Abstract
Objective
The contralateral knee of those with unilateral endstage hip OA is known to be at greater risk for endstage knee OA compared to the ipsilateral, same side knee. Likewise, in endstage hip OA, this contralateral knee is known to have increased dynamic joint loads compared to the ipsilateral knee. Here, we study a population with unilateral hip OA, who are asymptomatic at the knees, for early asymmetries in knee loading.
Methods
Data from 62 subjects with unilateral hip OA were evaluated. Subjects underwent gait analyses for evaluation of dynamic knee loads as well as dual energy X-ray absorptiometry for evaluation of bone mineral density (BMD) at both knees. Differences between knees were compared.
Results
Peak dynamic knee loads were significantly higher at the contralateral knee compared to the ipsilateral knee (2.46±0.71 vs 2.23±0.81 %BW*ht, p=0.029). Similarly, medial compartment tibial BMD was significantly higher at the contralateral knee compared to the ipsilateral knee (0.897±0.208 vs 0.854±0.206 gm/c2, p=0.033). Interestingly, there was a direct correlation between contralteral:ipsilateral dynamic knee load and contralateral:ipsilateral medial compartment tibial BMD (Spearman’s rho= 0.287, p=0.036).
Conclusions
This study demonstrates that at the contralateral knees of patients with unilateral hip OA, which are at higher risk of developing progressive symptomatic OA compared to the ipsilateral knees, loading and structural asymmetries appear early in the disease course, while the knees are still asymptomatic. These early biomechanical asymmetries may have corresponding long term consequences, providing further support for the potential role of loading in OA onset and progression.
Introduction
Osteoarthritis (OA) is the most common arthropathy worldwide and a major cause of disability and impaired quality of life. It is a chronic, slowly progressive arthropathy; hence, longitudinal studies evaluating its natural history are lengthy, costly, and often impractical to perform. Although several factors have been associated with incident OA and with OA disease severity, it has been difficult to establish both their sequence of onset and whether they are causally involved in OA pathophysiology.
The role of biomechanics has been an important area of investigation in OA, especially dynamic joint loading during physiologic activity(1). The peak external knee adduction moment (PaddM), a validated gait parameter that reflects the load at the medial compartment of the knee(2), has been associated with pain, radiographic severity, and progression of knee OA (3–5). Although one study demonstrated that high adduction moments preceded the onset of knee pain symptoms(5), most other studies to date have evaluated the relationship between dynamic joint loads and already established OA; as of yet, it has not been clearly shown that elevated peak dynamic loads precede the development of symptomatic knee OA.
In addition to gait analyses and dynamic loading, complementary information is provided by assessing subchondral areal bone mineral density (BMD, g/cm2) at the tibial plateau, which reflects load history across the joint (6–9), and can be assessed non-invasively using dual energy X-ray absorptiometry (DXA).
We previously observed that in endstage unilateral hip OA, the contralateral knee (opposite side from the affected hip) is substantially more likely to develop advanced OA than the ipsilateral knee(10); moreover, we also reported that the contralateral knee was subjected to substantially higher peak dynamic loads than the ipsilateral knee, and that these asymmetries remained constant several years after hip replacement.(11). These previous observations in unilateral hip OA in which the contralateral knee is known to be at elevated risk for developing OA relative to the ipsilateral knee, suggest a unique model to study factors involved in the pathogenesis of early (pre-symptomatic) knee OA.
Herein, using that model (Fig 1), we evaluate dynamic joint loading and proximal tibial BMD in subjects with unilateral hip OA who are asymptomatic at the knees to test the hypotheses that they have elevated dynamic loads and increased tibial BMD at the contralateral knee compared to the ipsilateral knee, and thus demonstrating that these loading asymmetries are present during an early pre-symptomatic state.
Figure 1. Unilateral hip OA study model.
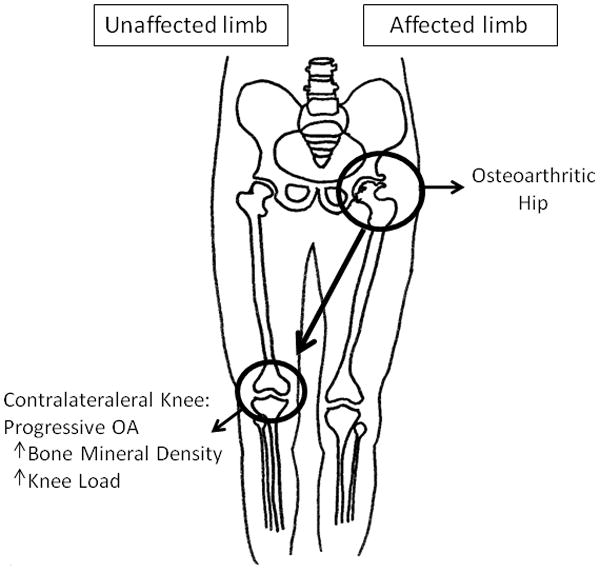
The knee contralateral to the affected hip was observed to have higher dynamic joint loads and higher medial tibial BMD compared to the ipsilateral knee.
Patients and Methods
Patient
This study was approved through the institution’s review board for studies involving human subjects and written informed consent was obtained from all subjects. Inclusion criteria included the presence of symptomatic OA of the hip, which was defined by the American College of Rheumatology’s Clinical Criteria for Classification and Reporting of OA of the hip(12) and by the presence of at least 30 mm of pain (on a 100 mm scale) while walking (corresponding to question 1 of the visual analog format of the hip-directed Western Ontario and McMaster Universities Arthritis Index (WOMAC))(13). Radiographic OA of the index hip was documented by anterior-posterior radiographs of the pelvis, of grade greater than or equal to 2 as defined by the Kellgren-Lawrence (KL) grading scale(14).
Subjects were excluded if they demonstrated symptomatic OA of the contralateral hip or of either knee, with presence of pain defined as a response of greater than 30 mm (of 100 mm) while walking (corresponding to question 1 of the VAS format of the site-directed WOMAC). Subjects were also excluded if they had evidence of radiographic OA of the contralateral hip or of either knee in excess of grade 3 according to the modified K-L scale. Other exclusion criteria included the inability to walk without assistance, presence of an inflammatory arthropathy, history of any lower extremity joint replacement, and history of trauma or arthroscopy to either knee within the preceding 6 months.
Clinical Assessment
Radiographs
All subjects underwent AP radiographs of the pelvis which were evaluated for KL grade at the hips. All subjects also underwent AP standing knee radiographs that were evaluated for KL grade at the knees. All KL evaluations were performed by a single trained observer (NS).
Pain assessment
Subjects completed the WOMAC visual analog scale for evaluation of pain at both knees and both hips. The WOMAC is the current standard in the analysis of pain and function in lower extremity OA(15–17) and site-directed adaptation of the WOMAC has proven useful and feasible(18;19). The WOMAC scores were normalized to a 100 mm scale.
Gait Analyses
All subjects underwent gait analysis. Gait assessment included collection of three-dimensional kinematics and ground reaction forces using four Qualisys (Innovision Systems, Inc., Columbiaville, MI) optoelectronic cameras with passive markers and a multi-component force plate with a sampling frequency of 120 Hz (Bertec, Columbus, OH). Passive markers were placed at the lateral most aspect of the superior iliac crest, the superior aspect of the greater trochanter, the lateral knee joint line, lateral malleolus, lateral calcaneus, and the head of the fifth metatarsal. For moment calculations, the joint centers of the hip, knee, and ankle were approximated in the lab frontal plane following previously published methods(20;21). The joint center of the ankle was determined to be the midpoint of the distance from the medial to lateral malleolus. The joint center of the knee was determined to be the midpoint of the distance between the medial and lateral joint lines of the tibio-femoral joint. The joint center of the hip was determined to be 2.5 cm distal to the midpoint of the distance between the anterior superior iliac spine and the pubic tubercle.
Subjects were instructed to walk at a self-selected normal speed with their conventional walking shoes on a 2-inch thick wooden pressboard covered with linoleum. Kinetic components calculated using processing software developed by CFTC (Computerized Functional Testing Corporation, Chicago, IL) included frontal plane external joint moments at the knee(22). Gait data from one of two normal walking runs of the unaffected limb was chosen and speed-matched to one of two normal walking runs from the affected limb for comparison. The individual choosing the runs was blinded to the affected side.
The position and force data were then utilized to assess sagittal range of motion at the joints and to calculate three-dimensional external moments using inverse dynamics. The external moments that act on a joint during gait are, according to Newton’s second law, equal and opposite to the net internal moments produced primarily by the muscles, soft tissues, and joint contact forces. The external moments are normalized to the subjects body weight (BW) multiplied by height (Ht) times 100 (%BW*Ht)(23). Peak forces on the lateral and medial compartments of the knees were calculated using gait data in conjunction with a statically determinate model(24).
BMD analyses
Dual energy absorptiometry (DXA, General Electric, Lunar Prodigy 7.53E, Madison, WI) was used to scan the bilateral proximal tibiae. Subjects were supine on the examination table and AP evaluations were performed. The subjects’ legs were internally rotated approximately 15 degrees and held in full extension to ensure that scanning was performed perpendicular to the tibial shaft. In this manner the fibula is clearly evident and tibial bone is not superimposed on fibular bone. Proximal tibial BMD was assessed using a method previously described by Clarke, et al.(9) The Lunar Prodigy software were used to assess the area (cm2), BMC (g), and BMD (g/cm2) for the proximal and lateral regions of interest (ROI) as well as for the distal region of interest in the tibial shaft. The height of each ROI was equal to 10% of the width of the tibial plateau to standardize the measurement for differences in bone size between subjects. The software automatically determined the subperiosteal surface of the tibia to which each ROI extended superiorly. The internal border of the medial and lateral ROI’s extended to intercondylar eminences. The cortical bone of the subchondral plate was excluded from the measurements as sclerosis in this region can affect BMD measurements. Care was taken to avoid including bone from the lip of the tibia, since osteophytes could affect BMD measurements. With the lateral ROI, care was taken not to include the fibula. The medial and lateral regions of interest therefore include subchondral trabecular bone. The repeatability of these measurements has previously been assessed in subjects with mild to moderate knee OA (n=10 subjects each scanned twice). Coefficients of variation after reposition of subjects on the table were 3.8%, 2.0%, 1.5% for the medial, lateral, and distal ROIs, respectively, and 3.0% for the medial-to-lateral ratio(6).
Study endpoints
The primary endpoints for the study were the PAddM (defined as the external adduction moment of greatest magnitude during the stance phase of the gait cycle) and total loading of the medial compartment, and medial compartment BMD as measured by DXA. Secondary endpoints included sagittal plane (flexion-extension) range of motion at the lower extremity joints, hip flexion, adduction, internal and external rotation moments, knee flexion moment, and lateral compartment BMD at the knees.
Statistical Analyses
Statistical analysis was performed using SPSS software. Paired samples t-test was used to compare dynamic joint loads and BMD at the ipsilateral and contralateral knees. Pearson and Spearman correlations were used to evaluate relationships between loading parameters and BMD.
Results
One hundred and twenty-four subjects were screened, and sixty-two fulfilled the inclusion/exclusion criteria and completed the study. Figure 1 illustrates the unilateral hip OA model, to relate the ipsilateral and contralateral associations with the results of this study. Table 1 summarizes general demographic data and baseline characteristics of the subjects. Subjects had a mean age (±SD) of 62±11 years. There were 26 males and 36 females. Complete WOMAC data were missing for one subject. The mean baseline pain scores ± SD (adjusted to 100 mm scale) were higher at the ipsilateral knee compared to the contralateral knee, but were extremely low in both cases, 10±18 mm and 5±8 mm, respectively (p = 0.006).
Table 1.
Subject characteristics
| Age (years) | 62±11 | ||
| Gender (M/F) | 26/36 | ||
| Body Mass Index (kg/m2) | 28±5 | ||
| WOMAC pain visual analog scale (0 to100 mm) | |||
| Affected hip | 37±24 | ||
| Unaffected hip | 6±11 | ||
| Ipsilateral knee | 10±18 | ||
| Contralateral knee | 5±8 | ||
| WOMAC stiffness visual analog scale (0 to 100 mm) | |||
| Affected hip | 42±29 | ||
| Unaffected hip | 9±17 | ||
| Ipsilateral knee | 12±18 | ||
| Contralateral knee | 7±14 | ||
| Kellgren Lawrence grade (0 to 4) | |||
| Affected hip | Unaffected hip | ||
| KL 1 | 0 | 15 | |
| KL 2 | 17 | 35 | |
| KL 3 | 21 | 12 | |
| KL 4 | 24 | 0 | |
| Ipsilateral knee | Contralateral knee | ||
| KL 0 | 20 | 16 | |
| KL 1 | 22 | 21 | |
| KL 2 | 18 | 21 | |
| KL 3 | 2 | 4 | |
Fifty-eight subjects had appropriate gait data available for analyses. Gait data from three of the subjects were excluded due to malfunction of the gait analysis equipment during their visits. Fifty-five subjects had evaluation of bone density at bilateral knees.
Table 2 summarizes gait results from the ipsilateral and contralateral limbs. As expected, dynamic hip range of motion was significantly greater at the contralateral hip compared to the affected hip (p<0.001). Peak hip moments, including the peak hip flexion (p=0.003), adduction (p=0.018), internal rotation (p<0.001) and external rotation moments (p=0.037) were significantly higher at the contralateral unaffected hip compared to the ipsilateral osteoarthritic hip.
Table 2.
Gait characteristics and bone density at the limbs
| Ipsilateral (Affected Hip) Limb | Contralateral (Unaffected hip) Limb | |
|---|---|---|
| Flexion-Extension ROM(degrees) | ||
| Hip ROM | 21±8 | 29±6* |
| Knee ROM | 60±6 | 62±4 |
| Peak Moments (%BW* ht) | ||
| Hip flexion | 4.75±1.49 | 5.66±2.09* |
| Hip adduction | 3.06±0.98 | 3.36±0.94* |
| Hip internal rotation | 0.45±0.25 | 0.57±0.23* |
| Hip external rotation | 0.37±0.26 | 0.46±0.22* |
| Knee flexion | 1.43±0.97 | 1.79±1.26 |
| Knee adduction | 2.23±0.81 | 2.46±0.71* |
| Compartmental loads (BW) | ||
| Medial compartment load | 1.96±0.63 | 2.23±0.52* |
| Lateral compartment load | 1.23±0.35 | 1.41±0.48* |
| Bone Mineral Density (g/c2) | ||
| Medial tibial plateau | 0.854±0.206 | 0.897±0.208* |
| Lateral tibial plateau | 0.726±0.190 | 0.744±0.195 |
p<0.05 pair-wise comparisons; all values are mean±standard deviation; ROM: range of motion; %BW*ht: percent body weight times height; BW: body weight; gm/c2: grams per centimeters squared
At the knees, both primary gait outcomes measures, the PAddM (p=0.029) and the total medial compartment knee load (p=0.003) were significantly higher at the contralateral knee relative to the ipsilateral knee, as was the lateral compartment load (p=0.008). In addition the peak knee flexion moment appeared to be higher at the contralateral knee, but did not reach statistical significance (p=0.052).
Medial tibial plateau BMD was significantly higher at the contralateral knee relative to the ipsilateral knee (p=0.033) while there were no significant differences at the lateral tibial plateau (p=0.469) (Table 2).
Bivariate correlations between contralateral knee to ipsilateral knee dynamic loading and BMD revealed that the ratio of contralateral:ipsilateral medial compartment knee BMD was directly correlated with contralateral:ipsilateral knee PaddM (Spearman’s rho= 0.287, p=0.036) and contralateral:ipsilateral knee medial compartment load (Spearman’s rho=0.351, p=0.009).
Considering that some participants had KL 3 radiographic changes at the contralateral hip, despite minimal clinical symptoms of pain, a separate analysis was performed that excluded these participants. The asymmetry between the contralateral knee and ipsilateral knee medial tibial BMD remained significant (0.892±0.199 vs 0.845±0.212 g/cm2, respectively, p=0.030, n=47). The asymmetry in the PAddM remained but lost significance (2.39±0.69 vs 2.18±0.75 %BW*ht, p=0.075, n=46). The correlation between contralateral:ipsilateral medial compartment knee BMD and knee PaddM (Spearman’s rho= 0.406, p=0.006) and contralateral:ipsilateral knee medial compartment load (Spearman’s rho=0.454, p=0.002) strengthened and remained significant.
Similarly, an analysis excluding knees with KL 2 and 3 was performed. Once again, the asymmetry between the contralateral knee and ipsilateral knee medial tibial BMD remained significant (0.901±0.217 vs 0.851±0.218 g/cm2, respectively, p=0.05, n=35). The asymmetry in the PAddM remained but lost significance (2.51±0.73 vs 2.31±0.69%BW*ht, p=0.109, n=36). The correlation between contralateral:ipsilateral medial compartment knee BMD with the knee PaddM (Spearman’s rho= 0.310, p=0.079) lost significance, however, that with the contralateral:ipsilateral knee medial compartment load (Spearman’s rho=0.452, p=0.008) remained significant.
Discussion
This study demonstrates that in unilateral hip OA, the contralateral knee is subjected to significantly higher dynamic joint loading, as assessed by PaddM and by total medial compartment loads, relative to the ipsilateral knee. Importantly, this asymmetry of knee loading is observed even though the knees are asymptomatic and do not have clinical evidence of OA. Moreover, the significant asymmetries observed in the proximal tibial BMD of the contralateral vs. ipsilateral knees provide evidence of substantially altered load history in the knees as well. This study, along with data from our previous study demonstrating asymmetric progression knee OA in those with unilateral hip OA(10), provides support in humans that alterations in dynamic joint loading may precede the onset of symptomatic knee OA. In addition, this study supports the relationship between dynamic loading and measures of load history in OA
In a study of community dwelling adults, Amin and colleagues demonstrated that the development of new chronic knee pain over three to four years was associated with significantly higher baseline knee adduction moments relative to those that did not develop pain(5). The current study provides further support to this concept that high dynamic loads may precede knee pain symptoms.
The asymmetry in knee loading in unilateral hip OA was first described in a population with endstage hip disease (in patients awaiting total hip replacement) and interestingly, was shown to persist for up to two years, even after hip replacement and complete resolution of hip pain (11). The current study demonstrates that loading asymmetries at the knees begin early in the disease course of hip OA, suggesting that the relative overload of the contralateral knee that places it at higher risk of developing advanced OA is likely already established years before the progression of hip OA to endstage disease. These results may have implications for interventional strategies targeted in those with unilateral hip OA in order to prevent or minimize these asymmetries early in the disease course.
Increased loading was observed at both the contralateral hip and the contralateral knee, which is presumably related to a pain response in the ipsilateral limb. This pain-loading relationship, which was previously demonstrated in knee OA where pain relief with analgesia actually increased loading at the affected joint (3;25), complicates the interpretation and evaluation of the relationship between loading and disease: whereas loading and disease severity are directly related, pain (the principal symptom of OA) actually may result in decreased loading. In contrast, a unique aspect of this study, and of the unilateral hip model, is the ability to assess the knees without the confounding influence of localized knee pain.
The asymmetries noted in the subchondral bone mineral density of the proximal tibiae, which reflected those observed in medial knee loads, provide evidence for the structural consequences of loading alterations, particularly since the relative loading and bone density asymmetries were directly associated. Although the relationship between dynamic knee loading and tibial BMD has previously been reported(6;26), the observation of asymmetric BMDs reflecting asymmetric knee loading and asymmetric risk of progressive knee OA is novel. Interestingly, this is consistent with previous reports in hip OA which suggested that increased hip BMD was associated with an increased risk of disease progression(27).
It should be noted that whereas the participants in this study were explicitly asymptomatic at the knee, several were found to have radiographic evidence of OA. Because radiographic OA is almost universal in this age group, these subjects are likely more representative of “normal” than if radiographic OA had been completely excluded. It is for this reason that this study employed a more clinically relevant definition of OA than purely structural degeneration. In addition, it has previously been demonstrated that asymptomatic individuals with radiographic KL grade 2 disease are biomechanically indistinguishable from normal people without structural degeneration(28), whereas those who have KL grade 2 knees and OA symptoms have significantly elevated peak adduction moments, suggesting that those with clinically evident OA are fundamentally different from those without clinical OA.
It should also be noted that increased loading at the contralateral knee and hip in this model of unilateral hip OA is “relative” to the ipsilateral side. It is not necessarily that the loading at the contralateral side is higher than a “normal” population. Nevertheless, the important concept is that this “relative” asymmetry in loading precedes the corresponding asymmetric progression of knee OA in this group.
This study provides some evidence that in the contralateral knees of patients with unilateral hip OA, which are at high risk of developing progressive symptomatic OA, loading and structural asymmetries appear early in the disease course, while the knees are still asymptomatic. Thus, this model suggests that these early biomechanical asymmetries may have corresponding long term consequences, providing further support for the role of loading in OA progression.
Acknowledgments
The authors acknowledge funding support for this study from the NIH/NIA AG024891, NIH/NIAMS K23AR049748 and the Schweppe Foundation.
Footnotes
Conflict of interest: none to declare
Reference List
- 1.Block JA, Shakoor N. The biomechanics of osteoarthritis: implications for therapy. Current Rheumatology Reports. 2009;11(1):15–22. doi: 10.1007/s11926-009-0003-7. [DOI] [PubMed] [Google Scholar]
- 2.Andriacchi TP, Natarajan RN, Hurwitz DE. Musculoskeletal Dynamics, Locomotion, and Clinical Applications. In: Mow VC, Wilson CH, editors. Basic Orthopaedic Biomechanics. Philadelphia: Lippincott-Raven Publishers; 1997. pp. 37–68. [Google Scholar]
- 3.Hurwitz DE, Ryals AR, Block JA, Sharma L, Schnitzer TJ, Andriacchi TP. Knee pain and joint loading in subjects with knee osteoarthritis. J Orthop Res. 2000;18(4):572–579. doi: 10.1002/jor.1100180409. [DOI] [PubMed] [Google Scholar]
- 4.Sharma L, Hurwitz DE, Thonar EJ, Sum JA, Lenz ME, Dunlop DD, et al. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum. 1998;41(7):1233–1240. doi: 10.1002/1529-0131(199807)41:7<1233::AID-ART14>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 5.Amin S, Luepongsak N, McGibbon CA, LaValley MP, Krebs DE, Felson DT. Knee adduction moment and development of chronic knee pain in elders. Arthritis Rheum. 2004;51(3):371–376. doi: 10.1002/art.20396. [DOI] [PubMed] [Google Scholar]
- 6.Thorp LE, Wimmer MA, Block JA, Moisio KC, Shott S, Goker B, et al. Bone mineral density in the proximal tibia varies as a function of static alignment and knee adduction angular momentum in individuals with medial knee osteoarthritis. Bone. 2006;39(5):1116–1122. doi: 10.1016/j.bone.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Hurwitz DE, Sumner DR, Block JA. Bone density, dynamic joint loading and joint degeneraton. A review. Cells Tissues Organs. 2001;169:201–209. doi: 10.1159/000047883. [DOI] [PubMed] [Google Scholar]
- 8.Wada M, Maezawa Y, Baba H, Shimada S, Sasaki S, Nose Y. Relationships among bone mineral densities, static alignment and dynamic load in patients with medial compartment knee osteoarthritis. Rheumatology. 2001;40:499–505. doi: 10.1093/rheumatology/40.5.499. [DOI] [PubMed] [Google Scholar]
- 9.Clarke S, Wakeley C, Duddy J, Sharif M, Watt I, Ellingham K, et al. Dual-energy X-ray absorptiometry applied to the assessment of tibial subchondral bone mineral density in osteoarthritis of the knee. Skeletal Radiol. 2004;33(10):588–595. doi: 10.1007/s00256-004-0790-x. [DOI] [PubMed] [Google Scholar]
- 10.Shakoor N, Block JA, Shott S, Case JP. Nonrandom evolution of end-stage osteoarthritis of the lower limbs. Arthritis Rheum. 2002;46(12):3185–3189. doi: 10.1002/art.10649. [DOI] [PubMed] [Google Scholar]
- 11.Shakoor N, Hurwitz DE, Block JA, Shott S, Case JP. Asymmetric knee loading in advanced unilateral hip osteoarthritis. Arthritis Rheum. 2003;48(6):1556–1561. doi: 10.1002/art.11034. [DOI] [PubMed] [Google Scholar]
- 12.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 13.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 14.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman BN, Aliabadi P, et al. The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1995;38(10):1500–1505. doi: 10.1002/art.1780381017. [DOI] [PubMed] [Google Scholar]
- 15.Bellamy N. Pain assessment in osteoarthritis: experience with the WOMAC osteoarthritis index. Semin Arthritis Rheum. 1989;18(4 Suppl 2):14–17. doi: 10.1016/0049-0172(89)90010-3. [DOI] [PubMed] [Google Scholar]
- 16.Theiler R, Sangha O, Schaeren S, Michel BA, Tyndall A, Dick W, et al. Superior responsiveness of the pain and function sections of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) as compared to the Lequesne-Algofunctional Index in patients with osteoarthritis of the lower extremities. Osteoarthritis Cartilage. 1999;7(6):515–519. doi: 10.1053/joca.1999.0262. [DOI] [PubMed] [Google Scholar]
- 17.Stucki G, Sangha O, Stucki S, Michel BA, Tyndall A, Dick W, et al. Comparison of the WOMAC (Western Ontario and McMaster Universities) osteoarthritis index and a self-report format of the self-administered Lequesne-Algofunctional index in patients with knee and hip osteoarthritis. Osteoarthritis Cartilage. 1998;6(2):79–86. doi: 10.1053/joca.1997.0097. [DOI] [PubMed] [Google Scholar]
- 18.McGrory BJ, Harris WH. Can the western Ontario and McMaster Universities (WOMAC) osteoarthritis index be used to evaluate different hip joints in the same patient? J Arthroplasty. 1996;11(7):841–844. doi: 10.1016/s0883-5403(96)80184-7. [DOI] [PubMed] [Google Scholar]
- 19.Pincus T, Koch GG, Sokka T, Lefkowith J, Wolfe F, Jordan JM, et al. A randomized, double-blind, crossover clinical trial of diclofenac plus misoprostol versus acetaminophen in patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2001;44(7):1587–1598. doi: 10.1002/1529-0131(200107)44:7<1587::AID-ART282>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 20.Andriacchi TP, Galante JO, Fermier RW. The influence of total knee-replacement design on walking and stair-climbing. J Bone Joint Surg Am. 1982;64(9):1328–1335. [PubMed] [Google Scholar]
- 21.Prodromos CC, Andriacchi TP, Galante JO. A relationship between gait and clinical changes following high tibial osteotomy. J Bone Joint Surg Am. 1985;67(8):1188–1194. [PubMed] [Google Scholar]
- 22.Andriacchi TP, Strickland AB. Gait analysis as a tool to assess joint kinetics. In: Berme E, Engin AE, Correia da Silva KM, editors. Biomechanics of Normal and Pathological Human Articulating Joints. Doordrecht: Martiuns Nijhoff; 1985. p. 83. [Google Scholar]
- 23.Moisio KC, Sumner DR, Shott S, Hurwitz DE. Normalization of joint moments during gait: a comparison of two techniques. J Biomech. 2003;36(4):599–603. doi: 10.1016/s0021-9290(02)00433-5. [DOI] [PubMed] [Google Scholar]
- 24.Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9(1):113–119. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 25.Schnitzer TJ, Popovich JM, Andersson GB, Andriacchi TP. Effect of piroxicam on gait in patients with osteoarthritis of the knee. Arthritis Rheum. 1993;36(9):1207–1213. doi: 10.1002/art.1780360905. [DOI] [PubMed] [Google Scholar]
- 26.Hurwitz DE, Sumner DR, Andriacchi TP, Sugar DA. Dynamic knee loads during gait predict proximal tibial bone distribution. J Biomech. 1998;31(5):423–430. doi: 10.1016/s0021-9290(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 27.Goker B, Sumner DR, Hurwitz DE, Block JA. Bone mineral density varies as a function of the rate of joint space narrowing in the hip. J Rheumatol. 2000;27(3):735–738. [PubMed] [Google Scholar]
- 28.Thorp LE, Sumner DR, Wimmer MA, Block JA. Relationship between pain and medial knee joint loading in mild radiographic knee osteoarthritis. Arthritis Rheum. 2007 Oct 15;57(7):1254–1260. doi: 10.1002/art.22991. [DOI] [PubMed] [Google Scholar]


