Abstract
The brain is the most cholesterol-rich organ in the body. Although most of the cholesterol in the brain is produced endogenously, some studies suggest that systemic cholesterol may be able to enter the brain. We investigated whether abnormal cholesterol profiles correlated with diffusion-tensor-imaging-based estimates of white matter microstructural integrity of lean and overweight/obese (o/o) adults. Twenty-two lean and 39 obese adults underwent magnetic resonance imaging, kept a 3-day food diary, and had a standardized assessment of fasting blood lipids. The lean group ate less cholesterol rich food than o/o although both groups ate equivalent servings of food per day. Voxelwise correlational analyses controlling for age, diabetes, and white matter hyperintensities, resulted in two significant clusters of negative associations between abnormal cholesterol profile and fractional anisotropy, located in the left and right prefrontal lobes. When the groups were split, the lean subjects showed no associations, whereas the o/o group expanded the association to three significant clusters, still in the frontal lobes. These findings suggest that cholesterol profile abnormalities may explain some of the reductions in white matter microstructural integrity that are reported in obesity.
Keywords: cholesterol, obesity, prefrontal lobes, MRI, fractional anisotropy
Introduction
The brain has the highest cholesterol concentration of any organ in the body [1]. Approximately 95% of the cholesterol contained in the brain is produced endogenously by astrocytes and oligodendrocytes [1, 2]. Cholesterol is important for neuronal communication as it is an important component of the myelin that encases axons, thus allowing for more efficient signal transduction [3]. Under normal conditions circulating cholesterol, which is distinct from brain cholesterol, is kept out of the brain by the blood brain barrier (BBB) [4, 5]. However, some studies in rodents suggest that it is possible for systemic cholesterol, specifically low-density lipoproteins (LDL), to enter the brain [6–8]. Endothelial cells in the BBB contain receptors that are capable of translocating LDL from the general circulation into the brain [6, 7].
Obese organisms, from rodents to humans, are more likely to have elevations in LDL or reductions in high density lipoprotein (HDL) concentrations. In addition, independent of obesity, there is an age-associated increase in brain cholesterol concentration in both mice and humans [9, 10]. While elevated LDL is strongly associated with cardiovascular disease, recent evidence suggests that elevated cholesterol levels may also have detrimental effects on the brain [1, 11]. With the exception of some studies in Alzheimer’s disease [12] and data from our laboratory on type 2 diabetes [13], very little is known about HDL and its effects on the brain.
Healthy adults [14, 15] and to a greater extent adults with metabolic syndrome [11], a condition characterized in part by obesity and elevated LDL or low HDL, have myelin loss, axon degeneration and white matter abnormalities. Additionally, during normal aging the frontal lobe exhibits decreases in fractional anisotropy (FA), a sensitive indicator of white matter integrity thought to reflect fiber density or axonal diameters [16]. The frontal lobe is involved in executive functions [17] therefore, damage to this area of the brain, as occurs in metabolic syndrome, may affect decision making and processing speed [11, 18]. Previous work from our laboratory demonstrated that orbitofrontal cortex volume is positively associated with healthy food choice [19]. Thus far, no data demonstrates associations between abnormal cholesterol profile, FA, and possible connections to decision making and food choice. This study sought to explore these associations.
We hypothesized that obese adults with an abnormal cholesterol profile will have lower white matter microstructural integrity and that these abnormalities will be found predominantly in the frontal lobe. By controlling for age and diabetes, two factors known to impact white matter integrity, as well as also controlling for overt white matter lesions expressed as hyperintensities on the FLAIR scan, we hope to demonstrate that in an overweight/obese adult population, abnormal cholesterol profile (high LDL, low HDL or use of statins) is associated with lower FA in the frontal lobe.
Materials and Methods
Subjects
A total of 61 adults (22 lean and 39 overweight and obese), matched on age, gender, race, and education were included in the study (Table 1). Subjects were consecutive cases evaluated at the Brain, Obesity, and Diabetes Laboratory (BODyLab), Department of Psychiatry, New York University School of Medicine as part of an NIH-sponsored study of the brain effects of obesity and type 2 diabetes. Participants were recruited via internet advertisement, referred by collaborating endocrinologists, or recruited from an ongoing study of normal aging. The study was approved by the local Institutional Review Board. All participants signed informed written consent and received compensation for their time and inconvenience. Participants were screened to rule out exclusionary preexisting medical conditions (other than hypertension, dyslipidemia, insulin resistance, or type 2 diabetes) and psychiatric conditions. Other exclusion criteria were a history of significant head trauma, stroke, hydrocephalus, lacunar infarcts, mental retardation, neurological disorders or less than a 12th grade education.
Table 1.
Group demographic data
| Lean | o/o | p-value | Cohen's d | |
|---|---|---|---|---|
| n=22 | n=39 | |||
| Gender (% Female) | 13 | 15 | Χ2= 2.41 | |
| Caucasian (%) | 80 | 53.8 | ||
| Hispanic (%) | 2.9 | 3.8 | ||
| Asian (%) | 2.9 | 11.5 | ||
| African American (%) | 14.3 | 30.8 | ||
| Age | 58.1 ± 1.5 | 58.4 ± 1.1 | 0.84 | 0.05 |
| Education (years) | 16 ± 2.5 | 15.5 ± 2.2 | 0.37 | 0.24 |
| BMI | 22.1 ± 0.5 | 31.7 ± 1.0 | 0.00* | 1.92 |
| Glucose | 82.1 ± 3.5 | 118.6 ± 7.4 | 0.00* | 0.95 |
| Insulin | 6.0 ± 0.9 | 13.9 ± 1.7 | 0.00* | 0.91 |
| HOMA-IR | 1.1 ± 0.2 | 4.3 ± 0.6 | 0.00* | 1.00 |
| % Hypertensive | 41 | 67 | Χ2= 1.85; NS |
Values with (*) are significantly different, p<0.05.
After an overnight fast, blood was drawn in a standardized fashion at 9:00 A.M. and a comprehensive panel was performed to assess blood count, blood chemistries, lipid and liver profiles, thyroid function, glucose and insulin levels, and inflammatory markers. Body mass index (BMI) was calculated using the following formula: weight (kg)/height (m)2. Lean individuals had a BMI between 19 and 24.9 kg/m2. Participants with a BMI of 25 kg/m2 or greater were assigned to the overweight/obese (o/o) group. We defined abnormal cholesterol as an LDL greater than 130 mg/dL, an HDL lower than 34 mg/dL, or use of statin medications to control elevated cholesterol. We used these criteria because low HDL is common in insulin resistance and high LDL is common in obesity and obesity-related diseases [20]. We classified subjects who were on a statin as having an abnormal cholesterol profile irrespective of whether as a result of the treatment they had cholesterol levels in the normal range.
Three-day dietary recall
Participants received a 3-day food diary form and were instructed to record everything they consumed (breakfast, lunch, dinner and snacks) with the exception of water. The participants’ self-reported diets were quantified into 13 different categories (produce, dairy, meat, fish, whole grains, refined carbohydrates, fried food, nuts, fast food, soda, junk food, alcohol, miscellaneous) in a blinded fashion by JIC, a Ph.D. in nutrition. Quantification of all foods was based upon serving sizes, as defined by the American Dietetic Association. The cholesterol rich food group was the sum of meat, dairy, fried food and fast food. By summing these categories we included both saturated and unsaturated fats as well as cholesterol. A second trained rater, a Ph.D. candidate in nutrition, independently assessed a randomly selected subset of 30 food diaries, to assess inter-rater reliability. These independent ratings showed excellent agreement with an inter-class correlation coefficient (ICC) of 0.91 for cholesterol rich foods.
Magnetic Resonance Imaging (MRI)
Image acquisition
All MR imaging was performed on a 1.5 T Siemens Avanto MRI System. A structural T1-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence was acquired with the following parameters: TR = 1300 ms, TE= 4.38 ms, TI 800ms, NEX 1 FOV 250×250, 196 coronal slices, slice thickness 1.2 mm, Flip angle 15°. The MPRAGE images were manually skull-stripped and normalized to standard space. The 3D warp field containing the MPRAGE to target transformation parameters was used to spatially-normalize the FA maps [21]. Fast fluid-attenuated inversion recovery (FLAIR) sequence (TR = 9000 ms, TE= 97 ms, FOV 210×210, 1 average, 2 concatenations, Flip angle 145°) were collected to rule out primary neurological disease and to account for white matter hyperintensities. A conventional T2-weighted sequence (TR = 9000ms, TE=94 ms, TI 2000ms, FOV 210×210 mm, 50 axial slices, slice thickness 3 mm) was acquired to correct the spatial distortion inherent in the echo planar DTI acquisition [21]. The DTI echo planar sequence was obtained in 6 directions (TR=6100ms, TE =75ms, delay in TR=0, b values 0 and 1000, FOV 210×210, 4 averages, 1 concatenation, 50 axial slices, voxel size 1.64×1.64×3 mm3). The FLAIR, T2-weighted, and DTI images were collected in the same orientation, slice thickness, and number of slices in order to optimize co-registration of the different image sets. The plane of acquisition for all these images was an axial orientation set up parallel to a plane that on the mid-sagittal view goes through the inferior aspect of the orbitofrontal cortex and the inferior aspect of the occipital cortex, the so-called pathological angle.
DTI voxelwise image processing and analyses
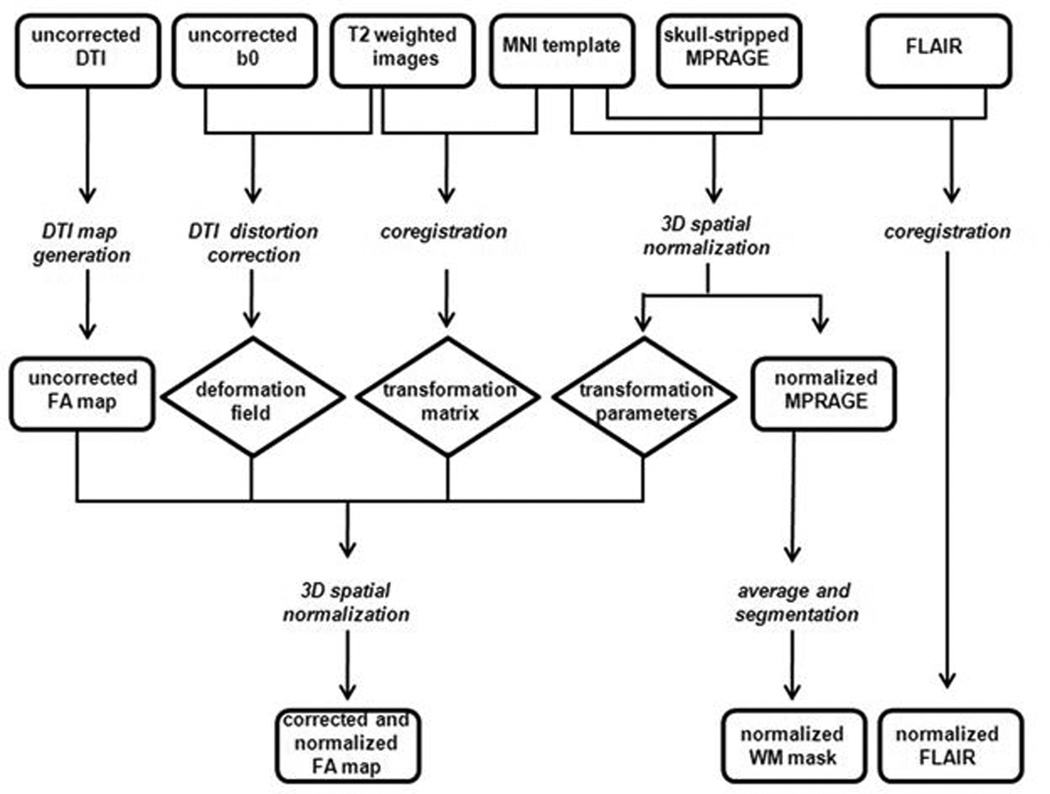
Images were processed using the in-house software Multimodal Imaging Data System (MIDAS) and the automated toolbox ART2 (Automated Registration Toolkit 2) [21]. FA maps were generated from the native DTI images, after correcting for distortions and spatially normalizing (Figure 1). As a result, FA maps ended up with a voxel size of 1×1×1 mm3. We checked the FA maps at the individual case level to ensure that there were no spatial distortions and that all images were high quality. To ascertain the possible associations between an abnormal cholesterol profile and reductions in white matter microstructural integrity, FA maps were subjected to a voxelwise correlational analysis [22], with age and diabetes as covariates. There was no difference in the prevalence of hypertension (defined as either blood pressure above the normal range (135/85 mmHg) or use of medication to control hypertension) between the two groups; therefore, we did not control for hypertension. We also used the FLAIR images, which were first normalized to the target template by applying the same transformation parameters that were applied to the FA maps, as a covariate to ensure that our results were not influenced by white matter hyperintensities. To confine FA analyses to within the white matter, we used the determined white matter of the average segmented MPRAGE image of all the subjects as a mask. To reduce the escalation of type 1 error due to multiple comparisons, only significant groups of voxels (clusters) of at least 100 contiguous voxels (0.1 cc) showing significant associations were defined as a significant cluster. We chose a false discovery rate (FDR) less than 1% according to the original Benjamini-Hochberg [23] procedure. Moreover, we selected a significance threshold of 0.001 to ensure that the FDR would be kept below 1%. For display purposes, the correlation map showing the significant clusters of association was registered to the standard Montreal Neurological Institute (MNI) T1 MRI template and visualized with AFNI (Analysis of Functional NeuroImages).
Figure 1. Generation of images for voxel wise analysis.
This is a pictorial depiction of how the originally acquired MR images were processed into a format where voxel wise analysis was possible. Rectangles represent images, italicized words represent procedures and diamonds represent the output from the procedures. Diffusion tensor imaging (DTI); Montreal Neurological Institute (MNI); magnetization-prepared rapid acquisition gradient echo (MPRAGE); fast fluid-attenuated inversion recovery (FLAIR); fractional anisotropy (FA); white matter (WM).
Statistical analyses
We analyzed the group demographic and endocrine data using SPSS for Windows version 18.0 (SPSS, Inc., Chicago, IL). We checked the normality of our data using the Shapiro-Wilk test. We performed two-tailed independent sample t-tests and chi-square tests to examine the group differences between lean and o/o characteristics. The effect sizes are reported as Cohen’d coefficient. For a description of the automated image analyses, please see the MRI section immediately above.
Results
Demographic and endocrine data
The participants’ demographic and endocrine characteristics are summarized in Table 1. As expected, relative to lean subjects, o/o individuals had significantly larger BMI (t[52]=−8.97, P<0.0001), as well as, higher fasting glucose level (t[52]=−4.44, P<0.0001) and fasting insulin level (t[55]=−4.02, P<0.0001). The groups did not differ in total cholesterol and LDL (Table 2). However, o/o individuals had significantly lower HDL (t[59]=4.09, P<0.0001) and higher triglyceride levels (t[59]=−2.51, P=0.02). In the lean group 23% of subjects were on a statin and in the o/o group 58% of participants were on statins.
Table 2.
Group lipid profile.
| Lean | o/o | p-value | Cohen's d | |
|---|---|---|---|---|
| HDL | 55.6 ± 3.1 | 42.7 ± 1.6 | 0.00* | 1.09 |
| LDL | 112.6 ± 5.9 | 110.0 ± 5.6 | 0.76 | 0.08 |
| Total Cholesterol | 187.1 ± 6.7 | 179.2 ± 6.1 | 0.41 | 0.22 |
| Triglycerides | 94.4 ± 8.7 | 132.4 ± 12.4 | 0.02* | 0.57 |
Values with (*) are significantly different, p<0.05.
Dietary data
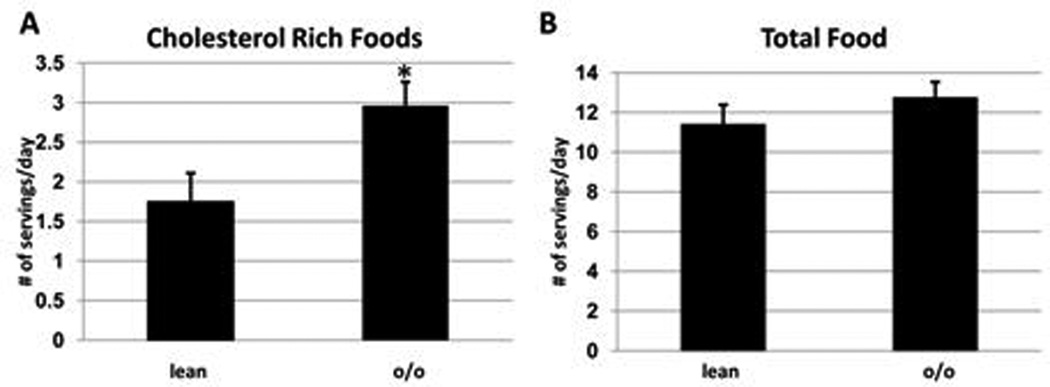
The o/o group consumed significantly more cholesterol-containing foods (dairy, meat, fried food, fast food) compared to the lean group (Figure 1A). However, because the o/o group consumed less low cholesterol foods, the total amount of food consumed (number of servings) was not different between the two groups (Figure 1B).
Voxelwise correlational analyses
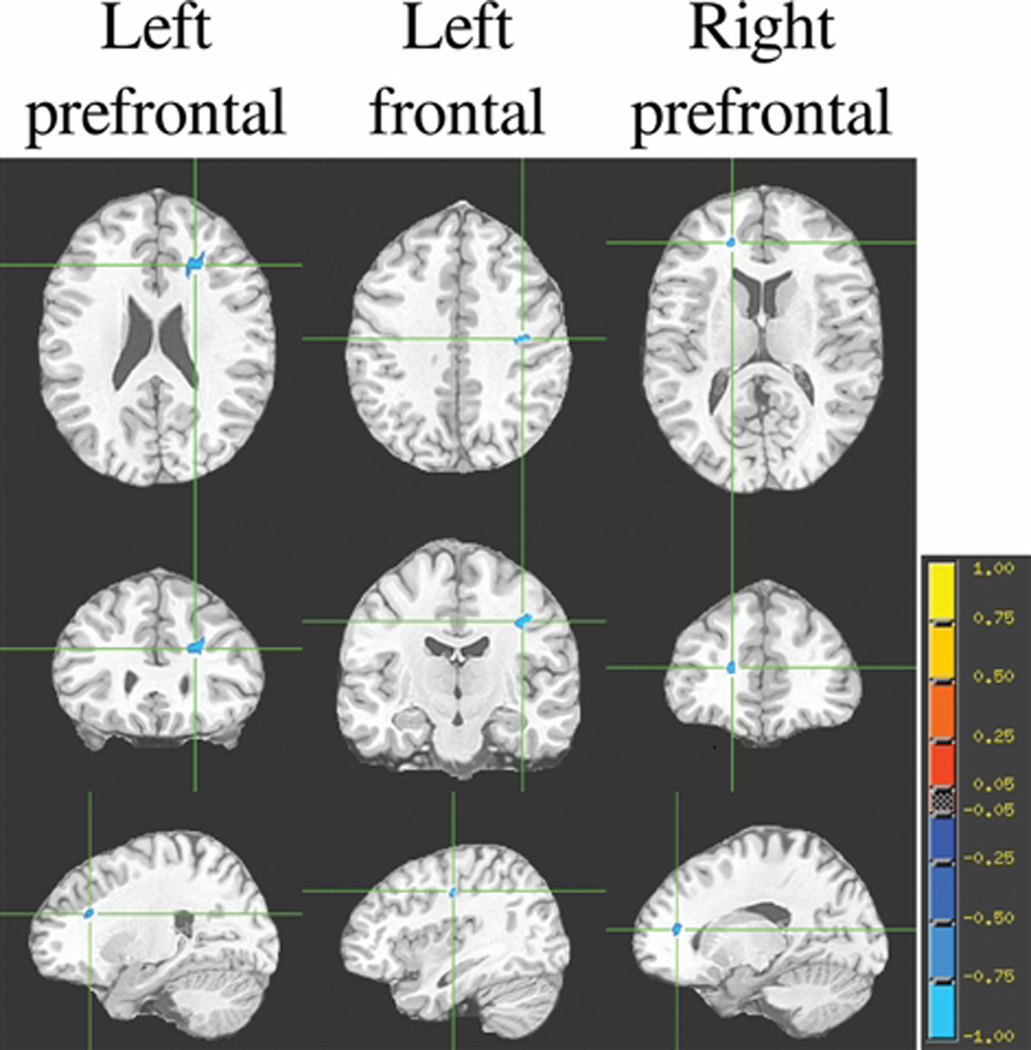
The voxelwise correlational analyses among all the participants, controlling for age, diabetes, and white matter hyperintensities, resulted in two significant (P< 0.001) clusters of negative associations between abnormal cholesterol and FA, located in the left and right prefrontal lobes (Table 3). However, when these analyses were carried out separately for each of the two groups, they showed that the groups behaved quite differently. For lean individuals, after adjusting for age, diabetes, and white matter hyperintensities, there were no significant association between abnormal cholesterol and FA. In contrast, among o/o individuals, after correcting for age, diabetes and white matter hyperintensities, we indentified three significant clusters (P< 0.001) showing an inverse association between FA and abnormal cholesterol, namely an abnormal cholesterol profile was associated with lower FA values. These three significant clusters were located in the left and right prefrontal lobes (see Table 3 and Figure 1). Furthermore, both groups had a high and comparable occurrence of hypertension. For this reason hypertension was not considered a confounder that needed to be accounted for in this study.
Table 3.
Cluster Size, t-value and Taliarach coordinates of associational voxelwise correlational analyses. Correlation between FA values and normal/abnormal cholesterol among overweight and obese, p<0.001
| Clusters | Size (voxels) |
Mean t value |
Talairach Coordinates |
||
|---|---|---|---|---|---|
| x | y | z | |||
| Left prefrontal | 487* | −4.0249 | 23.1 | −11.9 | 5.5 |
| Left frontal | 157* | −4.3928 | 38 | 31 | 20.8 |
| Right prefrontal | 134 | −4.0316 | −17.5 | −25 | −5.8 |
Clusters present in the analysis of total participant pool (lean and overweight/obese)
Discussion
We found that o/o adults had significantly lower HDL and higher triglyceride concentrations compared to lean controls, which is consistent with previous findings by other groups [24]. Both subject groups consumed the same number of food servings per day but the o/o group consumed more cholesterol rich food than the lean group. Interestingly, among the o/o group, but not the lean group, there was a strong inverse association between abnormal cholesterol (high LDL, low HDL and/or statin use) and FA values in prefrontal regions of the brain. Taken together, these data may suggest that an abnormal cholesterol profile may negatively affect the brains of overweight and obese adults.
It is common for people with type 2 diabetes to also exhibit dyslipidemia [25]. Individuals with type 2 diabetes may exhibit high concentrations of LDL, low concentrations of HDL and hypertriglyceridemia [26]. These impairments in cholesterol and lipid metabolism may precede the development of diabetes by years [20, 27]. Furthermore, obesity increases oxidative stress which can result in the formation of oxysterols, an end-product of cholesterol oxidation [28, 29]. Studies show that oxysterols can readily enter the brain and are associated with neurodegenerative diseases [30].
Our study found that the o/o group had lower HDL concentrations, the anti-atherogenic form of cholesterol, and higher concentrations of triglycerides compared to the lean group. The o/o group did not have higher concentrations of circulating LDL compared to the lean group, but this is likely due to the large percentage of individuals in the o/o group that are receiving statin medications.
To assess white matter microstructural integrity we used quantitative DTI MRI and generated FA maps [31, 32]. FA is a scalar value that ranges between 0.1 and 1.0 (peaking at ~0.3 for white matter) [32] with 0 representing perfectly isotropic diffusion (free movement of water). Although there are some limitations to the specificity of FA as a biomarker of microstructural integrity, nevertheless white matter FA is a highly sensitive marker of neuropathology [32, 33]. Consequently, our lower FA values associated with abnormal cholesterol profile in prefrontal areas of the brain in o/o, but not lean, participants may underlie white matter changes in the presence of dyslipidemia. This is consistent with recent results among patients with metabolic syndrome showing FA reductions mostly restricted to the frontal lobe [11].
Consistent with our findings, other studies show that obesity is associated with alterations in brain structural integrity in both adolescents [34] and adults [35, 36]. Obese adolescents with type 2 diabetes have decreased white matter volume and reduced white and grey matter microstructural integrity compared to lean controls [34]. Moreover, obese adults have reduced grey matter integrity in areas of the brain associated with the reward system [35]. These differences are associated with inflammation, a common correlate of obesity [37].
A major strength, but perhaps also a weakness of this study, was the use of food diaries, which allowed participants to record food items independent of finite list. However, food diaries may result in increased variability due to lack of standarization. There was variability in the amount of detail between diaries such as incomplete entries and lack of adequate descriptions, which led to the elimination of some participants. Additionally, due to the cross-sectional study design, we cannot determine if changes in white matter microstructural integrity preceded or are the result of obesity. Despite this limitation, weighted food diaries remain the gold standard that is used to validate other dietary assessment methods [38]. The imaging techniques utilized also provided strength and weakness to our approach. For example, although sophisticated techniques were used to correct for the spatial distortions that occur in the echo-planar acquisition of the DTI data, we cannot completely rule out that these distortions, despite corrections, did not influence our findings. However, this is likely only a theoretical concern since both hemispheres were affected; results from distortions would more likely be unilateral. We allowed only clusters that were at least 100 contiguous voxels and used an FDR of less than 1% and a p-value of <0.001 to denote associations between abnormal cholesterol profile and reductions in FA. In the future, this study should be repeated using a larger sample size in order to investigate if statin use and/or higher levels of physical activity in obese adults limits the decrease in white matter structural integrity observed in the this study.
Conclusions
Overall, we found a link between abnormal systemic cholesterol profile and low FA values in o/o adults but not lean adults. To our knowledge, this is the first report that suggests systemic cholesterol abnormalities can negatively impact white matter integrity. Specifically, lower FA values associated with abnormal cholesterol profile were observed in areas of the brain involved in executive functioning and decision making. These findings offer insight into how cholesterol dysregulation may mediate or exacerbate brain abnormalities that are associated with obesity. Further studies evaluating cognition, specifically executive functioning, would provide further insight into how the observed differences between the groups may be affecting brain function.
Figure 2. Differences in consumption of cholesterol rich foods in o/o and lean adults.
The total number of (A) cholesterol rich foods (meat, dairy, fried food and fast food) and (B) total food consumed per day was quantified. Values represent means ± SEM. Values with (*) are significantly different, p<0.05.
Figure 3. Brain regions showing clusters of association between DTI FA and abnormal cholesterol profile among o/o individuals.
Each column represents the 3 orthogonal orientations (axial, coronal, sagittal) for the significant inverse correlation clusters (analysis controlling for age, diabetes diagnosis and white matter hyperintensities; minimum cluster size is 100 voxels; p<0.001) overlaid on the normalized average T1 target image. The color bar represents the strength of the correlations.
Acknowledgments
This study was supported by a grant from the National Institutes of Health DK064087 and T32-DA007254-16 (JIC) and supported in part by grant1UL1RR029893 from the National Center for Research Resources.
Footnotes
Financial Disclosure(s): None of the authors have any financial/conflicting interests to disclose.
Contributor Information
Jessica I. Cohen, Email: cohenj25@nyumc.org.
Fanny Cazettes, Email: cazetf01@nuymc.org.
Antonio Convit, Email: antonio.convit@med.nyu.edu.
References
- 1.Bjorkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol. 2004 May;24(5):806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 2.Saito M, Benson EP, Rosenberg A. Metabolism of cholesterol and triacylglycerol in cultured chick neuronal cells, glial cells, and fibroblasts: accumulation of esterified cholesterol in serum-free culture. J Neurosci Res. 1987;18(2):319–325. doi: 10.1002/jnr.490180208. [DOI] [PubMed] [Google Scholar]
- 3.Snipes G, Suter U. In: Cholesterol and Myelin. R B, editor. New York: Plenum Press; 1998. [Google Scholar]
- 4.Waelsch H, Sperry W, Stoyanoff VA. A study of the synthesis and deposition of lipids in brain and other tissues with deuterium as an indicator. J Biol Chem. 1940;135:291–296. [Google Scholar]
- 5.Meaney S, Hassan M, Sakinis A, et al. Evidence that the major oxysterols in human circulation originate from distinct pools of cholesterol: a stable isotope study. J Lipid Res. 2001 Jan;42(1):70–78. [PubMed] [Google Scholar]
- 6.Dehouck B, Dehouck MP, Fruchart JC, Cecchelli R. Upregulation of the low density lipoprotein receptor at the blood-brain barrier: intercommunications between brain capillary endothelial cells and astrocytes. J Cell Biol. 1994 Jul;126(2):465–473. doi: 10.1083/jcb.126.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dehouck B, Fenart L, Dehouck MP, Pierce A, Torpier G, Cecchelli R. A new function for the LDL receptor: transcytosis of LDL across the blood-brain barrier. J Cell Biol. 1997 Aug 25;138(4):877–889. doi: 10.1083/jcb.138.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karmi A, Iozzo P, Viljanen A, et al. Increased brain fatty acid uptake in metabolic syndrome. Diabetes. 2010;59:2171–2177. doi: 10.2337/db09-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quan G, Xie C, Dietschy JM, Turley SD. Ontogenesis and regulation of cholesterol metabolism in the central nervous system of the mouse. Brain Res Dev Brain Res. 2003 Dec 19;146(1–2):87–98. doi: 10.1016/j.devbrainres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Lentner C. Geigy Scientific Tables. West Caldwell: Ciba-Geigy Corp.; 1981. [Google Scholar]
- 11.Segura B, Jurado MA, Freixenet N, Bargallo N, Junque C, Arboix A. White matter fractional anisotropy is related to processing speed in metabolic syndrome patients: a case-control study. BMC Neurol. 2010;10:64. doi: 10.1186/1471-2377-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reitz C, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch Neurol. 2010 Dec;67(12):1491–1497. doi: 10.1001/archneurol.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruehl H, Wolf OT, Sweat V, Tirsi A, Richardson S, Convit A. Modifiers of cognitive function and brain structure in middle-aged and elderly individuals with type 2 diabetes mellitus. Brain Res. 2009 Jul 14;1280:186–194. doi: 10.1016/j.brainres.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burzynska AZ, Preuschhof C, Backman L, et al. Age-related differences in white matter microstructure: region-specific patterns of diffusivity. Neuroimage. 2010 Feb 1;49(3):2104–2112. doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 15.Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH., Jr. Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp. 2010 Mar;31(3):378–390. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage. 2005 Jul 1;26(3):891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 17.Lezak MD, Howieson DB, Loring DW, editors. Neuropsychological assessment. New York: Oxford University Press; 2004. [Google Scholar]
- 18.Segura B, Jurado MA, Freixenet N, Falcon C, Junque C, Arboix A. Microstructural white matter changes in metabolic syndrome: a diffusion tensor imaging study. Neurology. 2009 Aug 11;73(6):438–444. doi: 10.1212/WNL.0b013e3181b163cd. [DOI] [PubMed] [Google Scholar]
- 19.Cohen JI, Yates KF, Duong M, Convit A. Obesity, orbitofrontal structure and function are associated with food choice: a cross-sectional study. BMJ Open. 2011;2 doi: 10.1136/bmjopen-2011-000175. (e000175). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruit JK, Brunham LR, Verchere CB, Hayden MR. HDL and LDL cholesterol significantly influence beta-cell function in type 2 diabetes mellitus. Curr Opin Lipidol. 2010 Jun;21(3):178–185. doi: 10.1097/MOL.0b013e328339387b. [DOI] [PubMed] [Google Scholar]
- 21.Ardekani BA, Guckemus S, Bachman A, Hoptman MJ, Wojtaszek M, Nierenberg J. Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. J Neurosci Methods. 2005 Mar 15;142(1):67–76. doi: 10.1016/j.jneumeth.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann SL, Russell DW, Goldstein JL, Brown MS. mRNA for low density lipoprotein receptor in brain and spinal cord of immature and mature rabbits. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6312–6316. doi: 10.1073/pnas.84.17.6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klipper-Aurbach Y, Wasserman M, Braunspiegel-Weintrob N, et al. Mathematical formulae for the prediction of the residual beta cell function during the first two years of disease in children and adolescents with insulin-dependent diabetes mellitus. Med Hypotheses. 1995 Nov;45(5):486–490. doi: 10.1016/0306-9877(95)90228-7. [DOI] [PubMed] [Google Scholar]
- 24.Francis GA. The complexity of HDL. Biochim Biophys Acta. 2010 Dec;1801(12):1286–1293. doi: 10.1016/j.bbalip.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Nicholls SJ, Lundman P, Tardif JC. Diabetic dyslipidemia: extending the target beyond LDL cholesterol. Eur J Cardiovasc Prev Rehabil. 2010 May;17 Suppl 1:S20–S24. doi: 10.1097/01.hjr.0000368195.09485.17. [DOI] [PubMed] [Google Scholar]
- 26.Brunzell JD, Davidson M, Furberg CD, et al. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008 Apr 15;51(15):1512–1524. doi: 10.1016/j.jacc.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 27.Kendall DM. The dyslipidemia of diabetes mellitus: giving triglycerides and high-density lipoprotein cholesterol a higher priority? Endocrinol Metab Clin North Am. 2005 Mar;34(1):27–48. doi: 10.1016/j.ecl.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Alkazemi D, Egeland G, Vaya J, Meltzer S, Kubow S. Oxysterol as a marker of atherogenic dyslipidemia in adolescence. J Clin Endocrinol Metab. 2008 Nov;93(11):4282–4289. doi: 10.1210/jc.2008-0586. [DOI] [PubMed] [Google Scholar]
- 29.Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sottero B, Gamba P, Gargiulo S, Leonarduzzi G, Poli G. Cholesterol oxidation products and disease: an emerging topic of interest in medicinal chemistry. Curr Med Chem. 2009;16(6):685–705. doi: 10.2174/092986709787458353. [DOI] [PubMed] [Google Scholar]
- 31.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996 Jun;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 32.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007 Jul;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundgren PC, Dong Q, Gomez-Hassan D, Mukherji SK, Maly P, Welsh R. Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology. 2004 May;46(5):339–350. doi: 10.1007/s00234-003-1114-x. [DOI] [PubMed] [Google Scholar]
- 34.Yau PL, Javier DC, Ryan CM, et al. Preliminary evidence for brain complications in obese adolescents with type 2 diabetes mellitus. Diabetologia. 2010 Nov;53(11):2298–2306. doi: 10.1007/s00125-010-1857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cazettes F, Cohen JI, Yau PL, Talbot H, Convit A. Obesity-mediated inflammation may damage the brain circuit that regulates food intake. Brain Res. 2010 Dec 9; doi: 10.1016/j.brainres.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanek KM, Grieve SM, Brickman AM, et al. Obesity Is Associated With Reduced White Matter Integrity in Otherwise Healthy Adults. Obesity (Silver Spring) 2010 Dec 23; doi: 10.1038/oby.2010.312. [DOI] [PubMed] [Google Scholar]
- 37.Vachharajani V, Granger DN. Adipose tissue: a motor for the inflammation associated with obesity. IUBMB Life. 2009 Apr;61(4):424–430. doi: 10.1002/iub.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrett-Connor E. Nutrition epidemiology: how do we know what they ate? Am J Clin Nutr. 1991 Jul;54(1 Suppl):182S–187S. doi: 10.1093/ajcn/54.1.182S. [DOI] [PubMed] [Google Scholar]