Abstract
A multicomponent reaction of 3-aminopyrazol-5-ones with substituted salicylic aldehydes and acetylacetic ester leading to the formation of novel 2,3-dihydrochromeno[4,3-d]pyrazolo[3,4-b]pyridine-1,6-diones was discovered. The elucidation of the reaction scope revealed that 5-aminopyrazoles, 3-amino-1,2,4-triazoles and 6-aminouracil could be used as the heterocyclic amine component. Selected heterocyclic products were found to possess notable antibacterial activities.
Keywords: MCR, aminopyrazole, antibacterial activity, benzopyranopyridines
Multicomponent reactions (MCRs) are a powerful synthetic tool. In this approach, three or more reactants are combined together in a single reaction flask to generate a product incorporating most of the atoms contained in the starting materials. MCRs have been widely exploited in combinatorial and medicinal chemistry and they are particularly useful for the preparation of polycondensed heterocyclic systems.1
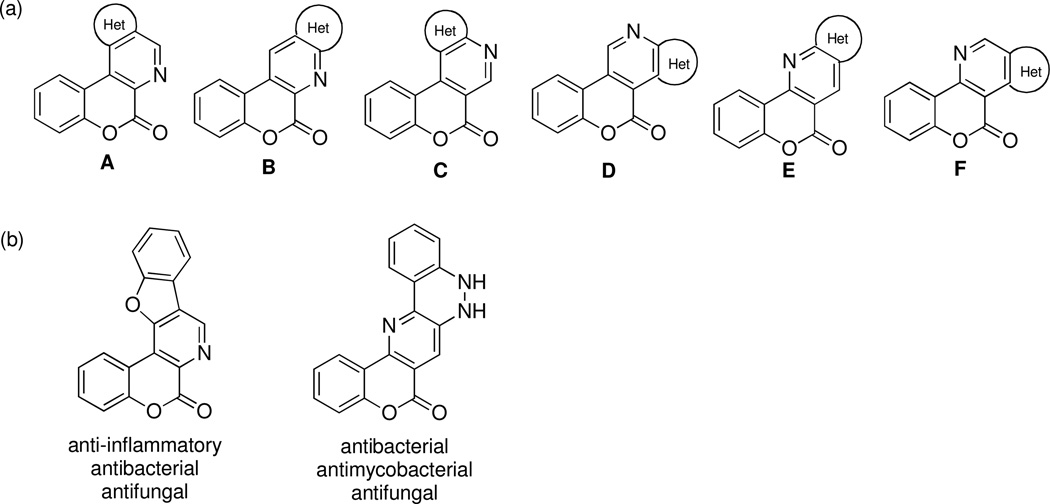
In this connection, hetero-fused benzopyranopyridines are a poorly studied class of polycondensed heterocycles (Figure 1a). Only four types of scaffolds (A, C, D, E) have been reported in the literature out of the six possible molecular frameworks (A–F) and the synthetic approaches used by researchers to access these structures have invariably involved multistep sequences.2–13 Importantly, compounds based on these heterocyclic systems have been reported to possess anti-inflammatory,2 antibacterial2,10 and antifungal10 activities (Figure 1b). As part of our efforts aimed at the discovery of MCRs to synthesize compounds with anticancer and antibacterial activities,14–25 we discovered a new approach to access the type C molecular framework. Herein, we describe this synthetic finding as well as the preliminary biological evaluation of compounds synthesized using this new method.
Figure 1.
(a) Six possible types of hetero-fused benzopyranopyridines (b) and representative compounds with biological activities
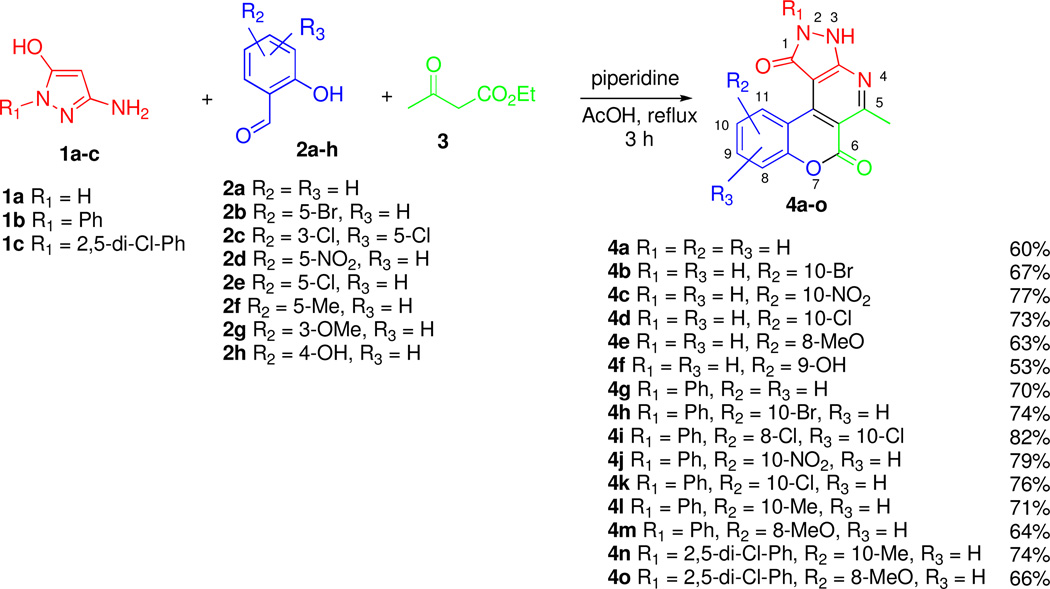
Specifically, we found that combining 3-aminopyrazol-5-ones 1a–c with substituted salicylic aldehydes 2a–h and acetylacetic ester (3) leads to the formation of 2,3-dihydrochromeno[4,3-d]pyrazolo[3,4-b]pyridin-1,6-diones 4a–o (Scheme 1). Generally good yields of these polyheterocyclic compounds are obtained when mixtures of the three starting components and one drop of piperidine are refluxed in acetic acid for 3 h. This three-component process works well any tested combination of substituted salicylic aldehydes 2 and 3-aminopyrazol-5-ones 1. The desired products precipitate upon cooling of the reaction mixtures and a simple filtration provides analytically pure material (> 95%).26
Scheme 1.
MCR synthesis of 2,3-dihydrochromeno[4,3-d]pyrazolo[3,4-b]pyridin-1,6-diones 4a–o
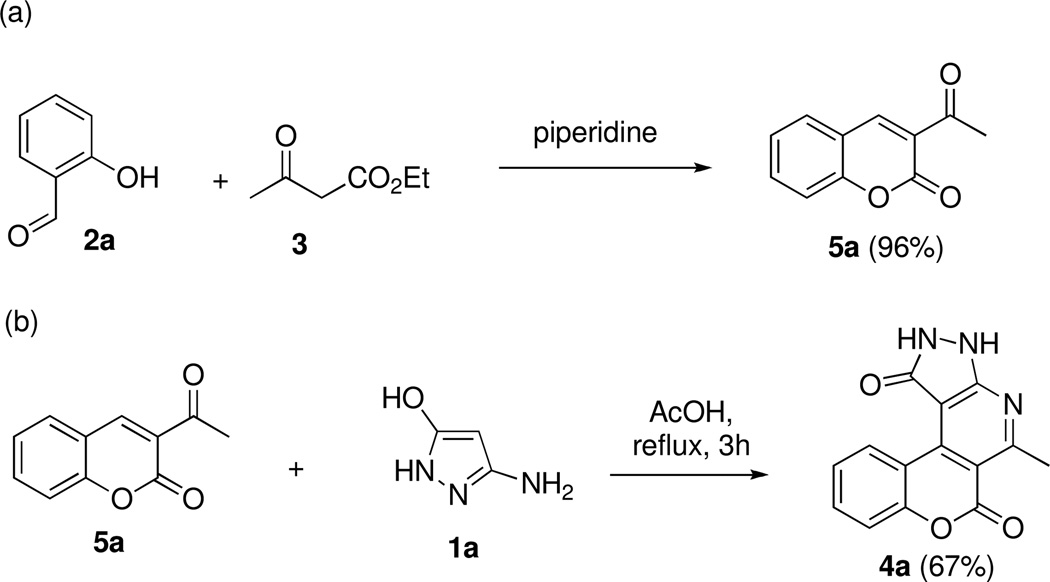
We propose that the mechanistic route for this transformation involves an in situ formation of 3-acetylcoumarins 5 and their subsequent condensation with aminopyrazolones 1 (Scheme 2). Indeed, we demonstrated that this process can be conducted stepwise by condensing salicylic aldehyde (2a) with acetylacetic ester (3) and isolating 3-acetylcoumarin (5a) in a quantitative yield (Scheme 2a). 5a was further reacted with 3-amino-1H-pyrazol-5(4H)-one (1a) under the same reaction conditions and gave a comparable yield of polyheterocycle 4a (Scheme 2b).
Scheme 2.
3-Acetylcoumarins as intermediates in the discovered MCR
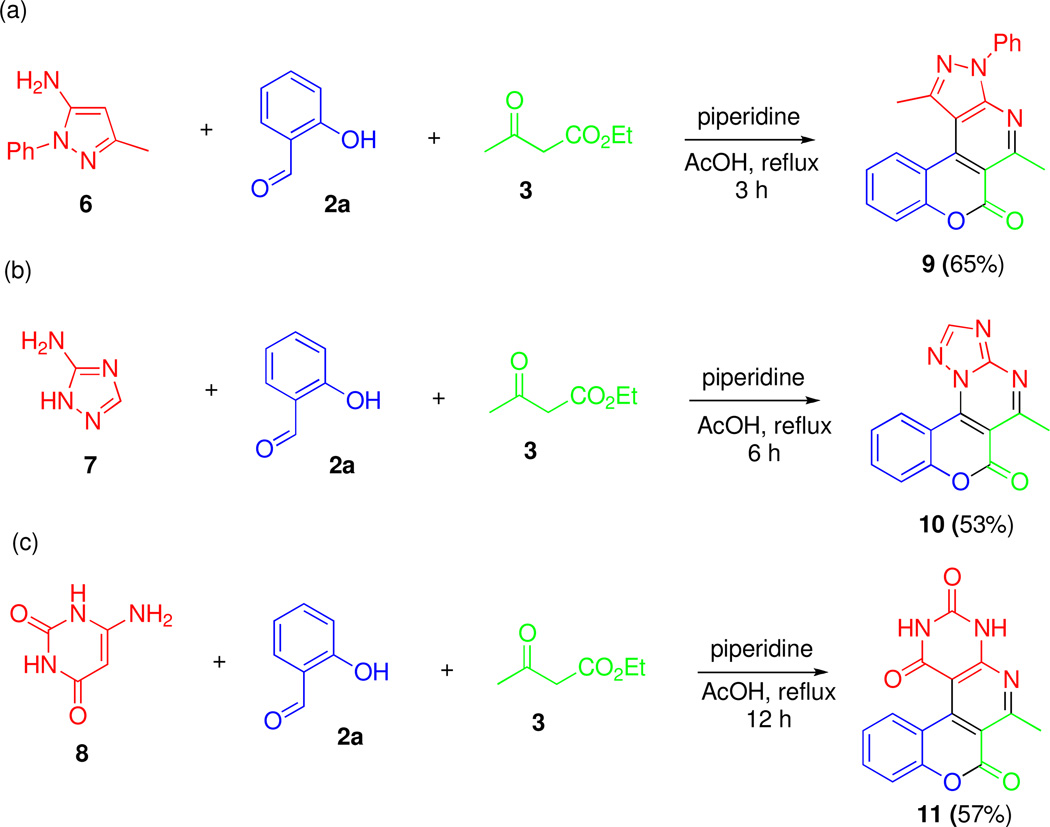
To investigate the scope of this process with respect to the acetylacetic ester component, we attempted to replace it with ethyl benzoylacetate. However, the reaction was unsuccessful in this case, possibly due to the change in electronic and steric environment of the ketone carbonyl. On the other hand, the replacement of the aminoheterocyclic component was much more forgiving. Thus, we explored the corresponding reactions of 5-amino-1-phenyl-3-methylpyrazole (6), 3-amino-1,2,4-triazole (7) and 6-aminouracil (8, Scheme 3). The desired polyheterocycles 9, 10 and 11 were obtained in acceptable yields, indicating the potential of this MCR to be used as a general method to access polyheterocyclic scaffolds C (see Figure 1).27
Scheme 3.
MCR synthesis of polyheterocycles 9, 10 and 11
The initial evaluation of the synthesized polyheterocycles for antimicrobial activities revealed significant antibacterial properties associated with several analogues specifically against Gram-(+) strains. Thus, compounds 4i and 4o inhibited the growth of S. epidermidis with the MIC values of 6.3 µM and 25 µM, respectively. Furthermore, heterocycle 4i was also effective against methicillin-resistant S. aureus (MRSA), inhibiting the growth of this clinically important nosocomial pathogen with an MIC value of 25 µM. Further exploration of the MCR scope and antimicrobial activities associated with these novel heterocyclic structures are underway and will be reported in due course.
Acknowledgments
US National Institutes of Health (grants RR-16480 and CA-99957) are gratefully acknowledged for financial support of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.For a recent review, see: Syamala M. Org. Prep. Proceed. Int. 2009;41:1.
- 2.Khan IA, Kulkarni MV, Gopal M, Shahabuddin MS, Sun C-M. Bioorg. Med. Chem. Lett. 2005;15:3584. doi: 10.1016/j.bmcl.2005.05.063. [DOI] [PubMed] [Google Scholar]
- 3.Frasinyuk MS, Gorelov SV, Bondarenko SP, Khilya VP. Chem. Heterocycl. Compd. 2009;45:1261. [Google Scholar]
- 4.Gohain M, Prajapati D, Gogoi BJ, Sandhu JS. Synlett. 2004:1179. [Google Scholar]
- 5.Ismail NA, Khalifa F, Magd ED, Asmaa A. Heterocycles. 1991;32:1101. [Google Scholar]
- 6.Gautam DR, Protopappas J, Fylaktakidou KC, Litinas KE, Nicolaides DN, Tsoleridis CA. Tetrahedron Lett. 2009;50:448. [Google Scholar]
- 7.Prasad KR, Darbarwar M. Org. Prep. Proceed. Int. 1995;27:547. [Google Scholar]
- 8.Prasad KR, Darbarwar M. Syn. Commun. 1992;22:2479. [Google Scholar]
- 9.Siddiqui ZN, Praveen S, Farooq F. Chem. Papers. 2010;64:818. [Google Scholar]
- 10.Ramalingam P, Ganapaty S, Rao CB, Ravi TK. Indian J. Heterocycl. Chem. 2006;15:359. [Google Scholar]
- 11.Brownsort PA, Paton RM, Stutherland AG. J. Chem. Soc., Perkin Trans. 1. 1989:1679. [Google Scholar]
- 12.Schurreit T. Arch. Pharm. 1987;320:500. [Google Scholar]
- 13.Mogilaiah K, Sreenivasulu B. Indian J. Chem., Sec. B. Org. Chem. Incl. Med. Chem. 1982;21B:582. [Google Scholar]
- 14.Evdokimov NM, Magedov IV, Kireev AS, Kornienko A. Org. Lett. 2006;9:899. doi: 10.1021/ol052994+. [DOI] [PubMed] [Google Scholar]
- 15.Evdokimov NM, Kireev AS, Yakovenko AA, Antipin MY, Magedov IV, Kornienko A. Tetrahedron Lett. 2006;47:9309. doi: 10.1016/j.tetlet.2006.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evdokimov NM, Kireev AS, Yakovenko AA, Antipin MY, Magedov IV, Kornienko A. J. Org. Chem. 2007;72:3443. doi: 10.1021/jo070114u. [DOI] [PubMed] [Google Scholar]
- 17.Magedov IV, Manpadi M, Rozhkova E, Przheval’skii NM, Rogelj S, Shors ST, Steelant WFA, Van Slambrouck S, Kornienko A. Bioorg. Med. Chem. Lett. 2007;17:1381. doi: 10.1016/j.bmcl.2006.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magedov IV, Manpadi M, Van Slambrouck S, Steelant WFA, Rozhkova E, Przhevalískii NM, Rogelj S, Kornienko A. J. Med. Chem. 2007;50:5183. doi: 10.1021/jm070528f. [DOI] [PubMed] [Google Scholar]
- 19.Magedov IV, Manpadi M, Evdokimov NM, Elias EM, Rozhkova E, Ogasawara MA, Bettale JD, Przeval’skii NM, Rogelj S, Kornienko A. Bioorg. Med. Chem. Lett. 2007;17:3872. doi: 10.1016/j.bmcl.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manpadi M, Uglinskii PY, Rastogi SK, Cotter KM, Wong Y-SC, Anderson LA, Ortega AJ, Van slambrouck S, Steelant WFA, Rogelj S, Tongwa P, Antipin MY, Magedov IV, Kornienko A. Org. Biomol. Chem. 2007;5:3865. doi: 10.1039/b713820b. [DOI] [PubMed] [Google Scholar]
- 21.Magedov IV, Luchetti G, Evdokimov NM, Manpadi M, Steelant WFA, Van slambrouck S, Tongwa P, Antipin MYu, Kornienko A. Bioorg. Med. Chem Lett. 2008;18:1392. doi: 10.1016/j.bmcl.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magedov IV, Manpadi M, Ogasawara MA, Dhawan AS, Rogelj S, Van slambrouck S, Steelant WFA, Evdokimov NM, Uglinskii PY, Elias EM, Knee EJ, Tongwa P, Antipin MY, Kornienko A. J. Med. Chem. 2008;51:2561. doi: 10.1021/jm701499n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frolova LV, Evdokimov NM, Hayden K, Malik I, Rogelj S, Kornienko A, Magedov IV. Org. Lett. 2011;13:1118. doi: 10.1021/ol103149b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evdokimov NM, Van slambrouck S, Heffeter P, Tu L, Le Calve B, Lamoral-Theys D, Hooten CJ, Uglinskii PY, Rogelj S, Kiss R, Steelant WFA, Berger W, Bologa CJ, Yang JJ, Kornienko A, Magedov IV. J. Med. Chem. 2011;54:2012. doi: 10.1021/jm1009428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magedov IV, Frolova L, Manpadi M, Bhoga UD, Tang H, Evdokimov NM, George O, Georgiou KH, Renner S, Getlik M, Kinnibrugh TL, Fernandes MA, Van slambrouck S, Steelant WFA, Shuster CB, Rogelj S, van Otterlo WAL, Kornienko A. J. Med. Chem. 2011;54:4234. doi: 10.1021/jm200410r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Synthetic procedure (4a–o, 9, 10, 11): To a mixture of acetylacetic ester, substituted salicylic aldehyde (2a–h) and aminoheterocycle (1a–c, 6, 7, 8) was added 1 drop of piperidine. The mixture was stirred for 15 min after which time acetic acid (5 mL) was added and the reaction mixture was refluxed for 3–12 hours. The desired products precipitate upon cooling of the reaction mixtures, and a simple filtration and washing with ethanol provides analytically pure material (> 95%). Selected characterization data: 4a: 60%; 1H NMR (DMSO-d6, 373 K) δ 9.95 (d, J=7.98 Hz, 1H), 7.67 (t, J=7.14 Hz, 1H), 7.42-7.36 (m, 2H), 2.96 (s, 3H); 13C NMR (DMSO-d6, 373 K) δ 164.9, 157.3, 152.9, 133.9, 131.9, 124.6, 117.6, 117.1, 113.7, 28.1; HRMS m/z (ESI) calcd for C14H9N3O3 – H− 266.0566, found 266.0562. 9: 65%; 1H NMR (DMSO-d6, 373 K) δ 8.33 (d, J=7.68 Hz, 1H), 8.19 (d, J=7.17 Hz, 2H), 7.73 (t, J=7.24 Hz, 1H), 7.59-7.30 (m, 6H), 3.36 (s, 3H), 3.02 (s, 3H); 13C NMR (DMSO-d6, 373 K) δ 162.9, 159.8, 152.9, 143.8, 139.2, 133.9, 130.4, 129.8, 129.6, 127.3, 124.8, 122.5, 122.2 117.6, 117.6, 111.2, 28.0, 19.3; HRMS m/z (ESI) calcd for C21H15N3O2 + Na+ 364.1062, found 364.1067. 10: 53%; 1H NMR (DMSO-d6, 373 K) δ 9.79 (d, J=8.25 Hz, 1H), 8.94 (s, 1H), 7.93 (t, J=7.68 Hz, 1H), δ 7.65 (d, J=8.25 Hz, 1H), 7.59 (d, J=7.98 Hz, 1H), 3.11 (s, 3H); 13C NMR (DMSO-d6, 373 K) δ 167.6, 158.3, 156.2, 154.7, 136.5, 130.6, 126.0, 118.0, 117.6, 112.3, 106.0, 27.9; HRMS m/z (ESI) calcd for C13H8N4O2 + Na+ 275.0545, found 275.0537. 11: 57%; 1H NMR (DMSO-d6, 323 K) δ 10.27 (bs, 1H), 9.87 (bs, 1H) 8.17 (d, J=8.22 Hz, 1H), 7.65 (t, J=8.22 Hz, 1H), 7.37 (d, J=8.22 Hz, 1H), 7.27 (t, J=8.25 Hz, 1H), 3.05 (s, 3H); 13C NMR (DMSO-d6, 323 K) δ 169.1, 166.3, 162.4, 159.1, 156.2, 152.9, 150.7, 134.4, 132.8, 123.6, 117.0, 116.1, 113.1, 103.2, 27.7; HRMS m/z (ESI) calcd for C15H10N3O4 + H+ 296.0671, found 296.0670.
- 27.As our work was in progress, a similar reaction between salicylic aldehyde, acetylacetic ester and 3-methyl-5-aminopyrazole leading to the formation 1,4-dihydropyridine analogs of 9, appeared in the literature: Svetlik J, Veizerova L, Mayer TU, Catarinella M. Bioorg. Med. Chem. Lett. 2010;20:4073. doi: 10.1016/j.bmcl.2010.05.085.