Abstract
Emerging studies from our laboratory demonstrate that interleukin-1 (IL-1) family members play a major role in impairing lacrimal gland functions. Here we have extended our investigations to observe the effects of IL-1 on aqueous tear production, lacrimal gland secretion, lacrimal gland histology, and acinar and ductal cell proliferation. We demonstrate that a single injection of IL-1 into the lacrimal glands inhibited neurally- as well as agonist-induced protein secretion resulting in decreased tear output. Meanwhile, IL-1 injection induced a severe, but reversible (7–13 days), inflammatory response that led to destruction of lacrimal gland acinar epithelial cells. Finally, we demonstrate that as the inflammatory response subsided and lacrimal gland secretion and tear production returned to normal levels, there was increased proliferation of acinar and ductal epithelial cells. Our work uncovers novel effects of IL-1 on lacrimal gland functions and the potential regenerative capacity of the mouse lacrimal gland.
Keywords: Inflammation, IL-1, dry eye, lacrimal gland, proliferation, regeneration, ocular surface, epithelial cells, secretion
The tear film, which is the interface between the external environment and the ocular surface, has several different functions. It forms a smooth refractive surface over the corneal surface and lubricates the eyelids (Lamberts, 1994, Tiffany and Bron, 1978). Moreover, it maintains an optimal extracellular environment for the epithelial cells of the cornea and conjunctiva because the electrolyte composition, osmolarity, pH, O2 and CO2 levels, nutrient levels, and concentration of growth factors in the tears are regulated within narrow limits (Lamberts, 1994, Tiffany and Bron, 1978). The tear film is a hydrated mucus gel covered by a lipid layer. Mucus are secreted by the cornea and conjunctiva (Gipson and Argueso, 2003). The aqueous component of the tear film is secreted by the main and accessory lacrimal glands (Dartt, 1994), whereas the meibomian glands secrete the lipid layer (Bron et al., 2004).
The lacrimal gland consists of acini, ducts, nerves, myoepithelial cells, and plasma cells (Dartt, 1994, Hodges and Dartt, 2003). Acinar cells compose about 80% of the gland and secrete electrolytes, water, and proteins to form primary fluid. As the primary fluid moves along the duct system, duct cells modify the primary fluid by secreting or absorbing electrolytes. The final lacrimal gland fluid is then secreted onto the surface of the eye. Lacrimal gland secretion is primarily under neural control, which is achieved through a neural reflex arc (Botelho, 1964). Stimuli to the ocular surface activate afferent sensory nerves in the cornea and conjunctiva (Botelho, 1964). This in turn activates efferent parasympathetic and sympathetic nerves in the lacrimal gland to stimulate secretion (Dartt, 1994, Hodges and Dartt, 2003). A decrease or lack of lacrimal gland secretion is the leading cause of aqueous tear deficient dry eye syndrome (Sullivan et al., 1997).
In several pathological instances, the lacrimal gland can become a target of the immune system and show signs of inflammation leading to inadequate tear secretion (Zoukhri, 2006). This can result from autoimmune diseases (Sjögren’s syndrome, sarcoidosis, diabetes) (Drosos et al., 1999, Fox, 1992, Nepp et al., 2000), organ transplantation (chronic graft versus host disease, cGVHD) (Ogawa and Kuwana, 2003), or simply as a result of aging (Rios et al., 2005). Inflammation of the lacrimal gland may lead to inadequate secretion of the aqueous layer of the tear film which is a leading cause of keratoconjunctivitis sicca (KCS) or dry eye syndromes (Schaumberg, Sullivan and Dana, 2002, Stern et al., 1998, Stern et al., 2004). To date, the mechanisms leading to lacrimal gland insufficiency during inflammation are not completely understood (Zoukhri, 2006). The hallmarks of lacrimal gland inflammation are the presence of focal lymphocytic infiltrates, increased production of proinflammatory cytokines, and destruction of the tear producing parenchymal cells (Zoukhri, 2006). Emerging studies from our laboratory suggest that interleukin-1 (IL-1) family members play a major role in impairing lacrimal gland functions (Zoukhri et al., 2002, Zoukhri et al., 2006).
IL-1 is a potent proinflammatory cytokine and consists of two isoforms, α and β, and a specific receptor antagonist, IL-1ra (Dinarello, 1996). There are two receptors for IL-1, a signaling receptor named IL-1RI and a decoy receptor named IL-1 RII (Dinarello, 1996). Activation of the IL-1RI triggers a signaling cascade leading to the transcription of several genes involved in acute and chronic inflammation and connective tissue diseases (Dinarello, 2000). IL-1 increases the expression of adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) on mesenchymal cells and vascular-cell adhesion molecule-1 (VCAM-1) on endothelial cells leading to the infiltration of inflammatory and immunocompetent cells into the extravascular space (Dinarello, 1996, Dinarello, 2000). IL-1 can be produced by many cell types, including macrophages, monocytes, lymphocytes, keratinocytes and fibroblasts in response to inflammation, infection, injury or other environmental challenges (Dinarello, 1996, Dinarello, 2000). Our previous research showed that lacrimal gland epithelial cells can produce IL-1β and that they express the IL-1RI (Zoukhri et al., 2002). Furthermore, using a murine model of human Sjögren’s syndrome, we showed that the amounts of IL-1β and IL-1RI proteins increased with age in the lacrimal gland in a disease specific manner (Zoukhri et al., 2002).
We previously reported that a single injection of IL-1 (β or α) into the lacrimal gland inhibited both neurally- as well as agonist-induced protein secretion measured 1 day after injection (Zoukhri et al., 2002). We also reported that IL-1 injection induced a severe inflammatory response in the lacrimal gland, which was manifested by an increase in the number of granulocytes and degranulating mast cells (Zoukhri et al., 2006). The purpose of the present studies was to extend those findings and determine the effect of IL-1α injection on aqueous tear production, lacrimal gland secretion, lacrimal gland histology, and acinar and ductal cell proliferation.
Materials and Methods
Chemicals
Phenylephrine, carbamylcholine chloride (carbachol), and hydrogen peroxide were from Sigma (St. Louis, MO). Amplex Red was from Molecular Probes (Eugene, OR). Recombinant human IL-1α was a generous gift from Dr. Craig W. Reynolds (Biological Resources Branch, National Cancer Institute Preclinical Repository, Rockville, MD).
Animals and treatment
Female BALB/c and C57BL/6mice (10–12 wk old) were purchased from Taconic (Germantown, NY). Animals were maintained in constant temperature rooms with fixed light/dark intervals of 12 hours’ length and were fed ad libitum. All experiments were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Tufts-New England Medical Center Animal Care and Use Committee. Animals were anesthetized and the exorbital lacrimal glands were left untreated (control) or were injected with either saline (vehicle) or rhIL-1α (1 µg) in a total volume of 2 µl.
Measurement of Aqueous Tear Production
Saline and IL-1α treated animals were lightly anesthetized (with a mixture of Ketamine and Xylazine) aqueous tear production was measured using phenol-impregnated cotton threads (Zone-Quick, Lacrimedics). The threads were held with jeweler forceps and applied to the ocular surface, on both eyes, in the lateral canthus for 10 (BALB/c) or 30 (C57BL/6) seconds. In preliminary experiments, we found that C57BL/6 mice produced less tears than the BALB/c strain. Wetting of the thread (which turns red in contact with tears) was measured in millimeters under a dissecting microscope.
Measurement of peroxidase secretion
Peroxidase secretion was measured as previously described (Zoukhri and Kublin, 2001, Zoukhri et al., 2006). Lacrimal glands were cut into small lobules (~ 2 mm diameter), placed in cell strainers, and incubated at 37°C in Krebs-Ringer bicarbonate buffer (KRB, containing in mM: 120 NaCl, 5 KCl, 1 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, and 25 NaHCO3) supplemented with 10 mM HEPES and 5.5 mM glucose (pH 7.4). The cell strainers containing lobules were transferred into fresh KRB solution every 20 min for a total of 60 min. The lobules were then incubated for 20 min in a total volume of 0.8 ml in normal KRB (referred to as spontaneous secretion) then in depolarizing KRB (evoked secretion) solution where the concentration of KCl was increased to 75 mM and that of NaCl was decreased to 50 mM to maintain isotonicity. Lacrimal gland lobules were further incubated for 20 min in 0.8 ml of normal KRB containing phenylephrine (an α1-adrenergic agonist, 10−4 M) or carbachol (a cholinergic agonist, 10−4 M). After incubation, the lobules were homogenized in 10 mM Tris-HCl (pH 7.5). The amount of peroxidase in the media and tissue homogenate was determined using Amplex Red. For the measurement of peroxidase, 0.1 ml of media and 0.01 ml of tissue homogenate were spotted in duplicate onto 96 well microplates. To each well was added 0.1 ml of assay buffer (50 mM Tris-HCl, pH 7.5) containing 0.2 M Amplex Red reagent and 0.2 M hydrogen peroxide. After incubation, the fluorescence was determined in a fluorescence microplate reader using 530 nm excitation wavelength and 590 nm emission wavelength. The amount of secreted peroxidase was expressed as percent of total: [peroxidase in media/(peroxidase in media + peroxidase in tissue)] × 100.
Histopathology and Immunocytochemistry
Lacrimal glands pieces were fixed, overnight at 4°C, in 4% formaldehyde made in phosphate buffered saline (PBS, containing in mM: 145 NaCl, 7.3 Na2HPO4, and 2.7 NaH2PO4 at pH 7.2). For histopathology experiments, paraffin sections of the lacrimal gland (6 µm) were stained with hematoxylin and eosin. For immunocytochemistry experiments, sections were deparaffinized and rehydrated using graded alcohols. Endogenous peroxidase was then quenched for 10 min with a hydrogen peroxide solution (3 %, in PBS). Slides were then subjected to microwave pretreatment (20 min) with antigen unmasking solution (Dako, Carpinteria, CA). After 3 washes in PBS, non-specific binding sites were blocked for 30 min using 10 % rabbit serum diluted in PBS. Slides were incubated overnight at 4°C with a rat monoclonal anti-Ki67 antibody (Dako) diluted 1:100 in PBS. After 3 washes in PBS, immunoreactivity was detected using the avidin-biotin peroxidase complex method (ABC kit; Vector Laboratories, Burlingame, CA). Diaminobenzidine (Vector Laboratories) was used as chromogen. Nuclei were lightly counterstained with hematoxylin before mounting.
Control experiments were performed to determine the specificity of Ki67 staining. Firstly, murine spleen was used as a positive control for Ki67 staining. Secondly, lacrimal gland sections from BALB/c and C57BL6 mice were incubated with irrelevant rat immunoglobulin, instead of the Ki67 antibody.
Data Presentation and Statistical Analysis
Data are expressed as means ± SEM. The data were statistically analyzed using Student's t-test for paired or unpaired values. Values of p < 0.05 were considered to be significant.
Results
1. Effect of IL-1α on aqueous tear production
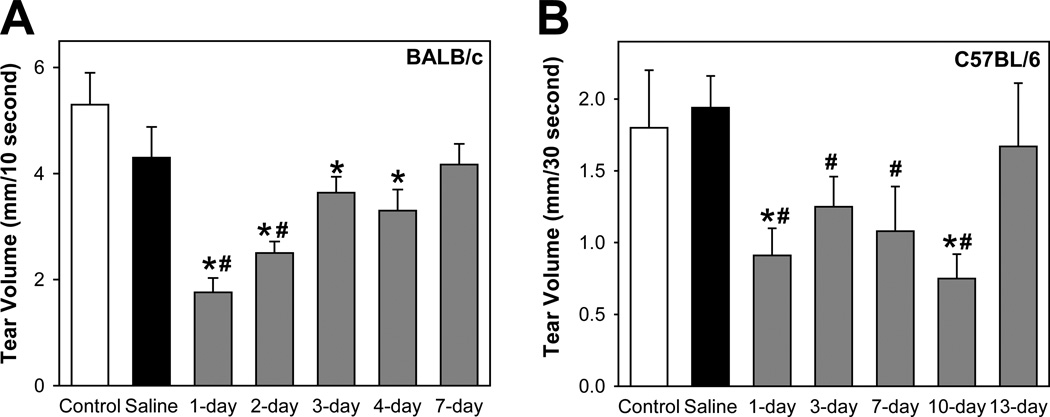
As shown in Figure 1A, in BALB/c mice, saline injection resulted in a slight, but not significant, reduction in tear production. In contrast, 1 day following injection of IL-1α, tear production was significantly decreased (1.8 ± 0.3 mm) compared to either control (5.3 ± 0.6 mm) or saline (4.3 ± 0.6 mm) treated animals (Fig. 1A). Tear production was still significantly lower in IL-1α treated animals at 2- (2.5 ± 0.2 mm), 3- (3.6 ± 0.3 mm), and 4- days (3.3 ± 0.4 mm) but returned to control levels by 7 days (4.3 ± 0.7 mm) (Fig. 1A).
Figure 1. Effect of IL-1α on aqueous tear production.
A. Female BALB/c mice were anesthetized and their exorbital lacrimal glands were either left untreated (control) or were injected with saline (vehicle) or IL-1α (1 µg). Aqueous tear production from both eyes was measured 1, 2, 3, 4, and 7 days post injection, using phenol-impregnated cotton threads for 10 seconds. Data are presented as mean ± SEM from 3 to 9 independent experiments. *Denotes statistically significant difference from saline. #Denotes statistically significant difference from control.
B. Female C57BL/6 mice were treated as described for BALB/c mice and aqueous tear production was measured 1, 3, 7, 10, and 13 days post injection, using phenol-impregnated cotton threads for 30 seconds. Data are presented as mean ± SEM from 3 to 6 independent experiments. *Denotes statistically significant difference from saline. #Denotes statistically significant difference from control.
To determine whether the transient and reversible inhibitory effects of IL-1α on tear production were inherent to the BALB/c strain, we conducted similar experiments on C57BL/6 mice, a strain with a different genetic background.
Untreated control female C57BL/6 mice produced significantly less tears (1.8 ± 0.4 mm, Fig. 1B) than BALB/c mice (5.3 ± 0.6 mm, Fig. 1). Nevertheless, 1 day following injection of IL-1α, tear production was significantly decreased (0.9 ± 0.2 mm) compared to either control (1.8 ± 0.4 mm) or saline (1.9 ± 0.2 mm) treated animals (Fig. 1B). Tear production was still significantly lower in IL-1α treated animals at 3- (1.2 ± 0.2 mm), 7- (1.08 ± 0.3 mm), and 10-days (0.8 ± 0.2 mm) but returned to control levels by 13 days (1.7 ± 0.4 mm) (Fig. 1B).
These results show that a single injection of IL-1α into the lacrimal gland resulted in decreased aqueous tear production. Inhibition of tear production was transient in both BALB/c and C57BL/6 mice albeit the inhibitory effect lasted longer in the latter strain.
2. Effect of IL-1α on lacrimal gland secretion
We previously reported that a single injection of IL-1α into the lacrimal gland inhibited both neurally- as well as agonist-induced protein secretion measured 1 day after injection. In the following series of experiments, we sought to determine if the reversible effects of IL-1α observed on tear production were paralleled on lacrimal gland secretion.
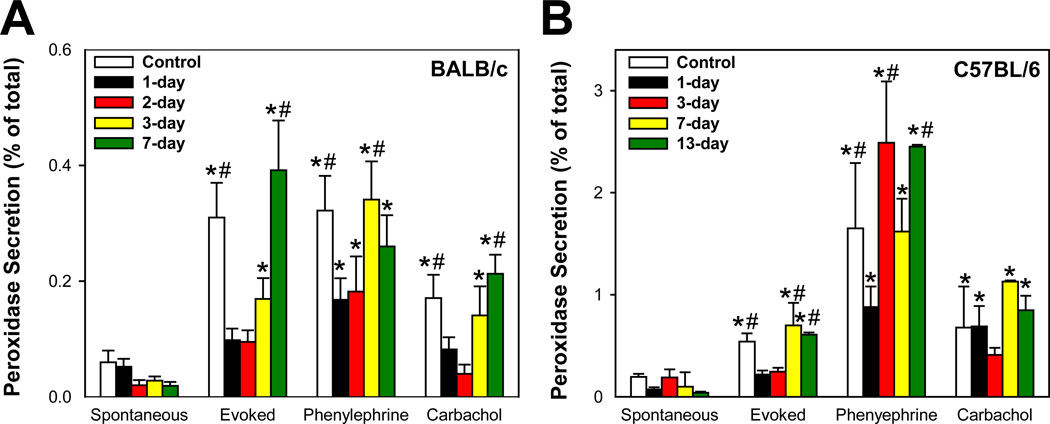
As shown in Figure 2A, compared to saline-injected BALB/c mice, KCl-induced lacrimal gland secretion was significantly inhibited by 82%, 70%, and 44% at 1-, 2-, and 3-days post IL-1α injection, respectively. KCl-induced lacrimal gland secretion (0.39 ± 0.09) measured 7 days post IL-1α injection was comparable to that measured in control animals (0.31 ± 0.06) (Fig. 2A). Similarly, compared to saline-injected mice, phenylephrine- and carbachol-stimulated protein secretion was significantly inhibited at 1- and 2-days post IL-1α injection and returned to control levels at 3- and 7-days post IL-1α injection (Fig 2A).
Figure 2. Effect of IL-1α on lacrimal gland protein secretion.
A. Female BALB/c mice were anesthetized and their exorbital lacrimal glands were either injected with saline (vehicle, control) or IL-1α (1 µg). Lacrimal gland lobules were prepared and protein secretion was measured 1, 2, 3, 4, and 7 days post injection. Lobules were incubated for 20 minutes each in normal KRB buffer (spontaneous), in depolarizing KRB (evoked) solution where the concentration of KCl was increased to 75 mM, then in KRB containing phenylephrine (an α1-adrenergic agonist, 10−4 M) or carbachol (a cholinergic agonist, 10−4 M). After incubation, the amount of peroxidase in the media and tissue homogenate was determined using Amplex Red, as described in the Methods section. Peroxidase secretion was expressed as percent of total (i.e.; [peroxidase in media/peroxidase media + peroxidase in tissue]*100). Data are the means ± SEM from 3 to 9 independent experiments. *Denotes statistically significant difference from spontaneous. #Denotes statistically significant difference from IL-1α.
B. Female C57BL/6 mice were treated as described for BALB/c mice and lacrimal gland protein secretion was measured 1, 3, 7, 10, and 13 days post injection. Data are the means ± SEM from 3 to 6 independent experiments. *Denotes statistically significant difference from spontaneous. #Denotes statistically significant difference from IL-1α.
Figure 2B shows that IL-1α injection into C57BL/6 mice lacrimal glands induced similar effects to those observed in BALB/c mice. KCl-induced protein secretion was significantly inhibited (compared to saline injected animals) at 1- and 3-days following IL-1α injection and returned to control levels 7–13 days post injection (Fig. 2B). However, the effects of IL-1 on phenylephrine and carbachol-induced protein secretion in C57BL/6 mice were less consistent (Fig. 2B). At present, we do not have an explanation for this discrepancy. Interestingly, compared to lacrimal glands from BALB/c mice, those from C57BL6 mice had higher peroxidase activity.
In both strains of mice, IL-1α injection did not affect the total amount of tissue peroxidase (Data not shown).
These results show that IL-1α induced impairment of KCl- and agonist-stimulated lacrimal gland secretion in both BALB/c and C57BL/6 mice strains is reversible and paralleled the reversal of the decreased aqueous tear output.
3. Effect of IL-1α on lacrimal gland histology
In a previous study, we showed that 24-hours after injection of IL-1 there was a severe inflammatory response in the lacrimal glands of BALB/c mice, which was manifested by an increase in the number of granulocytes and mast cells. Based on the results described above, we hypothesized that the recoveries of aqueous tear production and KCl- and agonist-stimulated protein secretion were due to the resolution of IL-1α induced lacrimal gland inflammation. To test this hypothesis, lacrimal gland removed from control and IL-1α injected animals were sectioned and stained with hematoxylin and eosin.
As shown in Figure 3A, lacrimal glands removed from saline-treated (or control, not shown) BALB/c mice presented a normal pattern of ductal and acinar cells containing secretory granules distributed in lobules separated by interlobular connective tissue. Mast cells and other immune cells were scarce and restricted to the connective tissue. In contrast, in IL-1α treated animals, there was a severe inflammatory response and edema with inflammatory cells invading the interlobular space and surrounding both acinar and ductal cells (Fig. 3A). This inflammatory response was evident 1-, 2-, and 3-days following IL-1α injection, with extensive tissue damage resulting in loss of acinar cells (Fig. 3A). Inflammation subsided between 4 and 7 days following IL-1α injection and lacrimal gland histology became comparable to that of saline treated and control non-injected animals (Fig. 3A).
Figure 3. Effect of IL-1α on lacrimal gland histology.
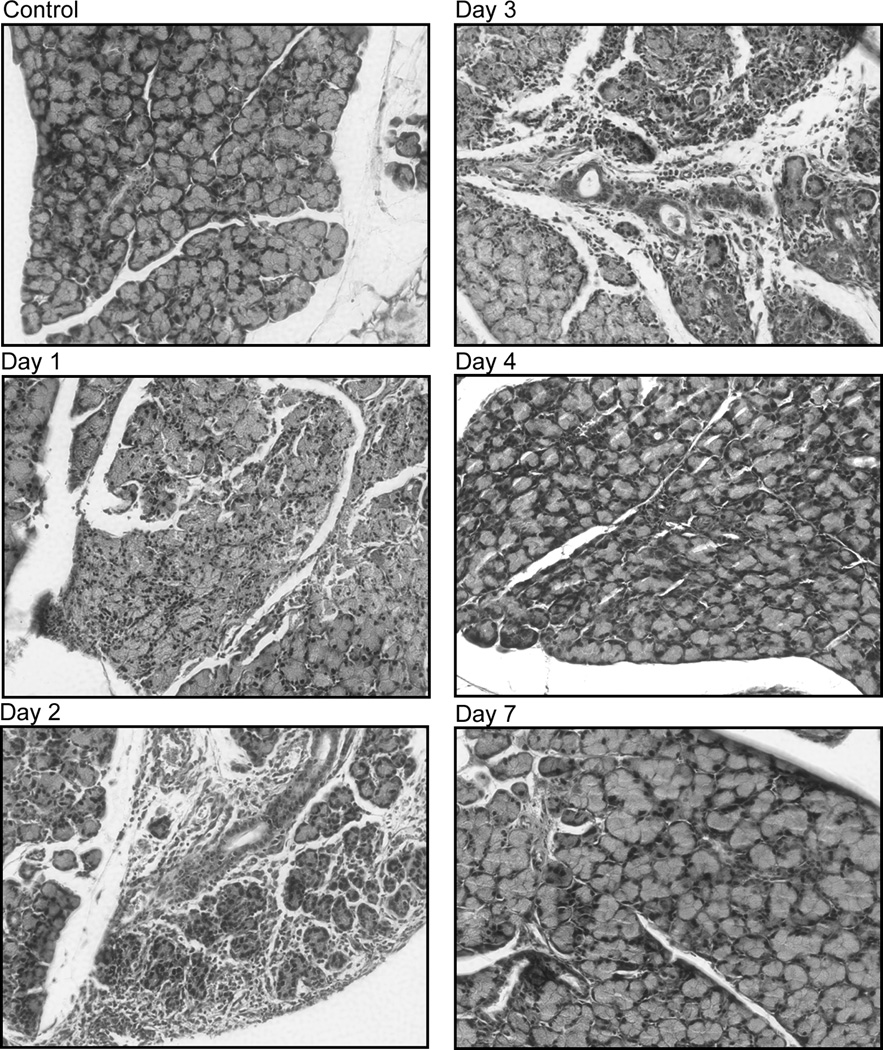
A. Female BALB/c mice were anesthetized and their exorbital lacrimal glands were either injected with saline (vehicle, control) or IL-1α (1 µg). Lacrimal glands were removed 1, 2, 3, 4, and 7 days post injection, sectioned and stained with hematoxylin and eosin. The experiment was repeated at least three times on lacrimal glands from separate animals. Magnification 200×.
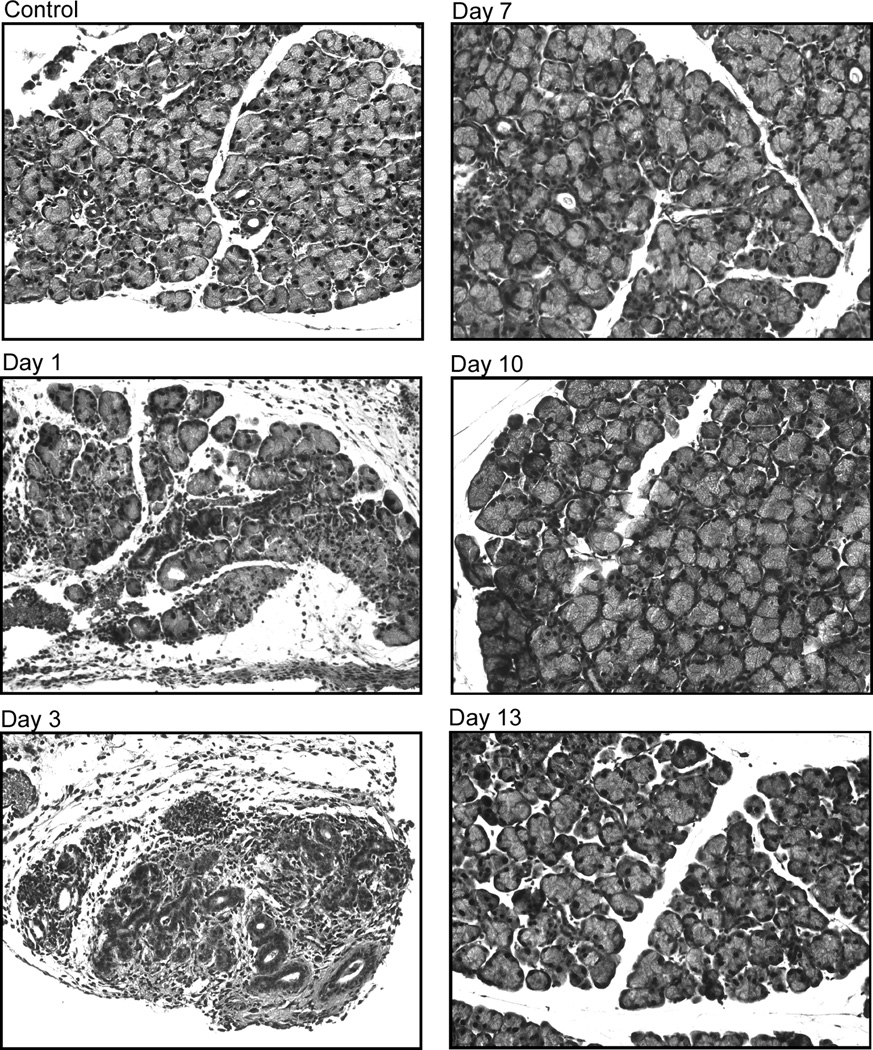
B. Female C57BL/6 mice were treated as described for BALB/c mice and lacrimal glands were removed 1, 3, 7, 10, and 13 days post injection and processed for hematoxylin and eosin staining. The experiment was repeated at least three times on lacrimal glands from separate animals. Magnification 200×.
Similar results were obtained in C57BL/6 mice with an inflammatory response that peaked between 1 and 7 days and subsided between 7 and13 days following IL-1α injection (Fig. 3B).
These results show that IL-1α injection into the lacrimal gland elicited a severe but reversible inflammatory response.
4. Effect of IL-1α on lacrimal gland epithelial cell proliferation
The results presented in Figure 3 suggest that, following trauma, murine lacrimal gland is capable of self-repair. To test this hypothesis, lacrimal gland sections from control and IL-1α injected animals, were stained with an antibody against Ki67, a proliferation-associated protein tightly bound by chromatin in all cells as they progress from S into G2 phase of the cell cycle (Brown and Gatter, 2002, Sawhney and Hall, 1992).
As depicted in Figure 4A, lacrimal glands from saline-injected (or control, not shown) BALB/c mice showed minimal to no Ki67 immunoreactivity. In contrast, 1 day after injection of IL-1α, there was intense Ki67 staining in the lacrimal gland that was almost exclusively associated with the infiltrating immune cells (Fig. 4A). By the second day following IL-1α injection, Ki67 immunoreactivity could be detected in the acinar and ductal epithelial cells and this pattern of staining was also evident in lacrimal glands from 3- and 4-day treated animals (Fig. 4A). Lacrimal gland ductal cells were stained more frequently when compared to the acinar cells. By the seventh day following IL-1α injection, Ki67 immunoreactivity declined slightly but could still be detected on acinar and ductal epithelial cells (Fig. 4A).
Figure 4. Effect of IL-1α on lacrimal gland prolifration.
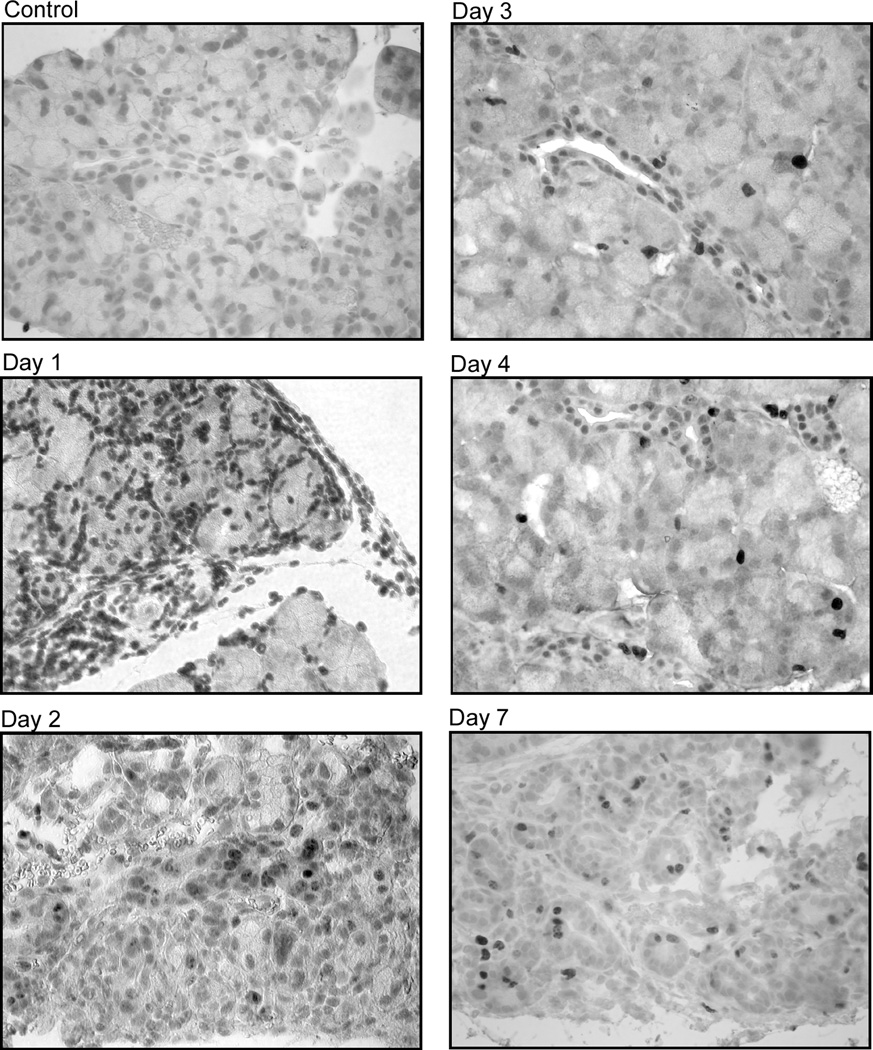
A. Female BALB/c mice were anesthetized and their exorbital lacrimal glands were either injected with saline (vehicle, control) or IL-1α (1 µg). Lacrimal glands were removed 1, 2, 3, 4, and 7 days post injection, sectioned and processed for immunocytochemistry using a rat monoclonal antibody against Ki67, as described in the Methods section. The experiment was repeated at least three times on lacrimal glands from separate animals. Magnification 400×.
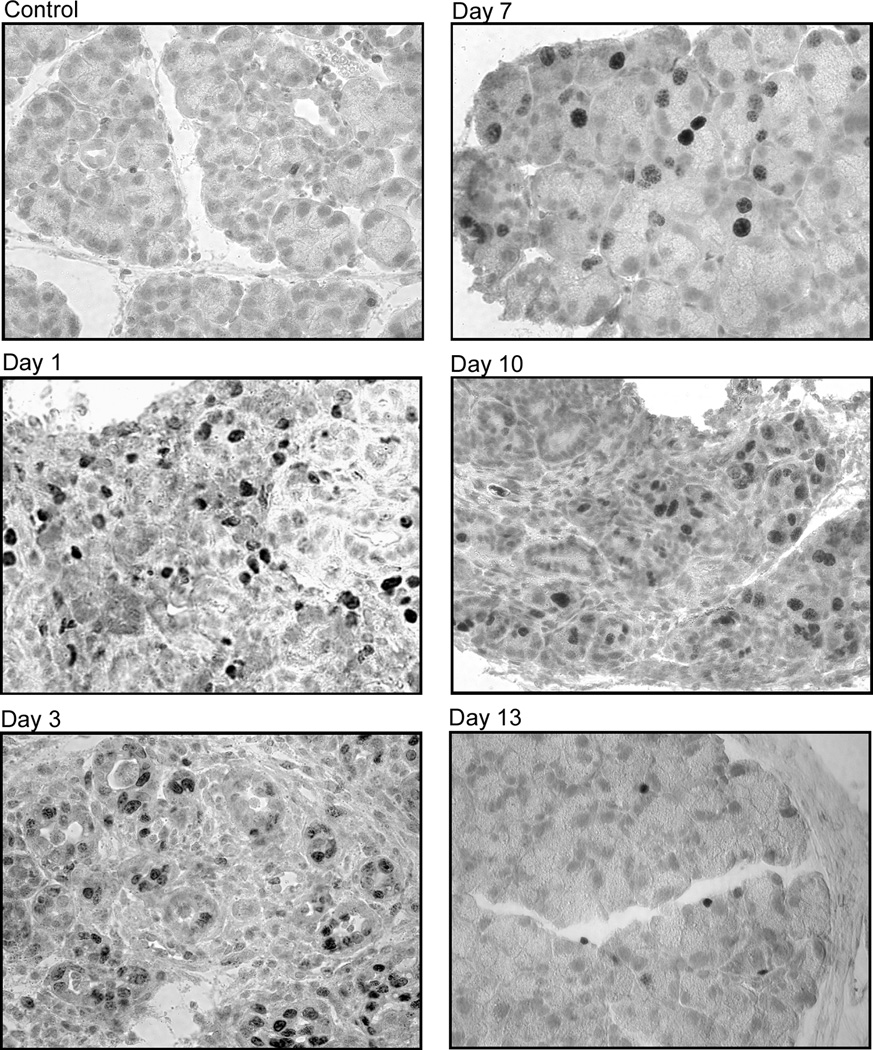
B. Female C57BL/6 mice were treated as described for BALB/c mice and lacrimal glands were removed 1, 3, 7, 10, and 13 days post injection and processed for Ki67 staining. The experiment was repeated at least three times on lacrimal glands from separate animals. Magnification 400×.
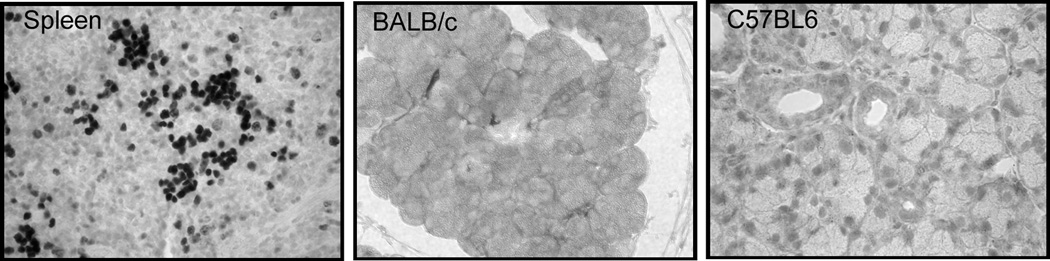
C. Spleen was removed from female BALB/c mice and processed for immunocytochemistry using a rat monoclonal antibody against Ki67, as described in the Methods section. Lacrimal gland sections from BALB/c and C57BL/6 mice were incubated with irrelevant rat immunoglobulin, instead of the Ki67 antibody. The experiment was repeated at least three times on lacrimal glands from separate animals. Magnification 200×.
Similar results were obtained when C57BL6 mice lacrimal glands were processed for Ki67 staining (Fig. 4B). Saline-injected (and control, not shown) lacrimal glands showed minimal or no staining whereas in those from IL-1α treated animals, Ki67 immunoreactivity was detected in acinar and ductal epithelial cells (Fig. 4B). The intensity of Ki67 staining declined with time but was still detectable on few acinar and ductal epithelial cells 13 days following IL-1α injection.
Control experiments were performed to determine the specificity of Ki67 staining. Firstly, murine spleen was used as a positive control for Ki67 staining (Fig. 4C). Secondly, when lacrimal gland sections from BALB/c and C57BL6 mice were incubated with irrelevant rat immunoglobulin, the immunostaining was abolished (Fig. 4C).
These results show that murine lacrimal glands are capable of self-repair following IL-1α induced inflammation.
Discussion
In previous reports we showed that injection of IL-1 into murine exorbital lacrimal glands lead to a severe inflammatory response and impaired protein secretion in response to both nerve as well as to exogenous agonist stimulations (Zoukhri et al., 2002). In the present studies, we extended those findings and showed that a single injection of IL-1α induced reversible aqueous-tear deficiency, inhibition of lacrimal gland secretion, lacrimal gland inflammation, and stimulated acinar and ductal cell proliferation.
In this study, we used to genetically different strains of mice to study the effect of IL-1 on lacrimal gland functions. BALB/c mice are described as Th2 prone whereas the C57BL6 mice are reported to be Th1 prone (Mosmann and Coffman, 1989). Our results show that the lacrimal glands of both strains are susceptible to the effects of IL-1. The only noticeable difference we found was that the time-course of lacrimal gland recovery was slower in the C57BL6 mice (13 days) compared to the 7 days it took for full recovery of lacrimal gland functions in BALB/c mice. This difference might prove to be instrumental when studying the molecular events associated with lacrimal gland repair in experimentally induced inflammation.
There is a large body of literature on the regenerative capacity of the salivary glands (Cummins et al., 1994, Dardick, Byard and Carnegie, 1990). Long term (7–21 days) ligation of the main excretory ducts of parotid (Burford-Mason et al., 1993, Burgess et al., 1996, Takahashi et al., 1999), submandibular (Takahashi et al., 2001, Takahashi et al., 2000, Takahashi et al., 2004, Takahashi et al., 2004), or sublingual salivary glands (Takahashi et al., 2005, Takahashi et al., 2003, Takahashi et al., 2002) leads to atrophy of these glands, and has been used as an experimental model of human diseases of the salivary glands. Salivary glands with ligated ducts show signs of inflammation, edema, and death of the acinar cells through apoptosis and/or necrosis (Burgess et al., 1996, Cummins et al., 1994, Takahashi et al., 2000, Takahashi et al., 2002, Takahashi et al., 2004). When the duct ligation is released, the salivary glands go through a process of repair through proliferation of acinar, ductal, and/or myoepithelial cells and synthesis and deposition of extracellular matrix (Burford-Mason et al., 1993, Burgess et al., 1996, Dardick et al., 1990, Takahashi et al., 1997, Takahashi et al., 2001, Takahashi et al., 2003, Takahashi et al., 2004). Similar findings were also reported in non-experimentally induced forms of chronic sialoadenitis (Ihrler et al., 2004).
Similarly, studies on the pancreas (Scoggins et al., 2000, Walker, 1987, Walker, Winterford and Kerr, 1992), liver (Giannelli, Quaranta and Antonaci, 2003, Kurosawa et al., 1997), intestine (Alison and Sarraf, 1994), and mammary glands (Walker, Bennett and Kerr, 1989) reported self-regenerating capabilities of these tissues. As for the salivary glands, these tissues go through a phase of inflammation/destruction of parenchymal cells followed by a period of proliferation/tissue repair. The studies described in this report show that murine lacrimal glands can also undergo self-repair when subjected to experimentally induced inflammation.
Several questions remain unanswered and will deserve further investigations. Firstly, through which process (i.e., apoptosis, necrosis, or both) are the lacrimal gland epithelial cells eliminated during experimentally induced inflammation? Studies on other exocrine tissues showed that following experimentally induced inflammation, the acinar cells are eliminated through apoptosis (Takahashi et al., 2000, Takahashi et al., 2002, Walker, 1987, Walker et al., 1989). In fact, it has been demonstrated that apoptosis of the pancreatic acinar cells is necessary for induction of cell proliferation in the regenerating tissues (Scoggins et al., 2000). Indeed, using p53 tumor suppressor gene (a gene involved in initiation of apoptosis)-null mice, Scoggins et al. (Scoggins et al., 2000) showed that, when compared to wild type mice, duct-ligated pancreatic cells not only did not undergo apoptosis but the associated proliferative response was inhibited. Secondly, which cells are responsible for the repair of the lacrimal gland following IL-1 induced inflammation and acinar cell loss? In the salivary glands, it was shown that all three cell types; acinar, ductal, and myoepithelial cells; actively proliferate during regeneration of these tissues (Burford-Mason et al., 1993, Burgess et al., 1996, Takahashi et al., 2005, Takahashi et al., 2003, Takahashi et al., 2004, Takahashi et al., 2004). Similar findings were reported in the regenerating pancreas and liver (Giannelli et al., 2003, Kelly, Reid and Walker, 1999, Kurosawa et al., 1997, Miyoshi et al., 1999). To date, it is still not completely understood if these proliferating cells arise from resident stem cells or if dedifferentiation of other cell types is responsible for tissue regeneration (Alison, Golding and Sarraf, 1997, Dardick et al., 1990, Giannelli et al., 2003). Thirdly, what factors are responsible for triggering lacrimal gland repair following experimentally induced inflammation? Remodeling of tissues following injury or trauma usually recapitulate the same cellular events that govern embryonic tissue development. It is therefore not surprising that a number of growth factors and cytokines known to regulate tissue development were found to also play a role during tissue regeneration (Okazaki et al., 2000).
In summary, our studies showed that experimentally induced inflammation of the lacrimal gland, by leading to loss of function, cell death, and proliferation of epithelial cells, mimics the effects of duct ligation on the salivary glands, the pancreas, and the liver pathophysiology. Therefore, we propose that IL-1 induced inflammation might offer a suitable experimental model to study the self-repair capacity of murine lacrimal glands. This model will allow investigations on the molecular mechanisms that govern lacrimal gland repair, which might unravel new strategies to promote repair of inflamed human lacrimal glands to restore adequate aqueous tear production.
Acknowledgements
The authors gratefully acknowledge and appreciate Darlene Dartt, Robin Hodges, and David Sullivan for critical reading of the manuscript, Dr. Craig Reynolds for the generous gift of recombinant human cytokines, and Dr. Fara Sourie for her invaluable contribution to this work. This study was supported by National Eye Institute grant RO1-EY12383.
References
- Alison MR, Golding M, Sarraf CE. Liver stem cells: when the going gets tough they get going. Int J Exp Pathol. 1997;78:365–381. doi: 10.1046/j.1365-2613.1997.500375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alison MR, Sarraf CE. The role of growth factors in gastrointestinal cell proliferation. Cell Biol Int. 1994;18:1–10. doi: 10.1006/cbir.1994.1001. [DOI] [PubMed] [Google Scholar]
- Botelho SY. Tears and the Lacrimal Gland. Sci Am. 1964;211:78–86. doi: 10.1038/scientificamerican1064-78. [DOI] [PubMed] [Google Scholar]
- Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78:347–360. doi: 10.1016/j.exer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Brown DC, Gatter KC. Ki67 protein: the immaculate deception? Histopathology. 2002;40:2–11. doi: 10.1046/j.1365-2559.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- Burford-Mason AP, Cummins MM, Brown DH, MacKay AJ, Dardick I. Immunohistochemical analysis of the proliferative capacity of duct and acinar cells during ligation-induced atrophy and subsequent regeneration of rat parotid gland. J Oral Pathol Med. 1993;22:440–446. doi: 10.1111/j.1600-0714.1993.tb00122.x. [DOI] [PubMed] [Google Scholar]
- Burgess KL, Dardick I, Cummins MM, Burford-Mason AP, Bassett R, Brown DH. Myoepithelial cells actively proliferate during atrophy of rat parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:674–680. doi: 10.1016/s1079-2104(96)80443-4. [DOI] [PubMed] [Google Scholar]
- Cummins M, Dardick I, Brown D, Burford-Mason A. Obstructive sialadenitis: a rat model. J Otolaryngol. 1994;23:50–56. [PubMed] [Google Scholar]
- Dardick I, Byard RW, Carnegie JA. A review of the proliferative capacity of major salivary glands and the relationship to current concepts of neoplasia in salivary glands. Oral Surg Oral Med Oral Pathol. 1990;69:53–67. doi: 10.1016/0030-4220(90)90269-x. [DOI] [PubMed] [Google Scholar]
- Dartt DA. Regulation of tear secretion. Adv Exp Med Biol. 1994;350:1–9. doi: 10.1007/978-1-4615-2417-5_1. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2174. [PubMed] [Google Scholar]
- Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- Drosos AA, Voulgari PV, Psychos DN, Tsifetaki N, Bai M. Sicca syndrome in patients with sarcoidosis. Rheumatol Int. 1999;18:177–180. doi: 10.1007/s002960050081. [DOI] [PubMed] [Google Scholar]
- Fox RI. Pathogenesis of Sjögren's syndrome. Rheum. Dis. Clin. North Am. 1992;18:517–538. [PubMed] [Google Scholar]
- Giannelli G, Quaranta V, Antonaci S. Tissue remodelling in liver diseases. Histol Histopathol. 2003;18:1267–1274. doi: 10.14670/HH-18.1267. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Argueso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003;231:1–49. doi: 10.1016/s0074-7696(03)31001-0. [DOI] [PubMed] [Google Scholar]
- Hodges RR, Dartt DA. Regulatory pathways in lacrimal gland epithelium. Int Rev Cytol. 2003;231:129–196. doi: 10.1016/s0074-7696(03)31004-6. [DOI] [PubMed] [Google Scholar]
- Ihrler S, Blasenbreu-Vogt S, Sendelhofert A, Rossle M, Harrison JD, Lohrs U. Regeneration in chronic sialadenitis: an analysis of proliferation and apoptosis based on double immunohistochemical labelling. Virchows Arch. 2004;444:356–361. doi: 10.1007/s00428-003-0964-2. [DOI] [PubMed] [Google Scholar]
- Kelly L, Reid L, Walker NI. Massive acinar cell apoptosis with secondary necrosis, origin of ducts in atrophic lobules and failure to regenerate in cyanohydroxybutene pancreatopathy in rats. Int J Exp Pathol. 1999;80:217–226. doi: 10.1046/j.1365-2613.1999.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa H, Que FG, Roberts LR, Fesmier PJ, Gores GJ. Hepatocytes in the bile duct-ligated rat express Bcl-2. Am J Physiol. 1997;272:G1587–G1593. doi: 10.1152/ajpgi.1997.272.6.G1587. [DOI] [PubMed] [Google Scholar]
- Lamberts DW. In: Physiology of the tear film in The Cornea. Smolin G, Thoft RA, editors. Boston: Little, Brown and Company; 1994. pp. 439–483. [Google Scholar]
- Miyoshi H, Rust C, Roberts PJ, Burgart LJ, Gores GJ. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology. 1999;117:669–677. doi: 10.1016/s0016-5085(99)70461-0. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Nepp J, Abela C, Polzer I, Derbolav A, Wedrich A. Is there a correlation between the severity of diabetic retinopathy and keratoconjunctivitis sicca? Cornea. 2000;19:487–491. doi: 10.1097/00003226-200007000-00017. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Kuwana M. Dry eye as a major complication associated with chronic graft-versus-host disease after hematopoietic stem cell transplantation. Cornea. 2003;22:S19–S27. doi: 10.1097/00003226-200310001-00004. [DOI] [PubMed] [Google Scholar]
- Okazaki Y, Kagami H, Hattori T, Hishida S, Shigetomi T, Ueda M. Acceleration of rat salivary gland tissue repair by basic fibroblast growth factor. Arch Oral Biol. 2000;45:911–919. doi: 10.1016/s0003-9969(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Rios JD, Horikawa Y, Chen LL, Kublin CL, Hodges RR, Dartt DA, Zoukhri D. Age-dependent alterations in mouse exorbital lacrimal gland structure, innervation and secretory response. Exp Eye Res. 2005;80:477–491. doi: 10.1016/j.exer.2004.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney N, Hall PA. Ki67--structure, function, and new antibodies. J Pathol. 1992;168:161–162. doi: 10.1002/path.1711680202. [DOI] [PubMed] [Google Scholar]
- Schaumberg DA, Sullivan DA, Dana MR. Epidemiology of dry eye syndrome. Adv Exp Med Biol. 2002;506:989–998. doi: 10.1007/978-1-4615-0717-8_140. [DOI] [PubMed] [Google Scholar]
- Scoggins CR, Meszoely IM, Wada M, Means AL, Yang L, Leach SD. p53-dependent acinar cell apoptosis triggers epithelial proliferation in duct-ligated murine pancreas. Am J Physiol Gastrointest Liver Physiol. 2000;279:G827–G836. doi: 10.1152/ajpgi.2000.279.4.G827. [DOI] [PubMed] [Google Scholar]
- Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78:409–416. doi: 10.1016/j.exer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Sullivan DA, Wickham LA, Krenzer KL, Rocha EM, Toda I. In: Aqueous tear deficiency in Sjögren's syndrome: possible causes and potential treatment, in Oculodermal Diseases. Pleyer U, Hartmenn C, editors. Buren: Aeolus Press; 1997. pp. 95–152. [Google Scholar]
- Takahashi S, Domon T, Yamamoto T, Wakita M. Regeneration of myoepithelial cells in rat submandibular glands after yttrium aluminium garnett laser irradiation. Int J Exp Pathol. 1997;78:91–99. doi: 10.1046/j.1365-2613.1997.d01-244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Nakamura S, Domon T, Yamamoto T, Wakita M. Active participation of apoptosis and mitosis in sublingual gland regeneration of the rat following release from duct ligation. J Mol Histol. 2005;36:199–205. doi: 10.1007/s10735-005-1764-6. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Nakamura S, Shinzato K, Domon T, Yamamoto T, Wakita M. Apoptosis and proliferation of myoepithelial cells in atrophic rat submandibular glands. J Histochem Cytochem. 2001;49:1557–1564. doi: 10.1177/002215540104901209. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Nakamura S, Suzuki R, Domon T, Yamamoto T, Wakita M. Changing myoepithelial cell distribution during regeneration of rat parotid glands. Int J Exp Pathol. 1999;80:283–290. doi: 10.1046/j.1365-2613.1999.00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Nakamura S, Suzuki R, Islam N, Domon T, Yamamoto T, Wakita M. Apoptosis and mitosis of parenchymal cells in the duct-ligated rat submandibular gland. Tissue Cell. 2000;32:457–463. doi: 10.1016/s0040-8166(00)80002-6. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Shinzato K, Domon T, Yamamoto T, Wakita M. Proliferation and distribution of myoepithelial cells during atrophy of the rat sublingual gland. J Oral Pathol Med. 2003;32:90–94. doi: 10.1034/j.1600-0714.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Shinzato K, Domon T, Yamamoto T, Wakita M. Mitotic proliferation of myoepithelial cells during regeneration of atrophied rat submandibular glands after duct ligation. J Oral Pathol Med. 2004;33:430–434. doi: 10.1111/j.1600-0714.2004.00234.x. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Shinzato K, Nakamura S, Domon T, Yamamoto T, Wakita M. The roles of apoptosis and mitosis in atrophy of the rat sublingual gland. Tissue Cell. 2002;34:297–304. doi: 10.1016/s0040816602000034. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Shinzato K, Nakamura S, Domon T, Yamamoto T, Wakita M. Cell death and cell proliferation in the regeneration of atrophied rat submandibular glands after duct ligation. J Oral Pathol Med. 2004;33:23–29. doi: 10.1111/j.1600-0714.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- Tiffany JM, Bron AJ. Role of tears in maintaining corneal integrity. Trans Ophthalmol Soc U K. 1978;98:335–338. [PubMed] [Google Scholar]
- Walker NI. Ultrastructure of the rat pancreas after experimental duct ligationI. The role of apoptosis and intraepithelial macrophages in acinar cell deletion. Am J Pathol. 1987;126:439–451. [PMC free article] [PubMed] [Google Scholar]
- Walker NI, Bennett RE, Kerr JF. Cell death by apoptosis during involution of the lactating breast in mice and rats. Am J Anat. 1989;185:19–32. doi: 10.1002/aja.1001850104. [DOI] [PubMed] [Google Scholar]
- Walker NI, Winterford CM, Kerr JF. Ultrastructure of the rat pancreas after experimental duct ligation. II. Duct and stromal cell proliferation, differentiation, and deletion. Pancreas. 1992;7:420–434. doi: 10.1097/00006676-199207000-00002. [DOI] [PubMed] [Google Scholar]
- Zoukhri D. Effect of inflammation on lacrimal gland function. Exp Eye Res. 2006;82:885–898. doi: 10.1016/j.exer.2005.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoukhri D, Hodges R, Byon B, Kublin L. Role of proinflammatory cytokines in the impaired lacrimation associated with autoimmune xerophthalmia. Invest Ophthalmol Vis Sci. 2002;43:1429–1436. [PMC free article] [PubMed] [Google Scholar]
- Zoukhri D, Kublin CL. Impaired neurotransmitter release from lacrimal and salivary gland nerves of a murine model of Sjögren's syndrome. Invest Ophthalmol Vis Sci. 2001;42:925–932. [PMC free article] [PubMed] [Google Scholar]
- Zoukhri D, Macari E, Choi SH, Kublin CL. c-Jun NH2-terminal kinase mediates interleukin-1β-induced inhibition of lacrimal gland secretion. J Neurochem. 2006;96:126–135. doi: 10.1111/j.1471-4159.2005.03529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]