Abstract
When human polymorphonuclear leukocytes (PMN) are placed on various surfaces, they attach and spread rapidly, increasing their diameter severalfold. The spreading is associated with extensive changes in the cytoskeleton. Since many cytoskeletal events are regulated by Ca2+, we measured the cytosolic free calcium concentration ([Ca2+]i) in individual human PMN as they spread. [Ca2+]i was measured in single cells by microspectrofluorometry using the fluorescent Ca2+-sensitive dye fura-2. Immediately before spreading, PMN exhibit a rapid increase in [Ca2+]i, from 69 +/- 51 nM to 547 +/- 190 nM (mean +/- SD, n = 12). [Ca2+]i returns to near resting levels during the next minute, as the cells spread. Neither the spreading nor the [Ca2+]i spike is blocked by removal of extracellular calcium, by verapamil, by calmodulin antagonists, or by mitochondrial or microtubule poisons. Spreading, but not the [Ca2+]i increase, is blocked by the microfilament inhibitor cytochalasin B. Both spreading and the [Ca2+]i spike are blocked by ATP depletion and reversibly blocked by placing the cells in medium containing hypertonic sucrose or sodium chloride. These data strongly suggest that an increase in [Ca2+]i, derived from nonmitochondrial intracellular pools, plays an important role in the microfilament-mediated process of PMN spreading.
Full text
PDF


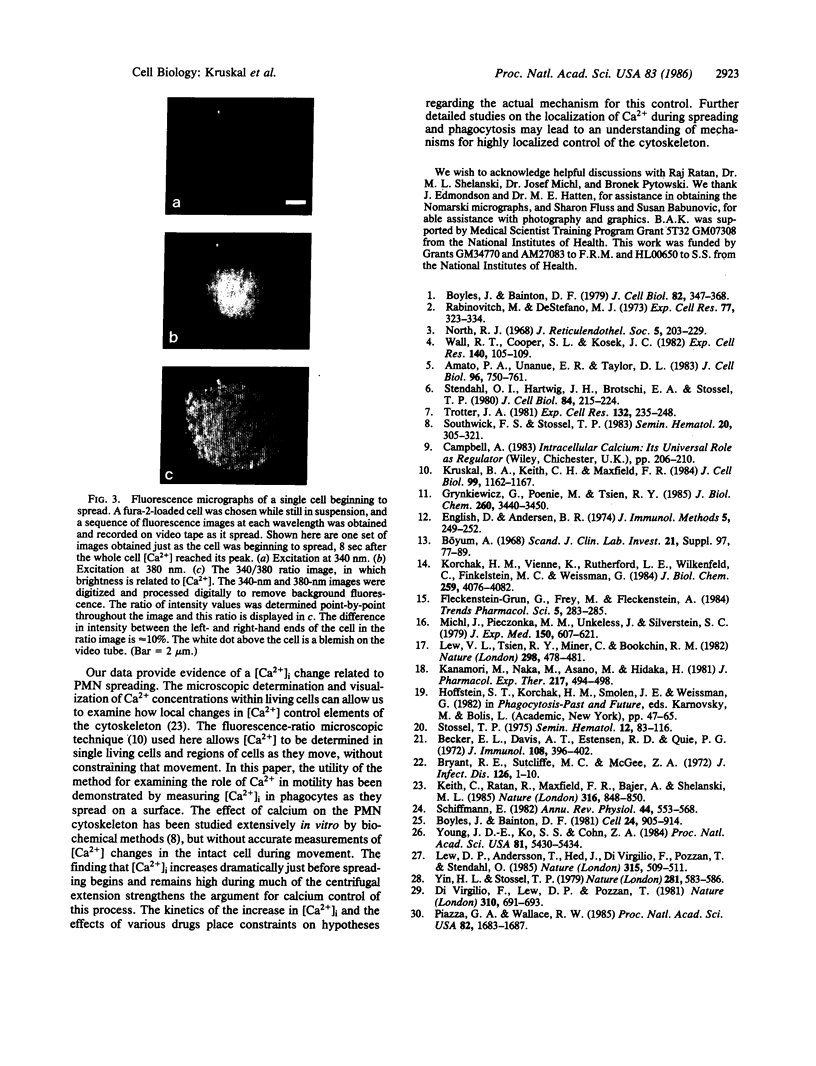
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amato P. A., Unanue E. R., Taylor D. L. Distribution of actin in spreading macrophages: a comparative study on living and fixed cells. J Cell Biol. 1983 Mar;96(3):750–761. doi: 10.1083/jcb.96.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. L., Davis A. T., Estensen R. D., Quie P. G. Cytochalasin B. IV. Inhibition and stimulation of chemotaxis of rabbit and human polymorphonuclear leukocytes. J Immunol. 1972 Feb;108(2):396–402. [PubMed] [Google Scholar]
- Boyles J., Bainton D. F. Changes in plasma-membrane-associated filaments during endocytosis and exocytosis in polymorphonuclear leukocytes. Cell. 1981 Jun;24(3):905–914. doi: 10.1016/0092-8674(81)90116-1. [DOI] [PubMed] [Google Scholar]
- Boyles J., Bainton D. F. Changing patterns of plasma membrane-associated filaments during the initial phases of polymorphonuclear leukocyte adherence. J Cell Biol. 1979 Aug;82(2):347–368. doi: 10.1083/jcb.82.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R. E., Sutcliffe M. C., McGee Z. A. Effect of osmolalities comparable to those of the renal medulla on function of human polymorphonuclear leukocytes. J Infect Dis. 1972 Jul;126(1):1–10. doi: 10.1093/infdis/126.1.1. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Lew D. P., Pozzan T. Protein kinase C activation of physiological processes in human neutrophils at vanishingly small cytosolic Ca2+ levels. Nature. 1984 Aug 23;310(5979):691–693. doi: 10.1038/310691a0. [DOI] [PubMed] [Google Scholar]
- English D., Andersen B. R. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974 Aug;5(3):249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Kanamori M., Naka M., Asano M., Hidaka H. Effects of N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide and other calmodulin antagonists (calmodulin interacting agents) on calcium-induced contraction of rabbit aortic strips. J Pharmacol Exp Ther. 1981 May;217(2):494–499. [PubMed] [Google Scholar]
- Keith C. H., Ratan R., Maxfield F. R., Bajer A., Shelanski M. L. Local cytoplasmic calcium gradients in living mitotic cells. 1985 Aug 29-Sep 4Nature. 316(6031):848–850. doi: 10.1038/316848a0. [DOI] [PubMed] [Google Scholar]
- Korchak H. M., Vienne K., Rutherford L. E., Wilkenfeld C., Finkelstein M. C., Weissmann G. Stimulus response coupling in the human neutrophil. II. Temporal analysis of changes in cytosolic calcium and calcium efflux. J Biol Chem. 1984 Apr 10;259(7):4076–4082. [PubMed] [Google Scholar]
- Kruskal B. A., Keith C. H., Maxfield F. R. Thyrotropin-releasing hormone-induced changes in intracellular [Ca2+] measured by microspectrofluorometry on individual quin2-loaded cells. J Cell Biol. 1984 Sep;99(3):1167–1172. doi: 10.1083/jcb.99.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D. P., Andersson T., Hed J., Di Virgilio F., Pozzan T., Stendahl O. Ca2+-dependent and Ca2+-independent phagocytosis in human neutrophils. Nature. 1985 Jun 6;315(6019):509–511. doi: 10.1038/315509a0. [DOI] [PubMed] [Google Scholar]
- Lew V. L., Tsien R. Y., Miner C., Bookchin R. M. Physiological [Ca2+]i level and pump-leak turnover in intact red cells measured using an incorporated Ca chelator. Nature. 1982 Jul 29;298(5873):478–481. doi: 10.1038/298478a0. [DOI] [PubMed] [Google Scholar]
- Michl J., Pieczonka M. M., Unkeless J. C., Silverstein S. C. Effects of immobilized immune complexes on Fc- and complement-receptor function in resident and thioglycollate-elicited mouse peritoneal macrophages. J Exp Med. 1979 Sep 19;150(3):607–621. doi: 10.1084/jem.150.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The uptake of particulate antigens. J Reticuloendothel Soc. 1968 Jun;5(3):203–229. [PubMed] [Google Scholar]
- Piazza G. A., Wallace R. W. Calmodulin accelerates the rate of polymerization of human platelet actin and alters the structural characteristics of actin filaments. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1683–1687. doi: 10.1073/pnas.82.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M., DeStefano M. J. Macrophage spreading in vitro. I. Inducers of spreading. Exp Cell Res. 1973 Mar 15;77(1):323–334. doi: 10.1016/0014-4827(73)90584-3. [DOI] [PubMed] [Google Scholar]
- Schiffmann E. Leukocyte chemotaxis. Annu Rev Physiol. 1982;44:553–568. doi: 10.1146/annurev.ph.44.030182.003005. [DOI] [PubMed] [Google Scholar]
- Southwick F. S., Stossel T. P. Contractile proteins in leukocyte function. Semin Hematol. 1983 Oct;20(4):305–321. [PubMed] [Google Scholar]
- Stendahl O. I., Hartwig J. H., Brotschi E. A., Stossel T. P. Distribution of actin-binding protein and myosin in macrophages during spreading and phagocytosis. J Cell Biol. 1980 Feb;84(2):215–224. doi: 10.1083/jcb.84.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis: recognition and ingestion. Semin Hematol. 1975 Jan;12(1):83–116. [PubMed] [Google Scholar]
- Trotter J. A. The organization of actin in spreading macrophages. The actin-cytoskeleton of peritoneal macrophages is linked to the substratum via transmembrane connections. Exp Cell Res. 1981 Apr;132(2):235–248. doi: 10.1016/0014-4827(81)90099-9. [DOI] [PubMed] [Google Scholar]
- Wall R. T., Cooper S. L., Kosek J. C. The influence of exogenous fibronectin on blood granulocyte adherence to vascular endothelium in vitro. Exp Cell Res. 1982 Jul;140(1):105–109. doi: 10.1016/0014-4827(82)90161-6. [DOI] [PubMed] [Google Scholar]
- Yin H. L., Stossel T. P. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979 Oct 18;281(5732):583–586. doi: 10.1038/281583a0. [DOI] [PubMed] [Google Scholar]
- Young J. D., Ko S. S., Cohn Z. A. The increase in intracellular free calcium associated with IgG gamma 2b/gamma 1 Fc receptor-ligand interactions: role in phagocytosis. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5430–5434. doi: 10.1073/pnas.81.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]