Abstract
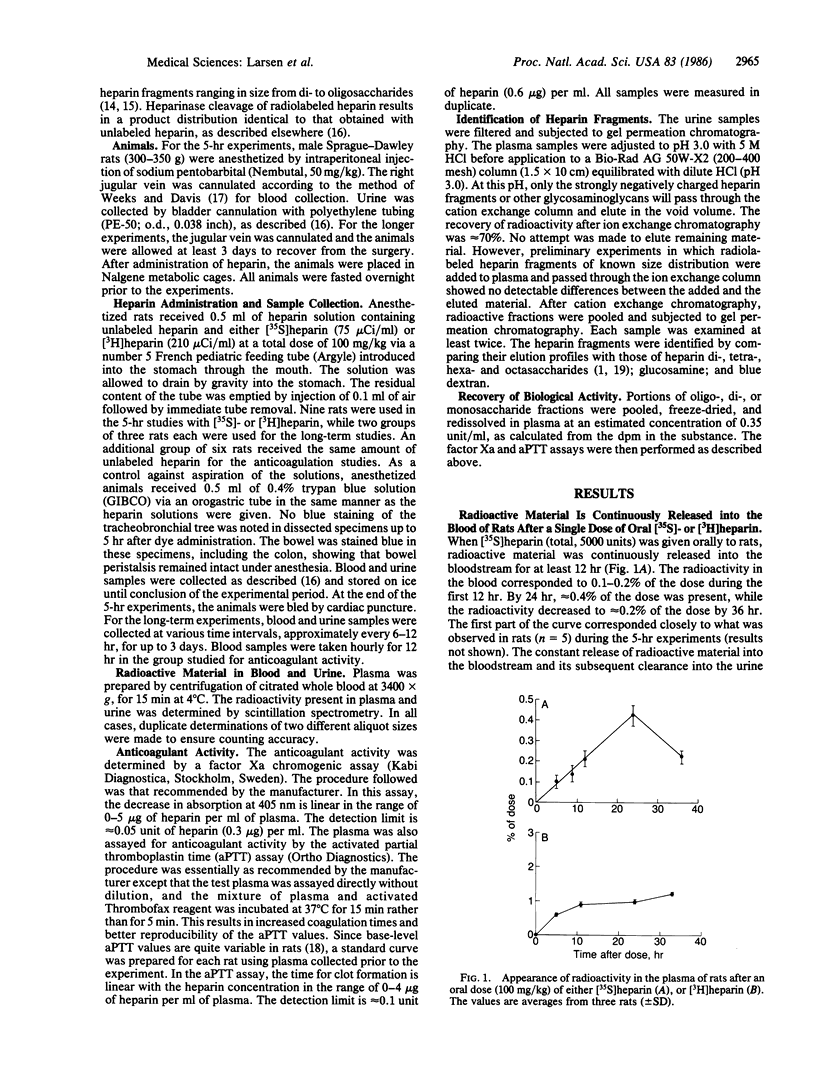
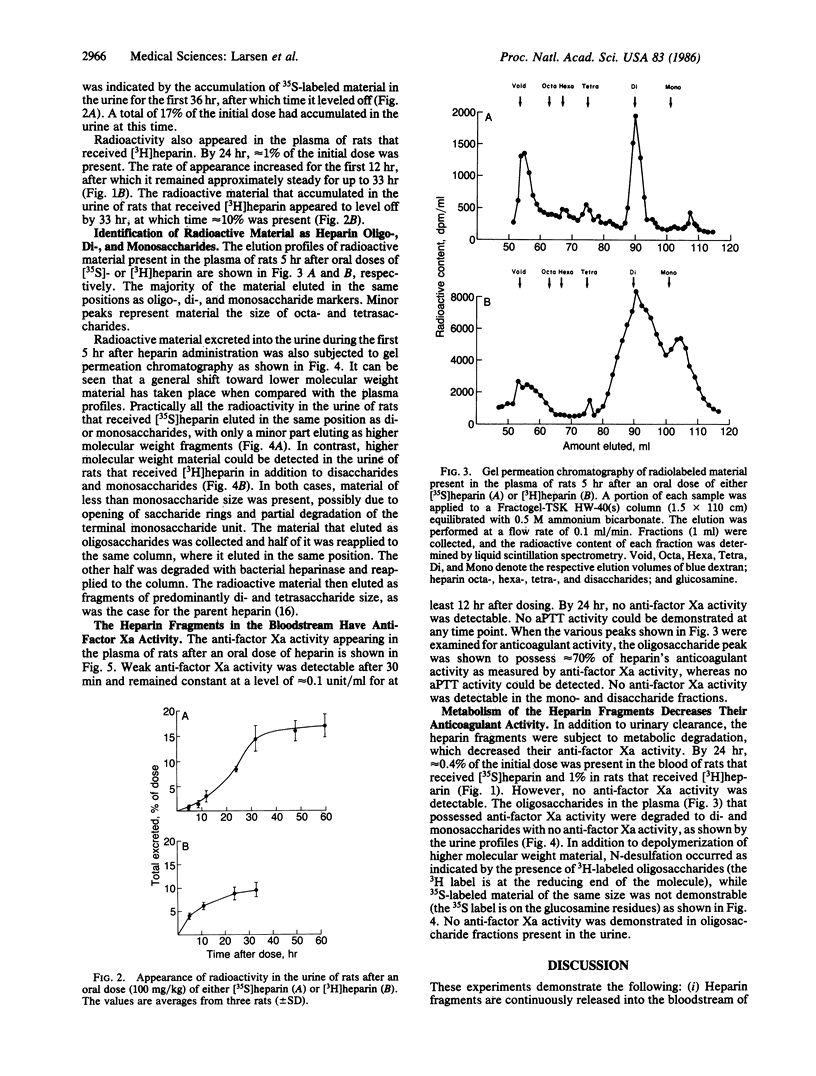
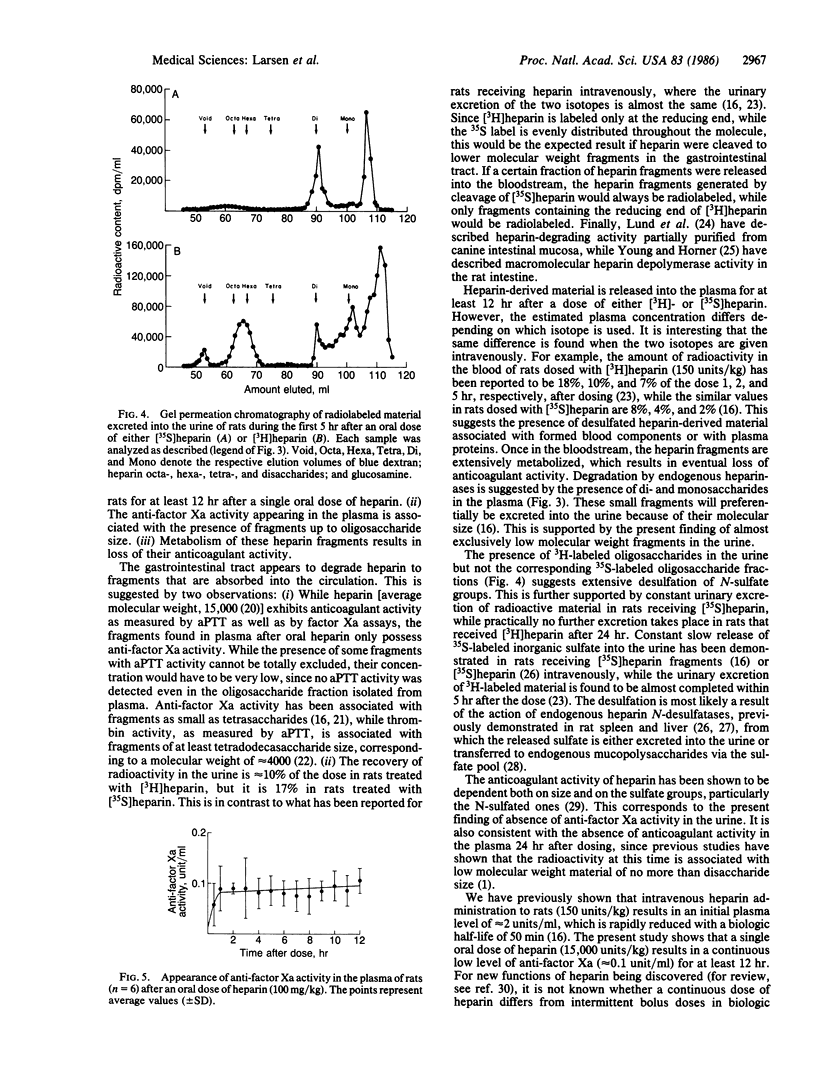
We have previously shown that angiogenesis inhibition and tumor regression can be accomplished by combinations of heparin or heparin fragments with cortisone [Folkman, J., Langer, R., Linhardt, R. J., Haudenschild, C. & Taylor, S. (1983) Science 221, 719-725]. Oral heparin was also effective in combination with cortisone. We now show that a single oral dose of [35S]heparin or [3H]heparin (15,000 units/kg) results in continuous release of radioactive material into the bloodstream for at least 12 hr. This is associated with the presence of anti-factor Xa activity at a level of approximately equal to 0.1 unit/ml. The radioactive material is identified as oligo-, di-, and monosaccharides by its behavior in chromatographic systems, its possession of anti-factor Xa activity, and the effect of treatment with bacterial heparinase. The heparin fragments are extensively metabolized to fragments without anti-factor Xa activity that are readily subject to urinary excretion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjornsson T. D., Levy G. Pharmacokinetics of heparin. I. Studies of dose dependence in rats. J Pharmacol Exp Ther. 1979 Aug;210(2):237–242. [PubMed] [Google Scholar]
- Dietrich C. P. Novel heparin degradation products. Isolation and characterization of novel disaccharides and oligosaccharides produced from heparin by bacterial degradation. Biochem J. 1968 Jul;108(4):647–654. doi: 10.1042/bj1080647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doutremépuich C., Masse A., Oca C., Maury M. O., Gestreau J. L., Toulemonde F., Lormeau J. C. Absorption duodénale d'une héparine de bas poids moléculaire chez le lapin. Pathol Biol (Paris) 1984 Jan;32(1):45–48. [PubMed] [Google Scholar]
- Ehrlich J., Stivala S. S. Chemistry and pharmacology of heparin. J Pharm Sci. 1973 Apr;62(4):517–544. doi: 10.1002/jps.2600620402. [DOI] [PubMed] [Google Scholar]
- Engel R. H., Fahrenbach M. J. Intestinal absorption of heparin in the rat and gerbil. Proc Soc Exp Biol Med. 1968 Dec;129(3):772–777. doi: 10.3181/00379727-129-33422. [DOI] [PubMed] [Google Scholar]
- Folkman J., Langer R., Linhardt R. J., Haudenschild C., Taylor S. Angiogenesis inhibition and tumor regression caused by heparin or a heparin fragment in the presence of cortisone. Science. 1983 Aug 19;221(4612):719–725. doi: 10.1126/science.6192498. [DOI] [PubMed] [Google Scholar]
- Folkman J., Langer R., Linhardt R. J., Haudenschild C., Taylor S. Angiogenesis inhibition and tumor regression caused by heparin or a heparin fragment in the presence of cortisone. Science. 1983 Aug 19;221(4612):719–725. doi: 10.1126/science.6192498. [DOI] [PubMed] [Google Scholar]
- Folkman J. Regulation of angiogenesis: a new function of heparin. Biochem Pharmacol. 1985 Apr 1;34(7):905–909. doi: 10.1016/0006-2952(85)90588-x. [DOI] [PubMed] [Google Scholar]
- Friedman Y., Arsenis C. Studies on the heparin sulphamidase activity from rat spleen. Intracellular distribution and characterization of the enzyme. Biochem J. 1974 Jun;139(3):699–708. doi: 10.1042/bj1390699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A. C., Linhardt R. J., Fitzgerald G. L., Park J. J., Langer R. Metachromatic activity of heparin and heparin fragments. Anal Biochem. 1984 Feb;137(1):25–32. doi: 10.1016/0003-2697(84)90341-5. [DOI] [PubMed] [Google Scholar]
- Hatton M. W., Berry L. R., Machovich R., Regoeczi E. Tritiation of commercial heparins by reaction with NaB3H4: chemical analysis and biological properties of the product. Anal Biochem. 1980 Aug;106(2):417–426. doi: 10.1016/0003-2697(80)90542-4. [DOI] [PubMed] [Google Scholar]
- Kambayashi J. Fate and anticoagulant activity of tritium labelled heparin in rats. Nihon Ketsueki Gakkai Zasshi. 1980 Feb;43(1):131–144. [PubMed] [Google Scholar]
- Koh T. Y. Intestinal absorption of heparin. Can J Biochem. 1969 Oct;47(10):951–954. doi: 10.1139/o69-149. [DOI] [PubMed] [Google Scholar]
- LEVY L., PETRACEK F. J. Chemical and pharmacological studies on N-resulfated heparin. Proc Soc Exp Biol Med. 1962 Apr;109:901–905. doi: 10.3181/00379727-109-27372. [DOI] [PubMed] [Google Scholar]
- Larsen A. K., Hetelekidis S., Langer R. Disposition and anticoagulant activity of biologically active heparin fragments in the rat. J Pharmacol Exp Ther. 1984 Nov;231(2):373–378. [PubMed] [Google Scholar]
- Linhardt R. J., Cooney C. L., Tapper D., Zannetos C. A., Larsen A. K., Langer R. An immobilized microbial heparinase for blood deheparinization. Appl Biochem Biotechnol. 1984 Feb;9(1):41–55. doi: 10.1007/BF02798373. [DOI] [PubMed] [Google Scholar]
- Linhardt R. J., Grant A., Cooney C. L., Langer R. Differential anticoagulant activity of heparin fragments prepared using microbial heparinase. J Biol Chem. 1982 Jul 10;257(13):7310–7313. [PubMed] [Google Scholar]
- Linker A., Hovingh P. Isolation and characterization of oligosaccharides obtained from heparin by the action of heparinase. Biochemistry. 1972 Feb 15;11(4):563–568. doi: 10.1021/bi00754a013. [DOI] [PubMed] [Google Scholar]
- Lloyd A. G., Fowler L. J., Embery G., Law B. A. Degradation of (35S) heparin by mammalian and bacterial sulphamidases. Biochem J. 1968 Dec;110(4):54P–55P. [PMC free article] [PubMed] [Google Scholar]
- Riensenfeld J., Hök M., Björk I., Lindahl U., Ajaxon B. Structural requirements for the interaction of heparin with antithrombin III. Fed Proc. 1977 Jan;36(1):39–43. [PubMed] [Google Scholar]
- Sagar S., Stamatakis J. D., Thomas D. P., Kakkar V. V. Oral contraceptives, antithrombin- III activity, and postoperative deep-vein thrombosis. Lancet. 1976 Mar 6;1(7958):509–511. doi: 10.1016/s0140-6736(76)90296-8. [DOI] [PubMed] [Google Scholar]
- Stau T., Metz J., Taugner R. Exogenous 35S-labeled heparin: organ distribution and metabolism. Naunyn Schmiedebergs Arch Pharmacol. 1973;280(1):93–102. doi: 10.1007/BF00505358. [DOI] [PubMed] [Google Scholar]
- Sue T. K., Jaques L. B., Yuen E. Effects of acidity, cations and alcoholic fractionation on absorption of heparin from gastrointestinal tract. Can J Physiol Pharmacol. 1976 Aug;54(4):613–617. doi: 10.1139/y76-084. [DOI] [PubMed] [Google Scholar]
- Ueno M., Nakasaki T., Horikoshi I., Sakuragawa N. Oral administration of liposomally-entrapped heparin to beagle dogs. Chem Pharm Bull (Tokyo) 1982 Jun;30(6):2245–2247. doi: 10.1248/cpb.30.2245. [DOI] [PubMed] [Google Scholar]
- WEEKS J. R., DAVIS J. D. CHRONIC INTRAVENOUS CANNULAS FOR RATS. J Appl Physiol. 1964 May;19:540–541. doi: 10.1152/jappl.1964.19.3.540. [DOI] [PubMed] [Google Scholar]
- WINDSOR E., CRONHEIM G. E. Gastro-intestinal absorption of heparin and synthetic heparinoids. Nature. 1961 Apr 15;190:263–264. doi: 10.1038/190263a0. [DOI] [PubMed] [Google Scholar]
- WINDSOR E., FREEMAN L. AN INVESTIGATION OF ROUTES OF ADMINISTRATION OF HEPARIN OTHER THAN INJECTION. Am J Med. 1964 Sep;37:408–416. doi: 10.1016/0002-9343(64)90197-4. [DOI] [PubMed] [Google Scholar]
- Young E., Horner A. A. The assay and partial characterization of macromolecular heparin depolymerase activity in rat small intestine. Biochem J. 1979 Jun 15;180(3):587–596. doi: 10.1042/bj1800587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv E., Eldor A., Kleinman Y., Bar-On H., Kidron M. Bile salts facilitate the absorption of heparin from the intestine. Biochem Pharmacol. 1983 Mar 1;32(5):773–776. doi: 10.1016/0006-2952(83)90575-0. [DOI] [PubMed] [Google Scholar]


