Abstract
Maternal obesity can influence susceptibility to obesity and type 2 diabetes in progeny. We examined the relationship of maternal insulin resistance (IR), a metabolically important consequence of increased adiposity, to adverse consequences of obesity for fetal development. We used mice heterozygous for a null allele of the insulin receptor (Insr) to study the contributions of maternal IR to offspring phenotype without the potential confound of obesity per se, and how maternal consumption of high-fat diet (HFD) may, independently and interactively, affect progeny. In progeny fed a 60% HFD, body weight and adiposity were transiently (5–7 weeks) increased in wild-type (+/+) offspring of Insr+/− HFD-fed dams compared to offspring of wild-type HFD-fed dams. Offspring of HFD-fed wild-type dams had increased body weight, blood glucose, and plasma insulin concentrations compared to offspring of chow-fed wild-type dams. Quantification of proopiomelanocortin (POMC) and neuropeptide-Y (NPY) populations in the arcuate nucleus of the hypothalamus (ARH) of offspring of wild-type vs. Insr+/− dams was performed to determine whether maternal IR affects the formation of central feeding circuits. We found a 20% increase in the number of Pomc-expressing cells at postnatal day 9 in offspring of Insr+/− dams. In conclusion, maternal HFD consumption—distinct from overt obesity per se—was a major contributor to increased body weight, adiposity, IR, and liver triglyceride (TG) phenotypes in progeny. Maternal IR played a minor role in predisposing progeny to obesity and IR, though it acted synergistically with maternal HFD to exacerbate early obesity in progeny.
INTRODUCTION
The prevalence of obesity and type 2 diabetes has increased to alarming levels over the past several decades. In 2005–2006, more than 34% of US adults (72 million people) were obese, and an additional 33% were considered overweight (1). Along with the rising prevalence in adults, childhood obesity rates have escalated dramatically. A recent study found that childhood obesity was a major predictor of premature death, underscoring the need to identify ways to prevent and treat this dangerous condition (2). Because the trajectory of the increase in childhood obesity is very steep, it is unlikely that genetic and conventional environmental factors alone are sufficient to explain these trends (3,4).
Intrauterine and early postnatal signals can influence the development of the hypothalamus, pancreatic islets, adipose tissue, and the liver, thereby potentially exerting a permanent impact on the metabolic status of the progeny (5–11). As the number of obese women of childbearing age has increased—it was estimated that in 2002, 23% of women aged 20–44 years were obese and an additional 24.5% were overweight (12)—so has the potential for detrimental influences of deranged maternal metabolic status during gestation and lactation on susceptibility to obesity and type 2 diabetes in progeny.
Studies in humans suggest that the intrauterine metabolic environment has long lasting effects on susceptibility to obesity and diabetes in progeny (7,8,13). This phenomenon has been studied in Pima Indians, where there is a higher incidence of diabetes in progeny of women who were diabetic during pregnancy vs. progeny of prediabetic and nondiabetic women (14). Similarly, the incidence of adolescent obesity in offspring exposed to diabetes in utero was greater than in their unexposed siblings (15).
Animal studies of maternal overnutrition—such as diet-induced obesity—have demonstrated that maternal obesity can influence offspring body weight, adiposity, and glucose homeostasis in varying ways (16–18). Maternal diet and obesity during the perinatal period has been shown to influence the development and function of the central nervous system circuits regulating energy balance, which may convey a long-term impact on the phenotype of the offspring (16,19–21).
To elucidate how obesity in a gravida could adversely affect fetal metabolic and neurodevelopment, we examined effects on progeny of two obesity-related maternal factors: insulin resistance (IR) and high-fat diet (HFD). We examined the effects of maternal HFD consumption—independently and interactively with IR—to assess additivity/interactions between these maternal phenotypes.
Additionally, we examined whether the maternal IR could affect the development of the hypothalamic feeding circuits. The arcuate nucleus of the hypothalamus (ARH) is comprised of at least two key neuronal populations involved in energy homeostasis: the anorexigenic proopiomelanocortin (Pomc)-expressing cells and the orexigenic Npy/AgRP-expressing cells. At midgestation in the mouse, Pomc is expressed in a broad population of immature neurons. Only half of these cells differentiate into mature Pomc neurons; the remainder extinguishes Pomc expression by the second postnatal week (22). We examined whether the maternal IR could affect the number of Pomc and Npy (neuropeptide-Y) neurons in the ARH, by influencing cell fate decisions during late gestation and the early postnatal period.
METHODS AND PROCEDURES
Animals and animal care
Mice with null alleles of Insr were previously generated (23). Animals were maintained on a mixed C57BL/6x129/Sv background that had been intercrossed for generations (N > 40), effectively creating a stable recombinant inbred line. Animals were housed within a pathogen-free barrier facility that maintained a 12-h light–dark cycle. Genotyping was performed using the following primer sets: (Insr+/+) 5′-TCTTTGCCTGTGCTCCACTCTCA-3′/5′-AGCTGTGCACTTCCCTGCTCAC-3′; (Insr−/−) 5′-TCTTTGCCTGTGCTCCACTCTCA-3′/5′-TTAAGGGCCAGCTCATTCCTCC-3′. Dams and offspring had ad libitum access to HFD (providing 60% kcal from fat, Research Diets, cat. no. D12492), unless otherwise noted as being provided a chow diet (12% kcal from fat, PicoLab Rodent Diet, cat. no. 5053). All procedures were approved by Columbia University’s Institutional Animal Care and Use Committee.
Metabolic phenotyping
Blood was collected in a random-fed state, in the third hour of the light phase. Blood glucose values were determined from tail blood of unanesthetized animals using an automatic glucose meter (Freestyle Flash; Therasense, Alameda, CA). Blood for plasma insulin was collected retro-orbitally into heparin-lined microtubes from mice anesthetized by isoflurane inhalation. Plasma insulin levels were determined by ELISA (Linco, St Charles, MO).
Breeding
All dams were at least 4 months of age, and fed the HFD 3 weeks prior to breeding (where indicated). Experimental crosses were performed as described in Figure 1. Mating was confirmed by sperm plug presence. Dam blood and body composition measures were taken on a subset of dams. Dam blood for glucose and insulin measurements was taken at embryonic days 17–19 (E17–E19). Dam body weight gain per pup was determined by subtracting the body weight at E17–E19 from pre-breeding body weight, then dividing by the number of pups in the litter. Offspring were weaned at 3 weeks of age, and fed the HFD. Offspring from chow-fed dams were followed through 16 weeks of age (n = 26). One group of offspring born to HFD-fed dams were followed through 16 weeks of age (n = 24 males; n = 29 females). A separate group born to HFD-fed dams was used to assess body composition at 5 and 7 weeks of age (n = 11 males). A subset of the offspring born to chow-fed dams was used to assess body composition (n = 11 males). All offspring were from a total of 37 litters. Average litter sizes were as follows: chow-fed wild-type dams: 7.7 pups; chow-fed Insr+/− dams: 7.1 pups; HFD-fed wild-type dams: 6.8 pups; HFD-fed Insr+/− dams: 6.9 pups. Because the pertinent offspring measured only represented 25% of each litter (Insr+/+ males = ~2 animals per litter), results represent data from individual offspring, rather than litters.
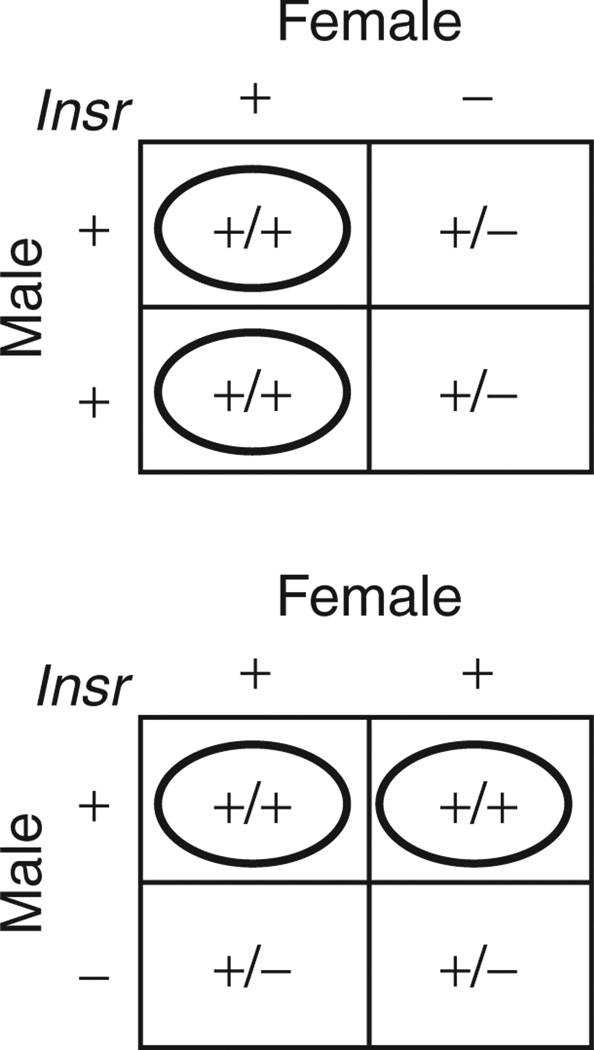
Figure 1.
Breeding strategy. Female Insr+/− mice were crossed to wild-type (+/+) males to produce an anticipated 1:1 ratio of Insr+/+ (wild-type) to Insr+/− offspring. A reciprocal cross of female +/+ and male Insr+/− mice was used to generate control animals. Wild-type (+/+) offspring from the crosses were studied. Insr, insulin receptor.
Body composition
Body composition was determined by magnetic resonance imaging (Bruker Minispec Lean/Fat Analyzer, Bruker Optics). Animals were placed in a clear, plastic cylinder (50 mm diameter) and kept immobile by insertion of a plunger. The tube was then lowered into the instrument for the duration of the scan (~2 min).
Sacrifice and tissue analysis
Mice were anesthetized by i.p. injection of 0.02 ml of 2.5% avertin per gram of body weight. A liver section (~200 mg) was immediately removed and snap frozen.
Liver triglyceride analysis
The weighed liver section (~100 mg) was saponified and assayed for glycerol concentration using Free Glycerol Reagent kit (Sigma, St Louis, MO) (24). Concentrations were calculated using a Glycerol Standard Solution (Sigma) and weight of the liver section.
Brain tissue processing
P0, P9, and P35 offspring (of chow-fed wild-type or Insr+/− dams) were anesthetized and perfused transcardially with 0.9% NaCl followed by 4% paraformaldehyde in phosphate buffer. Brains were post-fixed at 4 °C overnight and cryoprotected with 30% sucrose. For embryonic tissue, dams were anesthetized with avertin and embryos were dissected in cold phosphate-buffered saline/L15 media, fixed at 4 °C overnight and cryoprotected with 30% sucrose. Frozen 10 µm-thick coronal sections were collected across the rostral-caudal extent of the ARH on Superfrost Plus slides (Fisher, Pittsburgh, PA). Each group represents the average counts of at least six coronal hemisections per animal spanning the rostrocaudal extent of the presumptive ARH.
Fluorescent in situ hybridization
Frozen sections were processed as described in the TSA Plus Cy3 System manual (Perkin Elmer, Waltham, MA). Antisense digoxigeninor fluorescein-labeled riboprobes were generated from plasmids containing PCR fragments of Npy and Pomc using the following primers sets: (NPY) 5′-TGCTAGGTAACAAGCGAATGG-3′/5′-CAACAACAACAAGGGAAATGG-3′; (POMC) 5′-GTTAAGAGCAGTGACTAAGAGAGGC-3′/5′-CCTAACACAGGTAACTCTAAGAGGC-3′.
Imaging and cell number quantification
Fluorescent microscopy was performed using a Nikon Eclipse 80i equipped with a Retiga EXicamera and X cite 120 fluorescent illumination system. TIFF files were acquired using Q CapturePro software (Qimaging) and analyzed using Adobe Photoshop.
Statistical analysis
Factorial ANOVA was performed to estimate the effects of maternal genotype, maternal diet, and their interactions for each dependent variable. For circulating insulin, two additional analyses of covariance were performed, using insulin as the dependent variable, maternal genotype and diet as independent variables, and adiposity or blood glucose as covariates. Student’s t-tests were used to compare the effects of maternal genotype (offspring of dams within each diet group) and also the effects of maternal diet (offspring of wild-type dams) at individual time points. Student’s t-tests were also used to confirm that there were no differences in litter size number. Therefore, results represent data from individual offspring. P < 0.05 (two-tailed) was considered statistically significant. Analyses were performed using Statistica 6.0 (ANOVA) (Tulsa, OK) or Microsoft Office Excel (t-tests).
RESULTS
Breeding insulin-resistant dams
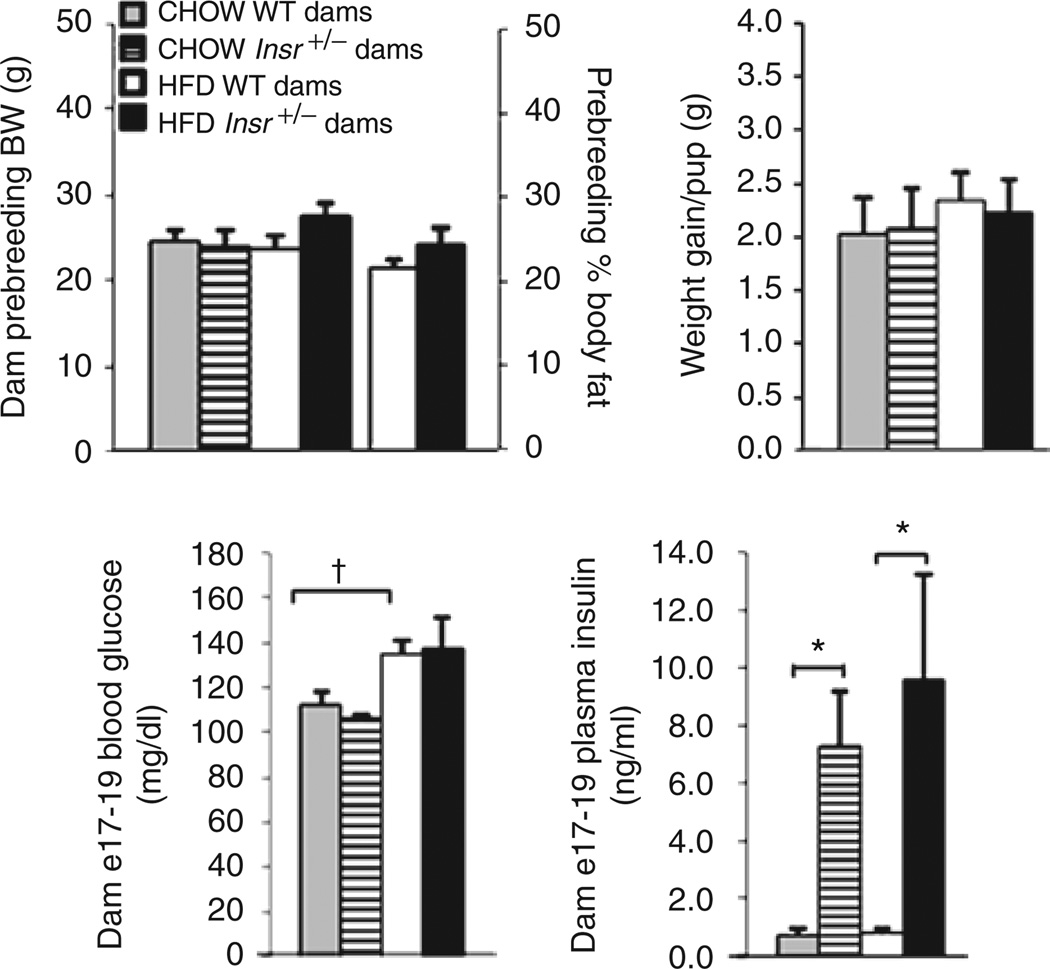
Three-month-old wild-type and Insr+/− dams maintained on a mixed C57BL/6x129/Sv background were fed chow (12% kcal from fat) or high fat (60% kcal from fat) diets 3 weeks prior to breeding, and throughout pregnancy and lactation. Insr+/− females were bred to wild-type (+/+) males to produce an anticipated 1:1 ratio of Insr+/+ (wild-type) to Insr+/− offspring (Figure 1) (only +/+ offspring were studied). Reciprocal crosses provided control animals. Insr+/− dams did not differ from wild-type dams in pre-pregnancy body weight, irrespective of diet. In HFD-fed dams, there was no difference in prepregnancy body composition between +/+ and Insr+/− dams (Figure 2). Body weight gain of dams per pup during gestation was also not different between Insr+/− and diet-matched +/+ dams, nor between wild-type dams fed chow vs. HFD. At E17–E19, blood glucose concentrations were not different between Insr+/− and diet-matched +/+ dams. Plasma insulin concentrations of Insr+/− dams at this date were approximately sevenfold higher than those of wild-type dams (P < 0.01).
Figure 2.
Dam phenotype. Wild-type dams did not differ in prebreeding body weight or weight gain during gestation compared to Insr+/− dams in diet-matched groups. Ingesting a high-fat diet (HFD), +/+ and Insr+/− dams did not have different fractional body fat content. Insr+/− dams showed increased circulating insulin concentrations at gestational day E17–E19, but not increased blood glucose. Gray bars: chow wild-type dams; hatched bars: chow Insr+/− dams; white bars: HFD wild-type dams; black bars: HFD Insr+/− dams. Data are expressed as mean ± s.e.m (*P < 0.01).
Effects of maternal IR and HFD on offspring body weight and composition
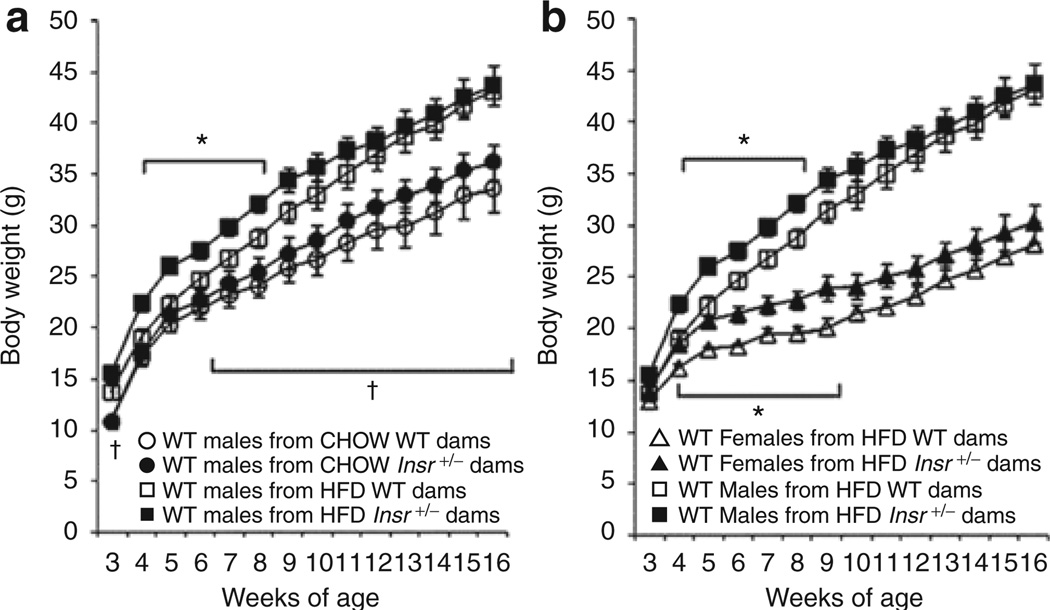
To assess the effects of maternal IR and HFD on progeny growth, HFD-fed wild-type offspring body weight was monitored weekly from weaning to 16 weeks of age. We detected an effect on male offspring body weight of maternal diet (F(14,33) = 4.48, P < 0.01), maternal genotype (F(14,33) = 2.96, P < 0.01), and an interaction between maternal diet and genotype (F(14,33) = 3.42, P < 0.01). At 4–8 weeks of age male wild-type offspring of HFD-fed Insr+/− dams exhibited ~14% higher body weight than wild-type males of HFD-fed +/+ dams (Figure 3, †P < 0.05). Similarly, female progeny of HFD Insr+/− dams had higher body weights than progeny of +/+ dams at 4–9 weeks of age; these differences were no longer apparent after 10 weeks (Figure 3, *P < 0.05). There was no difference in body weight between offspring born to chow-fed +/+ or Insr+/− dams at individual time points. Percent body fat in offspring of chow-fed Insr+/− dams trended higher than wild-type dam offspring (P = 0.09 at 16 weeks; data not shown). Male offspring born to HFD-fed +/+ dams had increased body weight (vs. progeny of chow-fed +/+ dams) throughout the entire period of study (3, 6–16 weeks of age); this difference reached 28% by 16 weeks, indicating an independent effect of maternal diet on offspring body weight.
Figure 3.
Wild-type offspring body weight. (a) Males. Male offspring of high-fat diet (HFD)-fed Insr+/− dams (n = 13) weighed more than male offspring of HFD-fed wild-type dams (n = 11) at 4 – 8 weeks of age (*P < 0.05). Male offspring of HFD-fed wild-type dams weighed more than male offspring of chow-fed wild-type dams at 3–16 weeks of age (n = 15). All offspring fed HFD (†P < 0.05). Open circles: wild-type male progeny of chow wild-type dams; filled circles: wild-type males of chow Insr+/− dams; open squares: wild-type males of HFD wild-type dams; filled squares: wild-type males of HFD Insr+/− dams. (b) Comparison of male and female offspring. Similar to males, female offspring of HFD-fed Insr+/− dams (n = 19) weighed more than females of HFD-fed wild-type dams (n = 10) at 4–9 weeks of age. All offspring fed HFD (*P < 0.05). Mean ± s.e.m. Open triangles: wild-type females of HFD wild-type dams; filled triangles: wild-type females of HFD Insr+/− dams; open squares: wild-type males of HFD wild-type dams; filled squares: wild-type males of HFD Insr+/− dams.
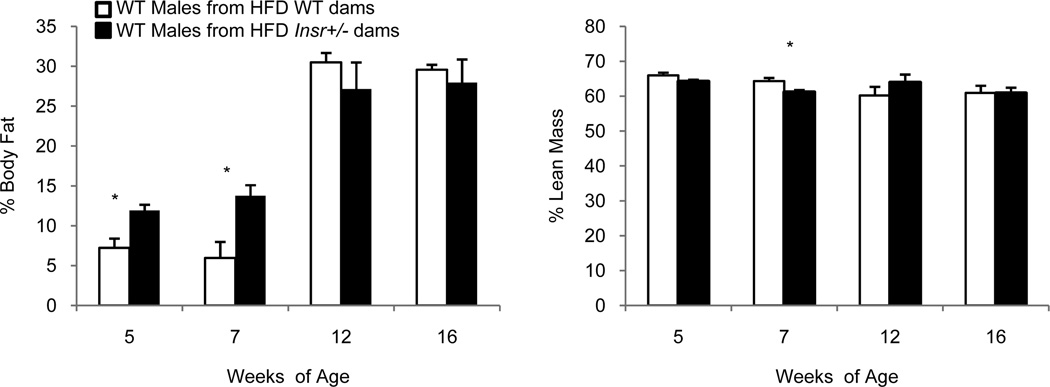
Male offspring of HFD-fed Insr+/− dams had a 6 percentage-point higher body fat percentage at 5 and 7 weeks compared to controls; this difference was no longer evident at 12 and 16 weeks of age (Figure 4, *P < 0.05).
Figure 4.
Male wild-type offspring body composition. Male offspring from high-fat diet (HFD)-fed Insr+/− dams had higher % body fat than males from HFD-fed wild-type dams (white bars) at 5 and 7 weeks of age, but not at later 12- and 16-week time points (*P < 0.05). Mean ± s.e.m. White bars: wild-type males of HFD wild-type dams; black bars: wild-type males of HFD Insr+/− dams.
Effects of maternal IR and HFD on offspring glucose and insulin concentrations
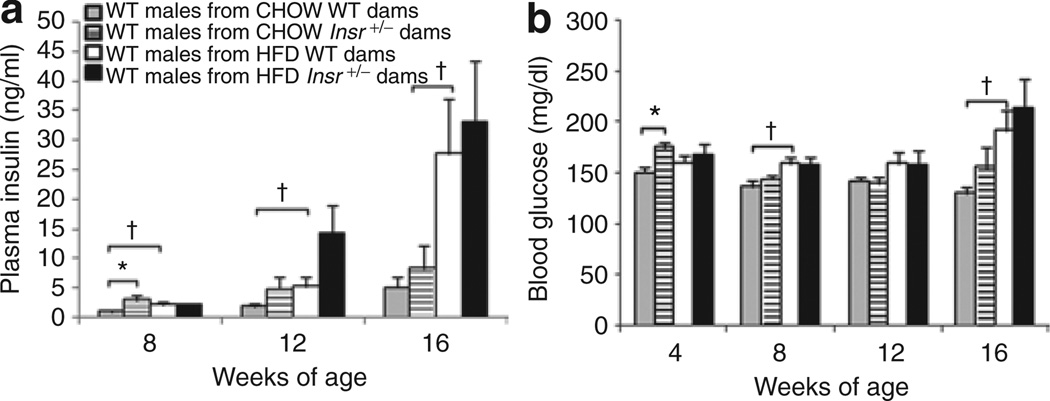
Blood glucose of ad libitum fed offspring was measured at 4, 8, 12, and 16 weeks of age, and an effect of maternal genotype was found (F(4,43) = 2.61, P < 0.05). However, when comparing males from HFD-fed wild-type and Insr+/− dams at individual time points, we did not find differences in blood glucose concentrations (Figure 5). Male offspring of chow-fed Insr+/− dams had increased blood glucose concentrations at 4 weeks of age compared to males from chow-fed +/+ dams, but not at later time points (Figure 5, **P < 0.01). Maternal diet affected offspring blood glucose concentrations (F(4,43) = 0.57, P < 0.01), but no significant interaction between maternal genotype and diet was found. Male progeny of HFD-fed wild-type dams had increased blood glucose at 8 and 16 weeks compared to males from chow-fed wild-type dams, suggesting that maternal HFD diet may impair glucose homeostasis in progeny (P < 0.05).
Figure 5.
Male wild-type offspring plasma insulin and blood glucose concentrations. (a) Plasma insulin. Plasma insulin concentrations did not differ between male offspring from high-fat diet (HFD)-fed wild-type and Insr+/− dams. Males from chow-fed Insr+/− dams had increased random-fed plasma insulin concentrations at 8 weeks of age, but these concentrations did not differ significantly at later time points (*P < 0.05). Plasma insulin concentrations were higher in males from HFD-fed wild-type dams at 8, 12, and 16 weeks compared to male progeny chow-fed wild-type dams. All offspring fed HFD (†P < 0.05). (b) Blood glucose. Blood glucose concentrations did not differ in male progeny of HFD-fed wild-type and Insr+/− dams. Male progeny of chow-fed Insr+/− dams had increased random fed blood glucose concentrations at 4 weeks of age compared to males from chow-fed wild-type dams (*P < 0.05). Blood glucose concentrations in male progeny of HFD-fed wild-type dams were increased at 8 and 16 weeks compared to males from chow-fed wild-type dams. All offspring fed HFD (†P < 0.05). Mean ± s.e.m. Gray bars: wild-type males of chow wild-type dams; hatched bars: wild-type males of chow Insr+/− dams; white bars: wild-type males of HFD wild-type dams; black bars: wild-type males of HFD Insr+/− dams.
Plasma insulin concentrations of offspring were significantly affected by maternal diet (F(3,44) = 3.67, P < 0.05), and there was also an interaction between maternal genotype and diet (F(3,44) = 2.91, P < 0.05). However, after controlling by analysis of covariance for offspring adiposity or blood glucose, the effects on offspring insulin concentrations were no longer statistically significant. Thus, it is likely that the maternal effects on offspring insulin homeostasis are indirect (e.g., caused by increased adiposity).
Effects of maternal HFD and IR on offspring liver triglyceride levels
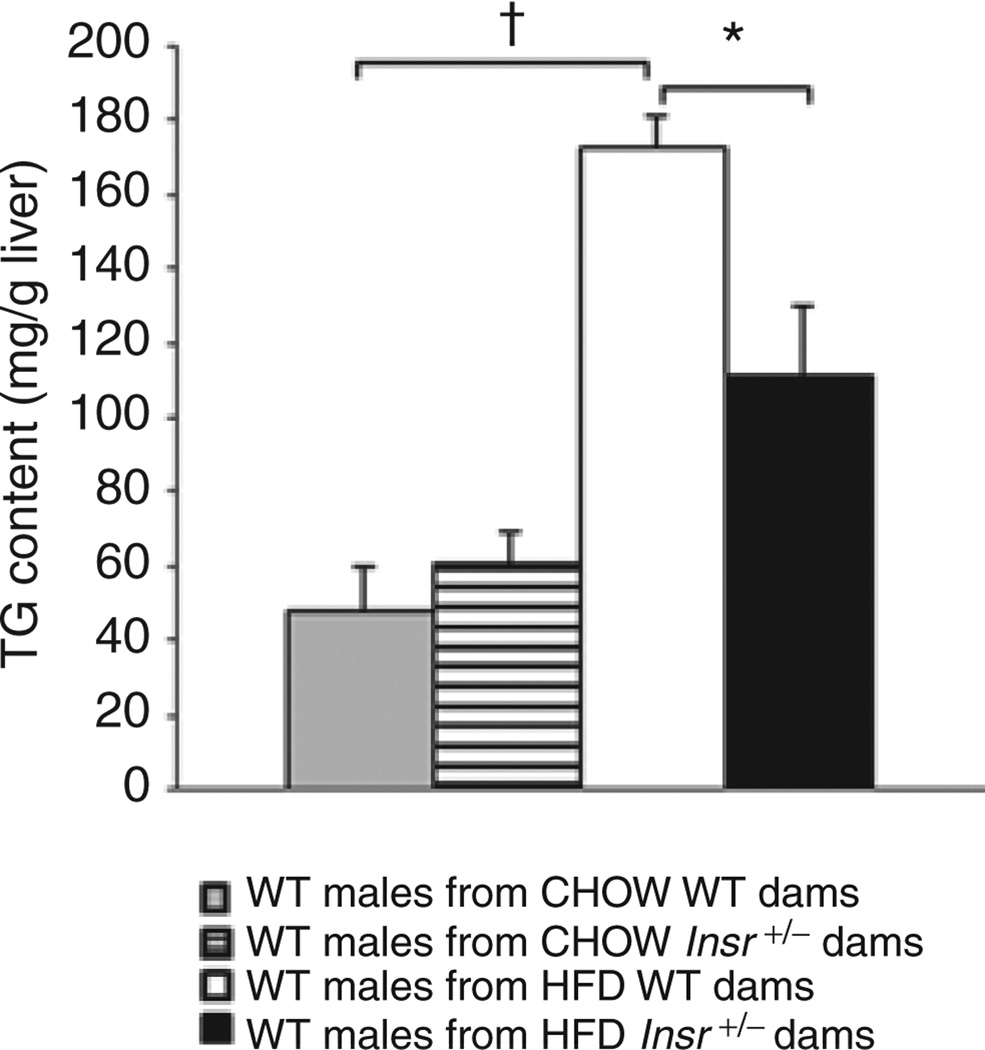
Liver triglyceride (TG) content is implicated in the etiopathogenesis of some of the metabolic consequences of obesity. Hepatic TG levels were measured in 16-week-old male wildtype offspring. There was an effect of maternal diet (F(1,43) = 41.18, P < 0.0001) and an interaction with maternal genotype (F(1,43) = 7.53, P < 0.01). In wild-type males of HFD-fed +/+ dams, liver TG levels were more than twice those in progeny of chow-fed +/+ dams, suggesting maternal diet can affect liver fat accumulation in offspring later in life (Figure 6). Male offspring of HFD-fed Insr+/− dams had decreased liver TG content vs. offspring born to HFD-fed +/+ dams, though levels were still higher than both chow-fed dam offspring groups (P < 0.05).
Figure 6.
Liver triglyceride (TG) concentration of male wild-type progeny. Liver TG concentration was higher in wild-type male progeny of wild-type dams fed a high-fat diet compared to progeny of wild-type dams fed a chow diet (†P < 0.05). Liver TG concentration was decreased in males born to HFD-fed Insr+/− dams vs. progeny of the HFD-fed wild-type dams (*P < 0.05). All offspring fed HFD. Mean ± s.e.m. Gray bars: wild-type males of chow wild-type dams; hatched bars: wild-type males of chow Insr+/− dams; white bars: wild-type males of HFD wild-type dams; black bars: wild-type males of HFD Insr+/− dams.
Effects of maternal IR on Pomc- and Npy-expressing cell number in the ARH
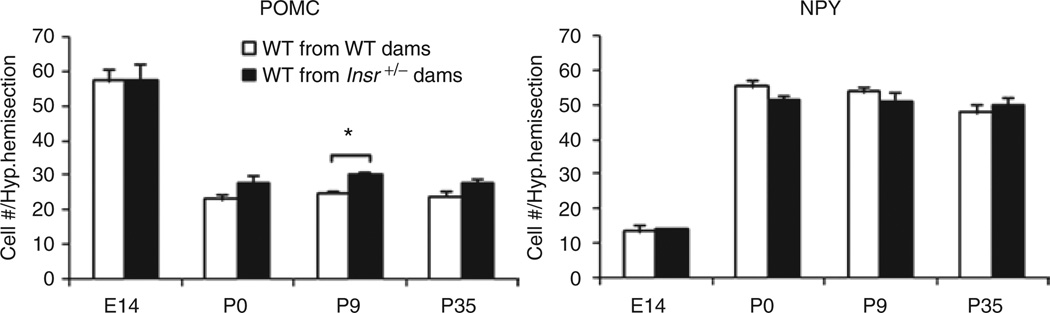
To investigate whether maternal IR could affect the cellular composition of critical neuronal populations in the ARH, we performed fluorescent in situ hybridization for Pomc and Npy on brain sections of offspring of wild-type vs. Insr+/− dams fed a chow diet at E14, P0, P9 and P35 (Figure 7). There was no difference in the number of Pomc cells at E14. At P0 and P35 there was a trend for increased number of Pomc cells, and a statistically significant (P < 0.01) increase Pomc-expressing cell number in offspring of Insr+/− dams at P9. There were no differences in the number of Npy-expressing cells between offspring of wild-type vs. Insr+/− dams.
Figure 7.
Pomc and Npy cell counts in the arcuate nucleus of the hypothalamus of offspring. There were no differences in the number of Pomc+ cells in the ARH at E14 in offspring born to wild-type vs. Insr+/− dams (n = 6). There was an increase in the number of Pomc-expressing cells at P9 in offspring born to Insr+/− dams, and a trend of an increase of Pomc+ cells at P0 and P35 (P0: P = 0.11, n = 9; P9: P = 0.007, n = 11; P35: P = 0.11, n = 9). P35 pups fed chow postweaning (*P < 0.05). Each group represents the average counts of at least 6 coronal hemisections sections per animal spanning the rostrocaudal extent of the presumptive ARH with error bars representing mean ± s.e.m. White bars: wild-type offspring of chow wild-type dams; black bars: wild-type offspring of chow Insr+/− dams.
DISCUSSION
Many investigators have used HFD-induced obesity to examine effects of maternal obesity on body weight/composition and metabolic health of progeny. These models generally provoke derangements in a variety of maternal metabolic processes, each with the potential to influence distinct aspects of developmental biology. To enable potential interventions in human pregnancy, it is important to understand how IR and diet composition interact in gravidas to affect specific aspects of body weight/composition, and metabolic/behavioral and central nervous system structural phenotypes in progeny. By imposing maternal IR genetically, rather than by acquired differences in adiposity, and by feeding dams HFD, we were able to isolate discrete and potentially critical features of obesity to study their independent and interactive effects on progeny phenotypes.
Offspring metabolic phenotypes: effect of maternal HFD
Our dams were fed HFD for only 3 weeks prior to gestation, which was not long enough to induce significant increases in prebreeding body weight or during gestation. Though, we cannot rule out the possibility that minor differences in adiposity may exist between dams fed the HFD vs. those fed chow (12% fat). However, even with potential small changes in levels of adiposity among the dams, the conclusion that HFD plays a greater role in influencing offspring metabolic phenotypes over maternal IR would remain true. In the situation in humans, diet and body composition differences are likely to covary and to interact. Offspring body weights of wild-type HFD-fed mothers were significantly increased compared to chow-fed dam offspring, and reached a maximum 28% difference by 16 weeks of age. Models addressing the effect of maternal HFD on offspring body weight have been diverse with regard to specific content of the HFDs, timing of maternal exposure to the diet, and age at which offspring phenotypes were evaluated. However, our finding of increased body weight in offspring of HFD-fed wild-type dams is consistent with other animal models of maternal HFD consumption (16,18,19,25,26).
We observed significant increases in circulating glucose and insulin concentrations in offspring of wild-type dams fed a HFD compared to offspring from chow-fed dams. This result is consistent with an earlier study in which offspring of HFD-fed mice (also in the absence of obesity) exhibited increased circulating insulin concentrations as adults (26).
Liver TG content was also increased in offspring of wild-type dams fed a HFD diet. This result is consistent with a study by McCurdy et al., reporting that maternal HFD results in increased liver TGs in nonhuman primate offspring (27). When these investigators restored the diet of the previously HFD-fed obese mothers to standard chow during pregnancy, offspring liver TG levels were reduced, highlighting the impact of diet per se as the primary factor influencing offspring hepatic lipid content. In mice, a maternal diet high in fat also increases liver TG accumulation in offspring (28,29). Overall, the finding that offspring phenotypes of our wild-type HFD-fed dams—regarding increased body weight, adiposity, glucose and insulin concentrations, and liver TG phenotypes—recapitulate phenotypes found in progeny of HFD-induced obese maternal models, suggests that maternal HFD consumption per se plays a major role in predisposing offspring to these adverse phenotypes.
Because maternal obesity per se, and HFD consumption, can impart distinct and/or synergistic effects on offspring phenotypes, several other groups have also tried to isolate and examine the discrete contributions of the HFD. Howie et al. demonstrated increased body weight, adiposity, and insulin concentrations in offspring of rat dams fed a HFD, regardless of whether the dam had been fed the HFD since her own weaning (resulting in increased body weight and adiposity by the time of breeding), or only during pregnancy and throughout lactation (therefore matching control body weights) (26). This result suggested that perinatal maternal diet, and not obesity, imparted the effects on offspring phenotype. Our results support this inference. However, other studies in which pair-feeding of dams fed a HFD to limit weight gain found no differences in offspring, raising the possibility that maternal obesity, amount of HFD consumed, and/or the composition of fats in the diet accounted for the influences on offspring phenotypes (17).
Offspring metabolic phenotypes: effect of maternal IR
Offspring of HFD-fed Insr+/− dams displayed a transient increase in body weight between weeks 4 and 8, which was accompanied by increased percent body fat at weeks 5 and 7. We conclude that maternal IR, interacting with HFD, can influence body weight and composition in offspring. Although maternal IR did not have as great of an effect as maternal HFD on progeny body weight, the fact that offspring of our Insr+/− dams fed a HFD showed increased body weight compared to HFD-fed dam offspring suggests that maternal IR, per se, is a minor contributor to offspring body weight determination in the obese models.
It is interesting that the differences in the body weight phenotypes of offspring born to HFD-fed +/+ vs. HFD-fed Insr+/− dams occurred between 4 and 8 weeks of age, a period equivalent to “childhood” or “adolescence” in mice. These results could have significant implications for humans, if maternal IR increases the risk of childhood obesity. Childhood obesity increases the risk of metabolic derangements—or even premature death—later in life (2).
The transient relative increase in body weight of offspring of HFD Insr+/− dams, which abated after 8 weeks, is important to note. One possibility is that the effects of the long-term feeding of HFD to all progeny overrode the gestational effects of maternal IR. If we had fed the progeny a lower fat diet, it is possible the phenotypic differences might have persisted or even increased.
Although the female offspring body weight data were comparable to those of the male offspring, overall, the male metabolic phenotypes were more pronounced. This dimorphism is a common feature of rodent metabolic studies, though the reasons are generally not clear (30). Sex-related differences in gonadal hormones, body fat distribution, and relationships between adipose tissue mass and insulin homeostasis may have influenced metabolic phenotypes in the male and female offspring in the present study.
Overall, there was no substantial effect of maternal IR, per se, on blood glucose and plasma insulin concentrations (corrected for adiposity) in the offspring. There was a significant maternal diet × maternal Insr genotype interaction, so that offspring of HFD-fed Insr+/− dams had increased concentrations of plasma insulin. However, when controlled for offspring glucose or adiposity, this impact diminished, suggesting that body composition differences were the main reason for the differences in circulating insulin among progeny. Previous studies using HFD-induced obese dams have found an effect of maternal diet on offspring glucose homeostasis (18,19). In these studies, offspring of obese rat dams exhibited increased circulating glucose and insulin concentrations, and/or glucose intolerance. One study, which reported reduced pancreatic β-cell number and volume in offspring born to dams fed a HFD throughout gestation, hypothesized that the HFD could be inhibiting β-cell replication pathways and/or inducing β-cell apoptosis during development in the offspring (31). Our finding that maternal IR per se did not impact offspring plasma glucose and insulin concentrations suggests that other features of the obese pregnancy, such as maternal consumption of HFD, may be responsible for conveying any effects on offspring glucose homeostasis beyond the secondary effects of progeny adiposity.
We were surprised to find that offspring of HFD-fed Insr+/− dams had significantly lower levels of liver TGs than in HFD-fed wild-type dam offspring. The decrease in liver TG levels occurred in the absence of any concurrent differences of body weight, adiposity, or circulating insulin concentrations. Because there was no difference in body weight or adiposity at sacrifice (week 16), we infer that both groups were consuming similar amounts of the HFD, and that differences found in liver TG levels reflected altered hepatic fatty acid and TG synthesis pathways (29,32).
Composition of ARH Pomc- and Npy-expressing neuronal populations: effects of maternal IR
We assessed the number of anorexigenic Pomc cells and orexigenic Npy cells in the ARH to begin to examine how the formation of circuits regulating energy homeostasis may be affected by maternal metabolic status. Indeed, maternal HFD and obesity during the perinatal period has been shown to influence the development of the central circuits regulating energy balance in rodents, which may convey a long-term impact on the phenotype of the offspring (16,19–21).
We compared the number of Pomc- and Npy-expressing cells in wild-type embryos from wild-type or Insr+/− dams at several perinatal time points. The onset of Pomc expression is at E10.5, and these cells proliferate and differentiate into neurons between E11.5–E12.5 (33). To see whether maternal IR could affect these developmental processes, we compared Pomc cell counts at E14 by fluorescent in situ hybridization, and did not find a difference. During the last week of gestation, Pomc is extinguished in half of the immature Pomc-expressing population. Finding an increase of 20% in the size of the POMC population at P9 (as estimated by counts spanning the rostral-caudal length of the arcuate) in offspring of Insr+/− dams compared to offspring of wild-type dams, supports the idea that maternal status can influence cell fate decisions within the Pomc-expression lineage. The Npy-expressing population was unaffected across these time points.
Although the observation that the maternal environment can influence the composition of neuronal circuits is intriguing, the functional significance of this increase in POMC neurons is not yet clear. Recent studies suggest that leptin- and insulin-sensing POMC neurons are independent populations that may serve opposite functions in the central circuits regulating energy balance (34,35). Once molecular markers that can distinguish between these POMC sub-populations are defined, we can examine whether the number of leptin-sensing and/or insulin-sensing POMC neurons are altered in our model. This knowledge would serve as the foundation for hypotheses regarding the relationship between neuronal composition in the ARH and maternal/progeny metabolic phenotypes.
Summary
The present study was designed to distinguish the independent and contingent effects of maternal IR and HFD per se, from overt obesity, in causing the metabolic consequences in progeny of maternal gestational obesity. Maternal HFD had the greatest effect on the metabolic phenotypes in progeny, whereas maternal IR—combined with a HFD—had effects on offspring body weight/adiposity phenotypes in the first 2 months of life that were greater than those associated with a HFD or maternal IR alone (Table 1). Maternal IR per se did not affect blood glucose and insulin homeostasis in offspring, suggesting that perhaps other features of an obese pregnancy (such as higher circulating free fatty acids/lipid levels, leptin, or inflammatory molecules, for example) are more likely candidates for mediating the impaired glucose homeostasis in offspring reported in maternal obesity studies.
Table 1.
Summary of offspring phenotypes by maternal genotype and diet
| Maternal status (diet and Insr genotype) |
Male offspring phenotypes | POMC neurons in ARH |
|||
|---|---|---|---|---|---|
| Weight | Adiposity | Glucose homeostasis | Liver TG | ||
| Chow: +/− vs. +/+ | No difference | No difference | Increased glucose (16%) at 4 weeks and insulin (150%) at 8 weeks | No difference | Increased by 20% at P9 |
| HFD: +/− vs. +/+ | Increased by ~17% up to 2 months | Increased by ~95% up to 2 months | Increased insulin (largely accounted for by adiposity) | Decreased by 35% at 16 weeks | Not done |
| +/+: HFD vs. chow | Increased by 28% at 16 weeks | Increased by 60% at 16 weeks | Increased glucose (70%) and insulin (200%), 4–16 weeks | Increased by 250% at 16 weeks | Not done |
Maternal high-fat diet (HFD) consumption results in prominent effects on progeny body weight, adiposity, glucose homeostasis, and liver triglyceride levels. Although the maternal Insr+/− genotype, per se, conveys minimal effects in progeny when dams are fed a chow diet, consumption of a HFD diet elicits interactive effects, resulting in increased body weight and adiposity in Insr+/− dam progeny. +/+, wild-type dam offspring; +/−, Insr+/− dam offspring; TG, triglycerides; ARH, arcuate nucleus of the hypothalamus.
Our mice had a mixed C57BL/6x129/Sv background (23); however, by intercrossing the line for many generations (N > 40), effectively a stable recombinant inbred line was created. Therefore, we do not think that differential segregation of C57BL/6 and 129/Sv alleles could account for the significant effects of maternal Insr genotype or diet.
What are the mechanisms by which maternal IR and HFD impart long-term effects in offspring? Free fatty acids cross the placenta, and it is possible that maternal consumption of a diet high in fat could lead to increased transport of these lipids, or affect placenta function, per se, which could subsequently affect development of critical organs regulating energy/glucose homeostasis (36,37). Maternal insulin does not cross the placenta or enter the fetal circulation. However, insulin receptors are located on the placenta, and higher levels of circulating insulin concentrations in maternal insulin-resistant states may alter placental growth and function (38). The placenta, of course, is a tissue derived from the fetus, so that in our model described here, placental insulin receptors were fully functional in +/+ progeny. The interaction of maternal HFD and IR led to the greatest effect on offspring body weight and composition, during the first two months of life, suggesting that if insulin does alter placental function, increased consumption/ambient concentrations of lipids are also required in order to impart an effect on offspring development/postnatal phenotypes. This does not exclude the possibility that IR, in the absence of obesity, could influence progeny body weight and composition phenotypes under different conditions; however, in our model we do not see an effect. The synergistic effects of the increased maternal insulin concentrations and consumption of a HFD in the perinatal period are likely critical factors in influencing offspring development, ultimately affecting metabolic phenotypes such as body weight and composition.
ACKNOWLEDGMENTS
This work was supported by RO1DK52431; P30DK63608; P30DK26687; T32 DK 007647; American Diabetes Association 7-07RA-195; and the Russell Berrie Foundation.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Prevalence of overweight obesity and extreme obesity among adults: United States, trends 1960–62 through 2005–2006. National Center for Health Statistics, National Health and Nutrition Examination Survey. www.cdc.gov/nchs/data/hestat/overweight/overweight_adults.html.
- 2.Franks PW, Hanson RL, Knowler WC, et al. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362:485–493. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 5.Fowden AL, Hill DJ. Intra-uterine programming of the endocrine pancreas. Br Med Bull. 2001;60:123–142. doi: 10.1093/bmb/60.1.123. [DOI] [PubMed] [Google Scholar]
- 6.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 7.Nathanielsz PW, Poston L, Taylor PD. In utero exposure to maternal obesity and diabetes: animal models that identify and characterize implications for future health. Clin Perinatol. 2007;34:515–526. doi: 10.1016/j.clp.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 8.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 9.Plagemann A. Perinatal programming and functional teratogenesis: impact on body weight regulation and obesity. Physiol Behav. 2005;86:661–668. doi: 10.1016/j.physbeh.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 10.Bouret SG. Early life origins of obesity: role of hypothalamic programming. J Pediatr Gastroenterol Nutr. 2009;48 Suppl 1:S31–S38. doi: 10.1097/MPG.0b013e3181977375. [DOI] [PubMed] [Google Scholar]
- 11.Grayson BE, Kievit P, Smith MS, Grove KL. Critical determinants of hypothalamic appetitive neuropeptide development and expression: species considerations. Front Neuroendocrinol. 2010;31:16–31. doi: 10.1016/j.yfrne.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vahratian A. Prevalence of overweight and obesity among women of childbearing age: results from the 2002 National Survey of Family Growth. Matern Child Health J. 2009;13:268–273. doi: 10.1007/s10995-008-0340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barker DJ. In utero programming of chronic disease. Clin Sci. 1998;95:115–128. [PubMed] [Google Scholar]
- 14.Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med. 2000;9:83–88. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<83::AID-MFM17>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 15.Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49:2208–2211. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- 16.Chen H, Simar D, Lambert K, Mercier J, Morris MJ. Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology. 2008;149:5348–5356. doi: 10.1210/en.2008-0582. [DOI] [PubMed] [Google Scholar]
- 17.White CL, Purpera MN, Morrison CD. Maternal obesity is necessary for programming effect of high-fat diet on offspring. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1464–R1472. doi: 10.1152/ajpregu.91015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivasan M, Katewa SD, Palaniyappan A, Pandya JD, Patel MS. Maternal high-fat diet consumption results in fetal malprogramming predisposing to the onset of metabolic syndrome-like phenotype in adulthood. Am J Physiol Endocrinol Metab. 2006;291:E792–E799. doi: 10.1152/ajpendo.00078.2006. [DOI] [PubMed] [Google Scholar]
- 19.Page KC, Malik RE, Ripple JA, Anday EK. Maternal and postweaning diet interaction alters hypothalamic gene expression and modulates response to a high-fat diet in male offspring. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1049–R1057. doi: 10.1152/ajpregu.90585.2008. [DOI] [PubMed] [Google Scholar]
- 20.Gupta A, Srinivasan M, Thamadilok S, Patel MS. Hypothalamic alterations in fetuses of high fat diet-fed obese female rats. J Endocrinol. 2009;200:293–300. doi: 10.1677/JOE-08-0429. [DOI] [PubMed] [Google Scholar]
- 21.Morris MJ, Chen H. Established maternal obesity in the rat reprograms hypothalamic appetite regulators and leptin signaling at birth. Int J Obes (Lond) 2009;33:115–122. doi: 10.1038/ijo.2008.213. [DOI] [PubMed] [Google Scholar]
- 22.Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat Med. 2010;16:403–405. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kido Y, Philippe N, Schäffer AA, Accili D. Genetic modifiers of the insulin resistance phenotype in mice. Diabetes. 2000;49:589–596. doi: 10.2337/diabetes.49.4.589. [DOI] [PubMed] [Google Scholar]
- 24.Norris AW, Chen L, Fisher SJ, et al. Muscle-specific PPARgamma-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest. 2003;112:608–618. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes. 2009;58:1116–1125. doi: 10.2337/db08-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howie GJ, Sloboda DM, Kamal T, Vickers MH. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol (Lond) 2009;587:905–915. doi: 10.1113/jphysiol.2008.163477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCurdy CE, Bishop JM, Williams SM, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119:323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckley AJ, Keserü B, Briody J, Thompson M, Ozanne SE, Thompson CH. Altered body composition and metabolism in the male offspring of high fat-fed rats. Metabolism. 2005;54:500–507. doi: 10.1016/j.metabol.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Bruce KD, Cagampang FR, Argenton M, et al. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009;50:1796–1808. doi: 10.1002/hep.23205. [DOI] [PubMed] [Google Scholar]
- 30.Woods SC, Gotoh K, Clegg DJ. Gender differences in the control of energy homeostasis. Exp Biol Med (Maywood) 2003;228:1175–1180. doi: 10.1177/153537020322801012. [DOI] [PubMed] [Google Scholar]
- 31.Cerf ME, Williams K, Nkomo XI, et al. Islet cell response in the neonatal rat after exposure to a high-fat diet during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1122–R1128. doi: 10.1152/ajpregu.00335.2004. [DOI] [PubMed] [Google Scholar]
- 32.Han S, Liang CP, Westerterp M, et al. Hepatic insulin signaling regulates VLDL secretion and atherogenesis in mice. J Clin Invest. 2009;119:1029–1041. doi: 10.1172/JCI36523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat Med. 2010;16:403–405. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams KW, Margatho LO, Lee CE, et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30:2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill JW, Elias CF, Fukuda M, et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varastehpour A, Radaelli T, Minium J, et al. Activation of phospholipase A2 is associated with generation of placental lipid signals and fetal obesity. J Clin Endocrinol Metab. 2006;91:248–255. doi: 10.1210/jc.2005-0873. [DOI] [PubMed] [Google Scholar]
- 37.Jones HN, Powell TL, Jansson T. Regulation of placental nutrient transport–a review. Placenta. 2007;28:763–774. doi: 10.1016/j.placenta.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Desoye G, Hauguel-de Mouzon S. The human placenta in gestational diabetes mellitus. The insulin and cytokine network. Diabetes Care. 2007;30 Suppl 2:S120–S126. doi: 10.2337/dc07-s203. [DOI] [PubMed] [Google Scholar]