Summary
Objective
The purpose of this study was to describe the pattern of dental plaque accumulation in mechanically ventilated adults. Accumulation of dental plaque and bacterial colonization of the oropharynx is associated with a number of systemic diseases including ventilator associated pneumonia.
Research Methodology/Design
Data were collected from mechanically ventilated critically ill adults (n=137), enrolled within 24 hours of intubation. Dental plaque, counts of decayed, missing and filled teeth and systemic antibiotic use was assessed on study days 1, 3, 5 and 7. Dental plaque averages per study day, tooth type and tooth location were analyzed.
Setting
Medical Respiratory, Surgical Trauma and Neuroscience ICU’s of a large tertiary care center in the southeast United States.
Results
Plaque: All surfaces > 60% plaque coverage from day 1 to day 7; Molars and Premolars contained greatest plaque average >70%. Systemic antibiotic use on day 1 had no significant effect on plaque accumulation on day 3 (p=0.73).
Conclusions
Patients arrive in critical care units with preexisting oral hygiene issues. Dental plaque tends to accumulate in the posterior teeth (molars and premolars) that may be hard for nurses to visualize and reach; this problem may be exacerbated by endotracheal tubes and other equipment. Knowing accumulation trends of plaque will guide the development of effective oral care protocols.
Keywords: Dental plaque, mechanical ventilation, oral health, oral care
Introduction
Oral health is an important topic in the critically ill population and has a profound effect on general health. The normal oral flora of healthy individuals includes gram positive organisms and dental pathogens, including the viridans streptococci. These microbial flora are concentrated in dental plaque, which is a biofilm that provides a microhabitat for organisms with opportunity for adherence either to the tooth surface or to other microorganisms (Munro and Grap 2004). The teeth are the only nonshedding surfaces in the body and bacterial levels can reach more than 1011 microorganisms per mg of dental plaque in healthy individuals (Gendron, et al. 2000, Li, et al. 2000, Lockhart and Durack 1999, Wilson 2001). The accumulation of dental plaque and bacterial colonization of the oropharynx has been associated with a number of systemic diseases including COPD (Scannapieco, et al. 1998), endocarditis (Carmona, et al. 2002, Hoen 2002, Kitten, et al. 2000, Munro and Macrina 1993, Seymour and Whitworth 2002) and bacteremia (Kerr 2000, Marron, et al. 2000).
Several factors influence the oral flora of the critically ill adult. Oral health in the ICU may be compromised by ICU equipment, medical conditions or treatments and the inability to attend to their own care (Munro and Grap 2004). Within 48 hours of hospital admission, the composition of oral flora in the critically ill adult can change to predominantly gram negative and virulent gram positive organisms including potential ventilator associated pneumonia (VAP) pathogens such as Staphylococcus aureus, Streptococcus pneumoniae, Acinetobacter baumanii, Haemophilus influenzae, and Pseudomonas aeruginosa (Abele-Horn, et al. 1997, Johanson Jr., et al. 1988, Jones, et al. 2010, Munro and Grap 2004). The pathophysiology underlying this change in microbial flora is unclear; systemic changes accompanying critical illness (for example, immunocompromise) as well as changes in the mouth itself (for example, mouth dryness and decrease in normal oral defense mechanisms) may contribute. Dental plaque may serve as a reservoir in critically ill patients for Methicillin Resistant S. aureus and P. aeruginosa (Heo, et al. 2008, Scannapieco, et al. 1992). Colonization of the oropharynx has been identified as a major risk factor for the development of VAP in mechanically ventilated adults (Craven and Driks 1987, Greene, et al. 1994, Torres, et al. 1993, Valles, et al. 1995).
Mechanically ventilated patients are dependent on health care providers, specifically nurses, to provide their oral care. Nurses provide a variety of oral interventions that are designed to address patients’ comfort but do not focus on dental plaque removal (Feider, et al. 2010, Fitch, et al. 1999, Grap, et al. 2003, Grap and Munro 2004). Although there are recommendations to focus oral care efforts on reducing dental plaque accumulation through toothbrushing and Chlorhexidine (AACN 2007) the most common tool used is the foam swab (Feider, et al. 2010). In surveys performed by Grap et al. (2003), ICU nurses reported 38.9% frequency for the use of a toothbrush to provide oral care to intubated patients. Foam swabs were reported to be used to provide mouth care for 91.5% of intubated patients (Grap, et al. 2003). The foam swabs provide mucosal stimulation but are not effective for plaque removal (Adams 1996, Buglass 1995, Holmes 1996, Moore 1995, Pearson 1996). Although the evidence regarding the use of Chlorhexidine to prevent VAP is inconclusive; recent reports have been published showing the positive effects of Chlorhexidine in VAP prevention (Chan, et al. 2007, Munro, et al. 2009), and a recommendation for daily Chlorhexidine has been added to the Institute for HealthCare Improvement ventilator bundle (Institute for Healthcare Improvement 2010). Chlorhexidine does not remove dental plaque but its bactericidal activity is the mechanism in decreasing VAP risks. Toothbrushing is generally considered the best tool for mechanical removal of dental plaque and can effectively aid in VAP prevention when Chlorhexidine is contraindicated (Fields 2008). Although the most recent Centers for Disease Control (CDC) recommendations (Tablan, et al. 2004) for prevention of nosocomial pneumonia in mechanically ventilated adults include decreasing oral microbial flora; and many healthcare facilities have adopted oral care policies; oral care practices often are not consistent with policies and recommendations (Feider, et al. 2010). Standardized protocols of oral care for mechanically ventilated patients based on empirical evidence are lacking (Berry and Davidson 2006).
Knowing where dental plaque tends to accumulate in the intubated patient could guide nurses in delivering effective oral care that targets removal of dental plaque and the bacterial embedded in the plaque. The purpose of this study was to understand the accumulation pattern of dental plaque over the first week following intubation in critically ill adults in a large tertiary care center in the southeastern United States.
Methods
Setting and sample
This was a descriptive correlational study examining dental plaque accumulation over time. The study was conducted in the Medical Respiratory, Surgical Trauma and Neuroscience intensive care units of a large tertiary care center in southeastern United States. The sample population was a subset of the control group of an NIH National Institute of Nursing Research funded study examining oral care interventions in mechanically ventilated adults. Subjects in the control group received usual oral care delivered by the bedside nurse; nurses were free to individualize the method of oral care, and the study units did not have protocols that specified tools and solutions for oral care. The sample population included subjects enrolled in the control group in the first year of data collection of the parent study (n=137). Data were analyzed regarding longitudinal trends in dental plaque accumulation. Consent was obtained from the subjects’ legally authorized representatives in accordance with IRB approval, within 24 hours of intubation and mechanical ventilation. Subjects remained in the study for 7 days or until extubation.
Data Collection
Demographic data including age, race, ethnicity & gender were collected. Length of present intubation and counts of decayed, missing and filled teeth (DMF) were recorded. Dental plaque was assessed by trained dental assessors, who were dental hygienists or senior dental hygiene students, using the University of Mississippi Oral Hygiene Index (UM-OHI) (Silberman, et al. 1998) (see Figure 1) with observations augmented by use of a dental plaque disclosing agent. Fluorescein was selected as the dental plaque disclosing agent because it was visible only under UV light; thus, nurses providing oral care were not aware of plaque assessment results. Because the UM-OHI assesses every tooth, and divides each tooth into more sections than standard tools used in dental practice, it enhances the ability to quantify dental plaque. Inter-rater reliability was reported at r=0.89 and the authors reported that scores for the tool were highly correlated (r=0.85 to 0.93) with scores on standardized clinical assessment tools (Silberman, et al. 1998). After staining with the dental plaque disclosing agent, each section of every tooth (total of 10 sections for each tooth) was scored for the presence or absence of dental plaque using a UV- penlight. Thus, the total score for each tooth could range from 0 (no dental plaque) to 10 (dental plaque in every section). The mean dental plaque score for the subject was then calculated by dividing the total score for all teeth by the number of teeth.
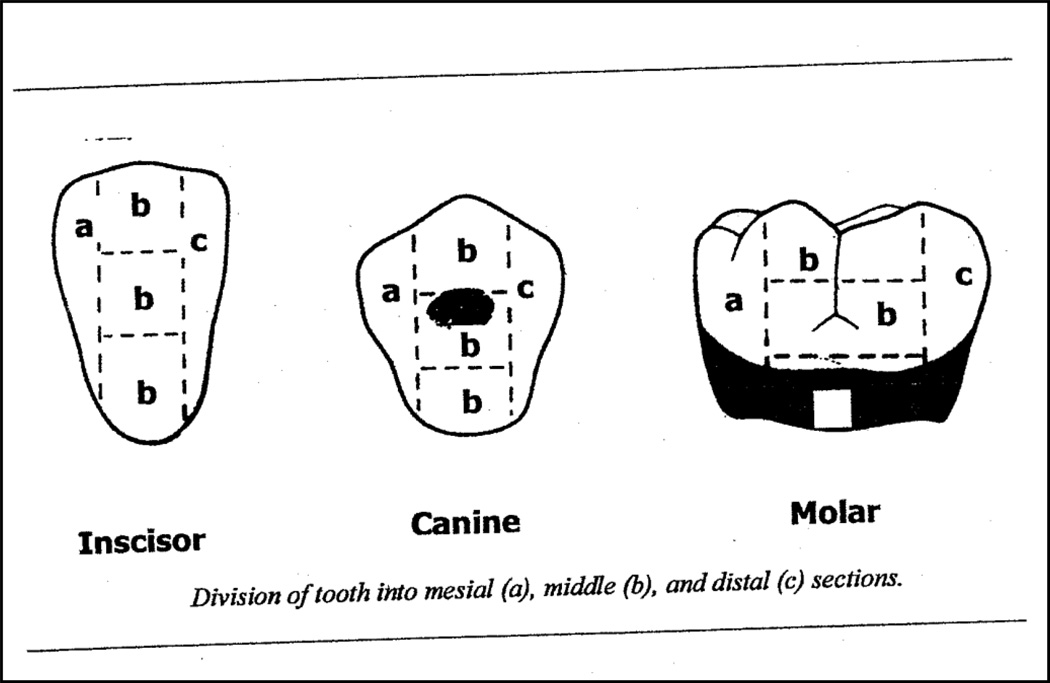
Figure 1.
Tooth subdivisions for UH-OMI. Dental plaque was assessed using the University of Mississippi Oral Hygiene Index (UM-OHI)35 Each tooth is divided into 5 sections for the buccal surface and 5 sections for the lingual surface. The 5 sections include the mesial, distal and middle sections each of which is further subdivided horizontally into gingival, middle and occlusal sections. If dental plaque was present in the section, the section received a score of 1; if no dental plaque was present, the section received a 0.
Figure 1 reproduced with permission. Original source: Silberman et al. J Periodontol 1998; 69(10):1176–1180. (Reference 35)
Each subject was assessed for the total number of decayed, missing and filled teeth (DMF) on initial study enrollment. In addition the trained dental assessors recorded the location of missing teeth. Each of the items (decayed, missing, filled) could be recorded in a range from 0 to 32. In adults with full dentition and no decayed or filled teeth the score would be recorded as 0.
Statistical Methods
JMP 6.0.2 statistical software was used for statistical analysis. Mean dental plaque scores obtained for each tooth on days 1, 3, 5 and 7 (see figures 2–5) appeared normally distributed and therefore an ANOVA was performed to look for differences in dental plaque averages per study day. Dental plaque averages per subject were analyzed on study days 1, 3, 5, and 7. A paired t-test was used to compare differences in dental plaque averages for the lingual and buccal surfaces.
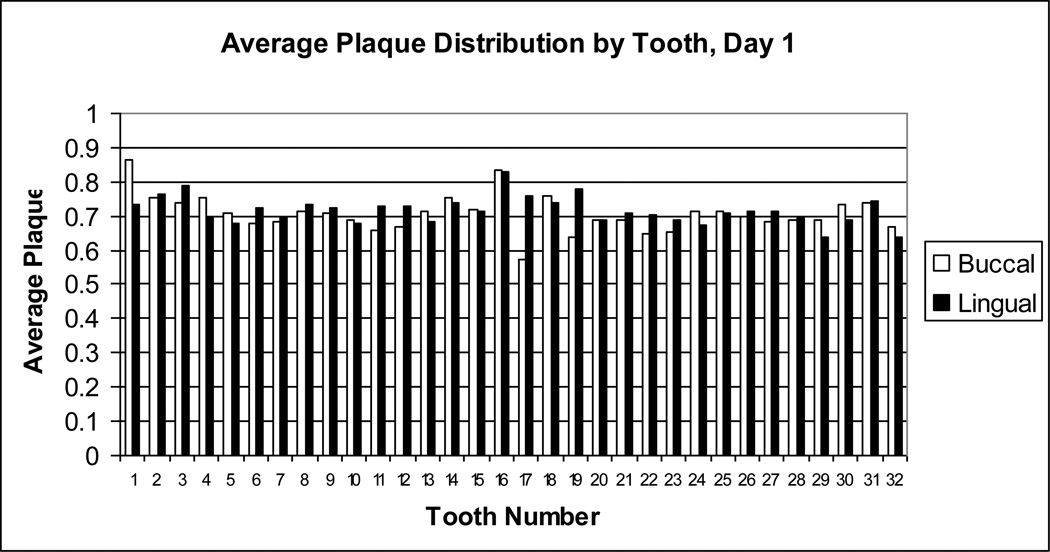
Figure 2.
Average dental plaque distribution by tooth number on Day 1. Dental plaque scores on day 1 for each buccal (white bar) or lingual (black bar) surface of each tooth were averaged for all subjects. Dental plaque scores could range from 0 (no dental plaque) to 1 (entire surface covered with dental plaque). Tooth numbers, displayed on the x-axis, are standard dental nomenclature. Tooth numbering begins with the right upper molar as tooth 1 and proceeds along the upper arch to 16, the left upper molar; the left lower molar is tooth 17, and numbering of the lower arch proceeds to 32, the right lower molar.
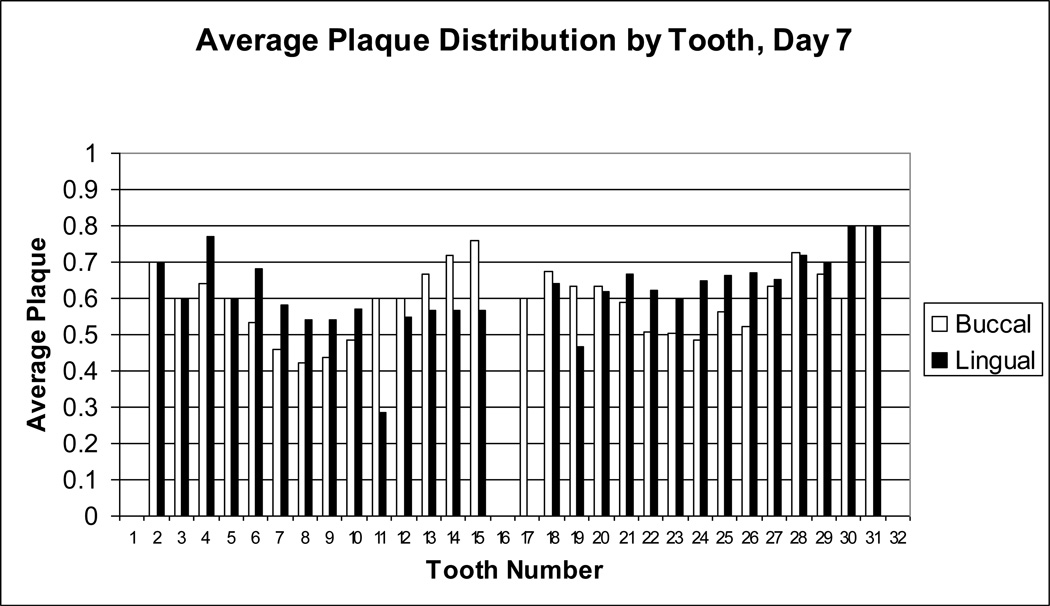
Figure 5.
Average dental plaque distribution for day 7. Dental plaque scores on day 7 for each buccal (white bar) or lingual (black bar) surface of each tooth were averaged for all subjects. Tooth numbers, displayed on the x-axis, are standard dental nomenclature.
Results
Demographic Characteristics
A summary of demographic characteristics are presented in Table 1. The mean (range) length of intubation was 5 (1 to 11) days and mean (range) DMF score was 9 (0 to 29). The sample was representative of this population in terms of race, age and gender.
Table 1.
Sample Characteristics. The analysis consisted of a sample of 137 mechanically ventilated adults.
| Ethnicity | |
| Hispanic | 1% |
| NonHispanic | 99% |
| Race | |
| Black/African American | 58% |
| White | 37% |
| Other | 5% |
| Gender | |
| Female | 36% |
| Male | 64% |
| Length of Intubation, mean (range) | 5 (1–11) days |
| Age, mean (range) | 46 (20–72) years |
| DMF, mean (range) | 9 (0–29) |
Dental Plaque
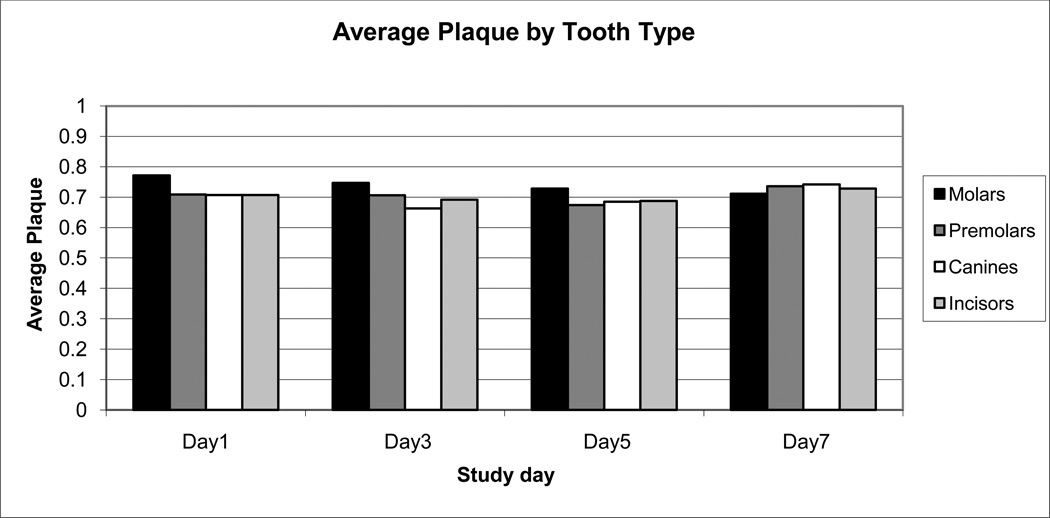
The subjects were found to have greater than 73% mean dental plaque coverage on day 1 and mean dental plaque remained greater than 60% through day 7. Dental plaque averages were significantly different between day 1 and day 7 (F (3, 227) = 3, p= .03). Day 1 had significantly higher plaque averages than day 7 (73% and 63%, respectively). There was no significant difference within other study days. Additionally, the pattern of dental plaque accumulation was examined by subsetting scores by location (buccal or lingual) and by tooth type (molars, premolars, canines, incisors). There was no significant difference found in dental plaque averages for the buccal (67%) and lingual (68%) surfaces (t= −.5494, df = 198, p-value=.71). The molars and premolar tooth types had the most dental plaque (greater than 70%) when compared to the other tooth types (Figure 6, tooth type).
Figure 6.
Dental plaque averages by tooth type. Average dental plaque scores were collapsed by tooth type (molars, premolars, canines, incisors), bars left to right within each assessment day (days 1, 3, 5, and 7).
Discussion
Our data indicate that patients arrive in critical care units with preexisting oral problems, including decayed and missing teeth and high levels of dental plaque. These preexisting problems increase their vulnerability to systemic diseases and complicate oral care management of the intubated patient.
Dental plaque averages on day 7 were statistically lower than averages on day 1 indicating current oral care methods may be beneficial in reducing plaque accumulation over time. However, the finding that dental plaque was present on more than 60% of the tooth surfaces on all days measured is important in relation to oral health of mechanically ventilated patients and nursing oral care practices. Ideally, oral care provided by nurses would consistently control dental plaque in the mouths of mechanically ventilated patients and plaque scores much lower than what was found in the current study would be the norm. The extensive amount of plaque that persisted in this population may be clinically important in developing an oral care protocol.
Data from this study reveal that dental plaque tends to accumulate in the posterior teeth (molars and premolars) that may be hard for nurses to visualize and reach; this problem may be exacerbated by endotracheal tubes and other equipment. Nurses should be aware of the tendency of plaque to accumulate in posterior teeth in order to focus plaque removal efforts on those areas that are usually inadequately cleaned. Adequate plaque removal may require repositioning of the endotracheal tube during the provision of oral care. This substudy did not examine the composition of the dental plaque (bacterial species represented) which may be an important component of risks for systemic disease associated with oral organisms. This substudy did not record the type of oral care being administered to the patients and the technique may have explained the differences in plaque accumulation per day per teeth. This was a study limitation. Tooth visualization depended on equipment position, patient position and patient condition; this limitation may have affected the average plaque distribution by tooth. Additional research to identify best practices for oral care in the critically ill is needed, and well-designed studies examining the effects of better dental plaque removal on outcomes in critically ill adult is essential. Knowing accumulation trends of dental plaque will guide the development of effective oral care protocols for further study. Such protocols may decrease length of stay, length of intubation and hospital costs in critically ill patients.
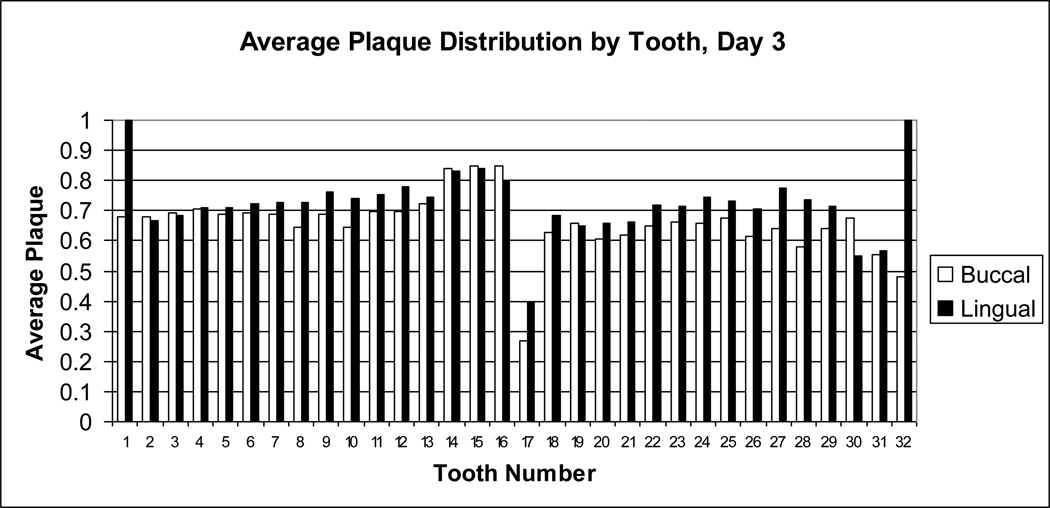
Figure 3.
Average dental plaque distribution for day 3. Dental plaque scores on day 3 for each buccal (white bar) or lingual (black bar) surface of each tooth were averaged for all subjects. Tooth numbers, displayed on the x-axis, are standard dental nomenclature.
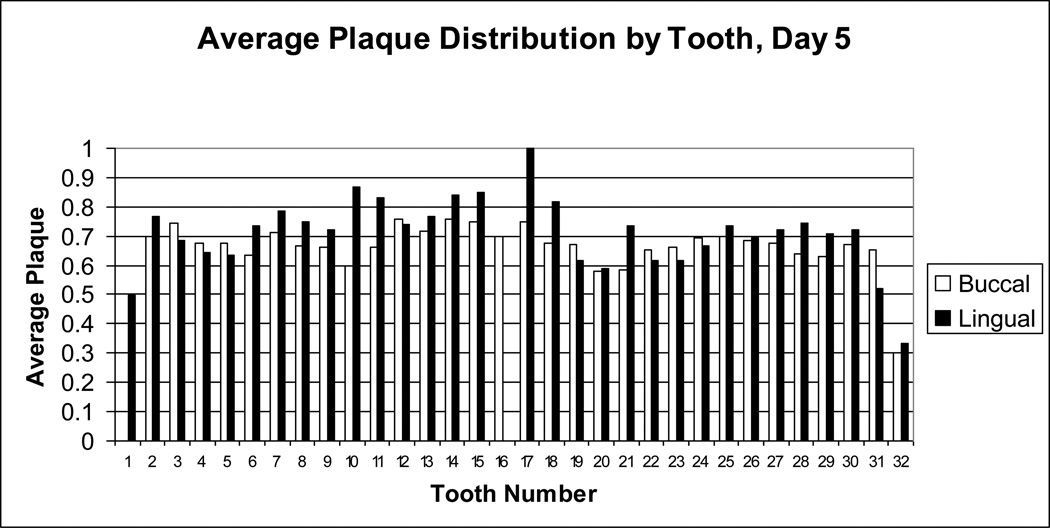
Figure 4.
Average dental plaque distribution for day 5. Dental plaque scores on day 5 for each buccal (white bar) or lingual (black bar) surface of each tooth were averaged for all subjects. Tooth numbers, displayed on the x-axis, are standard dental nomenclature.
Acknowledgments
Grant: “Oral Care Intervention in Mechanically Ventilated Adults” (Munro, C.L., R01 NR07652) and NIH/NINR 1F31 NR009596 (Jones, D. J.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Deborah J. Jones, University of Texas Health Science Center at Houston, School of Nursing, 6901 Bertner Ave, Houston, TX 77030, 713-500-2155 work, 713-500-2142 fax, Deborah.j.jones@uth.tmc.edu.
Cindy L. Munro, Virginia Commonwealth University, School of Nursing, 1100 East Leigh Street, PO Box 980567, Richmond, VA 23219, 804-828-3410 work, 804-828-7743 fax, cmunro@vcu.edu.
Mary Jo Grap, Virginia Commonwealth University, School of Nursing, 1100 East Leigh Street, PO Box 980567, Richmond, VA 23219, 804-828-0723 work, 804-828-7743 fax, mjgrap@vcu.edu.
References
- 1.AACN practice alert; oral care in the critically ill. Aliso Viejo, CA: American Association of Critical Care Nurses; 2007. [October 2007]. Available from: http://classic.aacn.org/AACN/practiceAlert.nsf/Files/OC/$file/Oral%20Care%20in%20the%20Critically%20Ill%20.pdf. [Google Scholar]
- 2.Abele-Horn M, Dauber A, Bauernfeind A, Russwurm W, Seyfarth-Metzger I, Gleich P, et al. Decrease in nosocomial pneumonia in ventilated patients by selective oropharyngeal decontamination (SOD) Intensive Care Med. 1997;23(2):187–195. doi: 10.1007/s001340050314. [DOI] [PubMed] [Google Scholar]
- 3.Adams R. Qualified nurses lack adequate knowledge related to oral health, resulting in inadequate oral care of patients on medical wards. J Adv Nurs. 1996;24(3):552–560. doi: 10.1046/j.1365-2648.1996.22416.x. [DOI] [PubMed] [Google Scholar]
- 4.Berry AM, Davidson PM. Beyond comfort: Oral hygiene as a critical nursing activity in the intensive care unit. Intensive Crit Care Nurs. 2006 Dec;22(6):318–328. doi: 10.1016/j.iccn.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Buglass EA. Oral hygiene. Br J Nurs. 1995 May 12–24;4(9):516–519. doi: 10.12968/bjon.1995.4.9.516. [DOI] [PubMed] [Google Scholar]
- 6.Carmona IT, Dios PD, Scully C. An update on the controversies in bacterial endocarditis of oral origin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93(6):660–670. doi: 10.1067/moe.2002.122338. [DOI] [PubMed] [Google Scholar]
- 7.Chan EY, Ruest A, Meade MO, Cook DJ. Oral decontamination for prevention of pneumonia in mechanically ventilated adults: Systematic review and meta-analysis. Br Med J. 2007;334(7599):889–893. doi: 10.1136/bmj.39136.528160.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craven DE, Driks MR. Nosocomial pneumonia in the intubated patient. Semin Respir Infect. 1987;2(1):20–33. [PubMed] [Google Scholar]
- 9.Feider LL, Mitchell P, Bridges E. Oral care practices for orally intubated critically ill adults. Am J Crit Care. 2010 Mar;19(2):175–183. doi: 10.4037/ajcc2010816. [DOI] [PubMed] [Google Scholar]
- 10.Fields LB. Oral care intervention to reduce incidence of ventilator-associated pneumonia in the neurologic intensive care unit. Journal of Neuroscience Nursing. 2008;40(5):291–298. doi: 10.1097/01376517-200810000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Fitch JA, Munro CL, Glass CA, Pellegrini JM. Oral care in the adult intensive care unit. Am J Crit Care. 1999 Sep;8(5):314–318. [PubMed] [Google Scholar]
- 12.Gendron R, Grenier D, Maheu-Robert L. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microb Infect. 2000;2(8):897–906. doi: 10.1016/s1286-4579(00)00391-9. [DOI] [PubMed] [Google Scholar]
- 13.Grap MJ, Munro CL. Preventing ventilator-associated pneumonia: Evidence-based care. Crit Care Nurs Clin North Am. 2004;16(3 SPEC. ISS.):349–358. doi: 10.1016/j.ccell.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Grap MJ, Munro CL, Ashtiani B, Bryant S. Oral care interventions in critical care: Frequency and documentation. American Journal of Critical Care. 2003;12(2):113–118. [PubMed] [Google Scholar]
- 15.Greene R, Thompson S, Jantsch HS, Teplick R, Cullen DJ, Greene EM, et al. Detection of pooled secretions above endotracheal-tube cuffs: Value of plain radiographs in sheep cadavers and patients. Am J Roentgenol. 1994;163(6):1333–1337. doi: 10.2214/ajr.163.6.7992723. [DOI] [PubMed] [Google Scholar]
- 16.Heo S, Haase EM, Lesse AJ, Gill SR, Scannapieco FA. Genetic relationships between respiratory pathogens isolated from dental plaque and bronchoalveolar lavage fluid from patients in the intensive care unit undergoing mechanical ventilation. Clinical Infectious Diseases. 2008;47(12):1562–1570. doi: 10.1086/593193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoen B. Special issues in the management of infective endocarditis caused by gram-positive cocci. Infect Dis Clin North Am. 2002;16(2):437–452. doi: 10.1016/s0891-5520(01)00004-6. [DOI] [PubMed] [Google Scholar]
- 18.Holmes S. Nursing management of oral care in older patients. Nurs Times. 1996;92(9):37–39. Feb 28–Mar 5. [PubMed] [Google Scholar]
- 19.Implement the ventilator bundle: Daily oral care with Chlorhexidine. Cambridge, MA: Institute for Healthcare Improvement; 2010. May, Available from: http://www.ihi.org/IHI/Topics/CriticalCare/IntensiveCare/Changes/IndividualChanges/DailyOralCarewithChlorhexidine.htm. [Google Scholar]
- 20.Johanson WG, Jr., Seidenfeld JJ, De Los Santos R, Coalson JJ, Gomez P. Prevention of nosocomial pneumonia using topical and parenteral antimicrobial agents. Am Rev Respir Dis. 1988;137(2):265–272. doi: 10.1164/ajrccm/137.2.265. [DOI] [PubMed] [Google Scholar]
- 21.Jones DJ, Munro CL, Grap MJ, Kitten T, Edmond M. Oral care and bacteremia risk in mechanically ventilated adults. Heart and Lung: Journal of Acute and Critical Care. 2010;39(6):S57–S65. doi: 10.1016/j.hrtlng.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerr V. Bacteraemia. Br Dent J. 2000 Apr 8;188(7):355. author reply 355–6. [PubMed] [Google Scholar]
- 23.Kitten T, Munro CL, Michalek SM, Macrina FL. Genetic characterization of a streptococcus mutans LraI family operon and role in virulence. Infect Immun. 2000;68(8):4441–4451. doi: 10.1128/iai.68.8.4441-4451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13(4):547–558. doi: 10.1128/cmr.13.4.547-558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lockhart PB, Durack DT. Oral microflora as a cause of endocarditis and other distant site infections. Infect Dis Clin North Am. 1999;13(4):833–850. doi: 10.1016/s0891-5520(05)70111-2. [DOI] [PubMed] [Google Scholar]
- 26.Marron A, Carratalà J, González-Barca E, Fernández-Sevilla A, Alcaide F, Gudiol F. Serious complications of bacteremia caused by viridans streptococci in neutropenic patients with cancer. Clinical Infectious Diseases. 2000;31(5):1126–1130. doi: 10.1086/317460. [DOI] [PubMed] [Google Scholar]
- 27.Moore J. Assessment of nurse-administered oral hygiene. Nurs Times. 1995;91(9):40–41. [PubMed] [Google Scholar]
- 28.Munro CL, Grap MJ. Oral health and care in the intensive care unit: State of the science. American Journal of Critical Care. 2004;13(1):25–34. [PubMed] [Google Scholar]
- 29.Munro CL, Grap MJ, Jones DJ, McClish DK, Sessler CN. Chlorhexidine, toothbrushing, and preventing ventilator-associated pneumonia in critically ill adults. American Journal of Critical Care. 2009;18(5):428–437. doi: 10.4037/ajcc2009792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munro CL, Macrina FL. Sucrose-derived exopolysaccharides of streptococcus mutans V403 contribute to infectivity in endocarditis. Mol Microbiol. 1993;8(1):133–142. doi: 10.1111/j.1365-2958.1993.tb01210.x. [DOI] [PubMed] [Google Scholar]
- 31.Pearson LS. A comparison of the ability of foam swabs and toothbrushes to remove dental plaque: Implications for nursing practice. J Adv Nurs. 1996;23(1):62–69. doi: 10.1111/j.1365-2648.1996.tb03136.x. [DOI] [PubMed] [Google Scholar]
- 32.Scannapieco FA, Papandonatos GD, Dunford RG. Associations between oral conditions and respiratory disease in a national sample survey population. Ann Periodontol. 1998;3(1):251–256. doi: 10.1902/annals.1998.3.1.251. [DOI] [PubMed] [Google Scholar]
- 33.Scannapieco FA, Stewart EM, Mylotte JM. Colonization of dental plaque by respiratory pathogens in medical intensive care patients. Crit Care Med. 1992;20(6):740–745. doi: 10.1097/00003246-199206000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Seymour RA, Whitworth JM. Antibiotic prophylaxis for endocarditis, prosthetic joints, and surgery. Dent Clin North Am. 2002;46(4):635–651. doi: 10.1016/s0011-8532(02)00033-2. [DOI] [PubMed] [Google Scholar]
- 35.Silberman SL, Le Jeune RC, Serio FG, Devidas M, Davidson L, Vernon K. A method for determining patient oral care skills: The university of mississippi oral hygiene index. J Periodontol. 1998;69(10):1176–1180. doi: 10.1902/jop.1998.69.10.1176. [DOI] [PubMed] [Google Scholar]
- 36.Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R. Guidelines for preventing health-care--associated pneumonia, 2003: Recommendations of CDC and the healthcare infection control practices advisory committee. MMWR.Recommendations and reports : Morbidity and mortality weekly report.Recommendations and reports / Centers for Disease Control. 2004;53(RR-3):1–36. [PubMed] [Google Scholar]
- 37.Torres A, El-Ebiary M, Gonzalez J, Ferrer M, De la Bellacasa JP, Gene A, et al. Gastric and pharyngeal flora in nosocomial pneumonia acquired during mechanical ventilation. Am Rev Respir Dis. 1993;148(2):352–357. doi: 10.1164/ajrccm/148.2.352. [DOI] [PubMed] [Google Scholar]
- 38.Valles J, Artigas A, Rello J, Bonsoms N, Fontanals D, Blanch L, et al. Continuous aspiration of subglottic secretions in preventing ventilator- associated pneumonia. Ann Intern Med. 1995;122(3):179–186. doi: 10.7326/0003-4819-122-3-199502010-00004. [DOI] [PubMed] [Google Scholar]
- 39.Wilson M. Bacterial biofilms and human disease. Sci Prog. 2001;84(Pt 3):235–254. doi: 10.3184/003685001783238998. [DOI] [PMC free article] [PubMed] [Google Scholar]