence suggest that gustatory signals, which guide ingestive behaviors impacting nutritional status, may influence the relationship between ethanol and sweet preference. In humans, ethanol elicits oral sensations described as having a sweet taste component (Scinska et al. 2000). Rodents also perceive similarities in the oral properties of ethanol and sweet-tasting stimuli, as conditioned aversions to the orosensory features of ethanol generalize to sucrose mixtures in rats (Di Lorenzo et al. 1986; Kiefer et al. 1990; Kiefer and Lawrence 1988; Kiefer and Mahadevan 1993) and taste aversions to sucrose alone generalize to ethanol in C57BL/6J (B6) mice (Blizard and McClearn 2000). Furthermore, neurophysiological studies across multiple species have demonstrated positive associations between responses to oral alcohol and sweet stimuli in peripheral gustatory fibers (Danilova and Hellekant 2000; Diamant et al. 1963; Hellekant et al. 1997; Sako and Yamamoto 1999) and central taste-sensitive neurons (Brasser et al. 2010; Di Lorenzo et al. 1986; Lemon et al. 2004; Lemon and Smith 2005). Selective antagonism of sweet taste receptors also directly suppresses oral responses to alcohol by gustatory-sensitive sensory neurons (Lemon et al. 2004; Sako and Yamamoto 1999), implicating sweet receptors in the transduction of alcohol taste. These data strongly support the idea that ethanol's oral sensory characteristics are detected and registered in part by neural pathways that process appetitive sweet taste and implicate the gustatory system as a potential contributor to the well-established relationship between ethanol and sweet intake.
The ability of ethanol to stimulate neural substrates for sweet taste is not trivial given evidence that activation of such circuitry engages brain reinforcement (i.e., dopaminergic, opioidergic) mechanisms that may motivate subsequent intake (Kelley et al. 2002; Norgren et al. 2006). Prior genetic mapping studies in mice have revealed a common chromosomal locus contributing to alcohol and sweetener consumption (the Ap3q alcohol preference/Sac locus; Bachmanov et al. 2002) that contains the gene for the T1r3 taste receptor protein involved in sweet taste transduction (Bachmanov et al. 2001; Damak et al. 2003; Kitagawa et al. 2001; Max et al. 2001; Montmayeur et al. 2001; Nelson et al. 2001; Sainz et al. 2001; Zhao et al. 2003). Knockout mice lacking the T1r3 receptor display substantially reduced intake of and preference for alcohol and sweet stimuli, but normal intake of other tastants, relative to B6 wild-type mice (Blednov et al. 2008; Brasser et al. 2010). Moreover, central taste-sensitive neurons in T1r3 receptor-deficient mice show suppressed electrophysiological responses to oral ethanol and sweet stimuli, but not other tastants, relative to B6 control animals (Brasser et al. 2010). The reduced behavioral intake and gustatory neural responses to ethanol following deletion of the T1r3 taste receptor protein suggest a direct influence of taste substrates in ethanol preference.
Several key questions to delineate the involvement of taste circuits in ethanol preference remain to be explored. For one, it is unknown whether oral sensory neural responses to ethanol differ in animals genetically selected to prefer ethanol compared with nonselected control animals. Here, we made electrophysiological recordings from individual taste-sensitive neurons in the nucleus of the solitary tract (NTS) of anesthetized selectively bred ethanol-preferring (P) rats (Lumeng et al. 1977) and genetically heterogeneous Wistar (W) rats, the progenitor strain from which the P line was developed. Neurons were stimulated by oral application of an ethanol concentration series and tastants representative of sweet, sodium salt, and aversive gustatory sensations, including acidic and bitter stimuli. Data were analyzed by using higher-order factorial, correlational, and multivariate techniques to probe the relationship between oral sensory neural responses to ethanol and genetically mediated ethanol preference. We hypothesized that neural taste responses to ethanol and preferred stimuli should show heightened similarity in P rats if ethanol's taste features positively influence genetically mediated alcohol preference. However, we found that while ethanol responses in W neurons strongly and selectively correlated with activity to sweet stimuli, the pattern of activity to ethanol across gustatory-sensitive neurons in the P line did not strongly align itself with activity to any taste class tested. These data show differential neural representation of oral ethanol by taste circuits in genetically heterogeneous rats and selectively bred alcohol-preferring P rats. Implications of these findings for alcohol preference are discussed.
METHODS
Animals and preparation.
Adult male selectively bred ethanol-preferring P rats (n = 30) and nonselected W rats (n = 27) were used. P rats were bred at the Indiana Alcohol Research Center at the Indiana University School of Medicine and shipped to our animal housing facility. The majority of P rats used were from breeding generations 66 through 70. W rats were purchased from Harlan Laboratories (Indianapolis, IN). All animals were naive to experimentation and did not have any prior experience with the ethanol and taste solutions used here. Rats were housed in a vivarium that maintained a 12:12-h light-dark cycle and an ambient temperature of ∼23°C. Food and water were available ad libitum. Selectively bred ethanol-nonpreferring (NP) rats, which show a low ethanol-drinking phenotype (Lumeng et al. 1977), were also initially included for neurophysiological recordings at the beginning of the project. However, during the NTS preparation, NP rats displayed unusual bleeding due to a genetic hemophilia that made recordings from this line untenable. Thus the use of the NP line was discontinued and data from NP rats were not used. P and W rats did not display this issue.
Preparation and electrophysiological recording procedures were performed in accordance with protocols reviewed and approved by the Institutional Animal Care and Use Committee and National Institutes of Health guidelines. Rats were anesthetized with urethane (∼1.7 g/kg ip) and pentobarbital (∼50 mg/kg ip). Once rats were anesthetized, a tracheal cannula was inserted to facilitate ease of breathing during solution flow into the mouth. Rats were positioned in a nontraumatic head holder that angled the snout ∼25° downward. A portion of the occipital bone was removed, and parts of the cerebellum were gently aspirated to expose the brain stem surface and allow vertical access to the NTS. Body temperature was held at ∼37°C by a heating pad.
Single-unit electrophysiology.
Our methods for recording from medullary taste-sensitive neurons in rodents have been described in detail previously (Lemon et al. 2003, 2004; Lemon and Margolskee 2009; Lemon and Smith 2005). Briefly, the rostral, gustatory-sensitive region of the NTS was targeted with the use of vascular landmarks present on the dorsal surface of the brain stem (Lemon and Margolskee 2009). Extracellular action potentials were recorded from neurons with etched tungsten microelectrodes insulated except for the tip [z = 2–5 MΩ (10 nA at 1 kHz); FHC, Bowdoinham, ME]. Electrophysiological activity was band-pass (0.3–10 kHz) filtered, AC amplified (Grass P511, high-z probe), and monitored on a storage oscilloscope and loudspeaker. A hydraulic micromanipulator was used to slowly advance the electrode ventrally through brain tissue. Given the focus of this study on oral ethanol and appetitive taste processing, the taste-sensitive region of the NTS, identified by a change in neural activity following gustatory stimulation, was largely sought out with oral application of (in M) 0.5 sucrose, although 0.1 NaCl and 0.01 quinine presented individually or mixed with 0.5 sucrose were also used. NTS search stimuli were presented and rinsed from the mouth with our stimulus delivery system (described below). Individual neurons were recorded pseudorandomly: On a given electrode advancement data were collected from the first taste-sensitive unit showing very good isolation, regardless of whether the cell showed strong responsiveness to the NTS search stimulus. This technique yielded a broad sample of different types of NTS units (cf. Fig. 2), including pools of neurons with differential sensitivity to sucrose (cf. Fig. 3). Unit activity was digitally sampled (25 kHz), and spikes generated by single neurons were identified by waveform consistency and a template-matching algorithm (Spike2 with Power 1401 analog-to-digital converter; CED, Cambridge, UK). Digitized single-unit activity was saved to storage media for off-line analysis.
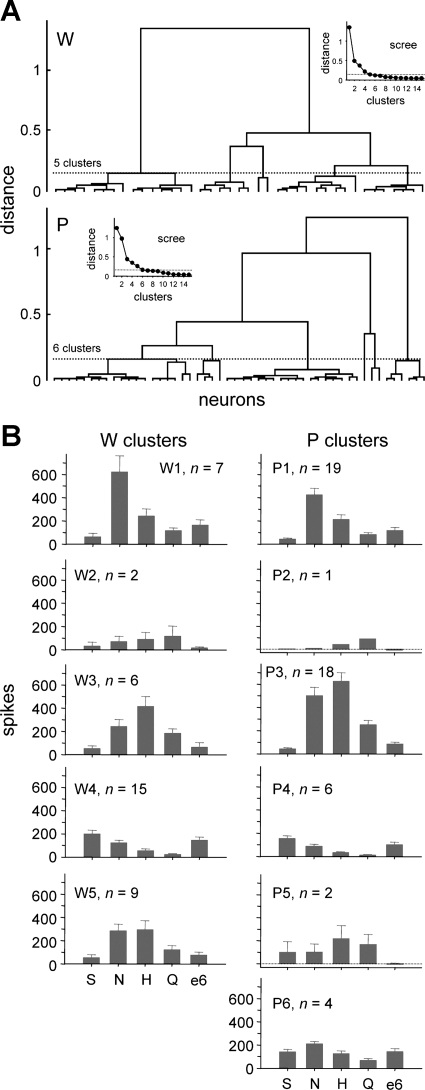
Fig. 2.
A: dendrograms representing the outcome of hierarchical cluster analyses used to define general trends in responding to prototype stimuli (group I, Table 1) among neurons sampled from Wistar (W) and ethanol-preferring P rats. Insets: scree plots used to “cut” tuning clusters from each tree. B: mean + SE responses to 0.5 M sucrose (S), 0.1 M NaCl (N), 0.01 M HCl (H), 0.01 M quinine (Q), and 40% ethanol (e6) observed in the 5 W and 6 P neural clusters identified in A. The number of neurons in each cluster is given.
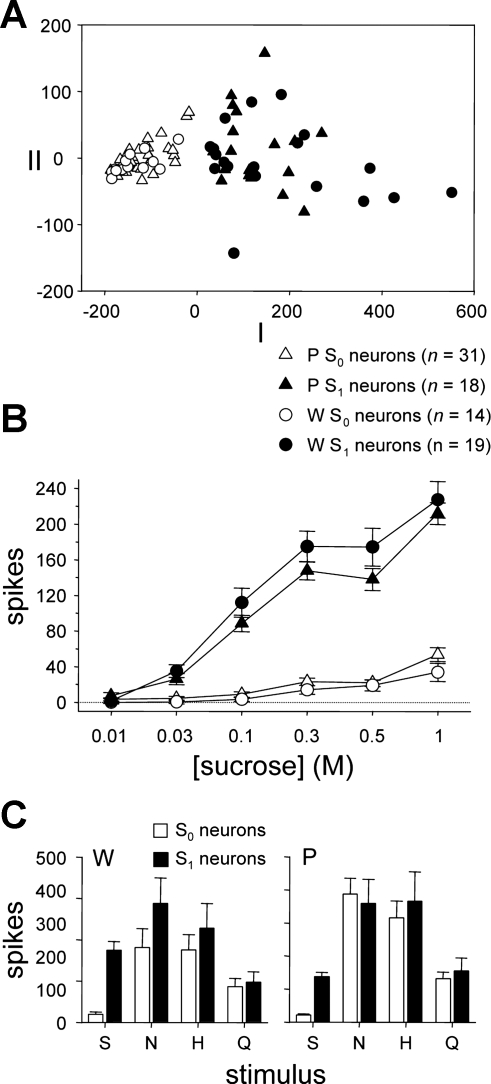
Fig. 3.
A: outcome of multidimensional scaling (MDS) of neurons recorded from W (circles) and ethanol-preferring P rats (triangles) according to their responses to 0.01, 0.03, 0.1, 0.3, 0.5, and 1 M sucrose. The first 2 dimensions of 3-dimensional metric MDS applied to a matrix of Euclidean distances between neurons are shown. Open symbols denote neurons showing low sensitivity to the sucrose series (S0 cells), and filled symbols indicate neurons with high sensitivity to sucrose (S1 cells), as identified by k-means clustering. The number of neurons in each category is indicated. MDS and k-means gave similar partitions of cells. B: mean ± SE responses to the sucrose series by S0 and S1 neurons recorded from W and P rats. Key is shared with A. C: mean + SE responses to prototype stimuli (group I, Table 1) by S0 and S1 neurons recorded from W and P rats.
Taste stimuli.
Well-isolated neurons showing a change in firing rate to oral stimulation with chemical taste stimuli were subsequently tested with oral application of six concentrations of ethanol, ranging from 3% to 40% (vol/vol), and 19 taste stimuli including several concentrations of sucrose, other sweet stimuli, sodium salts, halide salts, acidic stimuli, and bitter tastants (Table 1). The majority of stimuli and selected concentrations were comparable to those used in our prior investigation of ethanol taste processing by brain stem neurons in Sprague-Dawley (SD) rats (Lemon et al. 2004). All stimuli were of high purity and dissolved in reverse osmosis-purified water. Ethanol solutions were diluted from a 95% beverage-grade ethanol stock. All stimuli and water rinse solutions were presented at room temperature. All chemicals were purchased from Sigma (St. Louis, MO).
Table 1.
Stimuli, concentrations, and abbreviations
| Stimulus | Class | Concentration | Abbreviation |
|---|---|---|---|
| Group I | |||
| Sucrose | Sweet | 0.5 M | S |
| NaCl | Salt | 0.1 M | N |
| HCl | Acidic | 0.01 M | H |
| Quinine-HCl | Bitter | 0.01 M | Q |
| Group II | |||
| Ethanol | Alcohol | 3% | e1 |
| Ethanol | Alcohol | 5% | e2 |
| Ethanol | Alcohol | 10% | e3 |
| Ethanol | Alcohol | 15% | e4 |
| Ethanol | Alcohol | 25% | e5 |
| Ethanol | Alcohol | 40% | e6 |
| Group III | |||
| Sucrose | Sweet | 0.01 M | s1 |
| Sucrose | Sweet | 0.03 M | s2 |
| Sucrose | Sweet | 0.1 M | s3 |
| Sucrose | Sweet | 0.3 M | s4 |
| Sucrose | Sweet | 1 M | s5 |
| Group IV | |||
| d-Fructose | Sweet | 1 M | f |
| d-Glucose | Sweet | 1 M | g |
| Na+-saccharin | Artificial sweet | 0.005 M | sa |
| Sucrose + quinine-HCl | Mixture | 0.1 + 0.0001 M | sq |
| Na+-nitrate | Salt | 0.1 M | nn |
| Citric acid | Acidic | 0.01 M | c |
| Potassium chloride | Salt | 0.2 M | k |
| Magnesium chloride | Salt | 0.01 M | m |
| Nicotine | Bitter | 0.01 M | ni |
| Quinine-HCl | Bitter, weak | 0.0001 M | q |
For sequencing during data collection, stimuli were assigned to one of four groups (Table 1: group I, prototype taste stimuli; group II, ethanol series; group III, sucrose series; group IV, additional taste stimuli) tested in numeric order and stimulus ordering within a group was varied. The ordering of stimuli in groups I and IV was randomized for each cell. Concentration series in groups II and III were always presented in ascending order. This procedure intended to first collect a common basic set of taste (group I) and ethanol (group II) data from neurons in the event that cells were lost later in a recording session.
During taste trials, stimuli were applied to the anterior tongue and palate, consistent with our prior neurophysiological study on ethanol taste in randomly bred rats (Lemon et al. 2004). Taste receptors on the anterior tongue and palate are innervated by afferents of cranial nerve VII, which is known to contribute to circuits serving taste discriminative function (Spector and Grill 1992; Spector et al. 1997; St. John and Spector 1998). Stimulus solutions were flowed into the mouth at a constant rate (∼4 ml/s) with a gravity flow system, as described previously (Lemon et al. 2003, 2004; Lemon and Smith 2005). Stimuli entered and exited the mouth, falling into a drain positioned beneath the rat's mandible; the solutions were not ingested. The delivery tubing was positioned in the mouth such that stimuli first contacted the palate and then the anterior tongue. Visual inspection indicated that any lag between palatal and tongue stimulation was minimal and <1 s. Stimulus trials were 30 s long and began by flowing purified water vehicle into the rat's mouth for 10 s. This initial water rinse was intended to preadapt and control for any mechanical response component to oral stimulation. At 10 s into a trial, solution flow into the mouth changed immediately from water to a taste stimulus presented for 10 s. An inline three-way electronic solenoid fluid valve positioned near the rat's mouth and controlled by the data acquisition system achieved precisely timed switching between water and taste stimuli. For the remaining 10 s of the trial and longer, stimuli were rinsed from the mouth by switching solution flow back to purified water. All solution delivery tubing and the fluid passageways of the solenoid valve were thoroughly rinsed between trials with ∼125 ml of purified water. The animal's mouth was also bathed by this intertrial water rinse through the delivery system to ensure removal of the stimulus on the preceding trial from oral epithelia, precluding adaptation effects. The intertrial interval was 1.5–2 min and was sufficient to allow neurons to return to baseline levels of spontaneous spike discharge. Some stimulus trials were replicated to determine the reliability of evoked activity.
Data analysis.
A custom Spike2 script calculated and saved response parameters from template-matched single-unit spike trains recorded on each trial. Oral responses by individual cells were defined as the number of spikes arising during the 10-s stimulus presentation minus the number of spikes during the 10-s prestimulus (water) period. Responses on replicate trials taken from individual neurons were averaged. This method of quantifying neural activity was selected to be consistent with our prior investigation of oral sensory activity to ethanol in rat NTS (Lemon et al. 2004). Statistical analyses of neural response data were performed with MATLAB (v. 7.7, The MathWorks, Natick, MA), the Statistics Toolbox for MATLAB (v. 7.0, The MathWorks), and SPSS (v. 17.0, IBM, Somers, NY) and manually with a spreadsheet program.
Hierarchical cluster analysis was used to identify general trends in gustatory responding across W and P neurons. This analysis suggested groups of neurons based on taste profile dissimilarity, as given by 1 minus the Pearson coefficient of correlation (r) among responses to prototype taste stimuli (group I, Table 1). Cluster analysis was performed with an unweighted average distance amalgamation algorithm. Dendrograms describing dissimilarity (and similarity) relationships among neurons and neural groups within the W and P lines were constructed from the resulting cluster distances. The correct number of clusters for each solution was determined by inspection of a “scree plot” of cluster distances against amalgamation steps (cf. Geran and Travers 2006; Lemon and Margolskee 2009) and noting where the plot “elbowed.” Cluster analysis suggested neural groupings based on responses to multiple stimuli, which is appropriate as individual NTS neurons can show sensitivity to several types of taste stimuli.
Prior recordings from taste-sensitive NTS neurons in rodents revealed that oral responses to ethanol are larger in cells showing significant activity to sucrose relative to sucrose-unresponsive neurons (Brasser et al. 2010; Lemon et al. 2004). In the present study, the association between sensitivity to sucrose and ethanol was examined for W and P neurons by dividing the neural sample into groups showing relatively low (S0) or high (S1) sensitivity to sucrose and evaluating activity to ethanol across and within these neuronal groups and rat lines. The k-means algorithm (Hartigan and Wong 1979) applied to responses to several concentrations of sucrose partitioned neurons into S0 and S1 types. In brief, k-means assigned neurons to one of two clusters (i.e., k = 2) in a manner that minimized dissimilarity among the sucrose response properties of neurons within a cluster. Pairwise dissimilarity among the sucrose response profiles of neurons was measured using squared Euclidean distance, a metric sensitive to response magnitude differences. The endpoint of k-means clustering as applied here was that neurons in one cluster (e.g., S0) were as similar to one another as possible by their responses to sucrose and as different as possible from neurons in the other cluster (e.g., S1). To avoid local minima, k-means was replicated at least 50 times using random starting configurations to identify among replicates the best clustering solution (i.e., that which returned the lowest total sum of distances). The best solution was used for data interpretation, and the same best solution emerged over multiple runs of the analysis.
Taste responses to concentration series of sucrose and ethanol were analyzed with higher-order factorial ANOVAs that included as factors the sucrose sensitivity status of neurons (given by k-means clustering), stimulus concentration, and rat line. Simple interactions and simple effects were further analyzed with lower-order ANOVAs. Where applicable, post hoc comparisons of means for repeated factors were carried out with Dunn's multiple-comparison test, which provides appropriate control of α for pairwise comparisons in repeated-measures designs (Toothaker 1991).
Correlation and multivariate analyses were used to determine relationships among across-neuron responses to ethanol and other taste stimuli. Across-neuron responses associate with qualitative features of tastants: Stimuli with similar tastes produce similar across-neuron patterns of activity, and dissimilar tastes evoke disparate patterns (Erickson 1963; Smith et al. 1983). Pearson's r indexed the degree of correlation between across-neuron responses (Erickson 1963; Gill and Erickson 1985). Fisher-transformed r values for responses to ethanol and tastants were compared between lines with a z-statistic (Howell 2010). Metric multidimensional scaling (MDS; Kruskal and Wish 1978) was applied to further explore correlations among activity to ethanol and all tastants in each rat line. MDS produced a visual representation capturing dissimilarity (1 − r), and conversely similarity, among across-neuron responses to stimuli. To avoid local minima, each application of MDS was repeated 50 times with random starting configurations, and the solution showing the overall least stress (i.e., badness of fit to the correlation data) was used for interpretation. Similar solutions and stress values were achieved on the majority of the replicate runs.
RESULTS
Neural sampling characteristics.
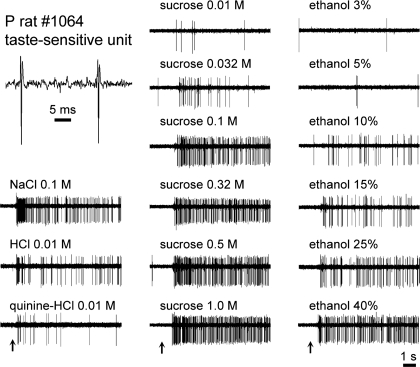
Trains of action potentials recorded from 89 taste-sensitive neurons were analyzed. Fifty neurons were recorded from P rats, and 39 neurons were recorded from W rats. Analyses of P neurons included data from 1,311 completed trials, where the mean number of trials sampled from a single neuron was 26.2 ± 0.8 (SE) (minimum = 10; maximum = 41). For W cells, 872 trials were acquired and an average of 22.4 ± 1.1 trials (minimum = 10; maximum = 35) were sampled from each unit. Stimuli tested repeatedly at the same concentration were presented twice on average. No difference was found between initial and subsequent responses to stimuli tested more than once [not significant (n.s.) paired samples t-test: P cells, t110 = 1.1, P = 0.3; W cells, t42 = 1.9, P = 0.07], suggesting that the initial response to a stimulus provided a reliable estimate of evoked magnitude. Mean prestimulus (baseline) activity during prototype stimulus trials (group I, Table 1) was 2.5 ± 0.2 (SE) spikes/s for P neurons and 1.4 ± 0.1 spikes/s for W neurons. Isolated neurons remained viable and stable during data collection. In cells tested with group I stimuli both initially and again after completion of all group IV trials, responses to prototype stimuli did not change over time (13 cells, repeated-measures ANOVA, n.s. time × stimulus interaction, F3,36 = 0.2, P = 0.9). Moreover, prestimulus baseline spike discharge rates did not differ between the first and last trials in a unit recording session (n.s. paired samples t-test: P cells, t49 = −0.4, P = 0.7; W cells, t38 = −0.7, P = 0.5). The quality of the recording from a sample neuron (P rat) is shown in Fig. 1.
Fig. 1.
Digital oscilloscope records showing activity by an individual taste-sensitive neuron recorded from an ethanol-preferring (P) rat. Top left: magnified view of spikes generated by this cell when driven by gustatory stimulation. Upward arrows below response traces indicate the onset of a given taste stimulus (denoted above each trace).
To evaluate the distributions of neurons sampled in W and P rats, groupings of cells sharing similar tuning profiles were identified in each line by hierarchical cluster analyses of responses to group I stimuli (Table 1), which included prototypical sweet, sodium salt, acidic, and bitter tastants. W and P neurons were clustered separately. Five clusters were identified for W neurons, and 6 clusters were found for P cells. These neural groupings included cells that were oriented toward but not specifically tuned to NaCl (clusters W1 and P1; Fig. 2), responded the strongest to quinine (W2 and P2), were broadly sensitive to NaCl, HCl, and quinine (W3 and P3), and were oriented toward sucrose and NaCl (W4 and P4). A wide array of common types of taste-sensitive neurons was sampled from W and P rats. Other clusters included P neurons broadly sensitive across prototype stimuli (P5 and P6) and W neurons oriented to HCl and NaCl (W5). As observed in many other neurophysiological studies of the NTS, taste-sensitive neurons recorded here were generally multisensitive: They responded to stimuli from multiple taste categories.
Central neural responses to oral sucrose and ethanol in ethanol-preferring P and Wistar rats.
k-means clustering applied to responses to 0.01, 0.03, 0.1, 0.3, 0.5, and 1 M sucrose identified W and P neurons showing low (S0) or relatively high (S1) sensitivity to sucrose (Fig. 3A). This multivariate grouping technique could be applied only to neurons tested with the entire sucrose series, which included almost all (33 W and 49 P) recorded cells. For W neurons, k-means identified 42% of analyzed cells as S0 and 58% as S1. For P cells, k-means typed 63% of units as S0 and 37% as S1. Factorial ANOVA indicated that responses to sucrose were not influenced by rat line (n.s. main effect of rat line, F1,78 = 0.5, P = 0.5; n.s. rat line × sucrose concentration interaction, F5,390 = 1.3, P = 0.2; n.s. rat line × sucrose sensitivity interaction, F1,78 = 3.0, P = 0.08; n.s. rat line × sucrose sensitivity × sucrose concentration interaction, F5,390 = 1.4, P = 0.2) but, expectedly, were dependent on concentration and a neuron's sucrose sensitivity status (sucrose sensitivity × sucrose concentration interaction, F5,390 = 99.6, P < 10−3; Fig. 3B). For S1 neurons, responses to sucrose were influenced by concentration (simple effect of concentration, F5,180 = 163.7, P < 10−3), with mean responses to 0.03, 0.1, 0.3, and 1 M sucrose significantly larger than the response to lower concentrations of sucrose; responses to 0.5 M sucrose were greater than activity to 0.01, 0.03, and 0.1 M sucrose (Dunn multiple-comparison tests, all P < 0.05) but not 0.3 M (P > 0.05). Activity to sucrose by S0 neurons also depended on concentration (simple effect of concentration, F5,220 = 36.3, P < 10−3), with responses to 0.3 and 1 M sucrose larger than activity to lower concentrations of sucrose and responses to 0.5 M sucrose exceeding activity to 0.01, 0.03, and 0.1 M sucrose (Dunn's multiple-comparison tests, all P < 0.05) but not 0.3 M (P > 0.05). Comparing across neuronal types, mean responses to 0.03, 0.1, 0.3, 0.5, and 1 M sucrose were significantly larger in S1 neurons compared with S0 cells (simple effects of sucrose sensitivity, F1,80 ≥ 35.3, P < 10−3), confirming that neurons classified as S1 indeed display significantly greater activity to sucrose. On average, S0 and S1 neurons in both lines also show activity to nonsweet taste stimuli (Fig. 3C; cf. Lemon et al. 2004).
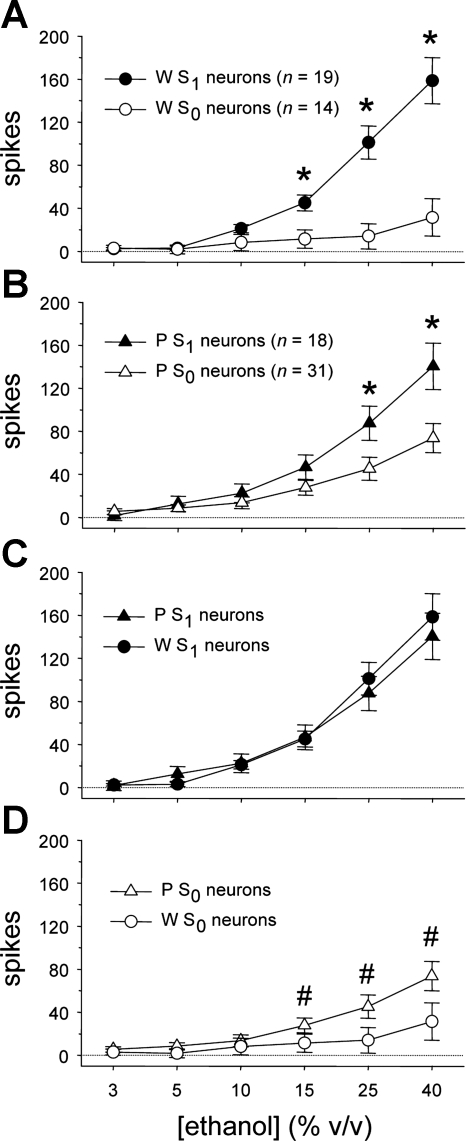
Although the overall neural response to oral ethanol was similar between W and P rats (n.s. main effect of rat line, F1,78 = 0.7, P = 0.4; n.s. rat line × ethanol concentration interaction, F5,390 = 0.3, P = 0.9), ethanol activity carried by S1 and S0 cell types varied across rat line and ethanol concentration (rat line × sucrose sensitivity × ethanol concentration interaction, F5,390 = 3.0, P = 0.01). This higher-order effect was probed by using simple two-way ANOVAs to examine how ethanol responses were influenced by concentration and neural sensitivity to sucrose within each line. For neurons recorded from W rats, responses to ethanol were dependent on concentration and a neuron's sucrose sensitivity status (main effect of ethanol concentration, F5,155 = 38.9, P < 10−3; sucrose sensitivity × ethanol concentration interaction, F5,155 = 20.0, P < 10−3; Fig. 4A). Mean responses to 15%, 25%, and 40% ethanol were larger in W S1 neurons compared with W S0 cells (simple effects of sucrose sensitivity, F1,31 > 8.9, P ≤ 0.005). Thus, in taste-sensitive neurons of W rats, activity to higher concentrations of ethanol was stronger in cells with relatively high sensitivity to sucrose.
Fig. 4.
A: mean ± SE responses to the ethanol concentration series by neurons with low (S0) and high (S1) sensitivity to sucrose recorded from Wistar (W) rats. *Simple effect of neural sucrose sensitivity on ethanol activity (P < 0.05). B: mean ± SE responses to ethanol by S0 and S1 neurons in ethanol-preferring P rats. *Simple effect of neural sucrose sensitivity on ethanol activity (P < 0.05). C: mean ± SE responses to ethanol by S1 neurons in W and P rats. D: mean ± SE responses to ethanol by S0 neurons in W and P rats. #Response to ethanol by S0 neurons in P rats that is greater than activity to each lower ethanol concentration measured in this line (P < 0.05).
Activity to oral ethanol by taste-sensitive neurons recorded from P rats was also influenced by concentration and neural sucrose sensitivity (main effect of ethanol concentration, F5,235 = 71.7, P < 10−3; sucrose sensitivity × ethanol concentration interaction, F5,235 = 8.2, P < 10−3; Fig. 4B). Mean responses to 25% and 40% ethanol were larger in P S1 neurons compared with P S0 cells (simple effects of sucrose sensitivity, F1,47 > 5.1, P < 0.03). Thus, within the P line, activity to the two highest concentrations of ethanol was greater in cells showing heightened sensitivity to sucrose.
Additional two-way line × concentration ANOVAs were used to directly compare any differences in the ethanol responses of W and P neurons within S1 and S0 neuron types. Responses to ethanol by S1 neurons did not differ between W and P rats (n.s. main effect of line, F1,35 = 0.07, P = 0.8; n.s. rat line × concentration interaction, F5,175 = 0.7, P = 0.6), although they were, as expected, influenced by concentration (main effect of ethanol concentration, F5,175 = 87.3, P < 10−3; Fig. 4C). For S1 cells, responses to 10%, 15%, 25%, and 40% ethanol were significantly larger than responses to all lower ethanol concentrations (Dunn's multiple-comparison test, all P < 0.05). These results indicate that ethanol evokes concentration-dependent responses in S1 neurons that are of similar magnitude in W and P rats.
For S0 neurons, ethanol concentration response functions varied by rat line (main effect of ethanol concentration, F5,215 = 17.8, P < 10−3; rat line × concentration interaction, F5,215 = 3.4, P = 0.006; Fig. 4D). Although responses at each individual concentration of ethanol did not significantly differ between S0 neurons from W and P rats (n.s. simple effects of rat line, F1,43 < 3.3, P > 0.07), line determined the degree to which S0 neurons increased their firing rate with rising ethanol concentration. Specifically, ethanol activity of S0 cells in the P line was influenced by concentration (simple effect of ethanol concentration, F5,150 = 25.1, P < 10−3), with mean responses to 15%, 25%, and 40% ethanol significantly greater than responses to all lower ethanol concentrations (Dunn's multiple-comparison test, all P < 0.05). Thus S0 neurons in P rats showed concentration-dependent responses to higher concentrations of ethanol that increased in magnitude with increasing concentration. For W rats, although a simple effect of concentration was detected for ethanol responses by S0 neurons (F5,65 = 3.5, P = 0.008), follow-up comparisons did not reveal any significant differences among responses to 3%, 5%, 10%, 15%, 25%, and 40% ethanol within W S0 cells (Dunn's multiple-comparison test, all P > 0.05). These results indicate that S0 neurons in P, but not W, rats display increased firing rates to increasing concentrations of orally applied ethanol.
For stimuli other than sucrose and ethanol, analyses of neurons where all taste trials could be completed (W cells, n = 25; P cells, n = 42) showed that activity to some stimuli differed across rat line (rat line × stimulus interaction, F12,780 = 4.4, P < 0.001), with larger mean responses in P rats (simple effects of rat line, F1,65 > 4.08, P < 0.05) to saccharin [P cells, 132.9 ± 17 (SE) spikes; W cells, 77.7 ± 15.2 spikes], NaCl (P cells, 381.2 ± 43.1 spikes; W cells, 234.3 ± 48.3 spikes), NaNO3 (P cells, 371.1 ± 44.2 spikes; W cells, 222.8 ± 47.3 spikes), HCl (P cells, 349.1 ± 50.7 spikes; W cells, 192.8 ± 41.2 spikes), and citric acid (P cells, 315 ± 44.7 spikes; W cells, 181.7 ± 40.6 spikes).
Central oral sensory representation of ethanol in ethanol-preferring P and Wistar rats.
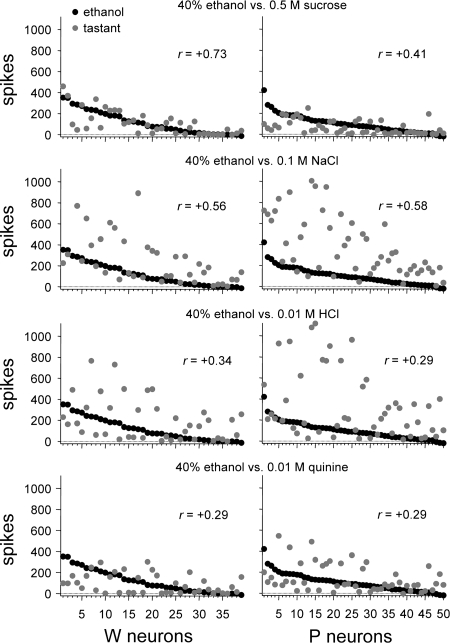
The above analyses of ethanol responses revealed that ethanol strongly activated S1 neurons, showing high sensitivity to sucrose, in both rat lines. Yet in P, but not W, rats ethanol also effectively stimulated S0 neurons, which showed low sucrose sensitivity. These findings could reflect that oral ethanol is differentially represented by taste-sensitive neurons in W and P rats. To explore this, Pearson coefficients of correlation (r) among across-neuron responses to 40% ethanol (the concentration producing the largest magnitude response) and prototype tastants (group I, Table 1) were calculated and compared across lines. For W neurons, responses to 40% ethanol showed the highest correlation with activity to (in M) 0.5 sucrose (r = +0.73) and lower correlations with responses to 0.1 NaCl (r = +0.56), 0.01 HCl (r = +0.34), and 0.01 quinine (r = +0.29; Fig. 5). A linear relationship could account for just over half (53%) of the variance between responses of W neurons to 40% ethanol and 0.5 sucrose (r2 = 0.53). A different result was observed in P neurons, where 40% ethanol produced an activity pattern that correlated most strongly with the response to 0.1 NaCl (r = +0.58), followed by activity to 0.5 sucrose (r = +0.41), 0.01 HCl (r = +0.29), and 0.01 quinine (r = +0.29). For P neurons, a linear function could explain only 34% (r2 = 0.34) of the response variance to 40% ethanol and 0.1 NaCl and only 17% (r2 = 0.17) of the variance in activity to 40% ethanol and 0.5 sucrose. The coefficient of correlation between responses to 40% ethanol and 0.5 M sucrose was significantly larger in W neurons compared with P cells (|zobt| = 2.28, P < 0.05). Correlations between responses to 40% ethanol and 0.1 NaCl, 0.01 HCl, and 0.01 quinine did not differ between lines (|zobt| ≤ 0.25, P > 0.05). Thus W neurons displayed responses to 40% ethanol that correlated most robustly with activity to 0.5 M sucrose, and the strength of this correlation was significantly greater than that observed for P cells, which displayed only moderately or minimally correlated responses between ethanol and prototype taste stimuli in general.
Fig. 5.
Across-unit responses to each prototype stimulus (group I, Table 1) relative to activity to 40% ethanol for neurons recorded from W (left) and alcohol-preferring P (right) rats. Neurons are rank-ordered along the x-axis by their response to 40% ethanol. The Pearson coefficient of correlation (r) calculated between the across-neuron response pattern to 40% ethanol and each stimulus is given.
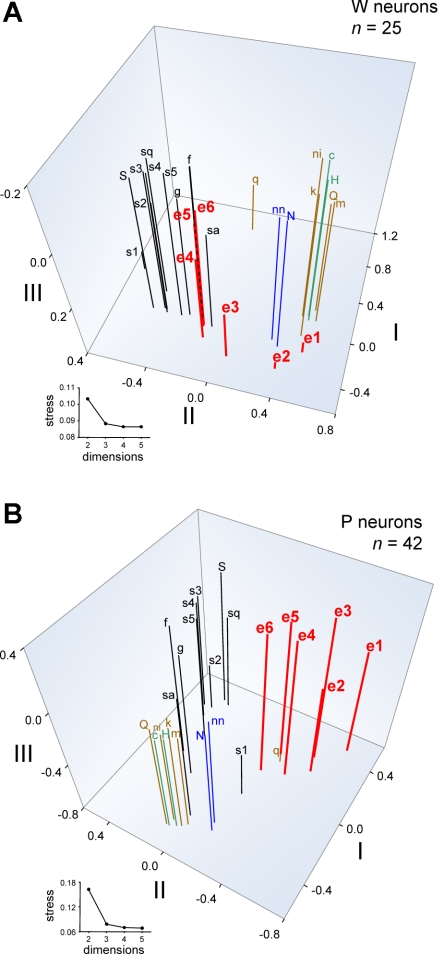
Coefficients of correlation provide a useful index of pairwise similarity between neural activity to ethanol and selected stimuli. However, a more thorough description of dissimilarities and similarities among responses to all ethanol concentrations and all tastants can be obtained through MDS analysis. For each line, MDS was used to visually represent relationships between across-neuron responses to all inputs and was applied to response data from 25 W and 42 P neurons tested with all 25 stimuli (Table 1). Although not all cells could be used for MDS, the relative proportions of neurons from each taste profile class (Fig. 2) did not differ between the neural subsets analyzed by MDS and all sampled neurons (W cells: χ2 = 9.3, df = 4, P = 0.053; P cells: χ2 = 0.8, df = 5, P = 1). Furthermore, the proportions of S0 and S1 neurons used in MDS [W cells: n = 11 S0 (44%), 14 S1 (56%); P cells: 26 S0 (62%), 16 S1 (38%)] and in the overall neuronal sample did not differ in either rat line (W cells, χ2 = 0.2, df = 1, P = 0.7; P cells, χ2 = 0.04, df = 1, P = 0.8). MDS captured meaningful relationships among across-neuron responses to tastants. The MDS plot for W cells in Fig. 6A shows that activity to sweet, sodium salt, and aversive halide, acidic, and bitter stimuli (Table 1) were distinguished by the scaling space. Based on positioning in the MDS solution, where stimuli producing similar response patterns are placed near one another, responses to increasing concentrations of ethanol became increasingly more similar to those evoked by sweet stimuli and unlike activity to salts or aversive tastants. Specifically, responses to 15%, 25%, and 40% ethanol by W neurons were highly similar to activity to fructose, glucose, and saccharin. These findings replicate previous data showing strong and selective similarity between oral responses to sweet stimuli and high concentrations of ethanol by NTS neurons in randomly bred SD rats (Lemon et al. 2004).
Fig. 6.
Three-dimensional spaces showing the outcome of metric MDS analyses of across-unit responses to all taste stimuli and all concentrations of ethanol by neurons recorded from W (A) and ethanol-preferring P (B) rats. An abbreviation (Table 1) marks the position of each stimulus response in the scaling spaces. The number of neurons in each analysis is indicated. Insets: stress functions for 2- to 5-dimensional solutions. Stress, an index of the badness of fit of the scaling solution to the data, is dramatically reduced with 3-dimensional MDS but is not further appreciably reduced with 4- or 5-dimensional analyses. Thus 3-dimensional MDS was used. Axes represent arbitrary units. Stimuli are color coded according to their general taste category: black, sweet; red, ethanol; blue, sodium salt; green, acid; brown, bitter or aversive.
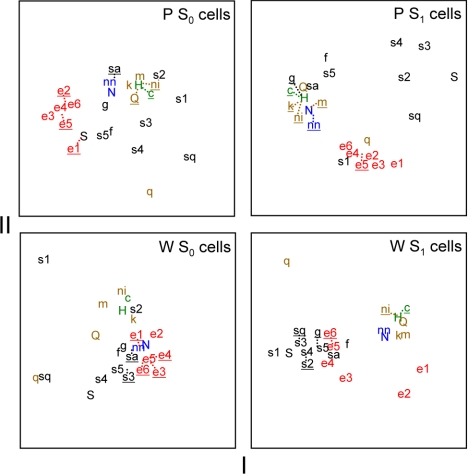
In P neurons, MDS also differentiated activity evoked by sugars, sodium salts, and aversive stimuli, the latter of which strongly clustered in scaling space (Fig. 6B). MDS positioned responses of P neurons to all concentrations of ethanol in a unique region of the scaling space unpopulated by activity to other classes of taste stimuli. This segregation of ethanol by MDS suggests that ethanol's oral sensory neural signal in P rats is not strongly associated with activity to any of the tested taste chemicals, which included various sweet stimuli, sodium salts, and aversive salt, acidic, and bitter tastants. This differs from the pattern observed in W neurons, where responses to moderate and high concentrations of ethanol showed strong and selective similarity to activity evoked by sweet tastants (Fig. 6A). Pearson coefficients of correlation between responses to 40% ethanol, the most effective ethanol concentration, and sweet stimuli including saccharin, fructose, glucose, and several concentrations of sucrose were significantly greater in W neurons compared with P cells (|zobt| > 2.19, P < 0.05; Table 2), further supporting differential neural representation of ethanol between lines. Additional MDS analyses of across-neuron responses by S1 or S0 neurons alone still revealed a relatively distinct clustering of response patterns to ethanol in the P line (Fig. 7).
Table 2.
Pearson coefficients of correlation between activity to 40% ethanol and tastants for 25 W and 42 P neurons tested with all stimuli
| Stimulus | r, vs. 40% Ethanol, W Neurons | r, vs. 40% Ethanol, P Neurons |
|---|---|---|
| S | +0.72 | +0.42 |
| N | +0.60 | +0.56 |
| H | +0.34 | +0.33 |
| Q | +0.23 | +0.33 |
| s1 | +0.18 | +0.27 |
| s2 | +0.78* | −0.04 |
| s3 | +0.79* | +0.27 |
| s4 | +0.80* | +0.40 |
| s5 | +0.86* | +0.61 |
| f | +0.87* | +0.49 |
| g | +0.90* | +0.58 |
| sa | +0.91* | +0.47 |
| sq | +0.69 | +0.34 |
| nn | +0.65 | +0.63 |
| c | +0.30 | +0.28 |
| k | +0.40 | +0.47 |
| m | +0.26 | +0.42 |
| ni | +0.34 | +0.33 |
| q | −0.18 | −0.01 |
Values are Pearson coefficients of correlation between activity to 40% ethanol and tastants for 25 Wistar rat (W) and 42 alcohol-preferring rat (P) neurons tested with all stimuli.
Correlation for an ethanol-stimulus pair that is significantly greater in W neurons compared with P cells (P < 0.05). Stimuli are abbreviated as in Table 1.
Fig. 7.
Plots showing the outcome of metric MDS applied to taste responses by neurons showing either low (S0 cells) or high (S1 cells) sensitivity to sucrose in W and P rats. Each plot shows the first 2 dimensions of solutions resulting from 3-dimensional MDS to reduce complexity. All plots are scaled identically along the x- and y-axes. An abbreviation (Table 1) marks the position of each stimulus response in the scaling spaces. Dotted lines are used to offset overlapping abbreviations (underlined) from their actual position to improve clarity. Stimuli are color coded as in Fig. 6.
DISCUSSION
This study analyzed associations between oral sensory responses to ethanol and taste stimuli by gustatory-sensitive medullary neurons in selectively bred ethanol-preferring P rats and their genetically heterogeneous Wistar control strain. Analyses of W neurons showed that the oral sensory signal for ethanol is predominantly distributed to cells with heightened sensitivity to sucrose (S1 neurons) relative to units showing low sucrose responses (S0 neurons; Fig. 4A). Across W neurons, increasing concentrations of ethanol induced increasing firing rates in S1, but not S0, cells (Fig. 4, C and D). Furthermore, robust and selective similarities between population responses to ethanol and sweet stimuli by W neurons emerged from correlation and multivariate analyses (Figs. 5–7 and Table 2). These data indicate that oral ethanol elicits responses in taste-sensitive neurons in W rats that closely approximate activity to appetitive sweet stimuli.
Neural activity to oral ethanol in P rats was, as in W rats, larger in S1 neurons compared with S0 cells (Fig. 4B), and responses to ethanol by S1 neurons did not differ between the P and W lines (Fig. 4C). Yet, unlike W neurons, P S0 cells showed significant increases in firing rate to increasing concentrations of oral ethanol (Fig. 4D). Across-neuron responses to ethanol and sweet stimuli by P neurons displayed a significantly lower correlation than that observed for W cells (Fig. 5 and Table 2). Multivariate analysis indicated that the population response to ethanol across P cells was not easily associated with activity to any of the taste stimuli tested, which included representative sweet, sodium salt, and aversive halide salt, acidic, and bitter stimuli (Figs. 6 and 7). The above data suggest that the neural representation of oral ethanol by taste-sensitive neurons in alcohol-preferring P rats differs from that of nonselected W rats. Furthermore, these findings raise the possibility that in P rats the overall oral sensory signal for ethanol conveyed by gustatory-responsive neurons is distinct from representations of stimuli from existing sweet, salt, acidic, and bitter taste classes.
The strong and selective association found between responses to sweet stimuli and increasing concentrations of ethanol by taste-sensitive neurons in outbred W rats indicates that these stimuli elicit shared oral sensory features in this strain. Similar results have been found in neurophysiological studies of taste-sensitive brain stem neurons in randomly bred SD rats, where responses to sweet tastants and high concentrations of oral ethanol are strongly and selectively correlated (Lemon et al. 2004; Lemon and Smith 2005). These data also agree with several other reports across primate and rodent species showing similarities in the gustatory neurophysiological processing of oral alcohol and sweet stimuli (Brasser et al. 2010; Danilova and Hellekant 2000; Di Lorenzo et al. 1986; Hellekant et al. 1997; Sako and Yamamoto 1999). A selective, positive relationship between neural responses to salient concentrations of ethanol and appetitive sweet stimuli in some ways appears counterintuitive given that nonselected rats often show initial avoidance toward drinking highly concentrated ethanol (Richter and Campbell 1940). Such avoidance has commonly been attributed to an unpalatable “bitter” taste component to ethanol, as has been self-reported in human studies (Scinska et al. 2000). Nevertheless, we are not aware of any direct physiological evidence to date showing a strong, positive relationship between gustatory neural activity to salient concentrations of oral ethanol and aversive taste inputs, including bitter or sour stimuli. Rather, prior work measuring the activity of central taste-sensitive neurons in outbred rats to oral applications of 40% ethanol and an array of intensity-matched stimuli including aversive salts, acids, and 10 different bitter-tasting chemicals found that responses to ethanol were, again, positively associated with activity to only sweet stimuli, such as sucrose and fructose (Lemon and Smith 2005). Similarly, no reliable association has been found between behavioral alcohol preference and chemosensory responses to quinine (Brasser et al. 2011) or intake of the bitter substance sucrose octaacetate (Stewart et al. 1994) in genetically selected ethanol-preferring and -nonpreferring rat lines. It is possible that the aversive sensory component of ethanol may result primarily from its interaction with the oral trigeminal system, which detects and processes noxious irritant input from the mouth. In rodents, oral ethanol induces concentration-dependent activation of lingual nociceptive neurons in the medullary dorsal horn that receive afferent input from the trigeminal nerve (Carstens et al. 1998). Targeted deletion of the transient receptor potential vanilloid type 1 receptor (TRPV1), the sensory receptor for capsaicin expressed on trigeminal nerve endings and activated by ethanol (Caterina et al. 1997; Trevisani et al. 2002), also reduces ethanol avoidance in mice (Blednov and Harris 2009; Ellingson et al. 2009), indicating that trigeminal substrates contribute to oral ethanol aversion. Moreover, psychophysical studies in humans have shown that noxious oral sensations processed by the trigeminal system, such as the “burning” of capsaicin, can be confused with bitter taste (Lim and Green 2007). Potentially, such cross-modal generalization could account for ethanol's “bitter” component to humans, as well as conditioned taste aversion generalization reported between ethanol and sweet-bitter mixtures in rodents (Kiefer and Lawrence 1988; Kiefer and Mahadevan 1993). Nonetheless, the oral perception of ethanol likely arises from a sensory-integrative process in which its ability to stimulate taste circuitry reflects only one facet of its sensory code. The present work suggests that the gustatory representation of ethanol in nonselected W rats strongly approximates activity to appetitive sweet stimuli.
An important consideration in interpreting the present findings is that the neural responses primarily reflect afferent input from the anterior tongue and palate innervated by the VIIth nerve, implicated in discriminative taste function (Spector and Grill 1992; Spector et al. 1997; St. John and Spector 1998). It is possible that the oral stimulation technique used did not effectively bathe taste receptors on the posterior tongue innervated by the IXth nerve. This nerve is implicated in oral reflexive behaviors (Travers et al. 1987) and importantly contributes to unconditioned avoidance responses to bitter stimuli in oral sensory behavioral paradigms (Geran and Travers 2011). The IXth nerve drives a unique population of NTS units, some selective toward bitter tastants (Geran and Travers 2006), and it would be worthwhile to investigate how afferent input from IX would contribute to ethanol taste representations in W and P rats. Gustatory recordings from IX in primates show that oral ethanol suppresses taste responses to quinine in mixtures, and that mixtures of ethanol and sucrose give a larger response in this nerve than sucrose alone (Danilova and Hellekant 2000). These mixture interactions induced by ethanol, the suppression of bitter (cf. Lawless 1979) and enhancement of sweet taste activity, would be expected of a stimulus encoded as an appetitive input by the IXth nerve.
In both W and P lines ethanol similarly and strongly activated S1 cells (Fig. 4), and patterns of activity across S1 neurons alone, selected for their heightened sensitivity to sucrose, can generally distinguish sucrose from nonsweet tastants (Fig. 7). In light of this, we hypothesized that the distinct neural representation of ethanol observed across P neurons may be composed of a sucrose-like component contributed by S1 cells, with other features combining to form a unique overall representation. If so, across-unit responses by P S1 neurons alone should logically represent ethanol and sucrose similarly relative to other taste inputs. However, this prediction was not fully realized. As shown in Fig. 7, a plot of the first two dimensions of three-dimensional MDS revealed that across-unit activity by S1 neurons in P rats distinguished ethanol from nonsweet sodium salt and aversive halide, acidic, and bitter stimuli but also differentiated ethanol from concentrations of sucrose that evoked robust activity (0.03–1 M; cf. Fig. 3B) and other effective sweet stimuli; the third dimension of MDS applied to P S1 cells (not shown in Fig. 7) further separated ethanol from relatively weakly effective concentrations of sucrose (0.01 M) and quinine (0.0001 M). In contrast, S1 neurons in W rats showed responses to ethanol that were strongly associated with activity to salient concentrations of sucrose and other sweet tastants (Fig. 7). Ethanol additionally produced concentration-dependent activation of S0 neurons in P rats (Fig. 4). Although MDS analysis of responses by S0 cells alone yielded less clear distinctions of taste stimuli in both rat lines, patterns of response to ethanol across P S0 cells still showed a noticeable degree of separation from taste categories (Fig. 7), as based on positioning in the scaling space.
Although one interpretation of these data is that the neural message for ethanol carried by taste-sensitive neurons in P rats does not clearly track features of stimuli from known taste categories, other possibilities exist. Defining associations between ethanol's oral sensation and taste is ideally achieved by testing stimuli representative of all possible permutations of taste quality, including mixtures, as well as stimuli of variable intensity. The present study in P and W rats was designed a priori to specifically examine in these lines relationships between neural activity to ethanol and sweet stimuli, given prior data from rodent NTS showing robust associations between neural representations for these stimuli (Brasser et al. 2010; Di Lorenzo et al. 1986; Lemon et al. 2004; Lemon and Smith 2005). Although a broad array of sweet and other stimuli, some of varying concentration, were tested here, umami stimuli were not evaluated and only one mixture was tested: a mixture of a salient concentration of sucrose and weak concentration of quinine, which has previously been shown to generalize to ethanol in outbred rats (Di Lorenzo et al. 1986; Kiefer and Mahadevan 1993). It is possible that further investigation using a larger set of stimuli and their mixtures might yet reveal a strong association between responses by P neurons to ethanol and a specific taste input. Such an experiment, however, would be challenging to conduct given a nearly unlimited number of possible taste inputs to test. Nevertheless, the present study indicates that there is not a robust association between oral responses to ethanol and common taste categories (sweet, sodium salt, acidic, or bitter) in taste-sensitive neurons of ethanol-preferring P rats.
That population responses to ethanol robustly associate with activity to sweet stimuli in W neurons but diverge from responses to stimuli of prototypical taste qualities in P cells could also potentially be influenced by line differences in the processing of oral somatosensory attributes of ethanol in the NTS. Regions of the NTS associated with taste also receive projections from trigeminal afferents (Felizardo et al. 2009; Hamilton and Norgren 1984; Marfurt and Rajchert 1991; Whitehead and Frank 1983), which transmit lingual chemesthetic information about ethanol to the brain stem (Carstens et al. 1998). Thus trigeminal processes may potentially contribute a portion of the oral responses to ethanol measured in the NTS. Future investigation of differences in oral somatic processing of ethanol between W and P rats may shed light on a possible contribution of somatosensory variables to the disparate sensory neural representations of ethanol in these lines.
Nevertheless, the present work indicates that oral ethanol is a powerful stimulant of brain stem circuitry for taste in selectively bred ethanol-preferring P and heterogeneous W rats and that the overall oral processing of ethanol by taste-sensitive medullary neurons differs between these lines. This differential neural processing is consistent with findings that P and W rats exhibit differing initial behavioral chemosensory responses to ethanol in brief-access assays (Brasser et al. 2011) designed to index orally guided behavior and mitigate postabsorptive influences on ingestion. Specifically, P rats display elevated short-term lick rates to a range of ethanol concentrations and a concentration-dependent increase in frequency to sample high ethanol concentrations relative to W rats (Brasser et al. 2011). The present data indicate that differences in the representation of ethanol chemosensory information between these lines as early as brain stem gustatory circuits may contribute to such behavioral differences in sensory responding to ethanol, although the precise nature of the NTS neural signal for ethanol in P rats remains to be determined. In brief-access tests, enhanced orosensory responding for ethanol in the P line is also accompanied by a heightened orally mediated avidity for sucrose compared with NP or W lines (Brasser et al. 2011). However, oral neural responses to a concentration series of sucrose between P and W lines in the present study were similar at the level of the NTS. These latter data suggest that differences in behavioral chemosensory responses to sucrose and to ethanol between these lines may also be mediated by variation in neural substrates in downstream gustatory pathways.
Neural pathways conveying appetitive tastes are known to engage mesolimbic dopamine (DA) circuits that may track the reinforcing value of sapid substances (Norgren et al. 2006). For example, licking and intake of sucrose, but not water, induces an immediate concentration-dependent rise in DA overflow in the nucleus accumbens (NAc) in rats (Hajnal and Norgren 2001). This increase in NAc DA flux occurs during sucrose sham ingestion procedures in which the stimulus is consumed orally but removed from the body by gastric fistula, suggesting that the rise in accumbal DA levels is mediated via an oral sensory route (Hajnal et al. 2004). Moreover, selective lesions of brain stem parabrachial subnuclei associated with taste processing can reduce the NAc DA response induced by sucrose consumption (Hajnal and Norgren 2005). These data indicate that neural circuits encoding appetitive tastes engage mesolimbic dopaminergic pathways that process orally derived reinforcement. P rats are known to display innate differences in mesolimbic DA system function relative to W and NP lines (Bell et al. 2005). Compared with W rats, for example, ventral tegmental DA neurons projecting to the NAc in P rats burst more frequently (Morzorati 1998) and voluntary oral ethanol intake induces greater NAc DA flux in the P line (Weiss et al. 1993). Differences between P and W rats in the gustatory processing of ethanol within the brain stem and in downstream DA circuits involved with taste-guided ingestive reinforcement may impart differential hedonic value to oral ethanol sensory stimulation between these rat lines. It is important to note that neural processes contributing to taste are positioned to induce rapid physiological and pharmacological changes in forebrain circuits associated with ingestive reinforcement prior to any potential influence of postabsorptive feedback on these systems. Heightened immediate behavioral avidity for ethanol by alcohol-preferring P rats (Brasser et al. 2011) suggests that the oral sensory neural signal for ethanol in this line may carry greater appetitive salience relative to nonselected or nonpreferring lines. These and the present data warrant further investigation of the sensory and hedonic neural codes for oral ethanol in animals bred for divergent ethanol preference.
GRANTS
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grants AA-015741 (S. M. Brasser) and AA-015512 (Indiana Alcohol Research Center) and also by St. Louis University seed funds (C. H. Lemon).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.H.L. and S.M.B. conception and design of research; C.H.L. and D.M.W. performed experiments; C.H.L. and D.M.W. analyzed data; C.H.L. and S.M.B. interpreted results of experiments; C.H.L. prepared figures; C.H.L. drafted manuscript; C.H.L. and S.M.B. edited and revised manuscript; C.H.L., D.M.W., and S.M.B. approved final version of manuscript.
REFERENCES
- Anstrom KK, Cromwell HC, Markowski T, Woodward DJ. Effect of baclofen on alcohol and sucrose self-administration in rats. Alcohol Clin Exp Res 27: 900–908, 2003 [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, Chatterjee A, Ross DA, Beauchamp GK. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses 26: 925–933, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Li X, Li S, Beauchamp GK, Tordoff MG. Voluntary ethanol consumption by mice: genome-wide analysis of quantitative trait loci and their interactions in a C57BL/6ByJ x 129P3/J F2 intercross. Genome Res 12: 1257–1268, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R, Rodd Z, Murphy J, McBride W. Use of selectively bred alcohol-preferring rats to study alcohol abuse, relapse and craving. In: Comprehensive Handbook of Alcohol Related Pathology, edited by Preedy V, Watson R. New York: Academic, 2005, p. 1515–1533 [Google Scholar]
- Blednov YA, Harris RA. Deletion of vanilloid receptor (TRPV1) in mice alters behavioral effects of ethanol. Neuropharmacology 56: 814–820, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav 7: 1–13, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizard DA, McClearn GE. Association between ethanol and sucrose intake in the laboratory mouse: exploration via congenic strains and conditioned taste aversion. Alcohol Clin Exp Res 24: 253–258, 2000 [PubMed] [Google Scholar]
- Brasser SM, Norman MB, Lemon CH. T1r3 taste receptor involvement in gustatory neural responses to ethanol and oral ethanol preference. Physiol Genomics 41: 232–243, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasser SM, Silbaugh BC, Ketchum MJ, Olney JJ, Lemon CH. Chemosensory responsiveness to ethanol and its individual sensory components in alcohol-preferring, -nopreferring, and genetically heterogeneous rats. Addict Biol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens E, Kuenzler N, Handwerker HO. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J Neurophysiol 80: 465–492, 1998 [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997 [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301: 850–853, 2003 [DOI] [PubMed] [Google Scholar]
- Danilova V, Hellekant G. The taste of ethanol in a primate model. II. Glossopharyngeal nerve response in Macaca mulatta. Alcohol 21: 259–269, 2000 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Kiefer SW, Rice AG, Garcia J. Neural and behavioral responsivity to ethyl alcohol as a tastant. Alcohol 3: 55–61, 1986 [DOI] [PubMed] [Google Scholar]
- Diamant H, Funakoshi M, Ström L, Zotterman Y. Electrophysiological studies on human taste nerves. In: Olfaction and Taste, edited by Zotterman Y. Oxford: Pergamon, 1963, p. 193–203 [Google Scholar]
- Dyr W, Kostowski W. Animal model of ethanol abuse: rats selectively bred for high and low voluntary alcohol intake. Acta Pol Pharm 57, Suppl: 90–92, 2000 [PubMed] [Google Scholar]
- Ellingson JM, Silbaugh BC, Brasser SM. Reduced oral ethanol avoidance in mice lacking transient receptor potential channel vanilloid receptor 1. Behav Genet 39: 62–72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RP. Sensory neural patterns and gustation. In: Olfaction and Taste, edited by Zotterman Y. Oxford: Pergamon, 1963, p. 205–213 [Google Scholar]
- Felizardo R, Boucher Y, Braud A, Carstens E, Dauvergne C, Zerari-Mailly F. Trigeminal projections on gustatory neurons of the nucleus of the solitary tract: a double-label strategy using electrical stimulation of the chorda tympani and tracer injection in the lingual nerve. Brain Res 1288: 60–68, 2009 [DOI] [PubMed] [Google Scholar]
- Foroud T, Bice P, Castelluccio P, Bo R, Ritchotte A, Stewart R, Lumeng L, Li TK, Carr L. Mapping of QTL influencing saccharin consumption in the selectively bred alcohol-preferring and -nonpreferring rat lines. Behav Genet 32: 57–67, 2002 [DOI] [PubMed] [Google Scholar]
- Freedland CS, Sharpe AL, Samson HH, Porrino LJ. Effects of SR141716A on ethanol and sucrose self-administration. Alcohol Clin Exp Res 25: 277–282, 2001 [PubMed] [Google Scholar]
- Geran LC, Travers SP. Single neurons in the nucleus of the solitary tract respond selectively to bitter taste stimuli. J Neurophysiol 96: 2513–2527, 2006 [DOI] [PubMed] [Google Scholar]
- Geran LC, Travers SP. Glossopharyngeal nerve transection impairs unconditioned avoidance of diverse bitter stimuli in rats. Behav Neurosci 125: 519–528, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JM, Erickson RP. Neural mass differences in gustation. Chem Senses 10: 531–548, 1985 [Google Scholar]
- Hajnal A, Norgren R. Accumbens dopamine mechanisms in sucrose intake. Brain Res 904: 76–84, 2001 [DOI] [PubMed] [Google Scholar]
- Hajnal A, Norgren R. Taste pathways that mediate accumbens dopamine release by sapid sucrose. Physiol Behav 84: 363–369, 2005 [DOI] [PubMed] [Google Scholar]
- Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol 286: R31–R37, 2004 [DOI] [PubMed] [Google Scholar]
- Hamilton RB, Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol 222: 560–577, 1984 [DOI] [PubMed] [Google Scholar]
- Hartigan JA, Wong MA. A k-means clustering algorithm. Appl Stat 28: 100–108, 1979 [Google Scholar]
- Hellekant G, Danilova V, Roberts T, Ninomiya Y. The taste of ethanol in a primate model. I. Chorda tympani nerve response in Macaca mulatta. Alcohol 14: 473–484, 1997 [DOI] [PubMed] [Google Scholar]
- Howell DC. Statistical Methods for Psychology (7th ed.). Belmont, CA: Wadsworth Cengage Learning, 2010, p. 275–276 [Google Scholar]
- Kampov-Polevoy A, Garbutt JC, Janowsky D. Evidence of preference for a high-concentration sucrose solution in alcoholic men. Am J Psychiatry 154: 269–270, 1997 [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Garbutt JC, Davis CE, Janowsky DS. Preference for higher sugar concentrations and Tridimensional Personality Questionnaire scores in alcoholic and nonalcoholic men. Alcohol Clin Exp Res 22: 610–614, 1998 [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Tsoi MV, Zvartau EE, Neznanov NG, Khalitov E. Sweet liking and family history of alcoholism in hospitalized alcoholic and non-alcoholic patients. Alcohol Alcohol 36: 165–170, 2001 [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav 76: 365–377, 2002 [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Bice PJ, Orr MR, Dopp JM. Similarity of taste reactivity responses to alcohol and sucrose mixtures in rats. Alcohol 7: 115–120, 1990 [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Lawrence GJ. The sweet-bitter taste of alcohol: aversion generalization to various sweet-quinine mixtures in the rat. Chem Senses 13: 633–641, 1988 [Google Scholar]
- Kiefer SW, Mahadevan RS. The taste of alcohol for rats as revealed by aversion generalization tests. Chem Senses 18: 509–522, 1993 [Google Scholar]
- Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem Biophys Res Commun 283: 236–242, 2001 [DOI] [PubMed] [Google Scholar]
- Kruskal JB, Wish M. Multidimensional Scaling. Beverly Hills, CA: Sage, 1978 [Google Scholar]
- Lawless HT. Evidence for neural inhibition in bittersweet taste mixtures. J Comp Physiol Psychol 93: 538–547, 1979 [DOI] [PubMed] [Google Scholar]
- Lemon CH, Brasser SM, Smith DV. Alcohol activates a sucrose-responsive gustatory neural pathway. J Neurophysiol 92: 536–544, 2004 [DOI] [PubMed] [Google Scholar]
- Lemon CH, Imoto T, Smith DV. Differential gurmarin suppression of sweet taste responses in rat solitary nucleus neurons. J Neurophysiol 90: 911–923, 2003 [DOI] [PubMed] [Google Scholar]
- Lemon CH, Margolskee RF. Contribution of the T1r3 taste receptor to the response properties of central gustatory neurons. J Neurophysiol 101: 2459–2471, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon CH, Smith DV. Neural representation of bitter taste in the nucleus of the solitary tract. J Neurophysiol 94: 3719–3729, 2005 [DOI] [PubMed] [Google Scholar]
- Lim J, Green BG. The psychophysical relationship between bitter taste and burning sensation: evidence of qualitative similarity. Chem Senses 32: 31–39, 2007 [DOI] [PubMed] [Google Scholar]
- Lumeng L, Hawkins T, Li T. New strains of rats with alcohol preference and non-preference. In: Alcohol and Aldehyde Metabolizing Systems, edited by Thurman R, Williamson J, Drott H, Chance B. New York: Academic, 1977, p. 537–544 [Google Scholar]
- Marfurt CF, Rajchert DM. Trigeminal primary afferent projections to “non-trigeminal” areas of the rat central nervous system. J Comp Neurol 303: 489–511, 1991 [DOI] [PubMed] [Google Scholar]
- Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet 28: 58–63, 2001 [DOI] [PubMed] [Google Scholar]
- Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci 4: 492–498, 2001 [DOI] [PubMed] [Google Scholar]
- Morzorati SL. VTA dopamine neuron activity distinguishes alcohol-preferring (P) rats from Wistar rats. Alcohol Clin Exp Res 22: 854–857, 1998 [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet 32: 363–388, 2002 [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell 106: 381–390, 2001 [DOI] [PubMed] [Google Scholar]
- Norgren R, Hajnal A, Mungarndee SS. Gustatory reward and the nucleus accumbens. Physiol Behav 89: 531–535, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Kampov-Polevoy AB, Rezvani AH, Murrelle L, Halikas JA, Janowsky DS. Saccharin intake predicts ethanol intake in genetically heterogeneous rats as well as different rat strains. Alcohol Clin Exp Res 17: 366–369, 1993 [DOI] [PubMed] [Google Scholar]
- Reid LD. Endogenous opioids and alcohol dependence: opioid alkaloids and the propensity to drink alcoholic beverages. Alcohol 13: 5–11, 1996 [DOI] [PubMed] [Google Scholar]
- Richter CP, Campbell KH. Alcohol taste thresholds and concentrations of solution preferred by rats. Science 91: 507–508, 1940 [DOI] [PubMed] [Google Scholar]
- Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. J Neurochem 77: 896–903, 2001 [DOI] [PubMed] [Google Scholar]
- Sako N, Yamamoto T. Electrophysiological and behavioral studies on taste effectiveness of alcohols in rats. Am J Physiol Regul Integr Comp Physiol 276: R388–R396, 1999 [DOI] [PubMed] [Google Scholar]
- Scinska A, Koros E, Habrat B, Kukwa A, Kostowski W, Bienkowski P. Bitter and sweet components of ethanol taste in humans. Drug Alcohol Depend 60: 199–206, 2000 [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Kampov-Polevoy A, Stewart R, Li TK. Taste preferences in rat lines selected for low and high alcohol consumption. Alcohol 9: 155–160, 1992 [DOI] [PubMed] [Google Scholar]
- Smith DV, Van Buskirk RL, Travers JB, Bieber SL. Coding of taste stimuli by hamster brainstem neurons. J Neurophysiol 50: 541–558, 1983 [DOI] [PubMed] [Google Scholar]
- Spector AC, Grill HJ. Salt taste discrimination after bilateral section of the chorda tympani or glossopharyngeal nerves. Am J Physiol Regul Integr Comp Physiol 263: R169–R176, 1992 [DOI] [PubMed] [Google Scholar]
- Spector AC, Markison S, St. John SJ, Garcea M. Sucrose vs. maltose taste discrimination by rats depends on the input of the seventh cranial nerve. Am J Physiol Regul Integr Comp Physiol 272: R1210–R1218, 1997 [DOI] [PubMed] [Google Scholar]
- St. John SJ, Spector AC. Behavioral discrimination between quinine and KCl is dependent on input from the seventh cranial nerve: implications for the functional roles of the gustatory nerves in rats. J Neurosci 18: 4353–4362, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RB, Russell RN, Lumeng L, Li TK, Murphy JM. Consumption of sweet, salty, sour, and bitter solutions by selectively bred alcohol-preferring and alcohol-nonpreferring lines of rats. Alcohol Clin Exp Res 18: 375–381, 1994 [DOI] [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature 396: 366–369, 1998 [DOI] [PubMed] [Google Scholar]
- Toothaker LE. Multiple Comparisons for Researchers. London: Sage, 1991 [Google Scholar]
- Travers JB, Grill HJ, Norgren R. The effects of glossopharyngeal and chorda tympani nerve cuts on the ingestion and rejection of sapid stimuli: an electromyographic analysis in the rat. Behav Brain Res 25: 233–246, 1987 [DOI] [PubMed] [Google Scholar]
- Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ, Owen D, Smith GD, Randall AD, Harrison S, Bianchi A, Davis JB, Geppetti P. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci 5: 546–551, 2002 [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther 267: 250–258, 1993 [PubMed] [Google Scholar]
- Whitehead MC, Frank ME. Anatomy of the gustatory system in the hamster: central projections of the chorda tympani and the lingual nerve. J Comp Neurol 220: 378–395, 1983 [DOI] [PubMed] [Google Scholar]
- Woods JE, 2nd, McKay PF, Masters J, Seyoum R, Chen A, La Duff L, Lewis MJ, June HL. Differential responding for brain stimulation reward and sucrose in high-alcohol-drinking (HAD) and low-alcohol-drinking (LAD) rats. Alcohol Clin Exp Res 27: 926–936, 2003 [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol 42: 149–160, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 159: 415–423, 2002 [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255–266, 2003 [DOI] [PubMed] [Google Scholar]