Summary
Biopharmaceuticals are therapeutic products based on biotechnology. They are manufactured by or from living organisms and are the most complex of all commercial medicines to develop, manufacture and qualify for regulatory approval. In recent years biopharmaceuticals have rapidly increased in number and importance with over 4001 already marketed in the U.S. and European markets alone. Many companies throughout the world are now ramping up investments in biopharmaceutical R&D and expanding their portfolios through licensing of early-stage biotechnologies from universities and other non-profit research institutions, and there is an increasing number of license agreements for biopharmaceutical product development relative to traditional small molecule drug compounds. This trend will only continue as large numbers of biosimilars and biogenerics enter the market.
A primary goal of technology transfer offices associated with publicly-funded, non-profit research institutions is to establish patent protection for inventions deemed to have commercial potential and license them for product development. Such licenses help stimulate economic development and job creation, bring a stream of royalty revenue to the institution and, hopefully, advance the public good or public health by bringing new and useful products to market. In the course of applying for such licenses, a commercial development plan is usually put forth by the license applicant. This plan indicates the path the applicant expects to follow to bring the licensed invention to market. In the case of small molecule drug compounds, there exists a widely-recognized series of clinical development steps, dictated by regulatory requirements, that must be met to bring a new drug to market, such as completion of preclinical toxicology, Phase 1, 2 and 3 testing and product approvals. These steps often become the milestone/benchmark schedule incorporated into license agreements which technology transfer offices use to monitor the licensee’s diligence and progress; most exclusive licenses include a commercial development plan, with penalties, financial or even revocation of the license, if the plan is not followed, e.g., the license falls too far behind.
This study examines whether developmental milestone schedules based on a small molecule drug development model are useful and realistic in setting expectations for biopharmaceutical product development. We reviewed the monitoring records of all exclusive Public Health Service (PHS) commercial development license agreements for small molecule drugs or therapeutics based on biotechnology (biopharmaceuticals) executed by the National Institutes of Health (NIH) Office of Technology Transfer (OTT) between 2003 and 2009. We found that most biopharmaceutical development license agreements required amending because developmental milestones in the negotiated schedule could not be met by the licensee. This was in stark contrast with license agreements for small molecule chemical compounds which rarely needed changes to their developmental milestone schedules. As commercial development licenses for biopharmaceuticals make up the vast majority of NIH’s exclusive license agreements, there is clearly a need to: 1) more closely examine how these benchmark schedules are formed, 2) try to understand the particular risk factors contributing to benchmark schedule non-compliance, and 3) devise alternatives to the current license benchmark schedule structural model. Schedules that properly weigh the most relevant risk factors such as technology classification (e.g., vaccine vs recombinant antibody vs gene therapy), likelihood of unforeseen regulatory issues, and company size/structure may help assure compliance with original license benchmark schedules. This understanding, coupled with a modified approach to the license negotiation process that makes use of a clear and comprehensive term sheet to minimize ambiguities should result in a more realistic benchmark schedule.
Introduction
The mission of the National Institutes of Health (NIH) is to seek fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to enhance health, lengthen life, and reduce the burdens of illness and disability. The translational half of the mission, ‘the application of that knowledge,’ largely occurs through collaborative and licensing mechanisms. In order to help make therapeutics available to the public via commercialization and widespread availability, new inventions made at the NIH can be patented and licensed to commercial entities. Several hundred new inventions are reported each year, and patent applications are filed on a minority of these. There are many success stories involving therapeutics and diagnostics first discovered at the NIH; a partial list of approved and marketed products is shown below in Table 1. A far more extensive list which includes other approved products as well as those currently pursuing regulatory approval can be found at: http://www.ott.nih.gov/productpipeline/default.aspx.
Table 1.
Examples of Some Successes from the NIH Licensing Program
| PRODUCT | INDICATION | INSTITUTE | LICENSEE |
|---|---|---|---|
| Fludara® | Cancer | NCI | Schering AG |
| CONFIRM™ | Cancer | NCI | Ventana Medical Systems |
| Velcade® | Multiple myeloma | NCI | Millennium Pharmaceuticals |
| Zevalin® | Non-Hodgkin’s lymphoma | NCI | Coulter Corporation |
| Guardasil® | Human Papilloma Virus | NCI | Merck |
| Videx® | HIV | NCI | Bristol-Myers Squibb |
| Twinrix® | Hepatitis A & B viruses | NIAID | GlaxoSmithKline |
| Synagis® | Respiratory syncytial virus | NIAID | MedImmune |
| Taxus® | Cardiovascular | NIA | Angiotech Pharma/Boston Sci |
| Thyrogen® | Thyroid cancer | NIDDK | Genzyme Diagnostics |
| PreserVision® | Macular Degeneration | NEI | Bausch & Lomb |
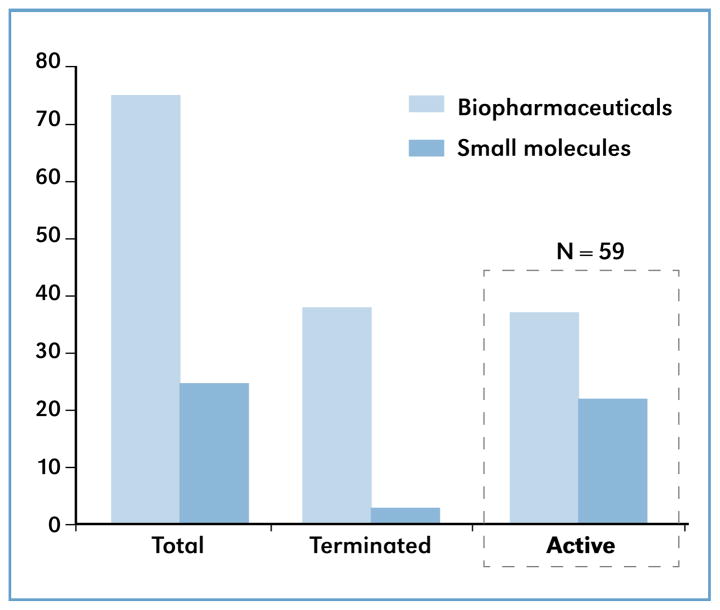
Negotiating a license to practice patented art for commercial product development includes designing appropriate benchmarks that weigh a variety of risks inherent to the development of a specific technology. To inform future negotiations and help advance this endeavor as it relates to licensing of and development of biopharmaceuticals, benchmark compliance of all of NIH’s exclusive agreements made between 2003 through 2009 was examined. A majority of agreements for commercializing biopharmaceutical products during this recent period were unable to meet at least one milestone on the negotiated schedule. Between 2003 through 2009 the National Institutes of Health Office of Technology Transfer (OTT) executed a total of 100 exclusive licensing agreements, 75 of which were for biopharmaceuticals and 25 for small molecule drugs (see Figure 1).
Figure 1.
Status of Exclusive License Agreements 2003–2009
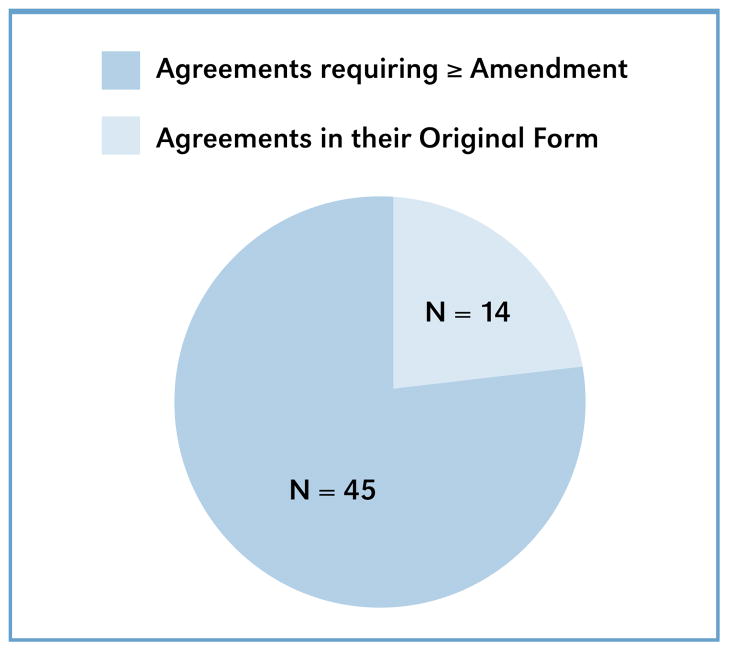
Of these 100 agreements, 59 are still active. However, 45 of those agreements require (or have required) amendment. A simple analysis indicates that 76 percent of the agreements belong to the group ‘Agreements requiring ≥ 1 amendment’ seen in Figure 2, illustrating that, for one reason or another, a strong majority of companies were unable to meet their milestones. This report briefly examines the standard negotiated benchmarks most often agreed upon, identifies risks associated with the most frequent reasons that licensees were not able to meet those benchmarks, and offers suggestions for assuring better benchmark schedule compliance in the future.
Figure 2.
Active License Agreements
Benchmarks Found in Past NIH/OTT Exclusive License Agreements
Appropriate data concerning the common developmental hurdles associated with the biopharmaceutical subgroup, the regulatory environment surrounding the ultimate product, as well as the current developmental stage of the technology are needed to help inform both licensors and licensees how to better structure the particulars of a benchmark agreement. Simply applying a standard benchmark schedule across diverse technologies, such as biopharmaceutical products, that face different scientific and regulatory obstacles is often not workable or optimal. When developing new and distinct cutting-edge technologies, it is often difficult to forecast the unanticipated financial, scientific and regulatory risks and hurdles that may arise.
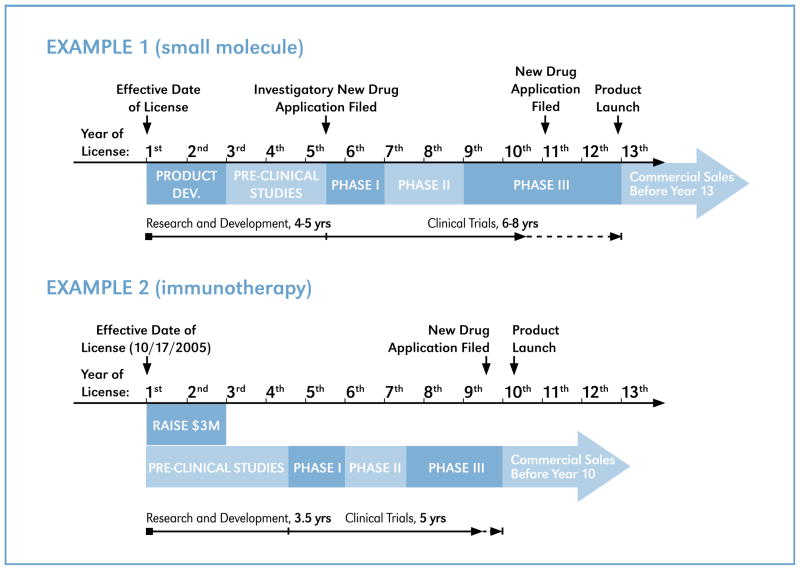
Immunotherapies, recombinant proteins, novel vaccines, gene-therapies and their therapeutic applications are all actively investigated by NIH researchers and being patented by the U.S. government. Those four categories of biologics account for roughly 75 percent of the exclusive licenses over the analyzed period, with small molecules largely filling the remaining portion (Figure 1). Still, small molecules have the most reliable and time-tested routes for first, clinical development, followed by approval from regulatory agencies, followed by commercialized product launch. When designing benchmark schedules, oftentimes a final timeline will closely resemble that which has been proposed by the licensee. Typical PHS license benchmark schedules for therapeutics mimic that found in agreements involving small molecules. Examples of schedules involving a small molecule (Example 1) and an immunotherapy (Example 2) at very early stages in development are shown in Figure 3.
Figure 3.
Benchmark Schedule For An Agreement Involving A Small Molecule Therapeutic And A Biopharmaceutical
In Example 1 the company was given 12 years from the effective date of the license to get a small molecule product to market, and the license remains active and annual progress reports indicate the company is currently working diligently to commercialize the product. This license has a generous schedule and has not required any amending or renegotiation to date, saving all involved valuable time. Furthermore, and importantly, no amending or renegotiation is overdue, as the company remains current with respect to the requirements of the benchmark schedule. Other benchmark timelines, such as that seen in Example 2, are more constricting. Here, the company was unable to secure financing to further develop the immunotherapy, and ultimately the license was terminated.
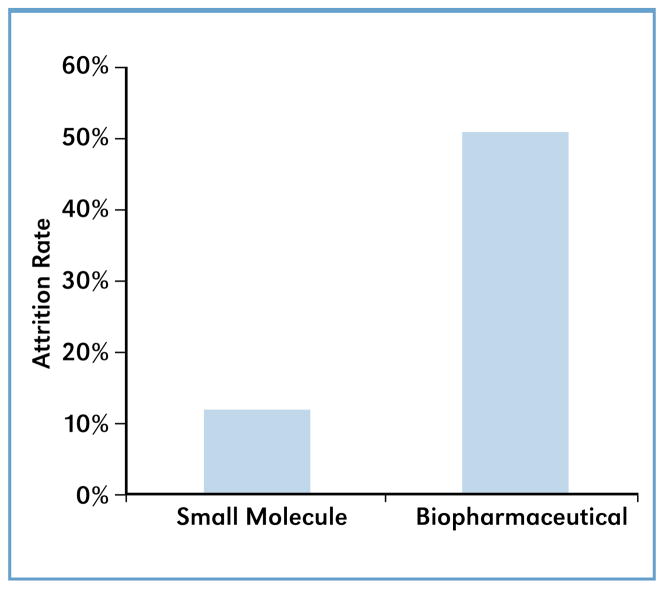
License termination for one reason or another, including a failure to meet benchmarks, is a normal occurrence; just over 40 percent of the exclusive license agreements between 2003–2009 have been terminated (see Figures 1 and 4), and license termination can be pursued by either party (see term sheet). Missed milestones will trigger the need for an amendment which may include financial penalties such as higher royalty rates. Noncompliance is the most common reason the NIH would terminate a license. Reasons for a licensee to pursue termination vary, but a common reason involves changes in company direction, often associated with acquisition. A clear difference was found between license terminations for small molecules and biopharmaceuticals. Exclusive licenses involving small molecules have a much lower attrition rate than biopharmaceuticals (Figure 4), and key factors that contribute to this difference are discussed in the following sections.
Figure 4.
Attrition Rates: Small Molecule vs. Biopharmaceutical
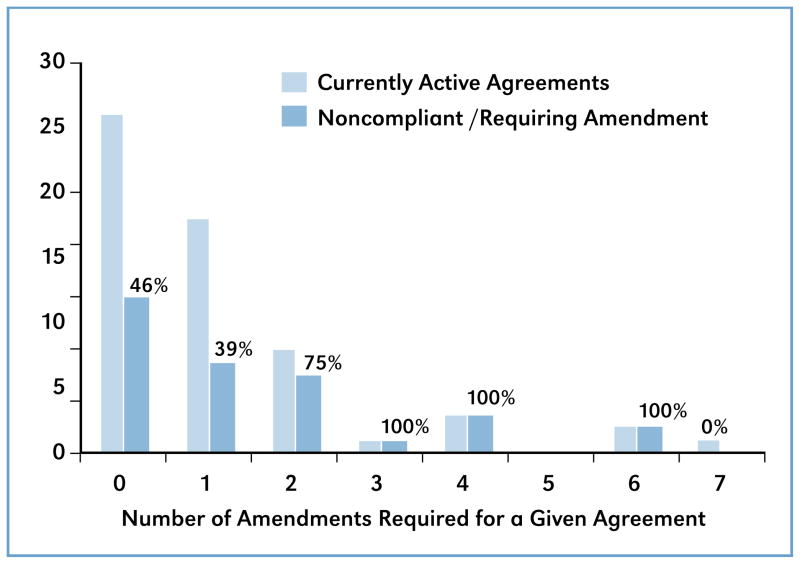
It is worth noting that the benchmark schedules through the regulatory phases are nearly identical in the two examples shown in Figure 3, even though normal commercialization timelines for small molecules and biopharmaceuticals are known to vary significantly. Average timelines for a small molecule are between 6–10 years while biopharmaceuticals can take up to 15 years, eight of which are generally spent in the clinic and in review.2 It is not uncommon for a PHS license agreement involving an early-stage technology to require a company to initiate a Phase I clinical trial before the start of the third year and have a complete marketed product within nine years. This schedule agrees better with a small molecule timeline. However, the majority of exclusive license agreements done over the past seven years have been for the development of biopharmaceuticals, which helps explain the need to continuously amend a significant amount of licenses (see Figure 5). For these agreements, a better understanding of the developmental risks associated with the technology could have favored smooth, amendment-free progression to product launch.
Figure 5.
License Amendments
The language used to describe benchmark schedules differs significantly between agreements, and can lead to misunderstandings, especially when dealing with start-up companies or new entrepreneurs. Some licenses require a particular developmental phase to ‘be completed’ while others require ‘initiation’ by a certain time. Some licenses tie the benchmark schedule to the effective date of the licensing agreement, while others have fixed dates written into the schedule. Some benchmarking schedules are so finely broken down that instead of the normal milestones written into a particular schedule, there are the usual eight or so typical benchmarks surrounded by 10 or more mini-milestones. In every company, technology and license are different and presents unique opportunities and risks. The challenge for licensors and licensees alike is for all to be on the same page with respect to milestone expectations that are realistic and attainable for the specific technology at hand. To minimize the risk of misunderstandings in communication, it would help to adopt a clear license term sheet.
Reasons Companies Fail To Meet Benchmarks
Of all the agreements analyzed in this study, approximately 50 percent of the active licenses involving biopharmaceuticals failed to meet benchmarks, whereas only 20 percent of licenses for small-molecule drugs were similarly behind. Negotiating a license agreement is fundamentally a human activity, involving different approaches and personalities. Many of the licenses negotiated by OTT are between PHS and a start-up or small biotechnology company. Their executives may have little previous business experience and limited capital. Over 80 percent of the exclusive licenses signed by PHS between 2003 and 2009 were with a ‘small business,’ defined as a business having fewer than 500 employees (13 CFR 121). In fact, the majority of those ‘small businesses’ have fewer than 50 employees, raising the question as to whether the current simple classification system of ‘small business’ vs. ‘large business’ is appropriate. Using more sector-relevant descriptors (i.e., start-up, virtual business, small business having <100 employees, large business) would be useful to better gauge the risks the business will face and understand the resources it has at hand. A large pharmaceutical company, for example, will have specialized staff devoted to technology development and regulatory approval. In contrast, a smaller business may be overly optimistic in their proposed timeline, especially in cases where the pathway to regulatory approval is murky. In cases such as this, the licensing officer could caution a smaller business against proposing overly optimistic schedules. However, from a public health perspective, it is important to get novel treatments and therapies made available to the population as soon as possible. Under conditions where more than one party is interested in licensing the technology, the NIH would tend to favor the company with the more successful track record and a more ambitious timeline. This might lead a company to knowingly underestimate the time required for development and propose an overly aggressive timeline. Furthermore, amended agreements can count the same as new agreements when it comes to evaluating licensing specialist productivity. Nevertheless, clearly it is better to agree on a longer, more realistic developmental schedule having a high probability of success than one which is more aggressive but has little chance of success.
In any case, a license may represent a scientist’s first attempt at being an entrepreneur. Often a new entrepreneur underestimates the time it takes to develop a product and simultaneously overestimates their company’s ability to move a product forward. For example, of all the agreements analyzed for this study, 36 involved recombinant proteins and, of those, 16 were late for the first benchmark involving pre-clinical research. Many recombinant protein-related agreements signed within the last 2–3 years (2007–2009, n=13 for recombinant proteins) still have time before the first scheduled milestone is due. The historical data suggests that well over half of these companies will not meet their first benchmark.
A close look at the agreements requiring repeated amendments corroborates this prediction, and suggests repeated misjudgment in predicting accurate benchmark schedules. A full 46 percent of the exclusive license agreements still in original form are in need of amending due to benchmark noncompliance (Figure 5). Those agreements having already undergone one or two amendments have noncompliance rates of 39 percent and 75 percent, respectively. A more realistic approach, involving a better understanding of the science underlying the patent and regulatory environment is needed.
Advancing a new biopharmaceutical to market requires not only excellent scientific skills, but also good business management skills. In addition, a thorough understanding of the regulatory environment for the particular product being developed is essential for speedy market approval. It is well known that for an excellent scientist to become an excellent business manager is the exception rather than the rule. Venture capital firms understand this well and it is one reason why the influx of venture capital money is often tied to an obligation for the scientist-entrepreneur to step aside from a business management role. This underestimation of the time required to develop a product which often reflects an overestimation of a company’s capabilities to move a product forward, usually creates a license compliance problem with regard to meeting the benchmark schedule. This can be addressed by amending the license, but this requires an additional expenditure of resources by both the licensee and the licensing office, and often results in penalizing terms for the licensee. For these reasons it is best to try and avoid license amendment and invest the time to establish a realistic and attainable benchmark schedule.
Lack of financing can also cause delay in achieving milestones. It goes without saying that in addition to hard work, determination and luck, it takes investment for a new biopharmaceutical business to move forward. Complicating matters is the fact that investment capital has become increasingly scarce over the past several years, especially for very early stage biopharmaceutical development companies. Many companies look to investment by venture capital (VC) or angel investors to move a pre-clinical stage technology into the clinic. A report released by PricewaterhouseCoopers (Jan 22, 2010)3 noted that total VC investment in 2009 ($17.7B) was down 30 percent from 2008, falling to 1997 levels. VC investment in biotechnology fell by 19 percent in terms of both dollars ($3.5B) and deals (406) over the same period. In the absence of capital, it is impossible for a company to make progress with any technology. Sourcing or preparing clinical-grade chemicals or biopharmaceuticals that are made according to current good manufacturing practice (cGMP) costs money. Moving from the pre-clinical phase into the clinic, or progressing from one clinical phase to the next all require money. As a consequence of the economic environment deteriorating over the last few years, many OTT licensees have been unable to secure capital, ultimately leading to non-fulfillment of their benchmark obligations. Therefore, relevant economic forecasts should be considered when structuring benchmarks to help mitigate the risk of benchmark non-compliance.
Another difficult factor to predict is the regulatory requirements a company will need to satisfy in the course of getting approval to market. For small molecules, there exists a clear path forward. For biopharmaceuticals, this is less the case. Though the FDA publishes general guides for biologics, to get a specific biopharmaceutical approved for even a Phase 1 clinical trial, extra testing may be necessary depending on the actions of the biopharmaceutical and the particular systems that may be affected. These tests, of course, take the essential resources of time, money and people, and a company working to develop a biopharmaceutical can never be completely sure what specific studies the FDA will require prior to filing their IND application.
Licensing officers themselves can also unwittingly contribute to creating an unrealistic benchmark schedule. One reason this happens is an incomplete understanding of the licensed technology and the issues involved in developing it into a product. At government laboratories and universities, any licensing officer may have a portfolio containing many different technologies. Each technology is itself complicated, likely evolving from several years of devoted study and much experimental research. It is unrealistic to expect a licensing officer to understand and foresee each challenge which needs to be overcome in the commercial development of their portfolio of technologies. However, this limited familiarity with the technology may lead a licensing officer to unintentionally neglect important factors and misjudge the time required to properly develop the technology.
Lastly, a licensor may negotiate for a benchmark schedule that has an unrealistic development schedule. A licensor typically wants to make sure that the technology being licensed is developed in a timely manner. After all, for NIH it is the taxpayer’s investments that underwrote the invention, and therapeutic products on the market are the returns on those investments. A licensing officer would then tend to want to establish a schedule that results in a product as fast as possible. At the same time, a savvy company may try to pad the benchmark schedule excessively, in order to buy time to pursue the technology in a more limited manner, and a licensing officer needs to protect against this. With these competing interests, reaching consensus on a realistic and appropriate schedule could be challenging.
PHS licenses for development of biopharmaceuticals or small molecule drugs, were found to be approximately 50 percent and 20 percent, respectively, late in meeting their benchmarks. This demonstrates how simply applying a classical small-molecule benchmark schedule (Example 1 in Figure 2) across varying technologies results in a significantly lower compliance rate. A licensing officer unaware of the different requirements for each type may seek to argue for a classical small molecule drug benchmark schedule which is generally unrealistic for a biopharmaceutical, and ultimately cause more work for everybody in the form of license amendments, renegotiations, etc.
Improving Benchmark Compliance
Improve classification scheme
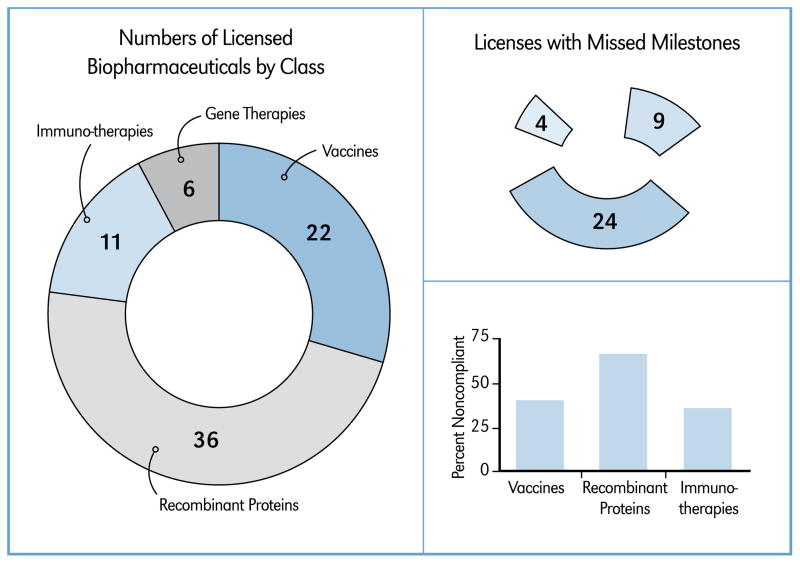
As mentioned above, it was observed that nearly 75 percent of the exclusive licenses analyzed in this report were for biopharmaceuticals. This category also had the most licenses with unmet or late benchmarks. The term ‘biopharmaceutical’ can be thought of as a catch-all phrase for products created by a biological process, such as antibodies, proteins, blood and blood components, tissues, and somatic cells. Within the category of ‘biopharmaceutical,’ NOH/OTT licenses include those for vaccines, recombinant proteins, immunotherapies and gene therapies. Among these four subtypes of biopharmaceuticals licenses, we see that between 2003 through 2009 there were 22, 36, 11 and 6 for vaccines, recombinant proteins, immunotherapies and gene therapies, respectively (see Figure 6, left panel). With only six licenses, the gene therapy group was left out of the following analysis due to the fact that three out of the six licenses have effective dates in late 2006 or more recently, and these licenses would tend to have several months to go before hitting their first benchmark. Additionally, though there were several gene therapies licensed in the 1990s, safety issues have significantly stalled this class of therapeutics and with only six exclusive licenses executed over the seven-year time span analyzed, a small sample size could suggest misleading conclusions. However, the number of exclusive licenses with missed milestones involving the other biopharmaceuticals: vaccines (9), recombinant proteins (24) and immunotherapies (4) is significant compared to small molecule drug development licenses, and non-compliance ranges from 36 percent to 67 percent with recombinant protein licenses showing the highest rate of missed milestones (Figure 6, right panel). This suggests that better awareness of the type of biopharmaceutical being licensed and the unique development, manufacturing and regulatory issues that may be encountered in bringing each type of product to market would help in establishing more realistic license benchmark schedules.
Figure 6.
Biopharmaceutical Licenses
As a case in point, consider that as much as the regulatory hurdles associated with recombinant proteins are a black-box, those associated with gene therapies are even more so. To date, no human gene therapy has been approved by the FDA. Nevertheless, hundreds4 of clinical trials involving gene therapies have registered with the NIH’s http://clinicaltrials.gov Web site. When structuring benchmark schedules for gene therapy, one should keep in mind the added risk associated with an evolving regulatory environment and resist putting only absolute dates into the schedule. Establishing fixed time points for projected completion of pre-clinical milestones may be important to ensure timely early stage development, but once the clinical stage is reached, license benchmark schedule compliance may be better served by not setting specific dates for milestone completion but rather setting timeframes for completing each subsequent clinical development step as the previous one is completed.
Building a benchmark schedule that also recognizes a licensee’s need to obtain adequate financial backing or to partner with a larger entity at certain stages in the development process would also be advisable, especially for start-up ventures. There is also a clear need to include a regulatory component. One possible example of a benchmark schedule for a biopharmaceutical that blends fixed time points with a broader sequential approach addressing regulatory issues could be shown in the following example:
Example of a Benchmark Schedule for a Novel Gene-Therapy
Proof of Concept in vivo: 2 years
Pre-IND meeting with FDA: Before end of 4th year
Total Developmental/Preclinical: Less than 6 years
Submission of IND: By end of 5th year
Develop updated clinical schedule: Before end of 6th year
Advantages of using a clear term sheet
Making the negotiation process clear benefits both licensor and licensee. Many technology-focused universities as well as government laboratories have term sheets on their Web sites that facilitate the transfer and development of technologies.5 The value of a term sheet is that it provides a clear starting point from which negotiations can go forward. With respect to a benchmark schedule for a specific technology, that too would need to be filled out in the term sheet. If either party has concerns or reservations about the adequacy of a proposed benchmark schedule, these can be discussed and refined before being rolled into the license agreement. Such discussions would help address the risk that the parties have different expectations with regard to product development timelines, and it would be more likely that benchmark schedule compliance will occur. An example of a possible term sheet for use is shown on the next page.
Conclusions
Exclusive biopharmaceutical product development licenses at OTT more frequently become non-compliant than exclusive small molecule drug development licenses due to failure to meet the original benchmark schedule for product development. A major contributing factor appears to be the use of a small molecule drug development benchmark schedule model for biopharmaceuticals. This model does not reflect important developmental risk factors inherent to bio-pharmaceutical product development. These include:
Business experience of the licensee,
Economic environment and company financing,
Technology-specific developmental hurdles, and
The evolving regulatory environment for each subclass of products within the category of biopharmaceuticals.
To help ensure that a licensee stays compliant with their benchmark schedule, it is important for the licensor to:
Be knowledgeable about normal preclinical and clinical developmental times for the specific class of biopharmaceutical (e.g. vaccine vs. recombinant protein vs. immune- therapy vs. gene therapy),
Make use of a term sheet to help assure that each party’s position during a license negotiation is understood and clear, and
Understand the financial limitations and expectations the license applicant has bringing the product to market.
A risk-informed approach to establishing benchmark schedules for biopharmaceuticals should help ensure better benchmark compliance and, with appropriate incentives, help keep a licensee on track to develop the biopharmaceutical. Ultimately, this should result in the delivery of more biopharmaceuticals on the market, resulting in improved public health while meeting the business goals of the companies.
Table 2.
Common Factors Affecting Milestone Fulfillment
| Issue | Notes |
|---|---|
| Overestimating abilities | A best-case scenario as opposed to a more realistic timeline may be suggested in a benchmark schedule |
| Lack of financing | A licensee will lose time if it is unable to raise and secure financing for technology development |
| Unclear regulatory path | When dealing with cutting-edge technologies, regulators may require extra unforeseen data sets and tests |
| Developmental hurdles | Licensors and licensees may be unaware of important challenges in developing a specific technology |
Table 3.
| Sample Term Sheet for a Commercial License | |
|---|---|
| FEATURE | TERMS |
| 1. License Agreement | NIH and its researchers will provide rights to use a selected technology for commercial product development, manufacture, use and sale. |
2. Scope of License
|
2. The company may, under certain circumstances, acquire exclusive rights to the technology to use in defined geographical markets (usually worldwide, subject to patent coverage) or specific fields of use. Rights to grant sublicenses to others may be provided under mutually agreeable terms. |
| 3. Filed of Use | 3. Rights are restricted to applications set forth by the company and in which the company is/will be able to develop and commercialize a new product or service based on the technology. Products sold in the United States will be substantially manufactured in the United States unless a waiver is granted. |
| 5. Performance Milestones | 5. NIH will require commitments from the company in the form of tailor-made performance milstones defined in terms of measurable events within the company’s own commercialization plans in addition to cash payments to NIH. For example:
|
| 6. Consideration | 6. Compensation
|
| 7. Government Use | 7. The NIH and U.S. government maintains a right to use and practice the technology. |
8. Patent Activity
|
|
| 9. Warranties and Indemnification | 9. NIH gives no warranties about the technology or the related patents. Licensee agrees to indemnify and hold harmless NIH in all cases involving issues with the licensed technology. |
10. Termination of License
|
|
Footnotes
There were several hundred biopharmaceuticals approved by the FDA between 1982–2009, see http://www.biopharma.com/approvals_2009.html also see: www.biopharma.com
http://www.innovation.org/index.cfm/toolsandresources/fact-sheets/innovation_by_the_numbers; DiMasi and Grabowski, 2007.
Currently 249 “gene therapy” trials registered at clincaltrials.gov.http://clinicaltrials.gov/ct2results?term=%22gene+therapy%22
Contributor Information
Todd A. Ponzio, Email: ponziot@mail.nih.gov, National Cancer Institute, Technology Transfer Center, Fellow, Rockville, MD, USA.
Hans Feindt, Email: feindth@mail.nih.gov, NIH Office of Technology Transfer, Chief, Monitoring and Enforcement Branch, Rockville, MD, USA.
Steven Ferguson, Email: sf8h@nih.gov, NIH Office of Technology Transfer, Deputy Director, Licensing & Entrepreneurship, Rockville, MD, USA.