Abstract
J Clin Hypertens (Greenwich). 2011;13:881–888. ©2011 Wiley Periodicals, Inc.
Environmental temperatures are inversely related to BP; however, the effects of short‐term temperature changes within a 24‐hour period and measured with high accuracy at the personal level have not been described. Fifty‐one nonsmoking patients living in the Detroit area had up to 5 consecutive days of 24‐hour personal‐level environmental temperature (PET) monitoring along with daily cardiovascular measurements, including BP, performed mostly between 5 pm and 7 pm during summer and/or winter periods. The associations between hour‐long mean PET levels during the previous 24 hours with the outcomes were assessed by linear mixed models. Accounting for demographics, environmental factors, and monitoring compliance, systolic and diastolic BP were positively associated with several hour–long PET measurements ending from 10 to 15 hours beforehand. During this time, corresponding mostly to a period starting from between 1am and 3 am to ending between 7 am and 9 am, an increase of 1°C was associated with a 0.81 mm Hg to 1.44 mm Hg and 0.59 mm Hg to 0.83 mm Hg elevation in systolic and diastolic BP, respectively. Modestly warmer, commonly encountered PET levels posed a clinically meaningful effect (eg, a 6.95 mm Hg systolic pressure increase per interquartile range (4.8°C) elevation at lag hour 10). Community‐level outdoor ambient temperatures were not related to BP. The authors provide the first evidence that personal exposure to warmer nighttime and early‐morning environmental temperatures might lead to an increase in BP during the ensuing day.
Many environmental factors such as air pollution, noise, and altitude affect blood pressure (BP). 1 , 2 Numerous studies have also demonstrated an inverse relationship between BP and outside environmental temperature during concurrent or recent days. 2 , 3 , 4 , 5 , 6 Longer‐term BP variations have been linked to seasonal changes, possibly due to colder temperatures during the winter. 4 , 6 Importantly, the magnitude of BP elevations have been shown to be of clinical relevance (ie, 5–10 mm Hg) 4 , 5 and could thus potentially contribute to an acute increase in risk for cardiovascular (CV) events. 7 , 8 , 9
Most previous studies have only investigated the BP associations with relatively prolonged outside temperature changes (eg, daily means) and by measurements performed at community sites. 2 , 3 , 4 , 5 , 6 In this fashion, only between‐day outside temperature effects on BP could be detected and patients were assumed to be equally exposed to the same prevailing levels. Even when additionally determined by a monitor at a fixed indoor location at the place of BP measurement, 4 this indoor temperature cannot adequately describe the numerous short‐term fluctuations uniquely encountered by each individual throughout a day. These limitations have likely resulted in significant exposure misclassifications. Only imprecise estimates of the environmental temperature–BP association from both temporal and spatial resolution standpoints have thus been provided by studies to date. The effects of shorter‐term variations (ie, occurring over hours) within a 24‐hour period during routine daily activity have not been reported. Moreover, the effects of alterations in the “true” environmental temperature, meaning the level actually encountered and unique to each individual, have not been described.
Given the complexities of this environment‐health interaction (eg, susceptibility, durations of exposure, timing of BP response), the relationship may be more complicated than a simple linear inverse association. In this context, we designed the CV substudy of the Detroit Exposure and Aerosol Research Study (DEARS) to elucidate the effects of several environmental factors, including temperatures, on health parameters such as arterial BP. Exposure parameters were characterized at both the community and personal levels (to enhance spatial resolution) and continuously throughout repeated 24‐hour periods (to enhance temporal resolution). The main CV study methods and results focusing on air pollution–mediated BP elevations are published elsewhere. 10 In this post hoc subanalysis, we performed a hypothesis‐generating evaluation of the associations between BP and short‐term variations in personal‐level environmental temperature (PET) exposures occurring within a 24‐hour period. We concomitantly explored whether outdoor and/or PET affected vascular endothelial function, also potentially affected by weather, 11 in a manner that could be a biological contributor (ie, altered vasodilatory responses) to any observed BP alteration.
Methods
This study was approved by the institutional review boards of the University of Michigan and the Human Subjects Approving Official of the US Environmental Protection Agency (EPA). Nearly 140 nonsmoking patients 18 years and older living in nonsmoking households among 6 neighborhoods in Wayne County, Michigan, were enrolled into the main DEARS, as described previously. 10 There were no exclusion criteria including for race, sex, medications, or health status. Field sampling occurred during 2 several‐week periods per year (winter and summer sessions) over 3 years (6 total sessions) and completed during the spring of 2007. All patients were invited without restrictions to also participate in the CV substudy during sessions 2 to 6. Volunteers underwent an additional visit in their home at which time written informed consent was obtained, a health interview was performed, and the average of the 2nd and 3rd dominant upper arm BP measurement was determined (Omron 780 monitor) after seated for 5 minutes. The 24‐hour PET measurements were measured and available for only sessions 4 to 6 (ie, winter in 2006 and 2007 and summer in 2006). The results of this paper are therefore related to data collected during these sessions only.
Exposure Assessments
Community‐level outdoor temperature was monitored daily at a nearby State of Michigan air‐quality site (Allen Park), which was 2.5 to 18 miles away from the 6 neighborhoods in DEARS. One member of each household underwent continuous personal exposure monitoring using the Personal Environmental Monitor (PEM) (PEM, SKC, Inc, Eighty Four, PA) on Tuesday through Saturday as previously described. 10 PEM sampling was initiated at a consistent time (9 am ± 2.5 hours) for a continuous 24‐hour period. Patients were instructed to wear the vest at all times except for periods of bathing and the changing of clothes, at which times the monitor was directed to be kept as close as possible to the patient. At night, the PEM was instructed to be kept at their bedside. During sessions 4 to 6, the PEM also had instruments to collect continuous measurements of PET. Data were collected and the results presented were related to this present analysis only during these sessions. The vest also contained sensors that monitored how compliant participants were with wearing it during the nonexclusion scenarios. Previous observations from the main DEARS led us to prespecify that results from patients meeting a conservative compliance rate of 60% would be analyzed. 10 Each participant’s exposure to secondhand smoke (SHS) was also measured by personal filter samples optically analyzed by a mass‐based estimate. The results from patients with a prespecified rate <1.5 μg/m3 of mean daily SHS exposure were included in this analysis to avoid its potential confounding effect on CV outcomes. 12
Cardiovascular End Points
CV study visits were performed at the participant’s home for up to 5 consecutive evenings, Tuesday through Saturday, mostly between 5 pm and 7 pm. A small portion of CV outcomes (26%) were measured on any given day either from 4 pm to 5 pm or between 7 pm and 10 pm for the convenience of the patients’ schedules. These visits took place on concurrent days while patients wore the vest monitors. The CV outcomes evaluated were: systolic and diastolic BP, heart rate, and brachial flow‐mediated dilatation (FMD). Participants were instructed to maintain their daily routine, including taking all medications, but to fast for at least 4 hours prior to the scheduled visits and to avoid unusual physical activity. During each visit, patients rested supine on a portable patient bed for 10 minutes prior to automated BP and heart rate measurement (Omron 780 monitor) that was obtained in triplicate with a 1‐minute lapse between measures. The average of the 2nd and 3rd BP and heart rate recordings was used for analyses. Next, vascular testing for FMD was performed using a portable Terason 2000 ultrasound system (Burlington, MA) as described previously in the main DEARS CV study. 10 Details of study analyses and reproducibility are described elsewhere and in accordance with guidelines. 13 , 14 , 15
Statistical Methods
Integrated hour‐long PET values during the preceding 24‐hour period, calculated for each individual starting immediately before the time of their CV outcome measurements, were determined from the vest instruments (ie, 24 individual hour‐long periods from lag 0 to 23 hours) with lag 0 representing the time between 0 and 60 minutes beforehand. Figure 1 illustrates a specific example of a measurement day. The associations for each hour‐long PET were made for the outcome variables. All of these CV outcomes were observed for 5 consecutive days for each season (winter vs summer) within a patient.
Figure 1.
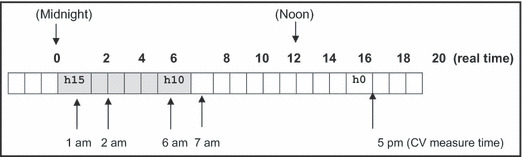
Example of a measurement day. An example of the time of cardiovascular (CV) outcome measurements and lag hours (h) for personal‐level environmental temperature (PET) monitoring in relation to the real time of day for an individual. For patients who had CV measurements (ie, BP) performed at 5 pm, lag hour 10 (h10) corresponds to a period between 6 am and 7 am, while lag hour 15 (h15) corresponds to a period between 1 am and 2 am during the same day.
The patients were considered to be selected at random from a population per the design and methods of the main DEARS cohort. 10 Considering the possibility that within‐subject errors were autocorrelated, a linear mixed model was employed since it was more appropriate when data were collected over time in the same patients. We thus assumed that the association between each of the CV outcomes evaluated and PET was linear with an intercept varying at random over individuals, but the slope representing the linear association was assumed to be the same for all patients. Several predictors of the response were included in the base model as fixed effects: age, sex, race, body mass index (BMI), same‐day community‐level outdoor temperature, and a time‐specific indicator. The relationship between these predictors and responses was assumed to be common to all patients. In equation (1),
where Y ij is the response, a CV outcome for patient i at study day j, PETij (k) the k‐hour lag personal‐level ET prior to the health outcome measurement time, and Temp the same‐day 24‐hour mean community‐level (outdoor) ET. Note that in the model (1) the k‐hour lag personal‐level ET, PETij (k), was included one at a time for k = 0,1,…23, while the outdoor ET, Temp, was included all the time with each PETij (k). Using previous‐day mean community‐level outdoor temperature did not alter the results. The model included a binary indicator variable, Time, assigning 1 if the time of day of the k‐hour lag was between 5 am and 9 am, thus controlling for the possible effect of this time of day itself on the measured BP outcome. This time window was chosen because it reflects a common period of BP surge upon awakening. Other time window periods were also evaluated without an effect on the results. All estimates in this study were obtained by the base model.
This model (1) includes fixed effects associated with the patient‐level covariates (β’s), random effects associated with the intercept for each patient (αi), and residuals associated with each observation (εij). The fixed effects describe the associations of the predictors to the CV outcomes for an entire population, while random effects are specific to patients within that population. 16 , 17
The random effects by patient were assumed to be independently distributed across patients with a normal distribution 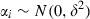 . The within‐subjects errors εij were assumed to be distributed
. The within‐subjects errors εij were assumed to be distributed 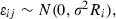 . The within‐subjects errors εij were assumed to be distributed
. The within‐subjects errors εij were assumed to be distributed 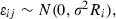 where R
i was the correlation matrix for the residuals. It was also assumed αi and εij were independent of each other.
where R
i was the correlation matrix for the residuals. It was also assumed αi and εij were independent of each other.
The first‐order autoregressive structure, denoted by AR(1), was explored for the covariance R i in the analysis, which implies observations closer to each other in time exhibit higher correlation than observations farther apart in time. 17 The likelihood ratio test showed the AR(1) correlation structure did not improve the fit. Other available covariates including season (ie, winter vs summer), neighborhood indicator, and the patient’s study day (eg, first vs second day of monitoring during the 5‐day period) were not included in the final model as they did not predict responses individually or alter the significance of any results. The analysis was performed by function linear mixed‐effects model in R–2.8.1 for Windows. Statistical significance was defined as P<.05.
Results
Fifty‐one patients participated (10 patients during 2 consecutive sessions [winter and summer], for a total of 61 patient‐observation periods). As patients could contribute up to 5 days of data per session, there were a total of 268 available observation days. Seventy‐six (28%) observations were not included due to patients not meeting the vest compliance rule. Among the 44 patients (192 observations) meeting vest compliance (vest group), 35 participants (103 observations) also met the SHS rule (vest‐low SHS group). We used this vest‐low SHS cohort for the main results in order to assure no confounding effect of SHS (Table). However, the PET‐BP associations from the vest group (larger cohort not accounting for SHS) were similar in magnitude and statistical significance. CV outcomes, beginning with BP measurement (mean time, 6:14 pm), were performed starting at 5 pm, 6 pm, and 7 pm in 22%, 26%, and 26% of patients, respectively. The other 26% of patients had measurements from 4 pm to 5 pm (10%) or 8 pm to 10 pm (16%).
Table TABLE.
Patient Characteristics (N=31) in the Vest and Low SHS Compliant Group
| Factor | Observations, No. | Mean or % | SD | Minimum | Q1 | Median | Q3 | Maximum |
|---|---|---|---|---|---|---|---|---|
| Age, y | 31 | 45.1 | 14.0 | 22 | 33 | 45 | 54 | 73 |
| Sex | 31 | |||||||
| Female | 26 | 84% | ||||||
| Male | 5 | 16% | ||||||
| Race | 31 | |||||||
| African American | 13 | 42% | ||||||
| Caucasian | 17 | 55% | ||||||
| American Indian | 1 | 3% | ||||||
| Body mass index, kg/m2 | 31 | 30.1 | 5.9 | 21.8 | 25.3 | 29.9 | 34.7 | 48.2 |
| SBP, mm Hg | 101 | 123.0 | 16.4 | 91 | 110 | 124 | 136 | 167 |
| DBP, mm Hg | 101 | 73.4 | 10.4 | 53 | 65 | 73 | 81 | 101 |
| HR beats per min | 101 | 73.8 | 10.2 | 51 | 67 | 74 | 79 | 100 |
| BAD, mm | 99 | 4.0 | 0.9 | 2.5 | 3.4 | 3.9 | 4.6 | 6.2 |
| FMD, % | 94 | 4.0 | 5.2 | −6.0 | 0.4 | 2.8 | 6.9 | 18.3 |
| NMD, % | 48 | 15.4 | 7.1 | 1.5 | 10.1 | 15.5 | 19.5 | 31.9 |
| Average of 24 hourly personal ET (celsius) | 103 | 26.5 | 3.0 | 19.5 | 24.4 | 27.4 | 28.6 | 33.7 |
| Daily average of community ET (celsius) | 103 | 17.6 | 11.9 | ‐12.9 | 5.0 | 23.3 | 24.8 | 30.8 |
Abbreviations: BAD, brachial artery diameter; BP, blood pressure; DBP, diastolic BP; ET, environmental temperature; FMD, flow‐mediated dilatation; HR, heart rate; NMD, nitroglycerin‐mediated dilatation; Q1, quartile 1 (25th percentile); Q3, quartile 3 (75th percentile); SBP, systolic BP; SD, standard deviation.
PET values were generally higher and with less variation than community levels (Table), likely because patients spent on average only 3.5% of each day outside per self‐report. However, there was still a broad range (19.5°C–33.7°C) in PET hourly values. On average, the levels were generally stable except for lower temperatures starting at the period that ended 9 to 12 hours before CV outcomes during the summer (Figure 2). This corresponded to the expected colder outside temperature penetrating indoor during summer nighttimes and early mornings. Similar variations were not observed during the winter. This pattern likely reflected the higher availability of home heating units (100%) compared with fewer households with air conditioning (29% central air: 77% some combination of central air and/or individual window units).
Figure 2.
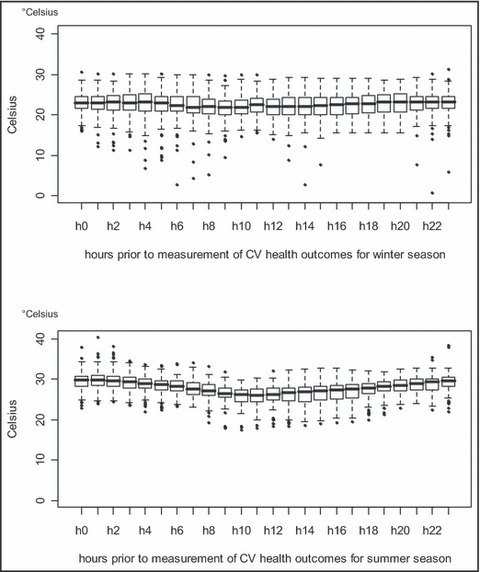
Twenty four–hour personal‐level environmental temperature (PET) values. Graph of the PET levels for the 24‐hour period preceding the cardiovascular (CV) outcome measurements. Lines and boxes represent the median and interquartile ranges, respectively. Graphed confidence intervals are set at 95% with individual outliers presented as data points. h indicates hour.
Season (winter vs summer), community‐level outdoor temperature during the same or preceding day, and PET averaged over the entire preceding 24‐hour period were not associated with changes in any outcome. Hourly PET values were also not significantly associated with heart rate or FMD (data not shown). On the other hand, several hour‐long PET values with measurements ending from 10 to 15 hours prior to obtaining the CV outcomes were significantly positively associated with systolic (Figure 3) and diastolic (Figure 4) BP levels (measured on the ensuing day mostly between 5 pm–7 pm). An increase in PET of 1°C during this corresponding time (starting mostly between 1 am–3 am and ending between 7 am–9 am) was associated with a 0.81‐mm Hg to 1.44‐mm Hg and 0.59‐mm Hg to 0.83‐mm Hg elevation in systolic and diastolic BP, respectively, in the fully adjusted multivariate mixed model. One interquartile range (IQR) increase in PET (4.8°C) had a significant effect on BP during many of these hourly periods (eg, a 6.95‐mm Hg systolic BP elevation for lag hour 10). The associations during this time window occurred without a threshold temperature value (ie, below or above which the positive significant association disappeared) and regardless of the absolute PET value, meaning increases from 25°C to 29.8°C caused similar BP elevations as increases from 30°C to 34.8°C.
Figure 3.
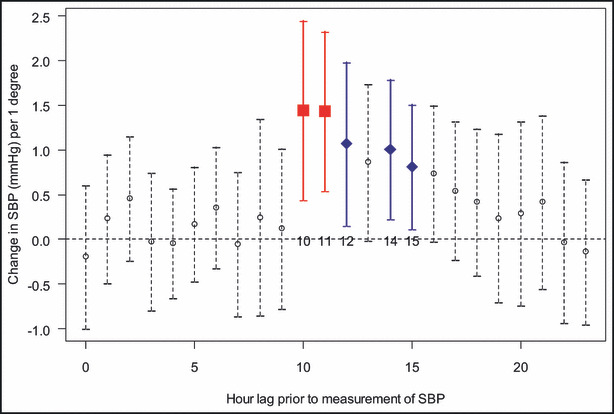
Associations between hourly personal‐level environmental temperature (PET) values and systolic blood pressure (SBP). The associations between systolic BP and each hourly (h) PET value measured during the preceding 24 hours. For example, lag hour 0=period from 0 to 60 minutes before CV outcomes were measured. Lag hour 10=period from 10 to 11 hours beforehand. Since BP was measured in most patients at a time between 5 pm and 7 pm, this means lag hour 10 corresponds to a 1‐hour period beginning from 6 am to 8 am and ending from 7 am to 9 am. Lag hour 15 corresponds to a 1‐hour period beginning from 1 am to 3 am and ending from 2 am to 4 am. Points indicate linear PET‐BP associations (coefficient β1, change in BP per 1°C) and vertical line segments are the 95% confidence intervals for each hourly time point from the full (multivariate) linear mixed model (1). Statistically significant time points are bolded in square shape for P<.01 and in diamond shape for P<.05.
Figure 4.
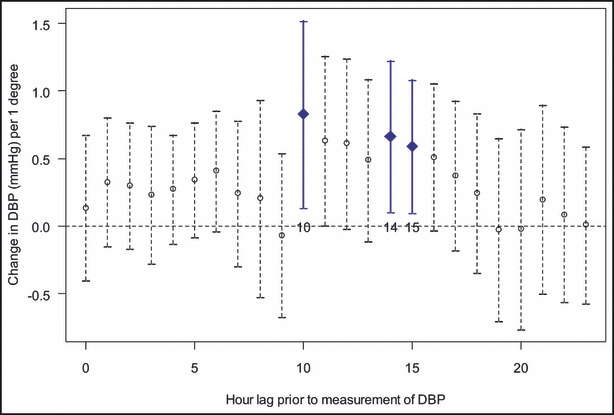
Associations between hourly personal‐level environmental temperature (PET) values and diastolic blood pressure (BP). The associations between diastolic BP and each hourly (h) PET value measured during the preceding 24 hours. Points indicate linear PET‐BP associations (coefficient β1, change in BP per 1°C) and vertical line segments are the 95% confidence intervals for each hourly time point from the full (multivariate) linear mixed model (1). Statistically significant time points are bolded in square shape for P<.01 and in diamond shape for P<.05.
These results accounted for daily average community‐level outside temperature and were not affected by adding any other covariate to the model (eg, season) or by the self‐reported time spent outdoors. The observed effect sizes were similar in magnitude when analyzed separately by winter and summer periods. Personal fine particulate matter levels during the same period did not alter the PET‐BP association. While technically no covariate was found to be a risk‐effect modifier (ie, nonsignificant interaction terms), the BP responses tended to be larger for older, more hypertensive, and overweight patients. For example, during lag hour 11 the effect of 1°C on systolic BP was greater (not significant due to lack of power) for patients above compared with those below the median values for age (44 years, 2.5 mm Hg vs 0.44 mm Hg), systolic BP (124 mm Hg, 2.2 mm Hg vs 0.81 mm Hg), and body mass index (29.9 mg/kg2, 1.8 mm Hg vs 0.6 mm Hg). Forty‐one patients (80%) were not taking any BP medications, while 2 (4%), 6 (12%), 1 (2%), and 1 patient (2%) were taking 1, 2, 4, and 5 medications, respectively. Overall, accounting for usage of any BP medication slightly enhanced the PET‐BP associations (data not shown). Importantly, there were no significant differences in mean PET levels (24‐hour average; lag hour 10–15; or from midnight to 6 am) between patients defined as hypertensive (basal systolic BP >140 mm Hg) or normotensive. This suggests that our observed PET‐BP association is not explained by hypertensive patients seeking to increase their PET exposure (ie, turning up indoor temperatures at night).
The evaluation of many different time intervals or durations or moving average time periods of exposures (eg, 2–8 hours epochs) did not provide additional insights. Finally, detailed exploration of the individual‐level data revealed that there were no specific individual patients, temperature values, or data points that heavily influenced or were responsible for the statistical significance of the results.
Discussion
Numerous studies have demonstrated an inverse relationship between outside community‐level outdoor temperature and BP. 2 , 3 , 4 , 5 , 6 Indoor temperature measured by stationary monitors placed within households has been shown to be even more strongly (inversely) related to BP, perhaps because people spend a large portion of time inside. 4 Hence, accurate estimation of the “true” environmental temperature actually encountered is critical in order to optimally assess the temperature‐BP relationship. This is the first study to evaluate the effect on BP of short‐term temperature exposures measured hourly and also at the personal level throughout a day during routine activities. Our hypothesis‐generating exploration found a novel positive association between higher nighttime and early‐morning PET exposures with an elevation in BP during the ensuing day. This response occurred without a threshold temperature, during winter and summer, and was not altered by outside weather. In clinical perspective, commonly encountered modestly warmer PET (interquartile range, 4.83°C) could raise systolic BP by 6.95 mm Hg. There was a trend for even larger elevations to occur in patients who were older and heavier and who had higher basal BP levels. We hypothesize that exposure to relatively warmer morning and nighttime PET values could adversely affect ensuing‐day BP control. Given the importance of this issue, follow‐up studies are warranted to corroborate this novel observation.
Biological Mechanisms
Cold‐induced elevations in BP have been ascribed to changes in sympathetic nervous system activity, endothelial function, thermoregulatory defenses, adrenal gland activity, and fluid shifts. 2 , 5 , 18 , 19 Additional pertinent changes accompanying longer‐term reductions during winter could include lower vitamin D, decreased physical activity, and weight gain. One potential biological mechanism we explored was temperature change–induced endothelial dysfunction. 11 As FMD was not related to either outdoor or PET, changes in endothelial‐dependent vasodilatation do not likely explain our findings.
At least one previous study has also reported a positive association whereby mean daily community‐level outdoor temperature was related to higher nocturnal BP among elderly patients treated for hypertension. 3 However, the authors suggested that lower daytime BP values during warmer weather (as they observed) may have lessened antihypertensive agent usage in these individuals and thus weakened 24‐hour BP control (exemplified by higher nighttime pressures). This cannot be the basis for our findings. While we explored more acute BP effects of briefer PET periods compared with previous studies, even rapid brief experimental cooling of skin or body temperature has been shown to typically lead to thermoregulatory vasoconstriction (and increased BP) within minutes. 18 , 19 In this regard, the most recently encountered hour‐long PET ending from 0 to 9 hours beforehand were unrelated to BP in our study. Hence, the distinctive temporal features of our study design alone cannot explain our results.
There appears to be some unique aspect and physiological response related to relatively warmer PET encountered >9 hours. We find it intriguing that this time window corresponds to a period during the early morning and nighttime in all patients. The mechanism(s) responsible must remain speculative. However, we hypothesize that perhaps there is an adverse effect of higher PET values during this time on sleep quality (or stages of sleep related to BP control), particularly during the early morning when the effects were slightly larger. There is a complex interaction between environmental temperature exposure, skin/core body temperature, and sleep. 20 , 21 Nonetheless, it has been demonstrated that higher nocturnal temperature exposures can impair sleep quality, particularly in elderly persons. 20 In this context, it is well‐established that reduced sleep duration and/or quality, even in the absence of overt sleep‐related breathing disorders, are capable of elevating daytime BP. 22 , 23 , 24 Recent findings from a sleep disorders breathing study in 7 US urban areas bolster our hypothesis. Indeed, higher ambient temperatures were predictive of impaired sleep quality as evidenced by worsening of the respiratory disturbance index. 25 Future studies are required to clarify whether this is the physiological mechanism involved.
Strengths and Limitations
It is unclear why we did not observe the expected inverse association between daily community‐level outdoor temperature and BP. It may be explained by our sample size being smaller than previous studies 3 , 4 , 5 , 6 or because patients spent the majority of time indoors. Nonetheless, our use of PET measurements likely greatly reduced exposure misclassification compared with earlier studies. In addition, the positive PET‐BP associations occurred at much shorter time windows of exposure than previous studies. There is no reason why a 24‐hour mean should be the environmental temperature most strongly associated with BP from a biological standpoint, hence the rationale for our study and a potential explanation for our findings. This is also the first study to explore the effect on CV outcomes of both outdoor‐community and PET values, with the latter measured hourly (with greater temporal resolution than ever before) and during routine daily activity. The CV outcomes were additionally determined in the households of patients, obviating extraneous activities/exposures that might have otherwise affected the CV outcomes (eg, stressor in traffic) encountered during the commute to a research laboratory. We were also able to evaluate and account for (as needed) the effects of personal and environmental covariates (eg, outdoor temperature, season, time of day, personal‐level particulate pollution, SHS). We believe it is unlikely that the findings represent a spurious association confounded by unmeasured factors. Moreover, detailed evaluation of individual‐level data does not demonstrate outlying patients, responses, or temperatures responsible for the overall positive association.
We did not have an hourly activity log and could not account for activities during the pertinent time window (approximately 7–9 am and 1–3 am). While conceivable that the positive PET‐BP associations were confounded by a relationship to temporally correlated changes in activities that raised BP 10 hours later, we believe this to be extremely unlikely. First, we controlled for the time period per se (5–9 am) associated with early‐morning activity (ie, the BP surge). Controlling for this time period or the actual time of day itself had no significant effect on the reported PET‐BP associations. It is important to clarify that we did not demonstrate BP during this early‐morning period itself to be elevated (eg, this is not a morning BP surge), rather we found that an increase in PET during this nighttime and early‐morning period was associated with higher BP 10 to 15 hours later. Second, the time window associated with elevated BP was consistent for most subsets of patients and during a period typically associated with similar day‐to‐day activity (ie, sleep). Last, physical activities do not generally cause elevations in BP that persist 10 hours later; if anything, BP is typically lower hours after increased activity.
This study was a post hoc exploratory analysis. While we cannot exclude the possibility of type I errors given the multiple comparisons, the consistency of the BP elevations that occurred during the consecutive hourly periods in the early‐morning and nighttime periods (ie, a coherent and plausible time window) during both the winter and summer, which also included several additional borderline nonsignificant associations, lends veracity to our interpretation of the findings. Regardless, this analysis was performed as a hypothesis‐generating study; hence, statistical significance is not a critical factor in this context without predetermined primary outcomes according to study power/design. As such, we recognize that future studies are needed to corroborate these initial results and better characterize the nature of the BP response (eg, onset, duration). Unfortunately, ambulatory BP monitoring was not part of the DEARS, which should be included in any future studies.
We do not have data regarding PET at actual skin level (ie, underneath clothing) because the thermometer was worn on a vest outside of clothing or kept by the bedside at nighttime. It is possible that we may have observed differing responses if actual skin‐level temperature were evaluated. However, this limitation does not likely alter the veracity of our reported findings. For example, the significant PET‐BP association occurred during both seasons (ie, regardless of differences in clothing) and was affected by temperatures determined indoors 96.5% of the time when clothing differences in response to relatively smaller environmental temperature changes will likely be comparatively smaller than during time spent outdoors. The effect of skin‐level temperature on BP may be complicated by the added relationship of body core temperature altering skin blood flow and thereby temperature (due to many factors such as activity or thermogenesis). Future studies are required to determine whether there are differences in skin‐level vs PET measured outside clothing as in our study on the association with BP.
Finally, PET was linearly positively associated with BP without a threshold during both seasons. This might potentially be explained by the limited range in PET compared with outdoor levels. It remains possible that the inclusion of more extreme values (particularly lower PET) could alter the shape of the PET‐BP relationship and produce a U‐shaped curve, whereby further lowering of nighttime and early‐morning PET causes an elevation in BP.
Conclusions
Our exploratory analysis illustrates that brief personal exposures to modestly warmer and commonly encountered early‐morning and nighttime temperatures could pose a clinically meaningful effect by raising BP during the ensuing day. This novel relationship could have important implications for the management of high BP and warrants more definitive follow‐up studies.
Disclosure: The US EPA, through its Office of Research and Development, partially funded and conducted the research under contract 68‐D‐00‐012 (RTI International), EP‐D‐04‐068 (Battelle Columbus Laboratory), 68‐D‐00‐206, and EP‐05‐D‐065 (Alion Science and Technology). It has been subjected to agency review and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. This study was also supported by the Electric Power Research Institute (Contract EP‐P15887/C7915) and from a National Institutes of Health General Clinical Research Center Grant: M01‐RR000042.
References
- 1. Brook RD, Rajagopalan S. Particulate matter, air pollution, and blood pressure. J Am Soc Hypertens. 2009;3:332–350. [DOI] [PubMed] [Google Scholar]
- 2. Hanna JM. Climate, altitude, and blood pressure. Hum Biol. 1999;71:4. [PubMed] [Google Scholar]
- 3. Modesti PA, Morabito M, Bertolozzi I, et al. Weather‐related change in 24‐hour blood pressure profile. Effects of age and implications for hypertension management. Hypertension. 2006;47:1–7. [DOI] [PubMed] [Google Scholar]
- 4. Barnett AG, Sans S, Salomaa V, et al. The effect of temperature on systolic blood pressure. Blood Press Monit. 2007;12:195–203. [DOI] [PubMed] [Google Scholar]
- 5. Alpérovitch A, Lacombe J‐M, Hanon O, et al. Relationship between blood pressure and outdoor temperature in a large sample of elderly individuals. The Three‐City Study. Arch Intern Med. 2009;169:75–80. [DOI] [PubMed] [Google Scholar]
- 6. Madsen C, Nafstsad P. Associations between environmental exposure and blood pressure among participants in the Oslo Health Study (HUBRO). Eur J Epidemiol. 2006;21:485–491. [DOI] [PubMed] [Google Scholar]
- 7. Wolf K, Schneider A, Breitner S, et al. Air temperature and the occurrence of myocardial infarction in Augsburg, Germany. Circulation. 2009;120:735–742. [DOI] [PubMed] [Google Scholar]
- 8. Dilaveris P, Synetos A, Giannopoulos G, et al. Climate impacts on myocardial infarction deaths in the Athens Territory: the CLIMATE study. Heart. 2006;92:1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gerber Y, Jacobsen SJ, Killian JM, et al. Seasonality and daily weather conditions in relation to myocardial infarction and sudden cardiac death in Olmsted County, Minnesota, 1979 to 2002. J Am Coll Cardiol. 2006;48:287–292. [DOI] [PubMed] [Google Scholar]
- 10. Brook RD, Bard RL, Burnett RT, et al. Differences in blood pressure and vascular responses associated with ambient fine particulate matter exposures measured at the personal versus community level. Occup Environ Med. 2011;68:224–230. [DOI] [PubMed] [Google Scholar]
- 11. Widlansky ME, Vita JA, Keyes MJ, et al. Relation of season and temperature to endothelium‐dependent flow‐mediated vasodilation in subjects withough clinical evidence of cardiovascular disease (from the Framingham Heart Study). Am J Cardiol. 2007;100:518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodes CE, Lawless PA, Thornburg JW, et al. DEARS particulate matter relationships for personal, indoor, outdoor, and central site settings for a general population. Atmos Environ. 2010;44:1386–1399. [Google Scholar]
- 13. Brook RD, Urch B, Dvonch JT, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brook RD, Grau M, Kehrer C, Rajagopalan S Intra‐subject variability of radial artery flow mediated dilatation: implications for use in prospective clinical trials. Am J Cardiol. 2005;96:1345–1348. [DOI] [PubMed] [Google Scholar]
- 15. Harris RA, Nishiyama SK, Wray W, Richardson RS Ultrasound assessment of flow‐mediated dilation. Hypertension. 2010;55:1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. West BT, Welch KB, Galecki AT. Linear Mixed Models, New York, NY: Chapman & Hall/CRC; 2007. [Google Scholar]
- 17. Shoukri MM, Chaudhary MA. Analysis of Correlated Data with SAS and R. 3rd ed. New York, NY: Chapman & Hall/CRC; 2007. [Google Scholar]
- 18. Greif R, Laciny S, Rajek A, et al. Blood pressure response to thermoregulatory vasoconstriction during isoflurane and desflurane anesthesia. Acta Anaesthesiol Scand. 2003;47:847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hess KL, Wilson TE, Sauder CL, et al. Aging affects the cardiovascular responses to cold stress in humans. J Appl Physiol. 2009;107:1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okamoto‐Mizuno K, Tsuzuki K, Mizuno K. Effects of mild heat exposure on sleep stages and body temperature in older men. Int J Biometerol. 2004;49:32–36. [DOI] [PubMed] [Google Scholar]
- 21. Raymann RJEM, Swaab DF, Van Someren EJW. Skin deep: enhanced sleep depth by cutaneous temperature manipulation. Brain. 2008;131:500–513. [DOI] [PubMed] [Google Scholar]
- 22. Friedman O, Bradley TD, Ruttanaumpawan P, Logan AG. Independent association of drug‐resistant hypertension to reduced sleep duration and efficiency. Am J Hypertens. 2010;23:174–179. [DOI] [PubMed] [Google Scholar]
- 23. Knutson KL, Van Cauter E, Rathouz PJ, et al. Association between sleep and blood pressure in midlife. The CARDIA sleep study. Arch Intern Med. 2009;169:1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gangwisch JE, Heymsfield SB, Boden‐Albala B, et al. Short sleep duration as a risk factor for hypertension. Hypertension. 2006;47:1–7. [DOI] [PubMed] [Google Scholar]
- 25. Zanobetti A, Redline S, Schwartz J, et al. Associations of PM10 with sleep and sleep‐disordered breathing in adults from seven U.S. urban areas. Am J Respir Crit Care Med. 2010;182:819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]


