Abstract
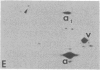
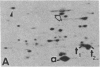
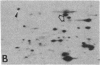
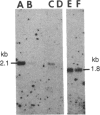
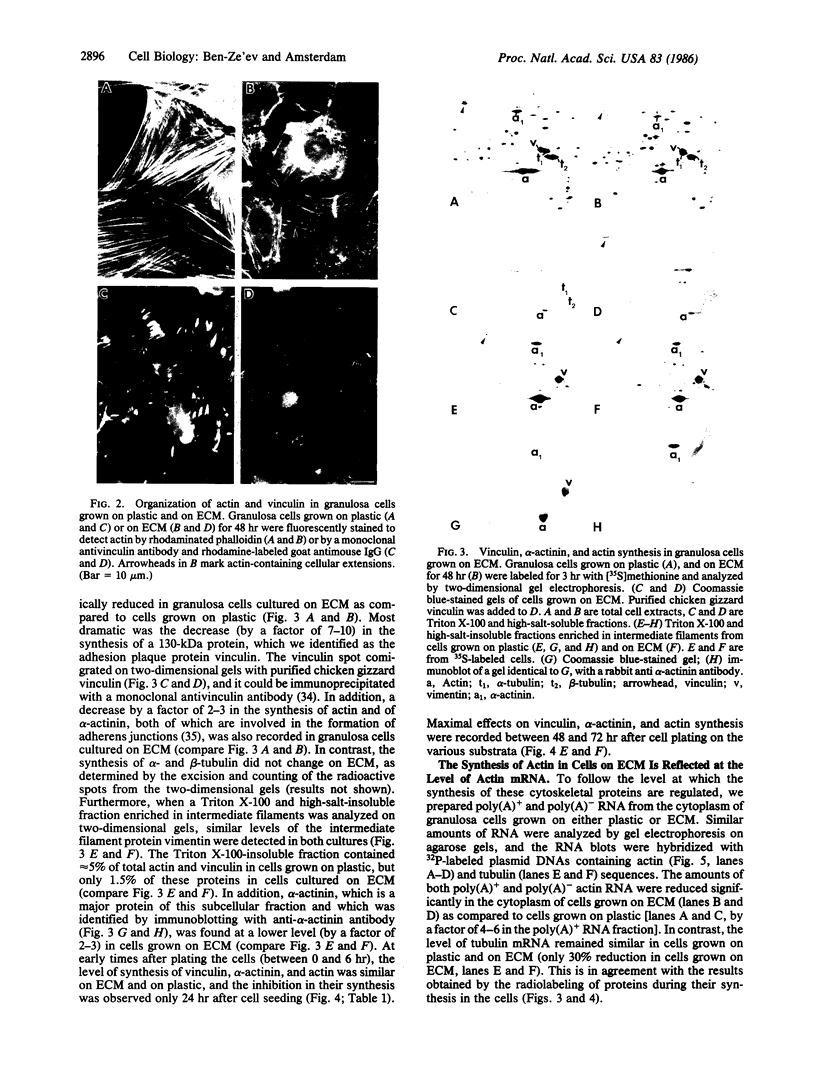
The organization and the expression of cytoskeletal proteins involved in determining cell contact and shape were analyzed in granulosa cells during their differentiation on extracellular matrix (ECM)-coated culture plates. Rat granulosa cells from preovulatory follicles displayed an epithelial shape on ECM and formed multilayered aggregates with numerous gap junctions between neighboring cells. These cells had few actin cables and often only a diffuse pattern of actin and a low amount of vinculin in very thin focal adhesion sites. In contrast, cells grown on plastic formed a monolayer of flat cells with a reduced number of gap junctions but with numerous stress fibers and abundant large vinculin-containing focal contacts. On ECM, the cells were stimulated to produce high levels of progesterone, while only trace amounts of the steroid accumulated in cells on plastic dishes. Two-dimensional gel electrophoretic analysis of [35S]methionine-labeled cells revealed a dramatic decrease in vinculin, alpha-actinin, and actin synthesis in cells grown on ECM, as compared to cells grown on plastic, while the synthesis of the tubulins and of the intermediate filament protein vimentin remained unchanged. RNA blot analysis showed a marked decrease in actin mRNA levels in cells from ECM plates, while the level of tubulin mRNA remained essentially unchanged. It is concluded that the differentiation of granulosa cells on ECM in vitro is associated with changes in cell shape and cell contacts and that such changes in cell morphology are accompanied by simultaneous alterations in the organization and expression of cytoskeletal proteins that are involved in determining these cellular structures.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini D. F., Anderson E. The appearance and structure of intercellular connections during the ontogeny of the rabbit ovarian follicle with particular reference to gap junctions. J Cell Biol. 1974 Oct;63(1):234–250. doi: 10.1083/jcb.63.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan M., Harrison P. Co-expression of differentiation markers in hybrids between Friend cells and lymphoid cells and the influence of the cell shape. Cell. 1980 Feb;19(2):437–447. doi: 10.1016/0092-8674(80)90518-8. [DOI] [PubMed] [Google Scholar]
- Amsterdam A., Josephs R., Lieberman M. E., Lindner H. R. Organization of intramembrane particles in freeze-cleaved gap junctions of rat graafian rollicles: optical-diffraction analysis. J Cell Sci. 1976 Jun;21(1):93–105. doi: 10.1242/jcs.21.1.93. [DOI] [PubMed] [Google Scholar]
- Amsterdam A., Knecht M., Catt K. J. Hormonal regulation of cytodifferentiation and intercellular communication in cultured granulosa cells. Proc Natl Acad Sci U S A. 1981 May;78(5):3000–3004. doi: 10.1073/pnas.78.5.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A., Koch Y., Lieberman M. E., Lindner H. R. Distribution of binding sites for human chorionic gonadotropin in the preovulatory follicle of the rat. J Cell Biol. 1975 Dec;67(3):894–900. doi: 10.1083/jcb.67.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A. Cell configuration-related control of vimentin biosynthesis and phosphorylation in cultured mammalian cells. J Cell Biol. 1983 Sep;97(3):858–865. doi: 10.1083/jcb.97.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A. Cell shape, the complex cellular networks, and gene expression. Cytoskeletal protein genes as a model system. Cell Muscle Motil. 1985;6:23–53. doi: 10.1007/978-1-4757-4723-2_2. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A. Differential control of cytokeratins and vimentin synthesis by cell-cell contact and cell spreading in cultured epithelial cells. J Cell Biol. 1984 Oct;99(4 Pt 1):1424–1433. doi: 10.1083/jcb.99.4.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Farmer S. R., Penman S. Mechanisms of regulating tubulin synthesis in cultured mammalian cells. Cell. 1979 Jun;17(2):319–325. doi: 10.1016/0092-8674(79)90157-0. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Farmer S. R., Penman S. Protein synthesis requires cell-surface contact while nuclear events respond to cell shape in anchorage-dependent fibroblasts. Cell. 1980 Sep;21(2):365–372. doi: 10.1016/0092-8674(80)90473-0. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Raz A. Multinucleation and inhibition of cytokinesis in suspended cells: reversal upon reattachment to a substrate. Cell. 1981 Oct;26(1 Pt 1):107–115. doi: 10.1016/0092-8674(81)90038-6. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A. Virus replication in infected epithelial cells is coupled to cell shape-responsive metabolic controls. J Cell Physiol. 1983 Feb;114(2):145–152. doi: 10.1002/jcp.1041140202. [DOI] [PubMed] [Google Scholar]
- Benecke B. J., Ben-Ze'ev A., Penman S. The control of mRNA production, translation and turnover in suspended and reattached anchorage-dependent fibroblasts. Cell. 1978 Aug;14(4):931–939. doi: 10.1016/0092-8674(78)90347-1. [DOI] [PubMed] [Google Scholar]
- Benya P. D., Shaffer J. D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982 Aug;30(1):215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bissell M. J., Hall H. G., Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982 Nov 7;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Campbell K. L. Ovarian granulosa cells isolated with EGTA and hypertonic sucrose: cellular integrity and function. Biol Reprod. 1979 Nov;21(4):773–786. doi: 10.1095/biolreprod21.4.773. [DOI] [PubMed] [Google Scholar]
- Chang C., Lin Y., O-Lee T. W., Chou C. K., Lee T. S., Liu T. J., P'eng F. K., Chen T. Y., Hu C. P. Induction of plasma protein secretion in a newly established human hepatoma cell line. Mol Cell Biol. 1983 Jun;3(6):1133–1137. doi: 10.1128/mcb.3.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. T., Singer S. J. Immunoelectron microscopic studies of the sites of cell-substratum and cell-cell contacts in cultured fibroblasts. J Cell Biol. 1982 Oct;95(1):205–222. doi: 10.1083/jcb.95.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson J. W., Hay E. D. Secretion of collagen by corneal epithelium. II. Effect of the underlying substratum on secretion and polymerization of epithelial products. J Exp Zool. 1974 Jul;189(1):51–72. doi: 10.1002/jez.1401890106. [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Burwen S. J., Pitelka D. R. Substrate properties influencing ultrastructural differentiation of mammary epithelial cells in culture. Tissue Cell. 1979;11(1):109–119. doi: 10.1016/0040-8166(79)90011-9. [DOI] [PubMed] [Google Scholar]
- Farmer S. R., Ben-Ze'av A., Benecke B. J., Penman S. Altered translatability of messenger RNA from suspended anchorage-dependent fibroblasts: reversal upon cell attachment to a surface. Cell. 1978 Oct;15(2):627–637. doi: 10.1016/0092-8674(78)90031-4. [DOI] [PubMed] [Google Scholar]
- Farmer S. R., Ben-Ze'av A., Benecke B. J., Penman S. Altered translatability of messenger RNA from suspended anchorage-dependent fibroblasts: reversal upon cell attachment to a surface. Cell. 1978 Oct;15(2):627–637. doi: 10.1016/0092-8674(78)90031-4. [DOI] [PubMed] [Google Scholar]
- Farmer S. R., Wan K. M., Ben-Ze'ev A., Penman S. Regulation of actin mRNA levels and translation responds to changes in cell configuration. Mol Cell Biol. 1983 Feb;3(2):182–189. doi: 10.1128/mcb.3.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- GROBSTEIN C. Morphogenetic interaction between embryonic mouse tissues separated by a membrane filter. Nature. 1953 Nov 7;172(4384):869–870. doi: 10.1038/172869a0. [DOI] [PubMed] [Google Scholar]
- Gebauer H., Lindner H. R., Amsterdam A. Synthesis of heparin-like glycosaminoglycans in rat ovarian slices,. Biol Reprod. 1978 Apr;18(3):350–358. doi: 10.1095/biolreprod18.3.350. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowacki J., Trepman E., Folkman J. Cell shape and phenotypic expression in chondrocytes. Proc Soc Exp Biol Med. 1983 Jan;172(1):93–98. doi: 10.3181/00379727-172-41533. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Delgado D., Vlodavsky I. Permissive effect of the extracellular matrix on cell proliferation in vitro. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4094–4098. doi: 10.1073/pnas.77.7.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Birdwell C. R. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978 Nov;38(11 Pt 2):4155–4171. [PubMed] [Google Scholar]
- Greenburg G., Hay E. D. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982 Oct;95(1):333–339. doi: 10.1083/jcb.95.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh A. J., Adashi E. Y., Jones P. B., Welsh T. H., Jr Hormonal regulation of the differentiation of cultured ovarian granulosa cells. Endocr Rev. 1984 Winter;5(1):76–127. doi: 10.1210/edrv-5-1-76. [DOI] [PubMed] [Google Scholar]
- Knecht M., Amsterdam A., Catt K. The regulatory role of cyclic AMP in hormone-induced of granulosa cell differentiation. J Biol Chem. 1981 Oct 25;256(20):10628–10633. [PubMed] [Google Scholar]
- Lawrence T. S., Ginzberg R. D., Gilula N. B., Beers W. H. Hormonally induced cell shape changes in cultured rat ovarian granulosa cells. J Cell Biol. 1979 Jan;80(1):21–36. doi: 10.1083/jcb.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S., Hay E. D. Control of corneal differentiation by extracellular materials. Collagen as a promoter and stabilizer of epithelial stroma production. Dev Biol. 1974 Jun;38(2):249–270. doi: 10.1016/0012-1606(74)90005-0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Spiegelman B. M., Farmer S. R. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell. 1982 May;29(1):53–60. doi: 10.1016/0092-8674(82)90089-7. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Ginty C. A. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983 Dec;35(3 Pt 2):657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- Sugrue S. P., Hay E. D. Response of basal epithelial cell surface and Cytoskeleton to solubilized extracellular matrix molecules. J Cell Biol. 1981 Oct;91(1):45–54. doi: 10.1083/jcb.91.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek J. J., Hay E. D., Fujiwara K. Collagen modulates cell shape and cytoskeleton of embryonic corneal and fibroma fibroblasts: distribution of actin, alpha-actinin, and myosin. Dev Biol. 1982 Jul;92(1):107–122. doi: 10.1016/0012-1606(82)90155-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller L. C., Weisz J. Identification of cytochrome P-450, and its distribution in the membrana granulosa of the preovulatory follicle, using quantitative cytochemistry. Endocrinology. 1978 Jul;103(1):310–313. doi: 10.1210/endo-103-1-310. [DOI] [PubMed] [Google Scholar]