Abstract
Mammalian antimicrobial peptides (AMPs) play an important role in host defense via direct antimicrobial activity as well as immune regulation. The mouse cathelin-related antimicrobial peptide (mCRAMP), produced from the mouse gene Camp, is the only mouse cathelicidin identified and the ortholog of the human gene encoding the peptide LL-37. This study tested the hypothesis that mouse B and T cells produce and respond to mCRAMP. We show that all mature mouse B-cell subsets, including follicular (FO), marginal zone (MZ), B1a, and B1b cells, as well as CD4+ and CD8+ T cells produce Camp mRNA and mCRAMP protein. Camp−/− B cells produced equivalent levels of IgM, IgG3, and IgG2c but less IgG1 and IgE, while Camp−/− CD4+ T cells cultured in Th2-inducing conditions produced more IL-4-expressing cells when compared with WT cells, effects that were reversed upon addition of mCRAMP. In vivo, Camp−/− mice immunized with TNP-OVA absorbed in alum produced an enhanced TNP-specific IgG1 response when compared with WT mice. ELISpot analysis revealed increased numbers of TNP-specific IgG1-secreting splenic B cells and FACS analysis revealed increased CD4+ T-cell IL-4 expression. Our results suggest that mCRAMP differentially regulates B- and T-cell function and implicate mCRAMP in the regulation of adaptive immune responses.
Keywords: Antibody, Cytokine, IgG1, mCRAMP
Introduction
Mammalian antimicrobial peptides (AMPs) include the gene families of defensins and cathelicidins. Defensins are characterized by six conserved cysteine residues and various disulfide bond configurations, while cathelicidins are characterized by the presence of a conserved cathelin-like domain, an N-terminal signal sequence, and a highly variable antimicrobial C-terminal domain [1, 2]. AMPs have direct lytic properties against a variety of organisms including bacteria, fungi, and viruses [1, 2]. In addition, it is becoming increasingly appreciated that AMPs are also immunomodulatory. For example, AMPs have been shown to act as chemoattractants [3–5], protect skin and mucosal surfaces against bacterial infections [6–10], promote wound healing [11–13], and modulate changes in cellular function [14–18]. The mechanism by which AMPs modulate immune trafficking and function is not completely understood, although a number of potential receptors have been suggested for the human cathelicidin LL-37. These include EGFR [11, 13, 19], FPRL1 [3, 5], P2X7 [20, 21], GAPDH [22], and CXCR2 [23].
The mouse cathelin-related antimicrobial peptide (mCRAMP) is encoded by the gene Camp and is the sole identified mouse cathelicidin. Camp is the mouse ortholog of the only human cathelicidin gene (CAMP), which encodes the peptide LL-37 [24]. mCRAMP forms a positively charged amphipathic α-helical structure [25, 26] and has direct antimicrobial properties through a number of proposed mechanisms [27]. While mCRAMP and other AMPs have been studied mainly for their role in regulating innate cell activation, their role in the adaptive immune response has been studied less extensively. LL-37 is expressed in human B and T cells [4, 28]; however, mCRAMP expression in mouse lymphocytes has not been investigated.
Mature B cells play an important role in the adaptive immune response through antigen presentation, T-cell-independent (TI) and -dependent (TD) antibody production, and regulatory functions [29, 30]. A TD antibody response is a tightly regulated process that needs T- and B-cell cooperation for an optimal antibody response. T-cell membrane-bound CD40L and secreted IL-4 interact with B-cell membrane-bound CD40 and IL-4R, respectively, to induce class switching to IgG1 [31, 32] and IgE [33], which are important antibody isotypes produced in a wide variety of immune responses. The ability of mouse B and T cells to produce and respond to mCRAMP and its role in an adaptive immune response is not fully known.
We hypothesized that mouse B and T cells express and respond to mCRAMP. In the current study, we show that all mature B-cell subsets tested, including marginal zone (MZ), follicular (FO), B1a, and B1b cells as well as all mature T-cell subsets tested express CAMP mRNA and mCRAMP protein directly ex vivo. CAMP mRNA was rapidly upregulated in mouse B and T cells following activation. Purified CAMP−/− B cells produced equivalent levels of IgM, IgG3, and IgG2c but less IgG1 and IgE, while purified CAMP−/− CD4+ T cells cultured in Th2-inducing conditions produced more IL-4+ cells when compared with WT B and T cells, effects that were reversed upon addition of exogenous mCRAMP. In addition, immunization of CAMP−/− mice with TNP-OVA, a TD antigen, showed an enhanced TNP-specific secondary IgG1 antibody response, increased IgG1 anti-body-secreting cells (ASCs), and increased IL-4-producing T cells. Overall, these results suggest an additional function of mCRAMP in the regulation of adaptive immune responses.
Results
B and T cells express CAMP mRNA and mCRAMP protein
The human cathelicidin LL-37 is expressed in neutrophils, epithelial cells, mast cells, B cells, γΔ cells, and gd T cells (reviewed in [1, 28]), while the detailed expression of mCRAMP is less well described. To determine whether splenic B and T cells express mCRAMP, splenocytes from C57BL/6 mice were sort-purified to obtain MZ (B220+, CD21hi, CD23low) B cells, FO (B220+, CD21int, CD23+) B cells, CD4+ and CD8+ T cells. In addition, total peritoneal lavage cells were sort-purified to obtain B1a (CD5+ Mac-1+ B220int), B1b (CD5+ Mac-1+ B220int), B2 (CD5− Mac-1− B220high), and T cells (CD5+ B220−). Post-sort analysis revealed greater than 95% purity for each B- and T-cell population (data not shown). Total RNA was isolated from each sort-purified cell population and RT-PCR was performed to detect CAMP, CD19, CD3e, and actin mRNA. All B and T cells tested expressed CAMP mRNA directly ex vivo (Fig. 1A). To determine whether B and T cells express the mCRAMP protein, total protein was isolated from purified B and T cells and analyzed using Western blot. Figure 1B confirms the expression of the immature mCRAMP protein in the total resting B and T cells. To determine whether B and T cells regulate the expression of CAMP following cell activation, total CD43− splenic B cells were sort-purified and activated with CD40L and IL-4 or IFN-γ, while purified CD4+ T cells were cultured in either Th1- or Th2-inducing conditions. Real-time PCR analysis for the relative expression level of CAMP, normalized to actin expression, revealed that both B and T cells increased CAMP expression following activation (Fig. 1C). Interestingly, B and T cells express less CAMP mRNA and mCRAMP protein relative to purified neutrophils (Fig. 1B and C). In addition, total numbers of B- and T-cell subsets as well as serum antibody levels were equivalent between C57BL/6 and CAMP−/− mice (data not shown). These data show that all B and T cells tested express CAMP mRNA and mCRAMP protein, suggesting that mCRAMP has the potential to regulate B- and T-cell functions.
Figure 1.
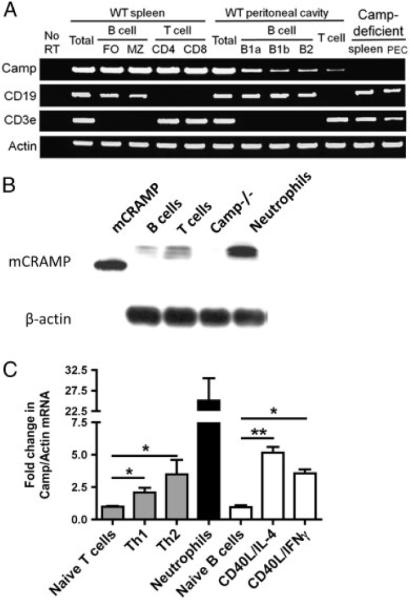
B and T cells express Camp and mCRAMP. MZ B cells, FO B cells, CD4+ and CD8+ T cells were purified from C57BL/6 spleens while B1a, B1b, B2, and total T cells were purified from peritoneal cavity washes. Total spleens and peritoneal cavity washes were also collected from Camp−/− (KO) mice. (A) Total RNA was isolated and analyzed using RT-PCR analysis for the mRNA levels of Camp, CD19, CD3e, and actin. (B) Total protein was isolated from synthetic mature mCRAMP, sort-purified CD43− B cells, CD4+CD62L+ naïve T cells, Camp−/− spleen, and neutrophils and analyzed by Western blot for the expression of mCRAMP and β-actin. (C) B and T cells were activated in vitro with the indicated stimuli and the level of Camp and actin mRNA was measured by real-time PCR and presented as fold change in Camp expression normalized to actin. Data represent the mean1SEM from three independent experiments. One representative gel and blot are shown. Data were analyzed by a one-way ANOVA followed by post hoc analysis. *p<0.05, *p<0.01.
CAMP-deficient T cells under Th2-inducing conditions produce more IL-4+ cells in vitro
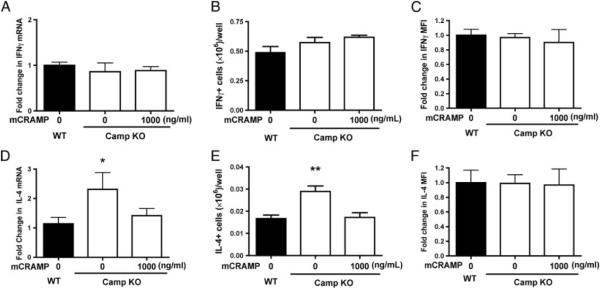
The ability of mCRAMP to directly regulate mouse T-cell cytokine production has not been fully investigated. WT and CAMP−/− naïve CD4+ T cells were sort-purified and cultured in either Th1 (anti-CD3, -CD28, and rIFN-γ) or Th2 (anti-CD3, -CD28, -IL-12, and rIL-4) inducing conditions. Under Th1-inducing conditions, WT and CAMP−/− T cells expressed equivalent amounts of IFN-γ mRNA (Fig. 2A), equivalent numbers of IFN-γ+ cells (Fig. 2B), and equivalent IFN-γ mean fluorescent intensity (MFI) (Fig. 2C). In contrast, CAMP−/− T cells cultured under Th2-inducing conditions expressed more IL-4 mRNA (Fig. 2D), more IL-4+ cells (Fig. 2E), and equivalent IL-4 MFI (Fig. 2F). The addition of mCRAMP to the CAMP−/− T-cell cultures had no effect on the level of IFN-γ mRNA or number of IFN-γ+ cells, while the level of IL-4 mRNA and IL-4+ cells returned to that of WT. Overall, these results suggest that mCRAMP also functions in the regulation of Th2 IL-4-producing cell differentiation.
Figure 2.
Camp-deficient T cells produce more IL-4 in vitro. Purified naïve CD4+CD62L+ T cells were cultured under Th1 (anti-CD3, -CD28, rIFN-γ)or Th2 (anti-CD3, -CD28, -IL-12, and rIL-4) driving conditions, in the presence or absence of mCRAMP. Total RNA was collected from WT (black bars) and Camp KO (white bars) cultures on day 4 and analyzed by RT-PCR for (A) IFN-γ and (D) IL-4. T cells were also collected and stained for intracellular (B and C) IFN-γ and (E and F) IL-4. (B and E) The total number and (C and F) fold change in MFI of IFN-γ and IL-4 are shown. Data represent the mean+ SEM from three independent experiments. Data were analyzed by a one-way ANOVA followed by post hoc analysis. *p<0.05, *p<0.01.
CAMP-deficient B cells produce less IgG1 and IgE antibody in vitro
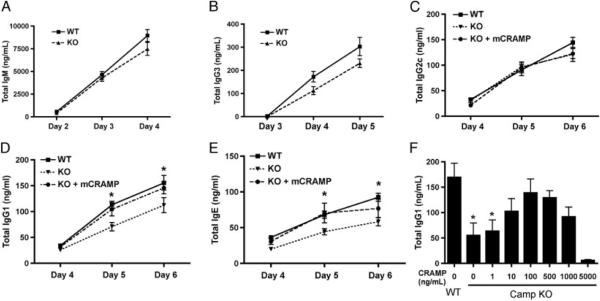
The role of mCRAMP during an antibody response to TI and TD antigens has not been fully investigated. Since B cells express CAMP/mCRAMP and CAMP is rapidly upregulated following B-cell activation, the possibility exists that mCRAMP directly regulates B cells during an antibody response. Furthermore, since LPS induces class switching to IgG3 [34] and IL-4 induces class switch recombination (CSR) to IgG1 and IgE [31], and IFN-γ induces CSR to IgG2a/2c [35], respectively, we hypothesized that mCRAMP mRNA upregulation during activation with these factors might affect the levels of specific antibody isotypes produced. Resting splenic B cells were sort-purified from WT and CAMP−/− mice and activated in vitro in the presence of LPS, CD40L/IL-4, and CD40L/IFN-γ. WT and CAMP−/− B cells produce similar amounts of IgM (Fig. 3A) and IgG3 (Fig. 3B) in response to LPS stimulation, while CD40L/IFN-γ induced equivalent amounts of IgG2c (Fig. 3C). However, CAMP−/− B cells produced significantly less IgG1 (Fig. 3D) and IgE (Fig. 3E) in response to CD40L/IL-4 when compared with WT B cells. To determine whether mCRAMP directly mediated these effects in vitro and the optimal peptide concentration, mCRAMP peptide (1 ng/mL–1 μg/mL) was added to CAMP−/− B-cell cultures on day 0 with CD40L/IL-4 and the level of IgG1 was measured on day 5. The addition of mCRAMP resulted in a dose-dependent increase in IgG1 with an optimal concentration of 100 ng/mL (Fig. 3F). CAMP−/− B cells cultures were repeated with the addition of 100 ng/mL of mCRAMP and the level of IgG2c (Fig. 3C) was unchanged while IgG1 (Fig. 3D) and IgE (Fig. 3E) returned to WT levels. Overall, these results suggest that mCRAMP functions to positively regulate the level of antibody produced by B cells in an IL-4-dependent manner.
Figure 3.
Camp-deficient B cells produce less IgG1 and IgE antibody in vitro. Resting CD43− WT and Camp−/− (KO) B cells were purified and cultured in the presence of LPS for the indicated number of days and cell culture supernatants were collected and analyzed by ELISA for the level of (A) IgM and (B) IgG3 antibody. B cells were also cultured with CD40L/IFN-γ or CD40L/IL-4, in the presence or absence of 100 ng/mL mCRAMP, and cell culture supernatant was collected and analyzed on days 4–6 for (C) IgG2c, (D) IgG1, and (E) IgE antibody, respectively. (F) CD40L/IL-4-activated Camp KO B cells were cultured in the presence of increasing concentrations of mCRAMP and total IgG1 was measured in culture supernatant on day 6. Data represent the mean+SEM from three independent experiments. Data with three or more groups were analyzed by a one-way ANOVA followed by post hoc analysis, while data with two groups were analyzed by a two-tailed unpaired t test. *p<0.05.
Camp-deficient B cells produce less IgG1 mRNA per cell
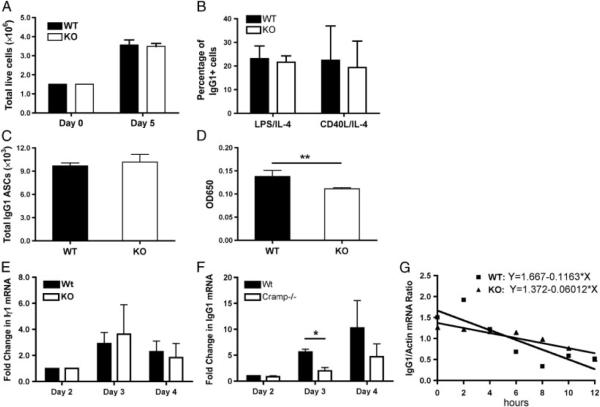
The mechanism by which Camp−/− B cells produce less IgG1 in comparison to WT B cells could be explained by a number of factors including differences in proliferation, survival, and CSR. To determine the mechanism by which Camp−/− B cells produce less IgG1, resting B cells were sort-purified and activated with CD40L/IL-4 or LPS/IL-4. The total live B-cell number (Fig. 4A), the percentage of surface IgG1+ B cells (Fig. 4B), and the cell cycle analysis (data not shown) were determined, showing no difference between WT and Camp−/− B cells. ELISpot experiments were performed on day 5 B-cell cultures and spots were enumerated to determine the number of IgG1-secreting B cells. Total spot counts were equivalent between WT and Camp−/− B cells (Fig. 4C), suggesting that CSR is not affected. However, visual inspection of the spot size of WT B cells appeared larger than that of Camp−/− B cell spots. Total ASC spots were dissolved with DMSO and the absorbance was measured at 650 nm (Fig. 4D), showing a significant decrease in absorbance in the Camp−/− B cells. Overall, these results suggest that the differences in IgG1 production between WT and Camp−/− B cells are not due to defects in proliferation, cell cycle progression, survival, or CSR, but are due to decreased IgG1 production per Camp−/− B cell.
Figure 4.
Camp-deficient B cells produce less IgG1 per cell, with no effect on proliferation, survival, or isotype switching. (A) Resting CD43− WT and Camp−/− (KO) B cells were sort-purified and total live CD40L/IL-4-activated B cells were enumerated on days 0 and 5. (B) The percentage of surface IgG1+ B cells in LPS/IL-4- and CD40L/IL-4-activated cultures was determined using flow cytometry. (C) The number of IgG1-secreting B cells was determined using ELISpot analysis on CD40L/IL-4-activated cells. (D) The IgG1 spots developed from the ELISpot procedure were dissolved using DMSO and the optical density was determined at 650 nm. (E and F) RT-PCR analysis was performed on days 2–4 after activation for the level of (E) Iγ1 and (F) IgG1 mRNA. (G) Actinomycin D was added to day 5 WT and Camp−/− B-cell cultures and total RNA was collected every 2 h for 12 h. RT-PCR was performed for the level of IgG1 mRNA at each time point and regression analysis was applied to estimate the stability of the mRNA transcript. Data represent the mean1SEM from three independent experiments. Data were analyzed by a two-tailed unpaired t test. *p<0.05, **p<0.01.
Differences in IgG1 production between WT and Camp−/− B cells could be explained if there was a change in CSR to IgG1 and a linear relationship has been shown between the amount of B-cell sterile Iγ1 transcript and CSR [36]. Alternatively, the amount of IgG1 mRNA production could be increased in the WT cells compared with Camp−/− cells. Therefore, to determine the amount of Iγ1 and IgG1 mRNA in WT and Camp−/− cells, B cells were sort-purified and activated as described earlier and total RNA was isolated on days 2–4. Semi-quantitative RT-PCR showed no significant difference in the levels of Iγ1 transcript over the time course analyzed (Fig. 4E), suggesting no change in CSR. However, the level of IgG1 mRNA was significantly higher in the WT compared with Camp−/− B cells (Fig. 4F), suggesting that mCRAMP was increasing either the rate or stability of the IgG1 mRNA. To determine the stability of the IgG1 mRNA, actinomycin D was added to the B-cell cultures on day 5 and total RNA was collected every 2 h for a total of 12 h. The stability of the IgG1 mRNA did not differ significantly between the WT and Camp−/− B cells (Fig. 4G). Thus, it appears that mCRAMP production by B cells increases the amount of IgG1 produced per cell by increasing the rate of IgG1 mRNA trancription, without affecting CSR or the stability of the IgG1 mRNA.
Camp-deficient mice produce more IgG1 in response to a TD antigen
Our data presented in Fig. 2 show that mCRAMP negatively regulates the level of T-cell IL-4 production in vitro, while our data presented in Fig. 3 show that mCRAMP positively regulates the level of B-cell IgG1 production in vitro. However, the antibody responses to TI-1, TI-2, and TD antigens have not been investigated extensively in Camp−/− mice to date. To investigate the antibody response in vivo to these three groups of antigens, WT and Camp−/− mice were immunized with either TNP-LPS (TI-1), S. pneumoniae (TI-2), or TNP-OVA absorbed to Alum (TD). The levels of IgM and IgG3 antibodies against TNP and phosphorylcholine(PC) were determined by ELISA and showed no significant difference between WT and Camp−/− mice (Fig. 5A–D), similar to our findings with LPS-activated B cells in vitro. Mice were also immunized i.p. and s.c. with TNP-OVA absorbed in alum on days 0 and 21 and the level of serum IgG1 antibody was measured. TNP-specific IgG1 was significantly higher in the Camp−/− mice following the second i.p. immunization (Fig. 5E) and first s.c. immunization (Fig. 5F). TNP-specific IgG2b and IgG2c were also determined and no differences were detected between WT and Camp−/− mice (data not shown). Overall, these results suggest that mCRAMP negatively regulates the TD antibody response in vivo, although the specific cell type responding to and affected by mCRAMP remains unknown.
Figure 5.
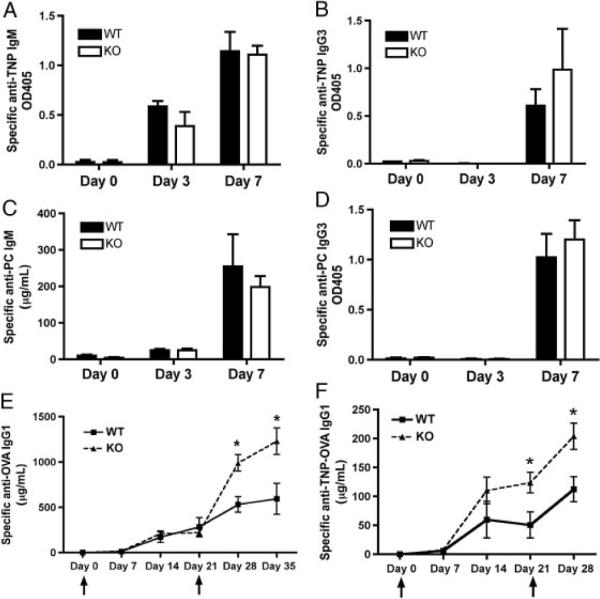
Camp-deficient mice produce more IgG1 in response to a T-cell-dependent antigen. (A–D) WT and Camp−/− (KO) mice were immunized with TNP-LPS or S. pneumoniae on day 0 and the level of serum antibody was measured using ELISA. The level of TNP-specific (A) IgM and (B) IgG3 as well as PC-specific (C) IgM and (D) IgG3 were measured on days 0, 3, and 7. (E and F) WT and Camp−/− (KO) mice were also immunized with TNPOVA/alum on days 0 and 21. The amount of TNP-OVA-specific IgG1 was measured following (E) i.p. or (F) s.c. immunizations. Data represent the mean1SEM from three independent experiments, five mice per group. ↑ injection. Data were analyzed by a two-tailed unpaired t test. *p<0.05.
CD4+ T cells produce more IL-4 and induce more IgG1+ASCs
The results of our in vivo immunization experiments in Fig. 5 suggest that mCRAMP is negatively regulating the antibody response to a TD antigen, TNP-OVA/Alum. Since our in vitro data suggest a differential regulation of B and T cells, we sought to determine the mechanism by which more TNP-specific IgG1 is made by Camp−/− mice compared with WT mice. ELISpot analysis of the spleens at 4 days after the second immunization with TNP-OVA/Alum shows that Camp−/− mice have more TNP-specific IgG1+ ASCs than WT (Fig. 6A). Since our in vitro data in Fig. 4 suggested that mCRAMP had no effect on isotype switching to IgG1, one potential explanation could be that the production of IL-4 was increased, similar to our findings in Fig. 2 with purified T cells in vitro. RT-PCR was performed to determine the level of total IL-4 mRNA in total spleen. Figure 6B shows that Camp−/− spleens contain more IL-4 mRNA than WT spleens. In addition, intracellular staining for IL-4 showed that the numbers of CD4+ IL-4+ T cells were significantly increased in the Camp−/− mice (Fig. 6C). Overall, these results suggest that mCRAMP negatively regulates TD antibody responses by regulation of T-cell IL-4 production.
Figure 6.
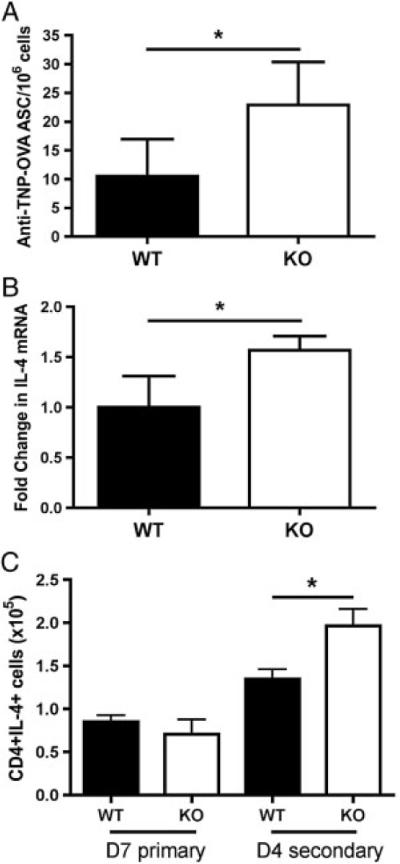
Camp-deficient mice produce more IL-4 and induce more antigen-specific IgG11 ASCs. Total splenic cells were isolated on days 7 and 4 following primary and secondary immunization with TNP-OVA/alum, respectively. (A) ELISpot analysis was performed on the splenic cells for anti-TNP-OVA IgG1 ASCs. (B) Total RNA was isolated and RTPCR analysis was performed for the level of IL-4 and actin mRNA, shown as fold change in IL-4 expression normalized to actin. (C) Flow cytometric analysis was performed and total CD41IL-41 T cells were determined by intracellular cytokine staining. Data represent the mean 1SEM from three independent experiments, five mice per group. Data were analyzed by a two-tailed unpaired t test. *p<0.05.
Discussion
Analysis of AMPs has shown that their cellular expression is widespread and their functions are diverse. Camp−/− mouse are more susceptible to, and fail to clear, numerous infections [1], supporting a role for AMPs in host defense and immune regulation. Our data showing that mouse B and T cells are capable of expressing and responding to mCRAMP further add to this complexity. Importantly, while the use of Camp−/− mice has aided in the study of AMP biology, it is not definitive in differentiating the direct antimicrobial activity from the immune regulation. In addition, our data show that mCRAMP has the ability to regulate B and T cells in vivo, although there is still no clarity as to the exact source of mCRAMP and the mechanism by which it regulates B- and T-cell function.
Using the Camp−/− mouse [24], we investigated the role of mCRAMP in regulating adaptive immune responses. Our data show that Camp−/− mice immunized with TNP-OVA/Alum produced more TNP-specific IgG1 antibody when compared with WT mice. In contrast, Kurosaka et al. showed that mCRAMP acted as an immune adjuvant and enhanced TD antibody production in WT mice [3]. The most obvious difference in the experiment design, which may contribute to the opposing findings, is that we studied effects of endogenously produced mCRAMP by comparing antibody responses in WT versus Camp−/− mice, while Kurosaka et al. [3] added additional exogenous mCRAMP to WT mice. The administration of exogenous mCRAMP to a WT mouse that is also making mCRAMP in response to the immunization may or may not accurately model the role of mCRAMP during an antibody response. In support of this possibility, previous studies have demonstrated that exogenous and endogenous mCRAMP function differently in macrophage activation [15]. An alternative explanation is the specific identity of the cathelicidin peptides produced by the Camp gene. It has been shown previously that alternative proteolytic processing is possible for endogenously expressed cathelicidin peptides, which may lead to different physiological effects in vivo [37]. Therefore, it is likely that the immunological response under investigation will be altered depending on the concentration, location, cell types, and the form of mCRAMP released during the response.
The role of AMPs in regulating the magnitude of the adaptive immune antibody responses has not been investigated extensively and the results to date are contradictory. LL-37 (20 μg/mL) was shown to decrease IgM and IgG2a production from mouse splenic B cells activated with LPS and IFN-γ, primarily through inhibition of cell activation and proliferation [16]. In contrast, another study demonstrated that LL-37 (6 μg/mL) increased the sensitivity of human peripheral B cells to CpG, enhancing B-cell activation and increasing IgM and IgG production [14]. Our data using mCRAMP (100 ng/mL) and purified mouse B cells agree with the latter study [14] and show that mCRAMP increases the amount of IgG1 and IgE antibody production in Camp−/− B cells. Of course, two obvious differences that may account for the discrepancies seen are the use of LL-37 versus mCRAMP peptides and mouse versus human B cells. In addition, another very important variable to consider is AMP concentration. Since it is nearly impossible to measure the physiological concentration within the splenic microenvironment where these responses are occurring, we titrated the mCRAMP concentration within our culture system ranging from 1 ng/mL to 10 μg/mL. Consistent with previous findings [38], our data showed that mCRAMP at the highest concentration tested induced cell apoptosis, while moderate concentrations increased IgG1 production, and the lowest concentration showed no effect on IgG1 production. These observations suggest that the AMP concentration within the microenviroment of an immune response may partially dictate the positive or negative effect on antibody production.
Our in vitro and in vivo data show that T cells exposed to mCRAMP produce less IL-4. However, the possibility exists that other cell types are affected by mCRAMP and secondarily affecting the T cells. LL-37 has been shown to drive mouse DC differentiation and enhance IL-6 and IL-12 production, while inhibiting IL-4 production. In addition, LL-37-exposed DCs increased IFN-γ production from T cells and polarize them to Th1 cells [39]. Our in vitro data clearly show that mCRAMP is capable of acting directly on purified T cells that were polarized to Th2 cells and decrease their IL-4 production. Similarly, our in vivo data show that T cells produced more IL-4 in the absence of mCRAMP expression. IL-4 is the critical cytokine for the IgG1 class switch, and its elevated expression in the Camp−/− spleen after secondary i.p. immunization is associated with an increased number of antigen-specific IgG1-secreting cells. These results suggest that endogenous mCRAMP regulates antigen-specific IgG1 production in vivo by suppressing CD4+ T-cell IL-4 expression, although whether this is a direct effect or indirect through another cell type is yet to be determined.
mCRAMP is an AMP that is beginning to be appreciated as a potent and important immunomodulatory molecule. While our data begin to elucidate the role of mCRAMP in the adaptive immune response, more information is needed to fully understand its role in the different microenvironments within the host. It is clear that the cell type producing and/or responding to mCRAMP will partially determine the effect observed. Additional studies are needed to fully understand the role of mCRAMP and other AMPs in the adaptive immune response.
Materials and methods
Animals
C57BL/6 mice were purchased from the Jackson Laboratory. Camp-deficient 129/SVJ mice (Camp−/−, KO) were backcrossed to B6 mice for ten generations and identified by PCR analysis as described previously [8]. All mice were maintained under pathogen-free conditions and under approved animal protocols from the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Peptide preparation and storage
The 38 amino acid mCRAMP peptide (ISRLAGLLRKGGEKIGEKLKKIGQKIKNFFQKLVPQPE) was synthesized by Alpha Diagnostic Int. (San Antonio, TX, USA) and the lyophilized peptides were resuspended in 0.01% acetic acid to generate 100 μM working stocks, which were stored at −80°C until time of use.
B-cell purification and activation
B-cell purification and activation was performed as described previously [40]. Purified splenic B cells were obtained using a CD43 magnetic bead depletion strategy (Miltenyi Biotec). B cells (5 × 104) were cultured in 96-well flat-bottom plates in 200μL of complete medium (cRPMI). B cells were stimulated with 20 μg/mL LPS (Sigma-Aldrich), 1 ng/mL recombinant mouse IL-4 (eBioscience), 10 ng/mL recombinant mouse IFN-γ (eBioscience), and/or CD40L-expressing Sf9 cells (a gift from Dr. Virginia Sanders, The Ohio State University) at a B cell-to-Sf9 ratio of 10:1. Culture supernatants were collected and stored at −80°C until further analysis.
Flow cytometry and cell sorting
Flow cytometry and cell sorting was performed as described previously [41]. Intracellular staining was performed using the Cytofix/Cytoperm kit (BD Biosciences). FITC-labeled anti-γ1, anti-CD23, anti-Mac-1; PE-labeled anti-CD5, anti-Mac 1, anti-IL-4; APC-labeled anti-B220, PE-Cy7-anti-CD4, PB-anti-B220, PE-anti-IL-4, and PE-rIgG1 isotype antibodies were purchased from BD Pharmingen. Anti-CD21 (clone 7G6) antibody was purified and labeled with PE in our laboratory. Cy5-labeled goat anti-mouse IgM antibody was purchased from Jackson ImmunoResearch. FcR blocker Ab93 was generated in our laboratory [42]. Experiments were performed on a FACSCalibur (BD Biosciences), cell sorting using a FACSAria (BD Biosciences), and analysis using FlowJo software (Tree Star).
Immunization
Seven- to nine-wk-old female mice were immunized i.v. with 1 × 108 heat-killed Streptococcus pneumonia (R36A) or i.p. with 100 μg TNP-LPS (Biosearch Technologies). Sera were collected on day 0 prior to immunization and days 3, 7, 14 after immunization. Mice were also immunized i.p. or s.c. with 100 μg TNP-OVA (Biosearch Technologies) absorbed in 4 mg alum (Sigma-Aldrich) on days 0 and 21. Sera were collected on day 0 prior to immunization and days 7, 14, 21, 28, and 35 after immunization.
ELISA
Total immunoglobulin levels were determined by ELISA, as described previously [43]. Briefly, total IgM, IgG3, IgG2c, IgG1, and IgE were captured by plate-bound goat anti-mouse IgM, IgG, or IgE and detected with alkaline phosphatase-conjugated goat anti-mouse IgM, IgG3, IgG2c, IgG1, and IgE (Southern Biotechnology Associates), respectively. A standard curve was prepared using known quantities of BH8 (anti-PC IgM, generated in our laboratory) or anti-TNP Ab (IgG1, eBioscience). To measure specific anti-PC or anti-TNP Abs concentration, plates were coated with PC-BSA or TNP-BSA. p-Nitrophenyl phosphate (Sigma-Aldrich) was added, and color development was determined on a Titertek Multiskan Plus reader (Labsystems, ICN Biomedicals) at 405 nm.
ELISPOT
The 96-well high-binding plates were coated with goat anti-mouse IgG or TNP-OVA and single-cell splenic suspensions were prepared 7 days after primary or secondary TNP-OVA/Alum immunization. In addition, 1 × 106 total splenocytes were seeded in each well containing 100 μL cRPMI followed by a 1:3 serial dilution. Cells were incubated at 37°C for 24 h before being lysed with PBS containing 0.05% Tween 20. Alkaline phosphatase-conjugated goat anti-mouse IgG1 was added and spots visualized by 5-bromo-4-chloro-3-indolyl phosphate (Sigma-Aldrich) and counted under a dissection microscope. Spots were then dissolved in 50 μL DMSO and absorbance of each well was measure with a spectrophotometer at 650 nm.
RT-PCR
RT-PCR was performed as described previously [41]. Briefly, total RNA was isolated using TRIzol (Invitrogen), cDNA was generated using the Omniscript RT-PCR kit (Qiagen), and PCR was performed using GoGreen Taq master mix (Promega) or SYBER green master mix (Invitrogen) at an annealing temperature of 60°C for 30–35 cycles. The following primer pairs were used: β-actin: 5′-TACAGCTTCACCACCACAGC-3′ and 5′-AAGGAAGGCTGGAAAAGAGC-3′; Camp: 5′-CGAGCTGTGGATGACTTCAA-3′ and 5′-CAGGCTCGTTACAGCTGATG-3′; CD19: 5′- GGAGGCAATGTTGTGCTGC-3′ and 5′- ACAATCACTAGCAAGATGCCC-3′; CD3e: 5′-ATGCGGTGGAACACTTTCTGG-3′ and 5′-GCACGTCAACTCTACACTGGT-3′; IL-4: 5′-ACCACAGAGAGTGAGCTCG-3′ and 5′-ATGGTGGCTCAGTACTACG-3′.
In vitro activation and differentiation of CD4+ T cells
Purified splenic naï CD4+ T cells (0.5 × 106 cells/mL) were obtained using negative selection followed by a CD62L+ magnetic bead selection (Miltenyi Biotec) and stimulated with 2 μg/mL plate-bound anti-CD3 and 2 μg/mL anti-CD28 (eBioscience). Cells were cultured in 96-well flat-bottom plates in 200μL of cRPMI with 1 ng/mL recombinant mouse IL-4, 10 ng/mL recombinant mouse IFN-γ, 5 μg/mL anti-IL-12 antibody (eBioscience), in the presence or absence of 100–1000 ng/mL mCRAMP peptide. Cells were incubated at 37°C for 4–7 days before analysis. For determination of in vivo IL-4 production, total splenocytes were isolated on days 7 and 4 following the primary and secondary immunizations. In total, 106 splenocytes were cultured in cRPMI in the presence or absence of 2.5 μg/mL ConA for 24 h. Brefeldin A was added after 19 h of stimulation, 5 h prior to analysis, and cells were collected and analyzed using flow cytometry.
Western blot
Western blot analysis was performed as described previously [43]. Briefly, protein samples (5–20 μg) were isolated and resolved by electrophoresis on a 4–20% gradient Tris-HCl gel, transferred to Immobilon-P polyvinylidene difluoride membrances (Millipore), probed with either anti-CRAMP (Santa Cruz) at a 1:200 dilution or anti-actin at a 1:10 000 dilution, detected with HRP-labeled secondary Ab at a 1:1000–1:10 000 dilution, and developed with the SuperSignal West Pico kit (Thermo Scientific).
Statistical analysis
Data with three or more groups were analyzed by a one-way ANOVA followed by post hoc analysis, while data with two groups were analyzed by a two-tailed unpaired t test. Statistically significant results were determined by a p value of *<0.05, **<0.01, ***<0.001.
Acknowledgements
This research is part of the dissertation research conducted by Yao Chen who is a pre-doctoral student in the Microbiology Graduate Program, University of Alabama at Birmingham, Birmingham, AL 35294, USA. The authors gratefully acknowledge Dr. Virginia M. Sanders (The Ohio State University) for generously sharing the Sf-9/CD40L cells, Dr. Mark Lisanby (University of Alabama at Birmingham) for backcrossing the Camp−/− mice to C57BL/6, and Dr. Tamer Mahmoud (University of Alabama at Birmingham) for critical reading of the manuscript. This work was supported by research funds from the National Institutes of Health (NIH) Grant AI14782 (J. F. K.), AR052728 (R. L. G.), and AI052453 (R. L. G.). J. F. K. is a recipient of a Senior Investigator Award from the American Asthma Foundation. N. W. K. is a recipient of an F32 NRSA Postdoctoral Fellowship Grant AI078662.
Abbreviations
- AMP
antimicrobial peptide
- ASC
antibody-secreting cell
- B6
C57BL/6
- Camp
cathelicidin gene
- CSR
class switch recombination
- FO
follicular
- LL-37
human cathelicidin
- mCRAMP
mouse cathelin-related antimicrobial peptide
- MZ
marginal zone
- TD
T-cell dependent
- TI
T-cell independent
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hancock RE, Scott MG. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA. 2000;97:8856–8861. doi: 10.1073/pnas.97.16.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurosaka K, Chen Q, Yarovinsky F, Oppenheim JJ, Yang D. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J. Immunol. 2005;174:6257–6265. doi: 10.4049/jimmunol.174.10.6257. [DOI] [PubMed] [Google Scholar]
- 4.Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- 5.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule-and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Garcia B, Lee PH, Yamasaki K, Gallo RL. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J. Invest. Dermatol. 2005;125:108–115. doi: 10.1111/j.0022-202X.2005.23713.x. [DOI] [PubMed] [Google Scholar]
- 7.Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, Hokfelt T, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 2006;12:636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 8.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 9.Lisanby MW, Swiecki MK, Dizon BL, Pflughoeft KJ, Koehler TM, Kearney JF. Cathelicidin administration protects mice from Bacillus anthracis spore challenge. J. Immunol. 2008;181:4989–5000. doi: 10.4049/jimmunol.181.7.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergman P, Johansson L, Wan H, Jones A, Gallo RL, Gudmundsson GH, Hokfelt T, et al. Induction of the antimicrobial peptide CRAMP in the blood-brain barrier and meninges after meningococcal infection. Infect. Immun. 2006;74:6982–6991. doi: 10.1128/IAI.01043-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin J, Yu FS. LL-37 via EGFR transactivation to promote high glucose-attenuated epithelial wound healing in organ-cultured corneas. Invest. Ophthalmol. Vis. Sci. 2010;51:1891–1897. doi: 10.1167/iovs.09-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heilborn JD, Nilsson MF, Kratz G, Weber G, Sorensen O, Borregaard N, Stahle-Backdahl M. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J. Invest. Dermatol. 2003;120:379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 13.Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, Yahata Y, et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J. Immunol. 2005;175:4662–4668. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 14.Hurtado P, Peh CA. LL-37 promotes rapid sensing of CpG oligodeoxynucleotides by B lymphocytes and plasmacytoid dendritic cells. J. Immunol. 2010;184:1425–1435. doi: 10.4049/jimmunol.0902305. [DOI] [PubMed] [Google Scholar]
- 15.Pinheiro da Silva F, Gallo RL, Nizet V. Differing effects of exogenous or endogenous cathelicidin on macrophage toll-like receptor signaling. Immunol. Cell Biol. 2009;87:496–500. doi: 10.1038/icb.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nijnik A, Pistolic J, Wyatt A, Tam S, Hancock RE. Human cathelicidin peptide LL-37 modulates the effects of IFN-gamma on APCs. J. Immunol. 2009;183:5788–5798. doi: 10.4049/jimmunol.0901491. [DOI] [PubMed] [Google Scholar]
- 17.Di Nardo A, Braff MH, Taylor KR, Na C, Granstein RD, McInturff JE, Krutzik S, et al. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J. Immunol. 2007;178:1829–1834. doi: 10.4049/jimmunol.178.3.1829. [DOI] [PubMed] [Google Scholar]
- 18.Morioka Y, Yamasaki K, Leung D, Gallo RL. Cathelicidin antimicrobial peptides inhibit hyaluronan-induced cytokine release and modulate chronic allergic dermatitis. J. Immunol. 2008;181:3915–3922. doi: 10.4049/jimmunol.181.6.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N, Rabe KF, Hiemstra PS. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J. Immunol. 2003;171:6690–6696. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 20.Nagaoka I, Tamura H, Hirata M. An antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and P2X7. J. Immunol. 2006;176:3044–3052. doi: 10.4049/jimmunol.176.5.3044. [DOI] [PubMed] [Google Scholar]
- 21.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J. Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 22.Mookherjee N, Lippert DN, Hamill P, Falsafi R, Nijnik A, Kindrachuk J, Pistolic J, et al. Intracellular receptor for human host defense peptide LL-37 in monocytes. J. Immunol. 2009;183:2688–2696. doi: 10.4049/jimmunol.0802586. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Cherryholmes G, Chang F, Rose DM, Schraufstatter I, Shively JE. Evidence that cathelicidin peptide LL-37 may act as a functional ligand for CXCR2 on human neutrophils. Eur. J. Immunol. 2009;39:3181–3194. doi: 10.1002/eji.200939496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallo RL, Kim KJ, Bernfield M, Kozak CA, Zanetti M, Merluzzi L, Gennaro R. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J. Biol. Chem. 1997;272:13088–13093. doi: 10.1074/jbc.272.20.13088. [DOI] [PubMed] [Google Scholar]
- 25.Pestonjamasp VK, Huttner KH, Gallo RL. Processing site and gene structure for the murine antimicrobial peptide CRAMP. Peptides. 2001;22:1643–1650. doi: 10.1016/s0196-9781(01)00499-5. [DOI] [PubMed] [Google Scholar]
- 26.Porcelli F, Verardi R, Shi L, Henzler-Wildman KA, Ramamoorthy A, Veglia G. NMR structure of the cathelicidin-derived human antimicrobial peptide LL-37 in dodecylphosphocholine micelles. Biochemistry. 2008;47:5565–5572. doi: 10.1021/bi702036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 28.Wah J, Wellek A, Frankenberger M, Unterberger P, Welsch U, Bals R. Antimicrobial peptides are present in immune and host defense cells of the human respiratory and gastrointestinal tracts. Cell Tissue Res. 2006;324:449–456. doi: 10.1007/s00441-005-0127-7. [DOI] [PubMed] [Google Scholar]
- 29.Martin F, Kearney JF. Marginal-zone B cells. Nat. Rev. Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 30.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4(+) T cell immunity. Nat. Rev. Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin SC, Wortis HH, Stavnezer J. The ability of CD40L, but not lipopolysaccharide, to initiate immunoglobulin switching to immunoglobulin G1 is explained by differential induction of NF-kappaB/Rel proteins. Mol. Cell. Biol. 1998;18:5523–5532. doi: 10.1128/mcb.18.9.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warren WD, Roberts KL, Linehan LA, Berton MT. Regulation of the germline immunoglobulin Cgamma1 promoter by CD40 ligand and IL-4: dual role for tandem NF-kappaB binding sites. Mol. Immunol. 1999;36:31–44. doi: 10.1016/s0161-5890(98)00114-x. [DOI] [PubMed] [Google Scholar]
- 33.de Vries JE, Punnonen J, Cocks BG, de Waal Malefyt R, Aversa G. Regulation of the human IgE response by IL4 and IL13. Res. Immunol. 1993;144:597–601. doi: 10.1016/s0923-2494(05)80009-4. [DOI] [PubMed] [Google Scholar]
- 34.Kearney JF, Cooper MD, Lawton AR. B cell differentiation induced by lipopolysaccharide. IV. Development of immunoglobulin class restriction in precursors of IgG-synthesizing cells. J. Immunol. 1976;117:1567–1572. [PubMed] [Google Scholar]
- 35.Snapper CM, Peschel C, Paul WE. IFN-gamma stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J. Immunol. 1988;140:2121–2127. [PubMed] [Google Scholar]
- 36.Lee CG, Kinoshita K, Arudchandran A, Cerritelli SM, Crouch RJ, Honjo T. Quantitative regulation of class switch recombination by switch region transcription. J. Exp. Med. 2001;194:365–374. doi: 10.1084/jem.194.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, Dorschner RA, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat. Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 38.Mader JS, Mookherjee N, Hancock RE, Bleackley RC. The human host defense peptide LL-37 induces apoptosis in a calpain- and apoptosis-inducing factor-dependent manner involving Bax activity. Mol. Cancer Res. 2009;7:689–702. doi: 10.1158/1541-7786.MCR-08-0274. [DOI] [PubMed] [Google Scholar]
- 39.Davidson DJ, Currie AJ, Reid GS, Bowdish DM, MacDonald KL, Ma RC, Hancock RE, Speert DP. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J. Immunol. 2004;172:1146–1156. doi: 10.4049/jimmunol.172.2.1146. [DOI] [PubMed] [Google Scholar]
- 40.Kin NW, Sanders VM. CD86 stimulation on a B cell activates the phosphatidylinositol 3-kinase/Akt and phospholipase C gamma 2/protein kinase C alpha beta signaling pathways. J. Immunol. 2006;176:6727–6735. doi: 10.4049/jimmunol.176.11.6727. [DOI] [PubMed] [Google Scholar]
- 41.Kin NW, Crawford DM, Liu J, Behrens TW, Kearney JF. DNA microarray gene expression profile of marginal zone versus follicular B cells and idiotype positive marginal zone B cells before and after immunization with Streptococcus pneumoniae. J. Immunol. 2008;180:6663–6674. doi: 10.4049/jimmunol.180.10.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliver AM, Grimaldi JC, Howard MC, Kearney JF. Independently ligating CD38 and Fc gammaRIIB relays a dominant negative signal to B cells. Hybridoma. 1999;18:113–119. doi: 10.1089/hyb.1999.18.113. [DOI] [PubMed] [Google Scholar]
- 43.Kin NW, Sanders VM. CD86 regulates IgG1 production via a CD19-dependent mechanism. J. Immunol. 2007;179:1516–1523. doi: 10.4049/jimmunol.179.3.1516. [DOI] [PubMed] [Google Scholar]