Abstract
Background:
The prevalence of chronic rhinitis is increasing rapidly; its pathogenesis is to be further understood; immune inflammation is one of the possible causative factors. Antigen specific CD8+ T cells play a critical role in the induction of chronic inflammation.
Aims:
This study aimed to investigate the role of antigen specific CD8+ T cells in the pathogenesis of chronic atypical allergic rhinitis.
Material and Methods:
Nasal mucosal epithelial surface scratching samples were obtained from patients with chronic obstruction atypical allergic rhinitis. Exosomes were purified from the scratching samples and examined by immune gold electron microscopy. The effect of exosomes on modulating dendritic cell's properties, the effect of exosome-pulsed dendritic cells on naïve T cell differentiation and the antigen specific CD8+ T cell activation were observed by cell culture models.
Results:
Exosomes purified from patients with chronic atypical allergic rhinitis carried microbial products, Staphylococcal enterotoxin B (SEB), and airborne antigen, Derp1. Dendritic cells pulsed by SEB/Derp1-carrying exosomes showed high levels of CD80, CD86 and the major histocompatibility class I (MHCI). Exosome-pulsed dendritic cells could induce the naïve CD3+ T cells to differentiate into CD8+ T cells. Upon the exposure to a specific antigen, the CD8+ T cells released granzyme B and perforin; more than 30% antigen specific CD8+ T cells proliferated.
Conclusions:
Antigen specific CD8+ T cells play an important role in the pathogenesis of chronic obstruction atypical allergic rhinitis.
Keywords: Rhinitis, CD8 T lymphocytes, Epithelium, Antigen specific response
Introduction
The nasal obstruction is a clinical symptom that substantially affects the life quality of human being; all most all the people have such an experience more or less in their life time. The symptom mostly appears in patients suffer from chronic idiopathic rhinitis or chronic allergic rhinitis; a large part of them usually try multiple therapeutic remedies to ameliorate the nasal obstruction; but finally ask for surgical intervention to remove a portion of the hypertrophic nasal mucosa to rebuild the airway in the nasal cavity [1,2].
The pathogenesis of chronic obstruction rhinitis is not fully understood [3]. It can be resulted from allergic rhinitis, vasomotor rhinitis, viral infection or bacterial infection [4]. Simulated by the secretion from chronic sinusitis is another common cause [5]. It is also reported that non-specific eosinophilia plays an important role in the pathogenesis of a subgroup of chronic rhinitis [6]. The eosinophils release a number of chemical mediators, including major basic protein, eosinophil cationic protein, eosinophil peroxidase and eosinophil-derived neurotoxin, etc [7]. It is true that these chemical mediators can induce inflammation in the tissue; however, the factors triggering eosinophils to release these mediators are to be further understood.
The cytotoxic CD8+ T cells can release a batch of cytotoxic molecules, such as granzyme B and perforin, to the microenvironment to interfere with cell and tissue physiological activities. The mediators can initiate the apoptotic pathway to induce cell death or cell injury. Recent reports indicate that antigen specific CD8+ T cells also play a role in the chronic inflammation, such as inflammatory bowel disease [8] , dermatitis [9] and diabetes [10]. In allergic rhinitis, investigators have also noted that CD8+ cells release interleukin (IL)-4 to be involved in the pathogenesis of the disease. Whether CD8+ T cells play a role in the chronic obstruction rhinitis is unclear.
In clinical practice, we have noted some patients with chronic rhinitis have positive skin test results to common allergens, but do not have typical symptoms of allergic rhinitis; they also only had low levels of antigen specific IgE in the serum. This subgroup of rhinitis may be designated atypical allergic rhinitis. We have also noted that many of these patients have chronic nasal obstruction. Therefore, we recruited a group of patients with chronic nasal obstruction and positive allergen skin test results in the present study. The results indicate that most of these patients have a high frequency of allergen specific CD8+ T cells in their nasal mucosa. Exposure to specific antigens can trigger these CD8+ T cells to release inflammatory molecules.
Materials and Methods
Reagents
Antibodies of beta 6, CD80, CD86, MHCI, porforin and granzyme B were purchased from Santa Cruz Biotech (Santa Cruz, CA, USA). Derp1 was obtained from BioWorld (Shanghai, China). Anti-Derp1 antibody was purchased from QCBio Ltc. (Shanghai, China). Staphylococcal enterotoxin B (SEB) was purchased from Sigma Aldrich (Shanghai, China).
Cell culture
Human nasal epithelial cell line, RPMI2650 cells (ATCC, CCL-30), was seeded into 75 cm2 flasks in RPMI1640 medium containing 10% FCS, 4 mM glutamine, 50 μg/ml penicillin /streptomycin. On day 3, when the cells reached 60% confluence, the mite antigen, Derp1, with or without SEB was added to the cultures. Culture media were collected; the exosomes were purified with gradient centrifugation.
Exosome preparation
The nasal epithelial cell line, RPMI2650 cells, were incubated with Derp1 or/and SEB for 24 h; the supernatants were collected and centrifuged at 300 × g (10 min), 1200 × g (20 min), and 10,000 × g (30 min) to remove cell debris. Exosomes were pelleted at 100,000 × g for 1 h and resuspended in PBS for further experiments. The protein in exosomes was quantified using a Bradford protein assay.
Electron microscopy
Exosomes were prepared following our previously established procedures [11]. The purified exosomes were fixed with fixatives that were added to the culture supernatant for 2 h before ultra-centrifugation at final concentrations of 0.75% (glutaraldehyde) and 2% (paraformaldehyde). The samples were dropped on grids that were pre-coated with formva and dried overnight, stained with anti-Derp1 or SEB antibodies followed by gold particle labeled second antibody (6 nm for Derp1; 12 nm for avb6). The grids were then stained with a negative contrast solution. Samples were observed with a JEOL JEM-1200 EX transmission electron microscope.
Flow cytometry
Cells were collected and incubated with primary antibodies on ice for 30 min (For the intracellular staining, cells were fixed with 1% paraformaldehyde on ice for 30 min and incubated with permealization reagents for 30 min on ice). The stained cells were analyzed using a FACSarray (BD Bioscience, San Jose, CA). Data were analyzed with software FlowJo.
Statistics
All values were expressed as the means ± SD of at least three separate experiments. The values were analyzed using the two-tailed unpaired Student's t-test when the data consisted of two groups or by ANOVA when three or more groups were compared. P<0.05 was accepted as statistically significant.
Results
Exosomes carrying protein Ag and SEB are identified in the human nasal mucosa
The nasal epithelial layer samples were collected from patients with chronic rhinitis. The patients had a typical nasal obstruction, inferior turbinate hypertrophy, skin scratch test showed positive response to one to several airborne antigens, but without typical symptoms of allergic rhinitis. The nasal epithelial samples were obtained from each patient. Exosomes were purified from the samples with our established procedures. The exosomes were stained with antibodies of SEB and Derp1, and processed for electron microscopy (EM). As shown by the EM pictures, Fig. 1, exosomes were successfully purified from the obtained samples from the nasal epithelium. The gold labels for SEB (the larger particles) and Derp1 (the smaller particles) were seen on most exosomes (Fig.1A) observed while the negative control exosomes (Fig.1B) did not show any gold particle labels. That exosomes carry Derp1 and SEB was confirmed by Western blotting (C, D).
Fig. 1.
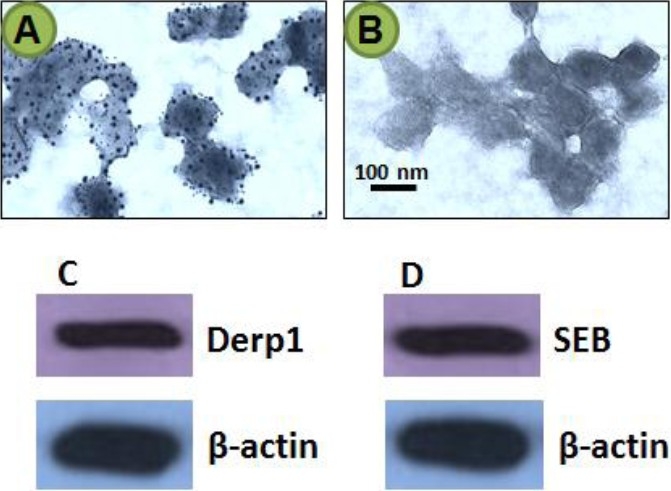
Exosomes carrying microbial products and protein antigens are localized in the nasal mucosa. The nasal epithelial layer was sampled from patients with chronic rhinitis as described above. The samples were processed for EM. The representative pictures show exosomes stained with antibodies of SEB and Derp1. The larger gold particles (12 nm) indicate the labels of avb6; the smaller gold particles (6 nm) indicate the labels of Derp1. C-D, the gels show the immune blots of Derp1 (C) and SEB (D). The blots of beta-actin were used as internal loading controls. The data represent 3 experiments.
Nasal epithelial cell-derived exosome generation and purification
To further investigate the significance of the nasal mucosa-derived exosomes carrying microbial products and protein antigens as shown in Fig. 1, we performed a cell culture study. Human nasal epithelial cell line, RPMI2650 cells, was in the presence of mite antigens, Derp1, and SEB overnight. The supernatant was collected and processed for purification of exosomes. As observed by immune gold EM, exosomes were observed that contained Derp1 and SEB (Fig. 2A–C). The data from Western blotting confirmed the Derp1 and SEB were entrapped by exosomes (Fig. 2D).
Fig. 2.
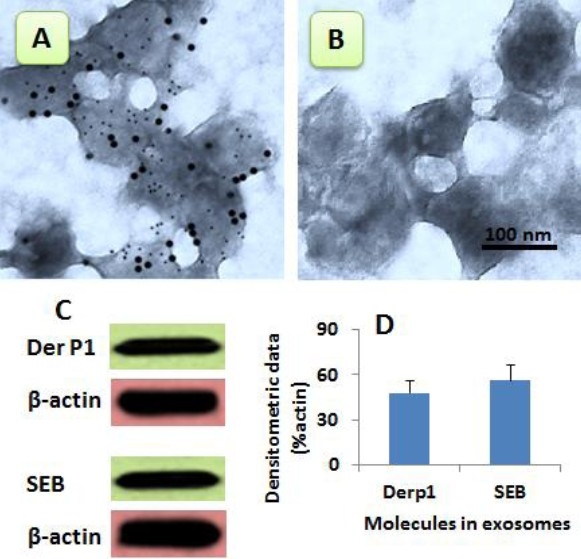
Generation of nasal mucosal cell-derived exosomes. A-B, the representative EM pictures show the nasal epithelial cell-derived exosomes carrying Derp1 and SEB (A) Panel B is an isotype control. C, proteins were extracted from purified exosomes and analyzed by Western blotting. The gel pictures show immune blots of Derp1 or SEB. The β-actin immune blots were used as internal control. D, bars indicate the summarized data of panel C. The data were presented as mean ± SD (from 3 experiments).
DCs capture nasal epithelial cell-derived exosomes
To determine if DCs could capture the epithelial cell-derived exosomes, we generated DCs from peripheral blood mononuclear cells (PBMC). The DCs were exposed to purified exosomes that carrying both SEB and Derpl. As examined by flow cytometry and immunocytochemistry, DCs only captured either SEB or Derpl if adding SEB and Derpl to the culture separately (Fig. 3A) while DCs captured both SEB and Derpl if adding SEB/Derpl-carrying exosomes to the culture (Fig. 3B).
Fig. 3.
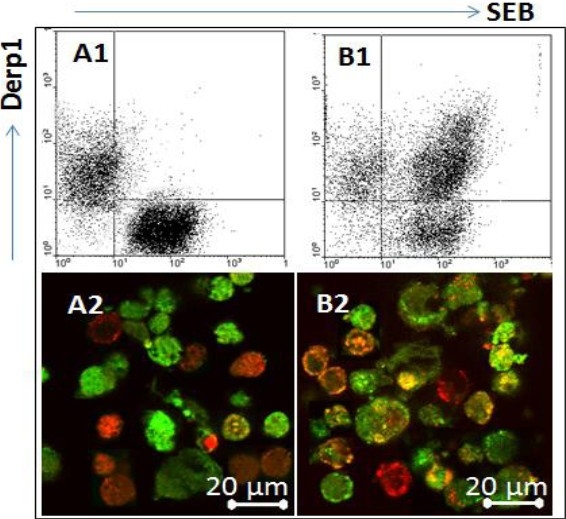
DCs can capture the nasal epithelial cell-derived exosomes. DCs were generated from isolated PBMC and cultured in RPMI1640 medium. Avb6, or Derp1, or SEB/Derp1-carrying exosomes were added to the culture. The cells were collected 6 h later and analyzed by flow cytometry and immunocytochemistry respectively. A1-B1, the flow cytometry dot plots show SEB+ or Derp1+ DCs. A2-B2, the representative confocal images show SEB+ staining (in green) and Derp1+ cells (in red). The yellow color was merged by green and red colors. The data represent 3 experiments.
DerplVSEB-carrying exosomes regulate the properties of DC
Since DCs disperse immediately under the epithelial layer, they certainly encounter the epithelial cell-released exosomes. How the exosomes regulate the properties of DCs is to be further understood. We therefore generated exosomes as described in Fig. 2. DCs were prepared with isolated human peripheral blood mononuclear cells as we reported previously [12]. The DCs were cultured in the presence of exosomes at graded concentrations for 5 days. The DCs were collected at the end of culture and analyzed by flow cytometry. The data showed that the stimulation of exosomes significantly increased the levels of CD80, CD86 and the major histocompatibility complex I (MHCI). The data indicate that the exposure to SEB/Derpl-carrying exosomes.
Nasal epithelial cell-derived exosomes from patients with chronic obstruction rhinitis promote antigen specific CD8+ cell differentiation
The finding that exosome-pulsed DCs expressed high levels of MHCI indicates that the DCs can facilitate the development of CD8+ T cells. To test the hypothesis, we isolated the CD3+ CD25- T cells from human PBMC; the cells were cultured with exosome-pulsed DCs for 3 rounds (each round consisted of 3 days). The cells were analyzed by flow cytometry. As shown by Fig.5, abundant CD8+CD25+ cells were generated. The cells were activated upon exposure to a specific antigen, Derpl, manifesting increase in perforin and granzyme B in culture supernatant.
Figure 5.
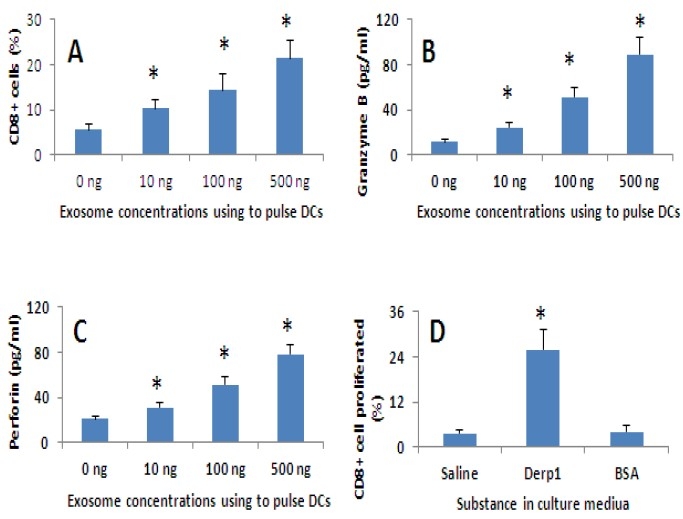
Exosome-pulsed DCs facilitate antigen specific CD8+ T cell differentiation. A-C. Human peripheral DCs were prepared from PBMC as described in the text. The DCs were pulsed with SEB/Derp1-carrying exosomes at the concentrations as indicated in panels A-C. Peripheral CD3+ CD25- T cells were isolated from PBMC. The DCs were cultured with CD3+ CD25- T cells at a ratio of 1:10 for 3 rounds (3 days/round). Cells were collected at the end of culture and analyzed by flow cytometry. A, bars indicate the frequency of CD8+ T cells. B-C, bars indicate the levels of granzyme B (B) and porforin (C) in culture medium. D. The cell culture procedures in panel D were the same as that in A-C except the CD3+ CD8+ T cells were labeled with CFSE prior to the culture. The specific antigen, Derp1, was added to the culture in the last culture round. The cells were collected at the end of culture and analyzed by CFSE-dilution assay. The bars indicate the frequency of proliferated CD8+ T cells. The data were presented as mean ± SD from 3 experiments. *, p<0.05, compared with the “0 ng” group in panels A-C and the saline group in panel D.
Fig. 4.
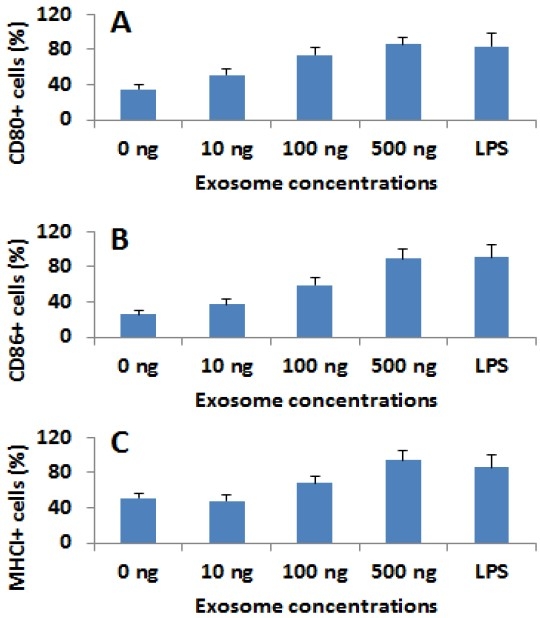
Nasal epithelial cell-derived exosomes modulate the properties of DCs. Human peripheral DCs were cultured in the presence of the nasal epithelial cell-derived exams at the indicated concentrations for 5 days. The cells were analyzed by flow cytometry. The bars indicate the percentage of cells of CD80 (A), CD86 (B) and MHCI (C). *, p<0.05, compared with the “0 ng” group. The data were presented as mean ± SD from 3 experiments. LPS: DCs were stimulated by LPS at 50 ng/ml in culture.
CD8+ T cells are increased in the nasal mucosa of patients with chronic obstructive rhinitis
The data in Fig.5 prompted us to observe the CD8+ T cells in the nasal mucosa. We collected surgically removed nasal mucosal specimens from 12 patients with chronic rhinitis. These patients had several skin test positive responses for one to several antigens; all of them were sensitized to mite Derpl. The patients did not have typical clinical symptoms of allergic rhinitis. Single cells were prepared from the collected nasal mucosa and analyzed for the frequency of CD8+ T cells by flow cytometry. The data showed that the frequency of CD8+ T cells was 22.8% ± 5.6% in the patients with chronic rhinitis while only 4.4% ± 3.2% (p<0.01, compared with the group of chronic rhinitis) was detected in the nasal mucosa collected from the marginal non-cancer tissue from patients with nasal cancer. To clarify if the CD8+ T cells were antigen specific, a portion of the isolated single cells were labeled with CFSE and cultured in the presence of specific antigens, Derpl for 3 days. As shown by the CFSE-dilution assay, 33.3% ± 11.5% cells proliferated. With the gating technique, 88.6% ± 15.4% proliferated cells were CD3+ CD8+ T cells. The results indicate that there are abundant antigen-specific CD8+ T cells in the nasal mucosa of patients with atypical chronic allergic rhinitis.
Discussion
The present study reports a set of novel data about a subgroup of chronic rhinitis, the atypical allergic rhinitis. These patients have detectable but low levels of antigen specific IgE, relatively high level of antigen specific IgG in the sera. The major clinical symptom of these patients is the chronic nasal obstruction caused by the inferior turbinate hypertrophy. Based on our previous studies, SEB plays a critical role in the allergic rhinitis [12] and allergic disorders in other organs [13] , we detected the absorption of SEB by the nasal mucosa; an exosome system was found to carry microbial product, SEB, to the subepithelial region. The purified exosomes or generated exosomes can regulate the properties of DCs; the pulsed DCs express high levels of CD80, CD86 and MHCI. These DCs can induce antigen specific CD8+ T cell development. The antigen specific CD8+ T cells can release inflammatory mediators upon exposure to the specific antigens.
The pathogenesis of chronic hypertrophic rhinitis is incompletely understood. Viral or bacterial infection is suggested as the primary causal factors; pus secretions can be another. It is usually observed in patients with persistent allergic rhinitis [14], idiopathic rhinitis and in long-standing septal deviation. Immune inflammation can be one of the factors in the initiation and perpetuation of chronic rhinitis. Our results are in line with the previous studies and revealed that the S. aureus-derived SEB played a role in chronic rhinitis [15,16]. Our study also revealed a previously undescribed mechanism in the pathogenesis of chronic hypertrophic rhinitis; the skewed antigen specific CD8+ T cell response might play a role.
DCs are the professional antigen presentation cells. DCs capture antigens to process and transport the antigen information to T cells. One of the features of DC capturing antigens is that once taking in one substance, the DCs mature quickly and do not capture another antigen anymore. Our results also confirmed this notion. The present study unraveled a novel aspect of DC in capturing antigens; DCs could capture exosomes. The exosomes released from the nasal epithelial cells entrapped microbial products and antigens, which enables a DC to capture more than one substance. Since the absorbed microbial products, such as SEB in the present study, promote the expression of MHCI, high levels of MHCI/antigen information may be passed to CD3+ T cells. The subsequent experimental data have supported this deduction; a large number of antigen specific CD8+ T cells were generated.
The CD8+ T cells are also called cytotoxic T cells. Upon activation, the CD8+ T cells release cytotoxic molecules, such as granzyme B and porforin, to the microenvironment. The molecules can cause other cells or invaded microbe apoptosis in a non-specific way; those bystander cells can be also killed or injured. The epithelial barrier function can be compromised in this way [17]. Since the epithelial barrier plays a critical role in maintaining the homeostasis in the tissue, the dysfunction of epithelial barrier can be one of the causes of chronic inflammation [18].
In summary, the present data indicate that nasal epithelial cell-derived exosomes contain microbial products and protein antigens; such exosomes can be detected in the patients with chronic atypical allergic rhinitis. Exosomes carrying microbial products and airborne antigens can promote DC maturation and express high levels of MHCI; the DCs can induce antigen specific CD8+ T cell development. Re-exposure to specific antigens trigger the antigen specific CD8+ T cells to release cytotoxic molecules, such as granzyme B and porforin, having the potential to initiate and perpetuate inflammation in the nasal mucosa.
Acknowledgments
This study was supported by grants from the Shenzhen-Hong Kong Innovation circle of Shenzhen Technology Program (SG200810220171A) and the Canadian Institutes of Health Research (CIHR #191063, #220058, #177843), Natural Science & Engineering Research Council of Canada (#371268).
References
- 1.Suh JD, Kennedy DW. Treatment Options for Chronic Rhinosinusitis. Proc Am Thorac Soc. 2011;8:132–140. doi: 10.1513/pats.201003-028RN. [DOI] [PubMed] [Google Scholar]
- 2.Timperley D, Schlosser RJ, Harvey RJ. Chronic rhinosinusitis: An education and treatment model. Otolaryngol Head Neck Surg. 2010;143:S3–S8. doi: 10.1016/j.otohns.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 3.Tomassen P, Zele TV, Zhang N, Perez-Novo C, Bruaene NV, Gevaert P, Bachert C. Pathophysiology of Chronic Rhinosinusitis. Proc Am Thorac Soc. 2011;8:115–120. doi: 10.1513/pats.201005-036RN. [DOI] [PubMed] [Google Scholar]
- 4.Greiner AN, Meltzer EO. Overview of the Treatment of Allergic Rhinitis and Nonallergic Rhinopathy. Proc Am Thorac Soc. 2011;8:121–31. doi: 10.1513/pats.201004-033RN. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Cutting GR. Chronic rhinosinusitis. Adv Otorhinolaryngol. 2011:114–21. doi: 10.1159/000322487. [DOI] [PubMed] [Google Scholar]
- 6.Sok J, Ferguson B. Differential diagnosis of eosinophilic chronic rhinosinusitis. Curr Allergy Asthma Rep. 2006;6:203–214. doi: 10.1007/s11882-006-0036-1. [DOI] [PubMed] [Google Scholar]
- 7.Sin B. Togias A: Pathophysiology of Allergic and Nonallergic Rhinitis. Proc Am Thorac Soc. 2011;8:106–114. doi: 10.1513/pats.201008-057RN. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Yadav PK, Xu X, et al. The increased expression of IL-23 in inflammatory bowel disease promotes intraepithelial and lamina propria lymphocyte inflammatory responses and cytotoxicity. J Leuko Biol. 2011;89:597–606. doi: 10.1189/jlb.0810456. [DOI] [PubMed] [Google Scholar]
- 9.Hennino A, Jean-Decoster C, Giordano-Labadie F, Debeer S, Vanbervliet B, RoziΦres A, Schmitt AM, Nicolas JF. CD8+ T cells are recruited early to allergen exposure sites in atopy patch test reactions in human atopic dermatitis. J Allergy Clin Immunol. 2011;127:1064–1067. doi: 10.1016/j.jaci.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Ye Z, Ye Z, Ahmed KA, et al. Active CD4+ helper T cells directly stimulate CD8+ cytotoxic T lymphocyte responses in wild-type and MHC II gene knockout C57BL/6 mice and transgenic RIP-mOVA mice expressing islet beta-cell ovalbumin antigen leading to diabetes. Autoimmunity. 2008 Jan 1;41:501–511. doi: 10.1080/08916930802069256. [DOI] [PubMed] [Google Scholar]
- 11.Luketic L, Delanghe J, Sobol PT, et al. Antigen Presentation by Exosomes Released from Peptide-Pulsed Dendritic Cells Is not Suppressed by the Presence of Active CTL. J Immunol. 2007;179:5024–5032. doi: 10.4049/jimmunol.179.8.5024. [DOI] [PubMed] [Google Scholar]
- 12.Liu T, He SH, Zheng PY, Zhang TY, Wang BQ, Yang PC. Staphylococcal enterotoxin B increases TIM4 expression in human dendritic cells that drives naive CD4 T cells to differentiate into Th2 cells. Mol Immunol. 2007;44:3580–3587. doi: 10.1016/j.molimm.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Yang PC, Xing Z, Berin CM, et al. TIM-4 Expressed by Mucosal Dendritic Cells Plays a Critical Role in Food Antigen-Specific Th2 Differentiation and Intestinal Allergy. Gastroenterology. 2007;133:1522–1533. doi: 10.1053/j.gastro.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Broide DH. Allergic rhinitis: Pathophysiology. Allergy Asthma Proc. 2010;31:370–374. doi: 10.2500/aap.2010.31.3388. [DOI] [PubMed] [Google Scholar]
- 15.Liu T, Song CH, Liu AM, et al. Forkhead box P3+ T cells express interleukin-17 in nasal mucosa of patients with both allergic rhinitis and polyposis. Clin Exp Immunol. 2011;163:59–64. doi: 10.1111/j.1365-2249.2010.04278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang N, Holtappels G, Gevaert P, et al. Mucosal tissue polyclonal IgE is functional in response to allergen and SEB. Allergy. 2011;66:141–148. doi: 10.1111/j.1398-9995.2010.02448.x. [DOI] [PubMed] [Google Scholar]
- 17.Epple H, Jr, Allers K, Tr÷ger H, et al. Acute HIV Infection Induces Mucosal Infiltration With CD4+ and CD8+ T Cells, Epithelial Apoptosis, and a Mucosal Barrier Defect. Gastroenterology. 2010;139:1289–300. doi: 10.1053/j.gastro.2010.06.065. [DOI] [PubMed] [Google Scholar]
- 18.Salim SY, Soderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362–381. doi: 10.1002/ibd.21403. [DOI] [PubMed] [Google Scholar]


