Abstract
Aim
To assess the performance of automated disease detection in diabetic retinopathy screening using two field mydriatic photography.
Methods
Images from 8,271 sequential patient screening episodes from a South London diabetic retinopathy screening service were processed by the Medalytix iGrading™ automated grading system. For each screening episode macular-centred and disc-centred images of both eyes were acquired and independently graded according to the English national grading scheme. Where discrepancies were found between the automated result and original manual grade, internal and external arbitration was used to determine the final study grades. Two versions of the software were used: one that detected microaneurysms alone, and one that detected blot haemorrhages and exudates in addition to microaneurysms. Results for each version were calculated once using both fields and once using the macula-centred field alone.
Results
Of the 8,271 episodes, 346 (4.2%) were considered unassessable. Referable disease was detected in 587 episodes (7.1%). The sensitivity of the automated system for detecting unassessable images ranged from 97.4% to 99.1% depending on configuration. The sensitivity of the automated system for referable episodes ranged from 98.3% to 99.3%. All the episodes that included proliferative or pre-proliferative retinopathy were detected by the automated system regardless of configuration (192/192, 95% confidence interval 98.0% to 100%). If implemented as the first step in grading, the automated system would have reduced the manual grading effort by between 2,183 and 3,147 patient episodes (26.4% to 38.1%).
Conclusion
Automated grading can safely reduce the workload of manual grading using two field, mydriatic photography in a routine screening service.
Introduction
Diabetic retinopathy is one of the most common causes of vision loss in the developed world. Approximately three million people are thought to have diabetes in England. Since timely treatment is effective in reducing vision loss[1], everyone known to have diabetes and aged over twelve is invited for annual retinal screening using digital photography.
Screening generates a large amount of image data requiring grading. As the prevalence of diabetes continues to rise, there is a concomitant increase in the grading burden[2]. Furthermore, as in other screening programmes, the majority of images are normal. Automated grading has been proposed as a method to remove normal images from the manual grading queue, so reducing the overall manual workload.
A fully automated system using macular-centred images has been tested on a number of datasets, one of which included 33,535 consecutive patient episodes[3]–[5]. These studies were based on a one-field, staged mydriasis photographic protocol, hence confirmation was required that the results were applicable to other photographic protocols, such as two-field, mydriatic photography.
We report here the results of a study testing automated disease detection including quality assessment using images acquired and graded using two-field, mydriatic photography.
Methods
Data collection
Images from 8,500 consecutive patient episodes (approximately 6 months of screening data) were extracted from the Wandsworth, Richmond and Twickenham diabetic retinopathy screening service (DRSS), South London in 2009. Duplicate visits, non-screening follow-up visits and test images were excluded, leaving 8,271 unique patient episodes and 36,236 retinal photographs. The retinal photographs were nominally 45 degree fields of view acquired using a non-mydriatic fundus camera: 68% were from Topcon NW6 fundus cameras with Nikon D80 digital single lens reflex camera (DSLR) backs, while the remainder were acquired using Canon CR6 non-mydriatic fundus cameras with a Canon D30, 30D or 40D DSLR back. The resulting colour images were between 3.1 and 10.1 megapixels and were stored using high quality JPEG compression. All patients underwent routine mydriasis with Tropicamide 1% and, where present, both eyes were imaged. Two fields were taken per eye: a macula-centred and a disc-centred view. Where appropriate, more than one image of each field was taken at the discretion of the retinal screener.
Manual Image grading
Images from all the patient episodes were routinely graded by the local DRSS, independently of this study, following the standard grading Pathway 2 (“full disease grading”) of the English National Screening Programme for diabetic retinopathy[6]. Where the photographic quality was deemed adequate, the eye was graded for both retinopathy and maculopathy, as per Table 1. The grade for each eye was a combined assessment of the macular and disc fields. Where multiple images were taken of a field, these were all included in the assessment; the grade for the eye was taken as the worst grade of all the fields acquired for the eye. The grade for the patient episode was taken from the eye with the more serious disease. Disagreements between the DRSS grade and the automated result were sent for internal arbitration. Disagreements pertaining to an episode originally graded as referable by the DRSS were also sent for external arbitration.
Table 1. Retinopathy and maculopathy grading scheme for the English National Screening Programme for Diabetic Retinopathy.
| Retinopathy (R) | ||
| R0 | None | No visible retinopathy |
| R1 | Background | Any microaneurym, haemorrhage or exudates |
| R2 | Pre-proliferative | Venous beading, loop or reduplication Intraretinal microvascular abnormality (IRMA)Multiple deep, round or blot haemorrhages |
| R3 | Proliferative | New vessels on the disc (NVD)New vessels elsewhere (NVE)Pre-retinal or vitreous haemorrhagePre-retinal fibrosis (with or without detachment) |
Automated image analysis
Automated grading software was purchased for this project (Medalytix iGrading™ system, 7 Water Street, Liverpool, L2 0RD) and run on a HP Proliant DL380 server at St George's Hospital. The software is designed to remove normal images from the manual grading queue. It does this by first checking the image for adequate clarity and field of view[3] before looking for the early signs of retinopathy. It may be operated in two modes: the first looks only for microaneurysms (MA)[3], while the second (MA/BH/EX) also looks for blots and exudates in addition to microaneurysms[4]. The software was evaluated here using both fields from each eye, and also using only the macula-centred field from each eye. Hence there were four automated strategies tested: MAs only on macular field alone, MA/BH/EX on macular field alone, MAs on the macular and disc fields, and MA/BH/EX on the macular and disc fields. As for manual grading, where more than one image of a field was acquired these were all included in the automated analysis: if any of the images were positive then that eye was treated as positive.
Internal arbitration of disease/no-disease discrepancies
Images where there was a discrepancy between the manual and automated systems regarding whether disease was present were internally arbitrated by one of two “arbitration level” graders within the screening programme (SN and AC). Occasionally images were re-graded jointly. The arbitration outcomes were simply disease or no-disease. Since full grading was not performed the severity of any disease missed by the original DRSS grading could not be categorized.
External arbitration of referable/non-referable discrepancies
Where there was a discrepancy between the DRSS grade and an automated strategy regarding whether an episode was referable these images were re-graded externally. Graders working in different screening programmes in England were invited to participate at the British Association of Retinal Screeners annual meeting in 2010. The grading took place in February and March 2011, using a web-based grading system based on the features of the English grading scheme. Graders were able to practise and become familiar with the system prior to carrying out the arbitration grading. A similar number of control images, where the manual and automated system agreed on the presence or absence of retinopathy, were added so that the graders did not know whether they were grading a discrepancy. Graders were able to view all the available images for each eye and each grader was shown the eyes in a random order. Twenty-five graders from 16 different English screening programmes took part. Of these 11 were arbitration-level graders, 8 secondary-level graders and 6 primary-level graders.
A consensus criterion, based on the grading of the eleven arbitration level graders, was used to determine whether each eye was referable. An eye was considered referable if at least 8/11 of the arbitration-level graders indicated referable features, and non-referable when 3/11 or fewer of the arbitration-level graders indicated a referable feature. When between 4 and 7 graders indicated a referable feature it was deemed there was insufficient consensus to assign a grade.
Statistical comparison
The sensitivities of the four automated strategies were compared for the detection of any retinopathy, referable retinopathy, unassessable episodes, and for each grade of retinopathy listed in Table 1. The workload reduction was calculated as the proportion of images the automated system recorded as both gradeable and without disease. 95% confidence intervals were calculated on all measurements.
Information Governance
All the images were abstracted without patient identifiers or grading information. Manual grading was done prior to image analysis and without knowledge of the study. Arbitration grading was done without knowledge of either the software analysis or prior grading results. Only one person (LW), who was not involved in any grading or software analysis, had access to both grading results and image analyses. The study had written consent from the Chairman of the London Wandsworth Research Ethics Committee and the Institutional Caldicott Guardian. All participants in the English National Screening Programme for Diabetic Retinopathy agree to the anonymous use of their images for teaching and research. The study had no effect on the routine clinical care of patients within the screening programme. Where there was any question of the effect of grading on patient management the electronic records were accessed and the subsequent outcome examined.
Results
Manual grading
Episodes in which the manual and automated systems disagreed were passed to internal arbitration grading. If the disagreement involved a referable episode then these were also passed to external arbitration grading. Of the 8,271 patient episodes included in the study, four were removed following external arbitration as no consensus was reached regarding their grade, leaving 8,267 episodes. Of these, 4.2% were considered unassessable by manual grading. 58.7% were graded as having no retinopathy, 30.0% were graded as having background retinopathy (R1), 4.8% were graded as having maculopathy (M1), 1.6% were graded as having pre-proliferative retinopathy (R2), and 0.7% were graded as having proliferative retinopathy (R3). Overall, 587 (7.1%) were given referable grades (i.e. R2, R3 or M1).
Internal arbitration of disease/no disease discrepancies
Of the disease/no-disease discrepancies there were 48 episodes where the original DRSS grade was disease (R1 or above) but the arbitration grade was R0 (no disease). These downgraded discrepancies included eleven referable episodes: one originally graded R3 (proliferative retinopathy) and 10 graded as M1 (maculopathy). All of these episodes were included in the external arbitration. There were 326 episodes whose original DRSS grade was R0 (no disease) but arbitration found disease. Since full grading was not performed the disease missed by the DRSS grading could range from background (R1) to proliferative retinopathy (R3). The significant increase in the number of images categorised as having disease following internal arbitration suggests the arbitration process operated at a higher disease sensitivity than the routine screening service.
External arbitration of referable/non-referable discrepancies
Referable/non-referable discrepancies from any of the four automated grading strategies were arbitrated externally. All of discrepancies concerned maculopathy (R1M1), except for a case where the original DRSS grade was proliferative retinopathy (R3) but internal arbitration had downgraded it to no disease (R0). Combining the discrepancies from the four automated strategies resulted in 32 eyes for external arbitration. A further 48 eyes, in which the manual and automated grading agreed, were also included as control images. The median time for all levels of grader to complete the 80 eyes was 2.6 hours (interquartile range 2.0 to 3.4 hours).
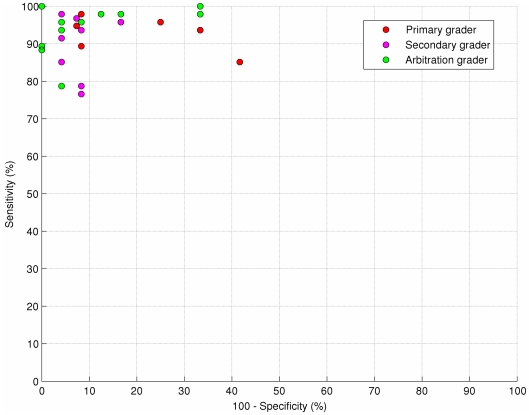
Figure 1 shows a receiver operator characteristic (ROC) plot for all the graders who completed the external arbitration. The three levels of grader (primary, secondary and arbitration) are indicated separately, since primary level graders may be expected to have a different sensitivity/specificity trade-off to arbitration level graders. Nevertheless, even amongst arbitration level graders there is a range of operating points from high specificity and lower sensitivity to high sensitivity and lower specificity.
Figure 1. Receiver operator characteristic (ROC) plot for all the graders who took part in the external arbitration.
The three levels of grader (primary, secondary and arbitration) are shown by ‘*’, ‘+’ and ‘o’ respectively. All the graders were compared against a consensus grading calculated from the results from all the arbitration level graders. Notice even amongst the arbitration level graders there is a range of operating points from high specificity and lower sensitivity to high sensitivity and lower specificity.
Of the 32 discrepancies presented to the graders, 28/32 achieved a consensus grade; the remaining 4 eyes were excluded from the analysis. Two of the episodes were considered unassessable by the arbitrators and nine were graded as non-referable (including the episode originally DRSS graded as having proliferative retinopathy which was internally arbitrated as R0). The remaining 17 eyes were graded as having maculopathy. However, three of these had only red lesions within the macula and normal visual acuity and so would have been graded as background retinopathy (R1) according to the English National Screening Programme for Diabetic Retinopathy grading scheme.
Hence, of the original 32 discrepancies, 2 unassessable eyes and 14 eyes with maculopathy were missed by the automated system. Of these, only 6/16 were unanimously graded as unassessable or referable by the arbitration level graders. Including the grading from the primary and secondary graders there were only 3/16 images which were unanimously graded as unassessable or referable by graders of all levels.
Automated grading
Table 2 lists the sensitivities for automated disease detection for each grade, as well as for any diabetic retinopathy and any referable diabetic retinopathy. The associated workload reduction of employing the automated strategy as a disease/no-disease grader is also shown. The sensitivity for detecting unassessable images ranged from 97.4% (MA only, macular field) to 99.1% (MA/BH/EX, both fields). The sensitivity for detecting any retinopathy ranged from 89.9% (MA/BH/EX, macular field) to 95.8% (MA only, both fields). The sensitivity for referable retinopathy (i.e. M1, R2 and R3) ranged from 98.3% (MA/BH/EX, single field) to 99.3% (MA only, both fields). The workload reduction ranged from 26.4% (MA/BH/EX, both fields) to 38.1% (MA only, macular field). The sensitivities for pre-proliferative and proliferative disease were 100% using all four automated strategies.
Table 2. Performance of the four automated strategies for detecting different grades of disease, together with the associated workload reduction.
| DRSS category | Number identified by the automated system | Proportion identified (%) [95% confidence interval] |
| MA/BH/EX (both fields) | ||
| Unassessable | 343/346 | 99.1 [97.5 to 99.7] |
| R0 | 3094/5141 | 60.2 [58.8 to 61.5] |
| R1 | 2065/2193 | 94.2 [93.1 to 95.1] |
| M1 | 390/395 | 98.7 [97.1 to 99.5] |
| R2 | 131/131 | 100 [97.2 to 100] |
| R3 | 61/61 | 100 [94.1 to 100] |
| Any retinopathy | 2647/2780 | 95.2 [94.4 to 95.9] |
| Referable | 582/587 | 99.1 [98.0 to 99.6] |
| (Workload reduction | 2183/8267 | 26.4 [25.5 to 27.4]) |
| MA(both fields) | ||
| Unassessable | 339/346 | 98.0 [95.9 to 99.0] |
| R0 | 2806/5141 | 54.6 [53.2 to 55.9] |
| R1 | 2080/2193 | 94.8 [93.8 to 95.7] |
| M1 | 391/395 | 99.0 [97.4 to 99.6] |
| R2 | 131/131 | 100.0 [97.2 to 100.0] |
| R3 | 61/61 | 100 [94.1 to 100] |
| Any retinopathy | 2663/2780 | 95.8 [95.0 to 96.5] |
| Referable | 583/587 | 99.3 [98.3 to 99.7] |
| (Workload reduction | 2459/8267 | 29.7 [28.8 to 30.7]) |
| MA/BH/EX (macular field only) | ||
| Unassessable | 342/346 | 98.8 [97.1 to 99.5] |
| R0 | 2607/5141 | 50.7 [49.3 to 52.1] |
| R1 | 1921/2193 | 87.6 [86.2 to 88.9] |
| M1 | 385/395 | 97.5 [95.4 to 98.6] |
| R2 | 131/131 | 100.0 [97.2 to 100.0] |
| R3 | 61/61 | 100 [94.1 to 100] |
| Any retinopathy | 2498/2780 | 89.9 [88.7 to 90.9] |
| Referable | 577/587 | 98.3 [96.9 to 99.1] |
| (Workload reduction | 2820/8267 | 34.1 [33.1 to 35.1]) |
| MA(macular field only) | ||
| Unassessable | 337/346 | 97.4 [95.1 to 98.6] |
| R0 | 2229/5141 | 43.4 [42.0 to 44.7] |
| R1 | 1976/2193 | 90.1 [88.8 to 91.3] |
| M1 | 386/395 | 97.7 [95.7 to 98.8] |
| R2 | 131/131 | 100 [97.2 to 100] |
| R3 | 61/61 | 100 [94.1 to 100] |
| Any retinopathy | 2554/2780 | 91.9 [90.8 to 92.8] |
| Referable | 578/5877 | 98.5 [97.1 to 99.2] |
| (Workload reduction | 3147/8267 | 38.1 [37.0 to 39.1]) |
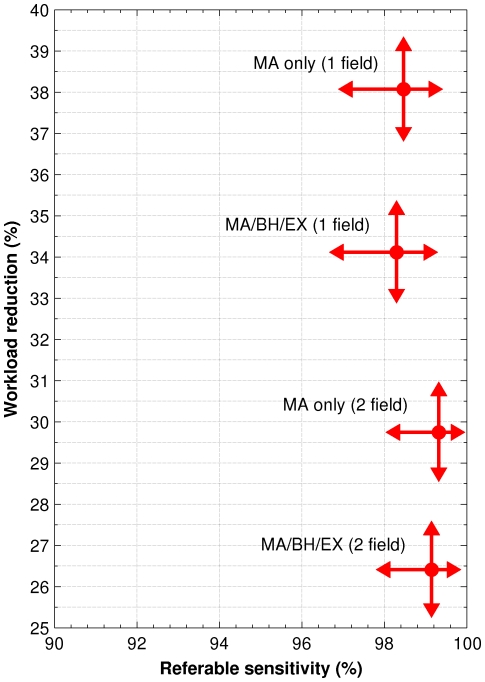
Figure 2 shows a plot of workload reduction versus referable disease sensitivity using the four automated strategies. The arrows indicate the 95% confidence intervals on the measurements. While including the disk centred field detected additional referable cases, it also resulted in many more non-referable cases being detected, which would require manual grading. For both the MA only and MA/BH/EX strategies, including the disk centred field picked up five additional referable maculopathy cases not detected using the macular field alone. However, in order to find these five additional cases an additional 688 non-referable cases were found using MAs only, or an additional 637 cases using MA/BH/EX. Hence, of the additional cases found by including the disk centred field, only 5/688 (0.73% [0.31% to 1.69%]) were referable using MAs only, and 5/637 (0.78% [0.34% to 1.82%]) were referable using MA/BH/EX.
Figure 2. Workload reduction versus referable sensitivity for the four automated strategies.
The arrows indicate the 95% confidence intervals on the measurements.
Discussion
There are three previous published reports of the performance of this automated system [3]–[5] using staged mydriasis and single-field photography. The present study assessed the performance of automated disease detection on an unselected population of 8,271 subjects from a South London retinal screening service using mydriatic, two-field photography. The sensitivities for referable disease using the four automated strategies are very similar to those published previously with the same techniques using a macular centred, mydriatic photographic protocol (Table 3).
Table 3. Comparison of per patient sensitivities and workload reduction from this study and three Scottish studies using the same software.
| MA (MAs only) | MA/BH/EX (MAs + blots + exudates) | |||||||
| Study | Unassessable (%) | Referable (%) | R2/R3 (%) | Workload reduction (%) | Unassessable (%) | Referable (%) | R2/R3 (%) | Workload reduction (%) |
| This study Macula field only | 97.4 [95.1,98.6](337/346) | 98.5[97.1,99.2](578/587) | 100[98.0,100](192/192) | 38.1[37.0,39.1](3147/8267) | 98.8[97.1,99.5](342/346) | 98.3[96.9,99.1](577/587) | 100[98.0,100](192/192) | 34.1[33.1,35.1](2820/8267) |
| This study Both fields | 98.0[95.9,99.0](339/346) | 99.3[98.3,99.7](583/587) | 100[98.0,100](192/192) | 29.7[28.8,30.7](2459/8267) | 99.1[97.5,99.7](343/346) | 99.1[98.0,99.6](582/587) | 100[98.0,100](192/192) | 26.4[25.5,27.4](2183/8267) |
| Philip et al. 2007 [3] | 99.8[99.0–100](552/553) | 98.0[95.3,99.1](241/246) | 100[94.6,100](67/67) | 45.7[44.5,46.9](3070/6722) | ||||
| Fleming et al. 2010a [4] | 98.6[97.4,99.3](634/643) | 95.0[93.5,96.1](1076/1133) | 97.6[95.9,98.6](488/500) | 39.2[38.1,40.3](2971/7586) | 98.8[97.6,99.4](635/643) | 96.9[95.7,97.8](1098/1133) | 98.2[96.6,99.1](491/500) | 38.9[37.8,40.0](2951/7586) |
| Fleming et al. 2010b [5] | 99.8[99.5,99.9](1824/1827) | 98.1[97.3,98.7](1603/1634) | 100[99.2,100](504/504) | 38.4[37.8,38.9](12154/31681) | ||||
Note that study [4] is the only one that apparently missed proliferative disease. However, subsequent re-grading of the six supposed proliferative cases downgraded all cases to non-referable (the six images in question are available as supplementary material from the BJO website).
The performance of automated grading should be compared with that achieved by manual grading of photographic images. Several studies have demonstrated the limited sensitivity and specificity of retinal photography for detecting referable retinopathy: some referable disease is missed before the eye is even graded[7]–[9]. Furthermore, image grading is not a trivial task and other studies have reported wide disagreement between graders[10]–[13]. The ROC plot in figure 1 indicates the range of performance that may be expected from different levels of grader within a screening service. Images are often difficult to interpret owing to subtle and inconclusive disease features, the presence of distracting artefacts or the borderline position of lesions. Finally, very occasionally, a grader makes a mistake and misses a clear feature. In the external arbitration exercise only 16 of the 32 eyes originally DRSS graded as referable, but graded negative by one or more of the automated strategies, were graded as referable by a consensus of the arbitration level graders. Of these only six were unanimously graded as referable, meaning that at least one arbitration level grader would also have missed each of the remaining 10 cases.
Clinical follow-up information was available for 11/14 episodes that were graded as normal by the automated system but as referable maculopathy by external arbitration. Of these, all but three went for optical coherence tomography (OCT). One of the patients had laser treatment 10 months after screening. Other studies have shown that maculopathy and macular oedema progresses slowly [14], [15].
There was a significant difference in workload reduction depending on whether the macular field alone or both fields were used (Figure 1). Automated grading using microaneurysms alone achieved a workload reduction of 38.1% (CI 37.0% – 39.1%) with the macular field alone and 29.7% (CI 28.8% – 30.7%) with both fields. The addition of the disc-centred field may be expected to increase sensitivity, since the manual and automated systems both have a second opportunity to spot retinopathy. However, it is also an additional opportunity to detect false positive disease which decreases the workload reduction. In this study less than 0.8% of the additional cases generated by including the disk centred field were referable. No advantage was found using MA/BH/EX over MAs alone. MA/BH/EX resulted in similar sensitivity but at a higher false positive rate that decreased the associated workload reduction.
No attempt has been made to estimate the cost-benefits associated with the workload reduction, as this will depend on the staffing numbers, salary grades and work throughput in individual screening services. However, it may be expected that a 38% workload reduction applied to a screening programme would have considerable benefits, especially given the increasing prevalence of diabetes.
In conclusion, an automated disease/no-disease grading system has been tested against data from a retinal screening service using a mydriatic two-field, photographic protocol. Automated grading can safely reduce the burden of manual grading with potential cost-benefits.
Acknowledgments
We thank Professor Derek Cook and Dr Alicja Rudnicka for the statistical power calculations that determined the number of episodes chosen for the study. We are indebted to Grant Duncan (Medalytix) for his help in software deployment.
We are extremely grateful to the following volunteers who took part in the external arbitration study: Stacey Barbaccia (Birmingham), Nicola Boult (Liverpool), Hayley Chambers (Birmingham), Raphael Cooper (Homerton), Trudi Evans (Barnet), Adele Farnsworth (Birmingham), Leon Gardner (Gloucestershire), Alyson Jaycock (Oxford), Vanessa Jones (Gloucestershire), Stuart Lark (Liverpool), Gerald Lewis (Southampton), Anna Lloyd (Wandsworth), Jenny Mackenzie (Gloucestershire), Brian Mealey (Liverpool), Jamili Miah (Birmingham), Mahi Muqit (Manchester), Tony Nicholls (Norfolk), Reg Parsons (Surrey), Clive Pledger (Bedfordshire), Nadine Rash (Croydon), Laura Reed-Peck (Herts East & North), Greg Russell (First Retinal), Althea Smith (North Yorkshire), Elizabeth Taylor (North Yorkshire), and Shelley Widdowson (North Yorkshire). We thank Peter Sharp, John Olson and Alan Fleming for their helpful comments on this manuscript.
Footnotes
Competing Interests: The authors have read the journal's policy and have the following conflict: KAG is employed by the University of Aberdeen who have licensed the automated grading software to Medalytix. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials. The remaining authors have declared that they have no competing interests.
Funding: KAG was supported by NIHR grant HTA 06/402/49 and the remaining authors were personally salaried by their institutions during the period of writing (though no specific salary was set aside or given for the writing of this paper). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Arun CS, Al-Bermani a, Stannard K, Taylor R. Long-term impact of retinal screening on significant diabetes-related visual impairment in the working age population. Diabetic medicine : a journal of the British Diabetic Association. 2009;26:489–492. doi: 10.1111/j.1464-5491.2009.02718.x. Available: http://www.ncbi.nlm.nih.gov/pubmed/19646188. Accessed 17 May 2011. [DOI] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Philip S, Fleming AD, Goatman KA, Fonseca S, McNamee P, et al. The efficacy of automated “disease/no disease” grading for diabetic retinopathy in a systematic screening programme. The British journal of ophthalmology. 2007;91:1512–1517. doi: 10.1136/bjo.2007.119453. Available: http://www.ncbi.nlm.nih.gov/pubmed/17504851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleming AD, Goatman KA, Philip S, Williams GJ, Prescott GJ, et al. The role of haemorrhage and exudate detection in automated grading of diabetic retinopathy. The British journal of ophthalmology. 2010;94:706–711. doi: 10.1136/bjo.2008.149807. Available: http://www.ncbi.nlm.nih.gov/pubmed/19661069. Accessed 16 May 2011. [DOI] [PubMed] [Google Scholar]
- 5.Fleming AD, Goatman K a, Philip S, Prescott GJ, Sharp PF, et al. Automated grading for diabetic retinopathy: a large-scale audit using arbitration by clinical experts. The British journal of ophthalmology. 2010:1–5. doi: 10.1136/bjo.2009.176784. Available: http://www.ncbi.nlm.nih.gov/pubmed/20858722. Accessed 4 October 2010. [DOI] [PubMed] [Google Scholar]
- 6.UK National Screening Committee. Essential elements in developing a diabetic retinal screening programme. Workbook version. 2009;4.3 [Google Scholar]
- 7.Harding S, Broadbent D, Neoh C, White M. Sensitivity and specificity of photography and direct ophthalmoscopy in screening for sight threatening eye disease: the Liverpool Diabetic Eye Study. BMJ. 1995;311:1131–1135. doi: 10.1136/bmj.311.7013.1131. Available: http://www.bmj.com/content/311/7013/1131.abstract. Accessed 24 March 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson JA, Strachan FM, Hipwell JH, Goatman KA, McHardy KC, et al. A comparative evaluation of digital imaging, retinal photography and optometrist examination in screening for diabetic retinopathy. Diabetic Medicine. 2003;20:528–534. doi: 10.1046/j.1464-5491.2003.00969.x. Available: http://www.ncbi.nlm.nih.gov/pubmed/12823232. [DOI] [PubMed] [Google Scholar]
- 9.Scanlon PH, Malhotra R, Thomas G, Foy C, Kirkpatrick JN, et al. The effectiveness of screening for diabetic retinopathy by digital imaging photography and technician ophthalmoscopy. Diabetic Medicine. 2003;20:467–474. doi: 10.1046/j.1464-5491.2003.00954.x. Available: http://www.ncbi.nlm.nih.gov/pubmed/12786681. [DOI] [PubMed] [Google Scholar]
- 10.Ruamviboonsuk P, Teerasuwanajak K, Tiensuwan M, Yuttitham K. Interobserver agreement in the interpretation of single-field digital fundus images for diabetic retinopathy screening. Ophthalmology. 2006;113:826–832. doi: 10.1016/j.ophtha.2005.11.021. Available: http://www.ncbi.nlm.nih.gov/pubmed/16650679. Accessed 24 September 2010. [DOI] [PubMed] [Google Scholar]
- 11.Stellingwerf C, Hardus PLLJ, Hooymans JMM. Assessing diabetic retinopathy using two-field digital photography and the influence of JPEG-compression. Documenta ophthalmologica Advances in ophthalmology. 2004;108:203–209. doi: 10.1007/s10633-004-5733-2. Available: http://www.ncbi.nlm.nih.gov/pubmed/15573944. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez CI, Niemeijer M, Dumitrescu AV, Suttorp-Schulten MSA, Abràmoff MD, et al. Evaluation of a computer-aided diagnosis system for diabetic retinopathy screening on public data. Investigative ophthalmology & visual science. 2011;52:4866–4871. doi: 10.1167/iovs.10-6633. Available: http://www.ncbi.nlm.nih.gov/pubmed/21527381. Accessed 11 October 2011. [DOI] [PubMed] [Google Scholar]
- 13.Goatman KA, Philip S, Harvey RD, Swa KK, Styles C, et al. Diabetic Medicine In Press; n.d.. External quality assurance for image grading in the Scottish diabetic retinopathy screening programme. [DOI] [PubMed] [Google Scholar]
- 14.Aiello LP, Davis MD, Girach A, Kles K a, Milton RC, et al. Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology. 2006;113:2221–2230. doi: 10.1016/j.ophtha.2006.07.032. Available: http://www.ncbi.nlm.nih.gov/pubmed/16989901. Accessed 21 July 2010. [DOI] [PubMed] [Google Scholar]
- 15.Gardner TW, Larsen M, Girach A, Zhi X. Diabetic macular oedema and visual loss: relationship to location, severity and duration. Acta Ophthalmologica. 2009;87:709–713. doi: 10.1111/j.1755-3768.2009.01545.x. Available: http://doi.wiley.com/10.1111/j.1755-3768.2009.01545.x. Accessed 24 August 2011. [DOI] [PubMed] [Google Scholar]