Abstract
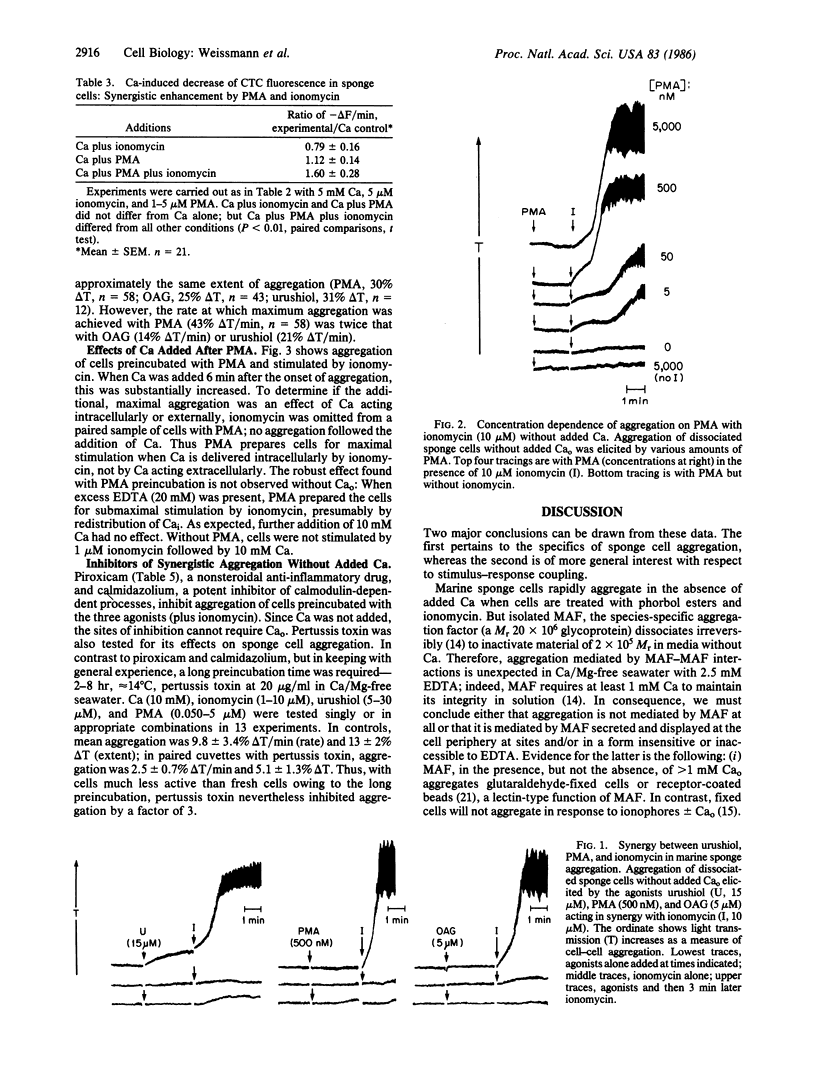
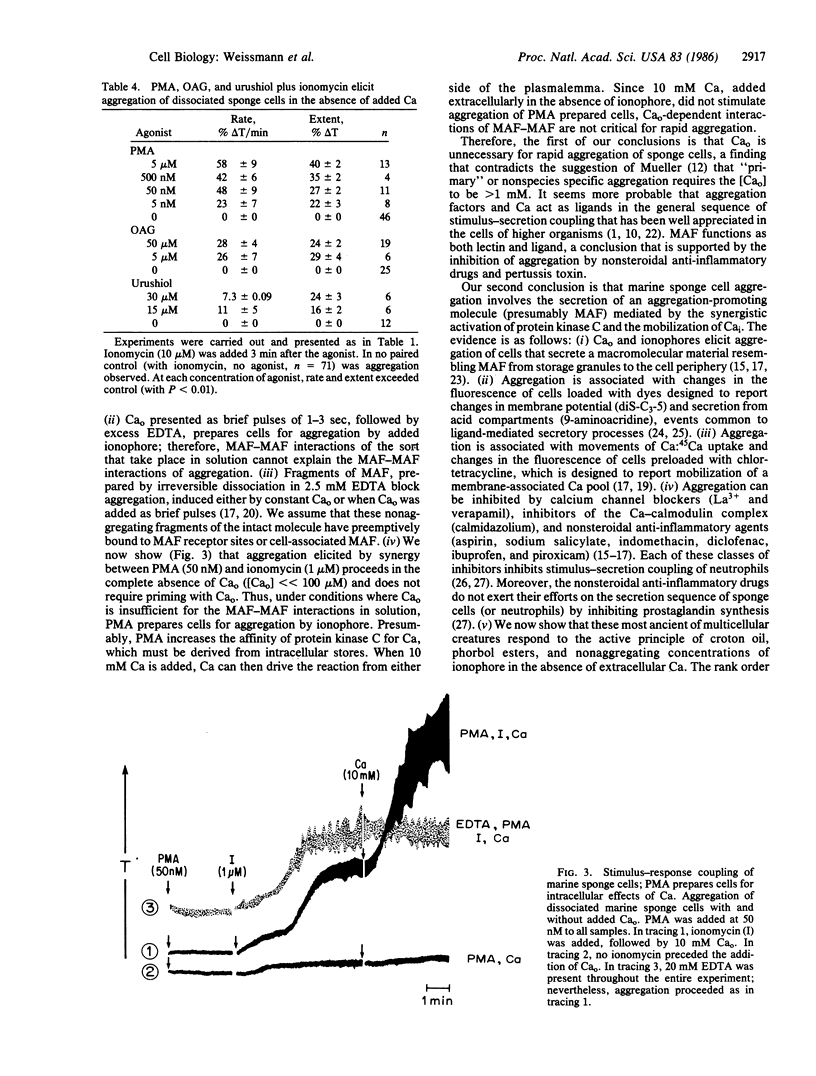
Aggregation of marine sponge cells (Microciona prolifera) resembles stimulus-response coupling of higher organisms in which activation of protein kinase C and movements of intracellular Ca provide twin signals. We now report that activators of protein kinase C (phorbol esters) and ionomycin act synergistically to aggregate sponge cells. Surprisingly--since extracellular Ca is required for integrity of the species-specific aggregation factor--synergistic aggregation proceeded in the complete absence of added extracellular Ca (2.5-20 mM EDTA). The order of activity of phorbol esters and related compounds was that of their effect on protein kinase C (phorbol myristate acetate, phorbol dibutyrate greater than phorbol diacetate much greater than phorbol, 4 alpha-phorbol). 1-Oleyl, 2-acetylglycerol a synthetic activator of protein kinase C, also showed synergy with ionomycin. Phorbol esters and 1-oleyl, 2-acetylglycerol acted in synergy with ionomycin to liberate membrane Ca as detected by decreased fluorescence of chlortetracycline in prelabeled cells. Moreover, urushiol, the toxic principle of poison ivy, but not pentadecanylcatechol, its inert analogue, showed synergy with ionomycin. Synergistic aggregation was inhibited by calmidazolium (10 microM), piroxicam (20-100 microM), and pertussis toxin (20 micrograms/ml). The data not only confirm that marine sponge cell aggregation follows the general sequence of stimulus-response coupling in the cells of higher organisms but also support, in this most ancient of multicellular creatures, the hypothesis that mobilization of intracellular Ca and activation of protein kinase C provide the twin signals for cell activation in the absence of added extracellular Ca.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Korchak H., Ludewig R., Edelson H., Haines K., Levin R. I., Herman R., Rider L., Kimmel S., Weissmann G. Modes of action of aspirin-like drugs. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7227–7231. doi: 10.1073/pnas.82.21.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert P. R., Tashjian A. H., Jr Dual actions of phorbol esters on cytosolic free Ca2+ concentrations and reconstitution with ionomycin of acute thyrotropin-releasing hormone responses. J Biol Chem. 1985 Jul 25;260(15):8746–8759. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M. In vitro studies on the mode of action of the phorbol esters, potent tumor promoters: part 1. Crit Rev Toxicol. 1980 Dec;8(2):153–197. doi: 10.3109/10408448009037493. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Hewlett E. L., Gilman A. G. Identification of the predominant substrate for ADP-ribosylation by islet activating protein. J Biol Chem. 1983 Feb 25;258(4):2072–2075. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D., White J. G., Dalmosso A. P., Jacob H. S. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J Clin Invest. 1977 Jul;60(1):260–264. doi: 10.1172/JCI108763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale M. M., Penfield A. Synergism between phorbol ester and A23187 in superoxide production by neutrophils. FEBS Lett. 1984 Sep 17;175(1):170–172. doi: 10.1016/0014-5793(84)80592-x. [DOI] [PubMed] [Google Scholar]
- Dunham P. B., Vosshall L. B., Bayer C. A., Rich A. M., Weissmann G. From Beaumont to poison ivy: marine sponge cell aggregation and the secretory basis of inflammation. Fed Proc. 1985 Nov;44(14):2914–2924. [PubMed] [Google Scholar]
- Dunham P., Anderson C., Rich A. M., Weissmann G. Stimulus-response coupling in sponge cell aggregation: Evidence for calcium as an intracellular messenger. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4756–4760. doi: 10.1073/pnas.80.15.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Hoffstein S. T., Weissmann G. Mechanisms of lysosomal enzyme release from human polymorphonuclear leukocytes. Effects of phorbol myristate acetate. J Cell Biol. 1975 Sep;66(3):647–652. doi: 10.1083/jcb.66.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K., Sano K., Hoshijima M., Takai Y., Nishizuka Y. Phosphatidylinositol turnover in platelet activation; calcium mobilization and protein phosphorylation. Cell Calcium. 1982 Oct;3(4-5):323–335. doi: 10.1016/0143-4160(82)90020-3. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. The phorbol ester TPA increases the affinity of exocytosis for calcium in 'leaky' adrenal medullary cells. FEBS Lett. 1983 Aug 22;160(1-2):98–100. doi: 10.1016/0014-5793(83)80944-2. [DOI] [PubMed] [Google Scholar]
- Kojima I., Lippes H., Kojima K., Rasmussen H. Aldosterone secretion: effect of phorbol ester and A23187. Biochem Biophys Res Commun. 1983 Oct 31;116(2):555–562. doi: 10.1016/0006-291x(83)90559-4. [DOI] [PubMed] [Google Scholar]
- Koo C., Lefkowitz R. J., Snyderman R. Guanine nucleotides modulate the binding affinity of the oligopeptide chemoattractant receptor on human polymorphonuclear leukocytes. J Clin Invest. 1983 Sep;72(3):748–753. doi: 10.1172/JCI111045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak H. M., Rich A. M., Wilkenfeld C., Rutherford L. E., Weissmann G. A carbocyanine dye, DiOC6(3), acts as a mitochondrial probe in human neutrophils. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1495–1501. doi: 10.1016/s0006-291x(82)80076-4. [DOI] [PubMed] [Google Scholar]
- Korchak H. M., Roos D., Giedd K. N., Wynkoop E. M., Vienne K., Rutherford L. E., Buyon J. P., Rich A. M., Weissmann G. Granulocytes without degranulation: neutrophil function in granule-depleted neutroplasts. Trans Assoc Am Physicians. 1983;96:356–364. [PubMed] [Google Scholar]
- Korchak H. M., Vienne K., Rutherford L. E., Wilkenfeld C., Finkelstein M. C., Weissmann G. Stimulus response coupling in the human neutrophil. II. Temporal analysis of changes in cytosolic calcium and calcium efflux. J Biol Chem. 1984 Apr 10;259(7):4076–4082. [PubMed] [Google Scholar]
- Misevic G. N., Jumblatt J. E., Burger M. M. Cell binding fragments from a sponge proteoglycan-like aggregation factor. J Biol Chem. 1982 Jun 25;257(12):6931–6936. [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Rice D. J., Humphreys T. Two Ca2+ functions are demonstrated by the substitution of specific divalent and lanthanide cations for the Ca2+ required by the aggregation factor complex from the marine sponge, Microciona prolifera. J Biol Chem. 1983 May 25;258(10):6394–6399. [PubMed] [Google Scholar]
- Rich A. M., Weissmann G., Anderson C., Vosshall L., Haines K. A., Humphreys T., Dunham P. Calcium dependent aggregation of marine sponge cells is provoked by leukotriene B4 and inhibited by inhibitors of arachidonic acid oxidation. Biochem Biophys Res Commun. 1984 Jun 29;121(3):863–870. doi: 10.1016/0006-291x(84)90757-5. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Korchak H. M., Weissmann G. The kinetics of lysosomal degranulation of human neutrophils as measured by 9-aminoacridine quenching. Biochim Biophys Acta. 1983 Apr 5;762(2):145–153. doi: 10.1016/0167-4889(83)90066-6. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Korchak H. M., Weissmann G. The roles of extracellular and intracellular calcium in lysosomal enzyme release and superoxide anion generation by human neutrophils. Biochim Biophys Acta. 1981 Nov 5;677(3-4):512–520. doi: 10.1016/0304-4165(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Noble P., Freed R., Weissmann G. Metabolic requirements for maintenance of the chlortetracycline-labeled pool of membrane-bound calcium in human neutrophils. J Cell Physiol. 1983 Dec;117(3):415–422. doi: 10.1002/jcp.1041170317. [DOI] [PubMed] [Google Scholar]
- Wilson C. R., Skinner S. E., Shaw W. V. Analysis of two chloramphenicol resistance plasmids from Staphylococcus aureus: insertional inactivation of Cm resistance, mapping of restriction sites, and construction of cloning vehicles. Plasmid. 1981 May;5(3):245–258. doi: 10.1016/0147-619x(81)90002-0. [DOI] [PubMed] [Google Scholar]


