Abstract
Improvements in protocol-driven clinical trials and supportive care for children and adolescents with cancer have reduced mortality rates by more than 50% over the past three decades. Overall, the 5-year survival rate for pediatric cancer patients has increased to approximately 80%. Recognition of the biological heterogeneity within specific subtypes of cancer, the discovery of genetic lesions that drive malignant transformation and cancer progression, and improved understanding of the basis of drug resistance will undoubtedly catalyze further advances in risk-directed treatments and the development of targeted therapies, boosting the cure rates further. Emerging new treatments include novel formulations of existing chemotherapeutic agents, monoclonal antibodies against cancer-associated antigens, and molecular therapies that target genetic lesions and their associated signaling pathways. Recent findings that link pharmacogenomic variations with drug exposure, adverse effects, and efficacy should accelerate efforts to develop personalized therapy for individual patients. Finally, palliative care should be included as an essential part of cancer management to prevent and relieve the suffering and to improve the quality of life of patients and their families.
Introduction
Major advances have been made in understanding the pathogenesis and treatment of pediatric cancers since the introduction of modern oncology half a century ago. The growing ability to analyze the genetic and epigenetic abnormalities in tumor cells is driving the discovery of somatic mutations and their role in the development and progression of cancer. Mutations of more than 1.6% of the approximately 22,000 protein-coding human genes have been implicated in carcinogenesis and, to date, the number of known cancer genes has grown to 437, many of which are involved in childhood cancer (www.sanger.ac.uk/genetics/CGP/Census).1 The next generation of highly parallel, single-molecule DNA-sequencing platforms will hopefully identify many more genetic alterations in tumor cells.2
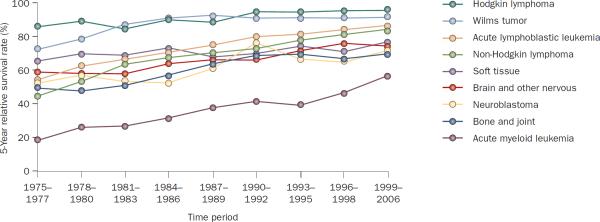
In parallel to these biological advances, there has been a remarkable improvement in the survival rates for pediatric patients with cancer. Among children from 0 to 19 years of age, the 5-year relative survival rate for all cancer combined has increased from 61.7% in 1975–1977 to 81.4% in 1999–2006, as estimated by the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute.3 With the exception of gliomas, the 5-year relative survival rate has now exceeded 55% for all major types of childhood cancer (Table 1). This advance can be attributed to the enrollment of large numbers of patients in well-designed prospective clinical trials, improved risk assessment and supportive care, and the development of new drugs directed at specific targets, which has partly been achieved through the use of preclinical models. Between 1975 and 2007 the mortality rates for all childhood cancers combined decreased by more than 50%. This decrease was led by a 75% reduction in the mortality rate (from 0.4 to 0.1 per 100,000) of non-Hodgkin lymphoma and a 60% reduction (from 1.0 to 0.4 per 100,000) of acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML).3 As a result, non-Hodgkin lymphomas and ALL are now among the most curable childhood cancers (Figure 1). By contrast, the survival rates for children with other solid tumors, particularly those with disseminated disease, and most brain tumors have not improved significantly over the past three decades with the exception of gonadal cancer, neuroblastoma and bone cancer.3 Between 2003 and 2007, solid tumors, leukemias, brain tumors, and lymphomas accounted for 38%, 31%, 27%, and 4%, respectively, of all pediatric cancer deaths in the USA.3
Table 1.
Pediatric patients aged 0–19 years and outcome by tumor type3
| Subgroup | n* | 5-year relative survival‡ (%) |
|---|---|---|
| Acute lymphoblastic leukemia | 2,466 | 84.0 |
| Acute myeloid leukemia | 622 | 58.0 |
| Hodgkin lymphoma | 882 | 95.1 |
| Non-Hodgkin lymphoma | 961 | 81.7 |
| Ependymomas and choroid plexus tumor | 197 | 71.0 |
| Astrocytoma | 1,150 | 82.8 |
| Intracranial and intraspinal embryonal tumors | 481 | 61.8 |
| Other gliomas | 457 | 54.9 |
| Neuroblastoma and ganglioneuroblastoma | 685 | 72.5 |
| Retinoblastoma | 236 | 97.6 |
| Nephroblastoma and other non-epithelial renal tumor | 433 | 88.7 |
| Hepatoblastoma | 126 | 71.3 |
| Osteosarcoma | 355 | 68.1 |
| Ewing tumor and related sarcomas of bone | 213 | 63.2 |
| Rhabdomyosarcoma | 378 | 62.1 |
| Intracranial and intraspinal germ-cell tumor | 134 | 84.0 |
| Malignant gonadal germ-cell tumor | 591 | 94.6 |
| Thyroid carcinoma | 496 | 98.8 |
| Malignant melanoma | 504 | 94.7 |
The numbers of patients are calculated based on the incidence rates that are age adjusted to the 2000 US standard population (19 age groups) taken from Census P25-1130.
Based on SEER data for cases diagnosed between 1999 and 2006 with follow-up of patients into 2007; SEER 13 areas contributed cases for the entire period 1999–2006, and SEER 17 areas contributed cases for years 2000–2006.
Figure 1.
Survival rates for different cancers among adolescents and young adults. 5-year relative survival rates for selected primary cancers according to year of diagnosis (1975–2006) among children younger than 20 years of age. Data obtained from Surveillance, Epidemiology, and End Results Registries based on follow-up into 2007 of patients from SEER 9 areas (San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, and Atlanta).
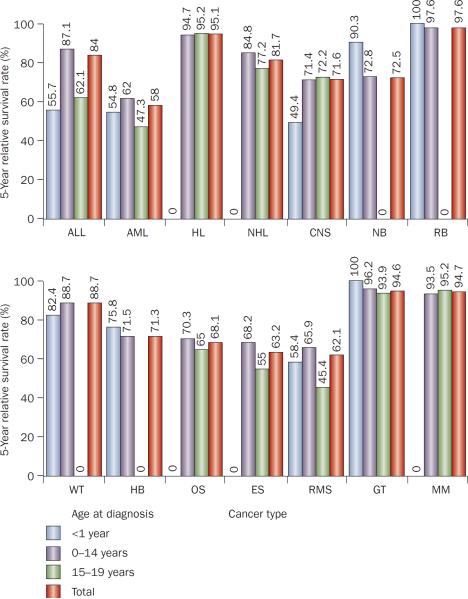
Of note, there is marked heterogeneity within each type of pediatric cancer, such that the survival rate varies substantially according to the disease stage, acquired genetic abnormalities, and age at clinical presentation (Figure 2). The current challenge is to improve not only the cure rates for high-risk (difficult-to-treat) subtypes of childhood cancer, but also the overall quality of life of patients. In this Review, we describe the newly discovered genetic subtypes of cancers, the development of therapy targeted against molecular lesions, and major issues facing pediatric oncologists. We focus on the subtypes for which substantial advances in the understanding of biology and treatment have been made.
Figure 2.
Survival rates for different cancers in children. 5-year relative survival rates for children with ALL, AML, HL, NHL, CNS tumor, NB, RB, WT, HB, OS, ES, RMS, GT and MM by age group. Data obtained from Surveillance, Epidemiology, and End Results Registries based on follow up into 2007 of patients from SEER 17 areas (SEER 9 areas plus San Jose-Monterey, Los Angeles, Alaska Native Registry, Rural Georgia, California,—excluding San Francisco, San Jose-Monterey and Los Angeles—Kentucky, Louisiana and New Jersey) who were diagnosed between 1999 and 2006. Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CNS, central nervous system; ES, Ewing sarcoma; GT, gonadal germ-cell tumor; HB, hepatoblastoma; HL, Hodgkin lymphoma; MM, malignant melanoma; NB, neuroblastoma and ganglioneuroblastoma; NHL, non-Hodgkin lymphoma; OS, osteosarcoma; RB, retinoblastoma; RMS, rhabdomyosarcoma; WT, Wilms' tumor.
Acute lymphoblastic leukemia
As the 5-year survival rates in childhood ALL improved to more than 84%,3,4 many conventional factors that have guided prognosis—such as gender and race—are no longer useful. Historically, compared with younger children, adolescents had a much worse treatment outcome because of an increased prevalence of high-risk leukemia and a poorer tolerance and adherence to therapy. Now, adolescents have similar high cure rates to those of younger children owing to the use of risk-adjusted intensive chemotherapy including glucocorticoids, vincristine, asparaginase and triple intrathecal therapy, provided compliance is closely monitored.5,6
Philadelphia chromosome-positive ALL—that results in the gene fusion BCR–ABL1 and was once associated with a dismal prognosis—is no longer considered to be an absolute indication for allogeneic stem-cell transplantation during first remission. In 2009, the Children's Oncology Group reported a 3-year event-free survival of 80% (95% CI 64–90%) in patients treated with continuous imatinib—a tyrosine kinase inhibitor (TKI)—and intensive chemotherapy, compared with 31–39% for historical controls.7 There are, however, limited studies on the use of TKIs in patients who experience relapse after transplantation. Although the impact of this treatment on long-term event-free survival is unknown, many investigators reserve allogeneic transplantation for therapy after relapse in children with Philadelphia chromosome-positive ALL. Ongoing studies—such as the AALL0622 trial of the Children's Oncology Group—will determine whether the new and more potent TKIs (for example dasatinib)8 will further improve outcome. Moreover, these studies will help to determine if chemotherapy intensity can be reduced in patients with good early responses. To this end, two studies published in 2009 showed that with effective intrathecal and systemic therapy,9 prophylactic cranial irradiation (once a standard treatment) can be safely omitted in all patients, regardless of the presented features.10,11 These two studies yielded excellent 5-year event-free survival rates of 85.6% and 81%, and low rates of isolated central nervous system (CNS) relapse (2.7% and 2.6%), respectively.10,11 In the first study, only one of 498 patients developed a secondary myelodysplastic syndrome.10
Several high-risk subtypes of ALL still pose therapeutic challenges (Table 2). Early T-cell precursor ALL—a new subset of T-cell ALL characterized in 2009 by immature genetic and immunophenotypic features—does not respond to conventional therapy, such as prednisone.12 Treatments under investigation for this subtype of ALL include nelarabine,8 allogeneic stem-cell transplantation,4 and high-dose dexamethasone (10 mg/m2 per day).13 Treatment with dexamethasone yielded a cumulative relapse of 4–8% compared with a relapse of 16–24% in T-cell ALL cases that were considered to respond favorably to prednisone. Despite intensive chemotherapy and allogeneic transplantation, infants with myeloid, lymphoid or mixed-lineage leukemia (MLL)-rearranged ALL continue to have a dismal prognosis due to relapse.14–16 Potential therapeutics for these infants include a TKI against FLT-3 —which has schedule-dependent synergistic effects with chemotherapy for MLL-rearranged leukemia in preclinical studies—17 and DNA methyltransferase inhibitors, because aberrant DNA methylation occurs in the majority of these cases.18
Table 2.
Selected challenging subtypes of childhood cancer
| Targets altered and disease subtype | Frequency (%) | 5-year event-free survival (%) | Potential therapy |
|---|---|---|---|
| Early T-cell precursor ALL4,8,12 | 1.5 | 20 | Nelarabine; allogenic transplantation |
| MLL-rearranged ALL in infants4,17,18 | 2 | 30 | FLT-3 inhibitors (lestaurtinib, midostaurin); DNA methyltransferase inhibitors (decitabine) |
| AML with internal tandem duplication of FLT34,28–30 | 10–15 | <35 | FLT-3 inhibitors; allogenic transplantation |
| AML with monosomy 7, 5q or t(6;9)3 | 3 | <40 | Immunotherapy; cellular therapy |
| Large cell and/or aplastic medulloblastoma with MYC amplification40 | 10–15 | <40 | To be determined |
| Notch pathway-driven ependymoma46 | 35 | <20 | Notch inhibitors |
| Diffuse intrinsic pontine glioma with PDGFR amplification53 | 40 | <5 | PDGFR antagonists; PARP inhibitors |
| Metastatic rhabdomyosarcoma71–76 | 16 | <30 | MET inhibitors; IGF-1R and mTOR inhibitors; FGFR-4 inhibitors; hedgehog pathway inhibitors; glycogen synthase kinase 3 (GSK-3) inhibitor; PDGFR antagonists |
| Gastrointestinal stromal tumor80 | <1 | <20 | IGF-1R inhibitors |
| High-risk neuroblastoma (MYCN amplification, 11q− , 17q+, 1p−)64 | 45 | ~50 | ALK inhibitors; I131 or I123 metaiodobenzylguanidine; aurora kinase inhibitors |
| Metastatic Ewing sarcoma family of tumors85 | 25 | <25 | IGF-1R inhibitors and mTOR inhibitors |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; FGFR, fibroblast growth factor receptor; FLT, fms-related tyrosine kinase; IGF-1R, insulin-like growth factor receptor; mTOR, mammalian target of rapamycin; PARP, poly(ADP) ribose polymerase.
IKZF1 (encoding the lymphoid transcription factor Ikaros) is deleted in approximately 80% of cases of BCR–ABL1 positive ALL.19 Large-scale genome-wide analyses have identified a high-risk subgroup of BCR–ABL1 negative B-cell precursor ALL that is also characterized by IKZF1 deletion,20,21 and another high-risk subtype of B-cell precursor ALL with overexpression of CRLF2 (encoding for cytokine receptor-like factor 2 [CRLF-2]).22,23 In the study by Mullighan et al.,20 the cumulative rates of relapse were 47.0% and 24.6% at 10 years in the original cohort of patients, and were 73.8% and 25.0% at 5 years in the validated cohort with or without IKZF1 deletion, respectively. High levels of expression of CRLF-2 were associated with a significantly higher 6-year cumulative risk of relapse (23–39% versus 10–12% in patients with low expression) in a study by Cario et al.23 The presence of activating mutations in the JAK family of protein kinases in both of these subtypes raises the possibility of treating these patients with JAK inhibitors. Identification of new mutations that affect transcriptional and epigenetic regulation as a mechanism of drug resistance raises the possibility of reversing the aberrant epigenetic alterations using drugs such as histone deacetylase inhibitors.24 However, no clinical data are currently available.
Hypodiploidy (<44 chromosomes), translocation t(17;19)(q22;p13.3) TCF3–HLF, and poor responses to early treatment—such as induction failure and a high level (1% or more) of minimal residual disease (MRD) at the end of remission induction—are features associated with a poor prognosis, despite the use of allogeneic transplantation.25 Promising novel therapeutics under investigation in this setting include natural killer-cell (NK-cell) transplantation using donor NK cells that express inhibitory killer-cell immunoglobulin-like receptors in the absence of cognate ligand in the recipient—that is, receptor–ligand mismatch—and immunotherapy with conjugated antibodies, bispecific T-cell-engager single-chain antibodies (for example, blinatumomab), and chimeric T-cell receptors.4,26
Intensive use of glucocorticoids, asparaginase and methotrexate in contemporary clinical trials has led to an increased prevalence of osteonecrosis, especially in adolescents aged 10–20 years, partly because of slower systemic clearance of glucocorticoids in this group.27 Studies are under way to determine whether early detection of this complication by prospective MRI27 or interrupted use of glucocorticoids—CCG 1961 trial—would decrease the severity of this complication.
Acute myeloid leukemia
As in the case of ALL, identification of specific genetic abnormalities and early treatment response represent ways to assess the risk of induction failure or relapse in current clinical protocols for AML. Subtypes of AML with t(8;21) ETO–AML1, inv(16) MYH11–CBFB, t(15;17) PML–RARα, or mutations of CEBPA (encoding for CCAAT/enhancer-binding protein alpha) or NPM (encoding for nucleophosmin) are highly curable without the use of allogeneic stem-cell transplantation.4,28 By contrast, monosomy 7, abnormalities in 5q or 12p, t(6;9)(p23;q34), and internal tandem duplications of FLT3—particularly when associated with a high mutant to wild-type allelic ratio—are associated with 5-year event-free survival below 40% despite the use of allogeneic stem-cell transplantation.4,28–30 Several clinical trials are testing various FLT-3 inhibitors with or without transplantation in 10–15% of the participating patients with internal tandem duplications of FLT3. Acute megakaryoblastic leukemias—with the exception of the 2–3% of those with t(1;22) RBM15–MKL1—continue to confer a poor prognosis.28
Response to induction therapy, as assessed by MRD assays, is one of the most reliable prognostic indicators of AML. In the AML02 study, which used MRD level to direct the intensity of treatment, patients with low levels of MRD (0.1% to <1%) after the first course of remission induction had a similar positive response to those with undetectable MRD.28 These results suggested that this treatment strategy has abrogated the adverse prognosis previously associated with a poor early treatment response. However, patients with MRD above 1% have an estimated 3-year event-free survival of only 32%, despite allogeneic transplantation. Thus, novel therapies are needed for this patient subgroup. One promising approach is the use of NK-cell transplantation, which yielded a 2-year event-free survival of 100% (95% CI 63.1–100%) in 10 patients treated after completion of chemotherapy in first complete remission.31
A major component of AML treatment is the use of an anthracycline; however, intensive use is associated with cardiomyopathy.32 Attempts to reduce the debilitating late effect of anthracyclines include reducing the dose or replacing some of the doses with other drugs such as cladribine, fludarabine, and clofarabine.33 Although many studies have shown that allogeneic transplantation improves the overall relapse-free survival rate in AML, there is no consensus on the indications for this treatment modality or on the most suitable donors or optimal preparative regimens. Less controversial is the omission of cranial irradiation in AML, a strategy that proved successful in the AML02 study.28
Infectious complications remain a major cause of morbidity and mortality for patients with AML. Prophylactic treatment with cefepime or vancomycin plus ciprofloxacin during periods of leukopenia, significantly reduced bacterial infection, febrile neutropenia and the duration of hospitalization without increasing fungal infection.34 Although controlled trials are still needed to firmly establish its efficacy, antifungal prophylaxis has been widely adopted. Among the available antifungal treatments, fluconazole and itraconazole are not recommended—not only because they inhibit cytochrome P450 enzymes, thereby affecting subsequent chemotherapy efficacy, but also because they are not effective against Aspergillus species.35 Finally, prophylactic granulocyte colony-stimulating factor (G-CSF) is not recommended in AML because it does not reduce morbidity or mortality and is associated with an increased risk of relapse in cases that overexpress differentiation-defective G-CSF receptor isoform IV.36
Central nervous system tumors
Medulloblastoma
Cure rates for patients with newly diagnosed medulloblastoma—the most common malignant CNS tumor of childhood—have steadily improved over the past three decades. Current forms of multimodality therapy can cure more than 80% of patients with standard-risk disease and more than 60% of those with high-risk presentations.37 Gene-expression profiling studies have demonstrated that medulloblastoma actually encompasses four or five molecular diseases with distinct clinical, pathological and prognostic features.38–40 The subgroup of adolescents with mutations in the components of the Wnt and β-catenin signaling pathway constitute about 15% of the patients with an excellent prognosis and a 5-year event-free survival rate of more than 95% with conventional therapy. The subgroup of patients with mutations in components of the hedgehog signaling pathway (HH)—which comprise about 20–25% of the patients with medulloblastoma and who have mutations in PTCH, Smoothened (SMO) and SUFU—have an intermediate prognosis with 5-year event-free survival rate of 75–85% when treated with conventional therapy.40 Introduction of targeted therapy directed to the HH pathway promises to improve the outcome for these patients by potentially reducing the need for cytotoxic chemotherapy41 The remaining 60–65% of patients—which include a small proportion of patients with MYC amplification—have an inferior prognosis (~60% 5-year event-free survival) when treated with conventional therapy.40 Current research efforts are focused on the development of targeted agents that can block specific signaling pathways associated with each molecular subtype of the disease, resulting in more-effective and less-toxic therapy.
Ependymoma
The extent of surgical resection, histological grade and the presence of metastatic disease are key prognostic factors for ependymomas—tumors that arise from ependymal cells that line the ventricles in the CNS.42 In 2009, a prospective study demonstrated that approximately 69% of the patients with ependymoma can be cured at 7 years of follow-up when treated with aggressive surgical resection and local conformal radiation therapy.43 Gain of chromosome 1q25 and homozygous deletion of CDKN2A (encoding for the tumor suppressor p16-INK4α) negatively impact the survival of these patients with overall survival of 30–82% and 10–76%, respectively, whereas gain of chromosomes 9q34, 15q22, 18q21 and loss of 6q23 are associated with excellent survival.44 A more-extensive genomic analysis with single nucleotide polymorphism data has classified ependymoma into nine molecular subtypes based on tumor location and chromosomal changes.45 On the basis of these data, several candidate genes (for example, THAP11, PSPH, EPHB2, KCNN1, RAB3A, NOTCH1, PTEN and CDKN2A) have emerged that may have a critical role in ependymoma pathogenesis.45 Novel therapeutic approaches that target signaling pathways critical to the malignant transformation of neural stem cells, such as Notch inhibitors, are being tested in clinical trials.46,47 No data are currently documented on approaches for patients with gain of 1q25 or loss of CDKN2A.
High-grade and diffuse-pontine glioma
Despite aggressive surgical resection, high-dose radiation therapy, chemotherapy, and autologous bone-marrow transplantation, the outcome of patients diagnosed with high-grade glioma and diffuse-pontine glioma remains dismal.48 Chemotherapeutic agents that have demonstrated some efficacy in adult gliomas (for example, temozolomide, irinotecan and bevacizumab) have showed disappointing results in pediatric gliomas.49,50 Genomic analysis of adult high-grade glioma offers insight into the molecular pathogenesis of the adult disease.51,52 Abnormalities in specific genes—such as IDH1 (encoding for isocitrate dehydrogenase 1), PDGFRA (encoding for platelet derived growth factor A), EGFR, NF1(encoding for neurofibromin 1), RB, TP53—and signaling pathways (for example RTK/RAS/PI3K) have been implicated in malignant transformation.52 Similar analyses of pediatric tumors have demonstrated key differences between adult and pediatric high-grade glioma. These include the absence of hot-spot mutations in IDH1 and the presence of focal amplification of PDGFRA in pediatric tumors that are not present in adults, and explain some of the different responses to chemotherapy.53,54 Despite efforts to devise novel translational approaches to therapy, effective treatment for these tumors remains elusive and presents a major challenge to the field.
Low-grade glioma
Low-grade gliomas that are localized in areas amenable to gross total resection can be cured by surgery alone with minimal morbidity.55 Tumors located in the midline (chiasmatic-hypothalamic) are often not surgically resectable, leading to significant morbidity—such as visual impairment or blindness and endocrine abnormalities. Chemotherapy and irradiation remain effective against low-grade glial tumors, but are often not curative.56 The major main molecular alterations in low-grade gliomas include duplication, activating mutations and fusion transcripts of BRAF, implicating the MAPK pathway in the pathogenesis of these tumors.57–60 In addition to advanced radiation therapy techniques, finding effective agents with minimal associated morbidity that target the MAPK pathway in the CNS should be a research priority for curing low-grade gliomas.
Infant brain tumors
The vulnerability of the infant brain to the adverse effects of surgery, chemotherapy and irradiation underscores the fundamental problem hindering the development of curative treatment approaches for this patient group. Desmoplastic medulloblastoma in infancy has a high cure rate with surgery and chemotherapy alone.61 Most of these tumors have mutations in the HH pathway and may be cured with targeted therapy in the future. Compared with older children, infants with high-grade gliomas and brain-stem tumors have an overall survival of 60%, suggesting that these tumors are molecularly different from the same tumors in older patients.62 Atypical teratoid rhabdoid tumors (ATRT) constitute a rare, but important, subgroup of infant brain tumors with a very poor prognosis (overall survival <20%).63 The molecular hallmark signature of ATRT is deletion of SMARCB1 (encoding the chromatin regulator BAF47), detected by presence of monosomy 22 and the absence of BAF47 staining on immunohistochemistry. A small subgroup of these patients presents with synchronous renal and ATRT. Current therapy for infant brain tumors focuses on tailoring the intensity of therapy to predicted outcome, hence reducing the risk of long-term toxic effects in the survivors. Genomic analysis of tumors from this patient group will further inform us of key differences in their pathogenesis as compared with their older counterparts.
Neuroblastoma
Neuroblastoma, the most common extracranial solid tumor in childhood, is readily cured in about 70% of patients.64 In 2010, Baker et al.65 reported overall 5-year survival rates in excess of 95% in children with intermediate-risk disease treated with reduced-intensity risk-based chemotherapy. By contrast, among patients with high-risk disease—defined by segmental chromosomal aberrations such as amplification of MYCN (also known as neuroblastoma MYC oncogene)—the cure rate is much lower with induction chemotherapy, myeloblative consolidation therapy, and maintenance therapy with isotrenitoin.64 The addition of ch14.18, the anti-GD2 antibody, with alternating cycles of interleukin-2 or granulocyte macrophage-CSF (GM-CSF) in addition to isotretinoin, has been reported to improve the 2-year event-free survival of these patients to 66%, a 20% increase over the 2-year event-free survival of patients who received isotretinoin alone.66 Promising results in the relapse setting suggested that radiolabeled I131-metaiodobenzylguanidine or I123-metaiodobenzylguanidine should also be explored as consolidation therapy in high-risk patients.64 Moreover, the discovery of ALK mutations or amplifications in about 15% of newly diagnosed neuroblastoma cases has prompted the development of treatment to inhibit this target.67 Finally, the Pediatric Preclinical Testing Program has identified an aurora kinase A inhibitor (MLN8237) and the replication-competent RNA virus Seneca Valley virus as potential active agents.68,69
Rhabdomyosarcoma
Around 75% of the children and adolescents with localized rhabdomyosarcoma survive. However, patients with metastatic disease and at least two adverse presenting factors (such as age 12 months or younger or 10 years or older, bone marrow involvement, and three or more metastatic sites) respond poorly, with a 3-year event-free survival of less than 20%.70 Indeed, an analysis of 542 children and adolescents with high-risk rhabdomyosarcoma enrolled in Intergroup Rhabdomyosarcoma Studies (IRS III, IV, IVp and D series) between 1986 and 2005 demonstrated a lack of improvement in clinical outcome over the two decades and a median time to progression of only 1.4 years (J. Anderson, personal communication, with permission from the Children's Oncology Group soft-tissue sarcoma committee).
The current challenge is to develop effective treatments that reduce or eliminate the use of radiation or alkylating agents for low-risk cases and incorporate novel therapies for high-risk cases. In cell lines and animal model systems, insulin-like growth factor 1 receptor (IGF-1R) has important roles in the proliferation, stress response and survival of rhabdomyosarcoma cells, via the AKT protein.71 Thus, the Children's Oncology Group is currently evaluating the feasibility of adding the IGF-1R monoclonal antibody IMC-A12 to standard chemotherapy in patients aged 10 years or older with metastatic embryonal rhabdomyosarcoma and in all patients with metastatic alveolar rhabdomyosarcoma (trial ARST08P1). In rhabdomyosarcoma xenograft models, the anti-IGF-1R antibody h7C10 synergized with rapamycin causing a reduction in tumor growth and phosphorylated AKT levels.71 Future trials should also explore this treatment combination. HH pathway activation has been documented in subgroups of patients with rhabdomyosarcoma and targeting this pathway may benefit patients with embryonal and fusion gene-negative tumors.72 Aberrant Met signaling has been identified in mouse models and gene-expression studies of rhabdomyosarcoma, suggesting that the inhibition of this pathway may also be useful in the treatment of the disease.73 Moreover, mutations in FGFR4 (encoding for fibroblast growth factor receptor 4) were found in 7% of primary rhabdomyosarcomas, providing a rationale for targeting this gene.74 Other potential targets include MYCN, the chemokine (C-X-C motif) receptor 4–stromal cell-derived factor 1 (CXCR-4–SDF-1) axis, PDGFR, cyclins D2 and D3, VEGF, and glycogen synthase kinase-3 (GSK-3).75,76 Finally, in a study of 120 rhabdomyosarcoma specimens, gene-expression analysis identified a 34-probe set model that was highly predictive of clinical outcome and correlated well with the current risk stratification system used by the Children's Oncology Group.77 This strategy may help improve risk-adapted therapies and identify additional targets for therapy.
Other soft-tissue sarcomas
Non-rhabdomyosarcoma soft-tissue sarcomas are a heterogeneous group of tumors that account for about 4% of all pediatric cancers.3 These tumors are relatively chemoresistant, more commonly affect adults and have been under studied in the pediatric population. Effective treatment of adults with soft-tissue sarcoma has been achieved by the use of targeted therapies, including imatinib for KIT-mutated gastrointestinal stromal tumor (GIST)78 and crizotinib for ALK-rearranged inflammatory myofibroblastic tumor.79 However, the histologic distribution and biology of some of these entities—including GIST and infantile fibrosarcoma—differ between childhood and adult cases.80,81 For example, activating mutations of KIT or PDGFRA are detected in over 90% of adult GISTs, but in only 11% of the childhood cases;82 thus, targeted therapies commonly used in adults—such as imatinib—are expected to be less effective in the pediatric population. By contrast, IGF-1R and the mitochondrial succinate dehydrogenase complex (DHSB) seem to be pivotal in the pathogenesis of pediatric and wild-type GISTs,82,83 suggesting that these pathways should be preferentially exploited in childhood cases.
Ewing sarcoma family of tumors
The Ewing sarcoma family of tumors—a group of bone and soft-tissue tumors derived from mesenchymal progenitor cells characterized by rearrangements of EWS—includes classic Ewing sarcoma, Askin tumor, and peripheral primitive neuroectodermal tumor. Although several different treatment regimens are available for the treatment of these tumors, they all use similar drugs (vincristine, doxorubicin, cyclophosphamide, ifosfamide and etoposide) as well as local control measures with radiation therapy, surgery or both. To date, 70–80% of the patients who present with localized disease are cured with this therapy but only about 25% of those with metastases survive, many of whom suffer from short-term and long-term treatment-related toxic effects.84
The EWS-ETS family fusion genes, their transcripts and protein products, and the pathways they activate all provide promising targets for therapy. The most attractive candidate agents are the IGF-1R inhibitors, which have produced responses as single agents in about 10% of patients with relapsed disease.85 Other potential agents include inhibitors of PDGFR, mTOR (in combination with IGF-1R inhibitors), SRC, VEGF, and CD99, as well as the Seneca Valley virus and the kinesin spindle protein inhibitor ispinesib.69,86
Osteosarcoma
Approximately 70% of patients with localized osteosarcoma are cured with the use of current treatment but less than 30% of those patients with metastatic disease survive.87 The search for specific genetic alterations in osteosarcoma—the most common primary bone tumor in children and adolescents—has yet to uncover any consistent lesions that could be used to develop targeted therapy. Instead, the tumors are characterized by a complex karyotype, aneuploidy, and dysregulation of numerous pathways and genes including RB, TP53, CDKN2A, RECQL4, MET, FOS, mTOR, WNT, NOTCH, IGF1, EGFR, VEGFR, PDGFR, MYC, HER2, and Ezrin.88 This characterization has greatly complicated efforts to devise effective targeted therapies. Promising new therapeutic approaches include administration of the immunostimulant muramyl tripeptide phosphatidylethanolamine, the combination of an IGF-1R and mTOR inhibitors, SRC kinase inhibition, immune targeting of HER2, angiogenesis inhibition (inhibition of VEGF), and agents that target the MET, receptor activator of nuclear factor κB ligand (RANKL), and Notch pathways.87–90
Retinoblastoma
Most children with retinoblastoma can be cured with enucleation and radiation, whereas patients with metastatic disease require systemic treatment.91 The current 5-year survival rate for all patients with retinoblastoma is 96.5%, compared with 92.3% 30 years ago.92 Therapeutic objectives in retinoblastoma include not only improved survival but also eye salvage with good vision and cosmesis.93 Advanced intraocular retinoblastoma poses a particular therapeutic challenge because current intensified therapy, especially radiation, increases the risk of secondary malignancies.94 Treatments under investigation include new chemotherapeutic agents such as topotecan,95 and different methods of local drug delivery, such as subconjunctival,95 intra-arterial,96 intra-vitreal,97 and episcleral implants for sustained release.98 The discovery of inactivation of the p53 pathway via MDMX amplification in promoting human retinoblastoma suggests that MDMX inhibitors, such as nutlin-3, might be effective for the treatment of this disease.99
Rare cancers
Rare cancers account for 9% of the childhood cancers and offer a unique opportunity to study novel mechanisms of disease. For example, adrenocortical carcinoma is a rare malignancy in childhood and adolescence. However, a high incidence of this disease has been observed in children in southern Brazil, a finding that has led to the discovery of unique germline TP53 mutations that contribute to its pathogenesis.100 Most children with adrenocortical tumors—particularly those under 4 years of age—have inherited mutations in TP53. The inherited mutations are less frequent in older children and adolescents and very rare in adult patients. Similarly, pleuropulmonary blastoma is a very rare malignant embryonal mesenchymal tumor of the lung and pleura that arises during fetal lung development; up to 40% of the patients may have genetic predisposition to this cancer. A family-based linkage study found that DICER1—a gene encoding ribonuclease III endonuclease that participates in the generation of small RNAs and small interfering RNAs—was mutated in this type of tumor.101
Palliative care
The most prevalent symptoms pediatric patients experience are pain, fatigue, nausea or vomiting, constipation and weight loss; however, the type and severity of symptoms vary by disease and their prevalence is highest for patients with solid tumors, patients who are hospitalized, undergo stem-cell transplantation, receive chemotherapy, or receive palliative care at the end of life.102,103 The parents of children with cancer report poorer quality of life compared with population norms;104 many parents experience distress from having to make difficult end-of-life care decisions, such as participating in phase I clinical trials, withholding or withdrawing life-sustaining treatments, foregoing cancer chemotherapy, talking to their children about death, or deciding on the location during the terminal phase.105–107 Bereaved parents are at increased risk of experiencing anxiety and depression for many years after the death of their child.108 Most parents of children with advanced-stage cancer desire both cancer-directed therapy and comfort-directed care. They also value effective communication and interpersonal relationships as important components of high-quality end-of-life care, and prefer to experience the death of their child at home.109,110
Palliative care that addresses the multiple physical, emotional, social, and spiritual issues should be provided concomitantly with cancer-directed treatment to improve the quality of care.111,112 Integration of palliative care into the ongoing care of children with cancer may be achieved by facilitating access to hospice and palliative care services early in the illness trajectory, promoting education, and developing policies and procedures that place greater emphasis on comfort and quality of life, particularly for patients with disseminated or advanced-stage disease.113 In this regard, palliative care may even improve life expectancy for some patients with incurable cancer.114 Health care providers involved in the care of children with cancer should establish the prognosis, negotiate goals, guide patients and/or their parents in the process of making difficult medical decisions, provide comfort, enhance quality of life, promote care coordination and continuity, optimize comfort at the end of life, and attend to the needs of bereaved family members (Box 1).115,116Despite unanimous recognition and support by national and international organizations for early incorporation of palliative care as a routine part of comprehensive cancer care, further efforts are needed to improve this discipline. There remains significant heterogeneity in the infrastructure of this service at cancer centers, and patients continue to be referred to palliative and hospice care late in the disease trajectory and in small numbers.117
Conclusions
Great strides have been made in the treatment of ALL, non-Hodgkin lymphoma and non-CNS germ-cell tumors. Indeed, it is likely that in the coming decade 90% of children with these cancers will be disease-free for 5 years, similar to the percentage of survivors with Hodgkin lymphoma and Wilms' tumors. By contrast, little or no progress has been made for children with rhabdomyosarcoma, osteosarcoma, Ewing sarcoma, malignant glioma and brain stem glioma over the past two decades, because of intractable drug resistance and because of the limited number of clinical trials being conducted for these tumors. Given that the intensity of chemotherapy has been pushed to the limit of tolerance, and that remarkable progress has been made in understanding cancer cell biology, the prospects for the development of effective targeted therapies for these pediatric tumors have never seemed brighter. Perhaps the greatest obstacle to achieving this goal is how to design and implement clinical trials that will identify effective strategies to improve outcome for progressively smaller subsets of drug-resistant cases that possess specific genetic alterations. With the growing number of potentially useful targeting agents, it will be necessary to cultivate greater international collaboration to test and refine therapies for these cases. It has become increasingly apparent that inter-individual differences in the pharmacodynamics of anticancer drugs that can be caused by both environmental and inherited genetic differences—that is, pharmacogenetics—can affect the efficacy and toxicity of treatment. Thus, it is essential to personalize therapy based on host pharmacodynamics and pharmacogenomics to avoid over treatment or under treatment. Finally, it must be remembered that even the most rational and well-tested treatment plans may fail, underscoring the need for comprehensive palliative care to relieve the suffering and improve the quality of life for both the patients and their families.
Key points
Prophylactic cranial irradiation can be omitted from the treatment of patients with acute lymphoblastic or myeloid leukemia, with the use of effective systemic and intrathecal therapy
Vigilant support care can reduce morbidity and mortality, and improve event-free survival of acute myeloid leukemia
The next generation of treatment for brain tumors will be tailored to specific molecular subtypes of disease to improve the cure rates and reduce the long-term sequela of therapy
The cure rates have improved dramatically over the past three decades for patients with localized solid tumors Patients with disseminated disease continue to fare poorly and some are now receiving targeted therapy against specific molecular alterations
Integration of palliative care into the ongoing care of children with cancer improves the quality of pediatric oncology care
Box 1 | Quality palliative and end-of-life care practices115,116
Understand the illness experience from the perspective of the child and the family
Practice advanced care planning and ensure ethical decision making
Provide expert control of pain and symptoms
Provide emotional, social, and spiritual support for the child and the family
Promote care coordination and continuity
Provide expert end-of-life care
Provide bereavement follow up for surviving family members
Acknowledgments
This project was supported in part by grant CA21765 from the National Institutes of Health, and by the American Lebanese Syrian Associated charities.
Footnotes
Competing interests The authors declare no competing interests.
Review criteria Information for this Review was compiled by searching the MEDLINE and PubMed databases for original articles and reviews published in English in the past 10 years, using the search terms “acute lymphoblastic leukemia”, “acute myeloid leukemia”, “medulloblastoma”, “ependymoma”, “glioma”, “infant brain tumor”, “osteosarcoma”, “Ewing sarcoma”, “neuroblastoma”, “rhabdomyosarcoma”, “soft tissue sarcoma”, “retinoblastoma”, and “palliative care”. Because of the large number of relevant articles, reviews were favored over original articles, and articles published in the past 5 years were preferentially cited.
Author contributions
All authors were involved in the research of data, discussion of content, writing the Review and to editing and revising the manuscript before submission.
References
- 1.The cancer genome census Welcome trust sanger institute [online] http://www.sanger.ac.uk/genetics/CGP/Census (XXXX)
- 2.Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719–724. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Cancer Institute Surveillance Epidemiology and End Results. Previous Version: SEER Cancer Statistics Review, 1975–2007 [online] 2010 http://seer.cancer.gov/csr/1975_2007/ based on November 2009 SEER data submission, posted to the SEER web site.
- 4.Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification and therapy of pediatric acute leukemias: an update. J. Clin. Oncol. 2011;29:551–565. doi: 10.1200/JCO.2010.30.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nachman JB, et al. Young adults with acute lymphoblastic leukemia have an excellent outcome with chemotherapy alone and benefit from intensive postinduction treatment: a report from the children's oncology group. J. Clin. Oncol. 2009;27:5189–5194. doi: 10.1200/JCO.2008.20.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pui CH, et al. Improved prognosis for older adolescents with acute lymphoblastic leukemia. J. Clin. Oncol. 2011;29:386–391. doi: 10.1200/JCO.2010.32.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz KR, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a Children's Oncology Group study. J. Clin. Oncol. 2009;27:5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pui CH, Jeha S. New therapeutic strategies for the treatment of acute lymphoblastic leukaemia. Nat. Rev. Drug. Discov. 2007;6:149–165. doi: 10.1038/nrd2240. [DOI] [PubMed] [Google Scholar]
- 9.Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9:257–268. doi: 10.1016/S1470-2045(08)70070-6. [DOI] [PubMed] [Google Scholar]
- 10.Pui CH, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N. Engl. J. Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veerman AJ, et al. Dexamethasone-based therapy for childhood acute lymphoblastic leukaemia: results of the prospective Dutch Childhood Oncology Group (DCOG) protocol ALL-9 (1997–2004) Lancet Oncol. 2009;10:957–966. doi: 10.1016/S1470-2045(09)70228-1. [DOI] [PubMed] [Google Scholar]
- 12.Coustan-Smith E, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10:147–156. doi: 10.1016/S1470-2045(08)70314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrappe M, et al. Dexamethasone in induction can eliminate one third of all relapses in childhood acute lymphoblastic leukemia (ALL): Results of an international randomized trial in 3655 patients (Trial AEIOP-BFM ALL 2000) [abstract] Am. Soc. Hematol. Ann. Meeting. 2008;112:a7. [Google Scholar]
- 14.Pui CH, et al. Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet. 2002;359:1909–1915. doi: 10.1016/S0140-6736(02)08782-2. [DOI] [PubMed] [Google Scholar]
- 15.Pieters R, et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet. 2007;370:240–250. doi: 10.1016/S0140-6736(07)61126-X. [DOI] [PubMed] [Google Scholar]
- 16.Dreyer ZE, et al. Analysis of the role of hematopoietic stem-cell transplantation in infants with acute lymphoblastic leukemia in first remission and MLL gene rearrangements: a report from the Children's Oncology Group. J. Clin. Oncol. 2011;29:214–222. doi: 10.1200/JCO.2009.26.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown P, Levis M, McIntyre E, Griesemer M, Small D. Combinations of the FLT3 inhibitor CEP-701 and chemotherapy synergistically kill infant and childhood MLL-rearranged ALL cells in a sequence-dependent manner. Leukemia. 2006;20:1368–1376. doi: 10.1038/sj.leu.2404277. [DOI] [PubMed] [Google Scholar]
- 18.Schafer E, et al. Promoter hypermethylation in MLL-r infant acute lymphoblastic leukemia: biology and therapeutic targeting. Blood. 2010;115:4798–4809. doi: 10.1182/blood-2009-09-243634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullighan CG, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453:110–114. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- 20.Mullighan CG, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N. Engl. J. Med. 2009;360:470–480. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Den Boer ML, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10:125–134. doi: 10.1016/S1470-2045(08)70339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullighan CG, et al. Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nat. Genet. 2009;41:1243–1246. doi: 10.1038/ng.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cario G, et al. Presence of the P2RY8-CRLF2 rearrangement is associated with a poor prognosis in non-high-risk precursor B-cell acute lymphoblastic leukemia in children treated according to the ALL-BFM 2000 protocol. Blood. 2010;115:5393–5397. doi: 10.1182/blood-2009-11-256131. [DOI] [PubMed] [Google Scholar]
- 24.Mullighan CG, et al. CREBBP mutations in relapsed acute lymphoblastic leukaemia. Nature. 2011;471:235–239. doi: 10.1038/nature09727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 26.Lee-Sherick AB, et al. Targeting paediatric acute lymphoblastic leukaemia: novel therapies currently in development. Br. J. Haematol. 2010;151:295–311. doi: 10.1111/j.1365-2141.2010.08282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawedia JD, et al. Pharmacokinetic, pharmacodynamic and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2011;117:2340–2347. doi: 10.1182/blood-2010-10-311969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubnitz JE, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 11:543–552. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrison CJ, et al. Cytogenetics of childhood acute myeloid leukemia: United Kingdom Medical Research Council Treatment trials AML 10 and 12. J. Clin. Oncol. 2010;28:2674–2681. doi: 10.1200/JCO.2009.24.8997. [DOI] [PubMed] [Google Scholar]
- 30.Pratz KW, et al. FLT3-mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML. Blood. 2010;115:1425–1432. doi: 10.1182/blood-2009-09-242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubnitz JE, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J. Clin. Oncol. 2010;28:955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hudson MM, et al. Noninvasive evaluation of late anthracycline cardiac toxicity in childhood cancer survivors. J. Clin. Oncol. 2007;25:3635–3643. doi: 10.1200/JCO.2006.09.7451. [DOI] [PubMed] [Google Scholar]
- 33.Jeha S, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute myeloid leukemia. J. Clin. Oncol. 2009;27:4392–4397. doi: 10.1200/JCO.2008.18.8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurt B, et al. Prophylactic antibiotics reduce morbidity due to septicemia during intensive treatment for pediatric acute myeloid leukemia. Cancer. 2008;113:376–382. doi: 10.1002/cncr.23563. [DOI] [PubMed] [Google Scholar]
- 35.Cornely OA, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 2007;356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 36.Ehlers S, et al. Granulocyte colony-stimulating factor (G.-CSF) treatment of childhood acute myeloid leukemias that overexpress the differentiation-defective G.-CSF receptor isoform IV is associated with a higher incidence of relapse. J. Clin. Oncol. 2010;28:2591–2597. doi: 10.1200/JCO.2009.25.9010. [DOI] [PubMed] [Google Scholar]
- 37.Gajjar A, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 38.Thompson MC, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J. Clin. Oncol. 2006;24:1924–1931. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 39.Kool M, et al. Integrated genomics identifies 5 medulloblastoma subtypes with distinct molecular profiles, pathway signatures and clinicopathological features. PLoS ONE. 2008;3:e3068. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Northcott PA, et al. Medulloblastoma comprises four distinct molecular variants. J. Clin. Oncol. 2011;29:1408–1414. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudin CM, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N. Engl. J. Med. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamburrini G, et al. Survival following treatment for intracranial ependymoma: a review. Childs Nerv. Syst. 2009;25:1303–1312. doi: 10.1007/s00381-009-0874-y. [DOI] [PubMed] [Google Scholar]
- 43.Merchant TE, et al. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10:258–266. doi: 10.1016/S1470-2045(08)70342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korshunov A, et al. Molecular staging of intracranial ependymoma in children and adults. J. Clin. Oncol. 2010;28:3182–3190. doi: 10.1200/JCO.2009.27.3359. [DOI] [PubMed] [Google Scholar]
- 45.Johnson RA, et al. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature. 2010;466:632–636. doi: 10.1038/nature09173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puget S, et al. Candidate genes on chromosome 9q33–34 involved in the progression of childhood ependymomas. J. Clin. Oncol. 2009;27:1884–1892. doi: 10.1200/JCO.2007.15.4195. [DOI] [PubMed] [Google Scholar]
- 47.Kilday JP, et al. Pediatric ependymoma: biological perspectives. Mol. Cancer Res. 2009;7:765–786. doi: 10.1158/1541-7786.MCR-08-0584. [DOI] [PubMed] [Google Scholar]
- 48.Broniscer A. Past, present, and future strategies in the treatment of high-grade glioma in children. Cancer Invest. 2006;24:77–81. doi: 10.1080/07357900500449702. [DOI] [PubMed] [Google Scholar]
- 49.Broniscer A, et al. Temozolomide after radiotherapy for newly diagnosed high-grade glioma and unfavorable low-grade glioma in children. J. Neurooncol. 2006;76:313–319. doi: 10.1007/s11060-005-7409-5. [DOI] [PubMed] [Google Scholar]
- 50.Gururangan S, et al. Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: a Pediatric Brain Tumor Consortium study. J. Clin. Oncol. 2010;28:3069–3075. doi: 10.1200/JCO.2009.26.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parsons DW, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zarghooni M, et al. Whole genome profiling of pediatric intrinsic diffuse pontine gliomas highlight platelet derived growth factor receptor α and poly (ADP-ribose) polymerase as potential therapeutic targets. J. Clin. Oncol. 2010;28:1337–1344. doi: 10.1200/JCO.2009.25.5463. [DOI] [PubMed] [Google Scholar]
- 54.Paugh BS, et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J. Clin. Oncol. 2010;28:3061–3068. doi: 10.1200/JCO.2009.26.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gajjar A, et al. Low-grade astrocytoma: a decade of experience at St. Jude Children's Research Hospital. J. Clin. Oncol. 1997;15:2792–2799. doi: 10.1200/JCO.1997.15.8.2792. [DOI] [PubMed] [Google Scholar]
- 56.Armstrong GT, et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol. 2011;13:223–234. doi: 10.1093/neuonc/noq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Forshew T, et al. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J. Pathol. 2009;218:172–181. doi: 10.1002/path.2558. [DOI] [PubMed] [Google Scholar]
- 58.Pfister S, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J. Clin. Invest. 2008;118:1739–1749. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones DT, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68:8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bar EE, Lin A, Tihan T, Burger PC, Eberhart CG. Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. J. Neuropathol. Exp. Neurol. 2008;67:878–887. doi: 10.1097/NEN.0b013e3181845622. [DOI] [PubMed] [Google Scholar]
- 61.Rutkowski S, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N. Engl. J. Med. 2005;352:978–986. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 62.Sanders RP, et al. High-grade astrocytoma in very young children. Pediatr. Blood Cancer. 2007;49:888–893. doi: 10.1002/pbc.21272. [DOI] [PubMed] [Google Scholar]
- 63.Tekautz TM, et al. Atypical teratoid/rhabdoid tumors (ATRT): improved survival in children 3 years of age and older with radiation therapy and high-dose alkylator-based chemotherapy. J. Clin. Oncol. 2005;23:1491–1499. doi: 10.1200/JCO.2005.05.187. [DOI] [PubMed] [Google Scholar]
- 64.Maris JM. Recent advances in neuroblastoma. N. Engl. J. Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baker DL, et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N. Engl. J. Med. 2010;363:1313–1323. doi: 10.1056/NEJMoa1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu AL, et al. Anti-GD2 Antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mossé YP, Wood A, Maris JM. Inhibition of ALK signaling for cancer therapy. Clin. Cancer Res. 2009;15:5609–5614. doi: 10.1158/1078-0432.CCR-08-2762. [DOI] [PubMed] [Google Scholar]
- 68.Maris JM, et al. Initial testing of the aurora kinase A inhibitor MLN8237 by the Pediatric Preclinical Testing Program (PPTP) Pediatr. Blood Cancer. 2010;55:26–34. doi: 10.1002/pbc.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morton CL, et al. Initial testing of the replication competent Seneca Valley virus (NTX-010) by the pediatric preclinical testing program. Pediatr. Blood Cancer. 2010;55:295–303. doi: 10.1002/pbc.22535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oberlin O, et al. Prognostic factors in metastatic rhabdomyosarcomas: results of a pooled analysis from United States and European cooperative groups. J. Clin. Oncol. 2008;26:2384–2389. doi: 10.1200/JCO.2007.14.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao L, et al. Addiction to elevated insulin-like growth factor I receptor and initial modulation of the AKT pathway define the responsiveness of rhabdomyosarcoma to the targeting antibody. Cancer Res. 2008;68:8039–8048. doi: 10.1158/0008-5472.CAN-08-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zibat A, et al. Activation of the hedgehog pathway confers a poor prognosis in embryonal and fusion gene-negative alveolar rhabdomyosarcoma. Oncogene. 2010;29:6323–6330. doi: 10.1038/onc.2010.368. [DOI] [PubMed] [Google Scholar]
- 73.Davicioni E, et al. Identification of a PAX-FKHR gene expression signature that defines molecular classes and determines the prognosis of alveolar rhabdomyosarcomas. Cancer Res. 2006;66:6936–6946. doi: 10.1158/0008-5472.CAN-05-4578. [DOI] [PubMed] [Google Scholar]
- 74.Taylor JG, 6th., et al. Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J. Clin. Invest. 2009;119:3395–3407. doi: 10.1172/JCI39703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wachtel M, Schäfer BW. Targets for cancer therapy in childhood sarcomas. Cancer Treat. Rev. 2010;36:318–327. doi: 10.1016/j.ctrv.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 76.Zeng FY, et al. Glycogen synthase kinase 3 regulates PAX3-FKHR-mediated cell proliferation in human alveolar rhabdomyosarcoma cells. Biochem. Biophys. Res. Commun. 2010;391:1049–1055. doi: 10.1016/j.bbrc.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davicioni E, et al. Gene expression profiling for survival prediction in pediatric rhabdomyosarcomas: a report from the children's oncology group. J. Clin. Oncol. 2010;28:1240–1246. doi: 10.1200/JCO.2008.21.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Demetri GD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 79.Butrynski JE, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N. Engl. J. Med. 2010;363:1727–1733. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agaram NP, et al. Molecular characterization of pediatric gastrointestinal stromal tumors. Clin. Cancer Res. 2008;14:3204–3215. doi: 10.1158/1078-0432.CCR-07-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loh ML, et al. Treatment of infantile fibrosarcoma with chemotherapy and surgery: results from the Dana-Farber Cancer Institute and Children's Hospital, Boston. J. Pediatr. Hematol. Oncol. 2002;24:722–726. doi: 10.1097/00043426-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 82.Pappo AS, Janeway KA. Pediatric gastrointestinal stromal tumors. Hematol. Oncol. Clin. North Am. 2009;23:15–34. doi: 10.1016/j.hoc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 83.Katherine A, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutation. Proc. Natl Acad. Sci. USA. 2011;108:314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Balamuth NJ, Womer RB. Ewing's sarcoma. Lancet Oncol. 2010;11:184–192. doi: 10.1016/S1470-2045(09)70286-4. [DOI] [PubMed] [Google Scholar]
- 85.Pappo AS, et al. Activity of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF1R) in patients with recurrent or refractory Ewing's sarcoma family of tumors (ESFT): results of a phase II SARC study [abstract] J. Clin. Oncol. 2010;28:a10000. doi: 10.1200/JCO.2010.34.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carol H, et al. Initial testing (stage 1) of the kinesin spindle protein inhibitor ispinesib by the pediatric preclinical testing program. Pediatr. Blood Cancer. 2009;53:1255–1263. doi: 10.1002/pbc.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meyers PA, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J. Clin. Oncol. 2005;23:2004–2011. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 88.Khanna C. Novel targets with potential therapeutic applications in osteosarcoma. Curr. Oncol. Rep. 2008;10:350–358. doi: 10.1007/s11912-008-0054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kolb EA, et al. R1507, a fully human monoclonal antibody targeting IGF-1R, is effective alone and in combination with rapamycin in inhibiting growth of osteosarcoma xenografts. Pediatr. Blood Cancer. 2010;55:67–75. doi: 10.1002/pbc.22479. [DOI] [PubMed] [Google Scholar]
- 90.Ahmed N, et al. Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression. Mol. Ther. 2009;17:1779–1787. doi: 10.1038/mt.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dunkel IJ, et al. Intensive multimodality therapy for patients with stage 4a metastatic retinoblastoma. Pediatr. Blood Cancer. 2010;55:55–59. doi: 10.1002/pbc.22504. [DOI] [PubMed] [Google Scholar]
- 92.Broaddus E, Topham A, Singh AD. Survival with retinoblastoma in the USA: 1975–2004. Br. J. Ophthalmol. 2009;93:24–27. doi: 10.1136/bjo.2008.143842. [DOI] [PubMed] [Google Scholar]
- 93.Shields CL, Shields JA. Retinoblastoma management: advances in enucleation, intravenous chemoreduction, and intra-arterial chemotherapy. Curr. Opin. Ophthalmol. 2010;21:203–212. doi: 10.1097/ICU.0b013e328338676a. [DOI] [PubMed] [Google Scholar]
- 94.Woo KI, Harbour JW. Review of 676 second primary tumors in patients with retinoblastoma: association between age at onset and tumor type. Arch. Ophthalmol. 2010;128:865–870. doi: 10.1001/archophthalmol.2010.126. [DOI] [PubMed] [Google Scholar]
- 95.Nemeth KM, et al. Subconjunctival carboplatin and systemic topotecan treatment in preclinical models of retinoblastoma. Cancer. 2010;117:421–434. doi: 10.1002/cncr.25574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abramson DH, Dunkel IJ, Brodie SE, Marr B, Gobin YP. Superselective ophthalmic artery chemotherapy as primary treatment for retinoblastoma (chemosurgery) Ophthalmology. 2010;117:1623–1629. doi: 10.1016/j.ophtha.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 97.Chévez-Barrios P, et al. Response of retinoblastoma with vitreous tumor seeding to adenovirus-mediated delivery of thymidine kinase followed by ganciclovir. J. Clin. Oncol. 2005;23:7927–7935. doi: 10.1200/JCO.2004.00.1883. [DOI] [PubMed] [Google Scholar]
- 98.Carcaboso AM, et al. Episcleral implants for topotecan delivery to the posterior segment of the eye. Invest. Ophthalmol. Vis. Sci. 2010;51:2126–2134. doi: 10.1167/iovs.09-4050. [DOI] [PubMed] [Google Scholar]
- 99.Laurie NA, et al. Inactivation of the p53 pathway in retinoblastoma. Nature. 2006;444:61–66. doi: 10.1038/nature05194. [DOI] [PubMed] [Google Scholar]
- 100.DiGiammarino EL, et al. A novel mechanism of tumorigenesis involving pH-dependent destabilization of a mutant p53 tetramer. Nat. Struct. Biol. 2002;9:12–16. doi: 10.1038/nsb730. [DOI] [PubMed] [Google Scholar]
- 101.Hill DA, et al. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325:965. doi: 10.1126/science.1174334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Collins JJ, et al. The measurement of symptoms in children with cancer. J. Pain Symptom Manage. 2000;19:363–377. doi: 10.1016/s0885-3924(00)00127-5. [DOI] [PubMed] [Google Scholar]
- 103.Ullrich CK, et al. End-of-life experience of children undergoing stem cell transplantation for malignancy: parent and provider perspectives and patterns of care. Blood. 2010;115:3879–3885. doi: 10.1182/blood-2009-10-250225. [DOI] [PubMed] [Google Scholar]
- 104.Klassen AF, et al. Impact of caring for a child with cancer on parents' health-related quality of life. J. Clin. Oncol. 2008;26:5884–5889. doi: 10.1200/JCO.2007.15.2835. [DOI] [PubMed] [Google Scholar]
- 105.Maurer SH, et al. Decision making by parents of children with incurable cancer who opt for enrollment on a phase I trial compared with choosing a do not resuscitate/terminal care option. J. Clin. Oncol. 2010;28:3292–3298. doi: 10.1200/JCO.2009.26.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mack JW, et al. Parents' views of cancer-directed therapy for children with no realistic chance for cure. J. Clin. Oncol. 2008;26:4759–4764. doi: 10.1200/JCO.2007.15.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dussel V, et al. Looking beyond where children die: determinants and effects of planning a child's location of death. J. Pain Symptom Manage. 2009;37:33–43. doi: 10.1016/j.jpainsymman.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kreicbergs U, et al. Anxiety and depression in parents 4-9 years after the loss of a child owing to a malignancy: a population-based follow-up. Psychol. Med. 2004;34:1431–1441. doi: 10.1017/s0033291704002740. [DOI] [PubMed] [Google Scholar]
- 109.Bluebond-Langner M, Belasco JB, Goldman A, Belasco C. Understanding parents' approaches to care and treatment of children with cancer when standard therapy has failed. J. Clin. Oncol. 2007;25:2414–2419. doi: 10.1200/JCO.2006.08.7759. [DOI] [PubMed] [Google Scholar]
- 110.Vickers J, Thompson A, Collins GS, Childs M, Hain R. Place and provision of palliative care for children with progressive cancer: a study by the Paediatric Oncology Nurses' Forum/United Kingdom Children's Cancer Study Group Palliative Care Working Group. J. Clin. Oncol. 2007;25:4472–4476. doi: 10.1200/JCO.2007.12.0493. [DOI] [PubMed] [Google Scholar]
- 111.Wolfe J, et al. Easing of suffering in children with cancer at the end of life: is care changing? J. Clin. Oncol. 2008;26:1717–1723. doi: 10.1200/JCO.2007.14.0277. [DOI] [PubMed] [Google Scholar]
- 112.Ferris FD, et al. Palliative cancer care a decade later: accomplishments, the need, next steps—from the American Society of Clinical Oncology. J. Clin. Oncol. 2009;27:3052–3058. doi: 10.1200/JCO.2008.20.1558. [DOI] [PubMed] [Google Scholar]
- 113.Kane JR. Pediatric palliative care moving forward: empathy, competence, quality, and the need for systematic change. J. Palliat. Med. 2006;9:847–849. doi: 10.1089/jpm.2006.9.847. [DOI] [PubMed] [Google Scholar]
- 114.Temel J, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N. Engl. J. Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 115.Baker JN, Barfield R, Hinds PS, Kane JR. A process to facilitate decision making in pediatric stem cell transplantation: the individualized care planning and coordination model. Biol. Blood Marrow Transplant. 2007;13:245–254. doi: 10.1016/j.bbmt.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 116.Baker JN, et al. Integration of palliative care practices into the ongoing care of children with cancer: individualized care planning and coordination. Pediatr. Clin. North Am. 2008;55:223–250. doi: 10.1016/j.pcl.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hui D, et al. Availability and integration of palliative care at US cancer centers. JAMA. 2010;303:1054–1061. doi: 10.1001/jama.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]