Abstract
We and others have demonstrated the ability of granulocyte-macrophage colony-stimulating factor (GM-CSF) to suppress autoimmunity by increasing the number of CD4+CD25+ regulatory T cells (Tregs). In the current study, we have explored the critical role of induced antigen specific Tregs in the therapeutic effects of GM-CSF in murine experimental autoimmune myasthenia gravis (EAMG). Specifically, we show that Tregs from GM-CSF treated EAMG mice (GM-CSF/AChR-induced-Tregs) adoptively transferred into animals with EAMG suppressed clinical disease more potently than equal numbers of Tregs from either GM-CSF untreated EAMG mice or healthy mice treated with GM-CSF. In addition, GM-CSF/AChR-induced-Tregs selectively suppressed antigen specific T cell proliferation induced by AChR relative to that induced by an irrelevant self antigen, (thyroglobulin) and failed to significantly alter T cell proliferation in response to an exogenous antigen (ovalbumin). These results are consistent with the hypothesized mechanism of action of GM-CSF involving the mobilization of tolerogenic dendritic cell precursors which, upon antigen (AChR) capture, suppress the anti-AChR immune response through the induction/expansion of AChR-specific Tregs.
Keywords: Myasthenia gravis, experimental autoimmune myasthenia gravis, autoimmune disease, GM-CSF, regulatory T cells, dendritic cells, acetylcholine receptor
1. Introduction
Myasthenia gravis (MG) is a B cell-mediated, T cell-dependent autoimmune disease in which the primary autoantigen is the skeletal muscle acetylcholine receptor (AChR) (Meriggioli and Sanders, 2009). The immunopathogenesis of myasthenia gravis (MG) involves the production of high-affinity anti-AChR antibodies whose synthesis is modulated by anti-AChR CD4+ T cells (Vincent, 2002). The activation of anti-AChR T cells is, in turn, determined by their interactions with antigen-presenting cells (APCs), consisting primarily of dendritic cells (DCs). DCs promote autoantibody synthesis by activation of T cells which provide cytokines and “help” to B cells, but also directly enhance antibody production and isotype switching (Cerutti et al., 2005; MacPherson et al., 1999). Experimental autoimmune MG (EAMG) can be induced in mice by immunization with AChR purified from the electric organs of the electric ray, Torpedo californica (tAChR) (Christadoss et al., 2000). In EAMG, anti-Torpedo AChR antibodies cross-react with mouse AChR and cause myasthenic symptoms (Lindstrom, 1999). In both MG and EAMG, AChR-specific B cells produce anti-AChR antibodies that bind to the AChR at the neuromuscular junction, activate complement, and accelerate AChR destruction, culminating in neuromuscular transmission failure and fatigable muscle weakness.
GM-CSF, a pleiotrophic immune modulator and a potent dendritic cell (DC) growth factor, (Hamilton, 2002), has been shown to be capable of both stimulating the immune response, endowing DCs with improved antigen presenting capacity, or alternatively suppressing the immune response by favoring the development of immature DCs that recruit Tregs (Parmiani et al., 2007; O’Keefe et al., 2002; Pulendran et al., 2000). We and others have demonstrated the ability of low-dose GM-CSF to maintain semi-mature, tolerogenic DCs in vivo, induce Foxp3+ regulatory T cells (Tregs), and suppress autoimmunity (Gangi et al., 2005; Ganesh et al., 2009; Gaudreau et al., 2007; Vasu et al., 2003). We have previously reported that GM-CSF effectively ameliorates EAMG in both pretreatment (Sheng et al., 2006) and therapeutic protocols (Sheng et al., 2008, Meriggioli et al., 2008), by down-modulating anti-AChR T cell and antibody responses. GM-CSF-induced suppression of EAMG was associated with a selective expansion of CD4+CD25+Foxp3+ Tregs that suppressed anti-AChR immune responses in vitro (Sheng et al., 2008). More recently, we have shown that the predominant tolerogenic effects of GM-CSF are mediated through mobilization of bone marrow DC precursors that develop into tolerogenic DCs, which not only expand Foxp3+ Tregs, but also facilitate adaptive conversion of CD4−CD25− T cells into Foxp3-expressing Tregs (Bhattacharya et al., 2011; Ganesh et al., 2011). Conversion of these “induced” or “adaptive” Tregs (iTregs) required T cell receptor (TCR) activation, suggesting that these cells may mediate antigen-specific suppression.
Therefore, in the current study, we investigated the functional properties of antigen-specific Tregs induced by GM-CSF in the treatment of EAMG. We demonstrate that adoptively transferred Tregs from GM-CSF treated animals (GM-CSF/AChR-induced Tregs) are endowed with potent suppressive properties selectively down-modulating anti-AChR immune responses. Specifically, we show that GM-CSF-induced Tregs from EAMG mice selectively suppress AChR-induced T cell proliferation, but suppress T cell proliferation in response to an irrelevant endogenous antigen (mouse thyroglobulin) to no greater extent than Tregs obtained from untreated, non-AChR-immunized donors, and do not significantly suppress T cell responses induced by an irrelevant exogenous antigen (ovalbumin). This enhanced AChR-specific potency may be explained by the induction/expansion of AChR-specific Tregs due to AChR derived peptide -presentation by tolerogenic DCs mobilized by GM-CSF.
2. Materials and Methods
2.1. Mice and Purification of tACHR
Eight-week old female C57BL6/J mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Mice were housed in the Biologic Resources Laboratory facilities at the University of Illinois (Chicago, IL) and provided food and water ad libitum. All mice were cared for in accordance with the guidelines set forth by the University of Illinois Animal Care and Use committee.
Torpedo AChR (tAChR) was purified from the electric organs of T. californica by affinity chromatography using a conjugate of neurotoxin coupled to agarose, as previously described (Sheng et al., 2006). The purified tAChR was used to induce EAMG and as antigen for in vitro testing of immune responses.
2.2. Induction and clinical scoring of EAMG
Eight-week old female C57BL6/J mice were immunized with 40 μg of tAChR/CFA, 200 μl, s.c, and boosted with 20 μg of tAChR emulsified in IFA in 200 μl volume injected in the flanks and tail base every 30 days. Mice were observed and scored every other day. For clinical examination, mice were evaluated for myasthenic weakness and assigned clinical scores as previously described (Sheng et al., 2006; Sheng et al., 2008). Briefly, mice were observed on a flat platform for a total of 2 min. They were then exercised by gently dragging them suspended by the base of the tail across a cage top grid repeatedly (20–30 times) as they attempted to grip the grid. They were then placed on a flat platform for 2 min and again observed for signs of EAMG. Clinical muscle weakness was graded as follows: grade 0, mouse with normal posture, muscle strength, and mobility at baseline and after exercise; grade 1, normal at rest but with muscle weakness characteristically shown by a hunchback posture, restricted mobility, and difficulty in raising the head after exercise; grade 2, grade 1 symptoms without exercise during observation period; grade 3, dehydrated and moribund with grade 2 weakness; and grade 4, dead. The evaluator was blinded to treatment status for all clinical evaluations.
2.3. GM-CSF treatment and adoptive transfer experiments
Eight-week old female C57BL6/J mice were immunized with tAchR/CFA as described above, and treated with either GM-CSF (2 μg daily for 10 days), or PBS administered by intravenous injection daily for 10 days. Treatment was administered 10 days after the second booster tAChR immunization, and animals were boosted again 28 days after initiation of treatment. For the adoptive transfer experiments, animals received the initial priming tAChR immunization and were then treated with GM-CSF (2 μg daily IP for 10 days) or PBS. A group of animals also received GM-CSF without tAChR immunization. These mice were used as donor animals and sacrificed 14 days after the treatments (24 days after the initial immunization). Splenic CD4+ CD25+ and CD4+CD25− T cells were isolated from mice by negative selection using magnetic beads (Miltenyi Biotec, Auburn, CA). Purity of isolated cells was typically >93% for both cell types.
Isolated cells CD25+ and CD25− (2 million) cells from donor mice (treated with GM-CSF or untreated) were injected intravenously into the tail veins of recipient EAMG mice in four experimental groups (n = 8 per group): 1.CD25+ cells from GM-CSF-treated mice; 2.CD25+ cells from untreated mice; 3.CD25− cells from GM-CSF-treated mice; 4. CD25− cells from untreated mice. Recipient mice in all four groups had been immunized with tAChR 24 days prior and were then boosted at 7 days after the adoptive transfer. Clinical scores were then followed daily with the day of the adoptive transfer designated day 0. The experiment was repeated twice to ensure reproducibility. Similar experiments were performed using isolated CD25+ cells from GM-CSF-treated, tAChR-immunized mice and GM-CSF-treated, non-AChR immunized mice.
2.4. CD4+CD25+/CD4+CD25− T cell co-culture and AChR-specific T cell proliferation
Parallel to the adoptive transfer experiments, CD4+CD25+ cells from donor mice in the PBS/tAChR group (tAChR-immunized mice receiving PBS) and in the GM-CSF/tAChR group (tAChR immunized mice receiving GM-CSF) were collected and co-cultured with responder T cells from immunized mice for the analysis of AChR-specific T cell proliferation. Responder CD4+ cells were isolated from mice in the PBS/tAChR group and mice in the GM-CSF/tAChR group using magnetic cell sorting (Miltenyi Biotec) and were stained with CFSE at a concentration of 1 μM for 10 min at 37 °C. Cells were washed three times and plated into 96-well, flat-bottom plates at 0.5 × 106 cells/well. Spleen CD4+CD25+ T cells were isolated from mice in the GM-CSF/tAChR group and the PBS/tAChR group (magnetic cell sorting; Miltenyi Biotec). Isolated CD4+CD25+ T cells from both groups were added to cultures at 0.25:1, 0.5:1, and 1:1 Treg:Tresponder ratios. T cell-depleted enriched DCs (0.1 × 106 cells/well) (also accomplished by magnetic cell sorting; Miltenyi Biotec) were used as feeder cells in these studies. Responder CD4+ cells were cultured either alone, or co-cultured with the isolated CD4+CD25+ cells from mice in the PBS/tAChR group or in the GM-CSF/tAChR group. Cells were stimulated with tAChR (5 μg/ml). Cells were harvested after 7 days in culture and were tested for CFSE dilution using a FACS analyzer (BD Biosciences). These experiments were repeated twice to ensure reproducibility.
2.5. ELISA for anti-mouse AChR antibody isotypes
Mice were bled on days 0 prior to the adoptive transfer and day 30 after adoptive transfer. Affinity-purified mouse AChR (0.5 ug/ml) was used to coat 96-well microtiter plates (Corning Costar 96 wells plate, eBioscience, San Diego, CA) with 0.1 M carbonate bicarbonate buffer (pH 9.6) overnight at 4 °C. Serum samples diluted 1:2000 to 1:10,000 in blocking buffer were added and incubated at 37 °C for 90 min. After four washes, horseradish peroxidase-conjugated (HRPO) goat anti-mouse IgG, IgG2b, diluted in 1:2000 (Caltag Laboratories, Burlingame, MA) in blocking buffer were added and incubated at 37 °C for 90 min. Subsequently, TMB substrate solution (eBioscience) was added, and color was allowed to develop at room temperature in the dark for 15 min. The reaction was stopped by adding 2 M H2SO4 and absorbance values were measured at a wavelength of 450 nm using a Bio-Rad microplate-reader (model 550) and the results were expressed as OD values.
2.6. Flow cytometry
Three mice from each of experimental groups were treated and immunized as described above. Single cell suspensions of splenocytes were prepared from mice sacrificed 48 h after the completion of the second GM-CSF/PBS treatment regimen for DC and Treg analysis. Cells were washed with PBS supplemented with 0.05% BSA, blocked with antiCD16/CD32 Fc-Block (BD PharMingen, San Jose, CA) on ice for 30 min. FITC-conjugated anti-CD11c and PE-conjugated anti-I-Ab (MHC class II), anti-CD8α, anti-CD80, anti-CD86, anti-PDL1, anti-PDL2, anti-CD40 and isotype control Abs (BD PharMingen), and FITC-conjugated anti-CD4, PE-conjugated anti-CD25 (Caltag Laboratories) Abs were used in flow cytometry and analyzed using a FACS analyzer (BD Biosciences). Mouse regulatory T cell staining kit (w/PE FOXP3 FJK-16s, FITC CD4, APC CD25) (eBioscience) Abs were used for intracellular staining for FOXP3 expression. PE-conjugated anti-IL10,. IL-6, IL-17, and IFNr were uses as Intracellular staining of T cells (BD PharMingen, San Jose, CA).
2.7. Determination of mouse muscle AChR content
Mice were sacrificed at day 30 after adoptive transfer and limb muscle was collected and muscle AChR was extracted. Briefly, mouse muscle (20 mg) was homogenized in 4 volumes of Tris buffer (25mM Tris HCL, 150mM NaCl, pH 7.2) using a Polytron-equipped homogenizer (Model PT 3000, Kinematica, Littau, Switzerland) on ice. Mechanical homogenization was achieved using a 7 mm tip (generator). Each sample was processed for 1 min. at 10 rpm, then centrifuged at 100,000×g for 30 minutes (4°C). Supernatants (100μl) were used to coat 96-well microtiter plates with coating buffer overnight at 4 °C. Torpedo AChR (0.5μg/ml) with double dilution was coated in the plates as the standard control. Biotin conjugated alpha bungarotoxin (Invitrogen, Carlsbad CA) (1μg/ml) in blocking buffer was added and incubated at 37 °C for 90 min. After four washes, horseradish peroxidase-conjugated (HRPO) Streptavidin (Invitrogen, Carlsbad CA) (0.1ug/ml) in blocking buffer was added and incubated at 37 °C for 90 min. Subsequently, TMB substrate solution (eBioscience) was added, and color was allowed to develop at room temperature in the dark for 15 min. The reaction was stopped by adding 2 M H2SO4 and absorbance values were measured at a wavelength of 450 nm using a Bio-Rad microplate-reader (model 550) and the results were expressed as OD values. The muscle content (ng/ml) was measured from the OD value according to the standard curve from Torpedo AChR.
2.8. In vitro, antigen specific T cell proliferation experiments
Five groups of mice (n=8) were immunized with tAChR (40 μg), CFA, ovalbumin (OVA, 100 μg) or mouse thyroglobulin (mTg, 100 μg) and boosted at day 30. The first two groups of mice that were immunized either with CFA or tAChR were treated with GM-CSF (2ug/ml) for 10 days. The remaining three groups of mice were immunized with tAChR, mTg and OVA, and then were boosted with the same respective antigen 30 days later. Responder CD4+ cells were isolated from mice in the PBS/tAChR group, PBS/Tg group, PBS/OVA group using magnetic cell sorting (Miltenyi Biotec) and were stained with CFSE at a concentration of 1 μM for 10 min at 37 °C. Cells were washed three times and plated into 96-well, flat-bottom plates at 0.5 × 106 cells/well. Spleen CD4+CD25+ T cells were isolated from mice in the GM-CSF/tAChR group and the GM-CSF/CFA group (magnetic cell sorting; Miltenyi Biotec). Isolated CD4+CD25+ T cells from both groups were added to cultures at a 2:1 effector:Treg ratio. Cells were harvested after 5 days in culture and were tested for CFSE dilution using a FACS analyzer (BD Biosciences).
2.9. Statistical analysis
All statistical analyses were performed using the SPSS software application. Nonparametric Wilcoxan signed rank test, Fisher’s exact test, and Student’s t test were utilized as appropriate. Significance levels were set at p ≤ 0.05. Error bars depict standard error of the mean (SEM).
3. Results
3.1. GM-CSF suppresses chronic EAMG and autoantibody responses
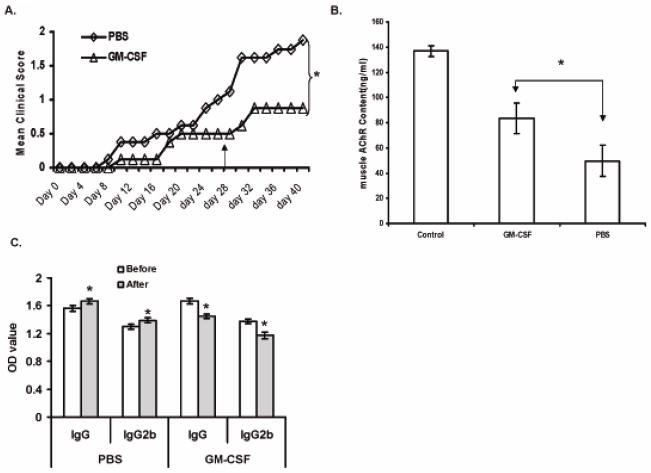
To confirm the efficacy of GM-CSF in active EAMG, mice were treated with GM-CSF (2 μg daily for 10 days). Treatment initiation was designated as day 0, after all animals had received a priming AChR immunization. A booster tAChR immunization was administered on day 28. Control mice were treated with PBS. Compared to controls, GM-CSF-treated mice developed less severe disease (Figure 1A). Mice were sacrificed and muscle AChR concentrations were determined; muscle AChR content was preserved in GM-CSF-treated animals relative to untreated control mice (Figure 1B). To evaluate the humoral immune response to AChR in treated vs. untreated mice, circulating anti-mouse AChR antibody levels were determined by ELISA. On day 40, circulating anti-AChR IgG and IgG2b antibody levels were reduced in animals receiving GM-CSF (Figure 1C).
Figure 1. Clinical Effects of GM-CSF.
Clinical severity of EAMG in GM-CSF-treated, compared to PBS-treated, tAChR-immunized mice. Mice were evaluated as described in Materials and Methods beginning at the time of initiation of treatment (Day 0). A) Average clinical score during days 0–40. Day 0 corresponds to initiation of treatment (GM-CSF vs. PBS). All mice had received a priming immunization; an additional tAChR booster immunization was given on day 28. B) Muscle AChR content in GM-CSF-treated vs PBS-treated and healthy control animals. C) Serum anti-mouse AChR IgG and IgG2b subclasses for GM-CSF-treated, and PBS-treated mice. Serum was analyzed for anti -mouse AChR IgG Ab and IgG2b isotype by ELISA (n=10 per group) on day 0 and day 40. Statistical comparisons were made between IgG levels before and after treatment; *statistically significant (p < 0.05) differences are indicated.
3.2. Immune effects of GM-CSF treatment
3.2a. Dendritic cell phenotype
In vitro studies have demonstrated that DC function and phenotype may be modified directly by GM-CSF (Zou and Tam, 2002). We have previously shown that GM-CSF treatment leads to an expansion of CD8α− DCs that produce less pro-inflammatory cytokines (Ganesh et al., 2009). Therefore, as expected, we observed a shift in the spleen-derived DC phenotype in GM-CSF-treated animals, characterized by a decrease in expression of CD8α, but also demonstrating reduced expression of co-stimulatory molecules (CD80, CD86) compared to controls (PBS-treated EAMG animals) (Figure 2A). During antigen presentation, the interaction of CD40 on DCs with CD40L on T cells induces interleukin-12 (IL-12) production by the DC, which leads to T helper type 1 (Th1) differentiation of the T cells (Cella et al., 1996; Moser and Murphy, 2000). We observed a reduction of expression of CD40 on DCs isolated from splenocytes in GM-CSF treated mice compared to controls (Figure 2A). Finally, programmed cell death 1 ligand-1 (PDL-1) and programmed cell death ligand-2 (PDL-2) are up-regulated on DCs during maturation and in response to inflammatory stimuli (Yamazaki, et al, 2002). We found a reduced expression of both PDL-1 and PDL-2 in splenic DCs from GM-CSF-treated mice compared to controls. Taken together, these findings are consistent with the idea that GM-CSF induces DCs with an immature or tolerogenic phenotype (Banchereau et al., 1999; Lutz and Schuler, 2002).
Figure 2. Immunological effects of GM-CSF.
A) Effect of GM-CSF treatment on DC phenotype in EAMG. Splenocytes were collected and stained with FITC-labeled anti-mouse CD11c and various other antibodies to cell surface proteins as described. DCs from GM-CSF treated-animals expressed lower levels of CD80, CD86, PDL-1, PDL-2 and CD40 and had an increase in the CD8α-negative population. B) Effects of GM-CSF on the population of FOXP3+ T cells. Representative plots indicating the percentage of FOXP3+ regulatory T cells in splenocytes are shown. C) T cell proliferative response to AChR stimulation. Responder CD4+ cells from GM-CSF-treated and untreated EAMG mice were labeled with CFSE and stimulated with AChR. The proliferative response was determined by CFSE dilution. D) B cell proliferative response to AChR stimulation. B cells were isolated based on expression of CD19 and cells from GM-CSF-treated and untreated mice were cultured and stimulated with AChR. The proliferative response was quantified by CFSE dilution as above. E. Effects of GM-CSF on cytokine profile in T cells. Mice were immunized with tAChR and treated with GM-CSF or PBS. CD4+ cells from mice immunized with tAChR and treated with GM-CSF or PBS were used to detect various cytokines by intra-cytoplasmic staining.
3.2b. T cells, B cells, and cytokines
We also examined relative numbers of CD4+ cells expressing Foxp3 in splenocytes from GM-CSF-treated and untreated animals by FACS analysis (Figure 2B). As in our previous studies GM-CSF treatment expanded the population of Foxp3-expressing cells (11.2% of CD4+ cells in GM-CSF-treated vs 5.8% in control animals). Accordingly, T cell proliferation in response to AChR was suppressed in CD4+ splenocytes from GM-CSF-treated mice compared to controls (Figure 2C). Interestingly, GM-CSF also reduced B cell proliferation in response to stimulation with AChR peptide (Figure 2D). Analysis of T cell cytokine profiles by intra-cytoplasmic staining revealed that GM-CSF treament suppressed IL-17, interferon gamma, and IL-6 responses, while enhancing IL-10 (Figure 2E).
3.3. GM-CSF/AChR-induced Tregs suppress EAMG upon adoptive transfer
Our previously published studies and the above results suggested that Tregs were critical for the suppressive clinical and immunological effects produced by GM-CSF in EAMG. We therefore proceeded to examine the in vivo suppressive effects of GM-CSF/AChR-induced Tregs by performing adoptive transfer experiments. We induced EAMG in a large group of mice and these animals were divided into four groups (n = 8 per group), serving as recipients of the following isolated cells. We isolated CD4+CD25+ cells (Tregs) and CD4+CD25- T cells from splenocytes of EAMG mice that were either treated with GM-CSF or PBS. The animals in all four groups received an additional booster immunization on day 7 after the adoptive transfer. GM-CSF/AChR-induced Tregs adoptively transferred into animals with EAMG suppressed clinical disease more potently than equal numbers of Tregs from untreated mice. In contrast, CD4+ CD25− T cells from either group failed to confer any protection. We calculated the difference in mean clinical score in each experimental group (post-treatment score – pre-treatment score) with a negative result indicating clinical improvement (lower post-treatment score) and noted a significant difference on day 30 after the adoptive transfer (Figure 3A) In further support of this, muscle AChR content was also determined at the time of sacrifice and was found to be preserved most prominently in mice receiving GM-CSF/AChR-induced Tregs compared to the various control groups (Figure 3B).
Figure 3. Adoptive transfer of GM-CSF/AChR-induced Tregs.
A) Change (post-adoptive transfer score minus baseline score) in clinical severity in 4 groups of EAMG mice adoptively transferred with either Tregs (CD25+) from GM-CSF (◇) or PBS-treated (□) EAMG mice vs. non-Tregs (CD25−) from GM-CSF (△) or PBS-treated (X) EAMG mice. EAMG severity was significantly reduced in mice receiving Tregs from GM-CSF treated donors compared to Tregs from PBS-treated EAMG mice (*p < 0.05). B) Muscle AChR content (ng/ml) at the end of study in the four experimental groups. AChR content was more significantly preserved in mice receiving CD4+CD25+ (Tregs) from GM-CSF-treated EAMG donors when compared to mice receiving Tregs from untreated donors (p < 0.05).
3.4. GM-CSF/AChR-induced Tregs suppress anti-AChR antibody levels
To specifically examine the effects of GM-CSF/AChR-induced Tregs on autoantibody production in vivo, we determined circulating anti-AChR antibody levels by ELISA. Anti-mouse AChR antibody production was assessed on day 0 (prior to adoptive transfer) and day 30 (after adoptive transfer) in the four experimental groups. Serum mouse anti-AChR total IgG and IgG2b levels were increased on day 30 compared to day 0 in mice receiving non-Tregs (CD4+CD25− cells), correlating with the observed clinical worsening. Serum mouse anti-AChR total IgG and IgG2b levels in mice receiving CD4+CD25+ cells from untreated mice were reduced mildly, but a significant reduction in autoantibody levels occurred only in mice receiving GM-CSF/AChR-induced Tregs (Figure 4).
Figure 4. Anti-AChR antibody levels in adoptively transferred EAMG mice.
Bar graphs showing anti-AChR IgG (A) And IgG2b (B) antibody levels as determined in the four experimental groups by an ELISA assay. Significant reductions in both anti-AChR total IgG and IgG2b were observed only in mice receiving CD4+CD25+ (Tregs) from GM-CSF-treated EAMG donors. *Statistically significant value (p < 0.05).
3.5. Adoptively transferred GM-CSF/AChR-induced Tregs increase the absolute numbers of splenic FOXP3+ Tregs to a comparable degree as Tregs from untreated animals
To begin to examine the possible reasons for the observed enhanced potency of GM-CSF/AChR-induced Tregs, we first assessed the percentage of Foxp3-expressing cells in the spleens of animals in all four groups of recipient mice. Interestingly, while animals receiving adoptively transferred GM-CSF/AChR-induced-Tregs had increased numbers of splenic Foxp3-expressing cells up to 30 days after the adoptive transfer, this observed increase (compared to animals receiving non-Tregs) was not significantly different than that observed in animals receiving Tregs from untreated donors (Figure 5a). Therefore, the enhanced efficacy of suppression could not be explained on the basis of increased numbers of Tregs.
Figure 5. Effects of Treg adoptive transfer on the splenic population of FOXP3+ T cells, and potency of GM-CSF-induced Tregs suppression of the AChR-stimulated T cell proliferative response.
The percentage of FOXP3+ regulatory T cells in splenocytes are shown for the four experimental groups (A). No significant difference is apparent in the expansion of Foxp3-expressing splenocytes in mice receiving CD4+CD25+ cells. Despite this, GM-CSF-induced Tregs suppressed the AChR-induced proliferation of responder T cells more potently compared to Tregs from PBS-treated animals; GM-CSF-induced Tregs maintain their suppressive potency at lower Treg/Teff ratios (B). Results are representative of three separate experiments with each column representing the mean ± SEM (*p < 0.05).
3.6. GM-CSF/AChR induced Tregs exhibit a potent AChR-selective suppressive effect
The above results suggested that GM-CSF treatment somehow endowed Tregs with more potent disease-suppressing capacity. To confirm this, we examined the potency of GM-CSF/AChR-induced Tregs more closely by performing in vitro AChR specific T cell proliferation assays using cells from GM-CSF treated and untreated EAMG mice. GM-CSF/AChR-induced Tregs maintained their suppressive potencies at lower Treg concentrations (i.e. Treg to Tresponder ratio of 0.25:1), while the suppressive ability was essentially lost even at a ratio of 0.5:1 when Tregs from untreated mice were used (Figure 5b).
These findings suggested the intriguing possibility that Tregs from GM-CSF-treated mice contained a higher percentage of antigen (AChR)-specific cells. We pursued this hypothesis by testing the capacity of GM-CSF-induced-Tregs in suppressing not only AChR-induced proliferation, but also proliferation induced by “irrelevant” antigens. For these experiments, we first immunized different groups of mice with an exogenous antigen, ovalbumin (OVA, 100 μg) and an irrelevant self antigen, mouse thyroglobulin (mTg, 100 μg ), or AChR (40 μg), followed by a booster immunization with the corresponding antigen. T cells obtained from these mice served as responder cells. Similarly, we immunized another group of mice with AChR (tAChR (40 μg [to induce EAMG]). followed by a booster immunization, and then treated them with GM-CSF (2 μg IP X 10 days). We harvested T cells and APCs from all three groups of mice, and isolated Tregs from AChR-primed GM-CSF treated mice. We set up co-cultures to determine the extent of T cell proliferation upon stimulation with AChR, mTg, or OVA, assessing the antigen specific suppressive capacity of GM-CSF-induced Tregs generated during in vivo exposure to AChR. As anticipated, although we found that Tregs from control mice (GM-CSF-treated but not exposed to AChR) suppressed AChR induced T cell proliferation (from 61% to 43%), GM-CSF/AChR-induced more potently and specifically suppressed T cell proliferation induced by AChR (from 61% to 22%) (Figure 6). However, these Tregs did not alter T cell proliferation in response to an irrelevant self antigen (thyroglobulin) to any greater extent than Tregs obtained from non-AChR immunized mice (68% to 54% versus 68% to 49%) (Figure 6). Furthermore, irrespective of the source, Tregs induced by GM-CSF did not significantly affect T cell proliferation in response to an exogenous (foreign) antigen (OVA) (75% to 67% versus 75% to 70%). These results indicated that the Tregs from AChR primed and GM-CSF treated mice have selective suppressive effects on T cell responses to AChR, compared to foreign and non-AChR self-Ags.
Figure 6. Antigen-specific suppression of T cell proliferation.
T cell proliferation in response to three different antigens (AChR, Tg (thyroglobulin), and ovalbumin (OVA). T responder (Tresp) cells were isolated from animals primed with the indicated antigen. A. Results from a representative experiment are shown. I. Baseline proliferation (without addition of Tregs) is shown in the top row; II. With the addition of Tregs from GM-CSF-treated, CFA-immunized mice; and III. With the addition of Tregs from GM-CSF-treated EAMG mice. B. Summary of T cell proliferation/suppression assays from three separate experiments, showing the mean ± SEM. *Statistically significant value (p < 0.05).
We surmised that this selective suppression derived from the fact that GM-CSF-induced, AChR-specific Tregs were selectively induced/expanded during active exposure (immunization) with AChR. To test this hypothesis we isolated Tregs from GM-CSF-treated, AChR-immunized and non-AChR immunized mice, and adoptively transferred these cells into mice with chronic EAMG. Mice receiving Tregs from GM-CSF-treated EAMG donors had more potent suppression of disease compared to mice receiving Tregs from donors not exposed to AChR but treated with GM-CSF (Figure 7A). This difference was maintained even after an additional tAChR boost was given 30 days after the adoptive transfer of cells. Additionally, while both groups of recipient mice showed a lowering of serum anti-AChR antibody levels after adoptive transfer, only mice receiving Tregs from GM-CSF-treated EAMG donors sustained the reduced anti-AChR antibody levels after the final tAChR boost (Figure 7B).
Figure 7. Adoptive transfer of GM-CSF expanded Tregs (exposed and not exposed to AChR).
Recipient mice were immunized and boosted with tAChR and divided into two treatment groups. Mice were adoptively transferred with Tregs (CD4+ CD25+ cells) from GM-CSF-treated EAMG donors (solid circles) or Tregs from GM-CSF-treated mice not exposed to AChR (solid squares). A. Serial determinations of mean clinical score are shown with day 0 corresponding to the day of adoptive transfer. B. Anti-AChR antibodies were determined using an ELISA assay at baseline (before adoptive transfer), prior to the tAChR boost (arrow) 30 days after adoptive transfer (After), and at the end of the study (post-boost). Mean OD values and standard errors are shown for three separate experiments. In each case, the post-treatment values were compared to the baseline value to determine significance (p < 0.05). *Statistically significant value (p < 0.05).
4. Discussion
CD4+FoxP3+ regulatory T cells (Tregs) play a crucial role in the maintenance of immune homeostasis against self-antigens (Sakaguchi et al., 2004). A prominent role for Tregs in the maintenance of immune tolerance has been emphasized recently (Brusko et al., 2008), with evidence suggesting a reduced function of these cells in autoimmune diseases (Torgerson, 2006; Venken et al., 2008; Moes et al., 2010), including MG (Huang et al., 2004; Fattorossi et al., 2005; Zhang et al., 2009; Masayuki et al., 2010). Tregs have been shown to be capable of suppressing Th cells that help autoantibody-producing B cells, thereby inhibiting antibody (and autoantibody) production (Yokoyama et al., 2011). Several methods have been investigated to directly target Tregs for the treatment of autoimmune disease, including expansion and induction of Tregs in vitro for subsequent adoptive immunotherapy (Hoffman et al., 2004; Earle et al., 2005). Despite experimental success with different methods of Treg induction and expansion, a safe, simple, and antigen-specific mode of inhibiting immune responses utilizing Tregs has not yet been achieved. Since Tregs make up only a very small percentage of the CD4+ T cell pool in both mice and humans, therapeutic applications have been limited by prohibitively low numbers of Tregs. Optimizing the expansion of Tregs in vivo using agents like GM-CSF may therefore be a more viable approach to developing Treg therapies that augment functional, antigen-specific CD4+CD25+Foxp3+ Treg populations.
We have shown in this study that adoptively transferred GM-CSF/AChR-induced Tregs are an effective treatment for EAMG, suppressing disease severity, preserving muscle AChR content, and reducing circulating levels of anti-AChR antibodies. GM-CSF/AChR-induced-Tregs from EAMG mice potently and specifically suppressed in vitro T cell proliferation induced by AChR, but did not alter T cell proliferation in response to an irrelevant self antigen (thyroglobulin) to any greater extent than Tregs obtained from GM-CSF-treated, non-AChR immunized mice. Perhaps even more importantly, GM-CSF/AChR-induced-Tregs did not significantly affect T cell proliferation in response to an exogenous antigen (OVA). This particular result may be very relevant from a translational point of view, suggesting that if effective in MG, GM-CSF treatment may not significantly affect the immune system’s ability to respond to exogenous (foreign) antigens (i.e., infections, malignancy, etc) unlike conventional immunosuppressant drugs.
While the adoptive transfer of Tregs at the time of experimental autoimmune disease induction has been consistently shown to decrease disease severity in a number of animal models (Brusko et al., 2008; Langier et al., 2010), this strategy has been reported to be less than successful in suppressing established, chronic autoimmune diseases, including EAMG (Nessi et al., 2010). Our findings in this study are in sharp contrast to the above report, as we have shown significant suppression of ongoing EAMG after transfer of GM-CSF/AChR-induced Tregs, and a less potent (but still present) suppression of established disease using Tregs isolated from untreated EAMG mice. This differential result may be explained by a consideration of the effects of Treg subsets on antigen-specific immune responses.
CD4+ FoxP3+ Tregs can be divided into two principal subsets: 1) naturally-occurring thymus-derived natural CD4+ cells that express the α chain of the interleukin-2 receptor (CD25) (nTregs), and 2) adaptive CD4+CD25+ cells that are induced from CD25− precursors in the peripheral lymphoid organs upon exposure to antigen (iTregs) (Horwitz et al., 2008; Bluestone and Abbas, 2003). Both nTregs and iTregs share a similar phenotype, expressing CTLA-4 (cytotoxic T lymphocyte 4), GITR (glucocorticoid-induced tumor necrosis factor receptor), CCR4 (chemokine receptor) and CD62L and requiring IL-2 and TGF-β (Bluestone and Tang, 2005; Askenasy et al., 2008). While our results suggested an AChR-selective effect of GM-CSF-induced Tregs, we did not know if this was explained by an expansion of the existing nTreg repertoire (only a small fraction of which are likely to be AchR specific) or if AChR-specific T cells were converted to iTregs. While it is difficult to differentiate nTregs from iTregs based on their in vitro properties alone, since they have a similar phenotype and both can produce suppressor cytokines that inhibit Teff function in a non-antigen specific manner, only iTregs would be expected to produce selective inhibition of AChR-specific versus irrelevant Ag specific responses as we have shown for GM-CSF/AChR-induced Tregs (Figure 6).
Previous studies (Nessi et al., 2010) indicating that Tregs prevent induction of EAMG but do not improve established disease, utilized CD4+CD25+ T cells isolated from naïve animals (nTregs). The specificity of this type of Treg cell population would be anticipated to be polyclonal and likely contain a relatively small percentage of AChR-specific Tregs. It can be predicted that these Tregs might exert some suppression of an initial immune response (priming immunization), but would likely not significantly suppress an established antigen-specific immune process. Since antigenic stimulation is required for the conversion of Teff to iTregs, Tregs obtained from EAMG animals would, on the other hand, be hypothesized to have a larger proportion of iTregs with specificity for the AChR, and would therefore mediate a more efficient suppression of established EAMG. While nTregs are likely to be distributed more randomly, the iTregs, because of similar antigen specificities, are likely to co-localize with the effector T cells and thus be more effective in suppressing antigen specific immune response. Finally, larger numbers of these AChR-specific iTregs are likely to be induced in EAMG mice treated with GM-CSF, through interactions with increasing proportions of tolerogenic DCs, accounting for the enhanced potency and specificity of GM-CSF-induced Tregs. Our findings showing an enhanced in vivo suppressive effect of Tregs from GM-CSF treated AChR-primed animals compared to Tregs from GM-CSF-treated, non-AChR immunized mice (Figure 7) provides support for this proposed mechanism.
We have also shown that adoptive transfer of GM-CSF/AChR-induced Tregs can potently suppress anti-AChR antibody production in vivo (Fig 4 and 7B). This finding is in agreement with previous work demonstrating that depletion of Tregs in rodents can lead to dysregulated antibody production (Morgan et al., 2003), and the adoptive transfer of Tregs into autoimmune animals can reduce pathogenic antibody responses (Seo et al, 2002). We do not know if this suppression is primarily mediated through Treg effects on T-helper cells or possibly by a direct suppression of autoreactive B cells as has recently been reported in systemic lupus erythematosus (SLE) (Iikuni et al., 2009). A direct effect on B cells in suggested by the suppression of B cell proliferation in GM-CSF-treated mice (Fig. 2D).
Based on our previous work and the results of this study, we hypothesize that the mechanism of action of GM-CSF in EAMG involves the mobilization of “semi-mature or tolerogenic” bone-marrow derived DC precursors (Bhattacharya et al., 2011) which, upon antigen (AChR) capture, initiate a suppression of the anti-AChR immune response, through the induction/expansion of AChR-specific Tregs. Our data indicate that a concomitant exposure to antigen (AChR) (as may be the case in active or recent onset MG) is required at the time of GM-CSF treatment for optimization of AChR-specific suppression. It is noteworthy that GM-CSF is currently used widely in the treatment of neutropenia in cancer patients receiving chemotherapy, and that its safety profile and toxicology/biodistribution are well-established (Arellano and Lonial, 2008). Therefore, a clinical trial of systemically-administered GM-CSF in active or recent onset human MG is quite feasible, and is planned for the near future. Furthermore, GM-CSF could also be used to induce/expand autologous Tregs in vitro for subsequent use as cellular immunotherapy of MG.
Acknowledgments
This work was supported by the NIH (National Institute of Neurologic Disorders and Stroke, K08NS058800, MNM; and National Institute of Allergy and Infectious Diseases, RO1 AI 058190, BSP); and the Muscular Dystrophy Association (MDA 134545, MNM and MDA 157286, JRS), This project was also supported by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS), Award Number UL1RR029879 from the National Center For Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Abbreviations
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- MG
myasthenia gravis
- EAMG
experimental autoimmune myasthenia gravis
- DC
dendritic cell
- AChR
acetylcholine receptor
- Treg
regulatory T cell
- Foxp3
forkhead box p3
Footnotes
Disclosure: Authors MNM and BSP are inventors on the following patent: Prabhakar BS, Holterman MJ, Vasu C, Meriggioli MN “Uses of Bispecific Antibody Coated Dendritic Cells Pulsed With Antigen and GM-CSF in Immune Regulation, ” U.S. Patent # 7,527,972B2, May 5, 2009.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arellano M, Lonial S. Clinical uses of GM-CSF, a critical appraisal and update. Biologics. 2008;2:13–27. doi: 10.2147/btt.s1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askenasy N, Kaminitz A, Yarkoni S. Mechanisms of T regulatory cell function. Autoimmun Rev. 2008;7:370–375. doi: 10.1016/j.autrev.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya P, Gopisetty A, Ganesh B, Sheng JR, Prabhakar BS. GM-CSF-induced, bone-marrow-derived dendritic cells expand natural Tregs by different mechanisms. J Leukoc Biol. 2011;89:235–49. doi: 10.1189/jlb.0310154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nature Rev. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 6.Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol. 2005;17:638–642. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 8.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerutti A, Qiao X, He B. Plasmacytoid dendritic cells and the regulation of immunoglobulin heavy chain class switching. Immunol Cell Biol. 2005;83:554–562. doi: 10.1111/j.1440-1711.2005.01389.x. [DOI] [PubMed] [Google Scholar]
- 10.Christadoss P, Poussin M, Deng C. Animal models of myasthenia gravis. Clin Immunol. 2000;94:75–87. doi: 10.1006/clim.1999.4807. [DOI] [PubMed] [Google Scholar]
- 11.Earle KE, Tang Q, Zhou X, Liu W, Zhu S, Bonyhadi ML, Bluestone JA. In vitro expanded human CD4+CD25+ regulatory T cells suppress effector T cell proliferation. Clin Immunol. 2005;115:3–9. doi: 10.1016/j.clim.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fattorossi A, Battaglia A, Buzzonetti A, Ciaraffa F, Scambia G, Evoli A. Circulating and thymic CD4+ CD25+ T regulatory cells in myasthenia gravis: effect of immunosuppressive treatment. Immunol. 2005;116:134–41. doi: 10.1111/j.1365-2567.2005.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganesh BB, Cheatem DM, Sheng JR, Vasu C, Prabhakar BS. GM-CSF-induced CD11c+CD8a− dendritic cells facilitate Foxp3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int Immunol. 2009;21:269–82. doi: 10.1093/intimm/dxn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganesh BB, Bhattacharya P, Gopisetty A, Sheng JR, Vasu C, Prabhakar BS. IL-1β promotes TGF-β1 and IL-2 dependent FoxP3 expression in regulatory T cells. PLoS One. 2011;6(7):e21949. doi: 10.1371/journal.pone.0021949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gangi E, Vasu C, Cheatem DM, Prabhakar BS. IL-10-producing CD4+CD25+ regulatory T cells play a critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis. J Immunol. 2005;174:7006–7013. doi: 10.4049/jimmunol.174.11.7006. [DOI] [PubMed] [Google Scholar]
- 16.Gaudreau S, Guindi C, Menard M, Besin G, Dupuis G, Amrani A. Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:3638–3647. doi: 10.4049/jimmunol.179.6.3638. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23:403–408. doi: 10.1016/s1471-4906(02)02260-3. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4+CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 19.Horwitz DA, Zheng SG, Gray JD. Natural and TGF-β-induced Foxp3+ CD4+ CD25+ regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29:429–435. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Huang YM, Pirskanen R, Giscombe R, Link H, Lefvert AK. Circulating CD4+CD25+ and CD4+CD25+Tcells in myasthenia gravis and in relation to thymectomy. Scand J Immunol. 2004;59:408–14. doi: 10.1111/j.0300-9475.2004.01410.x. [DOI] [PubMed] [Google Scholar]
- 21.Iikuni N, Lourenço EV, Hahn BH, La Cava A. Cutting edge: Regulatory T cells directly suppress B cells in systemic lupus erythematosis. J Immunol. 2009;183:1518–1522. doi: 10.4049/jimmunol.0901163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langier S, Sade K, Kivity S. Regulatory T cells: the suppressor arm of the immune system. Autoimmun Rev. 2010;10:112–115. doi: 10.1016/j.autrev.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Lindstrom JM. Experimental autoimmune myasthenia gravis: induction and treatment. In: Engel A, editor. Myasthenia Gravis and Myasthenic Disorders. Oxford University Press; New York: 1999. pp. 111–130. [Google Scholar]
- 24.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 25.MacPherson G, Kushnir N, Wykes M. Dendritic cells, B cells and the regulation of antibody synthesis. Immunol Rev. 1999;172:325–324. doi: 10.1111/j.1600-065x.1999.tb01376.x. [DOI] [PubMed] [Google Scholar]
- 26.Masayuki M, Matsumoto M, Tanaka S, Nakajima K, Yamada N, Ido N, Ohtsuka T, Nishida M, Hirano T, Utsumi H. Clinical implication of peripheral CD4+ CD25+ regulatory T cells and Th17 cells in myasthenia gravis patients. J Neuroimunol. 2010;225:123–131. doi: 10.1016/j.jneuroim.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Meriggioli MN, Sheng JR, Li L, Prabhakar BS. Strategies for treating autoimmunity: novel insights from experimental myasthenia gravis. Ann NY Acad Sci. 2008;1132:276–282. doi: 10.1196/annals.1405.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meriggioli MN, Sanders DB. Myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 2009;8:475–90. doi: 10.1016/S1474-4422(09)70063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moes N, Rieux-Laucat F, Begue B, Verdier J, Neven B, Patey N, Torgerson TT, Picard C, Stolzenberg MC, Ruemmele C, Rings EH, Casanova JL, Piloquet H, Biver A, Breton A, Ochs HD, Hermine O, Fischer A, Goulet O, Cerf-Bensussan N, Ruemmele FM. Reduced expression of FOXP3 and regulatory T-cell function in severe forms of early-onset autoimmune enteropathy. Gastroenterology. 2010;139:770–8. doi: 10.1053/j.gastro.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Morgan ME, Sutmuller RP, Witteveen HJ, van Duivevoorde LM, Zanelli E, Meleif CJ, Snijders A, Offringa R, de Vries RR, Toes RE. CD25+ depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452–1460. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- 31.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 32.Nessi V, Nava S, Ruocco C, Toscani C, Mantegazza R, Antozzi C, Baggi F. Naturally occuring CD4+CD25+ regulatory T cells prevent but do not improve experimental myasthenia gravis. J Immunol. 2010;185:5656–5667. doi: 10.4049/jimmunol.0903183. [DOI] [PubMed] [Google Scholar]
- 33.Parmiani G, Castelli C, Pilla L, Santiami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2007;18:226–232. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- 34.Pulendran B, Banchereau J, Burkeholder S, Kraus E, Guinet E, Chalouni C, Caron D, Maliszewski C, Davoust J, Fay J, Palucka K. Flt3-Ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J Immunol. 2000;165:566–572. doi: 10.4049/jimmunol.165.1.566. [DOI] [PubMed] [Google Scholar]
- 35.O’Keefe M, Hochrein H, Vremec D, Pooley J, Evans R, Woulfe S, Shortman K. Effects of administration of progenipoietin 1, Flt3 ligand, granulocyte colony-stimulating, and pegylated granulocyte colony-stimulating factor on dendritic cell subsets in mice. Blood. 2002;99:2122–2130. doi: 10.1182/blood.v99.6.2122. [DOI] [PubMed] [Google Scholar]
- 36.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologoic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 37.Seo SJ, Fields ML, Buckler JL, Reed AJ, Mandik-Nayak L, Nish SA, Noelle RJ, Turka LA, Finkelman FD, Caton AJ, Erickson J. The impact of T helper and T regulatory cells in the regulation of anti-double-stranded DNA B cells. Immunity. 2002;16:535–546. doi: 10.1016/s1074-7613(02)00298-4. [DOI] [PubMed] [Google Scholar]
- 38.Sheng JR, Li LC, Ganesh BB, Vasu C, Prabhakar BS, Meriggioli MN. Suppression of experimental autoimmune myasthenia gravis (EAMG) by Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) is associated with an expansion of FoxP3+ regulatory T cells. J Immunol. 2006;177:5296–306. doi: 10.4049/jimmunol.177.8.5296. [DOI] [PubMed] [Google Scholar]
- 39.Sheng JR, Li L, Ganesh BB, Prabhakar BS, Meriggioli MN. Regulatory T cells induced by GM-CSF suppress ongoing experimental myasthenia. Clin Immunol. 2008;128:172–180. doi: 10.1016/j.clim.2008.03.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torgerson TR. Regulatory T cells in human autoimmune diseases. Springer Semin Immunopathol. 2006;28:63–76. doi: 10.1007/s00281-006-0041-4. [DOI] [PubMed] [Google Scholar]
- 41.Vasu C, Dogan RE, Holterman MJ, Prabhakar BS. Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor-3 ligand, activates thyroglobulin-specific CD4+/CD25+ T cells and suppresses experimental autoimmune thyroiditis. J Immunol. 2003;170:5511–5522. doi: 10.4049/jimmunol.170.11.5511. [DOI] [PubMed] [Google Scholar]
- 42.Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens JL, Medaer R, Hupperts R, Stinissen P. Compromised CD4+CD25high regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2008;123:79–89. doi: 10.1111/j.1365-2567.2007.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vincent A. Unraveling the pathogenesis of myasthenia gravis. Nat Rev Immunol. 2002;2:797–804. doi: 10.1038/nri916. [DOI] [PubMed] [Google Scholar]
- 44.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M, Yagita H. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–45. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 45.Yokoyama T, Matsuda S, Takae Y, Wada N, Nishikawa T, Amagai M, Koyasu S. Antigen-indeppendnet development of Foxp3+ regulatory T cells suppressing autoantibody production in experimental pemphigus vulgaris. Int Immunol. 2011;23:365–373. doi: 10.1093/intimm/dxr020. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Wang H, Chi L, Wang W. The role of FoxP3+CD4+CD25hi Tregs in the pathogenesis of myasthenia gravis. Immunol Lett. 2009;122:52–57. doi: 10.1016/j.imlet.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 47.Zou GM, Tam YK. Cytokines in the generation and maturation of dendritic cells: recent advances. Eur Cytokine Netw. 2002;13:186–199. [PubMed] [Google Scholar]