Abstract
Until recently the mechanism for the enrichment of milk with calcium was thought to be almost entirely via the secretory pathway. However, recent studies suggest that a plasma membrane calcium ATPase, PMCA2, is the primary mechanism for calcium transport into milk, highlighting a major role for apical calcium transport. We compared the expression of the recently identified secretory calcium ATPase, SPCA2, and SPCA1, in the mouse mammary gland during development. SPCA2 levels increased over 35-fold during lactation with expression localized to luminal secretory cells, while SPCA1 increased only a modest 2-fold and was expressed throughout the cells of the mammary gland. We also observed major differences in the localization of PMCA2 and PMCA1. Our studies highlight the likely specific roles of PMCA2 and SPCA2 in lactation and indicate that calcium transport into milk is a complex interplay between apical and secretory pathways.
Keywords: Calcium, PMCA1, SPCA1, PMCA2, SPCA2, Mouse, Mammary gland, 3D mammary culture, Milk
Intracellular free Ca2+ levels are under tight regulation and control, with resting free cytosolic Ca2+ levels maintained at around 100 nM [1]. This regulation is achieved by proteins responsible for the translocation of Ca2+ ions, which include Ca2+ channels, Ca2+ exchangers, and Ca2+ pumps [1,2]. During lactation, the unidirectional transport of Ca2+ from the maternal blood supply to the milk must be achieved without causing major disturbances to cellular signaling within the epithelial cells of the mammary gland [3]. Concurrent with tightly maintaining intracellular Ca2+ levels, a large gradient between free and total Ca2+ between the maternal blood supply and milk is established. Indeed the total Ca2+ content of blood is 3 mM [3], while that of the milk is approximately 8 mM in humans, and over 60 mM in other species such as mice [4].
Active transporters of Ca2+ ions include the plasma membrane Ca2+ ATPases (PMCAs), the sarcoplasmic endoplasmic Ca2+-ATPases (SERCAs). and the secretory pathway Ca2+-ATPases (SPCAs) [2,3]. Consistent with a minor role for the endoplasmic reticulum in Ca2+ enrichment of milk, levels of SERCA isoform transcripts expressed in the rat mammary gland (SERCA2 and SERCA3) do not significantly increase during pregnancy or during the early stages of lactation [5]. In contrast, PMCA2, which has a highly restricted tissue distribution [6,7], increases approximately 30-fold during the early stages of lactation in the rat mammary gland as compared to the almost undetectable levels in nulliparous rats [5,8]. The presence of PMCA2 protein in milk fat globule membranes suggests a predominately apical distribution for PMCA2 during lactation, and a major role in the efflux of Ca2+ across the apical membrane into milk [4,8]. The reduction in the Ca2+ content of milk in PMCA2 null mice by 60% [9], when combined with only a comparatively modest increase (6–8-fold) in the expression of the secretory pathway pump SPCA1 [5,8], challenges the widely cited view of little to no involvement of direct apical transport of Ca2+ and the major role of the Golgi apparatus during lactation [4]. Although a possible role for PMCA2-mediated Ca2+ accumulation into the Golgi during trafficking to the membrane has been discussed [9], the recent report of PMCA2 localization to the apical membrane in mammary tissue from a lactating mouse [10] represents further evidence for its direct role in apical calcium transport.
The regulation of key Golgi enzymes involved in post-translational modification of milk proteins and lactose production by Ca2+ and Mn2+ [3,11], both substrates for the secretory pathway Ca2+ pump [12], still suggests a major role for Golgi Ca2+ accumulation in lactation, as does the secretion of Ca2+ in casein micelles derived from the Golgi [4]. In this study we sought to address the dichotomy arising from the modest increase in SPCA1 expression and the pronounced increase in PMCA2 during lactation, and the seemingly major role for the secretory pathway in Ca2+ secretion for the enrichment of milk with Ca2+ [4].
Subsequent to the studies discussed above regarding the expression of SPCA1 and PMCA2 in lactation, a second secretory pathway Ca2+/Mn2+ pump isoform has been identified, SPCA2 [13]. Similar to PMCA2, SPCA2 has a more restricted tissue distribution compared to SPCA1. SPCA2 is expressed in the brain and testis [13] as well as the cells of gastrointestinal and respiratory tract and mammary, salivary, and thyroid glands [14]. However, the possible physiological processes where SPCA2 may play a major role are still unclear. In these studies, we addressed the hypothesis that SPCA2 expression undergoes greater changes in expression than SPCA1 during pregnancy and lactation, and that PMCA isoforms and SPCAs have unique cellular distributions during lactation consistent with specific roles in Ca2+ homeostasis in the mammary gland and the transport of Ca2+ into milk.
Materials and methods
Animals
CBA × C57Bl6 mice were fed standard rodent chow and water ad libitum. Nulliparous animals were euthanized at 14 weeks of age. For all other developmental stages, dams were mated when 12 weeks old. Animals were then sacrificed at the following stages of mammary gland development: mid-pregnancy (∼day 10); lactation (day 1); and involution (48 h post-forced weaning).
RNA isolation and real-time RT-PCR
Total RNA was isolated from whole mammary glands using the Qiagen RNeasy mini Kit incorporating an on-column DNase treatment (Qiagen, Doncaster, Vic, Australia). SPCA1 and SPCA2 mRNA levels were quantified using real-time RT-PCR. The mRNA was reverse transcribed using the Omniscript® RT kit (Qiagen) while the real-time PCR step was performed using a TaqMan® universal PCR master mix (Applied Biosystems, Scoresby, Vic, Australia) and the following TaqMan® gene expression assays (Applied Biosystems): Mm00723486_m1 (SPCA1); and Mm01242899_m1 (SPCA2). Expression was normalized to endogenous 18S rRNA levels and is expressed relative to nulliparous animals. Reactions were cycled in an ABI PRISM 7500 Sequence Detector (Applied Biosystems) under the following conditions: 95 °C for 10 min; followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Threshold cycle (Ct) values were used for all analysis. Three separate PCR reactions were performed from each RNA sample from each mouse, to assess variability in Ct responses between replicates and between mice. The mean differences between Ct responses for the different targets, and their standard errors, were estimated using a random effects model, with an additional mouse effect, allowing for the variation in Ct responses between and within mice. Standard errors of further quantities derived from these estimates were determined using the delta technique. Significance was assessed using a z-test based on a normal distribution.
Immunofluorescence (in vivo samples)
Glands were sectioned at 5 μM thickness. Sections were fixed with 4% paraformaldehyde in phosphate buffered-saline (PBS). Slides were incubated with primary antibody for 1 h at room temperature. Primary antibodies used included: anti-PMCA1 (1:100, PA1-914; Affinity BioReagents, Golden, CO); anti-PMCA2 (1:800, PA1-915; Affinity BioReagents); affinity purified anti-SPCA1 (1:500); affinity purified anti-SPCA2 (1:100 [13]); and anti-smooth muscle actin (SMA) Cy3 Conjugate (1:400,1A4; Sigma–Aldrich, Castle Hill, NSW, Australia). Sections were incubated with the secondary antibody Alexa-Fluor®-488 goat anti-rabbit (1:500; Molecular Probes, Mt. Waverley, Vic, Australia) for 40 min, at room temperature. Cells were co-stained with 4,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich). Fluorescence images were captured with a Nikon Eclipse TE300 microscope (Nikon UK, Kingston, UK), equipped with a 40 × oil immersion NA 1.3 objective lens (Nikon), and with MetaFluor® imaging software (Universal Imaging, Downington, PA). All images were analyzed with MetaMorph®, version 6.2 r5 (Universal Imaging).
Cell culture
SCp2 mouse mammary epithelial cells [15] were routinely cultured in Dulbecco's modified Eagle's medium (DMEM)/Hams F-12 as previously described [16]. SCp2 were grown also as a three-dimensional (3D) culture on top of a layer of reconstituted basement membrane gel from Engelbreth–Holm–Swarm extracellular matrix extract, growth factor-reduced (Matrigel; BD Biosciences, Bedford, MA) as previously described [17].
Immunofluorescence (SCp2 cells)
Cells were grown in a monolayer (2D) to approximately 90% confluence or in 3D were fixed in an ice-cold methanol/acetone solution (1/1; v/v). Primary antibody dilutions were anti-PMCA1 (1:100), anti-PMCA2 (1:300) and anti-α6 integrin (1:100; MAB 1378; Chemicon International, Temecula, CA, USA). Slides were incubated with secondary antibodies: goat anti-rat Alexa-Fluor® 568 and goat anti-rabbit Alexa-Fluor® 488 (all 1:500; Molecular Probes). Cells were co-stained with DAPI. Confocal analysis was performed using a Solamere Technology Group (Salt Lake City, UT) spinning disk confocal system. Optical z-sections were acquired at 0.5 μm intervals and are an average of four exposures. All images were analyzed with MetaMorph®, version 6.2 r5.
Immunoblotting
Total cell lysates were isolated from SCp2 cells grown in 2D monolayers (in the presence of normal media or differentiation-inducing media) to reach approximately 90% confluence. For Western blotting antibodies used were anti-β-actin (1:1000, AC-15; Sigma–Aldrich), anti-β-casein (1:1000) prepared from a hybridoma kindly provided by Dr. Kaetzel, Institute of Pathology, Case Western Reserve University, Cleveland, OH [18], goat anti-mouse or goat anti-rabbit IgG horseradish peroxidase-conjugated (both 1:1000; Pierce, Rockford, IL).
Results
To ascertain which isoform, SPCA1 or SPCA2, is the predominant isoform up-regulated during lactation, mRNA levels were compared in mammary glands isolated from nulliparous, pregnant, lactating and involuting mice. Consistent with the increase seen in SPCA1 in rats [5,8], mouse SPCA1 levels increased modestly during pregnancy (∼1.5-fold at day 10) and lactation (∼2-fold at day 1) (Fig. 1). In contrast, a dramatic up-regulation of SPCA2 was observed in mouse mammary glands during lactation (∼35-fold at day 1) with an increase also seen during pregnancy (∼8-fold at day 10) compared to nulliparous animals (Fig. 1).
Fig. 1.
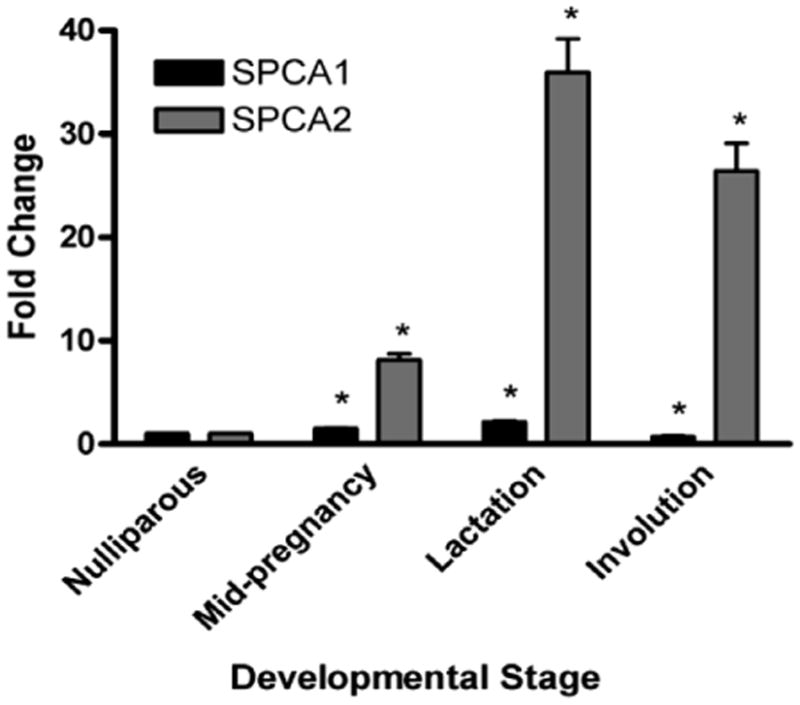
SPCA2 mRNA levels increase more than SPCA1 during murine mammary gland development. SPCA2 and SPCA1 mRNA levels during murine mammary gland development (relative to nulliparous). Bars represent means ± SEM (n = 3–4 mice) and are representative of two independent real-time RT-PCRs. The asterisk (*) denotes a significant difference (p < 0.05) compared to nulliparous gland, using a z-test based on a normal distribution.
Consistent with its more restricted tissue distribution [14], SPCA2 was expressed predominately in luminal epithelial cells of the mouse mammary gland during lactation (Fig. 2A), with no expression in cells outside the acini (arrows). SPCA1 staining (Fig. 2B) was seen in all cell types of the tissue section, including myoepithelial and stromal cells (arrows).
Fig. 2.
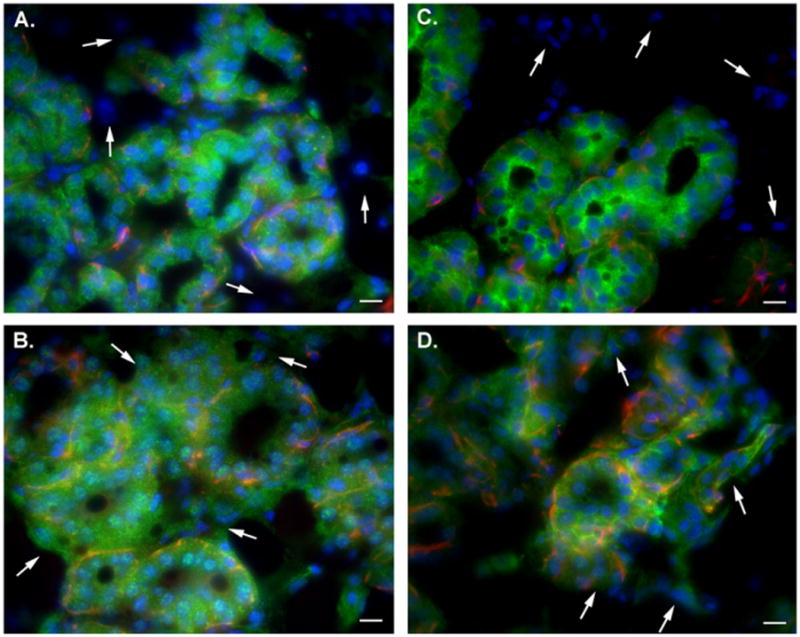
Immunolocalization of calcium ATPases in the lactating mouse mammary gland. Mammary gland sections isolated from lactating mice and immunostained for SPCA2 (A), SPCA1 (B), PMCA2 (C) or PMCA1 (D). All ATPases are shown in green with SMA (red) overlaid with the DAPI (blue) stain. (A) SPCA2. White arrows show cells which do not express SPCA2 outside the acini. (B) SPCA1. White arrows show cells not part of secretory acini that express SPCA1. (C) PMCA2. White arrows show cells which do not express PMCA2 outside the acini. (D) PMCA1. White arrows show cells not part of secretory acini that express PMCA1. Scale bars represent 10 μm in all panels. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
Analogous to SPCA1 and SPCA2, PMCA1, and PMCA2 exhibited differential isoform expression in the different cell types of the mammary gland during lactation, with distinct differences in sub-cellular distribution. Fig. 2C shows PMCA2 in the lactating mouse mammary gland. Consistent with its restricted distribution, PMCA2 expression appeared confined to the alveolus, whereas non-acinar cells did not express PMCA2 (arrows). PMCA2 also exhibited an apical localization in many of the acinar structures. Such a distribution suggests that PMCA2 may have a role in the direct translocation of Ca2+ across the apical membrane and is consistent with the recent work of VanHouten et al. [10]. In sharp contrast to PMCA2, PMCA1 appeared to be ubiquitously expressed in the mammary gland sections (Fig. 2D) with expression outside the acini (arrows). PMCA1 had a more basolateral distribution, consistent with a possible role in regulation of cytosolic free Ca2+ in cells of the mammary gland, rather than the active transport of Ca2+ into milk.
To confirm the distinct differences between PMCA1 and PMCA2 in sub-cellular distribution the mouse mammary SCp2 cell line was differentiated to produce 3D acini. These cells polarize and form a lumen and secrete the milk protein, β-casein (supplementary Fig. 1), analogous to primary cultures [19]. PMCA1 and PMCA2 exhibited pronounced differences in their sub-cellular localization in SCp2 cells. PMCA1 expression was basolateral (Fig. 3A and B and supplementary Fig. 2), whereas PMCA2 localization was more predominant towards the apical membrane and exhibited a cytosolic punctate staining (Fig. 3C and D and supplementary Fig. 3).
Fig. 3.
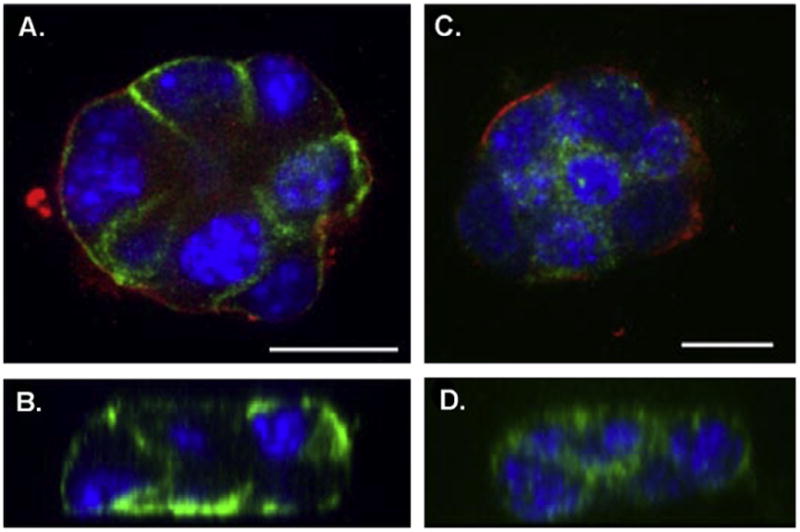
Localisation of PMCA1 and PMCA2 in SCp2 cells grown in 3D culture. (A) A representative acinus showing staining for PMCA1 (green), α6 integrin (red), and DAPI (blue). (B) The corresponding z–x plane through the acinus described in A. (C) A representative acinus showing staining for PMCA2 (green), a6 integrin (red), and DAPI (blue). (D) The corresponding z–x plane through the acinus described in C. Scale bars represent 10 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
Discussion
The modest increase in SPCA1 expression compared with PMCA2 expression during lactation in the rat [5,8], and the reduction of the Ca2+ content of milk in PMCA2-/- mice [9], has challenged the dogma of the greater importance of the Golgi compared to the apical membrane in the enrichment of milk with Ca2+. The data presented here suggest that SPCA2, rather than SPCA1, is the major secretory pathway Ca2+ ATPase important in lactation and is consistent with a major role of the Golgi in Ca2+ enrichment in milk.
The role of SPCA2 in mammary gland secretory cells during lactation is likely to be 2-fold: firstly, the up-regulation of SPCA2 may function to supply the secretory pathway with Ca2+ ions for packaging into casein micelles for calcium secretion into milk [4]. Indeed, the higher enzymatic turnover rate of SPCA2 compared to SPCA1 when over-expressed in HEK-293 cells and its high apparent affinity for Ca2+ [20], suggests that SPCA2 has an enhanced capability to replenish Golgi Ca2+ as Ca2+ is lost via secretion [20]. Since milk has a high Ca2+ content, lactation is an event where loss of Ca2+ from the tissue would be pronounced, and our observation of increased levels of SPCA2 during lactation is suggestive that this SPCA isoform has a major role in meeting the demand for Golgi Ca2+ replenishment. The second role for SPCA2 in lactation may go beyond the supply of Ca2+ to the Golgi for secretion into milk, and relate to the control of Ca2+ and Mn2+ ions in the Golgi for the regulation of Golgi-localized Ca2+ and Mn2+ sensitive enzymes, which are critical for post-translational modification of milk proteins and lactose production [3].
One key enzyme for which the regulation of Ca2+ and Mn2+ may be important is 1,4-b-galactosyltransferase I. This enzyme is a trans-Golgi enzyme [21] and is responsible for the transfer of galactose, a key event in the formation of some glycoconjugates [21,22]. 1,4-β-galactosyltransferase I is up-regulated during lactation, where it forms a heterodimer with α-lactalbumin to enable lactose production for secretion into milk [21]. The increase in both 1,4-b-galactosyltransferase I and SPCA2 during mid-pregnancy [21], combined with the sensitivity of 1,4-β-galactosyltransferase I to Mn2+ [23] suggests that SPCA2 may also be important in regulating the activity of this enzyme, and subsequently the lactose and glycoprotein profile of milk. The up-regulation of SPCA2 during mid-pregnancy is likely to be hormonally regulated as SPCA2 transcription is increased by prolactin in human MCF-7 breast cancer cells [24] and prolactin levels increase during pregnancy [25].
In addition to highlighting a physiological role for SPCA2 in lactation, this study provides further evidence for the isoform specific roles of PMCA2 and PMCA1 in the mammary gland, through the demonstration of their distinct sub-cellular and cellular locations during lactation. As expected, the ubiquitously expressed PMCA1 was present in the majority of cells in the lactating mammary gland with a basolateral localization both in vivo during lactation and in 3D culture. Such a distribution of PMCA1 is consistent with a role in the regulation of calcium fluxes in mammary gland epithelial cells, rather than direct transport of calcium into milk. Conversely, PMCA2 was localized to the secretory alveolus in vivo, with an apical localization, which is consistent with its proposed role in direct apical transportation of Ca2+ ions into milk [9,10]. PMCA2 localization in the 3D culture model appeared more pronounced towards the apical membrane, however, a vesicular distribution was also apparent, suggestive of a high turnover due to loss of PMCA2 during lactation as previous proposed [8], or indicative of a role for PMCA2 in the accumulation of Ca2+ into secretory vesicles [9] during particular periods of lactation. The reduction of total milk Ca2+ content by 60% in PMCA2 null mice, combined with the major contribution of casein micelles in milk Ca2+ enrichment, could also suggest a role for PMCA2 in Golgi Ca2+ accumulation [9]. However, the observation that PMCA2 heterozygous mice do not have reduced milk Ca2+ content, despite a 38% reduction in mammary PMCA2 levels [9], and our observation of pronounced up-regulation of SPCA2, suggests that more complex reasons may exist for the pronounced reduction of Ca2+ milk content in PMCA2 null mice, and that this result does not imply that 60% of Ca2+ transport into milk is mediated via PMCA2. SPCA1-null mice have only been characterized recently and are embryonic lethal [26]. SPCA2-null mice have not been characterized; however, we would predict from our studies that their phenotype may be associated with altered calcium secretion into milk, and an altered lactose content and glycoprotein profile of milk.
Despite our increasing knowledge of the transport of Ca2+ during lactation, important questions still remain. Our studies have, however, helped to clarify and highlight the importance of the secretory pathway ATPase, SPCA2, during pregnancy and lactation. They have also focused attention on the specific roles of the PMCA isoforms, PMCA1 and PMCA2 suggested by their different cellular and sub-cellular localization in the lactating mouse mammary gland.
Supplementary Material
Acknowledgments
We wish to thank Prof. Malcolm Faddy for help with statistical analysis. This work was funded by a Australian Postgraduate Award (H.F.), a University of Queensland GSRTA (H.F.), a QLD Cancer Fund travel award (H.F.), and the University of Queensland Foundation, and by grants from the Office of Biological and Environmental Research of the US Department of Energy (DE-AC03-76SF00098 and a Distinguish Fellow Award to M.J.B.) and the National Cancer Institute (CA57621 to M.J.B. and Zena Werb).
Footnotes
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbrc.2008.03.003.
References
- 1.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Carafoli E. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci USA. 2002;99:1115–1122. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee WJ, Monteith GR, Roberts-Thomson SJ. Calcium transport and signaling in the mammary gland: targets for breast cancer. Biochim Biophys Acta. 2006;1765:235–255. doi: 10.1016/j.bbcan.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Neville MC. Calcium secretion into milk. J Mammary Gland Biol Neoplasia. 2005;10:119–128. doi: 10.1007/s10911-005-5395-z. [DOI] [PubMed] [Google Scholar]
- 5.Reinhardt TA, Horst RL. Ca2+-ATPases and their expression in the mammary gland of pregnant and lactating rats. Am J Physiol Cell Physiol. 1999;276:C796–C802. doi: 10.1152/ajpcell.1999.276.4.C796. [DOI] [PubMed] [Google Scholar]
- 6.Kozel PJ, Friedman RA, Erway LC, Yamoah EN, Liu LH, Riddle T, Duffy JJ, Doetschman T, Miller ML, Cardell EL, Shull GE. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. J Biol Chem. 1998;273:18693–18696. doi: 10.1074/jbc.273.30.18693. [DOI] [PubMed] [Google Scholar]
- 7.Stauffer TP, Guerini D, Carafoli E. Tissue distribution of the four gene products of the plasma membrane Ca2+ pump. A study using specific antibodies. J Biol Chem. 1995;270:12184–12190. doi: 10.1074/jbc.270.20.12184. [DOI] [PubMed] [Google Scholar]
- 8.Reinhardt TA, Filoteo AG, Penniston JT, Horst RL. Ca(2+)-ATPase protein expression in mammary tissue. Am J Physiol Cell Physiol. 2000;279:C1595–C1602. doi: 10.1152/ajpcell.2000.279.5.C1595. [DOI] [PubMed] [Google Scholar]
- 9.Reinhardt TA, Lippolis JD, Shull GE, Horst RL. Null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2 impairs calcium transport into milk. J Biol Chem. 2004;279:42369–42373. doi: 10.1074/jbc.M407788200. [DOI] [PubMed] [Google Scholar]
- 10.VanHouten JN, Neville MC, Wysolmerski JJ. The calcium-sensing receptor regulates PMCA2 activity in mammary epithelial cells: a mechanism for calcium-regulated calcium transport into milk. Endocrinology. 2007;148:5943–5954. doi: 10.1210/en.2007-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navaratnam N, Ward S, Fisher C, Kuhn NJ, Keen JN, Findlay JB. Purification, properties and cation activation of galactosyltransferase from lactating-rat mammary Golgi membranes. Eur J Biochem. 1988;171:623–629. doi: 10.1111/j.1432-1033.1988.tb13833.x. [DOI] [PubMed] [Google Scholar]
- 12.Ton VK, Mandal D, Vahadji C, Rao R. Functional expression in yeast of the human secretory pathway Ca(2+), Mn(2+)-ATPase defective in Hailey–Hailey disease. J Biol Chem. 2002;277:6422–6427. doi: 10.1074/jbc.M110612200. [DOI] [PubMed] [Google Scholar]
- 13.Xiang M, Mohamalawari D, Rao R. A novel isoform of the secretory pathway Ca2+, Mn(2+)-ATPase, hSPCA2, has unusual properties and is expressed in the brain. J Biol Chem. 2005;280:11608–11614. doi: 10.1074/jbc.M413116200. [DOI] [PubMed] [Google Scholar]
- 14.Vanoevelen J, Dode L, Van Baelen K, Fairclough RJ, Missiaen L, Raeymaekers L, Wuytack F. The secretory pathway Ca2+/Mn2+-ATPase 2 is a Golgi-localized pump with high affinity for Ca2+ ions. J Biol Chem. 2005;280:22800–22808. doi: 10.1074/jbc.M501026200. [DOI] [PubMed] [Google Scholar]
- 15.Desprez P, Roskelley CD, Campisi J, Bissell MJ. Isolation of functional cell lines from a mouse mammary epithelial cell strain: the importance of basement membrane and cell–cell interaction. Mol Cell Differ. 1993;1:99–110. [Google Scholar]
- 16.Desprez PY, Hara E, Bissell MJ, Campisi J. Suppression of mammary epithelial cell differentiation by the helix–loop–helix protein Id-1. Mol Cell Biol. 1995;15:3398–3404. doi: 10.1128/mcb.15.6.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell–cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dode L, Andersen JP, Vanoevelen J, Raeymaekers L, Missiaen L, Vilsen B, Wuytack F. Dissection of the functional differences between human secretory pathway Ca2+/Mn2+-ATPase (SPCA) 1 and 2 isoenzymes by steady-state and transient kinetic analyses. J Biol Chem. 2006;281:3182–3189. doi: 10.1074/jbc.M511547200. [DOI] [PubMed] [Google Scholar]
- 21.Shaper NL, Charron M, Lo NW, Shaper JH. Beta1,4-galactosyltransferase and lactose biosynthesis: recruitment of a housekeeping gene from the nonmammalian vertebrate gene pool for a mammary gland specific function. J Mammary Gland Biol Neoplasia. 1998;3:315–324. doi: 10.1023/a:1018719612087. [DOI] [PubMed] [Google Scholar]
- 22.Hennet T. The galactosyltransferase family. Cell Mol Life Sci. 2002;59:1081–1095. doi: 10.1007/s00018-002-8489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snow DM, Shaper JH, Shaper NL, Hart GW. Determination of beta1,4-galactosyltransferase enzymatic activity by capillary electrophoresis and laser-induced fluorescence detection. Anal Biochem. 1999;271:36–42. doi: 10.1006/abio.1999.4104. [DOI] [PubMed] [Google Scholar]
- 24.Anantamongkol U, Takemura H, Suthiphongchai T, Krishnamra N, Horio Y. Regulation of Ca2+ mobilization by prolactin in mammary gland cells: possible role of secretory pathway Ca2+-ATPase type 2. Biochem Biophys Res Commun. 2007;352:537–542. doi: 10.1016/j.bbrc.2006.11.055. [DOI] [PubMed] [Google Scholar]
- 25.Neville MC, McFadden TB, Forsyth I. Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia. 2002;7:49–66. doi: 10.1023/a:1015770423167. [DOI] [PubMed] [Google Scholar]
- 26.Okunade GW, Miller ML, Azhar M, Andringa A, Sanford LP, Doetschman T, Prasad V, Shull GE. Loss of the ATP2C1 secretory pathway Ca2+-ATPase (SPCA1) in mice causes golgi stress, apoptosis, and mid-gestational death in homozygous embryos and squamous cell tumors in adult heterozygotes. J Biol Chem. 2007 doi: 10.1074/jbc.M703029200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


